Abstract
Introduction
Vitamin D is paramount to bone health and little is known about vitamin D’s role in the prevention of stress fractures in high-risk athletes. This study consists of a prospective, cross-sectional analysis accompanied by a retrospective review for control comparison of vitamin D3 supplementation in high-risk athletes. Our hypothesis is that supplemental vitamin D3 treatment will decrease the occurrence of stress fractures in high-risk collegiate athletes.
Materials and Methods
A total of 118 NCAA Division I athletes were recruited from 6 high-risk collegiate teams. Blood draws in August and February established baseline 25(OH)D levels. Subjects with serum 25(OH)D <30 ng/mL were supplemented with 50,000 IU of vitamin D3/week for 8 weeks. Treated subjects were re-tested to ensure serum 25(OH)D levels rose to sufficient status. All enrolled subjects were monitored for the development of stress fractures. A 5-year retrospective chart review of athletes from the same sports teams was conducted to determine the incidence of any reported stress fractures in the past.
Results
Prospective: 112 of the 118 enrolled subjects were tested in August. Sixty-one demonstrated vitamin D sufficiency (40.2 ng/mL ±8.28) and 51 were either insufficient or deficient (22.7 ng/mL ±4.89). Of the 118 enrolled subjects, 104 were tested in February. Fifty-six demonstrated vitamin D sufficiency (40.7 ng/mL ±9.47) and 48 were insufficient or deficient (21.6 ng/mL ±5.87). Two stress fractures were diagnosed amongst our cohort of 118 student athletes (1.69%). Retrospective: 34 stress fractures were diagnosed in 453 subjects from 01/2010-05/2015 (7.51%). Amongst our athletic teams, the cross-country team specifically demonstrated a statistically significant decrease in stress fracture incidence (p<0.05). We also found a statistically significant reduction in stress fracture incidence amongst the current overall cohort compared to our retrospective cohort (p<0.05).
Conclusion
In our population, almost half of the tested athletes proved to be vitamin D deficient. Hypovitaminosis D was prevalent throughout the winter months compared with the summer. With vitamin D3 supplementation, the stress fracture rate in our overall cohort demonstrated a statistically significant decrease from 7.51% to 1.65% (p=0.009).
Keywords: vitamin D, stress injuries, sports medicine, bone health
Introduction
Stress fractures represent debilitating injuries that can impair musculoskeletal function. These injuries commonly develop in active individuals as a result of sudden increased or prolonged training at a high level. Running, jumping, and other high-impact activities cause repetitive, cyclic skeletal loading and create microscopic damage to bone. This microdamage promotes cellular repair and remodeling, lending towards increased skeletal turnover and overall improved bone strength.1,2 However, when microdamage occurs more rapidly than the rate of the remodeling, the bone is unable to sustain the load and stress injuries occur. Due to their training regimen, stress fractures are common in elite athletes and military recruits. A study by Rizzone et al shows stress fracture rates are highest in athletes with repetitive lower extremity loading, such as cross-country, track/field, basketball, and soccer.3 Additionally, Tenforde et al demonstrated female cross-country runners had the highest incidence of bone stress injuries (64% of all bone stress injuries reported) and had a 4.0- to 5.7-fold increase in overall risk when compared to low-risk runners.4
Vitamin D is an endogenously produced lipophilic hormone that is imperative to maintaining proper skeletal health. There are several inactive precursors and an active form found in humans. Vitamin D3, known as cholecalciferol, is an inactive precursor produced by the skin during sun exposure.5 Vitamin D3 is then transported to the liver and hydroxylated to form 25-hydroxyvitamin D, or 25(OH)D.5 When the active metabolite is needed, 25(OH)D becomes hydroxylated in the kidney to 1,25-dihydroxyvitamin D, or 1,25(OH)2D.5
The physiologically active form 1,25(OH)2D functions primarily to increase dietary calcium uptake and regulate calcium ion levels in blood.6 It also plays a vital role in skeletal health by coordinating the functions of osteoblasts, cells that lay down new bone, and osteoclasts, cells that resorb old bone. Binding of 1,25(OH)2D to the vitamin D receptor on osteoblasts upregulates the production of specific signaling proteins that promote osteoclastogenesis, as well as osteoblast migration and survival.6–8 Osteocytes, the third major cell-type involved in bone remodeling, detect changes in skeletal forces and release other signaling proteins that initiate the remodeling process.9 Together, these cells deposit new bone and resorb old bone in areas of increased stress in order to strengthen the underlying microstructure. Therefore, it appears that adequate vitamin D status plays an important role in maintaining skeletal integrity and may be directly associated with the prevention of stress fractures.
