Abstract
BACKGROUND AND PURPOSE: A neuroimaging-based ischemic stroke classification system that predicts costs and outcomes would be useful for clinical prognostication and hospital resource planning. The Boston Acute Stroke Imaging Scale (BASIS), a neuroimaging-based ischemic stroke classification system, was tested to determine whether it was able to predict the costs and clinical outcomes of patients with stroke at an urban academic medical center.
MATERIALS AND METHODS: Patients with ischemic stroke who presented in the emergency department in 2000 (230 patients) and 2005 (250 patients) were classified by using BASIS as having either a major or minor stroke. Compared outcomes included death, length of hospitalization, discharge disposition, use of imaging and intensive care unit (ICU) resources, and total in-hospital cost. Continuous variables were compared by univariate analysis by using the Student t test or the Satterthwaite test adjusted for unequal variances. Categoric variables were tested with the χ2 test. Multiple regression analyses related total hospital cost (dependent variable) to stroke severity (major versus minor), sex, age, presence of comorbidities, and death during hospitalization. Logistic regression analysis was applied to identify the significant predictive variables indicating a greater likelihood of discharge home.
RESULTS: In both years, individuals with strokes classified as major had a significantly longer length of stay, spent more days in the ICU, and had a higher cost of hospitalization than patients with minor strokes (all outcomes, P < .0001). All deaths (8 in 2000, 26 in 2005) occurred in patients with major stroke. Whereas 73% of patients with minor stroke were discharged home, only 12.2% of patients with major stroke were discharged home (P < .0001); 61% of patients with major stroke were discharged to a rehabilitation or skilled nursing facility. Patients with major stroke cost 4.4 times and 3.0 times that of patients with minor stroke in 2000 and 2005, respectively. Making up less than one third of all patients, patients with major stroke accounted for 60% of the total in-hospital cost of acute stroke care.
CONCLUSIONS: BASIS, a neuroimaging-based stroke classification system, is highly effective at predicting in-hospital resource use, acute-hospitalization cost, and outcome. Predictive ability was maintained across the years studied.
Each year 500,000 people in the United States experience a new stroke, and 200,000 people experience a recurrent attack.1 Stroke is the third leading cause of death, resulting in more than 150,000 deaths per year.2 In 2005, 9.2% of all people older than 65 years of age were stroke survivors.3 Stroke is also the leading cause of serious long-term disability in the United States, with more than 1.1 million Americans reporting functional limitations and difficulty with activities of daily living resulting from stroke.4
Stroke has a large economic cost with an estimated annual direct and indirect cost of $62.7 billion in the United States.1 The estimated lifetime cost of an ischemic stroke in 1987 was $90,981 ($226,000 in 2005 dollars)5; however, between 1990 and 2000, the in-hospital charges, a proxy for cost, increased by 32% and 63% in rural and urban hospitals, respectively.6 Several studies of actual cost,7,8 estimated cost by using cost-to-charge ratios,9–11 and charges6,12–15 have been performed in the US health care system. However, these studies often combined patients of different stroke severity because the purpose of the analysis was to compare the care choices of physicians of different specialties, the effect of different hospital types (rural versus urban), or the cost of different types of stroke (ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, or transient ischemic attack [TIA]). Among the studies that did classify patients on the basis of stroke severity,10,13 clinical measures such as the National Institutes of Health Stroke Scale (NIHSS) or the Barthel index were used rather than a physiologically based measure of infarct.
We used the Boston Acute Stroke Imaging Scale (BASIS), a dichotomous neuroimaging-defined stroke severity classification system, to compare the costs and discharge outcomes of patients with major and minor stroke at a large urban teaching hospital. Using linear regression, we developed a statistical model for estimating the total hospital cost of a patient with stroke. Using logistic regression, we developed a statistical model estimating the likelihood of being discharged home. Outcomes, but not resource use or hospital cost, for the 2000 cohort have been previously reported.16
Materials and Methods
Patient Data
Patients with acute stroke symptoms presenting to the emergency department, admitted to the hospital, and subsequently discharged with a diagnosis of acute ischemic stroke between January and August 2000 and between January and December of 2005 were identified. The standard protocol of the hospital for evaluating patients with a potential new stroke includes a CT or MR imaging by using CT or MR angiography performed in ∼90% of studies. Imaging occurred within hours of the patient's arrival at the emergency department. When >1 neuroimaging study was performed, the images from the first study that included angiography were used for classification. Administrative data were collected on a prospective basis and analyzed in retrospect by using the hospital accounting system. The protocol (and all amendments) received institutional review board approval.
