Abstract
PURPOSE
In this multicenter phase II trial, we evaluated atezolizumab combined with bevacizumab in patients with advanced renal cell carcinoma (RCC) with variant histology or any RCC histology with ≥ 20% sarcomatoid differentiation.
PATIENTS AND METHODS
Eligible patients may have received previous systemic therapy, excluding prior bevacizumab or checkpoint inhibitors. Patients underwent a baseline biopsy and received atezolizumab 1,200 mg and bevacizumab 15 mg/kg intravenously every 3 weeks. The primary end point was overall response rate (ORR) by RECIST version 1.1. Additional end points were progression-free survival (PFS), toxicity, biomarkers of response as determined by programmed death-ligand 1 (PD-L1) status, and on-therapy quality-of-life (QOL) metrics using the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 and the Brief Fatigue Inventory.
RESULTS
Sixty patients received at least 1 dose of either study agent; the majority (65%) were treatment naïve. The ORR for the overall population was 33% and 50% in patients with clear cell RCC with sarcomatoid differentiation and 26% in patients with variant histology RCC. Median PFS was 8.3 months (95% CI, 5.7 to 10.9 months). PD-L1 status was available for 36 patients; 15 (42%) had ≥ 1% expression on tumor cells. ORR in PD-L1–positive patients was 60% (n = 9) v 19% (n = 4) in PD-L1–negative patients. Eight patients (13%) developed treatment-related grade 3 toxicities. There were no treatment-related grade 4-5 toxicities. QOL was maintained throughout therapy.
CONCLUSION
In this study, atezolizumab and bevacizumab demonstrated safety and resulted in objective responses in patients with variant histology RCC or RCC with ≥ 20% sarcomatoid differentiation. This regimen warrants additional exploration in patients with rare RCC, particularly those with PD-L1–positive tumors.
INTRODUCTION
Although conventional clear cell renal cell carcinoma (ccRCC) is the most common type of kidney cancer, up to 20% of all RCC cases are classified under the broad category of non-ccRCC or, more recently, categorized as rare histologic variants. This is a diverse group of malignancies that includes papillary, chromophobe, medullary, collecting duct, TFE3 translocation, and unclassified RCC. Each subtype is driven by a unique pathogenesis that in some cases is not fully understood, which likely accounts for the differing clinical presentation and response to therapy of these rare histologic variants. In addition, any RCC histology may be associated with sarcomatoid differentiation.1-3
Several large series have demonstrated that patients with variant histology RCC or RCC with sarcomatoid differentiation have a worse prognosis with lower response rates to targeted therapies than their counterparts with ccRCC or those who lack sarcomatoid differentiation, which underscores the need for improved treatments for these individuals.3-6 Given its heterogeneous nature, this group of diseases has historically been excluded from large phase III studies, which have focused primarily on patients with ccRCC (or component of clear cell features). Current management guidelines for patients with variant histology RCC are based on extrapolation of data from patients with ccRCC, smaller phase II trials that demonstrated superiority of sunitinib over everolimus,7,8 and subgroups analyses from phase III trials. While immunotherapy combinations have evolved as frontline regimens in clear cell histology, given their proven superiority over sunitinib in patients with ccRCC, sunitinib remains a preferred regimen by guideline panels for the initial management of variant histology RCC.9
The combination of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF) A, and atezolizumab, a monoclonal antibody targeting programmed death-ligand 1 (PD-L1), has only been studied in patients with ccRCC. Data from the phase I study of the combination suggested improvement in antitumor immunity given enhanced T-cell infiltration and decreased myeloid immunosuppression with the addition of bevacizumab to atezolizumab.10 The combination was further studied in patients with advanced ccRCC in phase II and III studies that showed enhancing antitumor activity with a favorable toxicity profile.10,11 We therefore conducted a multicenter, investigator-initiated, prospective phase II study of atezolizumab plus bevacizumab in patients with advanced variant histology RCC or RCC with at least 20% sarcomatoid differentiation.
PATIENTS AND METHODS
Patient Population
This study enrolled patients with histologically confirmed advanced variant histology RCC, including papillary, chromophobe, collecting duct, medullary, translocation, and unclassified RCC with or without sarcomatoid differentiation, in addition to ccRCC histology with ≥ 20% sarcomatoid differentiation. Pathology review conducted by a genitourinary pathologist was required at each institutional site to confirm histology. Advanced disease was defined as unresectable, locally recurrent, or metastatic by American Joint Commission on Cancer seventh edition staging system. Patients could have received any number of prior regimens provided that they had not received bevacizumab or any PD-1/PD-L1 inhibitors. Patients were required to have measurable disease per RECIST version 1.1, Eastern Cooperative Oncology Group performance status ≤ 2, adequate organ function, and controlled blood pressure. Patients with active brain metastases, active autoimmune disease, or a condition that required treatment with prednisone ≥ 10 mg/d or equivalent were excluded.
The study enrolled subjects at the Dana-Farber Cancer Institute; Beth Israel Deaconess Medical Center; University of California, San Diego, Moores Cancer Center; and Karmanos Cancer Center. The study was approved by the institutional review board at each participating institution. All patients provided written informed consent.
