Abstract
PURPOSE:
Effective enrollment and treatment of patients in cancer clinical trials require definition and coordination of roles and responsibilities among clinic and research personnel.
MATERIALS AND METHODS:
We developed a survey that incorporated modified components of the Survey of Physician Attitudes Regarding the Care of Cancer Survivors. Surveys were administered to clinic nursing staff and research personnel at a National Cancer Institute–designated comprehensive cancer center. Results were analyzed using χ2-tests, t tests, and analyses of variance.
RESULTS:
Surveys were completed by 105 staff members (n = 50 research staff, n = 55 clinic staff; 61% response rate). Research staff were more likely to feel that they had the skills to answer questions, convey information, and provide education for patients on trials (all P < .05). Both clinic and research staff reported receipt of communication about responsibilities in fewer than 30% of cases, although research staff reported provision of such information in more than 60% of cases. Among 20 tasks related to care of patients in trials, no single preferred model of responsibility assignment was selected by the majority of clinic staff for nine tasks (45%) or by research staff for three tasks (15%). Uncertainty about which team coordinates care was reported by three times as many clinic staff as research staff (P = .01). There was also substantial variation in the preferred model for delivery of care to patients in trials (P < .05).
CONCLUSION:
Knowledge, attitudes, and perception of care and responsibilities for patients on clinical trials differ between and among clinic and research personnel. Additional research about how these findings affect efficiency and quality of care on clinical trials is needed.
INTRODUCTION
Clarification and coordination of roles and responsibilities in the evaluation, treatment, and follow-up of patients with cancer have emerged as critical factors in the provision of quality care. These considerations persist throughout the entire disease course.1 During the evaluation of a suspected malignancy, primary care providers, pathologists, radiologists, surgeons, oncologists, nursing staff, clinic administrative staff, and patients must carry out distinct but interdependent tasks to ensure timely and accurate diagnosis, staging, and treatment planning.2-6 For multimodality treatment regimens, various oncology disciplines must agree upon and synchronize treatment plans and schedules.7-10 Throughout treatment, oncologists and primary care providers may comanage toxicities and comorbidities, and both may counsel patients through the process.11-15 After treatment, oncologists and primary care providers face the tasks of transferring and assigning responsibilities, including clinical and radiographic surveillance and the management of treatment-related toxicities, preventive care, and psychosocial support.16-21
Coordination and definition of roles and responsibilities may be particularly critical in oncology clinical trials.22 Screening, enrollment, and treatment require clear definition of responsibilities between team members and across teams.23-25 These responsibilities include scheduling appointments, communicating with patients, and providing status updates to clinicians. Because patients may participate in numerous clinical trials during the course of their disease, their longitudinal clinic team may interact with different research teams, each of which may approach responsibility assignment differently. Furthermore, trials are becoming increasingly complex, with more numerous eligibility criteria and study-related procedures.26,27 Added to the inherent complexities of modern-day combination cancer therapies, these requirements have resulted in increased demands on both clinic and research staff.26,28 Over time, an institution’s clinical trial portfolio changes, and ongoing trials undergo modifications. Clinic and research staff therefore must constantly adapt to these updates by adjusting expectations and practices.
The importance of explicit definition and understanding of team member roles and responsibilities has been demonstrated in multiple contexts, including corporate cultures and team sports.29 In recent years, team function and coordination also have been evaluated in medical scenarios, including the emergency department, operating room, and longitudinal multidisciplinary care.8,14,30-34 However, these issues remain poorly understood and essentially unstudied in the realm of clinical trials. In this study, we aimed to map this territory by understanding challenges in the definition and coordination of roles and responsibilities among clinic and research personnel. Specifically, we surveyed clinic and research staff at a National Cancer Institute–designated comprehensive cancer center to determine perceptions, preferences, and practices.
MATERIALS AND METHODS
Study Setting and Sample
The Harold C. Simmons Comprehensive Cancer Center at the University of Texas Southwestern Medical Center is a freestanding clinical, research, and educational facility in Dallas, Texas. Nursing clinic staff members are organized into hematology-oncology, radiation oncology, surgical oncology, and gynecologic oncology clinics. The Simmons Clinical Research Office is organized by cancer type and, at the time of this study, had 107 total staff members, who included clinical research coordinators, clinical research managers, protocol and regulatory team staff, administrative/compliance/financial support staff, and administrative managers.
In recent years, approximately 6,000 new adult patients with cancer have been seen annually within the Simmons Cancer Center. Of these, approximately 600 patients are enrolled in adult therapeutic clinical trials.
