Abstract
Purpose:
Luminal gastrointestinal tract cancers (LGC) are common malignancies, and many patients can achieve long-term responses with surgery, cytotoxic and/or targeted therapies, and radiation. The long-term follow-up for patients with durable disease control has not been fully characterized, including subsequent malignancies. Such cases have not been comprehensively described.
Patients and methods:
We identified patients evaluated for myeloid malignancies (MyM) who had a prior LGC at our institution over a 35-year period. Patient, disease, and treatment information was collected for analysis. Cytogenetic risk profiles were designated according to the Revised International Prognostic Scoring System for MDS and the European LeukemiaNet Guidelines for AML.
Results:
66 patients were included in our cohort with 71 prior LGC diagnoses, including three patients with multiple LGCs. 31 cases were treated with surgery alone, and 37 patients received chemotherapy. The median age at diagnosis of MyM was 71.8 years (range, 36.2 to 90.5), with median duration between initiation of treatment of LGC and diagnosis MyM of 7.9 years (range 0.005–38.8). Intermediate or adverse (AML)/poor-very poor (MDS) cytogenetic risk was common, occurring in 43% of MDS patients and 100% of AML patients; deletion 5q was the most common cytogenetic abnormality overall. DNMT3A mutations were the most common molecular alteration (6 patients with 7 mutations).
Conclusions:
Among patients with MyM following LGC, a high proportion harbored cytogenetic changes, many of which were adverse or poor-risk. Deletion 5q and mutated DNMT3A were the most common abnormalities identified.
Keywords: Treatment-related neoplasms, Cytogenetics, Molecular alterations, Survivorship
1. Introduction
Luminal gastrointestinal tract cancers (LGC), including those of the esophagus, stomach, and small/large intestine, comprise an epidemiologically large burden of disease, both in the United States and worldwide. For example, stomach and colorectal cancers are each counted among the top five causes of death worldwide leading to cancer according to the World Health Organization [1]. Depending on the primary site and extent of disease, treatment modalities are varied and have evolved, and can include a combination of various medical therapies, surgical resection, and radiation.
As medical therapies have been modified and advanced, survivorship has been prolonged in recent decades [2], warranting further attention towards the potential long-term sequelae of these treatments and other important aspects of survivorship care such as subsequent malignancies. Importantly, in a recent large registry-based analysis, Morton and colleagues [4] noted an increasing incidence of treatment-related myeloid neoplasia (tMN) following treatment for esophagus cancer since the early 2000’s. Although disease characteristics and clinical outcomes for tMN have been extensively studied in patients after treatment for breast cancer and lymphoma [5], only limited data exist for patients with a prior gastrointestinal malignancy, and all are confined to single case reports or small case series [6–8].
We conducted a retrospective analysis of all myeloid malignancy (MyM) patients evaluated at our institution who had previously been diagnosed for a LGC, irrespective of prior treatments, seeking to comprehensively characterize this patient cohort. We intentionally studied all patients who met these criteria at our center, including patients who had undergone only surgery as disease-directed treatment for their prior LGC, examining MDS and AML disease characteristics, treatment patterns, and long-term survival. Given the tremendous prognostic and treatment-related import to molecular and cytogenetic abnormalities [10, 11] in MDS and AML, we directed our attention especially towards those alterations present in these cases, utilizing results from next-generation sequencing (NGS) testing panels when available.
2. Methods
2.1. Patients
We performed a retrospective data inquiry for all patients seen at our institution with coexisting diagnosis codes corresponding to an LGC (esophagus, stomach, bowel, and rectum) and MyM defined as either MDS or AML (including acute promyelocytic leukemia [APL]) from 1980 until 2016. Patients with a confirmed or suspected underlying bone marrow failure disorder prior to the LGC were excluded, as were patients whose LGC was a lymphoma or stromal tumor and patients who had received cytotoxic chemotherapy for a different prior malignancy. The MDS subtype was assigned based on the revised 2016 WHO criteria [9]; cytogenetic risk profiles were designated according to the Revised International Prognostic Scoring System for MDS [10] and the European LeukemiaNet Guidelines for AML [11]. Cytogenetics, when available, was analyzed using karyotype or fluorescence in-situ hybridization.
