Abstract
PURPOSE
We undertook this study to evaluate the incremental cost per quality-adjusted life-year (QALY) gained with use of adjuvant trastuzumab as compared with chemotherapy alone among patients with nonmetastatic breast cancer in India.
METHODS
We used a Markov model to estimate the incremental cost of using trastuzumab (for 1 year, 6 months, or 9 weeks) as compared with chemotherapy alone using a societal perspective, excluding indirect productivity losses. Although the outcomes (QALYs) in the standard chemotherapy arm were estimated after calibrating the model as per survival data from 2 Indian cancer registries, effectiveness estimates from the HERA trial and a joint analysis of the NSABP B-31 and NCCTG N9831 trials were used to estimate the consequences of 1-year trastuzumab use. The cost of treatment was estimated using national standard treatment guidelines and real-world use estimates for different treatment modalities as per data from Indian cancer registries. Probabilistic sensitivity analysis was undertaken to evaluate parameter uncertainty.
RESULTS
For 1 year of trastuzumab use, the incremental benefit per patient, incremental cost per QALY gained, and probability of being cost effective using HERA trial estimates were 1.29 QALYs, 178,877 Indian national rupees (INRs; US$2,558), and 4%, respectively, whereas the corresponding figures using joint analysis estimates were 1.69 QALYs, INR 134,413 (US$1,922), and 57.3%, respectively.
CONCLUSION
Use of trastuzumab for 1 year is not cost effective in India at the current price. However, trastuzumab use for 9 weeks is cost effective and should be included in clinical guidelines and reimbursement policies. A price reduction of 15% to 35% increases the probability of 1-year trastuzumab use being cost effective, to 90%.
INTRODUCTION
Breast cancer is the most common cancer among women in India and accounts for 27% of all cancers in that country.1 Overexpression of the oncogene human epidermal growth factor receptor 2 (HER2/neu) is associated with poor prognosis and high risk of recurrence.2-4 Addition of the HER2-targeted monoclonal antibody trastuzumab to chemotherapy in adjuvant treatment has been shown to improve disease-free survival (DFS) by 50% and overall survival (OS) by 30%.5-7 However, trastuzumab is an expensive drug. It was reported to have been used in only 8.6% of eligible patients, half of whom were enrolled in a clinical trial.8
The low rate of trastuzumab use raises the important question of whether public resources should be used to make this treatment routinely accessible in India. This question is highly relevant because of the recently announced ambitious Indian health insurance program, Ayushman Bharat, which includes coverage of chemotherapy for cancer treatment under the Prime Minister’s Jan Aarogya Yojana (PMJAY) component.9,10
Many cost-effectiveness analyses of trastuzumab have been reported, with variable results.11-19 The variability in findings can be attributed to differences in perspective, modeling method, context, health care delivery structure, price, and other input parameters.
A major limitation of the existing literature is that a majority of these model-based cost-effectiveness analyses have based their outcome valuation on the interim results of clinical trials with relatively short follow-up. No cost-effectiveness analysis has yet been published taking into account the long-term clinical benefits based on the Herceptin Adjuvant (HERA) trial (ClinicalTrials.gov identifier: NCT00045032).7 Moreover, although a majority of previous economic evaluations have used effectiveness estimates from the HERA trial, the HERA trial protocol is not commonly followed in routine clinical practice by oncologists in India.20
CONTEXT
Key Objective
Is the use of Trastuzumab cost effective for patients with nonmetastatic breast cancer in low-income countries like India?
Knowledge Generated
Addition of trastuzumab to chemotherapy in the adjuvant setting for 1 year was not cost effective at the current price, but addition of trastuzumab for 9 weeks was cost effective. At the current price, 1-year trastuzumab use has just a 4% to 57% probability of being cost effective. In contrast, 9-week adjuvant trastuzumab therapy incurs an incremental cost per quality-adjusted life-year gained, ranging from 34,268 Indian national rupees (INRs; US$490) to INR 43,264 (US$619).