Survey Development
To develop survey questions, we modified content from the Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS), which assessed differences between oncologists and primary care physicians’ knowledge, attitudes, and practices related to care of patients after treatment.16,20,35,36 Similar to SPARCCS, we developed two versions (clinic team and research team) of the questionnaire, which differed only in the referent group label within survey items. Most items were measured using seven-or five-point Likert scales that referred to agreement (agree strongly [7], agree [6], somewhat agree [5], undecided/I don’t know [4], disagree somewhat [3], disagree [2], disagree strongly [1]) or frequency (always/almost always [5], often [4], sometimes [3], rarely [2], never [1]). Thus, higher scores indicated higher agreement with a statement or more frequent practice of a care model. Questions about care assignment provided a five-point scale: research team entirely responsible; research team mostly responsible; clinic team and research team share responsibility; clinic team mostly responsible; clinic team entirely responsible.
Survey Administration and Data Collection
One author (S.G.) distributed and collected surveys during a 2-week period. For research staff, the majority of surveys were distributed and completed individually and anonymously during regularly scheduled team meetings. For clinic nursing staff, surveys were completed anonymously and returned by individual staff members to a neutral party. Survey responses were entered into a Microsoft Excel (Microsoft, Redmond, WA) spreadsheet. Data accuracy was cross-checked with survey documents by two investigators (S.G. and D.E.G.).
Statistical Analysis
Likert scale responses were consolidated into binary categories (agree/disagree or usually/rarely) or a composite score for each participant was computed by averaging answers across items that measured the same construct. Responses related to team responsibility were consolidated into three categories: research team; shared; clinic team. As in previous studies,37-40 before an average score was computed across items assumed to measure the same construct, we determined Cronbach’s α as a measure of internal consistency. We inspected descriptive statistics, such as the mean and standard deviations of scales, across all participants and separately for research and clinic teams. To test for systematic differences between responses from the clinic and research teams, we conducted χ2 tests, t tests, and analyses of variance.
RESULTS
In total, 105 staff members participated in the study (n = 55 research and n = 50 clinic staff). Although all clinic staff had clinical degrees and/or certifications, only six research staff (11%) had clinical degrees (n = 4 RNs, n = 2 MSNs). The mean age of respondents was 38 years (standard deviation [SD], 11 years), and 80% were women. On average, participants had 10.8 years of professional experience (SD, 8.8 years) and had been in their current position for 3.3 years (SD, 4.1 years). The mean reported number of patients in clinical trials with whom staff had interacted was 