The BASIS classification methodology has been described in detail elsewhere and was established as independent of the imaging (CT or MR imaging) technique.16 Briefly, patients were classified as having a major stroke if by using either CT or MR angiography, a proximal cerebral artery occlusion was identified or when a large infarct was identified in a patient with patent major cerebral arteries. Proximal cerebral artery occlusion was defined as an occlusion of the distal (intracranial) internal carotid artery, the proximal (M1 or M2) middle cerebral artery, or the basilar artery. If these arteries were not occluded, the presence of parenchymal abnormalities, such as a significant ischemic lesion in the middle cerebral artery territory or any lesion of the bilateral pons and/or bilateral thalami identified with noncontrast CT or diffusion MR imaging, was classified as a major stroke. All those patients not meeting these criteria were classified as having a minor stroke.
Initial classification of stroke severity was based on the neuroradiologic interpretation in the medical record. The original imaging data for all patients were then reviewed by a neuroradiologist (R.G.G., J.H.) to confirm the initial interpretation and to clarify descriptions that were ambiguous with respect to the classification system used in this study. If there was conflict between the interpretations, both neuroradiologists reviewed the images and consensus between the radiologists was reached.
Patient characteristics, resource use, and cost information were collected from the hospital cost accounting system (Sunrise Decision Support Manager; Eclipsys, Atlanta, Ga), a data base into which all hospital use and financial information are entered. Patient information such as age, sex, length of stay, Charlson comorbidity score at admission, and discharge disposition were all extracted. The Charlson comorbidity score is an aggregate measure of the severity of chronic comorbid conditions weighted by their association with mortality and is the most widely accepted and validated quantitative measure of comorbidity.17
The accounting system identifies all itemized services provided to a particular patient through the billing system and then combines the itemized services into intermediate service-based products. Actual costs are assigned to these intermediate products, which are based on total hospital service volume of the product and relative value units. We retrieved the resource use and total hospital cost per patient, which included both direct (fixed and variable) and indirect (eg, building amortization and information systems) costs. Hospital costs include those for disposable supplies, labor, and major capital amortization and overhead. Disposable supply costs are based on actual acquisition costs. The labor costs for nurses, technicians, residents, and other personnel are derived directly from actual salaries and include benefits. Physician fees are not included in the cost accounting system data base and were not included in this analysis.
Use of diagnostic imaging was identified in the patient's accounting record through CPT4 codes. Where observed to have been listed separately, the cost of the contrast agent was added to the cost of the imaging procedure itself.
Use of intensive care unit (ICU) services was identified through a daily indicator for room type. Patients were classified as being in an observation room, private or semiprivate room, or in the ICU. On a day when the patient changed room type, the type was assigned by the room in which the patient spent the greater portion of the day. All expenses incurred on a day when the patient was indicated to have been in the ICU were included as ICU cost.
All costs were calculated per patient and expressed in US dollars. Costs from prior years were converted to 2005 dollars by using the medical component of the Consumer Price Index.18 Costs were indexed to the average cost of a minor stroke in 2000.
Statistical Analysis
Patients with major stroke were compared with patients with minor stroke. Among patients with major stroke, patients with proximal cerebral artery occlusion were compared with patients with parenchymal abnormalities only. Continuous patient characteristics and outcomes were compared by univariate analysis by using the Student t test or Satterthwaite test adjusted for unequal variances as indicated by the F-test of the variances. Categoric patient characteristics and outcomes were tested with the χ2 test. Data were pooled across years whenever the test for the interaction between each continuous variable and calendar year was not significant. Finally, the median Charlson comorbidity scores were compared across groups by using the Wilcoxon Rank Sum test. P values ≤.05 were considered significant.
Multiple regression analyses related total hospital cost (dependent variable) to stroke severity (major versus minor), sex, age, presence of comorbidities, and death during hospitalization. Stepwise regression was used to obtain parsimonious models. Total hospital cost was indexed to the average cost of a minor stroke in the year 2000. Logistic regression analysis was applied to identify the significant predictive variables indicating a greater likelihood of discharge home. All data were analyzed by using SAS, Version 9.1 (SAS Institute, Cary, NC).
