Abstract
BACKGROUND AND PURPOSE: CT angiography (CTA) is widely used and may be the only vascular imaging technique ordered for emergent evaluation of neurovascular disease. With thin-section multisection CTA, the resolution of vessel wall imaging has improved. We describe cases of acute vertebral artery dissection (VAD) in which the only abnormality on CTA was a characteristic thickening of the wall of the V3 portion of the vertebral artery (VA). The arterial lumen at the dissection site was normal in caliber. This type of dissection is easily overlooked if only lumen-opacifying studies such as contrast MR angiography (MRA) or conventional angiography are performed. We highlight the importance of recognizing this finding, the “suboccipital rind” sign, in the V3 portion, a segment commonly affected in VAD. The purpose of our study was to review the CTA imaging characteristics of patients with VAD in the V3 portion compared with normal controls.
MATERIALS AND METHODS: Our imaging data base was reviewed for cases of acute VAD and the presence of a “suboccipital rind” sign. A control group of 50 patients was randomly recruited from a group of patients undergoing CTA. The VA luminal diameter, the wall thickness (total diameter−luminal diameter), and the ratio of luminal diameter/total diameter were measured along 5 adjacent V3 segments and were compared between the 2 groups.
RESULTS: There was no evidence of luminal tapering or narrowing in the dissected VAs compared with controls (P = .1). The average wall thickness of the dissection group was 2.96 mm greater than that for the control group (P < .001; 95% confidence interval, 2.6–3.3). There was a significant difference in the ratio of lumen diameter/lumen+wall diameter in dissected segments compared with controls (P < .001).
CONCLUSIONS: Cross-sectional vascular imaging is often performed with multisection helical CTA for a variety of concerns, some without neurologic symptoms. Our study confirms that in cases of the “suboccipital rind” sign, the lumen appears normal in caliber, with wall thickening as the only imaging sign of VAD. In our center, this clinically occult VAD would influence management, with patients usually treated with antiplatelet agents. We caution against using only luminal-opacifying techniques such as contrast-enhanced MRA or conventional angiography to exclude VAD because they are limited in the evaluation of mural hematoma.
The imaging findings of acute vertebral artery dissection (VAD) are well known. Conventional angiography has long been considered the gold standard for imaging of vertebral artery dissections.1 This can demonstrate the typical angiographic findings of an intimal flap: irregularity and/or stenosis of the vessel, the string sign (arising as a result of a dissection that extends circumferentially around the lumen over a long segment), the double lumen sign, pseudoaneurysm formation, or complete occlusion.2-4 Stenosis is by far the most common finding resulting in luminal narrowing by subintimal hematoma formation.4
In our center and many others, noninvasive imaging for suspected dissections has complemented or even replaced conventional angiography. This may consist of MR imaging, MR angiography (MRA), and/or multisection helical CT angiography.5 The relative advantage of cross-sectional imaging over conventional angiography is that it may better demonstrate extraluminal abnormalities. Additionally, it has the ability to further evaluate patients for a variety of clinical conditions associated with VAD, including stroke, subarachnoid hemorrhage, and cervical spine abnormalities. In our center, CT angiography (CTA) has become the primary technique for evaluation of suspected acute neurologic vascular disorders.
VADs may often encroach on the arterial lumen, narrowing it and drawing one's attention to the area of abnormality. Occasionally, expansion of the lumen and wall from a pseudoaneurysm can occur. We have encountered cases of VAD in the segment between the C2 foramen transversaria and foramen magnum (V3 segment), in which the lumen diameter was normal in caliber. The only visible abnormality was a dorsal thickening of the arterial wall against the adjacent fat, the “suboccipital rind” sign (Figs 1–3). These findings resolved completely on follow-up imaging.
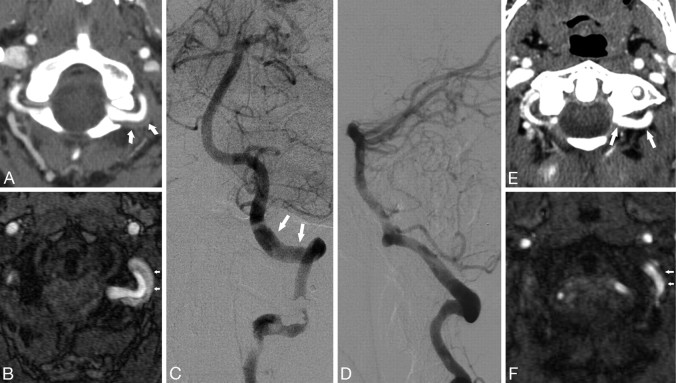
Fig 1.