39.5 for research personnel and 35.6 for clinic personnel (P = .70). There was no significant difference in age, sex, or professional experience between research staff and clinical staff. The response rate was 87% among research staff and was 46% among clinic nursing staff (61% overall). The higher response rate among research staff may reflect survey logistics. Research staff tended to complete surveys during weekly staff meetings, whereas clinic staff completed surveys individually.
Research Team Qualifications and Responsibilities
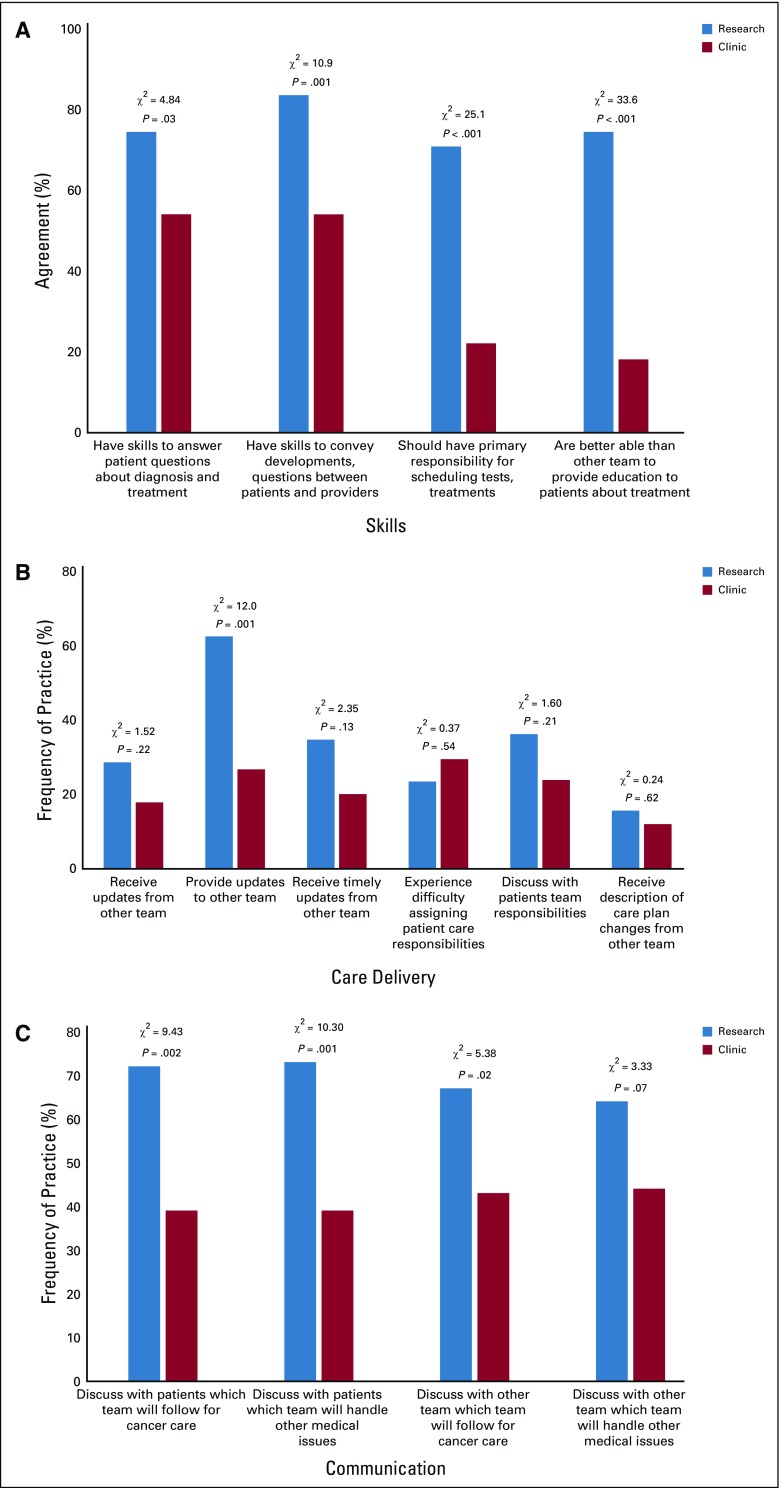
Four survey items addressed perceptions of research team qualifications and responsibilities (Fig 1A). Cronbach’s α was 0.75, which indicated acceptable internal consistency across the items. In assessment of their own skills, research team members were more confident in their skills than were their clinic counterparts (mean, 4.0 v 2.7; P < .001), and the greatest difference in perception was in the primary responsibility for scheduling diagnostics, referrals, and treatment of patients in trials.
Fig 1.
(A) Perceived skills and responsibilities of members of respondents’ own team. (B) Perceived delivery of care to patients on clinical trials. (C) Perceived communication practices. P value refers to 2 × 2 χ2.
Delivery of Care to Patients in Clinical Trials
Six survey items addressed perceived delivery of care to patients in trials (Fig 1B). Both the research and clinic teams reported receiving information/updates from the other team in only a minority of cases, although the research team felt that it provided information/updates to the clinic team in almost two thirds of cases. A minority of respondents from both teams reported difficulties in assigning patient care responsibilities or having discussions with patients about care team responsibilities.
Care Responsibilities for Patients in Clinical Trials
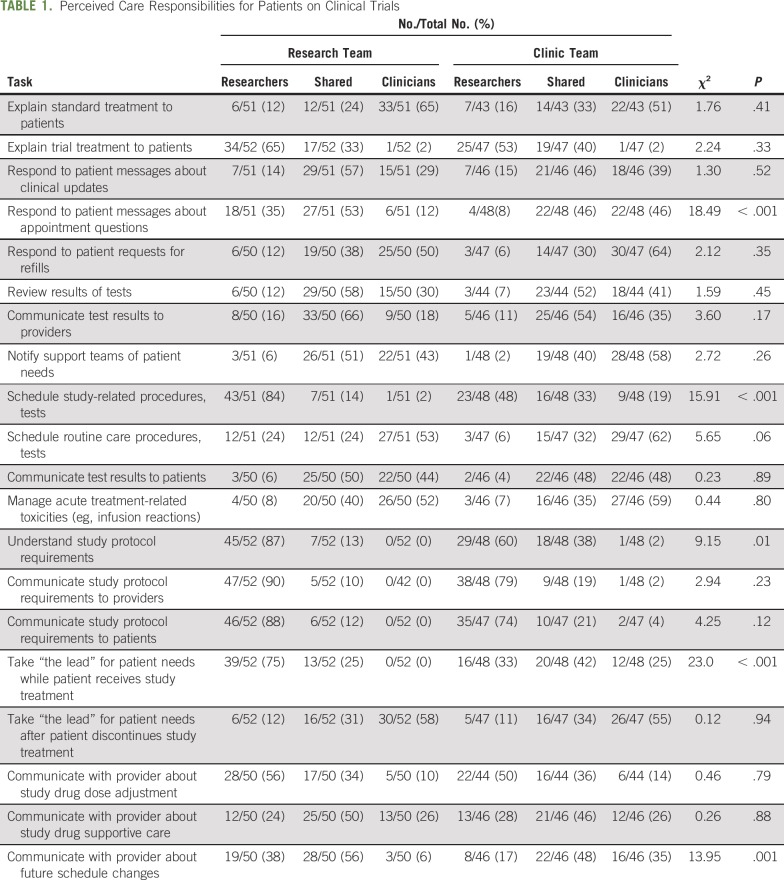
Respondents assigned primary responsibility (research team, clinic team, or shared) for 20 tasks related to the care of patients in clinical trials (Table 1). Tasks encompassed communicating with patients; reviewing and conveying test results; scheduling procedures; managing toxicities; understanding and communicating protocol requirements; and communicating with providers. In some cases, we observed lack of consensus within teams. For instance, for three tasks (15%), no single option (research team, clinic team, or shared) received more than 50% of responses from the research team. For nine tasks (45%), no single option received more than 50% of responses from the clinic team. Shared responsibility was the most common selection for eight tasks (40%) among research team responses and for eight tasks (40%) among clinic team responses. For tasks related to communicating with patients and providers and scheduling study-related procedures and tests, the research team was significantly more likely to assign responsibility to the research team. The clear majority of respondents from both teams felt that understanding and communicating study protocol requirements was the responsibility of the research team.