Results
Study Population
In 2000, we identified 230 patients with suspected acute nonhemorrhagic stroke: 127 men and 103 women between 19 and 97 years of age (mean, 69 ± 14 years). This population excluded TIA and is described in detail in Torres-Mozqueda et al.16 In 2005, 279 patients were identified who were admitted to the hospital from the emergency department with an admitting diagnosis of acute stroke. There were 15 patients who were excluded from the analysis because they had discharge diagnoses other than stroke (seizure, migraine, and neoplasia were the most common). There were 9 patients excluded because they had another unrelated acute illness or trauma that accounted for most of the hospitalization costs. There were 5 patients excluded because they remained in the hospital for elective procedures (4 underwent endarterectomy and 1, a patent foramen ovale closure). Among the 250 patients included in the analysis, there were 135 men and 115 women between 18 and 97 years of age (mean, 70 ± 14 years). Patients were classified into 2 groups: major stroke (2000: 59 patients; 2005: 86 patients) and minor stroke (2000: 171 patients; 2005: 164 patients). Patients classified as having a major stroke were further subclassified by those who had a proximal cerebral artery occlusion (2000: 52 patients; 2005: 67 patients) and those with parenchymal abnormalities.
Univariate Analysis
Table 1 summarizes the comparison of patients with major and minor strokes in each year and across years. In 2000, there was no significant difference in age between patients with minor and major strokes (P = .1846), whereas in 2005, patients with minor stroke were significantly younger than those with major stroke (P = .0029). The median comorbidity score was significantly less in patients with minor stroke than in those with major stroke in both years (2000: P < .0001; 2005: P < .0001).
Table 1:
Patient characteristics classified as a major or minor stroke by year, comparing across stroke classification and year
| 2000 |
2005 |
P† | |||||
|---|---|---|---|---|---|---|---|
| Major | Minor | P* | Major | Minor | P* | ||
| No. | 59 | 171 | 86 | 164 | |||
| % Female | 40.68 | 46.2 | 50 | 43.9 | .8974 | ||
| Mean age (yr) | 66.71 | 69.46 | .1846 | 73.91 | 68.45 | .0029 | |
| Median comorbidity | 3 | 1 | <.0001 | 3 | 2 | <.0001 | |
| Length of stay (days) | 12.58 | 3.16 | <.0001 | 7.67 | 3.40 | <.0001 | |
| Total cost of hospitalization‡ | 4.36 | 1.00 | <.0001 | 3.00 | 1.08 | <.0001 | |
| Total cost of hospitalization‡ (excluding in-hospital deaths) | 4.46 | 1.00 | <.0001 | 3.21 | 1.08 | <.0001 | |
| ICU use | |||||||
| Mean ICU days | 3.71 | 0.09 | <.0001 | 2.47 | 0.23 | <.0001 | |
| Mean ICU costs‡ | 2.19 | 0.05 | <.0001 | 1.45 | 0.12 | <.0001 | |
| % who spent any time in the ICU | 66.1 | 2.9 | <.0001 | 62.8 | 9.1 | <.0001 | |
| Among patients who spent any time in the ICU | |||||||
| Mean ICU days | 5.62 | 3.20 | 3.93 | 2.47 | .0921 | ||
| Mean ICU costs‡ | 3.31 | 1.70 | 2.31 | 1.26 | .0378 | ||
| Imaging use | |||||||
| No. of CT scans | 3.76 | 1.13 | 4.41 | 1.89 | <.0001 | ||
| No. of MRIs | 1.83 | 1.87 | 2.13 | 2.48 | .135 | ||
| Mean cost of CT‡ | 0.12 | 0.03 | 0.14 | 0.07 | <.0001 | ||
| Mean cost of MRI‡ | 0.19 | 0.15 | 0.16 | 0.15 | .0096 | ||
| Patient outcomes (%) | |||||||
| Deceased | 13.56 | 0 | 30.23 | 0 | <.0001 | ||
| Discharge to home | 18.64 | 76.61 | 8.14 | 70.12 | <.0001 | ||
| Discharge to rehabilitation facility | 59.32 | 17.54 | 15.12 | 6.71 | <.0001 | ||
| Discharge to facility | 1.69 | 2.92 | 45.35 | 22.56 | .0007 | ||
Note:—MRI indicates MR imaging; ICU, intensive care unit.
P values from least-squares means.
P value for interaction with year was ≤.05; therefore, data from each year were combined for statistical analysis.
Costs are in US dollars and indexed to the mean cost of a minor stroke in 2000.