Patient 3. A 32-year-old woman who presented with headache. A, CTA demonstrates abnormal thickening of the wall of the vertebral artery, the suboccipital rind sign (arrows). B, Time-of-flight MRA source image shows subacute blood in the area of wall thickening. C and D, Anteroposterior and lateral catheter angiograms demonstrate relatively normal lumen with no significant narrowing of the vessel (arrows). E, Follow-up CTA 7 months later shows resolution, compared with A, of soft tissue around the VA and re-appearance of normal fat planes (arrows). F, Follow-up MRA shows resolution, compared with B, of the intramural hematoma (arrows).
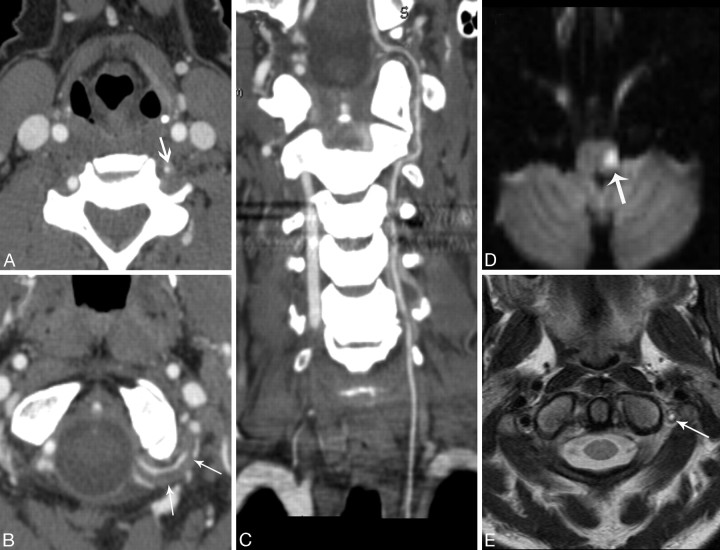
Fig 2.
Patient 6. A 46-year-old woman with a history of cocaine abuse who presented with left neck pain, Horner syndrome, ataxia, and left facial droop. A, CTA image through the lower cervical spine level shows a hypoplastic left vertebral artery (arrow). B, CTA shows the abnormal suboccipital rind sign (arrows) and relatively normal caliber of a hypoplastic VA. C, Curved planar reformatted image demonstrates the entire length of a uniform-caliber hypoplastic left VA. D, Diffusion-weighted MR imaging shows an acute lateral medullary infarct (arrow). E, T2-weighted MR imaging image shows slow-flow or clot (arrow).
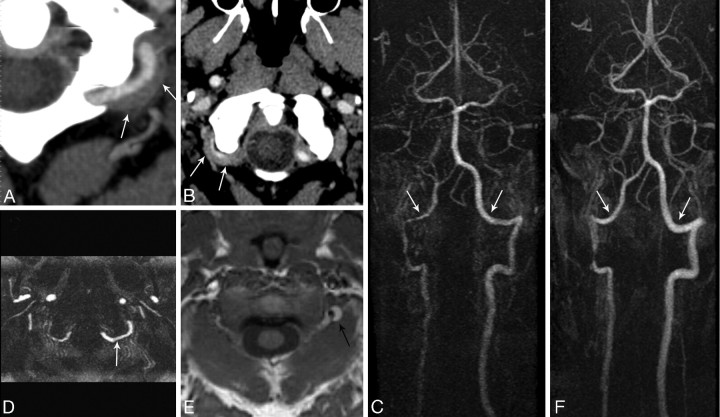
Fig 3.
Patient 4. A 41-year-old man who presented with occipital headache. A and B, CTAs demonstrate a bilateral suboccipital rind sign (arrows). C, CEMRA demonstrates normal VA caliber in the suboccipital portion (arrows). More proximally, there is narrowing of the right VA at the C2 vertical portion. D, Axial maximum-intensity-projection image shows a normal caliber of the left vertebral artery (arrow). E, T1-weighted MR image demonstrates subacute blood within the mural hematoma (arrow). F, Follow-up CEMRA shows both vertebral arteries after resolution of the hematoma (arrows). Note the normal lumen diameter especially in the suboccipital portion. The focal narrowing at the C2 level on the right has persisted.
