Abstract
BACKGROUND AND PURPOSE: A potential role of perfusion CT (PCT) in selecting patients with stroke for reperfusion therapies has been recently advocated. The purpose of the study was to assess the reliability of PCT in predicting clinical outcome of patients with acute ischemic stroke treated with intra-arterial thrombolysis (IAT).
MATERIALS AND METHODS: Twenty-seven patients with acute hemispheric ischemic stroke were investigated with PCT and treated with IAT between 3 and 6 hours of stroke onset. The infarct core was outlined on cerebral blood volume (CBV) maps by using accepted viability thresholds. The penumbra was defined as time-to-peak (TTP)-CBV mismatch. Clinical outcome was assessed by modified Rankin Scale (mRS) scores at 3 months and dichotomized into favorable (mRS score, 0–2) and unfavorable (mRS score, 3–6). Data were retrospectively analyzed by multiple regression to identify predictors of clinical outcome among the following variables: age, sex, National Institutes of Health Stroke Scale score, serum glucose level, thrombolytic agent, infarct core and mismatch size, collateral circulation, time to recanalization, and recanalization rate after IAT.
RESULTS: Patients with favorable outcome had smaller cores (P = .03), increased mismatch ratios (P = .03), smaller final infarct sizes (P < .01), higher recanalization rates (P = .03), and reduced infarct growth rates (P < .01), compared with patients with unfavorable outcome. The core size was the strongest predictor of clinical outcome in an “all subset” model search (P = .01; 0.96 point increase in mRS score per any increment of 1 SD; 95% confidence interval, +0.17 to +1.75).
CONCLUSIONS: PCT is a reliable tool for the identification of irreversibly damaged brain tissue and for the prediction of clinical outcome of patients with acute stroke treated with IAT.
Neuroimaging techniques have been proposed for selecting patients with acute ischemic stroke who might benefit from thrombolysis.1,2 Multimodal imaging may provide relevant information that improves the diagnosis of acute ischemic stroke by enabling assessment of vessel status, such as site of occlusion, tissue damaged and potentially at risk, and other features, with good accuracy and, in turn, may have an impact on treatment decisions and clinical outcome.
Intra-arterial thrombolysis (IAT) might be beneficial up to 6 hours from stroke onset, especially in patients with severe stroke caused by occlusion of major intracranial vessels such as the middle cerebral artery (MCA) stem, internal carotid artery (ICA), or basilar artery. The higher recanalization rates obtained with IAT, compared with intravenous thrombolysis,3 do not necessarily correlate with clinical outcome, probably because other factors such as depth and duration of the ischemia, baseline stroke severity, collateral circulation, lesion location, and lesion volume play a major role in recovery of function.
It has already been demonstrated that perfusion CT (PCT) is capable of accurately identifying the potentially salvageable tissue that can be rescued by thrombolysis,4 and the incidence of hemorrhagic complications after chemical thrombolysis can be reduced with a better selection of patients based on brain perfusion studies.5 Available data suggest that perfusion assessment with CT might also be used to identify effectively patients in whom thrombolysis can be safely administered after the typical 3-hour window.
The purpose of the present study was to assess the reliability of PCT in predicting follow-up clinical outcome in patients with acute ischemic stroke investigated beyond the 3-hour window and treated with IAT.
Materials and Methods
Patient Selection and Study Design
We retrospectively reviewed clinical and imaging findings of 42 patients consecutively admitted to the Department of Neurology, University of Brescia, between January 2002 and December 2006. Patients were eligible for inclusion in the study if they had received a clinical diagnosis of acute hemispheric stroke, a noncontrast cerebral CT (NCCT) had excluded an intracranial hemorrhage, they were able to receive IAT 3–6 hours after the onset of symptoms, and they had undergone first-pass double-section PCT. Imaging and treatment were performed according to the hospital guidelines for suspected acute stroke based on the current European Stroke Initiative recommendations.6
IAT was performed in those cases of acute cerebral ischemia fulfilling the following criteria: involvement of the MCA territory, early CT hypoattenuation area less than one third of the MCA territory, National Institutes of Health Stroke Scale (NIHSS) score on admission ≥10, and no contraindications to thrombolysis. NIHSS scores were recorded at the time of admission to the emergency department. Patient outcome was assessed by the modified Rankin Scale (mRS) 3 months after stroke onset by examiners blinded to image-volume analysis, and scores were dichotomized into favorable (mRS score, 0–2) and unfavorable (mRS score, 3–6). Written informed consent was obtained from the patients or next of kin. The study was approved by the local institutional ethics committee.