TABLE 1.
Perceived Care Responsibilities for Patients on Clinical Trials
Communication Practices
Four survey items addressed communication about responsibility for patients on trials (Fig 1C) and focused on discussing with patients and with the other team who will observe patients for cancer care and for other medical issues. For all items, the majority (64% to 73%) of research team members reported discussing these topics, whereas only a minority (39% to 44%) of clinic team members did. The research team tended to have these discussions more often with patients than with the clinic team; however, the clinic team reported having these discussions with the clinic team more often than with patients.
Problems Encountered in Clinical Trials
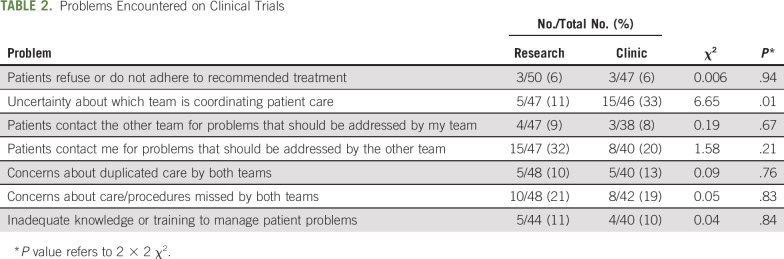
Seven survey items addressed perceived delivery of care of patients on trials (Table 2). These items related to patient adherence, coordination of care, patient contact, duplicated or missed care, and inadequate knowledge. None of the items was reported by a majority of respondents from either team. The particularly low rate (6%) of reported patient nonadherence may reflect the relatively motivated and informed population treated on clinical trials.41,42 Uncertainty about which team is coordinating care was reported by three times as many clinic team respondents as research team respondents (P = .01). Both research and clinic team respondents were more than twice as likely to report that patients contacted them for problems more appropriate for the other team than that patients contacted the other team for problems more appropriate for their own team.
TABLE 2.
Problems Encountered on Clinical Trials
Preferred Model for Care Delivery
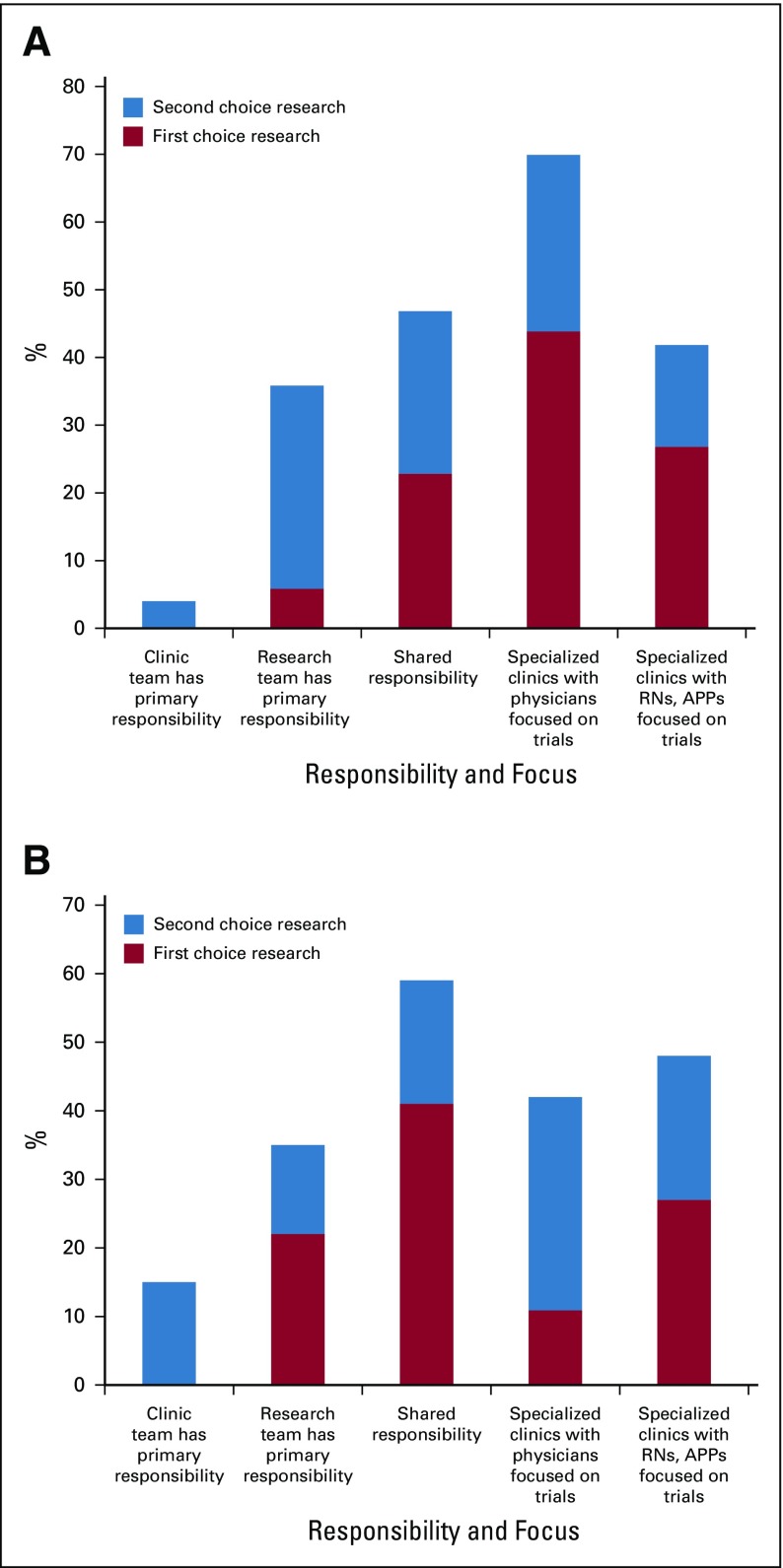
We surveyed research and clinic team members on their preferred model of care delivery for patients in clinical trials (Fig A1, online only). Options included (1) clinic team has primary responsibility, (2) research team has primary responsibility, (3) shared responsibility, (4) specialized clinics with physicians focused on trials, and (5) specialized clinics with registered nurses and advance practice providers focused on trials. There were significant differences between research team and clinic team preferences (P = .003 for first choice; P = .004 for first or second choice); the most common first choice among the research team was a specialized physician-run clinic, and the most common choice among the clinic team was a shared responsibility model. Among the research team, the most common second choice was “research team has primary responsibility,” whereas, among the clinic team, the most common second choice was a specialized physician-run clinic. Notably, neither the clinic nor research team had a single preferred model selected by the majority of respondents.
DISCUSSION
The challenges of clinical trial activation and enrollment have received considerable focus in recent years. These include increasingly complex and stringent eligibility criteria, escalating research costs, and lack of available trials.27,28 Yet, relatively little attention has been placed on study procedures after patients are enrolled. We previously framed the interface and interactions between clinic and research teams in cancer clinical trials as multiteam systems,22 which arise in situations in which members clearly identify with different teams when they collaborate on a joint task.43