Relevance
Nine weeks of adjuvant trastuzumab is an efficient option for use in India and other low-income countries where a large majority of patients do not experience the benefits of trastuzumab because of its cost.
We undertook this cost-effectiveness analysis of adjuvant trastuzumab in combination with standard chemotherapy compared with chemotherapy alone in the Indian context. The base case presents the analysis for 1-year use of trastuzumab, which is standard practice. Detailed subgroup analyses were also undertaken, and we present cost-effectiveness findings for 6-month and 9-week trastuzumab use.
METHODS
Model Overview
A Markov model was developed for HER2-positive breast cancer in Indian women (Fig 1). The 5 health states were as follows: disease-free state, locoregional recurrence (LR), metastasis, death resulting from breast cancer, and all-cause mortality. Ten percent of those who developed LR were assumed to revert back to a disease-free state in the subsequent year.21 Thereafter, no remission from LR to back to a disease-free state was possible. Transition probability from LR to metastasis was 3 times that of disease-free state to metastasis.
FIG 1.
Model schematic.
We modeled the lifetime costs and consequences of treating a cohort of patients with surgically resected HER2-positive breast cancer at age ≥ 50 years with adjuvant chemotherapy or adjuvant chemotherapy plus trastuzumab from a societal perspective. Both health system costs and out-of-pocket expenditures were estimated. Indirect costs resulting from productivity losses were not included. Outcomes were calculated on the basis of life-years (LYs) and quality-adjusted LYs (QALYs) gained. All future costs and consequences were discounted at 3% considering international best practices, as well as recently published Indian guidelines for economic evaluation.22-24 A cycle length of 1 year was considered appropriate based on available literature.16,18,19,25,26 Results are reported as incremental cost (Indian national rupee [INR]) per LY and QALY gained with use of trastuzumab. As per guidelines for health technology assessment in India, we used a threshold of per-capita gross domestic product (GDP) in 2019 to evaluate cost effectiveness.23
Intervention and Control
We considered 1 year of trastuzumab along with adjuvant chemotherapy as an intervention and adjuvant chemotherapy (comprising anthracycline and taxane-based drugs) as a counterfactual group in the base case analysis. The base case analysis is presented in 2 scenarios. In base case 1, we used the effectiveness evidence from the HERA trial, whereas in base case 2, the effect size of the joint analysis was used; everything else remained constant. Three alternative intervention scenarios were considered based on the duration of trastuzumab use: 1 year, 6 months, and 9 weeks, respectively. Patients in a disease-free, LR, or metastatic state were assumed to be managed as per standard international (National Comprehensive Cancer Network) and national (Indian Council of Medical Research) guidelines27,28 (Table 1).
TABLE 1.
Clinical Parameters for Assessing Cost Effectiveness of Adjuvant Trastuzumab Versus Chemotherapy
Cost
Trastuzumab infusion at 8 mg/kg for the first cycle and 6 mg/kg for the remaining 16 cycles was considered for all patients in the first year in the intervention arm, assuming an average weight of 60 kg. The average weight of women with breast cancer in India was assumed as per findings of previous studies.29,30 The cost for those with a disease-free health state in the intervention arm accounted for outpatient (OPD) oncology and cardiac consultation, electrocardiogram, echocardiography, mammography, and hormone therapy. For those with LR, the cost accounted for clinical examination (OPD consultation), routine diagnostic tests, and radiologic tests. Additionally, the costs of performing various procedures for patient management, such as local mastectomy, radiotherapy, chemotherapy, and hormone therapy, were included. Similarly, various diagnostic tests and management protocols (chemotherapy, radiotherapy, hormone therapy, and surgery) as per the Indian Council of Medical Research cancer registry were taken into account (Tables 1 and 2). In addition, the cost of management of cardiac complications was included in intervention arm.
TABLE 2.
