INTRODUCTION
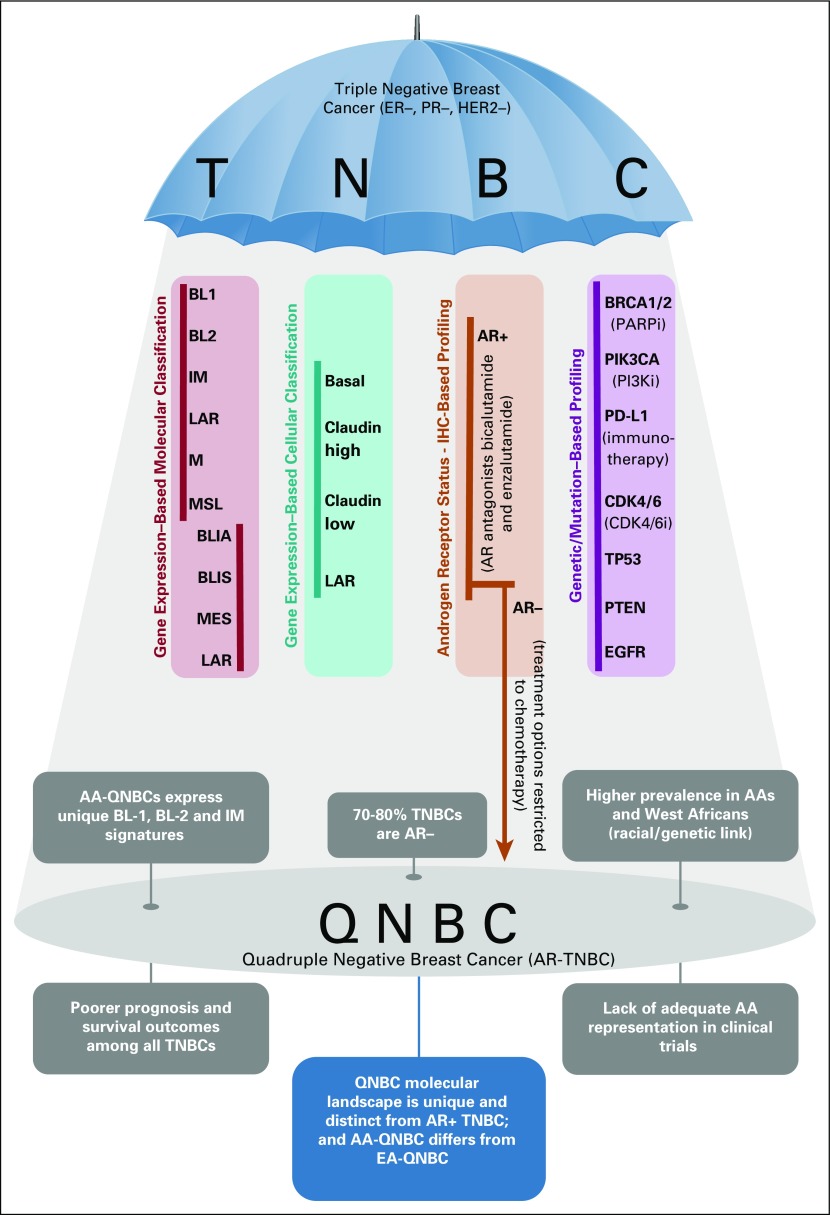
Triple-negative breast cancer (TNBC) is clinically defined as the absence of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The approximately 15%-20% of breast cancers (BCs) that are triple negative represent an aggressive subtype that is more likely to have a poor prognosis.1 Highly heterogeneous, the TNBC collective comprises multiple independent molecular subtypes, underpinned by unique biologic pathways. The chief TNBC subtyping systems are briefly outlined in Figure 1.2-4 Among these, the widely accepted Lehmann molecular classification categorizes TNBC into: basal-like (BL1 and BL2), immunomodulatory, luminal androgen receptor (LAR), mesenchymal, and mesenchymal stem-like.3 The subtypes also respond differently to available therapies. For example, the LAR tumors have a low proliferation rate and are less sensitive to standard chemotherapy, whereas the basal type is characterized by high proliferation and greater sensitivity to chemotherapy.5 Lumped with TNBC is the highly recalcitrant quadruple-negative breast cancer (QNBC), simplistically designated as a TNBC subset that lacks androgen receptor (AR) expression. In fact, 70%-80% of TNBCs are veritably QNBCs (ie, are AR negative).6,7
FIG 1.