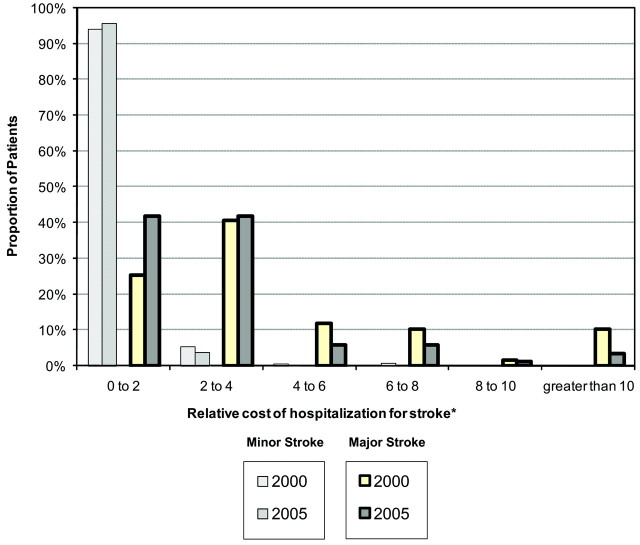
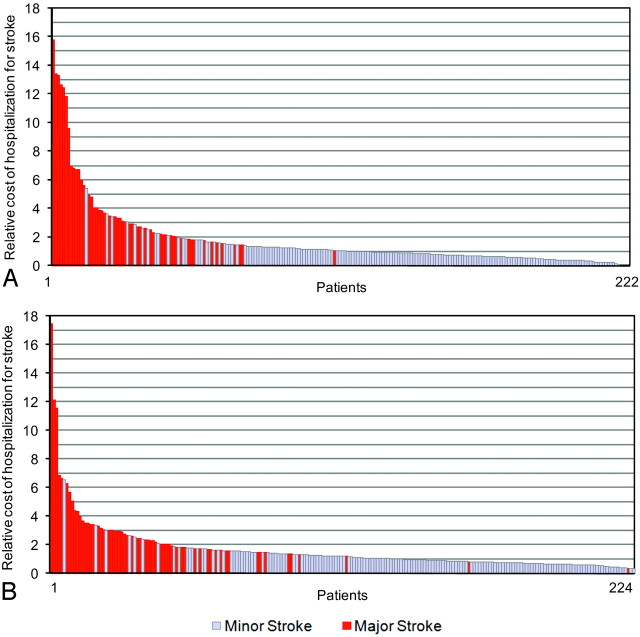
In both years studied, patients with a minor stroke had a shorter hospital length of stay (2000: P < .0001; 2005: P < .0001) and cost less than patients with major stroke (2000: P < .0001; 2005: P < .0001). The cost of stroke for patients with both major and minor strokes was positively skewed (Fig 1). In 2000, the most expensive 20% of patients had an average cost 4.5 times that of the average minor stroke and cumulatively represented 58% of all stroke costs (Fig 2). Thirty-nine (83%) of these patients had major stroke. In 2005, the most expensive 20% of cases had an average cost 3.9 times the average cost of a minor stroke in 2000 and cumulatively represented 50% of all stroke costs. Forty-four (88%) of these patients had major stroke.
Fig 1.
The distribution of the in-hospital costs in 2000 and 2005 for patients with major and minor strokes. Costs are indexed to the mean cost of minor stroke in 2000.
Fig 2.
The in-hospital costs in 2000 (A) and 2005 (B) for patients with (red) and without (light blue) proximal artery occlusion. In-hospital deaths were removed. Costs are indexed to the mean cost of minor stroke in 2000.
If we compared across years, the cost and length of stay for patients with minor stroke were not statistically distinguishable. Among patients with major stroke, however, between 2000 and 2005, the length of stay decreased 39% (P = .0008) and the total hospital cost decreased 31% (P = .014). Because a significantly larger number of patients with major stroke died during hospitalization in 2005 than in 2000 (P = .0333), we also considered total hospital cost excluding deceased patients from the analysis. If we excluded patients who died in-hospital, total hospital cost of patients with major stroke decreased 28% (P = .0471).
Overall, patients with minor stroke spent significantly fewer days in the ICU and incurred lower average ICU costs. However, in 2000 and 2005, respectively, only 2.9% and 9.1% of patients with minor stroke spent any time in the ICU compared with 66.1% and 62.8% of patients with major stroke. Among patients who spent any time in the ICU, the length of stay in the ICU was not significantly different across groups (P = .0921) but the average cost of the total time spent in the ICU remained 2 times greater for patients with major stroke (P = .0378).
Discharge outcomes for patients with stroke in 2000 were presented previously16 but are now combined and compared with outcomes observed in 2005. In both years, no patients with minor stroke died during hospitalization. However, 13.6% and 30.2% of patients with major stroke died during hospitalization in 2000 and 2005, respectively. Overall, 73.4% of patients with minor stroke were discharged home compared with only 12.4% of those with major stroke (P < .0001). Conversely, patients with major stroke were more likely to be discharged to a rehabilitation or skilled nursing facility than those with minor stroke (60.7% versus 24.8%, P < .0001).
