Abstract
BACKGROUND AND PURPOSE: Complete labyrinthine aplasia (CLA), also referred to as Michel aplasia, is a severe congenital anomaly of the inner ear, defined by the complete absence of inner ear structures. The purpose of this study was to document the imaging findings in a series of patients with CLA, with review of the literature, to better understand this anomaly.
MATERIALS AND METHODS: The CT and MR imaging findings of 9 patients (14 ears with CLA) were retrospectively evaluated. The audiologic tests and patient charts were also retrospectively reviewed.
RESULTS: CLA was bilateral in 5 and unilateral in 4 patients. The petrous bone was hypoplastic in all 14 ears, but the otic capsule was aplastic in only 5. The middle ear and mastoid volumes were decreased in most of the ears. The stapes was aplastic in 1 ear and was dysplastic in 5 ears. The internal acoustic canal was aplastic in 4 ears and markedly narrowed in 10 ears. The facial nerve canal showed a variety of anomalies and aberrant courses in 11/14 ears. The bony covering of the jugular bulb was defective in 9 ears. Tegmen tympani defects were seen in 3 patients, and there were several accompanying skull base and posterior fossa anomalies.
CONCLUSIONS: Although CLA is a rare developmental anomaly, its accurate diagnosis and its differential diagnosis from labyrinthine ossificans is crucial. Proper guidance of these patients for brain stem implantation in the critical period of brain development depends on the recognition of the characteristic imaging findings of CLA.
Complete labyrinthine aplasia (CLA, Michel anomaly) is a severe anomaly of the ear defined by total absence of inner ear structures.1,2 This rare anomaly was first described by Michel1 in 1863 and has only been reported as selected case reports in the literature.3–11 We report the first series of CLA cases consisting of 14 ears in 9 patients, emphasizing their distinguishing and unique features.
Materials and Methods
Patients
A data base search was performed to identify all patients with an imaging diagnosis of CLA among patients who were scanned between February 2000 and December 2007 in a single tertiary care health center. Nine patients were identified. High-resolution CT and MR imaging scans of these 9 patients (14 ears with CLA) were retrospectively evaluated by a neuroradiologist. An experienced audiologist (A.A.) performed a retrospective review of patients’ audiologic tests. The medical charts that were available in 6 patients were also noted for pertinent findings.
Imaging
Of 9 patients with CT scans, 6 had imaging on a 4-channel multidetector CT scanner (Somatom Plus 4 Volume Zoom; Siemens, Erlangen, Germany) in the axial plane. The images were obtained with 0.5-mm collimation and 0.5-mm thickness. In 3 patients (patients 5–7), CT was performed with an older generation helical CT scanner (Tomoscan AV; Philips Medical Systems, Eindhoven, the Netherlands), with 1-mm section thickness (direct axial and coronal scans), and only hard copy images were available for interpretation.
Seven patients had MR imaging examinations of the temporal bones. MR imaging was performed with either a 3T (Magnetom Allegra, Siemens) or a 1.5T scanner (Symphony, Siemens) by using a standard head coil. The standard temporal bone protocol included transverse T1-weighted imaging (T1WI), transverse T2-weighted imaging (T2WI), and axial and sagittal oblique 3D–constructive interference in steady state (3D-CISS) imaging.
Image Evaluation
The CT and MR images were retrospectively reviewed by a neuroradiologist with experience in head and neck imaging (B.O.), who assessed the following temporal bone structures: petrous apex, otic capsule, mastoid volume and level of the mastoid tip, middle ear volume and recesses, ossicles, facial canal and nerve, jugular bulb, carotid canal, internal auditory canal (IAC), and vestibulocochlear nerve. Additionally, skull base and posterior fossa structures were also evaluated.
Audiologic Assessments
Transient evoked oto-acoustic emission (TEOAE) was used for the first assessment in all subjects after otoscopic examination. Pure-tone audiometry, acoustic reflexes, and the auditory brain stem response (ABR) test were also performed in all patients.
Results
Of the 9 patients, 8 were female and 1 was male, with a mean age of 7.3 years (range, 3–17 years) at the time of the imaging.
None of the patients had any family history of consanguinity or sensorineural hearing loss. None of the patients were related. The prenatal, natal, and postnatal histories of the patients were unremarkable except for an episode of nephrotic syndrome in patient 4 and a history of adenoidectomy in patient 3. There was microtia in patient 5. Patient 3 had clinical findings consistent with autism and slightly decreased vision. Otherwise, the physical examination findings were unremarkable for the remainder of the patients.
The audiologic assessments did not reveal detectable responses in TEOAE, ABR, and acoustic reflexes in any of the subjects with bilateral CLA except for limited findings in pure-tone audiometry. Profound hearing loss was found in all subjects. In patients 4, 5, and 8, severely decreased hearing was also found in the contralateral ear.