Study Design
Before initiation of therapy in this multicenter, phase II, open-label, single-arm study, patients underwent a baseline tumor biopsy, unless medically not feasible. Eligible patients received treatment with atezolizumab (1,200 mg) and bevacizumab (15 mg/kg) intravenously every 3 weeks. Dose modifications were not permitted; however, dose delays were allowed. If 1 agent was discontinued, continuation of the other agent alone was permitted. Clinical and laboratory assessments were performed at the initiation of each 21-day cycle. Imaging assessments occurred every 6 weeks for the first 24 weeks and then every 12 weeks. Treatment was continued until disease progression, intolerable toxicity, or withdrawal of consent. Treatment beyond radiographic progression was permitted in patients who continued to derive clinical benefit as determined by the treating investigator. An optional tumor biopsy was performed in patients with an objective response to therapy and subsequent disease progression.
Study End Points
The primary end point was objective response rate (ORR), including rate of complete response (CR) and partial response as best overall response by RECIST version 1.1, by investigator assessment. Given the 6-week imaging period, confirmation of response was assessed at the subsequent imaging assessment time point. Progression-free survival (PFS) was defined as the time from treatment initiation to disease progression or death as a result of any cause. Overall survival (OS) was defined as the time from treatment initiation to death as a result of any cause. For both time-to-event outcomes, patients were censored at the time of last follow-up if an event had not occurred. Toxicity was assessed by CTCAE (version 4.0), and treatment-emergent adverse events were reported. Quality of life was assessed at baseline and every 6 weeks for the first 24 weeks then every 12 weeks using the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19) and the Brief Fatigue Inventory (BFI). The FKSI-19 is a 19-item questionnaire where each item is scored on a scale of 0-4 for a total score of 0-76, with higher scores indicating fewer symptoms.12 The BFI is a 9-item questionnaire where each item is scored on a scale of 0-10; scores are categorized as mild (1-3), moderate (4-6), or severe (7-10).13
PD-L1 Immunohistochemistry
Archival nephrectomy specimens were collected and used for PD-L1 tissue immunohistochemistry (IHC). Double IHC staining assay used a validated antibody against PD-L1 (405.9A11 mouse monoclonal antibody, 1:125, 13 μg/mL [a gift from G.J. Freeman, Dana-Farber Cancer Institute, and commercially available through Cell Signaling Technology, Danvers, MA])14-16 and a panel of antibodies recognizing immune cells consisting of anti-CD45 (1:400, D9M8I XP, rabbit monoclonal antibody; Cell Signaling Technology) and anti-CD163 (1:4,000, EPR19518, rabbit monoclonal antibody; Abcam, Cambridge, United Kingdom). Tumor sections were stained with BOND RX autostainer (Leica Biosystems, Buffalo Grove, IL) using the BOND Polymer Refine Detection Kit (DS9800; Leica Biosystems) and BOND Polymer Refine Red Detection Kit (DS9390; Leica Biosystems). Antigen retrieval was performed with BOND Epitope Retrieval Solution 2 (EDTA, pH = 9.0) for 30 minutes. Slides were scanned at ×200 magnification and analyzed by HALO platform multiplex IHC version 1.2 algorithm (Indica Labs, Albuquerque, NM). Results of the image analysis were validated through visual inspection by genitourinary pathologists A.F. and S.S. The percentage of PD-L1–positive tumor cells (score) was calculated using the equation PD-L1–positive tumor cells/total tumor cells. A score ≥ 1% was used to determine positivity (yes v no).14-16