Within patients with major stroke, we compared those with a proximal cerebral artery occlusion to those with parenchymal abnormalities on all factors. Combining data from both years, we found that patients with proximal cerebral artery occlusion were not significantly different from patients with parenchymal abnormalities on any measure except the proportion who were discharged home. No patients with a parenchymal abnormality were discharged home, compared with 15.13% of patients with a proximal cerebral artery occlusion (P = .0436).
Predicting Total Hospital Cost with Linear Regression Analysis
Using stepwise regression analysis, we evaluated which parameters significantly contributed to the prediction of total hospital cost (Table 2). The R2 of the final regression model is 0.3372. Statistically significant predictors of in-hospital cost were death (P = .0453), a comorbidity score of ≥1 (P = .0376), year (P < .0001), major-versus-minor stroke (P < .0001), and an interaction term between year and whether the patient had a minor stroke (P = .0006). In-hospital death, occurring only among patients with major stroke, decreased the total hospital cost.
Table 2:
Multiple regression analysis and total hospital cost indexed to the cost of total hospitalization of a patient with minor stroke in 2000
| Variable | Parameter Estimate | Standard Error | t Value | P |
|---|---|---|---|---|
| Intercept | 3.783 | 0.395 | 9.56 | <.0001 |
| Minor stroke | −3.346 | 0.272 | −12.31 | <.0001 |
| ≥1 Charlson comorbidity score | 0.669 | 0.321 | 2.08 | .0376 |
| Deceased | −0.698 | 0.348 | −2.01 | .0453 |
| Year* | −1.236 | 0.300 | −4.12 | <.0001 |
| Year × minor stroke | 1.238 | 0.357 | 3.47 | .0006 |
The variable “year”: in 2000, year = 0; in 2005, year = 1.
Predicting Discharge Home with Logistic Regression Analysis
Logistic regression revealed that controlling for a comorbidity score of ≥2 and patient age, patients with minor stroke have 18.6 times the odds of being discharged home compared with patients with major stroke (Table 3). Patients with a comorbidity score <2 are also more likely to be discharged home. If we controlled for these other factors, each additional year of patient age reduces the odds of being discharged home.
Table 3:
Logistic regression for discharge to home
| Variable | Odds Ratio | Lower 95% CI | Upper 95% CI | P |
|---|---|---|---|---|
| Minor stroke | 18.58 | 10.36 | 33.33 | <.0001 |
| Charlson comorbidity score <2 | 2.30 | 1.44 | 3.69 | .0005 |
| Age (yr) | 0.96 | 0.95 | 0.98 | <.0001 |
Note:—CI indicates confidence interval.
Discussion
We have shown that neuroimaging evidence of proximal cerebral artery occlusion, the first event in the chain of causality that leads to major neurologic symptoms, allows a meaningful dichotomous classification of ischemic strokes with respect to cost, resource use, and discharge disposition. According to the BASIS classification scheme,16 patients with major stroke have a higher risk of death, use more CT imaging services, are more likely to be admitted into the ICU, and have a longer stay and a higher total cost than patients with minor stroke. Risk of death among patients with major stroke was substantial (1 in 5 patients), and the likelihood of being discharged home was low (1 in 8). These observations emphasize the importance of biologic stratification. They also indicate a potential benefit if BASIS were to be used in concert with clinical rating instruments in the clinical and economic evaluation of ischemic stroke therapies and policy changes.
Clinical rating systems such as the NIHSS have been demonstrated to be predictive of the length of stay19 and outcome in patients with very low and very high scores.20 One previous study of hospital cost found that patients with stroke with a NIHSS score of >20 had a median cost 2.2 times the median cost of patients with a baseline NIHSS of <9.10 Another study found that “extreme” and “major” ischemic strokes cost 3.6 and 1.8 times more than “minor” strokes.7 However, clinical rating systems are limited, particularly because they are unable to identify the occluded artery. As argued by Caplan, 21 the lack of information on which, if any, artery is occluded is likely a major reason for the limited progress to date in the treatment of ischemic stroke. The predictive power of clinical rating instruments can be improved by augmenting them with neuroimaging data22; therefore, the approach described here can be considered complementary to clinical classification schemes already in use.