Imaging Techniques
Baseline NCCT was performed in all patients on admission before treatment, with coverage from the skull base to the vertex (5-mm section thickness). CT examinations were performed on a spiral CT scanner (Plus 4; Siemens, Erlangen, Germany) and on a 16-row multidetector system (Sensation 16; Siemens) with a 50% reduction of dose in comparison with a standard CT examination (80 kilovolt [peak], 165 mA). Section orientation of PCT was chosen individually.
PCT was performed before IAT and consisted of two 50-second series of a single 8-mm-thick section (first 16 patients) and of 4 adjacent 6-mm thick sections (last 11 patients) at the first interval during a bolus injection of 40 mL of iodinated contrast medium (300 mg iodine/mL; Iomeron, Bracco, Milan, Italy) in an antecubital vein with a power injector at constant flow (8 mL/s). Each patient, therefore, received two 40-mL boluses of contrast medium at an interval of 5 minutes for a total of 100 scans.
Follow-up CT was performed at 72 hours from the acute event. All neuroimaging data were independently reviewed by 2 neuroradiologists (D.M., M.F.) blinded to the patient's clinical history and outcome.
IAT
IAT was performed by 2 experienced interventional neuroradiologists, with manual injection of the thrombolytic agent. Because no form of thrombolytic therapy was approved in Italy until intravenous administration of recombinant tissue plasminogen activator (rtPA) was approved in August 2004 for the treatment of patients within 3 hours of onset from ischemic stroke, urokinase was the thrombolytic agent of choice until that date, whereas rtPA (0.9 mg per kilogram of body weight) was used thereafter.
The thrombolytic agent was directly injected into or near the proximal end of the occluding thrombus for 60–120 minutes. Aspiration or mechanical disruption of the clot or both were carried out in addition to pharmacologic thrombolysis. After completion of the 4-vessel diagnostic angiography, systemic heparinization was achieved with an intravenous bolus of heparin (2000 IU), followed by 500 IU/h. Antiplatelet agents were not regularly given before IAT, but some patients were on aspirin at admission to the hospital. The procedure was stopped after complete recanalization or after reaching the maximum dose of 1,200,000 IU of urokinase or 90 mg of rtPA. In 2 patients with no recanalization after reaching the maximal dose of the thrombolytic agent, a percutaneous transluminal angioplasty was performed by using a balloon dilation microcatheter (balloon diameter, 2.00 mm; Wordpass, Cordis, Miami Lakes, Fla). In 3 patients with ICA thrombosis and carotid T occlusion (CTO), a self-expandable stent was inserted in the carotid bifurcation at the end of thrombolysis.
On the basis of standard intra-arterial digital subtraction angiography (IADSA) imaging data obtained before treatment, the occluded levels were classified as the following: 1) isolated CTO or ICA thrombosis associated with CTO; 2) ICA thrombosis associated to M1 occlusion; and 3) MCA occlusion, proximal M1 occlusion, or M1 bifurcation occlusion. Collateral circulation was classified as leptomeningeal collaterals with the following: 1) complete retrograde filling up to M1 (type 1), 2) incomplete retrograde filling up to M2 branches (type 2), and 3) poor retrograde filling (type 3).7 Treatment effect was documented by standard IADSA at the end of the procedure. The rate of recanalization was assessed according to the Thrombolysis in Myocardial Infarction (TIMI) score.8 Patients were dichotomized into a subgroup with no recanalization (TIMI, 0–1) and a subgroup with successful recanalization (TIMI, 2–3). Collateral circulation was dichotomized into good (types 1 and 2) and poor (type 3).