2.2. Sequencing Methods
Patients who underwent molecular analysis were noted; some patient samples had targeted genetic testing for specific recurrent mutations, whereas others were evaluated using a 30-gene next-generation polymerase chain reaction (PCR)-based NGS panel; see Supplement 1 for full details of genetic studies contained in this panel.
2.3. Statistical Analysis
Descriptive statistics were used to compare and summarize characteristics of both the LGC and MyM disease. A Wilcoxon-rank sum test was used to compare the time from LGC to MyM by the type of LGC treatment among this cohort who all were diagnosed with MyM. Kaplan-Meier methods were used to estimate the overall survival from the time of MyM diagnosis; left truncation was used for patients who were first seen at MSKCC after MyM diagnosis. All analyses were done using the R statistical package.
3. Results
3.1. LGC Disease Characteristics
Table 1 displays characteristics for all LGC diagnoses; Three of these patients had multiple LGCs. The majority of patients were male (64%) and the median age of diagnosis of first LGC was 60 years (range 31–86); 6 patients had received external-beam radiation therapy for a prior malignancy besides LGC. The most common LGC diagnosis was colon (n=45, 63%) followed by rectum (n=12, 17%); among patients with treatment information available, a slight majority of LGC cases were treated with some form of medical therapy (53%), most commonly an antimetabolite (86%), whereas 31 patients (44%) only underwent surgery.
Table 1:
LGC Characteristics (N = 71 total LGC diagnoses)
| Variable | N (%) | |
|---|---|---|
| Type of LGC | ||
| Esophagus | 4 (6%) | |
| Stomach/gastroesophageal junction | 8 (11%) | |
| Small bowel | 2 (3%) | |
| Colon | 45 (63%) | |
| Rectum | 12 (17%) | |
| Stage of LGC* | ||
| I | 10 (18%) | |
| II | 17 (31%) | |
| III | 17 (31%) | |
| IV | 10 (19%) | |
| Unknown | 18 (25%) | |
| Treatment of LGC^ | ||
| Surgery alone | 31 (44%) | |
| Chemotherapy (with or without surgery/radiation) | 37 (53%) | |
| Radiation (with or without surgery/chemotherapy) | 20 (29%) | |
| Chemotherapy and radiation (with or without surgery) | 19 (27%) | |
| LGC medical treatment received (35 patients with treatment data) | ||
| 5-Fluorourocil/capecitabine | 30 (86%) | |
| Platinum-based agent | 20 (57%) | |
| Topoisomerase inhibitor | 8 (23%) | |
| Taxane | 4 (11%) | |
| Targeted agents (small molecule inhibitors, antibodies) | 4 (11%) | |
| Other | 2 (6%) | |
Percentages are of known stage; for unknown stage, this percentage is of all cases of LGC
Percentages are of all patients with known treatment
3.2. MyM Patient Characteristics
A total of 66 patients were diagnosed with MyM following a LGC diagnosis, which comprised approximately 1% of all MyM patients seen at our institution during this time period. Median age at diagnosis of MyM was 71.8 years (range, 36.2 to 90.5). The median latency time between LGC and MyM treatments was longer for patients who underwent surgery alone compared to patients who received other treatments (14.8 years vs. 6.0 years, respectively, P = 0.038). Table 2 summarizes the clinical characteristics for the MyM patients in our cohort. Our cohort includes 27 patients with AML (41%), including four with acute promyelocytic leukemia (APL), and 39 patients (59%) with MDS or MDS/MPN overlap syndrome.
