Abstract
PURPOSE
Patients with locally advanced prostate cancer have an increased risk of cancer recurrence and mortality. In this phase II trial, we evaluate neoadjuvant enzalutamide and leuprolide (EL) with or without abiraterone and prednisone (ELAP) before radical prostatectomy (RP) in men with locally advanced prostate cancer.
PATIENTS AND METHODS
Eligible patients had a biopsy Gleason score of 4 + 3 = 7 or greater, prostate-specific antigen (PSA) greater than 20 ng/mL, or T3 disease (by prostate magnetic resonance imaging). Lymph nodes were required to be smaller than 20 mm. Patients were randomly assigned 2:1 to ELAP or EL for 24 weeks followed by RP. All specimens underwent central pathology review. The primary end point was pathologic complete response or minimal residual disease (residual tumor ≤ 5 mm). Secondary end points were PSA, surgical staging, positive margins, and safety. Biomarkers associated with pathologic outcomes were explored.
RESULTS
Seventy-five patients were enrolled at four centers. Most patients had high-risk disease by National Comprehensive Cancer Network criteria (n = 65; 87%). The pathologic complete response or minimal residual disease rate was 30% (n = 15 of 50) in ELAP-treated patients and 16% (n = four of 25) in EL-treated patients (two-sided P = .263). Rates of ypT3 disease, positive margins, and positive lymph nodes were similar between arms. Treatment was well-tolerated. Residual tumors in the two arms showed comparable levels of ERG, PTEN, androgen receptor PSA, and glucocorticoid receptor expression. Tumor ERG positivity and PTEN loss were associated with more extensive residual tumors at RP.
CONCLUSION
Neoadjuvant hormone therapy followed by RP in locally advanced prostate cancer resulted in favorable pathologic responses in some patients, with a trend toward improved pathologic outcomes with ELAP. Longer follow-up is necessary to evaluate the impact of therapy on recurrence rates. The potential association of ERG and PTEN alterations with worse outcomes warrants additional investigation.
INTRODUCTION
Although radical prostatectomy (RP) can be curative for patients with high-risk prostate cancer, a subset experience prostate-specific antigen (PSA) recurrence and has an increased risk of prostate cancer mortality.1,2 Patients with prostate cancer with a Gleason score of 8 to 10 have a greater than 30% risk of 20-year prostate cancer mortality after RP.1,2 Despite this, the treatment paradigm for high-risk patients who choose RP has been unchanged. Consequently, an urgent need exists to develop novel multimodality strategies to improve outcomes for patients with prostate cancer at high risk of recurrence.
The efficacy of abiraterone, an irreversible potent inhibitor of CYP17, and enzalutamide, a potent second-generation androgen receptor (AR) inhibitor, in patients with metastatic prostate cancer provides a strong rationale to investigate these intense AR axis–targeting agents in localized disease before surgery.3-6 Neoadjuvant systemic therapy targets micrometastatic disease responsible for the majority of recurrences and has demonstrated improved outcomes for several solid tumors.7-10
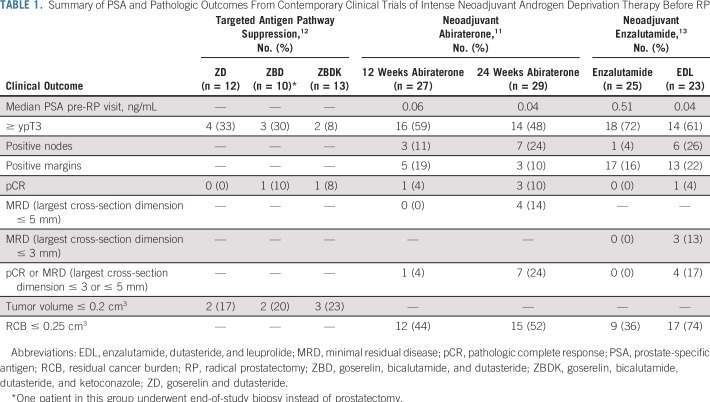
We have previously evaluated the effect of neoadjuvant abiraterone on intraprostatic androgens and RP pathologic outcomes11 (Table 1). We demonstrated that compared with luteinizing hormone–releasing hormone (LHRH) agonists alone, the combination of neoadjuvant abiraterone and an LHRH agonist results in significantly decreased tissue androgen levels and favorable tumor responses of 24%, with a pathologic complete response (pCR) or minimal residual disease (MRD; defined as ≤ 5 mm residual tumor) with the combination.11 We also observed that after 6 months of neoadjuvant abiraterone, molecular profiles of the largest residual tumors resembled castration-resistant prostate cancer.14,15 Transcriptome profiling of residual tumor foci demonstrated reduced, but persistent AR activity.13 Furthermore, although tissue androgens decreased from baseline, persistence of some androgen metabolites was observed.
TABLE 1.