Statistical Analysis
Patients who received at least 1 dose of either study treatment were included in the analysis. Assuming a null hypothesis of ORR of 10%6,17 compared with an alternative hypothesis of ORR of 25%, there is a 90% statistical power with the minimum sample size of 40 for an exact one-sided, one-sample binomial test with a type I error rate of 0.1 to assess the ORR of bevacizumab and atezolizumab. The sample size was increased to 60 patients to also explore the ORR according to histologic subtype. The number of patients who achieved an objective response was summarized for the overall population, by histologic subtype, by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group,18 and by prior treatment status as percentages with 80% CIs using the exact binomial method. The median with 95% CI of time-to-event end points, including PFS and OS, were summarized using Kaplan-Meier method. The association of PD-L1 positivity and ORR was assessed using Fisher’s exact test in a subset of patients. Statistical analysis was performed with SAS 9.4 software (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
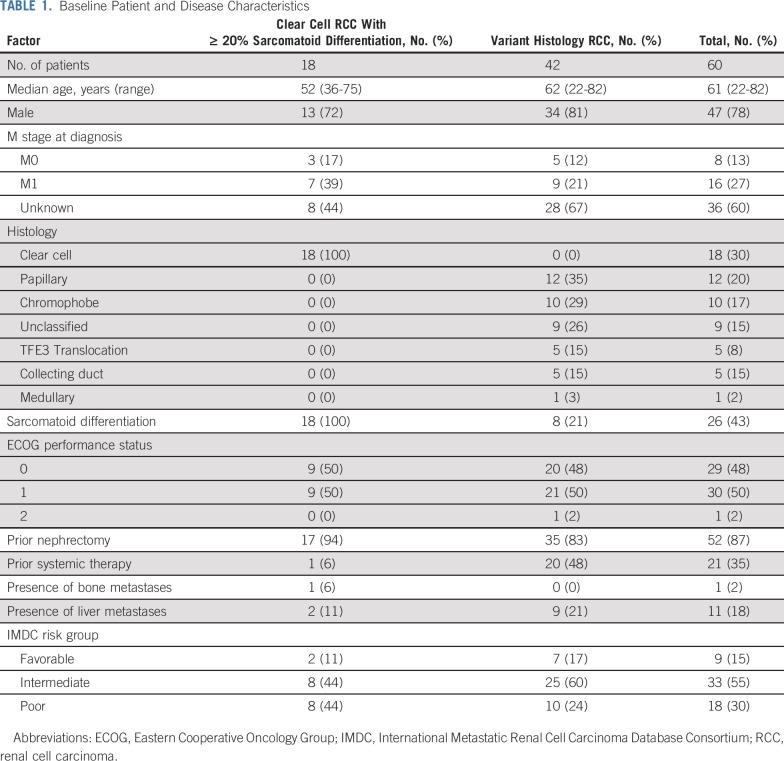
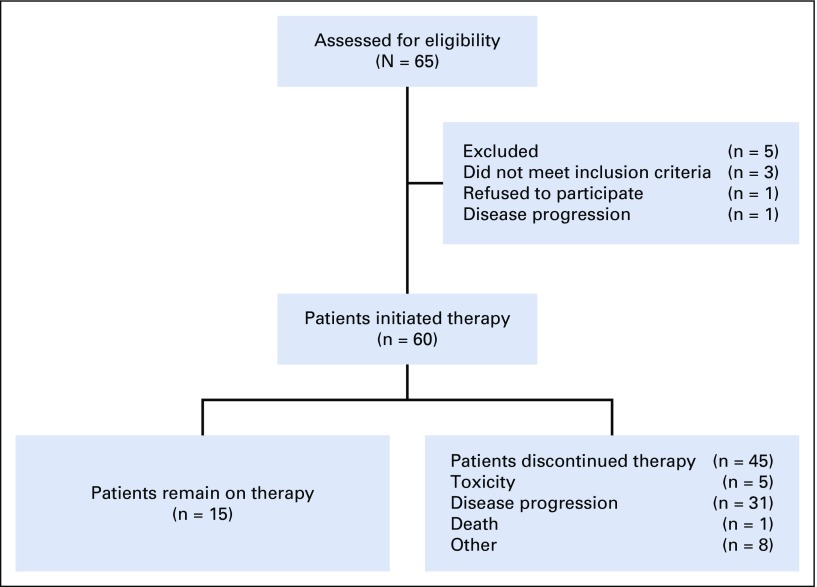
Between July 2016 and October 2018, 65 patients were enrolled from 4 centers in the United States. Five patients never initiated therapy and were excluded from the primary analysis cohort (n = 60). The study enrolled 42 patients (70%) with variant histology RCC and 18 patients (30%) with ccRCC with ≥ 20% sarcomatoid differentiation (Table 1). Variant histologies enrolled included papillary (n = 12), chromophobe (n = 10), unclassified (n = 9), TFE3 translocation (n = 5), collecting duct (n = 5), and medullary (n = 1) RCC. Most patients had IMDC intermediate-risk disease (n = 33; 55%) and had not received prior systemic treatment (n = 39; 65%).
TABLE 1.
Baseline Patient and Disease Characteristics
Treatment Exposure
Of the 60 patients who received at least 1 dose of treatment, the median number of cycles administered was 9.5 (range, 1-42 cycles). At the time of the analysis, 15 patients remained on therapy. Forty-five patients discontinued therapy: 32 because of disease progression or death, 5 because of toxicity, and 8 because of other reasons. Nine patients experienced atezolizumab dose delays, 3 as a result of adverse events. Six patients experienced bevacizumab dose delays, 3 as a result of adverse events.
Efficacy
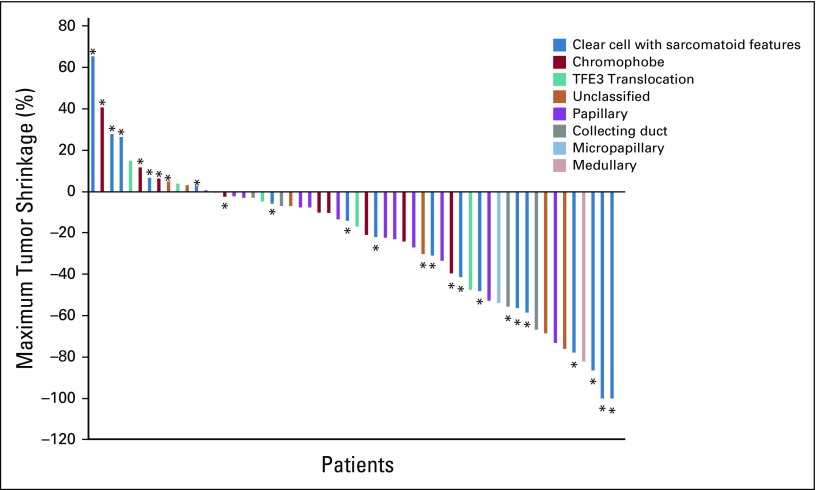
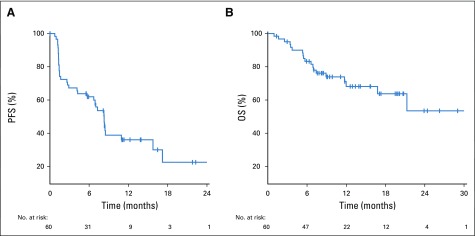
Data cutoff was May 15, 2019, and median time of follow-up was 13 months (range, 1-30.4 months). Fifty-six patients had at least 1 imaging assessment, 2 patients died, and 2 patients withdrew consent before the first imaging assessment (Appendix Fig A1, online only). Overall, the ORR was 33% (n = 20; 80% CI, 25% to 42%; Table 2). When stratified by histology, the ORR was 50% (n = 9 of 18) in patients with ccRCC with sarcomatoid differentiation and 26% (n = 11 of 42) in patients with variant histology RCC. There was no significant association between histology and response (P = .13; Fig 1). The ORR by IMDC risk group was 33% (n = 3 of 9), 45% (n = 15 of 33), and 11% (n = 2 of 18) in favorable-, intermediate-, and poor-risk patients, respectively. Treatment-naïve and previously treated patients had an ORR of 31% (n = 12 of 39) and 38% (n = 8 of 21), respectively. Median time to response in patients who achieved an objective response was 2.7 months (range, 1.2-11.1 months). Median duration of response in responders was 8.9 months (range, 1.4-29 months). Median PFS was 8.3 months (95% CI, 5.7 to 10.9 months), and median OS was not reached (Fig 2).