The purpose of our study was to review the CTA imaging characteristics of patients with VAD in the V3 portion compared with healthy controls. We sought to evaluate the normal relationship between the lumen and wall in the V3 portion of the vertebral artery. In VAD cases, we aimed to verify that the subjective appearance of a normal arterial caliber was no different from that in controls. In normal arteries, the ratio of the lumen diameter/lumen+wall diameter should approach unity because the normal wall has minimal thickness. We propose that in the rind sign cases, this ratio should significantly diminish, as a consequence of the wall hematoma thickness proportional to the lumen diameter.
Materials and Methods
This study received approval from our institutional review board. Our prospectively collected electronic data base was reviewed for cases categorized under “suboccipital rind” sign on CTA.
The scans were obtained on a 16- or 64-section volume CT (VCT) scanner (LightSpeed; GE Healthcare, Milwaukee, Wis). On the VCT, 1.25-mm images were obtained by using a helical acquisition of 0.625 mm and an interval of 0.4 mm. The helical pitch was 0.516:1 with a table speed of 20.62 mm/rotation. The technique was 120 KV, 0.4-second gantry rotation, by using Smart mA (GE Healthcare) dose modulation set at a maximum of 550 mA and a noise index of 16. On the LightSpeed 16, 1.25-mm images were obtained with an interval of 0.6 mm. The pitch was 0.93:1 and the table speed was 3.37 mm/rotation. The technique was 100 KV, 0.8-second rotation and an automated mA dose modulation set at a maximum of 350 mA and a noise index of 12. Seventy milliliters of iodinated contrast medium was injected, and scanning commenced after SmartPrep (GE Healthcare) bolus tracking indicated an increase of 100 HU measured at the aortic arch. Coronal, sagittal, and axial multiplanar reformats were created in maximum intensity projections of either 5 mm (VCT) or 25 mm (16-section CT) at 3-mm intervals.
The presenting symptoms, presence of wall hematoma, presence of luminal narrowing, and follow-up images were reviewed (Table). A control group of 50 patients was randomly recruited from a group of patients undergoing CTA for the evaluation of intracranial and extracranial carotid disease and/or unruptured cerebral aneurysms.
Baseline patient characteristics
| Patient | Sex | Presenting Symptoms | VAD | Imaging Follow-up |
|---|---|---|---|---|
| 1 | M | Trauma, neck pain | Left | Rind sign resolved, lumen normal |
| 2 | F | Posterior circ. stroke | Left | Narrowing of VAs bilateral |
| 3 | F | Headache, vertigo, occipital h/a | Left | Rind sign resolved, lumen normal |
| 4 | M | Occipital headache | Bilateral | Rind sign resolved, lumens normal |
| 5 | M | Posterior circ. stroke | Right | Rind sign resolved, lumen normal |
| 6 | F | Horner syndrome, posterior circ. stroke, neck pain | Left | Occlusion LVA |
Note:—circ. indicates circulation; h/a, headache; LVA, left vertebral artery; VAD, vertebral artery dissection; VA, vertebral artery.
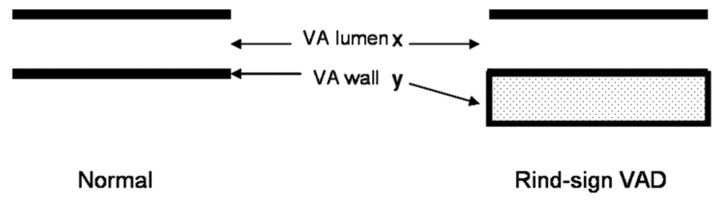
The V3 portion of the abnormal arteries was divided into the following 5 segments: C1 transverse foramen, proximal horizontal, midhorizontal, distal horizontal, and distal vertical before penetrating the dura. The total diameter, luminal diameter, and wall thickness (total diameter-luminal diameter) were measured. Mathematically, the relationship between vessel lumen and wall can be expressed as a ratio (Fig 4). We propose that the ratio of luminal diameter/total diameter should decrease between patients with the rind sign compared with controls.
Fig 4.
Diagram depicts a normal artery versus an artery with a rind sign. The lumen (x) in normal and dissected arteries is similar in caliber. The vessel wall (y) is thickened in patients with rind sign. The ratio of (x / x + y) will decrease in patients with rind sign as a result of wall thickening.