System Implications
We have reported, through linear regression, that the BASIS neuroimaging stroke classification predicts total hospital cost. However, from a broader perspective, we may have underestimated the magnitude of the cost difference between patients with major and minor stroke because we did not include the costs incurred at rehabilitation hospitals, skilled nursing and long-term care facilities, or in the home. One study observed that the average medical cost of stroke survivors up to 1 year poststroke hospitalization was $22,400 ($35,947 in 2005 dollars); however, patients with stroke discharged to institutional care (average stay, 28 days) had average institutional care costs of $47,613 ($76,408 in 2005 dollars).8
The difference in the cost of hospitalization for major and minor strokes is not fully reflected in the Medicare reimbursement policy. Historically, there were 2 reimbursement codes for patients with stroke: Diagnosis-Related Groups (DRG) 014 for hemorrhagic and ischemic strokes with infarction and DRG 015 for cerebral vascular occlusions without infarction (including TIA). In 2004, US Centers for Medicare and Medicaid Services introduced a new DRG (543), which included the new procedure code 39.74 to reimburse mechanical thrombectomy for acute ischemic stroke, including the use of the Merci retriever (Concentric Medical, Mountain View, Calif), at a level consistent with the cost of performing these procedures. In 2005, DRG 559 was introduced, increasing the reimbursement when thrombolysis is used.23 Thus, ischemic strokes are reimbursed on the basis of which treatment the patient receives: $23,107 when the patient receives mechanical thrombectomy, $11,950 when the patient receives thrombolysis, and between $5000 and $6430 otherwise. Therefore, the current reimbursement structure creates an incentive to use thrombolysis, which is readily available, in all eligible patients. Regardless of whether thrombolysis is used, DRG 559 reimbursement does not cover the cost of hospital care of patients with major stroke. In addition, intravenous thrombolytics have only been shown to provide a statistically significant improvement for strokes with a baseline NIHSS score between 6 and 10, and no significant benefit was observed in patients with severe ischemic stroke.24 In patients with major stroke, alternate therapies, such as those that focus on the recanalization of proximal cerebral artery occlusions, may improve outcomes25,26 but remain underused. Stratification based on imaging, which is readily obtained and is predictive of patient outcomes and acute hospitalization cost, can and should be used for patient severity−specific and treatment-specific reimbursement in acute ischemic stroke.
The findings reported here have another major implication for the current system of stroke care: the decisions made outside of the hospital as to where a potential stroke patient should be transported. Many states have emergency medical service (EMS) guidelines to take patients with potential stroke to the nearest qualified hospital.27 Given that the major stroke population may benefit from therapies that focus on the recanalization of proximal cerebral artery occlusions,25 being able to direct those patients to the right hospital, with advanced diagnostic and therapeutic capabilities,28 may improve outcomes and reduce overall costs. As such, a classification system such as BASIS may help improve EMS protocols.
Hospital Implications
Between 2000 and 2005, we observed a significant decrease in the length of stay and in-hospital cost in patients with major stroke. It is possible that this observation is due to undescribed patient factors, but there were no observed changes in the median number of comorbidities or the proportion of patients who required ICU admission to support that hypothesis. During this time period, economic forces caused our hospital administration (and hospitals across the country) to reduce the length of stay when possible. We observed a 50% reduction in the proportion of patients with major stroke who were discharged home between 2000 and 2005. We hypothesize that patients may have been discharged earlier in their recovery process in 2005 compared with 2000 and, therefore, more often required the care of rehabilitation or skilled nursing facilities. If we compare 2000 and 2005, the data indicate a significant reduction in discharge to rehabilitation facilities and a significant increase in discharge to skilled nursing facilities; we believe that the dramatic and opposite nature of these changes indicate that the use of these discharge codes or the classification of a common discharge facility has changed with time.
The use of an objective stroke classification instrument such as BASIS may help hospitals understand the costs of their stroke programs, year-to-year variations in those costs, and budgeting for stroke programs. Imaging-based classification data may also inform decisions on whether to make changes in hospital programs. For example, some hospitals may decide that it is beneficial to develop full-service capabilities, including endovascular treatment programs, whereas others may rationally decide to partner with other hospitals for the care of major stroke.
Medical Implications
The stratification of patients with stroke based on the underlying pathophysiology provides a foundation for new approaches to stroke care, not unlike the advances in the care of myocardial infarction based on acute ST-segment elevation.29 Improving outcomes in patients with severe stroke may require greater emphasis on rapid patient stratification and appropriate patient triage and transport policies. Appropriate use of recanalization procedures may also reduce hospital cost and system-wide cost by improving patient outcomes and, therefore, decreasing the burden on downstream health care facilities and families.