TABLE 2.
Overall and Subgroup ORRs
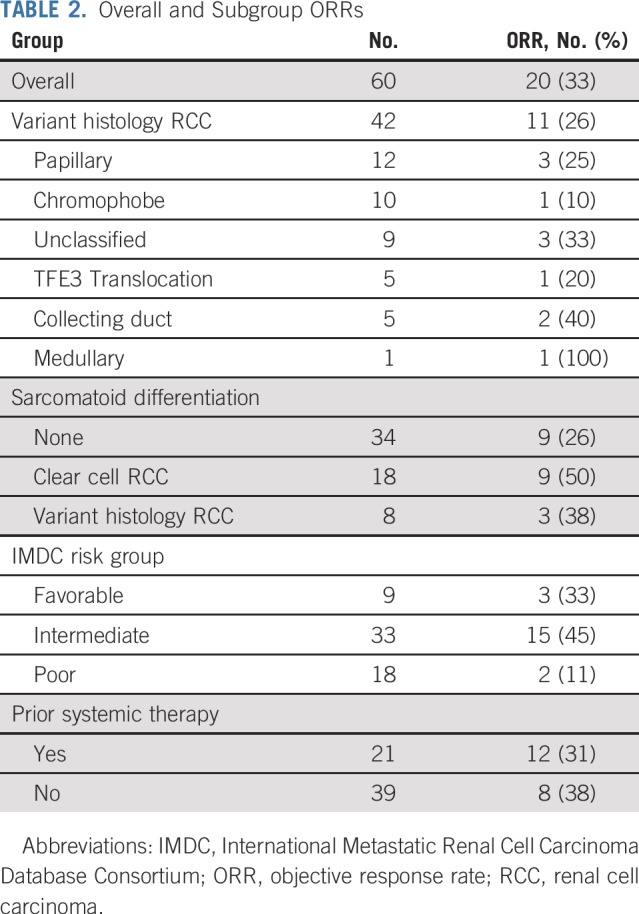
FIG 1.
Waterfall plot that shows maximum tumor shrinkage for all patients evaluable for response with at least 1 on-treatment imaging assessment time point. (*) Sarcomatoid differentiation.
FIG 2.
(A) Progression-free survival (PFS) Kaplan-Meier curve for the overall population. (B) Overall survival (OS) Kaplan-Meier curve for the overall population.
PD-L1 IHC
Tissue from mandatory biopsy was insufficient for PD-L1 staining. Subsequently, PD-L1 staining on archival nephrectomy tissue was successfully performed in 36 patients, whose characteristics were comparable with the total study population (Appendix Table A1, online only). Overall, 42% of patients (n = 15 of 36) were PD-L1 positive, and their ORR was 60% (n = 9 of 15) compared with 19% (n = 4 of 21) in PD-L1–negative patients (P = .01; Appendix Table A2, online only). In patients with ccRCC with sarcomatoid differentiation, the ORR was 50% (n = 3 of 6) in PD-L1–positive patients and 29% (n = 2 of 7) in PD-L1–negative patients (P = .84). In patients with variant histology RCC, the ORR was 67% (n = 6 of 9) in PD-L1–positive patients and 14% (n = 2 of 14) in PD-L1–negative patients (P = .02).
Toxicity
Adverse events were assessed in all 60 patients who received at least 1 dose of either therapy. The most common toxicities (any grade, any attribution) were fatigue (83%), musculoskeletal pain (82%), proteinuria (45%), diarrhea (45%), dyspnea (40%), nausea (40%), and hypertension (38%; Table 3). Thirty-four patients developed at least 1 grade ≥ 3 adverse event of any attribution. One on-protocol death occurred, which was unrelated to study treatment and was suspected to be related to disease progression.
TABLE 3.
Summary of Treatment-Emergent Adverse Events That Occurred in ≥ 10% of Patients
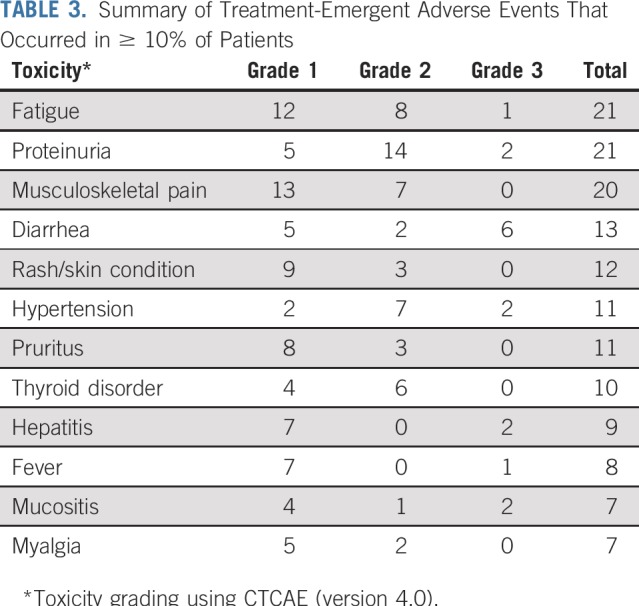
The most common treatment-related all-grade adverse events occurring in > 10% of patients were fatigue (35%), proteinuria (35%), musculoskeletal pain (33%), diarrhea (22%), rash (20%), hypertension (18%), pruritus (18%), thyroid dysfunction (17%), hepatitis (15%), fever (13%), and mucositis (12%; Table 3). Twenty patients (33%) experienced at least 1 treatment-related grade 3 adverse event. There were no treatment-related grade 4 or 5 toxicities. Six patients (10%) experienced adverse events of special interest that required high-dose corticosteroids (≥ 30 mg/d prednisone or equivalent).