Summary of PSA and Pathologic Outcomes From Contemporary Clinical Trials of Intense Neoadjuvant Androgen Deprivation Therapy Before RP
Subsequently, we investigated enzalutamide with or without dutasteride, a 5-α-reductase inhibitor, and an LHRH agonist before RP.13 No patient in the noncastrating enzalutamide arm experienced a pCR or MRD, whereas favorable pathologic responses were seen in patients who received combination therapy (17% with a pCR or MRD defined as residual tumor ≤ 3 mm). Furthermore, concentrations of intraprostatic testosterone and dihydrotestosterone and expression of nuclear AR and PSA in residual tumors were significantly lower in the enzalutamide, dutasteride, and LHRH agonist arm.
In summary, we learned that noncastrating therapy was ineffective in producing pathologic responses. Although abiraterone or enzalutamide combined with an LHRH agonist resulted in reductions in tissue androgens and AR activity, some androgen metabolites and AR activity persisted in the prostate tissue. Our prior studies provide the rationale for evaluating increased intensity androgen deprivation therapy (ADT) with abiraterone, enzalutamide, and leuprolide; therefore, we hypothesize that this triplicate would result in more pronounced inhibition of the AR axis and improved pathologic responses in unfavorable intermediate- or high-risk localized prostate cancer.
We report the results of a multicenter, open-label, randomized phase II trial of abiraterone, enzalutamide, leuprolide, and prednisone (ELAP) versus enzalutamide and leuprolide (EL) for 24 weeks followed by RP. Association of pathologic outcomes with tissue biomarkers are reported, including expression of Ki-67, ERG, PTEN, AR, PSA, and glucocorticoid receptor (GR).
PATIENTS AND METHODS
Patients
Eligible patients were candidates for RP with histologically confirmed prostatic adenocarcinoma with biopsy Gleason score 4 + 3 = 7 or greater (≥ 3 positive core biopsy specimens or > 1-cm tumor on prostate magnetic resonance imaging [MRI]), PSA greater than 20 ng/mL, or T3 disease (by prostate MRI). Patients had an Eastern Cooperative Oncology Group performance status of 0 to 1 and no evidence of metastatic disease on radiologic imaging, with lymph nodes smaller than 20 mm (assessed on local review). Baseline testosterone was 200 ng/dL or more. No prior prostate cancer treatment was allowed. Patients initiated on first-generation anti-androgens or an LHRH agonist within 4 weeks of study treatment were eligible. All patients provided written informed consent.
Design and Treatment
Patients were randomly assigned 2:1 to abiraterone (1,000 mg/d), enzalutamide (160 mg/d), leuprolide (22.5 mg every 12 weeks), and prednisone (5 mg/d) versus enzalutamide (160 mg/d) and leuprolide (22.5 mg every 12 weeks) for 24 weeks followed by RP, stratified by disease risk (intermediate v high defined by National Comprehensive Cancer Network criteria,16 Data Supplement).
Pathology and Immunohistochemistry
Two pathologists (H.Y., R.L.) blinded to clinical characteristics, treatment, and PSA outcomes centrally reviewed all RP slides. pCR and MRD (largest cross-sectional dimension of residual tumor measuring ≤ 5 mm) were tabulated. Residual cancer burden (RCB) was measured as the calculated tumor volume corrected by tumor cellularity.17 Surgical staging at RP was determined using the American Joint Committee on Cancer Prostate Cancer Staging Classification (8th edition).18 Presence of intraductal carcinoma was assessed. RP specimens with enough residual tumor for immunohistochemistry (IHC) were analyzed for ERG, PTEN, AR PSA, GR, and Ki-67 using established assays; semiquantitative scores were used to evaluate IHC stains (Data Supplement).
Statistical Analysis
The primary end point was pCR or MRD rate at RP. The sample size of 75 patients provided 84% power to distinguish a pCR or MRD rate of 35% in patients treated with ELAP (n = 50) from a rate of 10% in patients treated with EL (n = 25) using Fisher’s exact test with a one-sided type I error of 0.1. The primary comparison of pCR or MRD rate between arms was conducted using Fisher’s exact test, with a one-sided P ≤ .10 considered significant. The traditional two-sided 95% exact binomial CI was presented by treatment arms for descriptive purposes. Secondary end points were PSA response; surgical staging; positive margins; intra-, peri-, and postoperative adverse events; safety; quality-of-life assessments; and biomarkers associated with pathologic outcomes. A two-sided P < .05 was used to determine statistical significance for secondary and exploratory end points (Data Supplement).