Cost Parameters for Assessing Cost Effectiveness of 1-Year Adjuvant Trastuzumab SC
The cost for patients with a disease-free health state in the control arm included oncology OPD consultation, mammography, and hormone therapy. Similarly, for those in an LR or metastatic health state, an identical set of hematologic, diagnostic, and radiologic tests and recurrent breast cancer management guidelines were followed as for the intervention arm.
The treatment regimens and their use in the intervention and control arms (applicable to new or all health patients in respective health states) were followed as per standard treatment guidelines.27,28 To make the cost of treatment more in keeping with real data, we used the rates of use of various treatment options among patients in different health states, as reported in the pooled data from Indian cancer registries31 (Table 1).
Locally published studies were used to elicit the unit costs of various diagnostic and therapeutic services provided to these patients.32,33 For those services, where published cost studies were not available, we relied on provider payment rates under the national social insurance scheme for central government employees.34 Data on prices of medicine were obtained from procurement rates of the medical service corporation in Tamil Nadu state.35
Valuation of Consequences
Nearly 18 cost-effectiveness studies have been undertaken to evaluate trastuzumab.11-14,16-19,21,25,26,36-42 Eight studies modeled consequences using effectiveness estimates reported in the HERA trial, whereas 6 used the joint analysis of NSABP B-31 (ClinicalTrials.gov identifier: NCT00004067) and NCCTG N9831 (ClinicalTrials.gov identifier: NCT00898898) trials. The HERA trial reported OS and DFS over a longer follow-up period and reported hazard ratios (HRs) at multiple time points, but this protocol is not commonly practiced in India or elsewhere. Moreover, crossover of patients between study arms was likely to have led to an underestimation of the benefits of adjuvant trastuzumab. The joint analysis reported a greater benefit, with an HR of 0.60, and its protocol is commonly followed in routine practice. Therefore, we used the efficacy data from both analyses to separately report the outcomes and cost effectiveness of 1 year of trastuzumab in 2 separate base case analyses.6
The CONCORD study, which used data on survival outcomes from 2 Indian cancer registries, reported 5-year survival of 66.1%.43 Similarly, another Indian study that reported long-term outcomes found a 35% survival rate at 10 years.44 We calibrated the model in the control arm (because use of trastuzumab has been reported in India among only 8.6% of eligible patients) so that the survival rates were as reported for the Indian patient population. Furthermore, using the DFS HRs from the HERA trial at each of the 5 different time points, from the first to 11th year, we applied the year-wise HRs to the control arm transition probabilities to arrive at the intervention arm transition probabilities.7,45-48 For the 12th to 15th years, we assumed the same HR reported in the HERA trial for 11th year; beyond year 15, we did not assume any further trastuzumab effectiveness. For computing the transition probability in the intervention arm using the effectiveness estimate of the joint analysis, we used an HR of 0.60 for each year up to 15 years.
The risk of mortality resulting from metastatic breast cancer reported in published evidence from India44 was further calibrated to match the overall breast cancer survival trends reported in the CONCORD and long-term survival analysis studies. The same risk of mortality resulting from metastasis was applied to patients in both the intervention and control arms. Age-wise risk of mortality as per Indian sample registration survey life tables was applied to women in both the intervention and control groups.49 Utility values for the disease-free state in first and subsequent years, respectively, were 0.749 and 0.847, whereas for LR and metastatic health states, utility values were 0.484 and 0.810, respectively (Table 2).18
Sensitivity Analysis
A probabilistic sensitivity analysis using second-order Monte Carlo simulation was undertaken. The values for transition probability varied by 10%, whereas values for both utility and cost varied by 20% each around the base value. Beta distribution was used to parameterize transition probability and health state utility values. Similarly, gamma distribution was used for cost parameters. The number of iterations was restricted to 1,000.