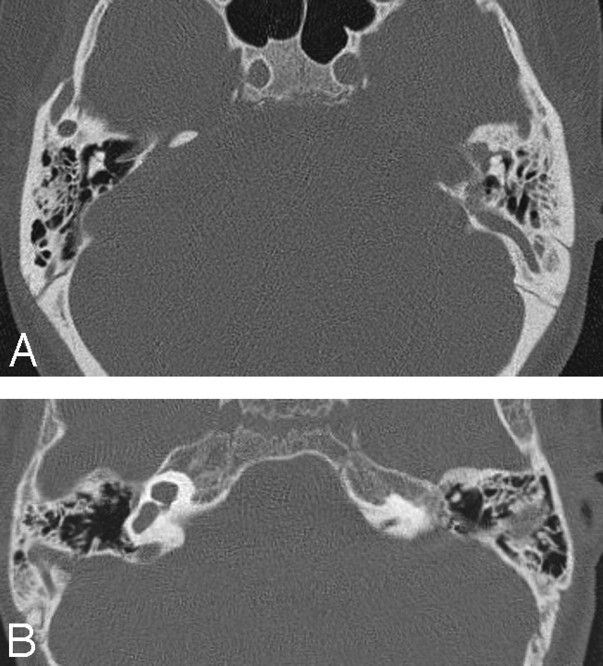
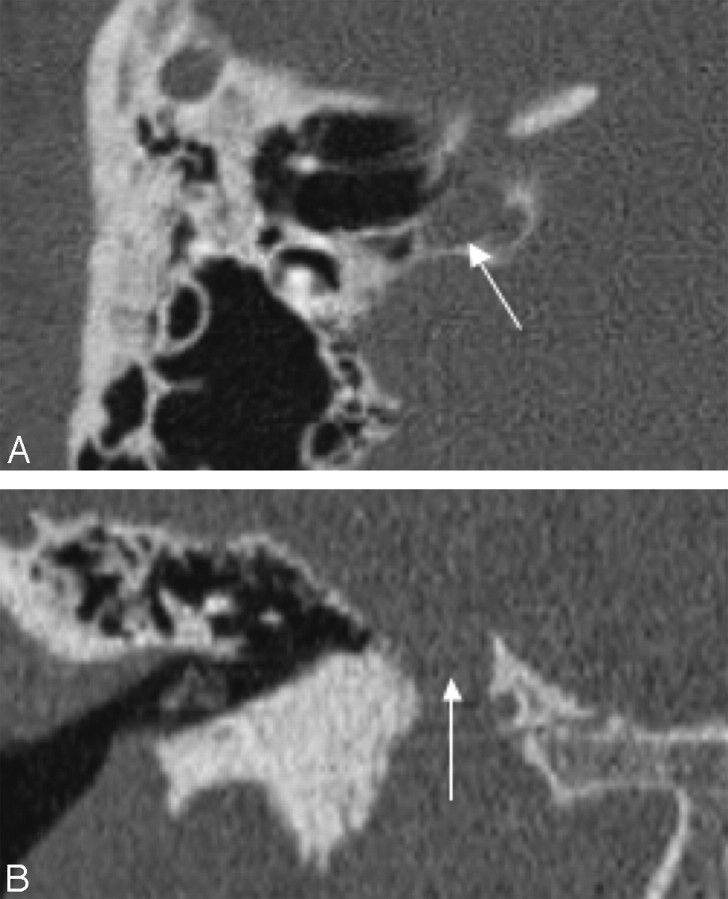
The imaging findings are summarized in the Table. Seven patients had both CT and MR imaging, but in 2 patients, only CT images were available for interpretation. CLA was bilateral in 5 and unilateral in 4 patients (Fig 1A, -B). The patients with bilateral CLA did not have more pronounced temporal bone or associated anomalies compared with those with unilateral CLA. The petrous bone was hypoplastic in all 14 ears with CLA, but the hypoplasia was mild in 5 (Fig 1B). The otic capsule was aplastic in 5 and hypoplastic in 9 ears.
Imaging findings of the ears with labyrinthine aplasia
| Patient | CT/MRI | CLA | Otic Capsule | Middle Ear | Ossicle Anomaly | IAC | Cochlear Nerve | Facial Nerve Course | Jugular Anomaly | Posterior Fossa Anomaly | Skull Base−Other Bone Anomaly |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +/+ | R | Apl | Hypopl | + | Apl | Apl | Aberr | + | Large CPA | Narrow clivus |
| L | Apl | Hypopl | + | Apl | Apl | Aberr | + | ||||
| 2 | +/− | L | Hypopl | Hypopl | + | Narrow | ? | Aberr | + | R CPA arachnoid cyst | Low tegmen tympani, narrow clivus |
| 3 | +/+ | R | Apl | Hypopl | + | Narrow | Apl | Aberr | + | – | Tegmental defect (L), low tegmen tympani (R), narrow clivus |
| L | Hypopl | Hypopl | – | Narrow | Apl | Aberr | – | ||||
| 4 | +/+ | L | Hypopl | Hypopl | + | Narrow | Apl | Aberr | + | Split brain stem | Narrow clivus |
| 5 | +/+ | R | Hypopl | Hypopl | ? | Narrow | Apl | Aberr | + | Wide 4th v lateral recess | Tegmental defect, narrow clivus |
| 6 | +/+ | R | Apl | Hypopl | ? | Narrow | Apl | Aberr | + | Pontine malformation | Encephalocele |
| L | Apl | Hypopl | + | Apl | Apl | Aberr | + | ||||
| 7 | +/− | R | Hypopl | N | ? | Apl | ? | ? | + | – | – |
| L | Hypopl | N | ? | Narrow | ? | Aberr | – | ||||
| 8 | +/+ | R | Hypopl | Hypopl | + | Narrow | Apl | Aberr | – | – | – |
| 9 | +/+ | R | Hypopl | Hypopl | – | Narrow | Apl | Aberr | – | Chiari 1 | – |
| L | Hypopl | Hypopl | + | Narrow | Apl | Aberr | + |
Note:—MRI indicates MR imaging; R, right; L, left; Apl, aplasia; Hypopl, hypoplasia; N, normal; ?, could not be evaluated in detail; Aberr, aberrant; CPA, cerebellopontine angle; 4th v, fourth ventricle; +/+, both present; +/−, MRI absent; –, not found; CLA, complete labyrinthine aplasia; IAC, internal auditory canal; +, present.