The SPARCCS assessed how primary care providers and oncology specialists perceive the assignment of care responsibilities for patients who had completed cancer treatment.16,18 In this study, we modified the SPARCCS to address care assignments for patients in cancer clinical trials. We targeted clinic nursing staff and research coordinators, because they serve as front-line first responders in the care of patients on clinical trials. We found multiple differences between clinic and research team perceptions and preferences. In general, the majority of research staff reported proficiency at education of and communication with patients on trials, assumption of responsibility for ordering tests and procedures for patients in trials, and management of clinical developments and questions between patients on trials and providers. By contrast, only a minority of clinic staff reported such skills. These observations are notable because (1) in contrast to clinic staff, many research staff may not necessarily have formal medical training; (2) depending on the trial, components or even the entirety of treatment may entail standard-of-care therapies; and (3) at some centers, recent regulatory changes, such as Medicare Meaningful Use requirements,44 have limited the ability of non-nurse research personnel to place orders in the electronic health record and have instead required them to depend on clinic staff to perform these tasks.
Communication practices differed considerably between teams. Both teams felt that they provided updates more often than they received them. We also observed clear differences in perceptions of the same process. For instance, more than 60% of research staff reported that they provided updates to the clinic team, but fewer than 20% of clinic staff reported receipt of these—a finding that stresses the relevance of perception in multiteam systems. Nevertheless, only approximately one third of respondents reported difficulty in assigning team responsibilities. The discussion of team responsibilities with patients and with the other team occurred significantly more frequently among research staff than among clinic staff. One possible explanation is that research staff members interact exclusively with patients in (or being screened for) clinical trials. Conversely, patients in trials may represent only a small minority of the total caseload for clinic staff. Therefore, research staff could incorporate mention of team responsibilities universally into patient discussions, whereas clinic staff would need to modify their discussion depending on whether patients were enrolled in trials. Clinic team members were three times as likely as research staff to report uncertainty about which team is coordinating care for their patients in trials, which perhaps also reflects less familiarity with trial requirements and structure.