We undertook a subgroup analysis to assess the cost effectiveness of 6-month and 9-week trastuzumab use compared with standard chemotherapy. The HRs for DFS and cardiac events with 6 versus 12 months of trastuzumab use were derived from estimates reported in 2 trials, PERSEPHONE and PHARE, respectively.50,51 Because the estimates of each of the 2 trials were slightly different, the incremental cost-effectiveness ratios (ICERs) were computed separately using the HR for DFS reported in each trial. The HRs for DFS of 1.07 and 1.08 as reported in the PERSEPHONE and PHARE trials, respectively, were applied to the transition probabilities of 1-year trastuzumab use as computed earlier in the base model to derive transition probabilities for 6-month trastuzumab use. The probability of dying with metastasis was similar to that of the base case. Similarly, transition probabilities for 9-week trastuzumab use were computed using hazard rates and cardiac events from 9 weeks versus 12 months of trastuzumab separately as reported in the Short HER (HR, 1.13) and FinHER trials.51-53
A threshold analysis was undertaken to ascertain the price at which the ICER value was below the per capita GDP. The threshold was justified based on economic evaluations conducted in India,22 Indian health technology assessment guidelines,23 and a recent oncologic cost-effectiveness analysis conducted in India.54-56
RESULTS
One-Year Trastuzumab: Base Case 1 (HERA trial effectiveness)
The lifetime discounted cost per patient for those receiving 1 year of adjuvant trastuzumab with chemotherapy was found to be INR 341,046 (US$4,878; Table 3). Similarly, patients receiving adjuvant chemotherapy alone incurred a lifetime cost of INR 110,151 (US$1,575). The incremental cost per patient of trastuzumab use was INR 230,895 (US$3,302; Table 3).
TABLE 3.
Deterministic Costs, Effects, and Cost Effectiveness of 1-Year Trastuzumab Use As Compared With SC
The number of QALYs lived per patient among those receiving trastuzumab and chemotherapy alone were 6.6 and 5.3 years, respectively. The incremental health benefits gained per patient after treatment with trastuzumab were 1.48 LYs and 1.29 QALYs.
Overall, our findings show that use of trastuzumab for 1 year would incur an incremental cost of INR 156,291 (US$2,235) per LY gained and INR 178,877 (US$2,558) per QALY gained (Table 3). The value of incremental cost per QALY gained would be more than the per capita GDP of India; therefore, use of trastuzumab for 1 year would not be considered cost effective in the Indian setting.
One-Year Trastuzumab: Base Case 2 (joint analysis effectiveness)
The lifetime and incremental costs per patient with trastuzumab were INR 3,37,935 (US$4,833) and INR 2,27,784 (US$3,258), respectively. The LYs and QALYs lived per patient using trastuzumab were 8.7 and 7.0, respectively. The incremental health benefits per patient were found to be 1.93 life-years and 1.69 QALYs gained. As a result, 1-year trastuzumab use would incur an additional cost of INR 1,18,096 (US$1,689) per LY and INR 1,34,413 (US$1,922) per QALY gained (Table 3).
Subgroup and Sensitivity Analyses
The incremental cost per QALY gained with 6-month trastuzumab use was found to be INR 110,455 (US$1,580) and INR 114,060 (US$1,631) when effectiveness estimates from the PERSEPHONE and PHARE trials, respectively, were used. The incremental cost of 9-week trastuzumab use per QALY gained was found to be INR 43,264 (US$619) and INR 34,268 (US$490) considering the effectiveness reported in the Short HER and FinHER trials, respectively. Each of these ICER estimates falls within the cost-effectiveness threshold of per capita GDP (Table 4).
TABLE 4.