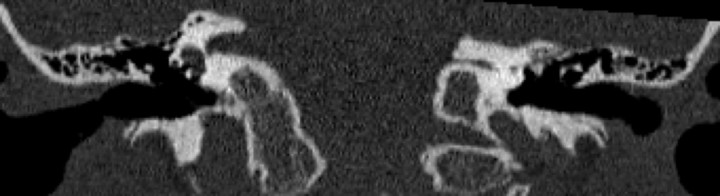
Fig 1.
A, Axial CT scan of the temporal bones of patient 1 shows bilateral absence of total inner ear structures with aplasia of the otic capsules bilaterally. Note a large emissary vein on the left. B, CT scan of patient 4 reveals unilateral CLA on the left with a type 1 incomplete cochlear partition on the right. The petrous bone and the otic capsule are hypoplastic on the left.
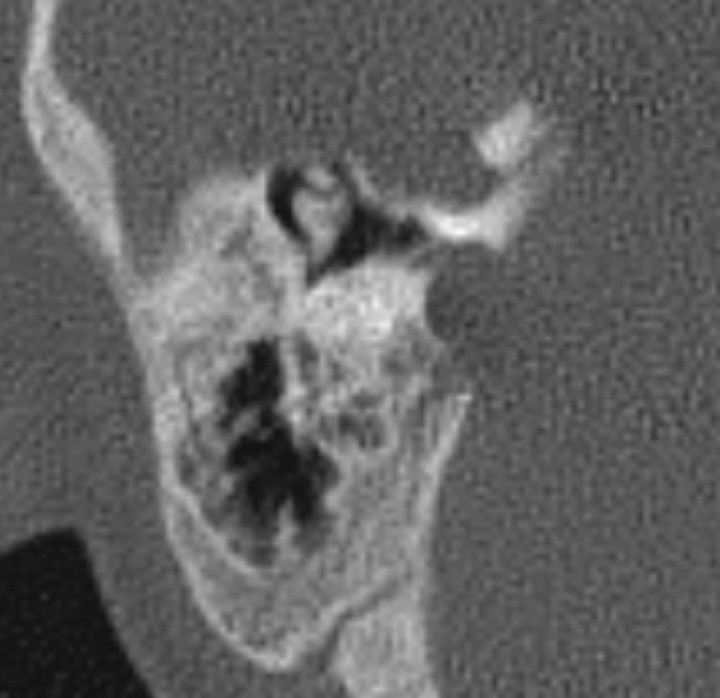
The flattening of the medial wall of the middle ear (cochlear promontory) was apparent in 9 of 14 ears (Fig 2) but subtle in the remaining 5. The middle ear volume was decreased in 12 ears, and the hypoplasia mostly involved the hypotympanum (Fig 3). The middle ear medial and posterior recesses were absent in all except 4 ears. Hypoplasia of the mastoid bone was observed in 11 ears, with the posterior air cells and mastoid antrum more frequently affected. In 8 ears, the mastoid bone height was minimally reduced, and the mastoid tips were hypoplastic and high-positioned (Fig 4).
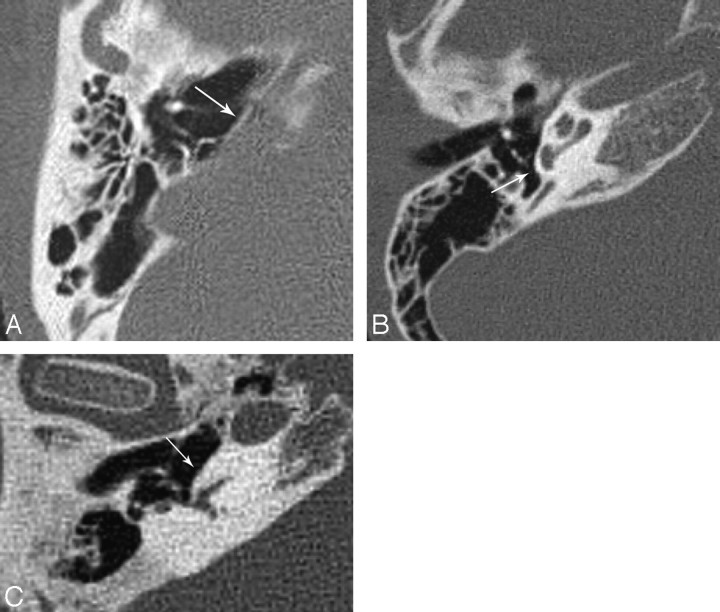
Fig 2.
A, Flattening of the cochlear promontory (arrow) in CLA. B, Normal appearance of the cochlear promontory (arrow) and inner ear in a healthy patient. C, Normal appearance of the promontory (arrow) in a patient with severe LO. Note the normal size of the petrous bone in the patient with LO.
Fig 3.
Narrowing of the middle ear and mastoid antrum.
Fig 4.