Vitamin D status is commonly established by measuring serum levels of the physiologically inactive form 25(OH) D. Hypovitaminosis D is prevalent among collegiate and professional athletes, ranging from 33% to 90%.10–13 Additionally, several important factors contribute to hypovitaminosis D, including skin color, residing at more polar latitudes, reduced sun exposure, seasonal variations, and poor nutrition.13–18 Supplemental options are effective at correcting hypovitaminosis D.19–21 One supplement option is the use of vitamin D3, an inactive precursor found in humans. However, very little data exists regarding skeletal overuse injuries and supplemental treatment as a preventive measure in athletes. Both military recruits and elite collegiate athletes are at a similarly increased risk for skeletal overuse injuries, like stress fractures, due to heightened physical demands of training regimens, training intensity, and overall performance expectations. To our knowledge, existing studies have focused on military recruits, with no studies prospectively evaluating vitamin D3 supplementation and its relation to stress fractures in athletes.22–24
This study functions as a pilot study to evaluate the effect of vitamin D3 supplementation on reducing stress fracture occurrence in National Collegiate Athletic Association Division I athletes on high-risk sports teams at a single institution. Our hypothesis is that supplementing vitamin D3 in high-risk collegiate athletes with hypovitaminosis D will decrease the occurrence of stress fractures.
Materials and Methods
Subjects
Institutional review board (IRB) approval was obtained for our study from both the hospital and university IRBs. This study was performed in accordance with the Declaration of Helsinki. Subjects were recruited from six high-risk NCAA Division I athletic teams at a single institution, including men’s and women’s track and field, men’s and women’s basketball, women’s soccer, and women’s cross-country. Recruitment took place during required pre-participation exams prior to the onset of the 2015–2016 academic year and each respective sport’s season. At a separate station, subjects were informed of the option to participate in the study, the goals and objectives of the study, the study timeline, and potential benefits and risks of participation. Each subject provided written informed consent to participate in the study. All participants of these teams were 18 years of age or older at the time of recruitment and consent. No subjects were excluded. A total of 118 athletes between the ages of 18 and 22 (19.7 ± 1.19) were enrolled in this study. Of the consenting subjects, 30 were male (25.4%, mean age 19.7 ± 1.26) and 88 were female (74.6%, mean age 19.6 ± 1.16). The subject-reported racial/ethnic distribution was 45.8% Caucasian, 36.4% African American, 1.7% Hispanic, 1.7% Asian, 4.2% multiracial, and 10.2% unreported.
Questionnaire
Prior to testing and supplementation, all subjects were asked to complete a brief questionnaire regarding the following demographics: height, weight, age, race, and specific sport. BMI was subsequently calculated based on subject responses for use in statistical analysis. All athletes were asked yes or no questions regarding whether or not they worried about their weight, were trying to or have been given a recommendation to gain or lose weight, followed a special diet or avoided certain foods or food groups, had a history of an eating disorder, had a history of stress fractures, had a history of low bone density, and if they were currently taking any over-the-counter vitamin D3 or multivitamin supplementation. Additionally, female subjects were asked to respond to an additional set of questions regarding having ever had a menstrual period, the age at onset of their first menstruation, their most recent menstrual period, the number of periods within the last 12 months, whether or not they were taking female hormones (i.e. birth control), and if so, which form of hormone.
Vitamin D Testing and Supplementation Procedure
Subjects’ vitamin D status was established by measuring serum 25-hydroxyvitamin D levels [25(OH)D] at two time points: once in August and once in February. Both August and February test results were used to determine seasonal baseline vitamin D status. Tests were completed by certified phlebotomists from the affiliated hospital using blood samples drawn from veins in the arm. Vitamin D statuses were specified as follows: sufficiency with serum levels ≥30 ng/mL and hypovitaminosis D with serum 25(OH)D levels <30 ng/mL.