Quality-of-Life Metrics
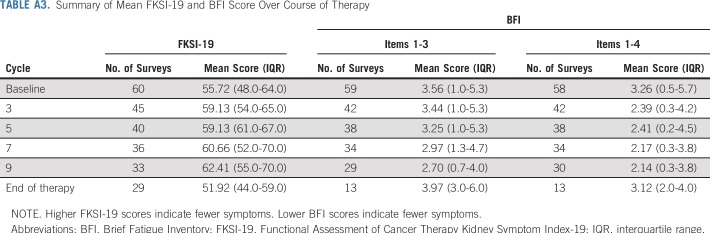
All 60 patients who started study treatment submitted at least 1 FKSI-19 questionnaire. Fifty-nine submitted at least 1 BFI questionnaire. Overall, during treatment with atezolizumab and bevacizumab, FKSI-19 and BFI scores were stable throughout treatment (Appendix Table A3, online only), which suggests no appreciable detriment to quality of life.
DISCUSSION
Our findings demonstrate that the combination of atezolizumab and bevacizumab is well tolerated with meaningful clinical efficacy in variant histology RCC and RCC with sarcomatoid differentiation. The combination demonstrated responses across several subtypes of RCC, including collecting duct and medullary carcinoma, histologies that are often treated with cytotoxic chemotherapy. This is notable given the generally poor prognosis and low response rates associated with variant histology RCC in trials to date. This work highlights the need for ongoing clinical trials to evaluate immune checkpoint inhibitor combinations in this diverse patient population given the unmet clinical need to improve outcomes for patients with variant histology RCC.
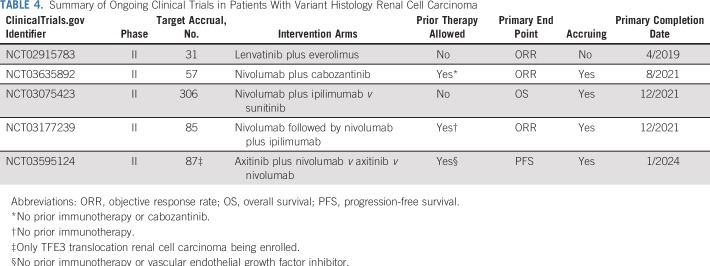
Sunitinib had been the standard of care for variant histology RCC despite low response rates in prospective trials.7,8 Recently, some noteworthy clinical trials and retrospective series have investigated the efficacy of additional therapies for patients with variant histology RCC. A prospective phase II trial of the immune checkpoint inhibitor pembrolizumab demonstrated an ORR of 25% (n = 41 of 165) in treatment-naïve variant histology RCC, but histologies were limited to papillary, chromophobe, or unclassified RCC.19 In a multicenter retrospective analysis, cabozantinib, a multitargeted tyrosine kinase inhibitor against VEGF receptor, MET, and AXL, demonstrated an ORR of 27% and median PFS of 6.7 months in a spectrum of patients with variant histology RCC.20 Furthermore, responses in our study were seen independent of prior therapy, including VEGF-targeting agents. However, response rates in several subtypes were ≤ 20%, and the ORR of 10% (n = 1 of 10) in chromophobe RCC highlights the need for new therapeutic strategies for this patient population. Several clinical trials are currently assessing alternative treatment options for patients with variant histology RCC (Table 4).
TABLE 4.
Summary of Ongoing Clinical Trials in Patients With Variant Histology Renal Cell Carcinoma
The combination of bevacizumab plus atezolizumab compared with sunitinib was investigated in a phase III study of patients with treatment-naïve advanced RCC with a clear cell component (IMmotion 151).11 The trial demonstrated prolonged PFS in both the PD-L1–positive and intention-to-treat populations, and responses were observed in 37% of patients who received the combination. The regimen was generally well tolerated, with 40% grade 3-4 toxicities and 16% of patients requiring high-dose corticosteroids for immune-related adverse events. This safety profile was comparable to our study and reinforced by the quality-of-life data, which suggests a lack of decline in quality of life with treatment.
Although the regimen of atezolizumab and bevacizumab is not approved by regulatory agencies, VEGF inhibitor and immunotherapy combinations have been approved in the United States for patients with advanced RCC. VEGF inhibitor/immunotherapy combinations, including axitinib with either pembrolizumab (KEYNOTE-426) or avelumab (JAVELIN Renal 101), have shown improvements in PFS, with an OS benefit observed with axitinib combined with pembrolizumab compared with sunitinib.21,22 These regimens are being explored in patients with variant histology RCC (Table 4).