Imaging Processing
For the analysis of PCT data, 2 levels were explored in each patient: The first included the basal ganglia, anterior cerebral artery (ACA), MCA, and posterior cerebral artery territories; the second, located more cranially, included the distal MCA and ACA territories. The analysis was performed with syngo Neuro Perfusion CT software (Siemens), which is based on the “maximum slope model.” Cerebral blood flow (CBF) was calculated as the maximum slope of the time/attenuation curve derived from each tissue voxel divided by the maximum enhancement within the brain-supplying artery. To avoid partial volume averaging, one can replace the latter by the maximum enhancement within the superior sagittal sinus, which was, therefore, selected for the calculation of the arterial input function. The software does not require a venous output function, and the error caused by this approximation is relatively small.9 Cerebral blood volume (CBV) was calculated as the ratio between the peak tissue enhancement and the peak enhancement in the superior sagittal sinus. The time to peak (TTP) was defined as the time lag between the first arrival of the contrast agent within major arterial vessels included in the section and the local bolus peak in the brain tissue. The image scale was set at 0%–6%, 0–100 mL/100 g/min, and 0–20 seconds for CBV, CBF, and TTP color-coded maps, respectively.
To achieve a comparable evaluation of the PCT maps obtained with the 2 CT scanners, we selected only the 2 sections corresponding to the same levels from the 16-row CT examination.
The hypoperfused areas were outlined on CBV and TTP maps. Mirror regions in the normally perfused contralateral hemisphere were automatically outlined by the software and were used to calculate the mean CBV and CBF ratio of the affected brain tissue and the TTP delay as a difference (ΔTTP). CBV was assessed semiquantitatively by using the results from mirrored regions within the contralateral hemisphere as a reference. The infarct core was outlined on CBV maps as a severely hypoperfused area displayed by 2 colors in the color bar (eg, violet and blue), corresponding to a CBV ratio <60% of the contralateral normally perfused hemisphere as suggested by previous findings by using the same model.10
The region of interest was automatically transferred to CBF maps to display the absolute CBF values. A CBF threshold of <12.7 mL/100 g/min was used to discriminate between infarcted and noninfarcted tissue, according to the recently proposed validated thresholds, to separate the ischemic penumbra from the infarct core.11 If the mean absolute CBF values of the region of interest were above the threshold, the region of interest was outlined again to achieve a more precise delimitation of the core.
The total perfusion deficit, including ischemic penumbra and infarct core, was outlined on TTP maps, by using a ΔTTP value of >4 seconds compared with the contralateral side. This value has been demonstrated to be a useful estimate of ischemic tissue with a CBF value of <20 mL/100 g/min,12 a reliable threshold capable of discriminating oligemia from penumbra.
To identify the ischemic penumbra, we chose the TTP-CBV mismatch, which has recently been proposed as a reliable model to predict the development of brain infarcts.13 We defined a TTP-CBV mismatch as a TTP abnormality greater than its related CBV abnormality. Mismatch was quantitatively assessed as (TTP area − CBV area / TTP area) × 100 = percentage mismatch. The areas of abnormality on TTP and CBV maps were summed, then multiplied by the section thickness to derive lesion volumes in milliliters. Follow-up CT was coregistered to the initial plain CT, by using an affine algorithm (Rasband WS, ImageJ; National Institutes of Health, Bethesda, Md), and the infarcted area was outlined on the sections corresponding to the 2 levels scanned with PCT. An infarct growth rate was calculated by dividing the final infarct size by the initial perfusion deficit volume identified on TTP maps.
Statistical Analysis
We applied the Student t test for variables with normal distribution (age, serum glucose level, NIHSS score, final infarct size, infarct growth, total perfusion deficit, penumbra size, and time to recanalization), the Mann-Whitney U test for variables with non-normal distribution (infarct core size, and time to IAT) for continuous variables, and 2-sided Fisher exact test for categoric variables (sex, thrombolytic agent, dichotomized outcome, recanalization, collateral circulation, and occlusion type). Differences between >2 variables were analyzed with 1-way analysis of variance (ANOVA). A 2-tailed P value < .05 was considered statistically significant. Pearson correlation coefficients were calculated to evaluate bivariate associations between variables.
A multiple linear regression analysis with an “all subset” model-selection procedure was performed to select baseline predictors of clinical outcome. The model included mRS scores at 3 months as outcome measures and the following 10 potential predictors: age, sex, serum glucose level, and NIHSS score on admission, thrombolytic agent, time to recanalization, infarct core size (in milliliters), mismatch size (percentage), collateral circulation, and recanalization rate after IAT.