Table 2:
MyM Characteristics (N = 66 total MyM diagnoses)
| Variable | N (%) | ||||
|---|---|---|---|---|---|
| Type of MyM | |||||
| Acute myeloid leukemia, non-M3 | 23 (35%) | ||||
| Acute myeloid leukemia, M3 (APL) | 4 (6%) | ||||
| MDS with multi-lineage dysplasia | 10 (15%) | ||||
| MDS with excess blasts – 1 | 8 (12%) | ||||
| MDS with excess blasts – 2 | 7 (11%) | ||||
| MDS with ringed sideroblasts | 3 (5%) | ||||
| MDS with single-lineage dysplasia | 2 (3%) | ||||
| MDS with isolated del(5q) | 2 (3%) | ||||
| MDS-other (MDS with no available blast percentage, MDS/MPN overlap including CMML) | 7 (9%) | ||||
| LGC medical treatment received (N = 35 MyM patients with treatment data) | |||||
| 5-Fluorourocil/capecitabine | 30 (86%) | ||||
| Platinum-based agent | 20 (57%) | ||||
| Topoisomerase inhibitor | 8 (23%) | ||||
| Taxane | 4 (11%) | ||||
| Targeted agents (small molecule inhibitors, antibodies) | 4 (11%) | ||||
| Other | 2 (6%) | ||||
| Cytogenetic Abnormality (N = 64) | |||||
| Deletion 5q | 9 | ||||
| Deletion 20q | 6 | ||||
| Trisomy 8 | 6 | ||||
| Monosomy 7 | 6 | ||||
| Deletion 11q | 3 | ||||
| Deletion 12p | 3 | ||||
| Complex | 10 | ||||
| Cytogenetic Risk Category | |||||
| By MyM Diagnosis | MDS (N = 37) | Very good | 2 (5%) | ||
| Good | 19 (51%) | ||||
| Intermediate | 11 (30%) | ||||
| Poor | 2 (5%) | ||||
| Very poor | 3 (8%) | ||||
| AML | N = 22 at diagnosis | Favorable | 0 (0%) | ||
| Intermediate | 13 (59%) | ||||
| Adverse | 9 (41%) | ||||
| N = 1 subsequent | Adverse | 1 (100%) | |||
| By LGC Treatment** | Surgery alone (N = 26 with known treatments and cytogenetics results) | Good/very good | 12 (46%) | ||
| Intermediate | 10 (38%) | ||||
| Adverse/poor/very poor | 4 (15%) | ||||
| All other (N = 32 with known treatments and cytogenetics results) | Good/very good | 9 (28%) | |||
| Intermediate | 13 (41%) | ||||
| Adverse/poor/very poor | 10 (31%) | ||||
| Treatment of MyM (N = 60 with known treatments) | |||||
| Hypomethylating agent | 27 (45%) | ||||
| Chemotherapy | 19 (32%) | ||||
| Supportive care alone (includes symptom treatment, transfusion, hydroxyurea, and epoetin) | 18 (30%) | ||||
| Allogeneic transplantation | 6 (10%) | ||||
| Other | 17 (28%) | ||||
| Unknown (% of all patients) | 6 (10%) | ||||
SLD – single-lineage dysplasia; MLD – multi-lineage dysplasia; EB – excess blasts; RS – ringed sideroblasts; CMML – chronic myelomonocytic leukemia; APL – acute promyelocytic leukemia
One patient had insufficient aspirate material for blast quantification to distinguish MDS subtype
Refers to patients with known treatments
Includes symptomatic treatment, hydroxyurea, and epoetin
Percentage refers to all patients
P=0.082
Almost all MyM patients (95%) had informative cytogenetics results at diagnosis and 68% were characterized as either intermediate or adverse risk; this was more prominent for non-APL AML, in which all patients were classified as either intermediate (57%) or adverse (43%). Among MDS patients, 21 of 37 (57%) harbored very good- or good-risk cytogenetics. No differences were seen in cytogenetic risk group when we compared patients who underwent surgery alone vs. all other treatments (P = 0.082). Among 5 AML patients with requisite sequencing information for unified molecular-cytogenetic risk assessment based on ELN, 2 were re-classified as adverse risk, while the other 3 remained classified as intermediate. Overall, the most common karyotypic abnormality found was deletion of chromosome arm 5q (n=9), and 17% (n=12) had a complex karyotype (>2 cytogenetic abnormalities). The karyotype in 2 of 3 patients with mutated tumor protein 53 (TP53) was normal, whereas the other was complex, including deletion 7q. Three of four patients with APL harbored only t(15;17), the remaining had der(17)t(15;17(q22;q21)t(3;17).