In our study, patients with ccRCC with sarcomatoid differentiation demonstrated a noteworthy response rate of > 50% with the combination of atezolizumab and bevacizumab. This is consistent with data from subset analyses of IMmotion 151 where 49% of patients with sarcomatoid differentiation (n = 33 of 68) had an objective response.11 Additional post hoc analyses from the phase III study of axitinib and pembrolizumab and phase III study of nivolumab plus ipilimumab compared with sunitinib (CheckMate 214) demonstrated improved responses in patients with sarcomatoid differentiation: ORR of 57% (n = 30 of 51) with a CR of 12% with pembrolizumab plus axitinib and ORR of 57% (n = 34 of 60) with a CR of 18% with nivolumab plus ipilimumab.23,24 Of note, these analyses were not conducted with prospective pathology review and are exploratory in nature. Nonetheless, with ORR in the 50%-60% range and CR rates > 10%, patients with sarcomatoid differentiation, a group that has historically responded poorly to single-agent VEGF-targeting agents, seem to derive a significant benefit from immunotherapy-based treatments.
In our study, clinical outcomes varied by PD-L1 status, particularly in patients with variant histology RCC. Although our sample size is small, to our knowledge, this study is one of the largest to examine PD-L1 status specifically in patients with advanced variant histology RCC treated with contemporary immunotherapy-based therapy. Several studies in RCC demonstrated higher response rates in PD-L1–positive patients compared with PD-L1–negative patients.11,25 While PD-L1 status is prognostic in RCC, its role as a predictive biomarker continues to evolve given the dynamic nature of PD-L1 expression and lack of consensus methodologies on biomarker assessment. Contemporary trials differed in the assays used to defined PD-L1 positivity, with approximately 60% of patients defined as having PD-L1–positive disease in KEYNOTE-426 and JAVELIN Renal 101 and 24% in CheckMate 214. Nonetheless, our results suggest that PD-L1 is intriguing as a biomarker for response to atezolizumab and bevacizumab in variant histology RCC. We plan to conduct additional correlative work, including genomic profiling and assessment of the immune microenvironment, to better elucidate markers of response and resistance. Indeed, alternative predictive biomarkers of response to immunotherapy are continuously being explored, including tumor-infiltrating CD8 T cells, tumor mutation burden, tumor mutations, and novel gene signatures.26,27
Our study has limitations. It is a small, single-arm, phase II trial of 60 patients with diverse histologies and treatment histories. It is difficult to assess the role of the combination with other agents being used in variant histologies, such as cabozantinib or the combination of nivolumab and ipilimumab. Furthermore, delineation of the difference in activity among the different subtypes or in pretreated or treatment-naïve patients would require a larger trial.
Despite these limitations, our results shed important insight into the management of variant histology RCC and support the use of immunotherapy/VEGF combinations to improve outcomes for this understudied patient population. Although a larger confirmatory randomized study would be needed to evaluate the treatment effect of the combination of atezolizumab and bevacizumab, the current study provides support for the use of immunotherapy/VEGF combinations in the management of variant histology RCC and RCC with sarcomatoid differentiation.
ACKNOWLEDGMENT
We acknowledge the following investigators for their participation in this study: Guru Sonpavde (Dana Farber Cancer Institute), James W. Mier (Beth Israel Deaconess Medical Center), and James M. Randall (University of California, San Diego, Moores Cancer Center). We also acknowledge all the study patients and their family members as well as all the study team members, including co-investigators, research staff, regulatory personnel, study coordinators, managers, and research nurses.
Appendix
FIG A1.
Patient eligibility diagram of the clinical trial.
TABLE A1.
Patient and Disease Characteristics in Patients With and Without Availability of PD-L1 Testing
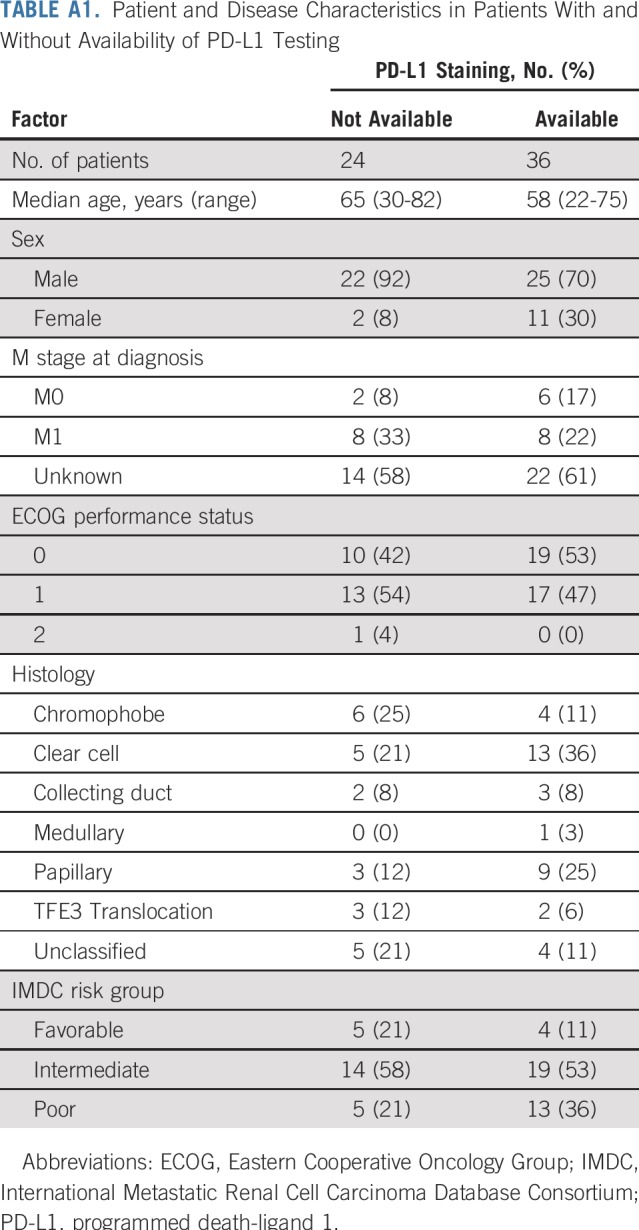
TABLE A2.