RESULTS
Baseline Characteristics
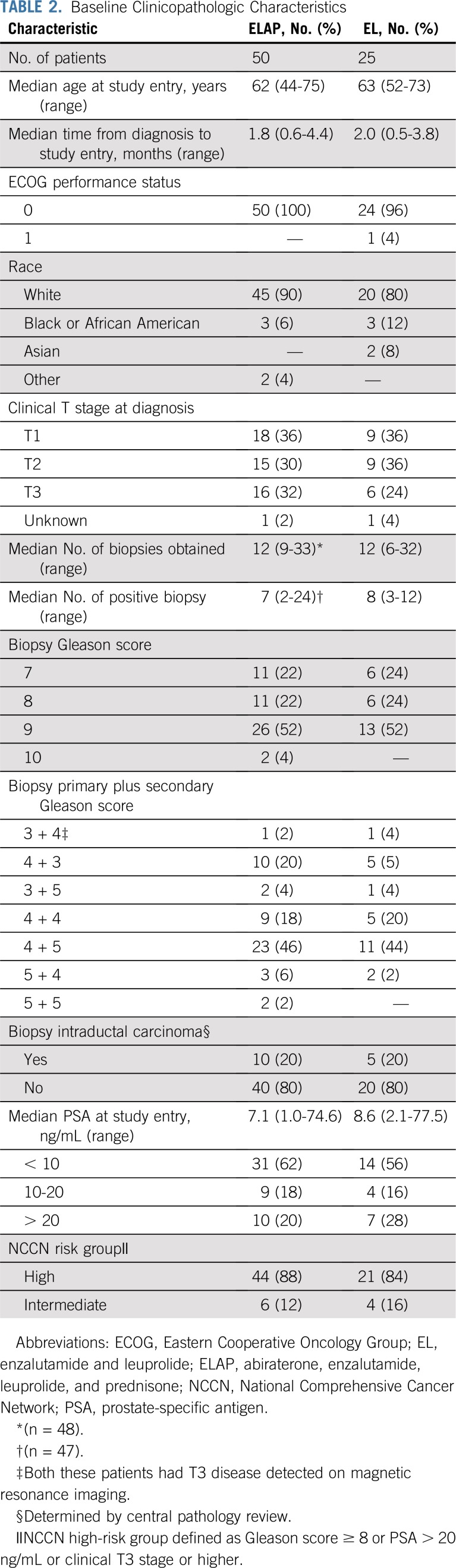
Seventy-five patients were enrolled (Data Supplement). The median age was 62 years, and 99% had an Eastern Cooperative Oncology Group performance status of 0. All patients had National Comprehensive Cancer Network intermediate-risk (n = 10; 13.3%) or high-risk (n = 65; 86.7%) disease. Baseline characteristics were balanced between arms (Table 2).
TABLE 2.
Baseline Clinicopathologic Characteristics
Treatment Exposure
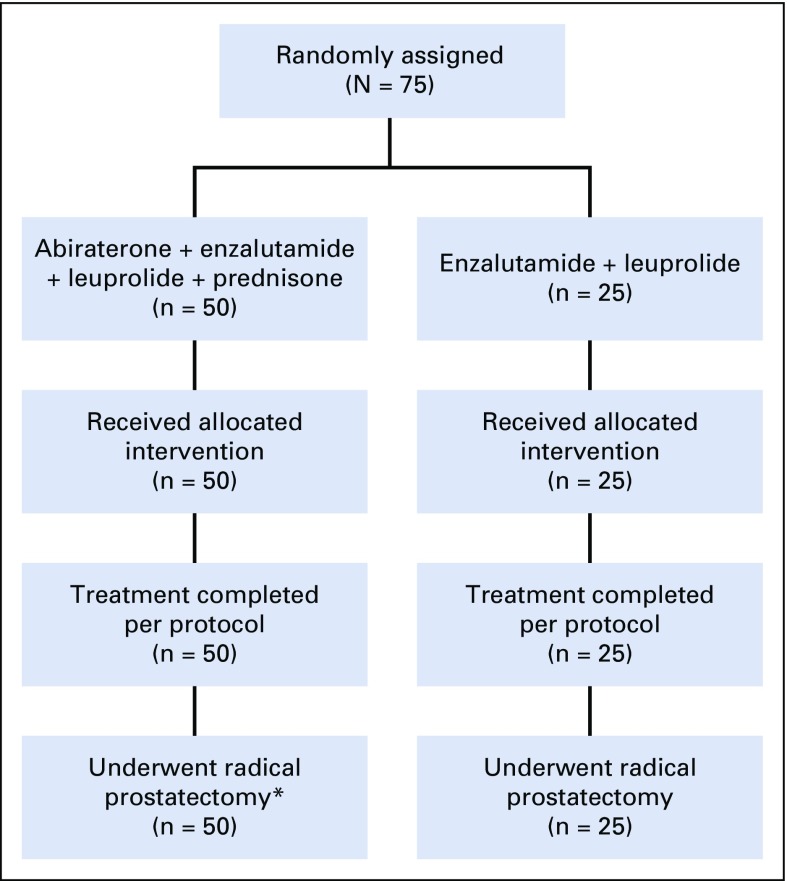
Fifty patients received ELAP, and 25 received EL. All patients completed six cycles of systemic therapy and underwent RP (Fig 1; Data Supplement).
FIG 1.
CONSORT diagram. Newly diagnosed unfavorable intermediate- and high-risk patients with prostate cancer were randomly assigned 2:1 to abiraterone, enzalutamide, leuprolide, and prednisone versus enzalutamide and leuprolide for 24 weeks followed by radical prostatectomy. (*) One patient allocated to the abiraterone, enzalutamide, leuprolide, and prednisone arm had a delay in radical prostatectomy by 2 months.