Seasonal baseline vitamin D statuses served as a way to categorize whether or not subjects were to be supplemented with vitamin D3. Following the August baseline testing phase, subjects with 25(OH)D levels <30 ng/mL began a supplemental vitamin D3 (Bio-Tech Pharmacal, Inc., Fayetteville, AR) treatment regimen. Subjects were provided one oral capsule of 50,000 IUs of vitamin D3 per week for a period of 8 weeks. Vitamin D3 was distributed to each subject via team athletic trainers. Alternatively, subjects with 25(OH)D levels >30 ng/mL were not supplemented. Additionally, following the February serum 25(OH)D testing phase, subjects with 25(OH)D levels <30 ng/mL began the same supplemental vitamin D3 regimen. Subjects with 25(OH)D levels <30 ng/mL following both August and February baseline testing phases completed two rounds of the supplemental regimen. Alternatively, subjects with 25(OH)D levels >30 ng/mL in each of the testing phases did not receive any supplementation.
For the duration of the academic year (August 2015-May 2016), all subjects were monitored during training and game performances for any injury occurrence. Injuries were initially assessed by each team’s certified athletic trainer, and appropriate care was provided by the physicians and medical staff. Stress fractures acquired during each team’s season and off-season training schedules were included in the analysis. Team physicians utilized the imaging modality of their choice to diagnose stress fractures, and this data was subsequently recorded for further analysis.
Retrospective Data Collection
No subjects were directly enrolled as a part of retrospective data collection. De-identified injury reports were obtained from the institution’s athletic department to determine the overall stress fracture incidence rates from January of 2010 through May 2015. Stress fracture diagnoses were initiated by the athletic training team and verified by appropriate physician input via physical exam and diagnostic imaging as needed on an individual basis. During this time at this specific institution, there were no strategies in place for testing athlete 25(OH)D status, and therefore vitamin D3 supplementation in the form of tablets or capsules for athletes with hypovitaminosis D was not a common practice. Additionally, due to the de-identified nature of this data collection, athlete background information (i.e. height, weight, age, race, body image, dietary habits, histories of low bone density or stress fracture, and current vitamin supplementation) were not available and were not included in the final analysis.
Data Analysis
Seasonal vitamin D characteristics were determined for the cohort and individual teams using mean serum 25(OH)D levels from August and February testing phases, as well as between subjects that received supplementation and subjects that did not. Fischer’s exact testing was used to compare questionnaire responses to vitamin D status. A log likelihood ratio of independence without correction (G-test) was used to determine significance between vitamin D status and sex, race (dichotomized to white and non-white), and sport, respectively. A Welch two-sample T-test was used to determine the significance between vitamin D status and body mass index (BMI) and age, respectively.
The occurrence of stress fractures overall and per team in the current supplemental cohort was compared to retrospective stress fracture injury reports of non-tested, non-supplemented athletes from January 2010 to May 2015. The cumulative incidence was compared between the current supplemental cohort and the retrospective collected data.
Results
Vitamin D Status and Supplementation
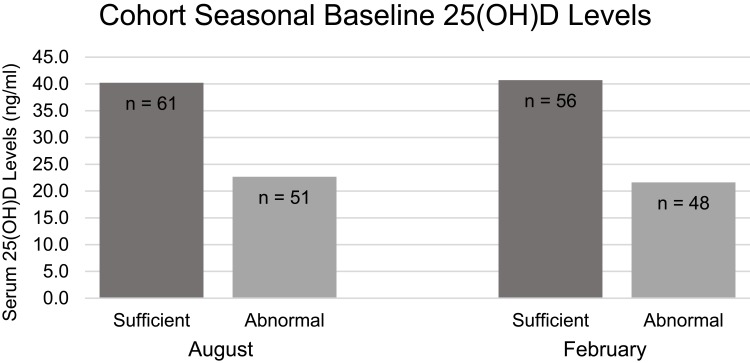
Of the 118 total enrolled subjects, 112 completed testing in August and 104 completed testing in February. Six subjects failed to complete testing in August and 14 failed to complete testing in February secondary to no longer being active with their respective teams. Seasonal baseline serum 25(OH)D levels for each team are depicted in Table 1. The cohort mean serum 25(OH)D levels in August were 32.2 ng/mL. Sixty-one subjects were of sufficient status (average = 40.2 ng/mL ± 8.28) and 51 were insufficient (average = 22.7 ng/mL ± 4.89). Mean serum 25(OH)D levels in February were 31.9 ng/mL. Fifty-six were of sufficient status (average = 40.7 ng/mL ± 9.47) and 48 were insufficient (average = 21.6 ng/mL ± 5.87). Figure 1 displays the seasonal baseline serum 25(OH)D levels of subjects with sufficient and abnormal vitamin D statuses in August and February. Our findings demonstrate a trend of decreasing 25(OH)D levels during winter months. Significant changes were found in the women’s basketball team (p≪0.001) and the women’s soccer team (p=0.025). Both of these teams had a significant number of supplemented subjects and may have helped to prevent further 25(OH)D decreases that were seen with other teams.