In addition, we observed considerable heterogeneity within teams. For almost half of the specific tasks for patients in trials, no single responsibility model (clinic, research, or shared) received a majority response from members of the clinic team. Furthermore, shared responsibility was selected for almost half of tasks, an option that inherently requires more discussion and clarification. These tasks included highly clinically oriented responsibilities, such as handling patient clinical updates, appointment questions, and refill requests; reviewing test results; and communicating with providers about schedule changes. How these and other tasks are best shared among clinic and research teams requires careful consideration to avoid duplication or overlooking of effort.
Ideally, cancer centers could clearly outline responsibility assignments for tasks, such as responding to patient questions, scheduling study-related tests, and communicating with providers. Determination of the most effective, appropriate, and efficient model will require additional study. Moreover, for cancer clinical trials, there may not be a one-size-fits-all template applicable across trials, because protocol requirements differ widely among studies and may change over time. Therefore, staff may not be able to develop, evaluate, and disseminate standard operating processes for clinical trials to the extent that they might for standard clinical care.
Divergent team perspectives of a preferred model of care delivery raise important concerns about how cancer centers organize the care of patients in clinical trials. First, it is noteworthy that no single model was the first choice for a majority of respondents, which suggests heterogeneous opinions among and between teams. The preference for specialized clinics expressed by the research team suggests a closed model, as used by some cancer centers for phase I clinical trials. However, the feasibility of such an approach is not clear when the entire spectrum of clinical research (eg, phase I to III trials, nontherapeutic studies) is considered. Second, preference for these closed clinics could imply isolation of research efforts from a larger institutional effort toward integrated, high-quality cancer care, which echoes our earlier findings that research staff perceive greater within-group identification but less identification with the cancer center compared with clinic staff.45 Finally, divergences also point to opportunities for training and education. Onboarding for new team members in both clinical and research roles could establish and increase alignment with site leadership expectations for care of patients in trials. Future research opportunities could test optimal implementation strategies for onboarding and team building to enhance these practices across the larger clinical trial enterprise.
The main limitations of this study are the nature of the study site and the heterogeneity of cancer clinical research programs nationally. With a clinical research office of more than 100 staff (many of whom work within a single disease group), our research operations may resemble those of other major academic centers but may be less relevant to smaller community practices. Furthermore, even among similarly sized programs, training, expertise and assigned responsibilities of research staff may differ substantially. Although we did collect data about the educational background of research staff, the numbers are too small to correlate (cross-tab) credentials with survey responses to indicate any interpretable trends. Nevertheless, because the interactions between clinic and research personnel have been essentially unstudied previously, by applying established tools to this new context, this cross-sectional study provides preliminary findings for future, multicenter, observational studies and interventions. Another limitation is the study sample, which represented almost 90% survey completion rate among research personnel but less than 50% completion rate for clinic personnel. This cohort could contribute a sampling bias to our findings, as responding clinic staff may represent a particularly motivated or concerned subset. At the same time, because they completed surveys in group settings, it seems plausible that some research personnel may have recorded more positive responses than they might have individually. Last, our sampling of clinic staff did not include input from physicians, whose practice patterns and preferences are likely to affect decisions about clinical trials.
In conclusion, like the provision of care for patients after completion of cancer treatment, care for patients in clinical trials requires high-level coordination within a complex multiteam system. Because every clinical trial has distinct requirements that may change with protocol amendments, individuals involved in the care of patients in trials must be highly adaptable and must maintain open lines of communication. Yet, research and clinic teams have clear differences in knowledge, attitudes, and practices related to clinical trials. There is also considerable heterogeneity within each of these groups. These findings may be relevant not only to cancer trials but across clinical research settings.
ACKNOWLEDGMENT
We thank Dru Gray for assistance with manuscript preparation and Helen Mayo, MLS, from the University of Texas Southwestern Medical Library, for assistance with literature searches.
APPENDIX
Fig A1.
Most preferred model for care delivery among (A) research personnel and (B) clinic personnel. Differences between research team and clinic team responses for first choice (P = .003) and for first or second choice (P = .004) were statistically significant. APP, advance practice provider; RN, registered nurse.
Footnotes
Presented in part at the 35th National Oncology Conference of the Association of Community Cancer Centers, Phoenix, AZ, October 17-19, 2018, and as a National Cancer Institute Division of Cancer Control and Population Sciences Healthcare Teams Cyber Discussion Series on July 19, 2019.
Supported by the National Cancer Institute (NCI)–ASCO Teams in Cancer Care Delivery Project, a National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Short-Term Institutional Research Training Grant (No. 5 T35 DK 66141-10 to S.G.), the University of Texas Southwestern Center for Patient-Centered Outcomes Research, Agency for Healthcare Research and Quality Grant No. 1R24HS022418-01 (to S.J.C.L.), an NCI Midcareer Investigator Award in Patient-Oriented Research (No. K24CA201543-01 to D.E.G.), the University of Texas Southwestern NCI National Clinical Trials Network Lead Academic Site (Award No. 5U10CA180870-02 to D.E.G.), and the Harold C. Simmons Comprehensive Cancer Center, which is supported in part by NCI Cancer Center Support Grant No. 1P30 CA142543-03.