PSA Outcomes
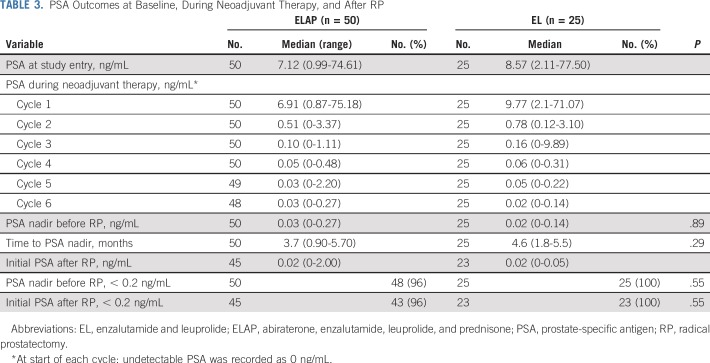
Median PSA at the start of cycle 1 was 6.91 ng/mL for patients who received ELAP and 9.77 ng/mL for those who received EL (Table 3). Median pre-RP PSA nadir was 0.03 ng/mL for patients who received ELAP and 0.02 ng/mL for those who received EL (P = .89). Nearly all patients had a pre-RP PSA nadir of less than 0.2 ng/mL (96% in ELAP arm; 100% in EL arm). Time to pre-RP nadir PSA was 3.7 and 4.6 months in the ELAP and EL arms, respectively. Median initial postoperative PSA measured at 1 month was 0.02 ng/mL (range, < 0.1 to 1.5) in both arms.
TABLE 3.
PSA Outcomes at Baseline, During Neoadjuvant Therapy, and After RP
Pathologic Outcomes at RP
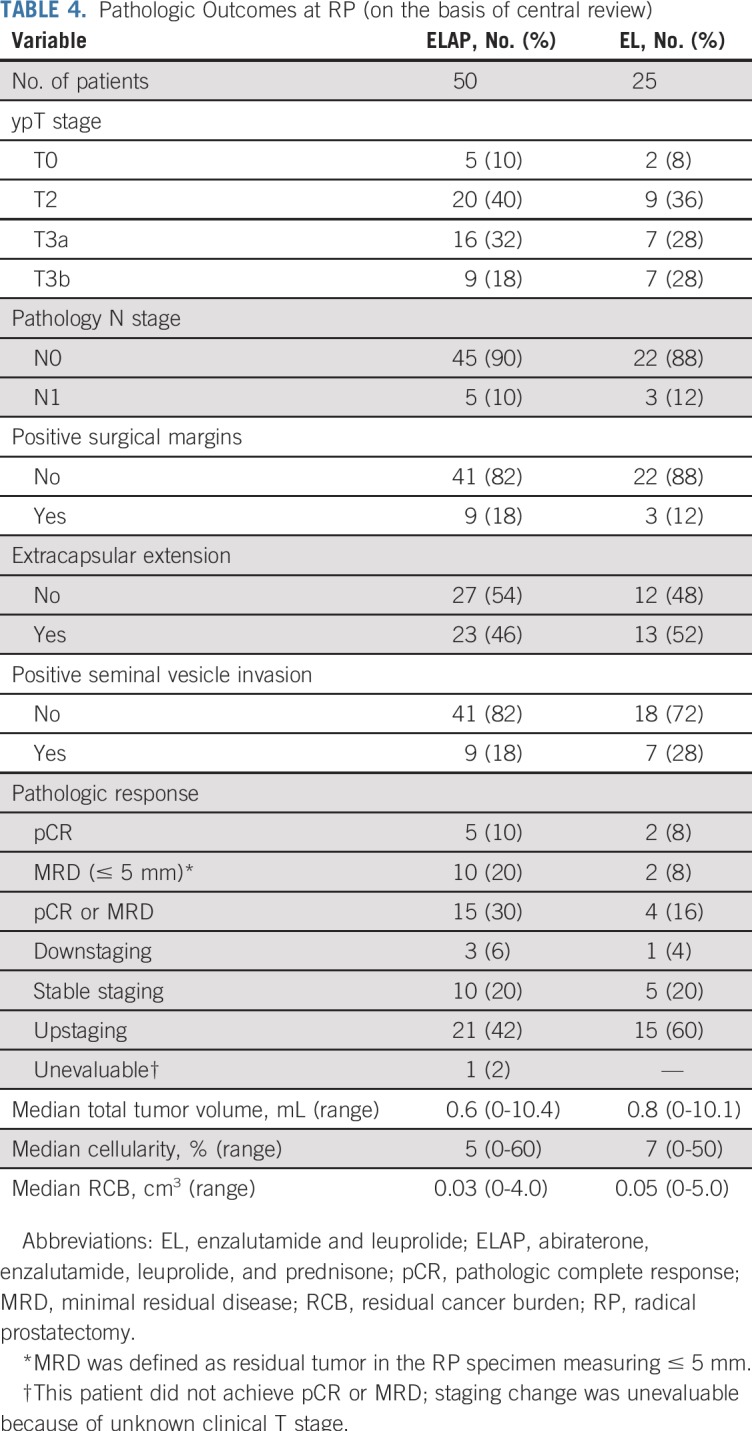
Institutional pathology review reported 26 patients with MRD (of 59 in whom residual tumor dimensions were recorded), of whom 10 had a pCR. Upon central pathology review, 19 of 26 had confirmed MRD with seven pCRs. The observed pCR or MRD rate was numerically higher with ELAP (n = 15 [30%] of 50; two-sided 95% CI, 18% to 45%) than with EL (n = 4 [16%] of 25; two-sided 95% CI, 5% to 36%); however, this difference was not statistically significant (Fisher’s exact test one-sided P = .151; two-sided P = .263; Table 4). The median percent tumor cellularity of the residual RP tumor was low in both arms (5% v 7% in ELAP v EL, respectively), although significant variability existed in tumor cellularity (range, 0% to 60%) among samples in both arms. The rate of positive surgical margins was 16% overall and similar between the arms. The majority of patients had significant residual tumor, with ypT3 status in 50% and 56% of patients in the ELAP and EL arms, respectively. Rates of lymph node involvement were similar between the arms (10% v 12% in the ELAP v EL arms, respectively).