The searching procedure identifies the best model among those obtained by all the combinations of the 9 potential predictors (ie, the sequence of all subsets with 1 predictor, all subsets with 2 predictors, and so on). The best model is the one that gives the lowest Cp, according to the Mallows’ Cp model selection criteria.14 Regression parameter and 95% confidence intervals (95% CIs) of the selected model were computed. P values of the null (zero) regression parameter estimate were evaluated by a t test. The regression parameter was expressed as the average change of the outcome measure per 1 SD change of the predictor variable. Inter-rater reliability was tested for measured volumes as a continuous variable by using an intraclass correlation coefficient, with 1-way ANOVA. Data analyses were performed with the Statistical Package for the Social Sciences for Windows, Version 15.0 (SPSS, Chicago, Ill) and XLStat 2007 (http://www.xlstat.com/en/products/xlstat-life/).
Results
After exclusion of 15 eligible patients who did not meet the established criteria for the study (3 incomplete imaging, 7 inadequate imaging, and 5 lost to follow-up), data from 27 patients were entered into the final analysis.
Angiography revealed CTO in 6 patients, ICA thrombosis in 5 patients (2 with associated CTO and 3 with associated MCA occlusion), and MCA occlusion in 16 patients (11 proximal, 5 distal to the origin of the lenticulostriate arteries). A mean dose of 605,000 IU of urokinase (range, 10,000 to 1,200,000 IU) was administered to the first 17 patients, and a mean dose of 45.5 mg of rtPA (range, 15–90 mg) was given to the last 10 patients. Recanalization in the 27 patients was categorized as TIMI 0 in 3 patients (11%), TIMI 1 in 4 (15%), TIMI 2 in 11 (40.7%), and TIMI 3 in 9 (33.3%). Two patients (7.4%) had a symptomatic intracerebral hemorrhage, and 7 (26%) had hemorrhagic transformations without clinical deterioration.
PCT showed a perfusion deficit in TTP maps in at least 1 of the 2 levels in all 27 patients. PCT values are reported in Table 1. The infarct core size correlated with mRS score (r = 0.604, P = .001) and final infarct size (r = 0.79, P < .001). The infarct growth correlated with mRS score (r = 0.49, P = .008) (Table 2).
Table 1:
Perfusion CT values in the infarct core and ischemic penumbra
| Core | Penumbra | P Value | |
|---|---|---|---|
| CBV ratio | 0.25 ± 0.09 | 0.81 ± 0.17 | <.001 |
| CBF (mL/100 g/min) | 6.9 ± 2.8 | 25.9 ± 2.7 | <.001 |
| CBF ratio | 0.14 ± 0.05 | 0.49 ± 0.06 | <.001 |
| ΔTTP (s) | 6.5 ± 2.63 | 4.32 ± 1.54 | <.01 |
Note:—CBV indicates cerebral blood volume; CBF, cerebral blood flow; TTP, time to peak.
Table 2:
Correlations between imaging data and clinical scores
| Core | Final Infarct | Infarct Growth | NIHSS Score | mRS Score | |
|---|---|---|---|---|---|
| Core | 1 | 0.793 | 0.401 | 0.408 | 0.604 |
| P < .001 | P = .038 | P = .035 | P = .001 | ||
| Final infarct | 0.793 | 1 | 0.788 | 0.56 | 0.73 |
| P < .001 | P < .001 | P = .002 | P < .001 | ||
| Infarct growth | 0.401 | 0.788 | 1 | 0.493 | 0.499 |
| P = .038 | P < .001 | P = .009 | P = .008 | ||
| NIHSS score | 0.408 | 0.56 | 0.493 | 1 | 0.545 |
| P = .035 | P = .002 | P = .009 | P = .003 | ||
| mRS score | 0.604 | 0.73 | 0.499 | 0.545 | 1 |
| P = .001 | P < .001 | P = .008 | P = .003 |
Note:—NIHSS indicates National Institutes of Health Stroke Scale; mRS, modified Rankin score.
CTO was characterized by a significantly larger infarct core (20.7 ± 17.1 mL; 95% CI, 9.3–32.2 versus 10.8 ± 7.4; 95% CI, 6.8–14.8; P = .05) and poorer outcome (mRS score, 3.4 ± 1.7 versus 2 ± 1.3, P = .028) compared with MCA occlusions. At 3 months, the median mRS score was 2.63. Twelve patients (44.4%) had favorable outcome (mRS score, 0–2), 15 (55.6%) had unfavorable outcome (mRS score, 3–6; 2 patients [7.4%] died, 1 from causes unrelated to the vascular event). Patients with favorable outcome were younger, had lower NIHSS scores, smaller cores, increased mismatch ratios, smaller final infarct sizes, reduced infarct growth rates, and higher recanalization rates compared with patients with poor outcome (Table 3 and Fig 1A).