The most common MyM therapy utilized (N = 60 with available treatment information) was hypomethylating agents (N = 27, 45%) followed by chemotherapy (n=19, 32%); 10% of patients underwent allogeneic transplant. The median survival after diagnosis of MyM was 16.6 months (95% CI: 10–32 months).
3.3. Molecular Sequencing Results
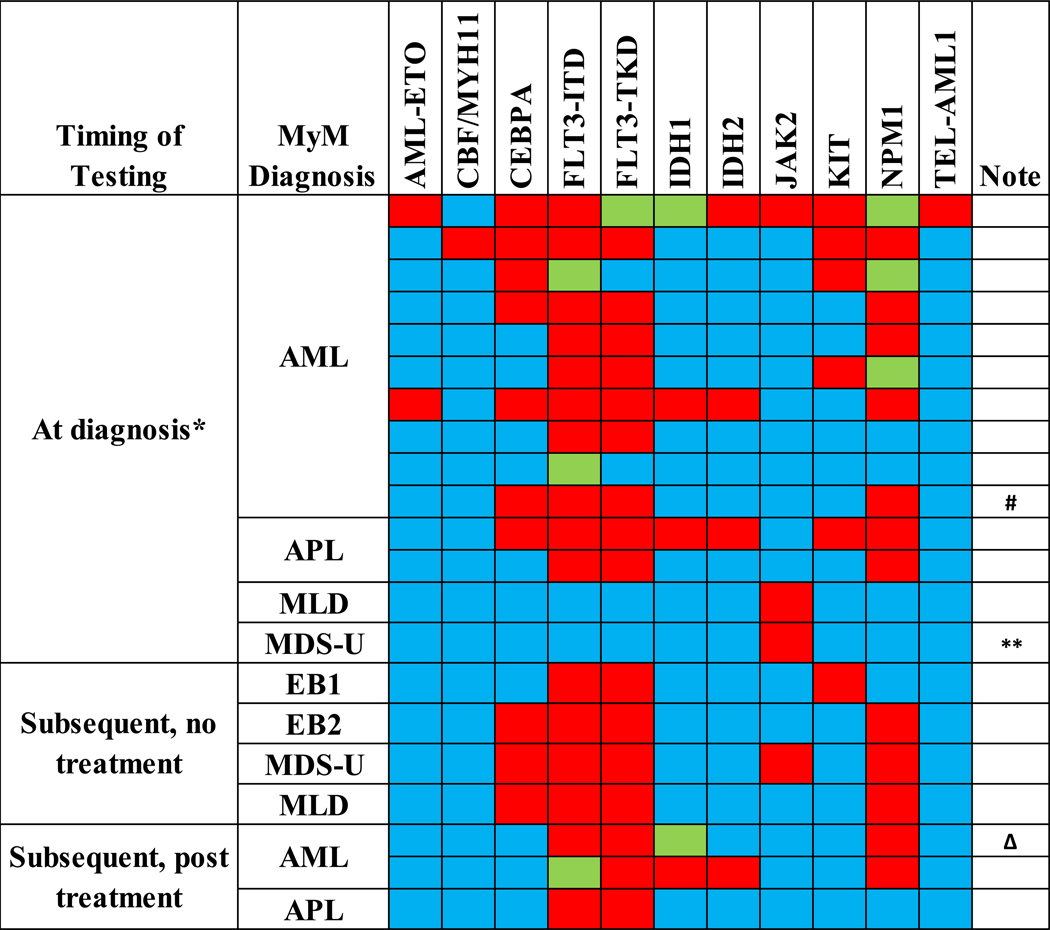
Sequencing results are displayed in Figure 1 (targeted sequencing) and Figure 2 (sequencing panels, N = 16 patients). Figure 2 also notes the treatment(s) rendered for the LGC. Given the paucity of existing data regarding this specific patient population, we included sequencing results collected after treatment of MyM had been initiated. Overall (including targeted and panel sequencing), the most commonly mutated gene was DNA cytosine-5-methyltransferase 3A (DNMT3A, 7 patients with 9 mutations) followed by isocitrate dehydrogenase isoform 1 (IDH1, exon 4, 6 patients). Besides these findings, there was also enrichment (4 patients each) for mutations in the enhancer of zeste homolog 2 (EZH2) and Tet methylcytosine dioxygenase 2 (TET2) genes; all but one patient had multiple gene mutations when any genetic mutation was present. Supplement 2 contains details regarding the specific genetic mutations present in each patient and assay coverage.