A, Sagittal reformatted image of the temporal bone CT scan of an ear with CLA, demonstrating slight hypoplasia of the mastoid tip. B, Normal appearance of the mastoid tip in a patient of the same age.
The stapes was aplastic in 1 ear and had a dysplastic appearance in 5 ears, characterized by the absence of the footplate and/or an increased angle between the crura. In 1 other patient, the stapes was rotated and displaced anterolaterally and did not contact the medial wall of the middle ear (Fig 5). The ossicles were completely normal in 2 ears. In the remaining 5 ears, the ossicles could not be evaluated in detail due to the section thickness and absence of digital images.
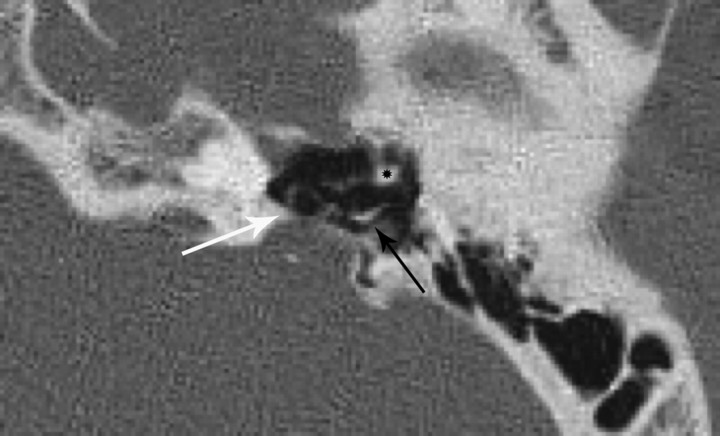
Fig 5.
Anterolateral displacement and rotation of the stapes (white arrow) in an ear with CLA. Note the short process of the incus (black arrow) and head of the malleus (asterisk).
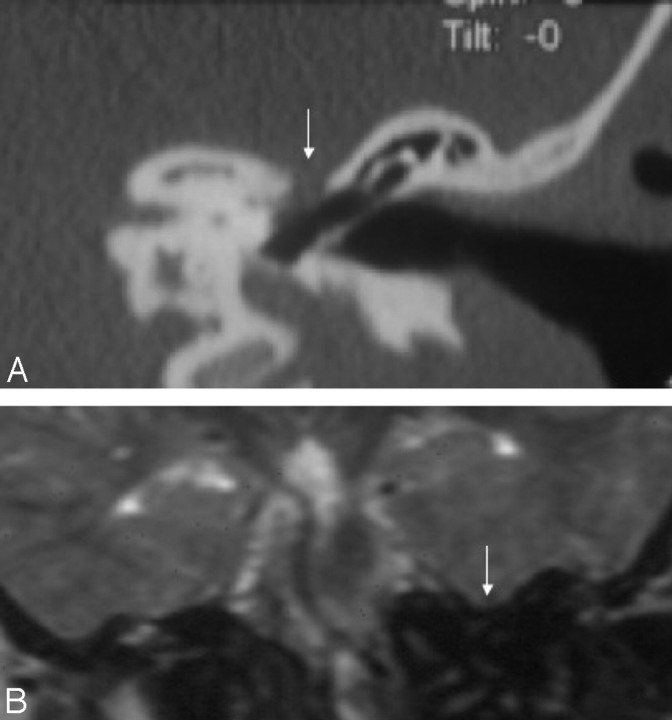
The IAC was aplastic in 4 ears and markedly narrow in 10 (Fig 6). The vestibular and cochlear nerves were aplastic in all patients with CLA who had MR imaging available for interpretation (Fig 7). The facial nerve canal showed a variety of anomalies and aberrant courses (Fig 8). The facial canal was not clearly visible in 1 patient, and its course could not be well appreciated.
Fig 6.
Coronal reformatted image of a patient with unilateral (left-sided) CLA reveals severe narrowing of the IAC on the left.
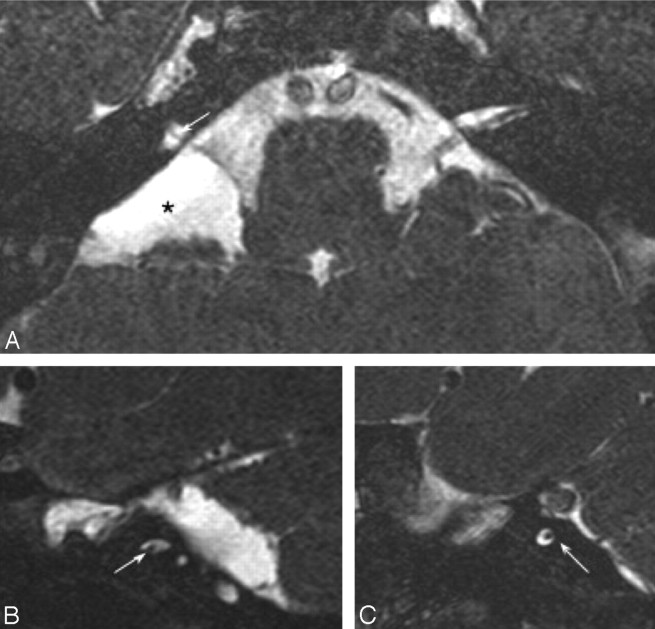
Fig 7.
Axial (A) and bilateral sagittal oblique (B and C) 3D-CISS images of a patient with bilateral CLA demonstrates narrowing of the IACs bilaterally, containing only facial nerves (arrows). An arachnoid cyst (asterisk) is seen within the right cerebellopontine angle (A).