Table 1.
Seasonal Differences in Baseline Mean Serum 25(OH)D Levels
| Baseline Serum 25(OH)D Levels | |||||||
|---|---|---|---|---|---|---|---|
| Team | Overall | Men’s Track/Field | Women’s Track/Field | Men’s Basketball | Women’s Basketball | Women’s Cross-Country | Women’s Soccer |
| August | |||||||
| N | 112 | 11 | 18 | 14 | 13 | 25 | 31 |
| Mean (ng/mL) | 32.2 | 27.4 | 27.3 | 35.3 | 21.4 | 35.7 | 37.1 |
| Std. Dev (ng/mL) | 11.2 | 7.02 | 7.59 | 10.2 | 8.70 | 14.7 | 7.35 |
| February | |||||||
| N | 104 | 15 | 18 | 15 | 12 | 22 | 22 |
| Mean (ng/mL) | 31.9 | 25.2 | 27.1 | 30.3 | 49.7 | 31.2 | 32.5 |
| Std. Dev (ng/mL) | 12.4 | 10.6 | 11.3 | 8.16 | 8.16 | 14.7 | 6.73 |
Figure 1.
Number of subjects with sufficient status vs abnormal status.
Ninety-eight of the 118 enrolled subjects completed both August and February phases of vitamin D status testing. Of the subjects that were tested twice, August baseline testing revealed 48 subjects with hypovitaminosis D (23.0 ng/mL ± 4.84) and 50 subjects with sufficient vitamin D status (40.9 ng/mL ± 8.85), shown in Table 2. Subjects with hypovitaminosis D in August that received vitamin D3 supplementation saw an increase in mean serum 25(OH)D levels of 11.2 ng/mL, and 68.8% were corrected to a sufficient vitamin D status. Alternatively, mean serum 25(OH)D levels decreased an average of 9.49 ng/mL in those subjects that were sufficient in August and did not receive supplementation. In this group, 56% developed hypovitaminosis D by February testing. This data demonstrates vitamin D3 supplementation is effective at correcting hypovitaminosis D.
Table 2.
Vitamin D3 Supplementation vs Non-Supplementation in the Prospective Cohort
| Hypovitaminosis D | Sufficient | |
|---|---|---|
| N | 48 | 50 |
| August mean | 23.0 | 40.9 |
| August Std. Dev. | 4.84 | 8.85 |
| February mean | 34.1 | 31.4 |
| February Std. Dev. | 13.1 | 11.1 |
| Average change | 11.2 | − 9.49 |
| Percent corrected to sufficient | 68.8% | |
| Percent developed hypovitaminosis D | 56.0% |
Correlations between vitamin D status and demographics are depicted in Table 3. A log likelihood ratio of independence without correction (G-test) was used to determine correlation between vitamin D status and sex, race (dichotomized to white and non-white), and sport. There was a significant difference in vitamin D status between race and sports. There was no significant difference between sexes. A Welch two-sample T-test was used to determine the correlation between BMI, age, and vitamin D status. There were no significant changes in vitamin D status based on BMI or age.
Table 3.
Vitamin D Status in Relation to Cohort Demographics
| Demographics | Vitamin D Status | P-values | ||
|---|---|---|---|---|
| Low | Normal | |||
| Sex | Male | 36.7% | 63.3% | 0.339 |
| Female | 45.5% | 54.5% | ||
| Race* | White | 20.4% | 79.6% | <<0.001 |
| Non-white | 65.4% | 34.6% | ||
| Sport* | Basketball | 53.6% | 46.4% | 0.036 |
| Soccer | 25.8% | 74.2% | ||
| Track and field | 57.6% | 42.4% | ||
| Cross-country | 36.0% | 64.0% | ||
Note: *Indicates statistical significance.
We performed a Fisher’s exact test to analyze questionnaire covariates in relation to vitamin D status. Only two of our questionnaire queries proved to have a statistically significant relationship to hypovitaminosis D: subjects that were taking female hormones (i.e. birth control), and subjects that had a history of previous stress fractures (Table 4).
Table 4.