Overall Response by PD-L1 Status According to Histology
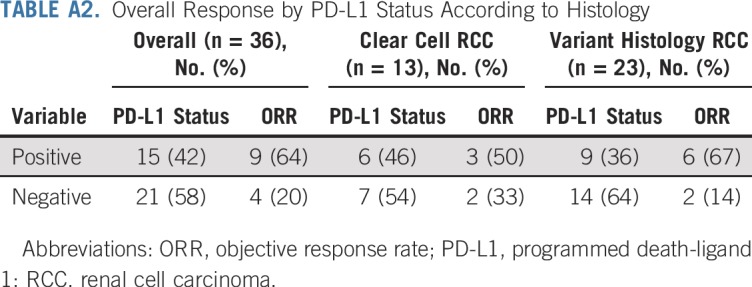
TABLE A3.
Summary of Mean FKSI-19 and BFI Score Over Course of Therapy
Footnotes
Supported in part by Roche and Genentech. T.K.C. is supported in part by the Dana-Farber/Harvard Cancer Center Kidney Specialized Programs of Research Excellence; the Kohlberg Chair at Harvard Medical School; and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at the Dana-Farber Cancer Institute. Medical writing and editorial assistance support may have been funded by communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, Oxford PharmaGenesis, and others). The institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter.
Clinical trials information: NCT02724878.
AUTHOR CONTRIBUTIONS
Conception and design: Bradley A. McGregor, Rana R. McKay, Kathryn Gray, Toni K. Choueiri
Financial support: Toni K. Choueiri
Administrative support: John A. Steinharter, Toni K. Choueiri
Provision of study material or patients: Bradley A. McGregor, Rana R. McKay, Michelle S. Hirsch, Xiao X. Wei, Atish Choudhury, Kerry Kilbridge, David F. McDermott, Ulka Vaishampayan, Toni K. Choueiri
Collection and assembly of data: Bradley A. McGregor, Lillian Werner, Kathryn Gray, Abdallah Flaifel, Sabina Signoretti, John A. Steinharter, Ronan Flippot, Atish Choudhury, Kerry Kilbridge, Gordon J. Freeman, David F. McDermott, Ulka Vaishampayan, Toni K. Choueiri
Data analysis and interpretation: Bradley A. McGregor, Rana R. McKay, David A. Braun, Lillian Werner, Kathryn Gray, Abdallah Flaifel, Sabina Signoretti, Michelle S. Hirsch, John A. Steinharter, Ziad Bakouny, Ronan Flippot, Xiao X. Wei, Gordon J. Freeman, Eliezer M. Van Allen, Lauren C. Harshman, David F. McDermott, Ulka Vaishampayan, Toni K. Choueiri
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for Patients With Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bradley A. McGregor
Consulting or Advisory Role: Bayer AG, Seattle Genetics, Astellas Pharma, Exelixis, AstraZeneca, Genentech, Roche, Nextar, Janssen Oncology, Pfizer, EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Exelixis (Inst), Calithera Biosciences (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst)
Rana R. McKay
Consulting or Advisory Role: Janssen Pharmaceuticals, Novartis, Tempus, Exelixis, Pfizer, Bristol-Myers Squibb, Astellas Pharma, Medivation, Dendreon
Research Funding: Pfizer (Inst), Bayer AG (Inst)
David A. Braun
Consulting or Advisory Role: Bristol-Myers Squibb, Octane Global, Defined Health, Dedham Group, Adept Field Solutions, Slingshot Insights, Blueprint Partnerships, Charles River Associates, Trinity Group, Insight Strategy
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Lillian Werner
Employment: Foundation Medicine
Kathryn Gray
Stock and Other Ownership Interests: DVAX, ArQule
Sabina Signoretti
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, MedImmune, Merck
Research Funding: Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Exelixis (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Biogenex
Other Relationship: American Association for Cancer Research, National Cancer Institute
Xiao X. Wei
Honoraria: OncLive
Travel, Accommodations, Expenses: Corvus Pharmaceuticals
Atish Choudhury
Employment: LeMaitre Vascular (I)
Honoraria: Bayer AG, Astellas Pharma, Janssen Pharmaceuticals
Research Funding: Janssen Pharmaceuticals (Inst), Bayer AG
Travel, Accommodations, Expenses: Genentech
Gordon J. Freeman
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Surface Oncology (I), Xios Therapeutics, Origmed, Elstar (I), SQ2 Biotech (I), Adaptimmune (I)
Research Funding: Novartis (I), Roche (I), Genentech (I), UCB (I), Ipsen (I), Quark (I), Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Roche, Genentech, Roche (I), Genentech (I), Pfizer (I), Bristol-Myers Squibb, Medarex, Amplimmune, AstraZeneca, Merck, EMD Serono, Boehringer Ingelheim, Novartis, Novartis (I), Dako
Eliezer M. Van Allen
Stock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx
Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda Pharmaceuticals, Novartis, Genome Medical, Invitae, Illumina, Tango Therapeutics, Ervaxx
Speakers’ Bureau: Illumina, Bristol-Myers Squibb, Novartis
Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst), patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst), patent on clinical interpretation algorithms using cancer molecular data (Inst)
Travel, Accommodations, Expenses: Roche, Genentech
Lauren C. Harshman
Consulting or Advisory Role: Pfizer, Genentech, Theragene, Corvus Pharmaceuticals, Merck, Exelixis, Bayer AG, Novartis, Jounce Therapeutics
Research Funding: Medivation (Inst), Astellas Pharma (Inst), Bayer AG (Inst), Sotio (Inst), Genentech (Inst), Roche (Inst), Dendreon (Inst), Bristol-Myers Squibb (Inst), Takeda Pharmaceuticals (Inst), Merck (Inst), Janssen Oncology (Inst), Pfizer (Inst), Endocyte (Inst)
Travel, Accommodations, Expenses: Bayer AG
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, Roche, Pfizer, Exelixis, Novartis, X4 Pharma, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Eli Lilly