Table 3:
Demographic, clinical, and imaging data according to clinical outcome
| Favorable Outcome (n = 12) | Unfavorable Outcome (n = 15) | P Value | |
|---|---|---|---|
| Age (year) | 46.4 ± 13.9 | 58.7 ± 15.2 | .034 |
| Sex (M/F) | 8/4 | 5/10 | n.s. |
| NIHSS score | 15 ± 4 | 21 ± 5 | <.01 |
| Serum glucose level (mg/dL) | 135.6 ± 38.1 | 136.7 ± 29.7 | n.s. |
| Core (mL) | 8.6 ± 7.8 | 19.8 ± 14.5 | .03 |
| TTP/CBV mismatch ratio (%) | 75.4 ± 19.8 | 55.8 ± 23 | .03 |
| Infarct volume (mL) | 11.3 ± 11.2 | 36.8 ± 15.7 | <.01 |
| Infarct growth (%) | 31.2 ± 28 | 90 ± 45 | <.01 |
| Time to IAT (min) | 61.2 ± 36.5 | 66.6 ± 35.9 | n.s. |
| Time to recanalization (min) | 338 ± 50 | 371 ± 36 | n.s |
| Recanalization rate (TIMI, 2–3) | 11/12 | 8/15 | .03 |
| Symptomatic hemorrhage | 1 | 1 | |
| Thrombolytic agent | n.s | ||
| UK | 6/17 | 11/17 | |
| rtPA | 6/10 | 4/10 | |
| ASPECTS | 9 | 8.6 | n.s. |
Note:—n.s. indicates not significant; UK, urokinase; IAT, intra-arterial thrombolysis; rtPA, recombinant tissue plasminogen activator; ASPECTS, Alberta Stroke Program Early CT Score.
Fig 1.
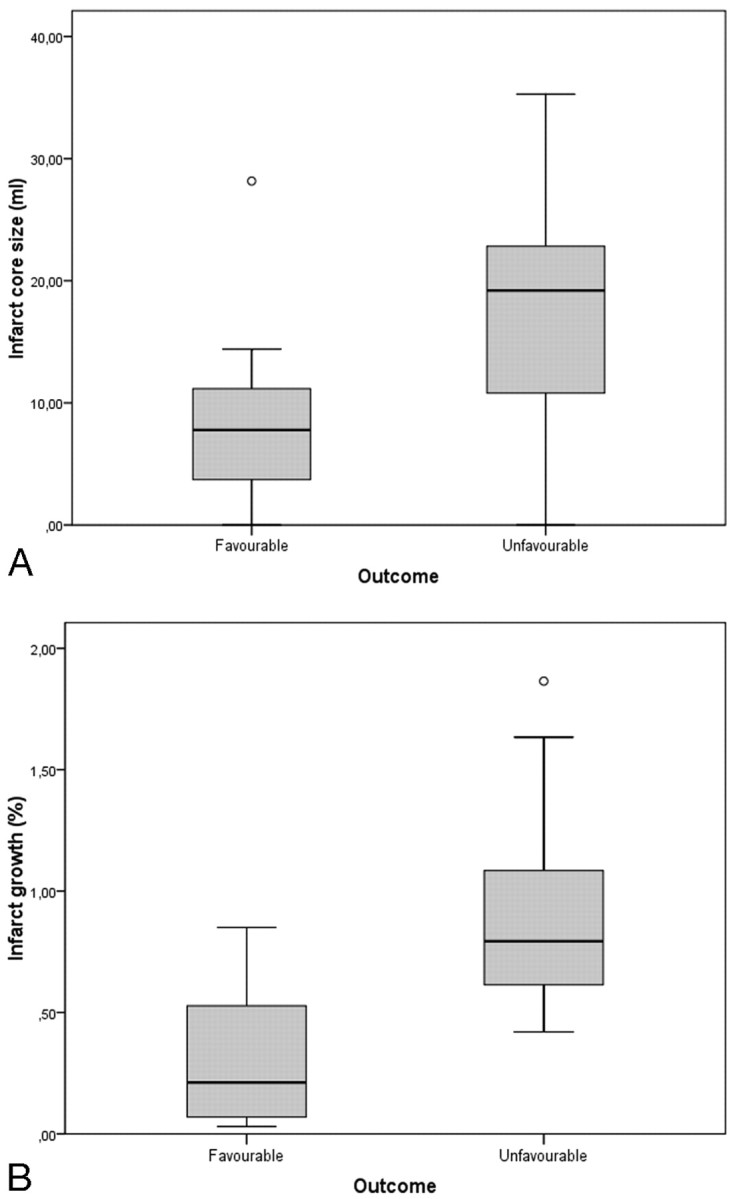
Box and whisker graphs of infarct core size according to clinical outcome (A) and infarct growth according to recanalization after IAT (B).