Vitamin D Status in Relation to Questionnaire Responses
| Questionnaire Topic | P-value | |
|---|---|---|
| Females only | Age of first menstruation | 0.457 |
| Menses in the past 12 months | 0.127 | |
| Taking female hormones* | 0.03 | |
| All subjects | Worrying about weight | 0.826 |
| Trying to change weight | 0.232 | |
| On a special diet | 0.452 | |
| Eating disorder | >0.99 | |
| History of stress fracture* | 0.046 | |
| Previous vitamin D supplementation | 0.085 |
Note: *Indicates statistical significance.
Stress Fracture Data
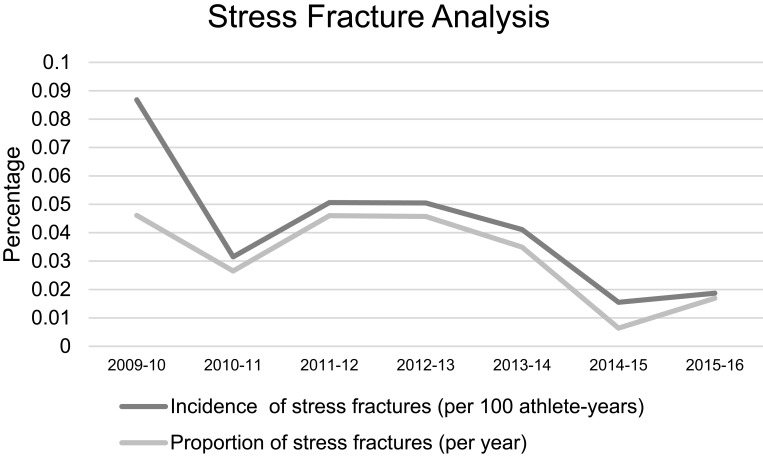
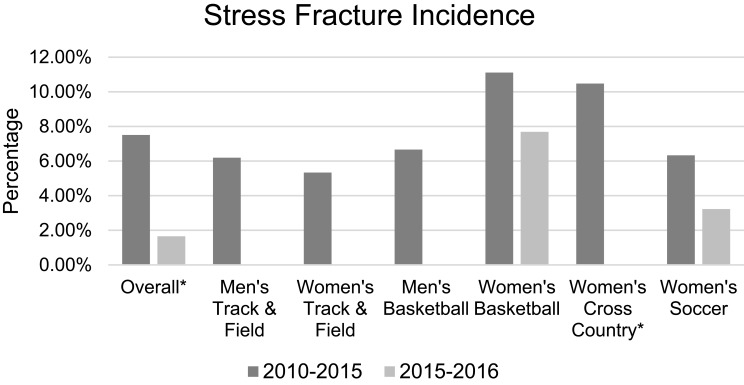
Stress fractures were diagnosed in 2 of the 118 currently enrolled subjects (1.69%). There were 34 stress fractures diagnosed in 453 subjects from January 2010 to May 2015 (7.51%). Figure 2 shows stress fracture incidence by year. The cumulative incidence of stress fractures per team from 2010 to 2015 was compared to the current 2015–2016 cohort. A significant reduction in stress fracture incidence was found in the current overall cohort (p=0.01) as well as in current cross-country subjects (p=0.046) compared to previous seasons (Figure 3). There was a decrease in stress fracture incidence between all other sports; however, none of these were statistically significant.
Figure 2.
Incidence and proportions of stress fractures.
Figure 3.
Current and historical stress fracture occurrences per team. *Indicates statistical significance.
Discussion
Vitamin D Status and Supplementation
Active 1,25-hydroxyvitamin D is a lipophilic hormone that functions via genomic pathways to promote synthesis of various signaling proteins.10 In bone, these proteins enhance osteoclastogenesis, leading to increased rates of bone resorption by osteoclasts and bone deposition by osteoblasts. In this way, 1,25(OH)2D plays an active role in bone’s response and adaptation to mechanical stress. Previous research evaluated hypovitaminosis D prevalence and its effects on health and performance in athletic cohorts, but data on vitamin D3 supplementation in the prevention of stress fractures are only beginning to emerge.25–27 To our knowledge, this research represents the first pilot study examining the effects of vitamin D3 supplementation on reducing stress fracture occurrences in NCAA Division I collegiate athletes.