Figure 1: Targeted Sequencing in MyM Patients.

*Includes up to 6 months after diagnosis, without treatment
**Insufficient aspirate material to distinguish MDS subtype
ΔThis patient later underwent NGS, see Table 4 for results (only results from the below NGS will be tallied towards the final summation of mutation frequencies)
#This patient later underwent NGS, see Table 4 for results (only results from the below NGS will be tallied towards the final summation of mutation frequencies)
Figure 2: Sequencing Panels in MyM Patients.

*Includes up to 6 months after diagnosis, without treatment
°This patient underwent NGS with a different sequencing panel than other patients
#This patient had previously undergone targeted sequencing, see Table 3. Additionally, two separate DNMT3A mutations were present (exons 4 and 19).
ΔThis patient had previously undergone targeted sequencing, see Table 3. Additionally, two separate DNMT3A mutations were present (exons 16 and 19).
4.1. Discussion
To our knowledge, this retrospective study characterizes the largest cohort of patients with MyM following prior LGC. We were able to capture detailed information regarding LGC and MyM disease characteristics, treatment modalities, and clinical outcomes for a majority of patients, and in a subset of patients, we report detailed molecular sequencing data. Most patients in our cohort received systemic or radiation therapy for colon cancer, which underscores the importance of studying the incidence of MyM in this population given the high incidence of colon cancer in the general population. Furthermore, few data currently exist regarding the potential for myelotoxicity specifically from platinum compounds and fluoropyrimidines compared to anthracyclines, topoisomerase inhibitors, and other alkylating agents such as cyclophosphamide. However, it should be noted that some patients did not receive potentially genotoxic therapies and therefore should be considered as de novo cases. Our data are exploratory in nature and should prompt further investigation into this specific area of survivorship care.
Most patients in our cohort developed MDS, as described in Table 2. The latency period for patients who underwent surgery alone was longer than those who received chemotherapy, and the median latency time for the treated cohort is similar to other large published cohorts specifically focusing on tMN that demonstrate an approximate 5-year latency time following alkylating agent-based or multimodal chemotherapy[12]. The shorter latency time for the chemotherapy plus radiation cohort and the similarity in latency time when compared to other tMN cohorts are suggestive of a link between therapy and onset of the MyM, although this is not definitive and warrants further investigation.
A majority of patients harbored intermediate or poor-risk cytogenetic patterns, a significant adverse prognostic factor, independent of whether they received prior therapy or not, although there was a trend toward a higher incidence of poor-risk karyotypes in those who received chemotherapy compared to those who underwent surgery alone. Molecular studies revealed frequent mutations in genes involved in transcriptional regulation, especially DNMT3A, EZH2, and TET2. Patients in our cohort who did not receive prior potentially myelotoxic therapies had similar patterns of molecular genetics, although they do not meet criteria for “therapy-related” MDS or AML per the WHO classification system, given the absence of these prior therapies. We emphasize that these represent preliminary data that require substantiation with confirmatory studies and should be interpreted in this context.
The frequency of APL cases among all AML types seen in our cohort (4 of 27, 15%) was generally consistent with prior data in tMN-focused cohorts, which also approximately mirrors the distribution among de novo AML diagnoses[13]. Studies investigating tAPL have mostly been comprised of patients having been treated for breast cancer, hematological malignancies (especially lymphoma), and genitourinary cancers, with a paucity of gastrointestinal primaries (Beaumont 2003 and Rashidi 2013). Of note, all but one of the patients with APL received cytotoxic chemotherapy, and all 3 of these received multiple lines of LGC treatment, including 5-FU. Although PML-RAR break point analysis was not undertaken, 3 of the 4 patients harbored the t(15;17)(q22;21) abnormality alone, and the final patient had der(17)t(15;17(q22;q21)t(3;17) and had received prior cytotoxic therapy. Conflicting data exist (Rashidi, Beaumont) regarding the patterns of cytogenetic abnormalities (non-t(15;17) PML-RAR variants and additional abnormalities beyond PML-RAR) comparing tAPL vs. de novo disease. None of our 4 patients underwent NGS for molecular characterization.