In patients with successful recanalization after IAT (TIMI, 2–3), the final infarct size was significantly smaller (P = .02) and the infarct growth rate was significantly reduced (P = .03), compared with patients with no recanalization (Fig 1B and Fig 2).
Fig 2.
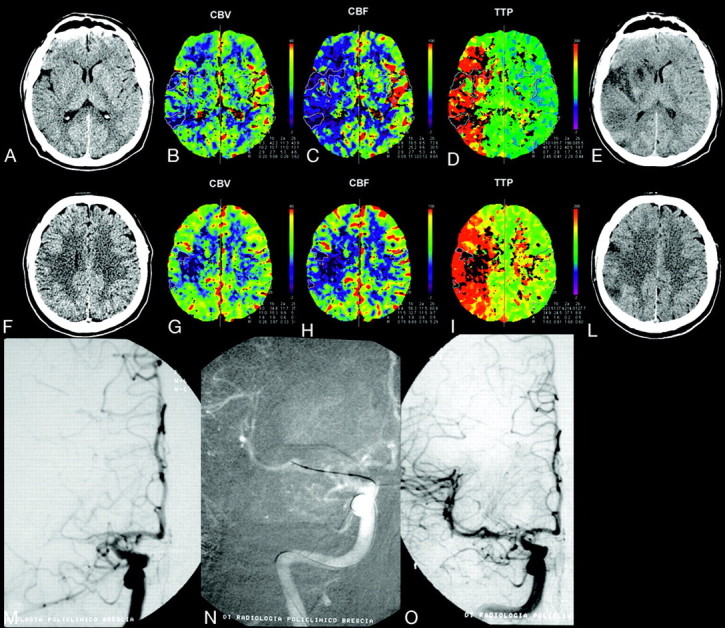
A 35-year-old man with left hemiplegia, imaged 3 hours after symptom onset. NCCT, CBV, CBF, and TTP maps, delayed NCCT (A−E, first level; F−L, second level); and IAT (M−O). NCCT shows mild hypoattenuation of the right lenticular nucleus. PCT color maps show small multiple areas with severely reduced CBV (CBV ratio <33%) and CBF (<11.5 mL/100 g/min) in the anterior third of the right lenticular nucleus; external capsule; and fronto-opercular, insular, posterior temporal and parietal cortices, corresponding to the infarct core (solid line). The infarct core is surrounded by a large perfusion deficit in the right MCA cortical territory, characterized by reduced CBF (color-coded blue) and increased TTP (color-coded red), indicating a TTP-CBV mismatch, which corresponds to the ischemic penumbra. IADSA, performed after PCT, shows proximal right M1 occlusion (M), with poor collateral leptomeningeals. The patient underwent intra-arterial thrombolysis with injection of rtPA and mechanical clot manipulation, followed by percutaneous transluminal angioplasty, with complete right MCA recanalization (N). A mild residual M1 stenosis is identifiable at the end of the procedure (O). Follow-up CT scans (E and L), obtained 2 days after stroke, show small multiple infarcts in the right caudate and anterior third of the lenticular nucleus; insular cortex; and temporal, frontal, and parietal cortices, which correspond to the infarct core, with consequent recovery of a large portion of the mismatch area. At 3 months, the patient was independent (mRS score, 1).
The time to recanalization was not significantly different in the 2 subgroups treated with different thrombolytic agents.
On the basis of the results of the “all subset” search procedure, the subset including core size, NIHSS score, and sex as predicting variables turned out to be the best predictor of clinical outcome (Table 4). The model equation explained 59.5% of the total variance of mRS scores. The infarct core was the strongest predictor of clinical outcome. In particular, any increment of 1 SD (13.2 mL) of the infarct core size increased the mRS score by 0.96 points (95% CI, +0.17 to +1.75), whereas any increment of 1 SD (5.8 points) of admission NIHSS score raised the mRS by 0.47 points (95% CI, 0.13–1.08). Male gender (95% CI, −2.1 to −0.1) decreased the mRS score by −1.06 points.