Statistical Analysis
The means of the 5 segments of each measured variable were analyzed. An independent samples t test was performed to estimate the average difference in the measurements between the 2 groups. Results were expressed as means ± SD (range) in the Table. All analyses were performed in SAS, Version 9.1 (SAS Institute, Cary, NC).
Results
Between November 2005 and July 2006 (9 months), 6 patients were identified with the suboccipital rind sign, accounting for a possible 12 vertebral artery (VA) measurements in the dissected cohort. One patient had bilateral suboccipital rind signs, whereas another patient had only 1 artery measured, because the contralateral VA was occluded. This accounted for 7 abnormal arteries from a total of 11 measurable arteries. These 7 abnormal arteries constituted the dissection group. In the control group, there were 50 patients accounting for 100 VAs plus 4 normal VAs from the group of patients with dissection, totaling 104 arteries in the control group.
Two of 6 (33%) patients presented with only neck pain or headache, without neurologic symptoms or occipital pain. Three of 6 (50%) presented with posterior circulation strokes. At imaging follow-up, 5 of 7 arteries (71%) returned to normal caliber. Seven of 7 (100%) of the rind signs resolved at follow-up (Table).
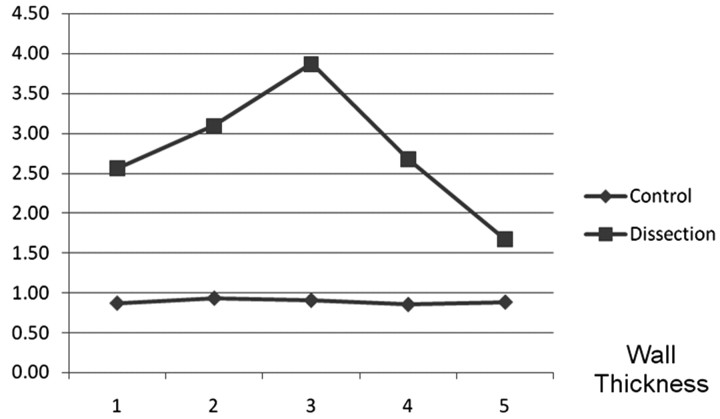
The lumen diameter at the 5 V3 segments in the VAD cohort was constant without evidence of tapering. Although there is variability in the vessel diameter within and between normal patients, there was no significant difference in the arterial lumen diameter between dissected VAs and controls (P = .1). The average wall thickness (Fig 5) of the dissection group was 2.96 mm greater than that for the control group (P < .001, 95% confidence interval, 2.6–3.3). There was a significant difference in the ratio of lumen diameter/lumen+wall diameter in dissected segments compared with controls (P < .001).
Fig 5.
The wall thickness is plotted at 5 V3 segments. There is a difference in mean wall thickness between the patients with rind sign and controls (P < .001).
Discussion
The vertebral artery can be classified into segments V1-V4.2 The V3 segment initially courses vertically between the C2 and C1 transverse foramina, then traverses horizontally over the atlas groove and finally obliquely upwards to pierce the dura.
Extracranial vertebral artery dissection can be spontaneous or traumatic. Most of the traumatic dissections involve the atlanto-occipital (V3) segment, which represents the segment of interest in our series. This may be secondary to the relative anchoring of the vessel at the C2 foramen transversarium and the dura.6
Clinically, 70% of patients with VAD may present with occipital headache and neck pain, whereas 60% of patients have symptoms of vertebrobasilar circulation ischemia.1,7 Although vertebral artery dissection accounts for only 0.4%–2.5% of the overall cases of cerebrovascular accident, it accounts for 4% of cases of ischemic stroke in patients younger than 45 years of age and 14% of cases of lower brain stem infarction.8 Two patients (33%) in our series underwent CTA for work-up of isolated suboccipital headaches. Because CTA is being widely used, recognition of the rind sign is helpful in imaging patients without clinical suspicion of dissection. In our center, this finding would influence clinical management. Patients with the rind sign, even without neurologic symptoms, would usually be treated with antiplatelet agents.
Both subjectively and objectively, our results demonstrated that there was no significant difference in the arterial lumen diameter in patients with rind sign compared with controls (P = .1). Thus, the more frequent finding of luminal tapering and narrowing seen in dissections was not evident in our series. There was, however, a significant thickening of the dissected vessel walls in our patients with rind sign compared with controls (P < .001). When the lumen and vessel wall were taken into account, there was a significant difference in the patients with rind sign compared with controls (P < .001). Therefore, the data support the subjective impression that in some cases of VAD, the vessel lumen is of normal caliber. Measurement of the vessel caliber in our dissected cohort was intended to further demonstrate to readers the true normal lumen diameter. However, in practice, recognition of the wall hematoma without direct lumen measurements is likely sufficient for diagnosis of VAD.