Triple-negative breast cancers (TNBCs) make up a highly heterogeneous group that can be classified variously, as outlined in the long columns (data adapted2-4). Quadruple-negative breast cancer (QNBC) is clinically defined as an androgen receptor (AR)–negative TNBC and is briefly characterized in the lower half of the schematic. There is a pressing need to extricate QNBC from the shadows of TNBC and classify it as a unique, clinically relevant BC subtype. AA, African American; BL, basal-like; BLIA, basal-like immune associated; BLIS, basal-like immune suppressed; CDK4/6i, CDK4/6 inhibitors; IHC, immunohistochemistry; IM, immunomodulatory; LAR, luminal androgen receptor; M, mesenchymal; ML, mesenchymal stem-like; PARPi, PARP inhibitor; PI3Ki, PI3K inhibitor.
African ancestry is one of the risk factors associated with TNBC. Among different populations, the incidence of TNBC is far greater in West African women (53.2%) and African American (AA) women (29.8%) compared with their European American (EA) counterparts (15.5%).8 This strongly suggests a genetic predisposition to the disease.9,10 AR-positive TNBCs are predominantly of a luminal subtype, whereas QNBCs tend to be the aggressive basal-like.2,11 A comprehensive AR assessment study by Davis et al12 reported that in all BC subtypes, AA women showed a propensity toward absence of AR expression, with the greatest frequency of loss observed in TNBCs. In addition, AA women with AR-negative TNBC experienced worse overall survival than EA women. The most cogent result from the study demonstrated that, relative to EAs, QNBCs in AA women express distinct enriched basal and immune (BL1, BL2, and immune modulatory) signatures.12 For example, PD-1, programmed death-ligand 1 (PD-L1), and CTLA-4 (immune checkpoint inhibitors), along with CD4 expression on T cells, were found to be significantly increased in both QNBC overall and in AA-QNBC as opposed to EA-QNBC. Thus, a lack of AR results in a difference in tumor-linked immune response, which depends on genetic ancestry.12 Although it is widely accepted that AR plays a role in BC progression,13 its function as a prognostic biomarker in TNBC remains ambiguous.14-19 A recent study found that AR-positive status displayed population-specific patterns, conferring a better prognosis in US and Nigerian cohorts and a poor prognosis in India, Ireland, and Norway cohorts and was of no prognostic value in a UK cohort.20 It has also been proposed that the ER status determines the prognostic role of AR; AR denotes good prognosis in ER-positive BC, but its role in ER-negative BC is indeterminate.21 In part, the equivocality surrounding the results can be attributed to differences in the anti-AR antibodies used, as well as the staining and scoring methods across studies, compounded by variable thresholds used to define AR positivity. In addition, small sample sizes and differences in the ethnic make-up of cohorts impair congruity in results.
The glaring racial disparity is not just confined to the prevalence and molecular portraiture of this intractable disease but spills over to treatment options as well, for the AA demographic. Regarding TNBC, the field of precision medicine is hamstrung, owing to its highly heterogeneous nature as well as a dearth of actionable targets. Typically, early-stage TNBC is treated with a combination of local (surgery and radiotherapy) and systemic therapy (chemotherapy), given the absence of targets responsive to endocrine or HER2 blockade. Chemoresistance is, however, fairly common in TNBC. A minority of patients with metastatic TNBC will have germline BRCA mutations and evidence of PD-L1 expression, enabling targeted treatment with poly ADP-ribose polymerase (PARP) inhibitors and immunotherapy, respectively.22,23
Drugs targeting the AR are a recent and promising line of investigation.22 Despite conflicting reports on its merits as a prognostic marker, AR antagonists such as bicalutamide and enzalutamide have exhibited encouraging results, principally in the subset of patients with TNBC who belong to the LAR molecular subtype (that is partly dependent on AR signaling).24-30 However, because only 20%-25% of TNBCs express AR, it leaves a vast majority of patients with TNBC who are in fact quadruple negative bereft of this class of drugs. In several BCs, tumor cell proliferation is marked by an overactive cyclinD-CDK4/6 holoenzyme, and, as such, inhibitors of CDK4/6 have proved successful in reining in cancer progression among patients with metastatic disease with hormone-positive BC.31 Among the TNBC subgroups, LAR has shown sensitivity to CDK4/6 inhibition; however, the basal-like subtype is resistant to it.32 PI3K inhibitors are another group of promising therapeutics that can be applied to PIK3CA-mutant TNBCs. Because the PIK3CA kinase mutations are more frequently found in AR-positive TNBCs (40%) as compared with AR-negative (4%),33 the QNBC group is deprived of this line of intervention as well. Most of the TNBC-combating therapeutics target AR-positive TNBCs, which ends up deepening the inequalities in treatment options. Currently, no targeted drugs are available or under development for patients with QNBC, and their treatment options are restricted to chemotherapy. Yet another consideration is a switch in hormone receptor status that often follows cancer recurrence, especially so in TNBC, which exhibits elevated metastatic potential. Angajala et al34 observed an increase in QNBC subtype at second profile status of TNBC that had progressed to a recurrent/metastatic stage. So although AR-positive TNBC exhibited a heterogeneous profile with a greater frequency of being diagnosed as AR negative at an advanced stage, QNBC displayed a more stable phenotype.34 In view of the complex role of AR in BC and the distinctive characterization its presence and absence effects in the TNBC landscape, it is imperative to include AR analysis in routine clinical practice (together with the assessment of ER, PR, and HER2 expression). Because there is little consensus on AR positivity cut-off as determined by immunohistochemistry, a more reliable means of assessment, such as an androgen-driven gene signature, should be depended on instead.