TABLE 4.
Pathologic Outcomes at RP (on the basis of central review)
Adverse Events
Overall treatment was well tolerated, and no grade 4 to 5 adverse events were observed (Data Supplement). Treatment-related adverse events were comparable between the arms, with the exception of increased any-grade and grade 3 hypertension, ALT, and AST in the ELAP arm compared with the EL arm.
No intra-operative complications were found (Data Supplement), and in-hospital complications (Data Supplement) were minimal. Postoperative complications were low and similar between arms. The most common 30-day post-RP complications were urine leak/urinoma (n = 9 [13.4%] of 67 patients) and urinary catheter malfunction (n = 4 [6.0%] of 67 patients; Data Supplement). The most common 90-day post-RP complication was urine leak/urinoma (n = 11 [16.9%] of 65 patients; Data Supplement).
Associations of Pathologic Reponses With Clinical Factors
Patients who achieved a pCR or MRD (responders) had a lower percentage of biopsy cores involved by cancer than nonresponders (median, 50% v 67%; P = .003). The mean percentage of biopsy tissue involved by cancer was also significantly lower in responders than in nonresponders (16% v 33%; P = .003), which together suggest that responders had a lower tumor volume at baseline. Baseline clinical stage, Gleason score, and PSA had no significant influence on pathologic outcomes. No patient with a pathologic response had intraductal carcinoma identified in RP tissue (P = .001; Data Supplement). Presence of intraductal carcinoma at RP was associated with worse pathologic T stage (P = .013), larger cross-sectional tumor dimension (P ≤ .001), and higher RCB (P ≤ .001). A similar association was detected for patients with intraductal carcinoma present in the baseline biopsy specimen.
IHC on RP Specimens
IHC was performed on 60 RP specimens. IHC profiles were similar between arms (Data Supplement). Of note, 20% of residual tumors (12 of 60) demonstrated undetectable AR staining in 50% or more tumor cells. The Ki-67 proliferation index was 1% or less in 91 % (53 of 58) residual tumors and more than 5% in only two (3%) of residual tumors.
Overall, the PTEN-loss rate was significantly higher in ERG-positive tumors (65% v 29%; P = .017). AR-low tumors were associated with significantly higher nuclear GR levels compared with AR-high tumors (59% v 28%; P = .03). Residual tumors with ERG positivity or PTEN loss were associated with significantly lower AR expression compared with tumors without ERG positivity or PTEN alterations (Data Supplement). Furthermore, dual ERG positivity and PTEN loss was associated with the lowest AR expression (Data Supplement).
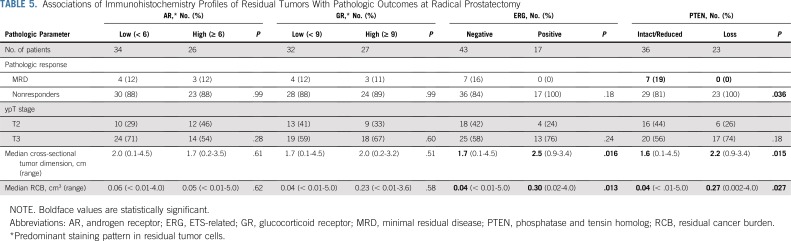
We investigated associations of RP IHC data with pathologic outcomes (Table 5). AR or GR expression levels showed no association with pathologic outcomes. ERG positivity or PTEN loss was significantly associated with larger residual tumors as determined by RCB (0.30 v 0.04 cm3 in ERG-positive v ERG-negative patients, respectively [P = .013]; 0.27 v 0.04 cm3 in PTEN loss v PTEN intact/reduced, respectively [P = .027]). A similar pattern was observed when assessing residual tumor size by cross-sectional tumor dimension. No MRD was observed in tumors with ERG positivity or PTEN loss. Tumors with dual ERG positivity and PTEN loss had the highest residual tumor volume compared with tumors with no or a single alteration (Data Supplement).