Table 4:
Multiple regression analysis of the best model from an “all subset” model selection (R2 = 74.6%)*
| Change X | Change Y | 95% CI | P Value | |
|---|---|---|---|---|
| Core (mL) | 1 SD = 13.21 | +0.96 | 0.17–1.75 | .01 |
| NIHSS | 1 SD = 5.8 | +0.47 | 0.13–1.08 | .05 |
| Sex (male) | 1 SD = 1 | −1.06 | −2.1 to −0.1 | .035 |
Note:—X indicates predictors (core, NIHSS score, sex); Y, outcome measure (mRS score).
The regression parameter is expressed as the average change of the outcome measure per 1 SD change of the predictor variable.
The inter-rater reliability was high for all the measured volumes (α = 0.92 for final infarct volume, α = 0.90 for core size, α = 0.85 for total perfusion deficit size).
Discussion
Because of the reliability of CT-based techniques in estimating infarct core and penumbra, it seems logical to assume a role for PCT in selecting patients with stroke for reperfusion therapies and in predicting recovery. The results of the present analysis support the assumption that a simple method to interpret PCT imaging, based on a multiparametric assessment of perfusion deficits on CBF, CBV, and TTP maps and characterized by high inter-rater reliability for the assessment of lesion volumes, is a reliable tool for predicting clinical outcome after IAT. In particular, the infarct core size, as defined in the present study, seems to be the strongest predictor of clinical outcome in patients with stroke treated with IAT, compared with the other PCT measures. Stated another way, our findings support the idea that the size of the infarct core is a more relevant determinant of the severity of clinical outcome than penumbra and total perfusion deficit. This is in agreement with the results of previous PCT-based studies showing that the core size identified on CT angiography source images, a surrogate marker of the perfused brain volume, is the most significant predictor of clinical outcome15 and with recent findings, based on the application of the Alberta Stroke Program Early CT Score (ASPECTS) to PCT maps suggesting that CBV is the best predictor of favorable outcome.16
The results of the present study also have a potential impact on interpreting the effect of reperfusion treatment in the acute phase of ischemic stroke. In one of the largest series of patients treated with IAT reported so far, a low NIHSS score on admission and vessel recanalization were independently associated with excellent or good outcome.17 Although early recanalization has consistently been demonstrated as a powerful predictor of favorable long-term outcome after thrombolysis,18,19 approaches based on recanalization as a primary outcome measure have been recently criticized.20 In line with these observations, our results prompt speculation that some patients with stroke experiencing little or no improvement despite induced recanalization might have a larger core size, which can be easily detected by PCT.
Finally, an indirect finding of the present analysis is the observation that IAT has an influence on infarct growth. As demonstrated by the relation between IAT and infarct growth rate, a useful measure of the efficacy of recanalization, the core size of patients with successful recanalization was not significantly different from that of patients with absent or poor recanalization, whereas the final infarct was significantly smaller and the infarct growth rate significantly reduced, indicating a successful effect of IAT in rescuing the ischemic penumbra (Fig 2).
Study Limitations
Although our study provides a great deal of information on the reliability of PCT in predicting clinical outcome in patients with acute ischemic stroke, several limitations should be noted. First, the number of patients is small. In this regard, a larger sample would likely reduce the variance seen in our study and make our results more stable.
Second, with a PCT based on the maximum slope model, the absolute quantification of CBF can be theoretically inaccurate compared with the other techniques currently used, which are based on the central volume principle and deconvolution analysis. The absolute CBF values of the core, CBV, and CBF ratios with respect to the normally perfused contralateral areas are lower than previously reported and might have been underestimated. However, taking into account that the outlined infarct core also included the severely hypoperfused white matter, we believe that our values are comparable with the results of prior studies, which have limited the PCT data analysis to the cortical areas10,21 or have demonstrated different ischemic thresholds for gray and white matter.11,22 The injection rate of 8 mL/s might have possibly contributed to the achievement of a more reliable CBF estimation. The TTP maps are also characterized by inaccurate quantification. In fact, with our simplified approach, the possibility of an overestimation of the ischemic penumbra cannot be ruled out.16
Third, because our PCT technique was based on a 2-section evaluation of the brain, the estimation of both penumbra and infarct may be an approximation of the real volume. However, because of the correlation between abnormal areas on PCT and abnormal volumes on MR perfusion images, PCT volumes may be retained as a good estimate of the full extent of the ischemic tissue.23
Overall, although the implications of these potential drawbacks are noteworthy, it seems unlikely that they have significantly altered the results of our study.