Probabilistic Costs, Consequences, and Probability of Being Cost Effective for 1-Year, 6-Month, and 9-Weeks Adjuvant Trastuzumab Use
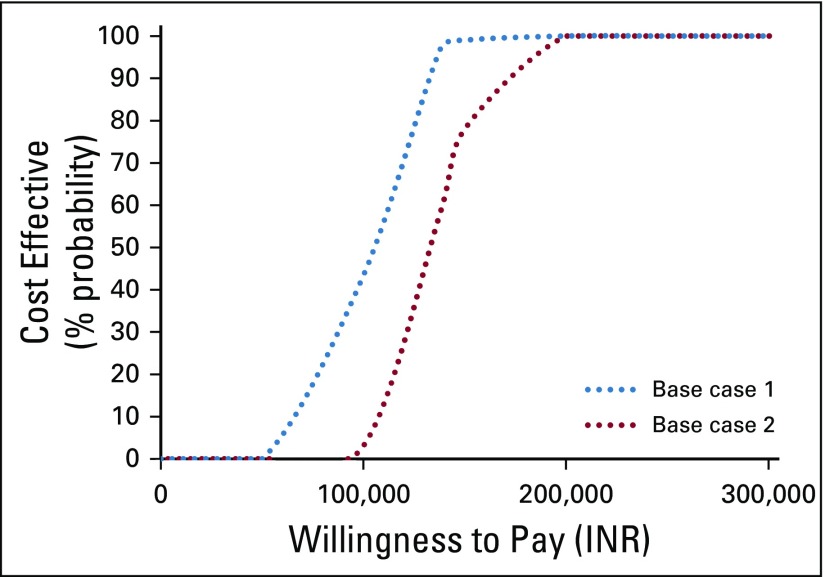
The findings of cost effectiveness are highly sensitive to the price of trastuzumab, DFS utility after 1 year, and transition probability from a disease-free to metastatic state in the chemotherapy arm. The findings of the probabilistic sensitivity analysis suggest that there is a 4% probability for 1-year trastuzumab use to be cost effective at a willingness-to-pay threshold equal to the per capita GDP (Figs 2 and 3). However, reducing the price by 15% to 35% increases the probability of 1-year trastuzumab use being cost effective to 90% (Fig 3).
FIG 2.
Probability of 1-year trastuzumab use being cost effective at varying willingness-to-pay thresholds. INR, Indian national rupee.
FIG 3.
Price sensitivity analysis for cost effectiveness of 1-year trastuzumab use. GDP, gross domestic product; INR, Indian national rupee.
DISCUSSION
Overall, our findings indicate that trastuzumab use for 1 year is not cost effective at its current price. However, with a 15% to 35% reduction of price, 1-year trastuzumab use would be cost effective. Use of trastuzumab for both 6 months and 9 weeks is cost effective. However, with a statistically similar number of QALYs gained, 9 weeks of trastuzumab use has a lower incremental cost and hence is the most efficient option.
We have presented our results using effectiveness data from a variety of different trials. Second, we used estimates of HRs as reported at different time points (as in the HERA trial) rather than a constant HR, which has been assumed in most of the previous economic evaluations. Third, we calibrated our model for the counterfactual scenario to predict survival based on breast cancer survival from 2 Indian cancer registries. Therefore, our findings are much more pragmatic and representative of the Indian population.
With regard to cost, our parameter values for the cost of management of breast cancer and its complications were obtained from locally published cost studies32,33 or reimbursement rates under 1 of India’s largest social insurance programs for provider payments.34,58 Similarly, the patterns of treatment use specific to each stage of disease were based on analysis of hospital-based cancer registries.31 Hence, our cost analysis seems realistic from the national viewpoint.
The incremental gain in LYs has ranged from 0.6 to 2.87 in various studies, whereas QALYs gained have varied from 0.49 to 2.83.11-14,16-19,21,25,26,36-42,59 We found the incremental health benefit after treatment with trastuzumab to be 1.48 LYs and 1.29 QALYs, both of which are well within the range of values in published evidence.
The incremental cost per QALY gained in terms of purchasing power parity ranges from 4,819 international dollars (Int$) to Int$110,283, with a median value of Int$40,998. Our study finding for an ICER (Int$8,954) fell within this range. The relatively lower ICER for trastuzumab use found in India could be attributable to India’s relatively lower drug prices and differences in health care delivery structure.