The first step in creating biologically driven stroke care is ensuring that every patient undergoes an imaging study that depicts the relevant anatomy and physiology, especially the status of major cerebral arteries. Modern MR imaging and CT are capable of providing this information rapidly. For example, a CT angiogram requires <2 minutes to acquire, and clinically useful reconstructions such as sliding thick-slab maximum intensity projection can be performed at the scanner in <5 minutes.30,31 This will provide highly relevant information that can be used to guide treatment decisions and improve the objective evaluation of stroke treatments across patient groups. Using this approach, hospital managers will be better able to evaluate the cost of patients with stroke based on a realistic expected cost structure.
Limitations
The NIHSS has been shown to be a predictor of length of stay, hospital cost, clinical outcomes, and hospital discharge disposition.19,20,32,33 The modified Barthel index has been demonstrated as a significant predictor of length of stay,19 and smoking status has been demonstrated as a possible predictor of acute hospitalization cost.34 Unfortunately, these patient characteristics were not available for our study, so we cannot compare the predictive value of these clinical characteristics to the predictive value of BASIS.
This was a retrospective cost analysis of observational data at a single institution and, thus, may not be generalizable to other institutions. Instead of being treated by using a standardized study protocol, patients received a normal standard of care. We did not collect or study the effect of physician variation on patient costs or outcomes. This study has a relatively small sample size, in which death and major complications were rare events and, as such, the frequency of these events may not be representative of the true frequency.
We have exclusively studied acute hospitalization cost from the hospital perspective; physician reimbursement was not included because it is not tracked by the hospital accounting system and is difficult to estimate. Costs of patient care after discharge, including outpatient visits, readmission, or rehabilitation and physiotherapy, are also excluded. In this study, we made no attempt to estimate the societal cost of acute stroke care, such as lost wages or lost productivity to the patient with stroke or their family members.
Future Directions
The relationship between BASIS classification and resource use and in-hospital cost indicates further investigation into the usefulness of BASIS for clinical and system planning purposes. Foremost is the inclusion of clinical rating scales in the statistical assessment of BASIS to determine the incremental benefit added by each in predictive models of outcome, resource use, and cost. In addition, the BASIS classification may be further developed by including perfusion data provided by either CT or MR imaging. Finally, we aim to use the BASIS classification method in ongoing evaluations of therapies such as recanalization. In future studies, we hope to include other outcome measures such as days in rehabilitation, readmissions, and postrecovery quality of life.
Conclusions
We demonstrated that the in-hospital cost of stroke and the discharge outcome of the patient can be predicted by using BASIS, a dichotomous classification of stroke severity based on neuroimaging evidence. Patients with minor stroke use fewer hospital resources, have a shorter length of stay, and are often discharged home. Patients with major stroke use more hospital resources, require longer hospitalization, cost >3 times more, and are frequently discharged to rehabilitation and skilled nursing facilities. The incorporation of an imaging-based classification such as BASIS as a routine part of the stroke care may provide a metric to predict and assess hospital costs related to this major disease.
Acknowledgments
We thank Elkan Halpern, PhD, of the Institute for Technology Assessment at Massachusetts General Hospital for his assistance guiding the statistical analysis and Julian He, MD, of the Neuroradiology Division at Massachusetts General Hospital for reviewing imaging data.
Footnotes
The work was funded by Stroke Pathways Project, M. Steinberg, Harvard Design School, and the National Institutes of Health Grant NS50041 (to R.G.G.).
Dr González reports receiving lecture fees from Bayer and from General Electric.