As exemplified by the study by Davis et al,12 the QNBC landscape diverges from that of TNBC, and the absence of AR manifests distinctively in AA versus EA women. The mechanistic action of AR is not yet clearly delineated in TNBC. Thus, identifying key AR-dependent proteins that may differ with race, BC subtypes, disease progression, and prior treatment would prove pivotal in mapping the TNBC landscape. This in turn will help discriminate QNBC from TNBC and the molecular nature of AA-QNBC compared with EA-QNBC, with the final goal of identifying potential druggable targets. However, this blueprint of TNBC/QNBC research, though robust, is bound to fail in the face of unequal racial composition that marks most study cohorts. Clinical trials suffer from an under-representation of racial minorities,35 and an adequate inclusion of AA women and women of African descent in these studies is indispensable to advance QNBC research.
To recapitulate, the major fraction of TNBCs are AR negative (ie, QNBC phenotype), which overwhelmingly afflicts AA women and expresses a unique racial fingerprint. Prevalence of the disease and poorer survival outcomes in AA and West African women strongly indicates a genetic predisposition. However, we cannot discount the role of socioeconomic factors, such as poverty and barriers to health care access,36 in contributing to mortality differences and exacerbating the disparities. Moreover, the treatment research focus on AR-positive TNBC (such as AR antagonists) inadvertently fuels these imparities. Relegating QNBC to a TNBC-subtype position is taking a parochial view of a complex disease, one that warrants an independent classification as a singular, clinically relevant BC subtype. Extricating it from the shadows of TNBC and extensively annotating its molecular features will aid in paring down the disparity gap that currently ails QNBC research (Fig 1).
Footnotes
Supported by National Cancer Institute Grant No. R01CA239120 (R.A.).
AUTHOR CONTRIBUTIONS
Conception and design: Geetanjali Saini, Shristi Bhattarai, Ritu Aneja
Provision of study material or patients: Shristi Bhattarai
Collection and assembly of data: Geetanjali Saini, Ritu Aneja
Data analysis and interpretation: Geetanjali Saini, Keerthi Gogineni, Ritu Aneja
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Keerthi Gogineni
Honoraria: Pfizer, Eli Lilly
Research Funding: Pfizer (Inst), Calithera Biosciences (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eli Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 2019;9:176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prado-Vázquez G, Gámez-Pozo A, Trilla-Fuertes L, et al. A novel approach to triple-negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci Rep. 2019;9:1538. doi: 10.1038/s41598-018-38364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santonja A, Sánchez-Muñoz A, Lluch A, et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget. 2018;9:26406–26416. doi: 10.18632/oncotarget.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang HS, Kuang XY, Sun WL, et al. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget. 2016;7:41285–41293. doi: 10.18632/oncotarget.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Pan B, Zhu H, et al. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget. 2016;7:46482–46491. doi: 10.18632/oncotarget.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiagge E, Jibril AS, Chitale D, et al. Comparative analysis of breast cancer phenotypes in African American, White American, and West versus East African patients: Correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol. 2016;23:3843–3849. doi: 10.1245/s10434-016-5420-z. [DOI] [PubMed] [Google Scholar]
- 9.Dietze EC, Sistrunk C, Miranda-Carboni G, et al. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat Rev Cancer. 2015;15:248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman LA, Jenkins B, Chen Y, et al. Hereditary susceptibility for triple negative breast cancer associated with Western Sub-Saharan African ancestry: Results from an International Surgical Breast Cancer Collaborative. Ann Surg. 2019;270:484–492. doi: 10.1097/SLA.0000000000003459. [DOI] [PubMed] [Google Scholar]