TABLE 5.
Associations of Immunohistochemistry Profiles of Residual Tumors With Pathologic Outcomes at Radical Prostatectomy
We evaluated associations between RP IHC profiles and baseline characteristics (Data Supplement). ERG-positive or PTEN-loss tumors demonstrated a trend toward lower baseline PSA (median: 5.5 v 9.4 ng/mL in PTEN loss v PTEN intact/reduced, respectively [P = .07]; 5.5 v 9.6 ng/mL in ERG-positive v ERG-negative patients, respectively [P = .058]). In addition, ERG-positive tumors were associated with significantly higher tumor volume in baseline biopsy cores. Similarly, PTEN-loss tumors showed a trend toward a higher biopsy tumor volume (P = .09). Tumors with dual ERG positivity and PTEN loss had an increased tumor biopsy volume and reduced baseline PSA level compared with tumors with a single alteration (Data Supplement). Prostate MRIs were not required, and thus not available, to measure baseline tumor volume more accurately. No association with baseline Gleason score and clinical T stage with ERG and PTEN status was found. IHC profiles of baseline biopsy specimens are detailed in the Data Supplement.
DISCUSSION
This study was designed to investigate whether neoadjuvant therapy with EL or ELAP would result in significant pathologic responses in patients with prostate cancer at high risk of recurrence. We demonstrated that neoadjuvant therapy with potent AR-directed therapies is feasible, safe, and results in favorable pathologic responses in a subset of patients with high-risk disease, although differences between arms were not statistically significant.
In our prior work, we investigated the efficacy of abiraterone or enzalutamide in separate trials11,13 (Table 1). Collectively, pCRs were observed in approximately 10% of patients, similar to the pCR rates observed herein (8% to 10%). Despite robust targeting of the androgen axis, the pCR rate has not increased. Although cross-trial comparisons have limitations, to our knowledge, the data presented here are of the most intense ADT regimen studied to date. We hypothesized that more robust inhibition of the AR axis with abiraterone and enzalutamide would result in improved pathologic and possibly improved clinical outcomes. A numerically higher pCR plus MRD rate of 30% with ELAP was observed compared with 16% with EL; this difference was not statistically significant. Prior studies of either agent (with an LHRH agonist) demonstrated a pCR plus MRD rate of 17% to 24%. In acknowledgment that pathologic outcomes may not be improved with more intense ADT, possible explanations for not demonstrating benefit are a decreased effect size and, therefore, an insufficient sample size to detect differences; a need for more-prolonged ADT; inherent resistance mechanisms; and a lack of a biomarker to target tumors most likely to benefit. A subset of patients may warrant alternative treatment strategies, potentially including ADT combined with chemotherapy or targeted therapy in predefined patients. The PUNCH trial (ClinicalTrials.gov identifier: NCT00430183) that is evaluating RP with or without neoadjuvant docetaxel and ADT will inform the utility of pre-RP chemohormonal therapy.
To get a sense of how our pathology outcomes could compare with RP alone, we entered the baseline patient characteristics into the publicly available Memorial Sloan Kettering Cancer Center pre-RP nomogram.19 The predicted rates of extracapsular extension, seminal vesicle invasion, and lymph node involvement for RP alone were 85% (interquartile range, 73% to 92%), 36% (interquartile range, 18% to 61%), and 34% (interquartile range, 17% to 57%), respectively. In our study, these rates were 48%, 21%, and 11%, respectively. This exercise was exploratory and not intended to prove benefit.
Neoadjuvant treatment with RP is not standard; thus, consensus criteria for the definition and measurement of benefit are not established. We used predefined criteria for measurement and reporting of pCR and MRD with expert central pathology review; 30% of pCRs were determined to harbor tumor after this review, which highlights its importance. We support ongoing efforts to standardize and validate pathology end points.
In this study, we observed that patients with ERG expression and PTEN aberrations had more extensive residual tumors. This outcome may be the consequence of either larger baseline tumor volumes, tumor heterogeneity, and/or low AR dependency, which suggests resistance to intense ADT. This is supported by our findings that ERG-positive and PTEN-loss tumors were associated with lower AR expression, a trend toward a lower baseline PSA, and higher tumor volume in baseline biopsy specimens. Patients with ERG-positive or PTEN-loss tumors did not experience MRD. In addition, AR-low tumors were associated with higher GR expression, which supports the previously reported mechanism that tumor cells can use GR to bypass AR and gain resistance to ADT.20-22 Taken together, poor pathologic outcomes of ERG-positive or PTEN-loss tumors can be attributed to both larger baseline tumors and insensitivity to intense ADT. We plan to validate this observation in an ongoing trial of neoadjuvant abiraterone and apalutamide that incorporates prostate MRI assessments.