The Endocrine Society describes hypovitaminosis D as serum 25(OH)D levels <30 ng/mL.28 Numerous studies have examined hypovitaminosis D prevalence in athletic cohorts of ranging specialties.10–13 Among these, a study by Maroon et al reported hypovitaminosis D in 68.7% of NFL players on a single professional football team.12 Additionally, Villacis et al reported 33.6% of the collegiate athletes had abnormal vitamin D status, ranging from 0.0% to 61.5%.13 In our study cohort, baseline vitamin D status was assessed twice: once in August and again in February. The prevalence of hypovitaminosis D was 45.5% in August (n=51, mean = 22.7 ng/mL ±4.89) and 46.2% in February (n=48, mean = 21.6 ng/mL ±5.58). Consistent with other findings from other athletic cohorts, our results further support poor vitamin D status is highly prevalent among collegiate athletes.
Clinicians commonly use serum 25(OH)D level as a marker to assess bone health. Screening for and correcting hypovitaminosis D to maintain appropriate 25(OH)D status may potentially reduce injury risks and may support bone health in collegiate athletes. Multiple studies found vitamin D3 supplementation significantly increased 25(OH)D levels in athletes, while levels in non-supplemented groups tended to decline.21,29,30 Our results show initial mean 25(OH)D levels in the hypovitaminosis D group were 23.0 ng/mL. Following supplementation, mean 25(OH)D levels increased to 34.1 ng/mL, with an average change of +11.2 ng/mL. Subjects with adequate vitamin D status in August dropped from 40.9 ng/mL to 31.4 ng/mL, with an average change of −9.49 ng/mL. This data supports previous findings from other studies that vitamin D3 supplementation is an effective method for correcting and preventing hypovitaminosis D in athlete populations.
Intrinsic and Extrinsic Factors on Vitamin D Status
Seasonal changes can contribute to endogenous vitamin D3 metabolism, and subsequent 1,25(OH)2D production, as athletes spend more time indoors and are exposed to relatively less sunlight during the winter. Therefore, it is common to see the rate of hypovitaminosis D increase during winter months.16,17,31,32 Our results demonstrate vitamin D3 supplementation is effective at correcting and preventing hypovitaminosis D. However, despite supplementation, serum 25(OH)D levels decreased as a whole in the winter months in our cohort, except for the women’s basketball team who represented a significant change from the normative trend. This change is likely due to the large percentage of athletes from this team treated (13 of 14 athletes). These results support the importance of screening athletes for hypovitaminosis D and providing supplementation as a protective measure.
Several intrinsic and extrinsic factors may contribute to vitamin D status, including skin color, location of sporting activities, and season. Dark-skinned athletes have been shown to have an elevated risk of poor vitamin D status compared to light-skinned athletes.13,14 Our regression analysis shows statistically significant differences in baseline serum 25(OH)D levels between races, with non-white athletes demonstrating hypovitaminosis D more frequently than white athletes.
Athletes of certain specialties may be at an increased risk for poor vitamin D status.13,15,18,30,33 Results from our cohort show significant differences in vitamin D status between athletic specialties. While some specialties may require heightened screening, it remains important to monitor vitamin D status in all athletic populations. In addition, our results indicate both use of female hormones (i.e. birth control) and a history of previous stress fractures were significantly correlated with hypovitaminosis D. This supports retrospective data analyzed in the previous literature.22,23 Although we found this corollary reduction in stress fractures in our cohort, it is certainly possible that adjustments to coaching staffing or training regimen could account for our findings as well.
Vitamin D3 Supplementation and Stress Fracture Occurrence
Correcting hypovitaminosis D can improve bone health and may reduce skeletal overuse injuries like stress fractures. Emerging evidence suggests supplementation is a feasible method for preventing these injuries; however, prospective data are limited and primarily focuses on military recruit populations. Lappe et al reported a significant decrease in stress fracture incidence among supplemented female navy recruits compared to non-supplemented recruits.24 Additionally, Davey et al and Burgi et al found stress fracture incidence and risk were inversely correlated to 25(OH)D status.22,23
Our study retrospectively analyzed stress fracture occurrence in 453 athletes of the six high-risk teams between 2010 and 2015. These athletes were neither screened for vitamin D status nor provided vitamin D3 supplementation in a standardized fashion. There were 34 diagnosed stress fractures, an overall cumulative incidence of 7.51%. In our current supplemented cohort, there were 2 stress fractures in 118 subjects (1.69%), a significant reduction (p=0.01). Although the two stress fractures were in subjects of sufficient vitamin D status, the overall significant reduction in stress fracture occurrence may be partially attributed to prospective screening and vitamin D3 supplementation.
Study Strengths and Weaknesses
Since our data compares current athlete stress fracture incidence to retrospective injury reports from athletes who were not prospectively provided vitamin D3 supplementation, we cannot definitively associate the reduction in injury occurrence in our treatment cohort to supplementation. Furthermore, in the current pilot study, we did not implement any means of accountability or compliance to ensure adequate vitamin D3 supplementation amongst the athletes.