Fig 8.
Axial CT scan of the temporal bones shows an aberrant facial canal with anterior displacement of the labyrinthine segment. The tympanic segment is short with a normal-appearing mastoid segment (not shown). The IAC is severely narrowed.
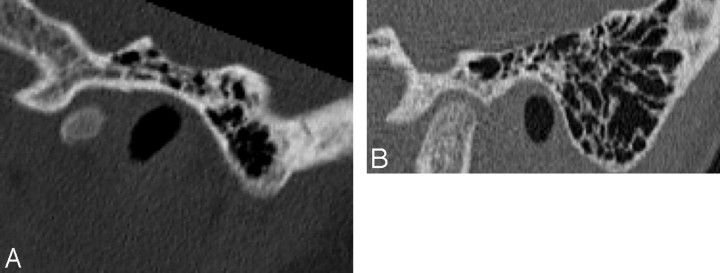
The images did not reveal an aberrant internal carotid artery (ICA), and there was no evidence of a persistent stapedial artery. However, the posterior genu of the petrous segment of the ICA was short in 3 patients. A defect of the jugular bulb bony covering was noted in 9 ears (Fig 9). The defect was located laterally in 1 patient, with protrusion of the bulb into the middle ear, consistent with a dehiscent jugular bulb. In addition, jugular diverticula were present in 7 ears.
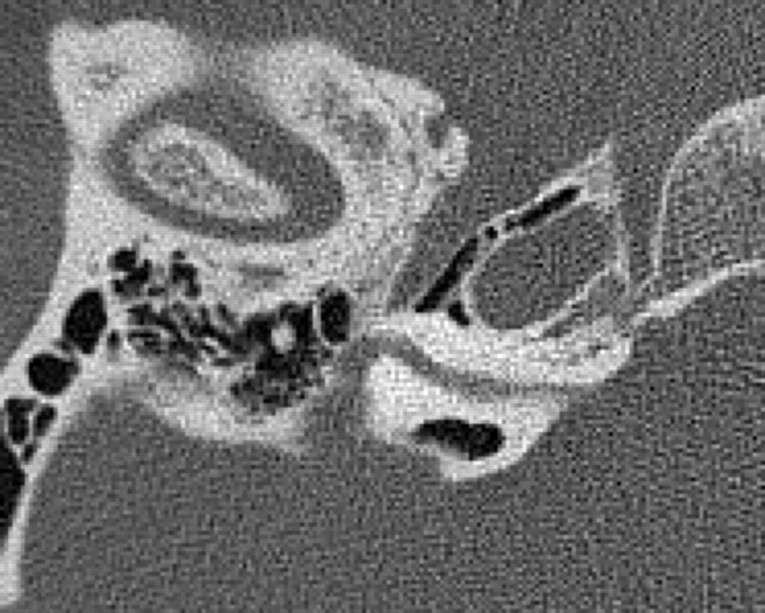
Fig 9.
Axial CT image (A) and coronal reformatted image (B) demonstrate a high-riding jugular bulb (arrow), with an absent bony covering superiorly.
In patients with unilateral CLA, the contralateral ears were also markedly dysplastic. The anomalies included the following: severe cochlear and vestibular hypoplasia with IAC narrowing in patient 2, type 1 incomplete cochlear partition (ICP) with cystic dilation of the vestibule and enlargement of the vestibular aqueduct in patients 4 (Fig 1B) and 8, and type 2 ICP with dysplastic vestibule and semicircular canals in patient 5.
There were several accompanying skull base anomalies in patients with CLA. The clivus and, in particular, its basisphenoid part, was narrow in 5 patients. The posterior aspect of the clivus had a more concave appearance with an accompanying J-type sella turcica in patient 1. Large bony defects at the tegmen were seen in 3 patients, with soft-tissue protrusions into the middle ear, suggestive of small skull base encephaloceles. However, the presence of the encephalocele could be verified by MR imaging in only a single patient (Fig 10). There was also no surgical confirmation of these probable encephaloceles. The posterior fossa was small in 5 patients. A small arachnoid cyst was present in the right cerebellopontine cistern of patient 5, and the right lateral recess of the fourth ventricle was asymmetrically wider in patient 3. Both cerebellopontine cisterns were prominent in 1 patient without evidence of a space-occupying lesion. One patient had findings consistent with incomplete split brain stem, 1 had Chiari 1 malformation, and 1 had a decreased anteroposterior diameter of the pons. In addition to the temporal bone, skull base and posterior fossa anomalies were present in 2 patients who had cervical vertebral anomalies with fusion defects in the C1 arches and cervical segmentation anomalies.
Fig 10.
A, Coronal CT image shows a bony defect along the tegmen tympani with soft-tissue protrusion (arrow) into the middle ear. B, Coronal T2WI image of the same patient reveals inferior displacement of the temporal lobe (arrow) through this bony defect, consistent with a small encephalocele.