Considering the huge disease and economic burden that cancer imposes, several publicly financed health insurance schemes have been implemented in India.60 The PMJAY, which is the largest tax-funded health insurance scheme for the poor in India, also includes cancer treatment in its benefit package.9,10 Given the evidence from our study, it is recommended that insurance schemes provide for 9-week trastuzumab treatment for patients with HER2/neu-positive breast cancer. Furthermore, the National Pharmaceutical Pricing Authority should consider reducing the price of trastuzumab by at least 35%, such that 1-year trastuzumab use would also become cost effective. The network of cancer hospitals as part of the National Cancer Grid could develop a mechanism for common procurement of chemotherapy drugs, which would likely bring down prices.20
There has been significant emphasis on the development of standard treatment guidelines based on evidence from health technology assessments.23,61 It is recommended that in addition to clinical evidence on effectiveness, evidence on cost effectiveness be considered while framing clinical guidelines.
Empirically derived evidence on transition probabilities and long-term survival to parameterize such cost-effectiveness models is currently lacking. More research is needed using longitudinal studies. Second, there is a lack of clinical data on quality of life at different stages of cancer survival. In the absence of such a study from India, we had to use a valuation study conducted elsewhere. Finally, we recommend generation of a cost database or reference cost menu that could be used by researchers to populate such economic models. This would help reduce the uncertainty.
In conclusion, our study findings show that 1-year use of trastuzumab is not cost effective, or there is significant uncertainty around its cost effectiveness. Reducing the price of the drug by 35% would make 1-year trastuzumab use cost effective. In the current scenario, use of trastuzumab for 9 weeks is the most efficient option. The clinical guidelines and provider payments for cancer treatment under health insurance schemes should be accordingly revised.
AUTHOR CONTRIBUTIONS
Conception and design: Nidhi Gupta, Shankar Prinja
Collection and assembly of data: Nidhi Gupta, Rohan Kumar Verma
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sudeep Gupta
Research Funding: Roche (Inst), Sanofi (Inst), Johnson & Johnson (Inst), Amgen (Inst), Celltrion (Inst), Oncostem Diagnostics (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Union for International Cancer Control: New global cancer data: GLOBOCAN 2018. https://www.uicc.org/news/new-global-cancer-data-globocan-2018.
- 2.Kumar N, Patni P, Agarwal A, et al. Prevalence of molecular subtypes of invasive breast cancer: A retrospective study. Med J Armed Forces India. 2015;71:254–258. doi: 10.1016/j.mjafi.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dogra A, Doval DC, Sardana M, et al. Clinicopathological characteristics of triple negative breast cancer at a tertiary care hospital in India. Asian Pac J Cancer Prev. 2014;15:10577–10583. doi: 10.7314/apjcp.2014.15.24.10577. [DOI] [PubMed] [Google Scholar]
- 4.Patnayak R, Jena A, Rukmangadha N, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience. Indian J Med Paediatr Oncol. 2015;36:117–122. doi: 10.4103/0971-5851.158844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh J, Gupta S, Desai S, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391–396. doi: 10.4103/0019-509X.92245. [DOI] [PubMed] [Google Scholar]
- 9.Das S, Jha AK. Getting coverage right for 500 million Indians. N Engl J Med. 2019;380:2287–2289. doi: 10.1056/NEJMp1901771. [DOI] [PubMed] [Google Scholar]
- 10.Angell BJ, Prinja S, Gupt A, et al. The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana and the path to universal health coverage in India: Overcoming the challenges of stewardship and governance. PLoS Med. 2019;16:e1002759. doi: 10.1371/journal.pmed.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrison LP, Jr, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110:489–498. doi: 10.1002/cncr.22806. [DOI] [PubMed] [Google Scholar]