Given our particular institutional trend toward vitamin D3 supplementation of athletes, our aim was to develop a pilot study to prospectively evaluate stress fracture data and vitamin D3 supplementation over the course of one school year. Our results suggest a statistically significant correlation with vitamin D3 supplementation and reduction of stress fractures, with a decrease in stress fracture cumulative incidence from 7.51% (2010–2015) to 1.69% (2015–2016).
Conclusion
Our study’s prospective design represents the first of its kind in evaluating stress fracture prevention with vitamin D3 supplementation in NCAA Division I collegiate athletes. In this pilot study, we noted a statistically significant reduction in the occurrence of stress fractures when compared to the historical data from our institution. We plan to assess the long-term results from our pilot study data and given the initial positive results on the health of our student athletes expand our supplementation to other teams. Given our study’s findings of new onset stress fractures in athletes with appropriate vitamin D status, we believe larger-scale prospective studies are necessary to confirm the decrease in stress fracture reduction seen in our athlete cohort and to establish the importance of other factors (i.e. coaching, strength and conditioning regimens) involved in the prevention of these injuries.
Acknowledgments
The study team would like to thank Dr Martin Durkin for his assistance with statistical analysis and planning. The team would also like to thank staff from the local university’s athletics department for facilitating athlete supplementation and injury management.
Funding Statement
Research funding was provided by the Grant in Aid for Resident Research from the Richland Memorial Hospital Research and Education Foundation.
Disclosure
Dr Christopher Mazoue reports grants from Palmetto Health, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Hadjidakis DJ, II A. Bone remodeling. Ann N Y Acad Sci. 2006;1092:285–296. [DOI] [PubMed] [Google Scholar]
- 2.Warden S, Burr D, Brukner P. Stress fractures: pathophysiology, epidemiology, and risk factors. Curr Osteoporos Rep. 2006;4:103–109. doi: 10.1007/s11914-996-0029-y [DOI] [PubMed] [Google Scholar]
- 3.Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The epidemiology of stress fractures in collegiate student-athletes, 2004–2005 through 2013–2014 academic years. J Athl Train. 2017;52(10):966–975. doi: 10.4085/1062-6050-52.8.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenforde AS, Carlson JL, Change A, et al. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med. 2017;45(2):302–310. doi: 10.1177/0363546516676262 [DOI] [PubMed] [Google Scholar]
- 5.Bikle D. Vitamin D: production, metabolism, and mechanisms of action. 2014 Jan 1 In: De Groot LJ, Beck-Peccoz P, Chrousos G, editors. Endotext (Internet). South Dartmouth, MA: MDText.com, Inc.; 2000;15–37. [Google Scholar]
- 6.Franceschi RT, Yan L. Vitamin D regulation in osteoblast function In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. Third ed. San Diego, CA: Academic Press; Elsevier, Inc; 2011:321–333. [Google Scholar]
- 7.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92(2):77–98. doi: 10.1007/s00223-012-9619-0 [DOI] [PubMed] [Google Scholar]
- 8.Donnelly E, Boskey AL. Mineralization. Vitamin D, Third Edition. Feldman D, Pike JW, Adams JS, Editors. San Diego, CA: Academic Press; Elsevier, Inc; 2011. [Google Scholar]
- 9.Qin L, Zhang M. Mechanical Testing for Bone Specimens. Current Topics in Bone Biology. Deng HW, Liu YZ. Hackensack, NJ: World Scientific Publishing Company; 2005. [Google Scholar]
- 10.Hamilton B, Grantham J, Racinais S, Chalabi H. Vitamin D deficiency is endemic in middle eastern sportsman. Public Health Nutr. 2010;13(10):1528–1534. doi: 10.1017/S136898000999320X [DOI] [PubMed] [Google Scholar]
- 11.Hamilton B, Whiteley R, Farooq A, Chalabi H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci Med Sport. 2014;17(1):139–143. doi: 10.1016/j.jsams.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Maroon JC, Mathyssek CM, Bost JW, et al. Vitamin D profile in national football league players. Am J Sports Med. 2015;43(5):1241–1245. doi: 10.1177/0363546514567297 [DOI] [PubMed] [Google Scholar]