Discussion
CLA, also called Michel anomaly or Michel aplasia, is a rare congenital anomaly of the inner ear, constituting 1% of cochlear bony abnormalities.2 CLA is defined by complete absence of inner ear structures due to a developmental arrest of the otic placode before the third gestational week.12 This anomaly was first reported by Michel in the autopsy report of a 12-year-old boy with a history of congenital deafness.1 The child described by Michel had bilateral complete absence of inner ear structures with markedly hypoplastic otic capsules and aplastic cochlear nerves. The report also included a detailed description of several associated anomalies.1 This anomaly was also documented in a few reports (9 case reports describing a total of 12 patients) in the English-language literature,3–11 all describing several associated anomalies. Until recently, the only treatment option for this developmental anomaly was vibrotactile hearing devices.9,13 However, with the development of implant technology, brain stem implantation is now an option for these patients who have functional cochlear nuclei despite cochlear nerve and inner ear aplasia.9 We aimed to evaluate the pertinent findings in our series with a review of the case reports appearing in the literature to date and to relate them to embryonic development to increase our understanding of this anomaly.
The inner ear anomalies are a complex group of disorders, the radiologic findings of which may be difficult to interpret. The diagnosis may be further complicated due to the usage of inappropriate terminology because different types of anomalies have been described by the same author in the literature (ie, “Michel aplasia” for CLA and “Michel dysplasia” for common cystic cavity14). Therefore, it may be better to adapt descriptive terminology such as CLA instead of Michel anomaly.
In the literature, “CLA” is sometimes defined as an anomaly with the absence of normal inner ear structures, which may be replaced by a small single cystic cavity or several small cystic cavities.15 However, none of our 14 ears and none of the reported cases in the literature had evidence of those cavities. When present, the finding of cystic cavity (or cavities) should imply a developmental arrest occurring after the development of the otocyst and should be considered as an entity separate from CLA.
Most (88.8%) of our patients were female; however, this anomaly was reported both in girls and boys, without a female dominance.2–10
Most of the reported cases of CLA in the literature,1,2,7,9,10 including the original article by Michel,1 described bilateral aplasia of the labyrinths. However, as also previously reported by Jackler et al,2 Isenberg and Tubergen,6 and Keeney et al,8 4 (44.4%) of our patients had unilateral CLA (as illustrated by Fig 1B); thus, the presence of unilateral aplasia should not exclude the diagnosis of CLA.
We have reviewed the imaging findings in our patients with respect to the anomalies described by Michel,1 and all the temporal bone structures (especially the ones that are embryologically related to the otic capsule) were evaluated.
The petrous bone was abnormal in all our patients, with complete aplasia-hypoplasia of the otic capsule and accompanying petrous apex hypoplasia. This finding was also described by Michel,1 Keeney et al,8 Marsot-Dupuch et al,9 and Daneshi et al,10 and though not mentioned in the text of the manuscript, it could be seen on the figures of case reports by Hersh et al7 and Gross et al.11 In several CLA definitions, the otic capsule is described as being entirely absent, and this is emphasized as a differential feature from labyrinthine ossificans (LO).16 The otic capsule may indeed be completely aplastic (and was the case in 5 of our imaged ears, as seen in Fig 1A); however as observed in 9 of our imaged ears (Figs 1B, 5, and 8), the otic capsule is not necessarily aplastic in labyrinthine aplasia.
The difference in the degree of otic capsule malformation may be related to the timing of the insult−developmental arrest. The otic capsule is formed by the differentiation of the mesenchyme surrounding the otic placode, and this differentiation is induced by the epithelium of the ectodermal otic placode.9,12,17 Thus, the anomalous formation of the otic capsule may also be related to the degree to which the periotic mesenchyme is affected by the arrested development of the otic placode, resulting in a spectrum of findings. Nevertheless, the malformation or underdevelopment of the otic capsule and petrous apex or both were invariably present in all our patients and in the cases previously published. We think that the petrous bone hypoplasia and especially otic capsule malformation (and not only otic capsule aplasia) might constitute a major distinguishing feature between CLA and LO. However, the presence of a cochlear malformation (and the accompanying otic capsule malformation) does not preclude coexistence of LO, which can also occur in malformed inner ears.
Flattening of the medial wall of the middle ear is also a well-described and known feature helping to differentiate CLA from LO (Fig 2A, -C).18 Although this finding was present in all of our patients, the flattening was subtle and hard to appreciate in 4 ears. It should, therefore, not be used as a pathognomonic feature (“must have”) of CLA, especially by the inexperienced eye.
Middle ear cavities were mildly hypoplastic in most of our patients (Fig 3), and the decreased volume was prominent in the hypotympanum. This finding may be simply related to the decreased height of the petrous bone, but it may also result from altered development of the otic capsule because the hypotympanum is partly formed by the latter.19 Similarly, the mastoid bone was slightly hypoplastic in most patients, and it had mildly decreased height in 8 ears (Fig 4A, -B). This association has not been reported since the initial article by Michel, who described total lack of mastoid air cells and the absence of the mastoid tip.1 If this appearance is indeed an underdevelopment of the mastoid bone accompanying CLA, it may again be due to the fact that the medial portion of the mastoid develops from the periosteal layer of the bony labyrinth and thus is dependent on proper otic capsule development.20 The above-described finding may of course be related to mastoid sclerosis (resulting from chronic otomastoiditis), and it has been previously demonstrated that the length of mastoid process is a correlate of mastoid pneumatization.21 However, mastoid sclerosis was present only in 5 ears (in 3/8 ears with decreased mastoid height). Mastoid process development occurs after the second year of age, and imaging performed before then may also give a false impression of a hypoplastic mastoid tip. Our findings are unlikely to result from this physiologic underdevelopment because the youngest patient in our series was 3 years old, and Michel's patient was 12 years old.1
Stapes aplasia-malformation is a previously reported feature of CLA1,9 and was also present in 7 (77.7%) of our patients. This association is not surprising because the medial surface of the stapes footplate arises from the otic capsule.20 The malformations in our patients included widening of the stapedial crura and rotation-displacement (Fig 5) and absence of the footplate. The obturator foramen is formed by the involution of the stapedial artery. The widening of this foramen is probably linked to the absence of the footplate or may also result from a delayed or altered involution of the stapedial artery in CLA. The annular ligament of the stapes also arises from the otic capsule, and lack of development—maldevelopment of this ligament may explain the displacement of the stapes in our patient.