We believe that the horizontal course of the V3 portion of the VA as it passes behind the C1 superior articular facet and in the sulcus arteriosus of C1 posterior arch renders detection of luminal narrowing more difficult because the horizontal course of the VA is parallel to the axially acquired imaging plane. Without complete cross-sectional imaging of the arterial wall, it is more difficult to identify the classic crescentic wall hematoma, which is normally perpendicular to the axial plane of noninvasive imaging techniques. Awareness of the typical location and appearance of the suboccipital rind sign may increase detection on multiplanar reformatted CTA images. This is especially true when the lumen is normal or imperceptibly reduced in caliber.
The MR imaging findings of VAD include an intramural hematoma, an intimal flap, and the enhancement of the arterial wall and septum. On the basis of the T1 and T2 signal intensity, the intramural hematoma can be characterized.9,10 Flow void within the true lumen signifies patency. Gradient- echo images show increased signal intensity in the true lumen when patent.11,12 MR imaging may be performed in cases in which VAD is highly suspected and can demonstrate the wall hematoma. However, in our center, CTA is more readily available and is the primary noninvasive vascular imaging technique in the emergent setting. Thus patients without a clinical suspicion of VAD may never undergo MR imaging.
Contrast-enhanced MRA (CEMRA) is now used in many centers for evaluation of carotid artery stenosis and vascular imaging. However, the vascular wall is poorly imaged because of the inherent strong background suppression. Other “lumen imaging” techniques such as digital subtraction conventional angiography provide high intraluminal spatial resolution; however, they have the same limitation as CEMRA, with limited evaluation of the vessel wall (Fig 3). With time-of-flight MRA in diagnosing VAD, some issues include confusing high signal intensity caused by the venous plexus or fat surrounding the VAs, the isointensity of an intramural hematoma in the acute phase, and the low resolution of visualizing relatively small VAs.11,13
Review of imaging should also consider the normal soft tissue and venous anatomy in the suboccipital region below the foramen magnum. The anatomy of the normal suboccipital cavernous sinus and its communications (VA venous plexus, anterior condylar vein, posterior condylar vein, internal vertebral venous plexus, and marginal sinus) requires adequate understanding to correctly interpret vascular abnormalities of the V3 portion of the VA. The VA venous plexus and suboccipital cavernous sinus intimately surround the V3 and should not be confused with abnormal VA wall thickening. The suboccipital cavernous sinus and its surrounding venous structures should enhance with contrast, whereas wall hematoma will typically not enhance.14,15
Several studies have highlighted the classic imaging features of VAD at MRA and CTA. Chen et al16 found that CTA was sensitive and accurate for diagnosis of VAD when compared with conventional angiography. However, in their series, the VA lumen was either stenotic or expanded. In other reports of VAD, the classic signs are discussed; however, there was little discussion around cases presenting with normal-appearing luminal caliber, as in our series.17-19
Our study has several limitations. In most cases, we did have MR imaging confirmation as a gold standard for demonstrating the intramural blood products. We recognize that the wall thickening could be secondary to some unknown disease. However, all patients, when further questioned, had clinical symptoms entirely compatible with VAD. We also believed, because of the typical location in the suboccipital portion where there are high rotational stresses and because of wall hematoma resolution on follow-up CTA, that these cases were true dissections. Bias during measurement of abnormal arteries may have been introduced because there was no blinding of the dissected-versus-normal arteries. Therefore, the relative normalcy of lumen caliber in the dissected cohort may be potentially overstated. Our study is one with relatively small numbers. A larger study population may be helpful in further verifying the suboccipital rind sign.
Caution must be exercised when abnormalities are detected in the V3 portion of the artery because the course of the obliquus capitis inferioris muscle passes directly posterior to the artery at the posterolateral aspect of the C1 arch. The proximity of the anterior aspect of the muscle to the posterior arterial wall could potentially lead to inaccuracies regarding arterial wall diameter in this location.6 We have seen a biopsy-proved case of giant cell arteritis in which there was extensive circumferential thickening of the vertebral arterial wall bilaterally. Therefore, a diffuse vasculitis is also a consideration in patients with wall thickening at CTA. The relative focality of VAD and the typical location and corroborating symptoms, if any, may help distinguish these entities.