AUTHOR CONTRIBUTIONS
Conception and design: Simon J. Craddock Lee, Torsten Reimer, Sandra Garcia, David E. Gerber
Collection and assembly of data: Simon J. Craddock Lee, Sandra Garcia, Erin L. Williams, Mary West, Tobi Stuart, David E. Gerber
Data analysis and interpretation: Simon J. Craddock Lee, Torsten Reimer, Mary West, Tobi Stuart, David E. Gerber
Administrative support: Simon J. Craddock Lee, Erin L. Williams, David E. Gerber
Financial support: David E. Gerber
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Definition and Coordination of Roles and Responsibilities Among Cancer Center Clinic and Research Personnel
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/site/ifc/journal-policies.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Torsten Reimer
Patents, Royalties, Other Intellectual Property: Royalties from Sage Publishing
Erin L. Williams
Consulting or Advisory Role: Lilly
Travel, Accommodations, Expenses: Lilly
Tobi Stuart
Speakers' Bureau: Merck
David E. Gerber
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Samsung Bioepis, Bristol-Myers Squibb
Speakers' Bureau: Bristol-Myers Squibb
Research Funding: Immunogen (Inst), ArQule (Inst), ImClone Systems (Inst), BerGenBio (Inst), Karyopharm Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: royalties from Oxford University Press from two books; royalties from Decision Support in Medicine from the Clinical Decision Support–Oncology online program.
Travel, Accommodations, Expenses: Lilly, ArQule, Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: Addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taplin SH, Weaver S, Chollette V, et al. Teams and teamwork during a cancer diagnosis: Interdependency within and between teams. J Oncol Pract. 2015;11:231–238. doi: 10.1200/JOP.2014.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: Absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518–1527. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: The importance of follow-up. Dis Colon Rectum. 2006;49:1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 5.Zapka J, Taplin SH, Price RA, et al. Factors in quality care: The case of follow-up to abnormal cancer screening tests—Problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010:58–71. doi: 10.1093/jncimonographs/lgq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partin MR, Burgess DJ, Burgess JF, Jr, et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: An observational study. Cancer Epidemiol Biomarkers Prev. 2015;24:422–434. doi: 10.1158/1055-9965.EPI-14-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osarogiagbon RU, Phelps G, McFarlane J, et al. Causes and consequences of deviation from multidisciplinary care in thoracic oncology. J Thorac Oncol. 2011;6:510–516. doi: 10.1097/JTO.0b013e31820b88a7. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, Rodriguez HP, Hicks D, et al. Deploying team science principles to optimize interdisciplinary lung cancer care delivery: Avoiding the long and winding road to optimal care. J Oncol Pract. 2016;12:983–991. doi: 10.1200/JOP.2016.013813. [DOI] [PubMed] [Google Scholar]
- 9.Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community healthcare setting. Transl Lung Cancer Res. 2015;4:456–464. doi: 10.3978/j.issn.2218-6751.2015.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osarogiagbon RU, Freeman RK, Krasna MJ. Implementing effective and sustainable multidisciplinary clinical thoracic oncology programs. Transl Lung Cancer Res. 2015;4:448–455. doi: 10.3978/j.issn.2218-6751.2015.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen MJ, Binz-Scharf M, D’Agostino T, et al. A mixed-methods examination of communication between oncologists and primary care providers among primary care physicians in underserved communities. Cancer. 2015;121:908–915. doi: 10.1002/cncr.29131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sada YH, Street RL, Jr, Singh H, et al. Primary care and communication in shared cancer care: A qualitative study. Am J Manag Care. 2011;17:259–265. [PMC free article] [PubMed] [Google Scholar]
- 14.Aubin M, Vézina L, Verreault R, et al. Patient, primary care physician and specialist expectations of primary care physician involvement in cancer care. J Gen Intern Med. 2012;27:8–15. doi: 10.1007/s11606-011-1777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Clark MA, Cox JV, et al. Achieving coordinated care for patients with complex cases of cancer: A multiteam system approach. J Oncol Pract. 2016;12:1029–1038. doi: 10.1200/JOP.2016.013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klabunde CN, Han PK, Earle CC, et al. Physician roles in the cancer-related follow-up care of cancer survivors. Fam Med. 2013;45:463–474. [PMC free article] [PubMed] [Google Scholar]