- 13.Villacis D, Yi A, Jahn R, et al. Prevalence of abnormal vitamin D levels among division I NCAA athletes. Sports Health. 2014;6(4):340–347. doi: 10.1177/1941738114524517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bescos Garcia R, Rodriguez Cuisado FA. Low levels of vitamin D in professional basketball players after wintertime: relation with dietary intake of vitamin D and calcium. Nutr Hosp. 2011;26(5):945–951. doi: 10.1590/S0212-16112011000500004 [DOI] [PubMed] [Google Scholar]
- 15.Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43(2):335–343. doi: 10.1249/MSS.0b013e3181eb9d4d [DOI] [PubMed] [Google Scholar]
- 16.Kopec A, Solarz K, Majda F, Slowinska-Lisowska M, Medras M. An evaluation of the levels of vitamin D and bone turnover markers after the summer and winter periods in Polish professional soccer players. J Hum Kinet. 2013;8(38):135–140. doi: 10.2478/hukin-2013-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37(4):789–802. doi: 10.1139/h2012-037 [DOI] [PubMed] [Google Scholar]
- 18.Peeling P, Fulton SK, Binnie M, Goodman C. Training environment and vitamin D status in athletes. Int J Sports Med. 2013;34(3):248–252. doi: 10.1055/s-0032-1321894 [DOI] [PubMed] [Google Scholar]
- 19.Close GL, Russell J, Cobley JN, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31(4):344–353. doi: 10.1080/02640414.2012.733822 [DOI] [PubMed] [Google Scholar]
- 20.Jastrzebska M, Kaczmarczyk M, Jastrzebski Z. The effect of vitamin D supplementation on training adaptation in well trained soccer players. J Strength Cond Res. 2016;30(9):2648–2655. doi: 10.1519/JSC.0000000000001337 [DOI] [PubMed] [Google Scholar]
- 21.Lewis RM, Redzic M, Thomas DT. The effects of season-long vitamin D supplementation on collegiate swimmers and divers. Int J Sport Nutr Exerc Metab. 2013;23(5):431–440. doi: 10.1123/ijsnem.23.5.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgi AA, Gorham ED, Garland CF, et al. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J Bone Miner Res. 2011;26(10):2371–2377. doi: 10.1002/jbmr.451 [DOI] [PubMed] [Google Scholar]
- 23.Davey T, Lanham-New SA, Shaw AM, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during royal marine recruit training. Osteoporos Int. 2016;27(1):171–179. doi: 10.1007/s00198-015-3228-5 [DOI] [PubMed] [Google Scholar]
- 24.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741–749. doi: 10.1359/jbmr.080102 [DOI] [PubMed] [Google Scholar]
- 25.Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013;41(2):461–464. doi: 10.1177/0363546513475787 [DOI] [PubMed] [Google Scholar]
- 26.Farrokhyar F, Tabasinejad R, Dao D, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365–378. doi: 10.1007/s40279-014-0267-6 [DOI] [PubMed] [Google Scholar]
- 27.Todd JJ, Pourshahidi LK, McSorley EM, Madigan SM, Magee PJ. Vitamin D: recent advances and implications for athletes. Sports Med. 2015;45(2):213–229. doi: 10.1007/s40279-014-0266-7 [DOI] [PubMed] [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. The endocrine society’s clinical guidelines: evaluation, treatment, and prevention of vitamin D deficiency. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 29.Silk LN, Greene DA, Baker MK, Jander CB. Tibial bone responses to 6-month calcium and vitamin D supplementation in young male jockeys: a randomised controlled trial. Bone. 2015;81:554–561. doi: 10.1016/j.bone.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Valtuena J, Dominguez D, Til L, Gonzalez-Gross M, Drobnic F. High prevalence of vitamin D insufficiency among elite spanish athletes; the importance of outdoor training adaptation. Nutr Hosp. 2014;1(31):124–131. [DOI] [PubMed] [Google Scholar]
- 31.Galan F, Ribas J, Sanchez-Martinez PM, Calero T, Sanchez AB, Munoz A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin Nutr. 2012;31(1):132–136. doi: 10.1016/j.clnu.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 32.Wolman R, Wyon MA, Koutedakis Y, Nevill AM, Eastell R, Allen N. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16(5):388–391. doi: 10.1016/j.jsams.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 33.Allison RJ, Farooq A, Hamilton B, Close GL, Wilson MG. No association between vitamin D deficiency and markers of bone health in athletes. Med Sci Sports Exerc. 2015;47(4):782–788. doi: 10.1249/MSS.0000000000000457 [DOI] [PubMed] [Google Scholar]