The normal development of the facial nerve is dependent on the normal formation of surrounding structures.22–27 The final position of the facial canal is determined by development of the stapes and membranous labyrinth; therefore, an anomalous course of the facial canal is a predictable finding in CLA. As expected, all of our patients with CLA (except 1) revealed an anomalous facial nerve course. In 7 ears, the aberrant course of the labyrinthine segment was directed more anteromedially (Fig 8), a course that was previously described with other cochlear malformations.25 In the remaining ears, the labyrinthine segment of the canal was either more inferior or posterior than its normal position as previously described by Michel1 and Marsot-Dupuch et al.9 Most interesting, none of our patients had any deficit in facial nerve function; this finding proves once again that function of the facial nerve is independent of the otic capsule formation.1,9
The eighth cranial nerve and its ganglion are formed by cells from the otic vesicle during the fourth gestational week.20 The otic vesicle also induces the persistence and growth of the eighth nerve via a growth factor.28 The vestibulocochlear nerve should then be aplastic in CLA by definition, due to the lack of otic vesicle development, and was indeed absent in all our patients scanned with MR imaging. The cartilage formation of the otic capsule is inhibited medially to form the internal acoustic canal. This inhibition is induced by the presence of the vestibulocochlear nerve.29 Consequently, in the absence of the eighth nerve, the IAC caliber decreases in size, only containing the facial nerve.30 Similarly, 10 ears in our series demonstrated marked narrowing of the canal, which probably only contained the facial nerve (proved in 8 ears with MR imaging and illustrated in Fig 7). However, the IAC was aplastic in 4 of our ears, similar to the CLA cases reported by Keeney et al8 and Marsot-Dupuch et al.9 The spectrum of atresia-hypoplasia may depend on the time of arrested development and/or the amount of tissue cells affected by a specific insult.
The jugular bulb had a defect in its bony covering in 9 ears (Fig 9), with marked ectasia and diverticula in most patients. A low tegmen tympani was seen in 2 patients. It is important to differentiate the low position of the tegmen from its true bony defects, because the latter can be associated with dural defects and encephaloceles. A low tegmen tympani rarely causes any obstacle to the middle ear surgery; however, bony defects at the tegmen and associated encephalocele may become surgical hazards if the dura is inadvertently penetrated. The fact that jugular bulb bony defects and ectasia are frequently associated with CLA may be related to the abnormal development of the jugular bulb with persistence of embryonic venous structures (such as the primary head sinus). Nevertheless, the possible occurrence of these anomalies is especially important in cases in which a mastoid or a middle ear intervention is planned because a dehiscent jugular bulb or encephalocele can become a hazard during such surgery.
Similarly, it is important to consider the increased incidence of posterior fossa anomalies in patients with CLA recommended for surgery. Although the asymmetric dilation of the lateral recess of the fourth ventricle has been described only in 1 case, it is of particular concern because the dilation may allow the migration or rotation of the electrode array following brain stem implantation.31
CLA has been reported to occur due to thalidomide exposure,3,5 but a prenatal insult could not be identified in any of our patients, and there was no genetic predisposition. However, given the associated anomalies in the skull base, cervical vertebrae, and brain, a critically timed insult around 2–3 weeks of gestational age or a genetic defect in a common precursor cell-molecule should be considered. Even in patients with unilateral CLA, the contralateral side is often dysplastic (as in all the cases in the literature except the patients with CLA in the series of Jackler et al,2 who had normal hearing in the contralateral ear).8
Conclusions
CLA is a rare developmental anomaly. Its accurate diagnosis and differential diagnosis from LO is fundamentally important in the current era of hearing-device implantation. Guiding these patients to brain stem implantation in the critical time period of the brain development also depends on recognition of imaging findings by the radiologists. In this context, it is important to acknowledge that this anomaly encompasses a spectrum of radiologic findings varying from petrous aplasia to otic capsule hypoplasia and may also be associated with anomalies of other temporal bone and skull base structures, such as tegmental defects, mastoid-middle ear hypoplasia, and jugular bulb variations.