- 11.Jézéquel P, Loussouarn D, Guérin-Charbonnel C, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: Importance of immune response. Breast Cancer Res. 2015;17:43. doi: 10.1186/s13058-015-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis M, Tripathi S, Hughley R, et al. AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PLoS One. 2018;13:e0196909. doi: 10.1371/journal.pone.0196909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannelli P, Di Donato M, Galasso G, et al. The androgen receptor in breast cancer. Front Endocrinol (Lausanne) 2018;9:492. doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano Y, Kashiwagi S, Goto W, et al. Expression and clinical significance of androgen receptor in triple-negative breast cancer. Cancers (Basel) 10.3390/cancers9010004. doi: 10.3390/cancers9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton VN, Christenson JL, Gordon MA, et al. Androgen receptor supports an anchorage-independent, cancer stem cell-like population in triple-negative breast cancer. Cancer Res. 2017;77:3455–3466. doi: 10.1158/0008-5472.CAN-16-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Peng R, Yuan Z, et al. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: A retrospective analysis based on a tissue microarray. Med Oncol. 2012;29:406–410. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 17.McGhan LJ, McCullough AE, Protheroe CA, et al. Androgen receptor-positive triple negative breast cancer: A unique breast cancer subtype. Ann Surg Oncol. 2014;21:361–367. doi: 10.1245/s10434-013-3260-7. [DOI] [PubMed] [Google Scholar]
- 18.Mrklić I, Pogorelić Z, Capkun V, et al. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 2013;115:344–348. doi: 10.1016/j.acthis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Yang F, Zhang W, et al. The androgen receptor promotes cellular proliferation by suppression of G-protein coupled estrogen receptor signaling in triple-negative breast cancer. Cell Physiol Biochem. 2017;43:2047–2061. doi: 10.1159/000484187. [DOI] [PubMed] [Google Scholar]
- 20.Bhattarai S, Klimov S, Mittal K, et al. Prognostic role of androgen receptor in triple negative breast cancer: A multi-institutional study. Cancers (Basel) 10.3390/cancers11070995. doi: 10.3390/cancers11070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elebro K, Bendahl PO, Jernström H, et al. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res Treat. 2017;165:645–657. doi: 10.1007/s10549-017-4343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebert JM, Lester R, Powell E, et al. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25(suppl 1):S142–S150. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019;17:90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arce-Salinas C, Riesco-Martinez MC, Hanna W, et al. Complete response of metastatic androgen receptor-positive breast cancer to bicalutamide: Case report and review of the literature. J Clin Oncol. 2016;34:e21–e24. doi: 10.1200/JCO.2013.49.8899. [DOI] [PubMed] [Google Scholar]
- 25.Barton VN, D’Amato NC, Gordon MA, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnefoi H, Grellety T, Tredan O, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1) Ann Oncol. 2016;27:812–818. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 27.Gerratana L, Basile D, Buono G, et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat Rev. 2018;68:102–110. doi: 10.1016/j.ctrv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R, Han J, Liang X, et al. Androgen receptor expression and bicalutamide antagonize androgen receptor inhibit β-catenin transcription complex in estrogen receptor-negative breast cancer. Cell Physiol Biochem. 2017;43:2212–2225. doi: 10.1159/000484300. [DOI] [PubMed] [Google Scholar]
- 30.Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernas S, Tolaney SM, Winer EP, et al. CDK4/6 inhibition in breast cancer: Current practice and future directions. Ther Adv Med Oncol 10.1177/1758835918786451. doi: 10.1177/1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar US, Barr AR, Cutts R, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angajala A, Mothershed E, Davis MB, et al. Quadruple negative breast cancers (QNBC) demonstrate subtype consistency among primary and recurrent or metastatic breast cancer. Transl Oncol. 2019;12:493–501. doi: 10.1016/j.tranon.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazha B, Mishra M, Pentz R, et al. Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39:3–10. doi: 10.1200/EDBK_100021. [DOI] [PubMed] [Google Scholar]
- 36.Siddharth S, Sharma D. Racial disparity and triple-negative breast cancer in African-American women: A multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel) 10.3390/cancers10120514. doi: 10.3390/cancers10120514. [DOI] [PMC free article] [PubMed] [Google Scholar]