- 12.Hedden L, O’Reilly S, Lohrisch C, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist. 2012;17:164–171. doi: 10.1634/theoncologist.2011-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2007;25:625–633. doi: 10.1200/JCO.2006.06.4220. [DOI] [PubMed] [Google Scholar]
- 14.Hall PS, Hulme C, McCabe C, et al. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: A UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. Pharmacoeconomics. 2011;29:415–432. doi: 10.2165/11588340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Lidgren M, Jönsson B, Rehnberg C, et al. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol. 2008;19:487–495. doi: 10.1093/annonc/mdm488. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009;12(suppl 3):S82–S84. doi: 10.1111/j.1524-4733.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 17.Shiroiwa T, Fukuda T, Shimozuma K, et al. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: Based on 2-year follow-up HERA trial data. Breast Cancer Res Treat. 2008;109:559–566. doi: 10.1007/s10549-007-9679-4. [DOI] [PubMed] [Google Scholar]
- 18.Aboutorabi A, Hadian M, Ghaderi H, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2014;7:98–106. doi: 10.5539/gjhs.v7n1p98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica. 2013;33:411–417. doi: 10.7705/biomedica.v33i3.832. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Grid National Cancer Grid Mumbai. https://tmc.gov.in/ncg/index.php/overview/about-us
- 21.Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: A lifetime model. Pharmacoeconomics. 2007;25:429–442. doi: 10.2165/00019053-200725050-00006. [DOI] [PubMed] [Google Scholar]
- 22.Prinja S, Chauhan AS, Angell B, et al. A systematic review of the state of economic evaluation for health care in India. Appl Health Econ Health Policy. 2015;13:595–613. doi: 10.1007/s40258-015-0201-6. [DOI] [PubMed] [Google Scholar]
- 23. Department of Health Research, Government of India: Health Technology Assessment in India: A Manual. New Delhi, India, Ministry of Health and Family Welfare, Government of India,, 2018, p 126. [Google Scholar]
- 24. Tan-Torres Edejer T, Baltussen R, Adam T, et al (eds): Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland, World Health Organization, 2003. [Google Scholar]
- 25.Pichon-Riviere A, Garay OU, Augustovski F, et al. Implications of global pricing policies on access to innovative drugs: The case of trastuzumab in seven Latin American countries. Int J Technol Assess Health Care. 2015;31:2–11. doi: 10.1017/S0266462315000094. [DOI] [PubMed] [Google Scholar]
- 26.Van Vlaenderen I, Canon JL, Cocquyt V, et al. Trastuzumab treatment of early stage breast cancer is cost-effective from the perspective of the Belgian health care authorities. Acta Clin Belg. 2009;64:100–112. doi: 10.1179/acb.2009.019. [DOI] [PubMed] [Google Scholar]
- 27. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology 2019. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 28. Indian Council of Medical Research: Consensus Document for Management of Breast Cancer. https://www.icmr.nic.in/sites/default/files/guidelines/Breast_Cancer.pdf.
- 29.Singh P, Kapil U, Shukla N, et al. Association of overweight and obesity with breast cancer in India. Indian J Community Med. 2011;36:259–262. doi: 10.4103/0970-0218.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony MP, Surakutty B, Vasu TA, et al. Risk factors for breast cancer among Indian women: A case-control study. Niger J Clin Pract. 2018;21:436–442. doi: 10.4103/njcp.njcp_102_17. [DOI] [PubMed] [Google Scholar]
- 31. National Centre for Disease Informatics and Research: Consolidated Report of Hospital Based Cancer Registries 2007-2011. https://icmr.nic.in/sites/default/files/reports/Preliminary_Pages_0.pdf. [Google Scholar]
- 32. Chauhan A, Prakash G, Gupta N, et al: Cost-effectiveness of rituximab for the treatment of non-Hodgkin’s lymphoma in India. XXXX (in press) [Google Scholar]
- 33. Prinja S, Sharma Y, Dixit J, et al: Cost of cardiac care at tertiary hospital in North India. Indian Heart J (in press) [Google Scholar]
- 34. Central Government Health Scheme: CGHS rate list. https://cghs.gov.in/index1.php?lang=1&level=3&sublinkid=5948&lid=3881.