References
- 1.Michel P. Mémoire sur les anomalies congénitales de l’oreille interne. Gazette Méd de Strasbourg 1863. :55–58
- 2.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 1987;97:2–14 [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen MB, Kristensen HK, Buch NH. Thalidomide induced aplasia of the inner ear. J Laryngol 1964;78:1095–101 [DOI] [PubMed] [Google Scholar]
- 4.Paparella MM, el-Fiky FM. Mondini's deafness. Arch Otolaryngol 1972;95:134–40 [DOI] [PubMed] [Google Scholar]
- 5.Lindsay JR. Profound childhood deafness: inner ear pathology. Ann Otol Rhinol Laryngol 1973;82:1–73 [PubMed] [Google Scholar]
- 6.Isenberg SF, Tubergen LB. Unilateral complete aplasia of the inner ear with associated tracheoesophageal fistula: report of a case. Otolaryngol Head Neck Surg 1979;87:435–39 [DOI] [PubMed] [Google Scholar]
- 7.Hersh JH, Ganzel TM, Fellows RA. Michel's anomaly, type I microtia and microdontia. Ear Nose Throat J 1991;70:155–57 [PubMed] [Google Scholar]
- 8.Keeney G, Gebarski S, Brinderg JA. CT of severe inner ear anomalies, including aplasia in a case of Wildervanck syndrome. AJNR Am J Neuroradiol 1992;13:201–02 [PMC free article] [PubMed] [Google Scholar]
- 9.Marsot-Dupuch K, Dominguez-Brito A, Ghasli K, et al. CT and MR findings of Michel anomaly: inner ear aplasia. AJNR Am J Neuroradiol 1999;20:281–84 [PMC free article] [PubMed] [Google Scholar]
- 10.Daneshi A, Farhadi M, Asghari A, et al. Three familial cases of Michel's aplasia. Otol Neurotol 2002;23:346–48 [DOI] [PubMed] [Google Scholar]
- 11.Gross M, Eliashar R, Elidan J. Michel's aplasia. Otol Neurotol 2005. :26:547. [DOI] [PubMed] [Google Scholar]
- 12.Sadler TW. Langman's Medical Embryology. 10th ed. Baltimore: Lippincott Williams & Wilkins;2006. :317–24
- 13.Brookhouser PE. Sensorineural hearing loss in children. In: Cummings CW, Fredrikson JM, Harker LA, et al, eds. Otolaryngology-Head and Neck Surgery. 2nd ed. St Louis: Mosby-Year Book;1993. :3080–102
- 14.Kavanagh KT, Magill HL. Michel dysplasia: common cavity inner ear deformity. Pediatr Radiol 1989;19:343–45 [DOI] [PubMed] [Google Scholar]
- 15.Romo VR, Casselman JW, Robson CD. Temporal bone: congenital anomalies. In: Som PM, Curtin HD, eds. Head and Neck Imaging. 4th ed. St. Louis: Mosby;2003. :1109–71
- 16.Jackler RK. Congenital malformations of the inner ear. In: Cummings CW, Flint PW, Harker LA, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 4th ed. Philadelphia: Elsevier Mosby;2005. :4413–14
- 17.Van de Water TR, McPhee JR. Determinants of otic capsule formation. Laryngoscope 1987;97 (3 pt 1):315–22 [PubMed] [Google Scholar]
- 18.Fisher NA, Curtin HD. Radiology of congenital hearing loss. Otolaryngol Clin North Am 1994;27:511–31 [PubMed] [Google Scholar]
- 19.Spector GJ, Ge XX. Development of the hypotympanum in the human fetus and neonate. Ann Otol Rhinol Laryngol Suppl 1981;90 (6 pt 2):1–20 [PubMed] [Google Scholar]
- 20.Gulya AJ. Developmental anatomy of the ear. In: Glasscock ME, Shambaugh GE, eds. Surgery of the Ear. 4th ed. Philadelphia: W.B. Saunders;1990. :5–33
- 21.Turgut S, Tos M. Correlation between temporal bone pneumatization, location of lateral sinus and length of the mastoid process. J Laryngol Otol 1992;106:485–89 [DOI] [PubMed] [Google Scholar]
- 22.Jahrsdoerfer RA. The facial nerve in congenital middle ear malformations. Laryngoscope 1981;91:1217–25 [DOI] [PubMed] [Google Scholar]
- 23.Anson BJ, Bast TH, Richany SF. Development of the second branchial arch (Reichert's cartilage), facial canal and associated structures in man. Q Bull Northwest Univ Med Sch 1956;30:235–49 [PMC free article] [PubMed] [Google Scholar]
- 24.Caparosa RJ, Klassen D. Congenital anomalies of the stapes and facial nerve. Arch Otolaryngol 1966;83:420–21 [DOI] [PubMed] [Google Scholar]
- 25.Romo LV, Curtin HD. Anomalous facial nerve canal with cochlear malformations. AJNR Am J Neuroradiol 2001;223:838–44 [PMC free article] [PubMed] [Google Scholar]
- 26.Altmann F. Malformations of the eustachian tube, the middle ear and its appendages: a critical review. Arch Otolaryngol 1951;54:241–66 [DOI] [PubMed] [Google Scholar]
- 27.Curtin HD, Vignaud J, Bar D. Anomaly of the facial canal in a Mondini malformation with recurrent meningitis. Radiology 1982;144:335–41 [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre PP, Leprince P, Weber T, et al. Neuronotrophic effect of developing otic vesicle on cochleo-vestibular neurons: evidence for nerve growth factor involvement. Brain Res 1990;507:254–60 [DOI] [PubMed] [Google Scholar]
- 29.McPhee JR, Van De Water TR. Epithelial-mesenchymal tissue interactions guiding otic capsule formation: the role of the otocyst. J Embryol Exp Morphol 1986;97:1–24 [PubMed] [Google Scholar]
- 30.Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol 2002;23:635–43 [PMC free article] [PubMed] [Google Scholar]
- 31.Lo WW. Imaging of cochlear and auditory brain stem implantation. AJNR Am J Neuroradiol 1998;19:1147–54 [PMC free article] [PubMed] [Google Scholar]