The patterns of cytogenetic abnormalities observed in our cohort are consistent with the poor-risk profiles previously described in tMN cohorts [14, 15]. As in these cohorts, and as was found in the large Chicago cohort [5], we found high rates of chromosome 5 and 7 abnormalities (11 patients each) and complex karyotypes (10 patients, 17% of those with available cytogenetic data). No patterns were seen with respect to recurring balanced translocations as had been seen previously [12].
As classified previously [16], mutations found in our cohort favored so-called “early events” in leukemogenesis, especially affecting DNMT3A and TET2. This same study also defined certain hallmark lesions strongly associated with secondary AML, including EZH2, which 4 patients harbored in our cohort. Mutational analyses in Shih et al. [17], conducted in 38 patients with secondary AML and MDS, also found a high rate of TET2 mutations, second only to TP53 in frequency. This high rate of mutated TP53, although not seen in our cohort (1 of 6 patients tested at diagnosis), was also seen in another large sequencing cohort[18]; following TP53, this group demonstrated lower rates of IDH1 (9.5%) and DNMT3A (7.1%) compared to our data; TET2 mutations were not tested for with their assay.
The number of DNMT3A and TET2 mutations in this patient group raises the possibility of pre-existing clonal hematopoiesis (CH) that could have been present prior to/during treatment for the LGC. Recent studies have highlighted the importance especially of DNMT3A mutations as possible founder events in CH[19]; clones harboring these specific mutations might be selected for by cancer-directed myelotoxic therapies, predisposing towards subsequent development of overt MDS or AML. Takahashi and colleagues conducted a retrospective case-control study of patients treated for cancer, finding a higher rate of subsequent tMN in patients with pre-existing CH as compared to those without. Mutations in TET2, DNMT3A, and IDH2 were common in this cohort. Moreover, it has been shown that such patients have an inferior outcome when compared to those without co-existing CH[20]. One can imagine patients with LGC diagnoses frequently have concurrent anemia; such patients likely would not undergo further evaluation of the anemia. Although the finding of CH in such a patient would not change management in current treatment paradigms for LGC, it would warrant close monitoring and follow-up. Furthermore, a diagnosis of CH could allow for enrollment in ongoing collaborative CH research registries aiming to comprehensively study CH disease progression and risk factors therein[21].
Results from our study raise additional questions that warrant evaluation in greater detail. A critical question is whether there is any population-level link between LGC and MyM beyond effects of prior therapies, such as a shared inherited predisposition or simply a similar age of onset. This could explain why patients in our cohort developed MyM with very similar MyM disease features but without an identifiable predisposition towards developing MyM such as receipt of chemotherapy. To properly address this question, large population-based studies would have to be conducted to calculate the standard incidence ratio for MyM among LGC survivors as compared to the general population. Furthermore, if such a link does exist, what is the strength of this effect and potential biologic basis for this connection?
There were limitations to our study. The retrospective nature of our study, along with the fact that many patients had been treated for their prior LGC at a different institution, meant that unfortunately our data are incomplete, and might be selected for patients seen at a referral center. Furthermore, we are unable to calculate the incidence of tMN following treatment for LGC at our center since many patients are no longer followed after approximately 2–3 years of treatment, and therefore, we cannot be confident we are capturing all tMN events in our MSKCC LGC cohort. Additionally, since many patients were treated for their LGC before mutational profiling became the standard of care at our institution, only a small proportion underwent comprehensive NGS testing at the time of diagnosis, and many underwent piecemeal testing for individual genes.
Our work characterizes the largest cohort of patients with MyM following prior LGC, and offers the unique opportunity to compare characteristics of MyM following LGC systemic therapy to those receiving surgery alone for their LGC. While the difference in the latency time for MyM treated with systemic therapy vs surgery alone suggested an effect of the systemic therapy on the development of the MyM, and the molecular studies showed enrichment for characteristic mutations of treatment-related MyM, the cytogenetics results demonstrated a high-rate of poor-risk features including those patients who did not receive chemotherapy or radiation therapy. We hope that future studies will continue to shed light on the molecular characteristics in this patient population. This emphasizes the difficulty in designating MyM as “therapy-related”. Although our results need to be replicated in larger cohorts from other centers, they offer novel and important insights into this growing patient population.