Conclusions
Cross-sectional vascular imaging is often performed with multisection helical CTA for a variety of concerns. We highlight a characteristic CTA imaging sign of vessel wall hematoma in the V3 portion, in which the imaging plane is parallel to the course of the VA. Awareness of this finding in its characteristic location may help in identifying clinically unsuspected VAD cases in which there is no visible narrowing of the lumen. We caution against using only luminal-opacifying techniques (lumenogram) such as CEMRA or conventional angiography to exclude VAD because they are limited for the evaluation of mural hematoma.
References
- 1.Mokri B, Houser OW, Sandok BA, et al. Spontaneous dissections of the vertebral arteries. Neurology 1988;38:880–85 [DOI] [PubMed] [Google Scholar]
- 2.Provenzale JM, Morgenlander JC, Gress D. Spontaneous vertebral dissection: clinical, conventional angiographic, CT, and MR findings. J Comput Assist Tomogr 1996;20:185–93 [DOI] [PubMed] [Google Scholar]
- 3.Hinse P, Thie A, Lachenmayer L. Dissection of the extracranial vertebral artery: report of four cases and review of the literature. J Neurol Neurosurg Psychiatry 1991;54:863–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagawa K, Touho H, Morisako T, et al. Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings. J Neurosurg 2000;93:19–25 [DOI] [PubMed] [Google Scholar]
- 5.Truwit C. CT angiography versus MR angiography in the evaluation of acute neurovascular disease. Radiology 2007;245:362–66 [DOI] [PubMed] [Google Scholar]
- 6.Bruneau M, Cornelius JF, George B. Anterolateral approach to the V1 segment of the vertebral artery. Neurosurgery 2006;58 (4 suppl 2):ONS-215–19, discussion ONS-219 [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI, Mokri B, O'Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med 1994;330:393–97 [DOI] [PubMed] [Google Scholar]
- 8.Hart RG, Miller VT. Cerebral infarction in young adults: a practical approach. Stroke 1983;14:110–14 [DOI] [PubMed] [Google Scholar]
- 9.Mascalchi M, Bianchi MC, Mangiafico S, et al. MRI and MR angiography of vertebral artery dissection. Neuroradiology 1997;39:329–40 [DOI] [PubMed] [Google Scholar]
- 10.Vieco PT, Maurin EE 3rd, Gross CE. Vertebrobasilar dolichoectasia: evaluation with CT angiography. AJNR Am J Neuroradiol 1997;18:1385–88 [PMC free article] [PubMed] [Google Scholar]
- 11.Bloem BR, Van Buchem GJ. Magnetic resonance imaging and vertebral artery dissection. J Neurol Neurosurg Psychiatry 1999;67:691–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soper JR, Parker GD, Hallinan JM. Vertebral artery dissection diagnosed with CT. AJNR Am J Neuroradiol 1995;16:952–54 [PMC free article] [PubMed] [Google Scholar]
- 13.Kitanaka C, Tanaka J, Kuwahara M, et al. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke 1994;25:571–75 [DOI] [PubMed] [Google Scholar]
- 14.Caruso RD, Rosenbaum AE, Chang JK, et al. Craniocervical junction venous anatomy on enhanced MR images: the suboccipital cavernous sinus. AJNR Am J Neuroradiol 1999;20:1127–31 [PMC free article] [PubMed] [Google Scholar]
- 15.Arnautovic KI, al-Mefty O, Pait TG, et al. The suboccipital cavernous sinus. J Neurosurg 1997;86:252–62 [DOI] [PubMed] [Google Scholar]
- 16.Chen CJ, Tseng YC, Lee TH, et al. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol 2004;25:769–74 [PMC free article] [PubMed] [Google Scholar]
- 17.Shin H, Suh DC, Choi CG, et al. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics 2000;20:1687–96 [DOI] [PubMed] [Google Scholar]
- 18.Provenzale J. Dissection of the internal carotid and vertebral arteries. AJR Am J Roentgenol 1995;165:1099–104 [DOI] [PubMed] [Google Scholar]
- 19.Vertinsky AT, Schwartz NE, Fischbein NJ, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol 2008;29:1753–60 [DOI] [PMC free article] [PubMed] [Google Scholar]