- 17.Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: Results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32:1578–1585. doi: 10.1200/JCO.2013.51.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403–1410. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nekhlyudov L, Aziz NM, Lerro C, et al. Oncologists’ and primary care physicians’ awareness of late and long-term effects of chemotherapy: Implications for care of the growing population of survivors. J Oncol Pract. 2014;10:e29–e36. doi: 10.1200/JOP.2013.001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe LP, Alfano CM, Leach CR, et al. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30:2897–2905. doi: 10.1200/JCO.2011.39.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virgo KS, Lerro CC, Klabunde CN, et al. Barriers to breast and colorectal cancer survivorship care: Perceptions of primary care physicians and medical oncologists in the United States. J Clin Oncol. 2013;31:2322–2336. doi: 10.1200/JCO.2012.45.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber DE, Reimer T, Williams EL, et al. Resolving rivalries and realigning goals: Challenges of clinical and research multiteam systems. J Oncol Pract. 2016;12:1020–1028. doi: 10.1200/JOP.2016.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raja-Jones H. Role boundaries: Research nurse or clinical nurse specialist? A literature review. J Clin Nurs. 2002;11:415–420. doi: 10.1046/j.1365-2702.2002.00597.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilkes L, Cert R, Beale B. Role conflict: Appropriateness of a nurse researcher’s actions in the clinical field. Nurse Res. 2005;12:57–70. doi: 10.7748/nr2005.04.12.4.57.c5959. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenberger HE, Lillington L. Development of a measure to delineate the clinical trials nursing role. Oncol Nurs Forum. 2004;31:E64–E68. doi: 10.1188/04.ONF.E64-E68. [DOI] [PubMed] [Google Scholar]
- 26.Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477:526–528. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- 27.Gerber DE, Lakoduk AM, Priddy LL, et al. Temporal trends and predictors for cancer clinical trial availability for medically underserved populations. Oncologist. 2015;20:674–682. doi: 10.1634/theoncologist.2015-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia S, Bisen A, Yan J, et al. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J Thorac Oncol. 2017;12:1489–1495. doi: 10.1016/j.jtho.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimer T, Park ES, Hinsz VB. Shared and coordinated cognition in competitive and dynamic task environments: An information-processing perspective for team sports. Int J Sport Exerc Psychol. 2006;4:376–400. [Google Scholar]
- 30.Tschan F, Semmer NK, Junziker S, et al. Decisive action vs. joint deliberation: Different medical tasks imply different coordination requirementsinDuffy V.Advances in Human Factors and Ergonomics in Healthcare Boca Raton, FL: Taylor & Francis; 2011. p191 [Google Scholar]
- 31.Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204:533–540. doi: 10.1016/j.jamcollsurg.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Reimer T, Russell T, Roland C.Decision making in medical teamsinHarrison T, Williams EA.Organizations, Health, and Communication New York: Routledge; 2015pp 65-81 [Google Scholar]
- 33.Taylor MA, Parekh SG. Optimizing outpatient total ankle replacement from clinic to pain management. Orthop Clin North Am. 2018;49:541–551. doi: 10.1016/j.ocl.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Escobar MA, Brewer A, Caviglia H, et al. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia. 2018;24:693–702. doi: 10.1111/hae.13549. [DOI] [PubMed] [Google Scholar]
- 35.Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: Associations with survivorship care. J Natl Cancer Inst. 2013;105:1579–1587. doi: 10.1093/jnci/djt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramanian BA, Jetelina KK, Bowen M, et al. Surveillance for colorectal cancer survivors in an integrated safety-net health system in the United States. Int J Care Coord. 2018;21:26–35. doi: 10.1177/2053434518764634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson NR, West MA. Measuring climate for work group innovation: Development and validation of the team climate inventory. J Organ Behav. 1998;19:235–258. [Google Scholar]
- 38.Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: A review of survey instruments. Med Care. 2015;53:e16–e30. doi: 10.1097/MLR.0b013e31827feef6. [DOI] [PubMed] [Google Scholar]
- 39.Leach CW, Zomeren M, Zebel S, et al. Collective self-definition and self-investment: A two-dimensional framework of group identification. J Pers Soc Psychol. 2008;95:144–165. doi: 10.1037/0022-3514.95.1.144. [DOI] [PubMed] [Google Scholar]
- 40.Gaertner SL, Dovidio JF. Reducing Intergroup Bias: The Common Ingroup Identity Model. Psychology Press; 2014. New York. [Google Scholar]
- 41.Nurgat ZA, Craig W, Campbell NC, et al. Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer. 2005;92:1001–1005. doi: 10.1038/sj.bjc.6602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson MH. Differences between clinical trial efficacy and real-world effectiveness. Am J Manag Care. 2006;12:S405–S411. [PubMed] [Google Scholar]
- 43.Mathieu JE, Marks MA, Zaccaro SJ. Multi-team systems, in Anderson N, Ones DS, Sinangil HK, et al (eds): Organizational Psychology (vol 2): Handbook of Industrial, Work and Organizational Psychology. London, Sage, 2001, pp 289-313. [Google Scholar]
- 44.Centers for Medicare and Medicaid Services Medicare & Medicaid EHR incentive program: Meaningful use stage 1 requirements overview, 2010. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/downloads/MU_Stage1_ReqOverview.pdf.
- 45.Reimer T, Lee SJC, Garcia S, et al. Cancer center clinic and research team perceptions of identity and interactions. J Oncol Pract. 2017;13:e1021–e1029. doi: 10.1200/JOP.2017.024349. [DOI] [PMC free article] [PubMed] [Google Scholar]