We postulated that ERG and PTEN status would not be significantly altered after 6 months of treatment. Although ERG intensity was reduced by treatment, ERG nuclear staining was still apparent in ERG-positive patients, which suggests ongoing ERG signaling. PTEN loss was defined by immunonegativity in 5% or more tumor cells. It is unlikely that any newly mutated PTEN-null subclones emerged and expanded to 5% or more of tumor volume during 6 months of ADT because the majority of residual tumors had an extremely low Ki-67 proliferation index. We attempted to validate our RP biomarker findings using available pretreatment biopsy materials. In agreement with our RP findings, none of the 10 responders tested showed ERG positivity or PTEN loss in baseline biopsy specimens, but 20% to 25% of nonresponders did. Future validation of the hypothesis that ERG or PTEN-altered expression confers resistance to intense ADT would provide a strategy to select patients unlikely to benefit from neoadjuvant ADT and offer them an alternative approach. Evaluation of markers of response and resistance by whole-exome sequencing and transcriptomic profiling is ongoing.
A fundamental principal of neoadjuvant treatment is the correlation between pathologic and long-term clinical outcomes. pCR has been correlated with improved long-term outcomes and survival in multiple solid tumors, including breast23 and bladder cancer.9 In addition, the prognostic significance of RCB has been validated in breast cancer.17 The impact of the pathologic response on recurrence and survival in prostate cancer has not been determined. An exploratory pooled analysis of prior contemporary neoadjuvant trials evaluated the post-RP outcomes of a subset of 72 patients treated with intense neoadjuvant ADT.24 At a median follow-up of 3 years after RP, 70% of patients remained free of disease, and no patient with pCR or MRD experienced a recurrence. Although longer follow-up is necessary, these data suggest that pathologic responses may be predictive of prostate cancer recurrence rates. Secondary end points of the current study include time to testosterone recovery and biochemical recurrence, which will be reported with longer follow-up. The impact of the pathologic response on long-term outcomes will be validated in a phase III trial of abiraterone and apalutamide to open in early 2019 (Taplin and Kibel). The phase III coprimary end points are pCR, and the study will evaluate pCR as a surrogate for metastasis-free survival.25
The limitations of our trial are inherent in the phase II design. Pathologic response was used as the primary end point, but correlation with long-term outcomes currently is limited, and larger studies are needed to validate this end point. Although central pathology review was performed, the definition and clinical significance of MRD is not established. In addition, the analysis of baseline biopsy specimens is limited given small size, tumor heterogeneity, incomplete sampling of the gland, and limitations of archival specimens.
Although efficacy is not proven, neoadjuvant therapy combined with RP holds promise for men with poor-prognosis prostate cancer. Our study demonstrates that a subset of patients had favorable pathologic responses and that ERG positivity and PTEN loss correlate with inferior pathologic outcomes. Our hypothesis will be tested in a phase III trial of intense ADT and RP for men with locally advanced prostate cancer.
ACKNOWLEDGMENT
We thank the patients and their family members who participated in this clinical trial.
Footnotes
Presented at the 2018 Genitourinary Cancers Symposium, San Francisco, CA, February 8-10, 2018.
Supported by Astellas Pharma, Medivation, and Pfizer. Also supported by the Fairweather Family Fund and Fat Boys/Slim Sisters Pan-Mass Challenge Fund at the Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute (M.-E.T.), Prostate Cancer Foundation Challenge Awards 16CHAS03 and 142016 (M.-E.T.), Prostate Cancer Clinical Trials Consortium (M.-E.T.), Dana-Farber Cancer Institute Prostate Specialized Program of Research Excellence (P50CA090), Prostate Cancer Foundation Young Investigator Award (H.Y.), Dana-Farber/Harvard Cancer Center Prostate Cancer Specialized Program of Research Excellence (National Cancer Institute grant P50CA090381), and Department of Defense Impact Award W81XWH-16-1-0433.
Clinical trial information: NCT02268175.