Research Funding: Prometheus Laboratories (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Novartis (Inst), Alkermes (Inst), Peloton Therapeutics (Inst)
Other Relationship: Beth Israel Deaconess Medical Center
Ulka Vaishampayan
Honoraria: Pfizer, Bayer AG, Sanofi, Bristol-Myers Squibb, Exelixis
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Exelixis, Bayer AG, EMD Serono
Speakers’ Bureau: Pfizer, Bayer AG, Bristol-Myers Squibb, Exelixis, Sanofi, Eisai
Research Funding: Astellas Pharma, Exelixis, Pfizer, Bristol-Myers Squibb
Toni K. Choueiri
Honoraria: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly, ASCO, ESMO
Consulting or Advisory Role: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest
Research Funding: AstraZeneca (Inst), Alexion (Inst), Bayer, Bristol Myers-Squibb/ER Squibb and Sons LLC (Inst), Cerulean (Inst), Eisai (Inst), Foundation Medicine Inc. (Inst), Exelixis (Inst), Ipsen (Inst), Tracon (Inst), Genentech (Inst), Roche (Inst), Roche Products Limited (Inst), F. Hoffmann-La Roche (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Peloton (Inst), Pfizer (Inst), Prometheus Labs (Inst), Corvus (Inst), Calithera (Inst), Analysis Group (Inst), Sanofi/Aventis (Inst), Takeda (Inst)
Stock and Other Ownership Interests: Pionyr, Tempest
Other Relationship: Medical writing and editorial assistance support may have been funded by communications companies funded by pharmaceutical companies, such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, PAREXEL
No other potential conflicts of interest were reported.
REFERENCES
- 1.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 2.Linehan WM. Genetic basis of kidney cancer: Role of genomics for the development of disease-based therapeutics. Genome Res. 2012;22:2089–2100. doi: 10.1101/gr.131110.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: An examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435–441. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1016/j.celrep.2018.03.075. Ricketts CJ, De Cubas AA, Fan H, et al: The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 23:313-326.e5, 2018 [Erratum Cell Rep 23:3698, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Velasco G, McKay RR, Lin X, et al. Comprehensive analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin Genitourin Cancer. 2017;15:652–660.e1. doi: 10.1016/j.clgc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119:2999–3006. doi: 10.1002/cncr.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17:378–388. doi: 10.1016/S1470-2045(15)00515-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol. 2016;69:866–874. doi: 10.1016/j.eururo.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network: Kidney Cancer, 2019. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 10. doi: 10.1038/s41591-018-0053-3. McDermott DF, Huseni MA, Atkins MB, et al: Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [Erratum: Nat Med 24:1941, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 12.Rao D, Butt Z, Rosenbloom S, et al. A comparison of the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) J Pain Symptom Manage. 2009;38:291–298. doi: 10.1016/j.jpainsymman.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 2017;3:256–259. doi: 10.1001/jamaoncol.2016.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney KM, Sun H, Liao X, et al. PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res. 2015;3:1308–1315. doi: 10.1158/2326-6066.CIR-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25:2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–1019. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 19. McDermott DF, Lee J-L, Ziobro M, et al: First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): Results from KEYNOTE-427 cohort B. J Clin Oncol 37, 2019 (suppl; abstr 546) [Google Scholar]
- 20.Martínez Chanzá N, Xie W, Asim Bilen M, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: A multicentre, retrospective, cohort study. Lancet Oncol. 2019;20:581–590. doi: 10.1016/S1470-2045(18)30907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 23. Rini BI, Plimack ER, Stus V, et al: Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol 37, 2019 (suppl; abstr 4500) [Google Scholar]
- 24. McDermott DF, Choueiri TK, Motzer RJ, et al: CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Oncol 37, 2019 (suppl; abstr 4513) [Google Scholar]
- 25.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choueiri TK, Albiges L, Haanen JBAG, et al: Biomarker analyses from JAVELIN Renal 101: Avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol 37, 2019 (suppl; abstr 101) [Google Scholar]
- 27. Gao J, Karam JA, Tannir NM, et al: A pilot randomized study evaluating nivolumab (nivo) or nivo + bevacizumab (bev) or nivo + ipilimumab (ipi) in patients with metastatic renal cell carcinoma (MRCC) eligible for cytoreductive nephrectomy (CN), metastasectomy (MS) or post-treatment biopsy (Bx). J Clin Oncol 36, 2018 (suppl; abstr 4520) [Google Scholar]