Supplementary Material
Acknowledgements:
We acknowledge with gratitude the assistance of Mr. Patrick Gonzales in obtaining patient records. This study was supported by the Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748).
Bibliography
- [1].http://www.who.int/en/news-room/fact-sheets/detail/cancer.
- [2].Hu C-Y, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival, JAMA surgery 150(3) (2015) 245–251. [DOI] [PubMed] [Google Scholar]
- [3].Almeida AM, Ramos F, Acute myeloid leukemia in the older adults, Leukemia research reports 6 (2016) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, Fraumeni JF Jr., Curtis RE, Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008, Blood 121(15) (2013) 2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA, Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series, Blood 102(1) (2003) 43–52. [DOI] [PubMed] [Google Scholar]
- [6].Wiggans RG, Jacobson RJ, Fialkow PJ, Woolley PV 3rd, Macdonald JS, Schein PS, Probable clonal origin of acute myeloblastic leukemia following radiation and chemotherapy of colon cancer, Blood 52(4) (1978) 659–63. [PubMed] [Google Scholar]
- [7].Hashimoto A, Takada K, Horiguchi H, Sato T, Iyama S, Murase K, Kamihara Y, Ono K, Tatekoshi A, Hayashi T, Miyanishi K, Sato Y, Furuhata T, Kobune M, Takimoto R, Hirata K, Kato J, Combination Chemotherapy of Azacitidine and Cetuximab for Therapy-Related Acute Myeloid Leukemia following Oxaliplatin for Metastatic Colorectal Cancer, Case Rep Oncol 7(2) (2014) 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shapiro S, Hughes G, Al-Obaidi MJ, O’Reilly E, Ramesh S, Smith J, Ahmad R, Dawson C, Riddle P, Sekhar M, Acute myeloid leukaemia secondary to treatment with capecitabine for metastatic colorectal cancer, Eur J Haematol 78(6) (2007) 543–4. [DOI] [PubMed] [Google Scholar]
- [9].Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW, The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia, Blood (2016) blood-2016–03-643544. [DOI] [PubMed] [Google Scholar]
- [10].Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Revised international prognostic scoring system for myelodysplastic syndromes, Blood 120(12) (2012) 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel, Blood (2016) blood-2016–08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Godley LA, Larson RA, Therapy-related myeloid leukemia, Semin Oncol 35(4) (2008) 418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rashidi A, Fisher SI, Therapy-related acute promyelocytic leukemia: a systematic review, Med Oncol 30(3) (2013) 625. [DOI] [PubMed] [Google Scholar]
- [14].Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T, Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML, Leukemia 18(1) (2004) 120–125. [DOI] [PubMed] [Google Scholar]
- [15].Kayser S, Döhner K, Krauter J, Köhne C-H, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K, The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML, Blood 117(7) (2011) 2137–2145. [DOI] [PubMed] [Google Scholar]
- [16].Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, Acute myeloid leukemia ontogeny is defined by distinct somatic mutations, Blood 125(9) (2015) 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shih AH, Chung SS, Dolezal EK, Zhang S-J, Abdel-Wahab OI, Park CY, Nimer SD, Levine RL, Klimek VM, Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia, Haematologica 98(6) (2013) 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ok CY, Patel KP, Garcia-Manero G, Routbort MJ, Fu B, Tang G, Goswami M, Singh R, Kanagal-Shamanna R, Pierce SA, Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases, Leukemia research 39(3) (2015) 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, Zhao L, Patel K, Neelapu S, Gumbs C, Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study, The Lancet Oncology 18(1) (2017) 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes, Cell Stem Cell 21(3) (2017) 374–382. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bolton K, Ptashkin R, Braunstein L, Kelly D, Devlin SM, Coombs CC, Moarii M, Patel M, Bernard E, Berthon A, Oncologic Therapy for Solid Tumors Alters the Risk of Clonal Hematopoiesis, Am Soc Hematology, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.