AUTHOR CONTRIBUTIONS
Conception and design: Rana R. McKay, Wanling Xie, Kenneth J. Pienta, Glenn J. Bubley, Adam S. Kibel, Mary-Ellen Taplin
Administrative support: Mary-Ellen Taplin
Financial support: Adam S. Kibel, Mary-Ellen Taplin
Provision of study material or patients: Lauren C. Harshman, Kenneth J. Pienta, Daniel W. Lin, William J. Ellis, Bruce Montgomery, Andrew A. Wagner, Glenn J. Bubley, Mary-Ellen Taplin
Collection and assembly of data: Rana R. McKay, Huihui Ye, Rosina Lis, Carla Calagua, Zhenwei Zhang, Steven L. Chang, Daniel W. Lin, Ashley E. Ross, William J. Ellis, Bruce Montgomery, Peter Chang, Andrew A. Wagner, Adam S. Kibel, Mary-Ellen Taplin
Data analysis and interpretation: Rana R. McKay, Huihui Ye, Wanling Xie, Rosina Lis, Quoc-Dien Trinh, Lauren C. Harshman, Kenneth J. Pienta, Bruce Montgomery, Adam S. Kibel, Mary-Ellen Taplin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide With or Without Abiraterone
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rana R. McKay
Consulting or Advisory Role: Janssen Pharmaceuticals, Novartis, Bristol-Myers Squibb, Pfizer, Tempus, Exelixis
Research Funding: Pfizer (Inst), Bayer AG (Inst)
Huihui Ye
Consulting or Advisory Role: Janssen Pharmaceuticals
Wanling Xie
Consulting or Advisory Role: Bayer AG
Quoc-Dien Trinh
Honoraria: Astellas Pharma, Bayer AG, Janssen Pharmaceuticals
Research Funding: Intuitive Surgical
Lauren C. Harshman
Consulting or Advisory Role: Pfizer, Genentech, Theragene, Corvus Pharmaceuticals, Merck, Exelixis, Bayer AG, Novartis, Jounce Therapeutics
Research Funding: Medivation (Inst), Astellas Pharma (Inst), Bayer AG (Inst), Sotio (Inst), Genentech (Inst), Roche (Inst), Dendreon (Inst), Bristol-Myers Squibb (Inst), Takeda Pharmaceuticals (Inst), Merck (Inst), Janssen Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Bayer AG
Ashley E. Ross
Stock and Other Ownership Interests: GenomeDx
Honoraria: HealthTronics
Consulting or Advisory Role: HealthTronics
Research Funding: Metamark Genetics
Kenneth J. Pienta
Leadership: Cue Biopharma
Stock and Other Ownership Interests: Cue Biopharma
Honoraria: Johnson & Johnson, Progenics
Consulting or Advisory Role: Cue Biopharma
Research Funding: Progenics
Travel, Accommodations, Expenses: Cue Biopharma
Daniel W. Lin
Consulting or Advisory Role: Astellas Pharma, Clovis Oncology, Dendreon
Research Funding: Genomic Health (Inst), GenomeDx (Inst), MDxHealth (Inst)
William J. Ellis
Research Funding: Progenics
Bruce Montgomery
Research Funding: ESSA Pharma, Medivation, Astellas Pharma, Janssen Pharmaceuticals, Ferring Pharmaceuticals, AstraZeneca
Adam S. Kibel
Honoraria: Profound, INSIGHTEC, Janssen Pharmaceuticals, Merck, Pfizer, Blue Earth Diagnostics
Mary-Ellen Taplin
Honoraria: Janssen Pharmaceuticals, Clovis Oncology, Astellas Pharma, Incyte, UpToDate, Research to Practice, Pfizer, Bayer AG
Consulting or Advisory Role: Janssen Pharmaceuticals, Bayer AG, Guidepoint Global, Best Doctors, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Incyte, Pfizer, AstraZeneca
Research Funding: Janssen Pharmaceuticals (Inst), Medivation (Inst), Bayer AG (Inst), Tokai Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Medivation, Janssen Pharmaceuticals, Tokai Pharmaceuticals, Astellas Pharma, Incyte, Pfizer, Clovis Oncology, Bayer AG
No other potential conflicts of interest were reported.
REFERENCES
- 1.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–875. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res. 2014;20:5359–5364. doi: 10.1158/1078-0432.CCR-14-1268. [DOI] [PubMed] [Google Scholar]
- 8.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur Urol. 2014;65:350–357. doi: 10.1016/j.eururo.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 11.Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostaghel EA, Nelson PS, Lange P, et al. Targeted androgen pathway suppression in localized prostate cancer: A pilot study. J Clin Oncol. 2014;32:229–237. doi: 10.1200/JCO.2012.48.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery B, Tretiakova MS, Joshua AM, et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res. 2017;23:2169–2176. doi: 10.1158/1078-0432.CCR-16-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;162:454. doi: 10.1016/j.cell.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 15.Sowalsky AG, Ye H, Bhasin M, et al. Neoadjuvant-intensive androgen deprivation therapy selects for prostate tumor foci with diverse subclonal oncogenic alterations. Cancer Res. 2018;78:4716–4730. doi: 10.1158/0008-5472.CAN-18-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Comprehensive Cancer Network: Prostate Cancer (Version 3.2018), 2018. https://www.nccn.org/professionals/physician_gls/recently_updated.aspx.
- 17.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 18.Bhindi B, Karnes RJ, Rangel LJ, et al. Independent validation of the American Joint Committee on Cancer 8th Edition Prostate Cancer Staging Classification. J Urol. 2017;198:1286–1294. doi: 10.1016/j.juro.2017.06.085. [DOI] [PubMed] [Google Scholar]
- 19. Memorial Sloan Kettering Cancer Center: Prediction Tools. https://www.mskcc.org/nomograms/prostate/pre-op.
- 20.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isikbay M, Otto K, Kregel S, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5:72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Alyamani M, Zhang A, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife. 2017;6:e20183. doi: 10.7554/eLife.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1038/s41391-017-0009-6. McKay RR, Montgomery B, Xie W, et al: Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis 21:364-372, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]