- 35. Tamil Nadu Medical Services, Government of Tamil Nadu: Essential drug list. https://www.tnmsc.tn.gov.in/user_pages/drugtender.php?drugcat=T18028.
- 36.Dedes KJ, Szucs TD, Imesch P, et al. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: A model-based analysis of the HERA and FinHer trial. Ann Oncol. 2007;18:1493–1499. doi: 10.1093/annonc/mdm185. [DOI] [PubMed] [Google Scholar]
- 37.Kurian AW, Thompson RN, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25:634–641. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- 38.Norum J, Olsen JA, Wist EA, et al. Trastuzumab in adjuvant breast cancer therapy: A model based cost-effectiveness analysis. Acta Oncol. 2007;46:153–164. doi: 10.1080/02841860601096841. [DOI] [PubMed] [Google Scholar]
- 39.Neyt M, Huybrechts M, Hulstaert F, et al. Trastuzumab in early stage breast cancer: A cost-effectiveness analysis for Belgium. Health Policy. 2008;87:146–159. doi: 10.1016/j.healthpol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Lang H-C, Chen H-W, Chiou T-J, et al. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016;19:923–927. doi: 10.1080/13696998.2016.1185013. [DOI] [PubMed] [Google Scholar]
- 41. Ansaripour A, Uyl-de Groot CA, Redekop WK: Adjuvant Trastuzumab therapy for early HER2-positive breast cancer in Iran: A cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics 36:91-103, 2018 [Erratum: Pharmacoeconomics 36:505, 2018] [DOI] [PMC free article] [PubMed]
- 42.Seferina SC, Ramaekers BLT, de Boer M, et al. Cost and cost-effectiveness of adjuvant trastuzumab in the real world setting: A study of the Southeast Netherlands Breast Cancer Consortium. Oncotarget. 2017;8:79223–79233. doi: 10.18632/oncotarget.16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care (Basel) 2008;3:21–27. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 46.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 47.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 48.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 49. Office of the Registrar General & Census Commissioner India: SRS Statistical Report 2015. http://www.censusindia.gov.in/vital_statistics/SRS_Reports_2015.html.
- 50.Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393:2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 52.Conte P, Frassoldati A, Bisagni G, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study. Ann Oncol. 2018;29:2328–2333. doi: 10.1093/annonc/mdy414. [DOI] [PubMed] [Google Scholar]
- 53.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 54.Gupta N, Verma RK, Prinja S, et al. Cost-effectiveness of sorafenib for treatment of advanced hepatocellular carcinoma in India. J Clin Exp Hepatol. 2019;9:468–475. doi: 10.1016/j.jceh.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prinja S, Kaur G, Malhotra P, et al. Cost-effectiveness of autologous stem cell treatment as compared to conventional chemotherapy for treatment of multiple myeloma in India. Indian J Hematol Blood Transfus. 2017;33:31–40. doi: 10.1007/s12288-017-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prinja S, Bahuguna P, Faujdar DS, et al. Cost effectiveness of human papillomavirus vaccination for adolescent girls in Punjab state: Implications for India’s universal immunization program. Cancer. 2017;123:3253–3260. doi: 10.1002/cncr.30734. [DOI] [PubMed] [Google Scholar]
- 57. Reference deleted. [Google Scholar]
- 58. Government of India: Rashtriya Swasthya Bima Yojana: Procedure list. https://www.india.gov.in/spotlight/rashtriya-swasthya-bima-yojana#rsby3. [Google Scholar]
- 59. Yalcin B: Staging, risk assessment and screening of breast cancer. Exp Oncol 35:238-245, 2013. [PubMed]
- 60.Prinja S, Chauhan AS, Karan A, et al. Impact of publicly financed health insurance schemes on healthcare utilization and financial risk protection in India: A systematic review. PLoS One. 2017;12:e0170996. doi: 10.1371/journal.pone.0170996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prinja S, Downey LE, Gauba VK, et al: Health technology assessment for policy making in India: Current scenario and way forward. Pharmacoecon Open 2:1-3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]