Preliminary results previously presented at: Annual Meeting of the American Society of Neuroradiology, June 2–5, 2008; New Orleans, La.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics: 2007 update—a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007;115:e69–171. Epub 2006 Dec 28 [DOI] [PubMed] [Google Scholar]
- 2.Adult Mortality by Cause: US, 1999–2004—Health Data for All Ages, Centers for Disease Control. US Department of Health and Human Services,2005
- 3.Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: national health interview survey, 2005 Vital Health Stat 10 2006. :1–153 [PubMed]
- 4.Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults: United States, 1999. MMWR Morb Mortal Wkly Rep 2001;50:120–25 [PubMed] [Google Scholar]
- 5.Taylor TN, Davis PH, Torner JC, et al. Lifetime cost of stroke in the United States. Stroke 1996;27:1459–66 [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AI, Suri MF, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke 2007;38:2180–84. Epub 2007 May 24 [DOI] [PubMed] [Google Scholar]
- 7.Reed SD, Blough DK, Meyer K, et al. Inpatient costs, length of stay, and mortality for cerebrovascular events in community hospitals. Neurology 2001;57:305–14 [DOI] [PubMed] [Google Scholar]
- 8.Tung CY, Granger CB, Sloan MA, et al. Effects of stroke on medical resource use and costs in acute myocardial infarction. Circulation 1999;99:370–76 [DOI] [PubMed] [Google Scholar]
- 9.Holloway RG, Witter DM Jr, Lawton KB, et al. Inpatient costs of specific cerebrovascular events at five academic medical centers. Neurology 1996;46:854–60 [PubMed] [Google Scholar]
- 10.Diringer MN, Edwards DF, Mattson DT, et al. Predictors of acute hospital costs for treatment of ischemic stroke in an academic center. Stroke 1999;30:724–28 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JB, Ballard DJ, Whisnant JP, et al. What role do neurologists play in determining the costs and outcomes of stroke patients? Stroke 1996;27:1937–43 [DOI] [PubMed] [Google Scholar]
- 12.Samsa GP, Bian J, Lipscomb J, et al. Epidemiology of recurrent cerebral infarction: a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999;30:338–49 [DOI] [PubMed] [Google Scholar]
- 13.Leibson CL, Hu T, Brown RD, et al. Utilization of acute care services in the year before and after first stroke: a population-based study. Neurology 1996;46:861–69 [PubMed] [Google Scholar]
- 14.Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–28 [DOI] [PubMed] [Google Scholar]
- 15.Mushinski M. Variations in average charges for strokes and TIAs: United States, 1995. Stat Bull Metrop Insur Co 1997;78:9–18 [PubMed] [Google Scholar]
- 16.Torres-Mozqueda F, He J, Yeh IB, et al. An acute stroke classification instrument that includes CT or MR angiography: The Boston Acute Stroke Imaging Scale (BASIS). AJNR Am J Neuroradiol 2008;29:1111–17. Epub 2008 May 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 18.Medical Component of the Consumer Price Index: 2007. Washington, DC; US Bureau of Labor Statistics,2007
- 19.Chang KC, Tseng MC, Weng HH, et al. Prediction of length of stay of first-ever ischemic stroke. Stroke 2002;33:2670–74 [DOI] [PubMed] [Google Scholar]
- 20.Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–31 [DOI] [PubMed] [Google Scholar]
- 21.Caplan LR. Treatment of acute stroke: still struggling. JAMA 2004;292:1883–85 [DOI] [PubMed] [Google Scholar]
- 22.Baird AE, Dambrosia J, Janket S, et al. A three-item scale for the early prediction of stroke recovery. Lancet 2001;357:2095–99 [DOI] [PubMed] [Google Scholar]
- 23.Demaerschalk BM, Durocher DL. How diagnosis-related group 559 will change the US medicare cost reimbursement ratio for stroke centers. Stroke 2007;38:1309–12. Epub 2007 Mar 1 [DOI] [PubMed] [Google Scholar]
- 24.Ingall TJ, O'Fallon WM, Asplund K, et al. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke 2004;35:2418–24. Epub 2004 Sep 2 [DOI] [PubMed] [Google Scholar]
- 25.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 26.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke 2008;39:1205–12. Epub 2008 Feb 28 [DOI] [PubMed] [Google Scholar]
- 27.Schwamm LH, Pancioli A, Acker JE 3rd, et al. Recommendations for the establishment of stroke systems of care. Stroke 2005;36:690–703 [DOI] [PubMed] [Google Scholar]
- 28.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the brain attack coalition. Stroke 2005;36:1597–616 [DOI] [PubMed] [Google Scholar]
- 29.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20 [DOI] [PubMed] [Google Scholar]
- 30.Pomerantz SR, Harris GJ, Desai HJ, et al. Computed tomography angiography and computed tomography perfusion in ischemic stroke: a step-by-step approach to image acquisition and three-dimensional postprocessing. Semin Ultrasound CT MR 2006;27:243–70 [DOI] [PubMed] [Google Scholar]
- 31.González RG, Hirsh JA, Koroshetz WJ, et al. Acute Ischemic Stroke: Imaging and Intervention. Heidelberg, Germany: Springer-Verlag;2006
- 32.Johnston KC, Connors AF Jr, Wagner DP, et al. Predicting outcome in ischemic stroke: external validation of predictive risk models. Stroke 2003;34:200–02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rundek T, Mast H, Hartmann A, et al. Predictors of resource use after acute hospitalization: the Northern Manhattan Stroke Study. Neurology 2000;55:1180–87 [DOI] [PubMed] [Google Scholar]
- 34.Chang KC, Tseng MC. Costs of acute care of first-ever ischemic stroke in Taiwan. Stroke 2003;34:e219–221 [DOI] [PubMed] [Google Scholar]