Conclusions
A simple method to interpret PCT imaging, based on a multiparametric assessment of perfusion deficits, may be of help in the management of patients with acute stroke, enabling a reliable assessment of the amount of irreversibly damaged brain tissue and, indirectly, of clinical outcome. In particular, the infarct core size, as defined in the present study, seems to be the most reliable predictor of clinical outcome in patients with stroke treated with IAT, when compared with the other PCT measures.
Acknowledgments
We thank Marco Ciccolella for his support in preparing the illustrations.
References
- 1.Thomalla G, Schwark C, Sobesky J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke 2006;37:852–58. Epub 2006 Jan 26 [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Meuli R, Browaeys P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology 2007;68:694–97 [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, del Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews. 2003. :CD000213. Available at: http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD000213/frame.html. Accessed January 9, 2009 [DOI] [PubMed]
- 4.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–32 [DOI] [PubMed] [Google Scholar]
- 5.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 [DOI] [PubMed] [Google Scholar]
- 6.Olsen TS, Langhorne P, Diener HC, et al, for the European Stroke Initiative Executive Committee and the EUSI Writing Committee. European Stroke Initiative recommendations for stroke management: update 2003. Cerebrovasc Dis 2003;16:311–37 [DOI] [PubMed] [Google Scholar]
- 7.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 2003;45:11–18. Epub 2002 Dec 7 [DOI] [PubMed] [Google Scholar]
- 8.Ganz W. The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med 1985;313:1018. [DOI] [PubMed] [Google Scholar]
- 9.Klotz E, Konig M. Perfusion measurements of the brain: using dynamic CT for the quantitative assessment of cerebral ischemia in acute stroke. Eur J Radiol 1999;30:170–84 [DOI] [PubMed] [Google Scholar]
- 10.Koenig M, Kraus M, Theek C, et al. Quantitative assessment of the ischemic brain by means of perfusion-related parameters derived from perfusion CT. Stroke 2001;32:431–37 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer PW, Roccatagliata L, Ledezma C, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. AJNR Am J Neuroradiol 2006;27:20–25 [PMC free article] [PubMed] [Google Scholar]
- 12.Sobesky J, Zaro WO, Lehnhardt FG, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke 2004;35:2843–47 [DOI] [PubMed] [Google Scholar]
- 13.Muir KW, Halbert HM, Baird TA, et al. Visual evaluation of perfusion computed tomography in acute stroke accurately estimates infarct volume and tissue viability. J Neurol Neurosurg Psychiatry 2006;77:334–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallows CL. Some comments on Cp. Technometrics 1973;15:661–75 [Google Scholar]
- 15.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 2001;32:2021–28 [DOI] [PubMed] [Google Scholar]
- 16.Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 2005;58:672–79 [DOI] [PubMed] [Google Scholar]
- 17.Arnold M, Schroth G, Nedeltchev K, et al. Intra-arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke 2002;33:1828–33 [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov AV, Burgin WS, Demchuk AM, et al. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation 2001;103:2897–902 [DOI] [PubMed] [Google Scholar]
- 19.Felberg RA, Okon NJ, El-Mitwalli A, et al. Early dramatic recovery during intravenous tissue plasminogen activator infusion: clinical pattern and outcome in acute middle cerebral artery stroke. Stroke 2002;33:1301–07 [DOI] [PubMed] [Google Scholar]
- 20.Wechsler LR. Does the Merci retriever work? Against. Stroke 2006;37:1341–42, discussion 1342–43. Epub 2006 Apr 6 [DOI] [PubMed] [Google Scholar]
- 21.Mayer TE, Hamann GF, Baranczyk J, et al. Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol 2000;21:1441–49 [PMC free article] [PubMed] [Google Scholar]
- 22.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 [DOI] [PubMed] [Google Scholar]
- 23.Eastwood JD, Lev MH, Wintermark M, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. AJNR Am J Neuroradiol 2003;24:1869–75 [PMC free article] [PubMed] [Google Scholar]


