Abstract
PURPOSE
We determined the prognostic factors and utility of allogeneic hematopoietic cell transplantation among children with newly diagnosed hypodiploid acute lymphoblastic leukemia (ALL) treated in contemporary clinical trials.
PATIENTS AND METHODS
This retrospective study collected data on 306 patients with hypodiploid ALL who were enrolled in the protocols of 16 cooperative study groups or institutions between 1997 and 2013. The clinical and biologic characteristics, early therapeutic responses as determined by minimal residual disease (MRD) assessment, treatment with or without MRD-stratified protocols, and allogeneic transplantation were analyzed for their impact on outcome.
RESULTS
With a median follow-up of 6.6 years, the 5-year event-free survival rate was 55.1% (95% CI, 49.3% to 61.5%), and the 5-year overall survival rate was 61.2% (95% CI, 55.5% to 67.4%) for the 272 evaluable patients. Negative MRD at the end of remission induction, high hypodiploidy with 44 chromosomes, and treatment in MRD-stratified protocols were associated with a favorable prognosis, with a 5-year event-free survival rate of 75% (95% CI, 66.0% to 85.0%), 74% (95% CI, 61.0% to 89.0%), and 62% (95% CI, 55.0% to 69.0%), respectively. After exclusion of patients with high hypodiploidy with 44 chromosomes and adjustment for waiting time to transplantation and for covariables in a Poisson model, disease-free survival did not differ significantly (P = .16) between the 42 patients who underwent transplantation and the 186 patients who received chemotherapy only, with an estimated 5-year survival rate of 59% (95% CI, 46.5% to 75.0%) versus 51.5% (95% CI, 44.7% to 59.4%), respectively. Transplantation produced no significant impact on outcome compared with chemotherapy alone, especially among the subgroup of patients who achieved a negative MRD status upon completion of remission induction.
CONCLUSION
MRD-stratified treatments improved the outcome for children with hypodiploid ALL. Allogeneic transplantation did not significantly improve outcome overall and, in particular, for patients who achieved MRD-negative status after induction.
INTRODUCTION
Hypodiploid acute lymphoblastic leukemia (ALL) with 44 chromosomes or fewer predicts a particularly poor prognosis.1-4 It comprises several distinct subtypes on the basis of modal chromosomal number: near haploidy (25 to 29 chromosomes) restricted to young children, low hypodiploidy (33 to 39 chromosomes) characterized by a higher incidence of structural abnormalities and older age at presentation, high hypodiploidy (42 to 43 chromosomes) with complex karyotypes, and high hypodiploidy with 44 chromosomes.5,6 In our previous study, 50 patients with 44 chromosomes had a mean ± standard error (SE) 8-year survival rate of 69% ± 6.7% that was superior to the eight with 40 to 43 chromosomes (50% ± 17.7%), which in turn had a better outcome than the 46 with low hypodiploidy (40% ± 10.1%) or the 46 with near haploidy (33.9% ± 7.6%).6 Near-haploid ALL is characterized by genetic alterations that target receptor tyrosine kinase signaling, Ras signaling, and the IKZF3 gene, whereas low-hypodiploid ALL has somatic genetic alterations that involve IKZF2; RB1; and, in particular, TP53 mutations, which often are inherited.7
Despite recent improvements in outcome of childhood ALL,8 patients with hypodiploid ALL have continued to fare poorly. In the Children’s Oncology Group AALL0031 (ClinicalTrials.gov Identifier: NCT0022737) study, the 4-year overall survival rate among the 41 patients with hypodiploid ALL was 54% ± 8%.9 Given the lack of obvious favorable prognostic indicators for hypodiploid ALL, many leukemia therapists continue to advocate allogeneic hematopoietic cell transplantation for these patients.10
Minimal residual disease (MRD) levels during induction and consolidation treatment have important prognostic and therapeutic implications, even within specific genetic subtypes of ALL.11-15 Of note, MRD-directed treatment in one clinical trial improved the outcome in two high-risk genetic subtypes6,9,16,17: Philadelphia chromosome–like18 and hypodiploid ALL.19 In that trial, all children with near-haploid or low-hypodiploid ALL achieved clinical remission with a 5-year event-free survival rate of 73.6%.19 Of the 13 patients with negative MRD (defined by a level < 10−4) at the end of a 6-week induction, 12 remained alive in remission after treatment with intensive chemotherapy only, with a 5-year event-free survival rate of 91.7%.19 In contrast, among the six patients with detectable MRD, only two remained in remission for more than 1.3 and more than 9.2 years. The latter was the only patient who underwent allogeneic transplantation on the basis of protocol recommendation for MRD greater than or equal to 10−2 at the end of induction.19 However, the results of this small study require validation. In the current study, we evaluated whether more recent treatments have improved outcome; identified potentially useful prognostic factors, including MRD; and assessed whether allogeneic transplantation benefits patients with hypodiploid ALL.
PATIENTS AND METHODS
Study Design and Patients
Data from patients with hypodiploid ALL up to 21 years of age and enrolled in clinical trials between 1997 and 2013 were collected from 16 cooperative study groups or single institutions (Appendix, online only; Appendix Table A1, online only). A DNA index (ratio of DNA content in leukemic G0/G1 cells v normal diploid G0/G1 cells) was used to define near haploidy and low hypodiploidy in the absence of karyotype if the result was less than 0.65 or greater than 0.65 to 0.82, respectively. Median follow-up time was 6.6 years (range, 105 days to 17 years). All clinical trials had been approved by review boards or ethics committees. Written informed consent was obtained from patients ages 18 years or older or from parents or guardians of younger patients.
Diagnosis and Risk Classification
Diagnosis of ALL was based on standard morphologic criteria, immunophenotype, and genetic features of leukemic blasts. All karyotypes were reviewed and classified by one of the authors (C.J.H.; Appendix; Appendix Table A2, online only) and designated according to the International System for Human Cytogenetic Nomenclature.20
Treatment
All patients were enrolled in intensive protocols. Eighteen of 183 patients with available information received prophylactic cranial irradiation, and 47 of 272 underwent allogeneic transplantation while in first remission, including both HLA-matched related donor (n = 11) and HLA-matched unrelated donor transplantations (n = 6); 30 patients had incomplete information on the type of transplantation.
Statistical Analysis
The Kaplan-Meier method was used to estimate the probabilities of event-free survival, disease-free survival, relapse-free survival, and overall survival (defined in the Appendix). Curves were compared with the log-rank test. The Cox proportional hazards regression model (stratified by groups) was used for multivariable analyses of prognostic factors. Estimated hazard ratios were reported with 95% CIs. Statistical methods were used to minimize potential bias in the comparison of outcome between patients treated with chemotherapy followed by transplantation and those who received intensive chemotherapy only. Kaplan-Meier curves were adjusted to account for the waiting time to transplantation with a landmark (median time to transplantation) and to account for delayed entry of patients into the transplantation group.21 To deal with the lack of proportional hazards, comparison between the two curves was performed at a predefined time point of 5 years on the basis of log-log transformation.22
To model the profile of the hazard ratio in time, we applied a piecewise Poisson model on disease-free survival (in intervals of 30 days), with transplantation treated as a time-dependent variable and with B-spline functions on two time scales (as detailed in the Appendix). Model-based disease-free survival estimates were derived with the major advantage of accounting for waiting times to transplantation on the basis of all available data without an arbitrary landmark time.23 On the basis of the result of the Poisson model, we applied a Cox model with treatment as a time-dependent variable both before and 1 year after complete remission to account for the nonproportionality of hazards and to adjust for relevant confounders (a frailty term accounts for the various groups involved).
RESULTS
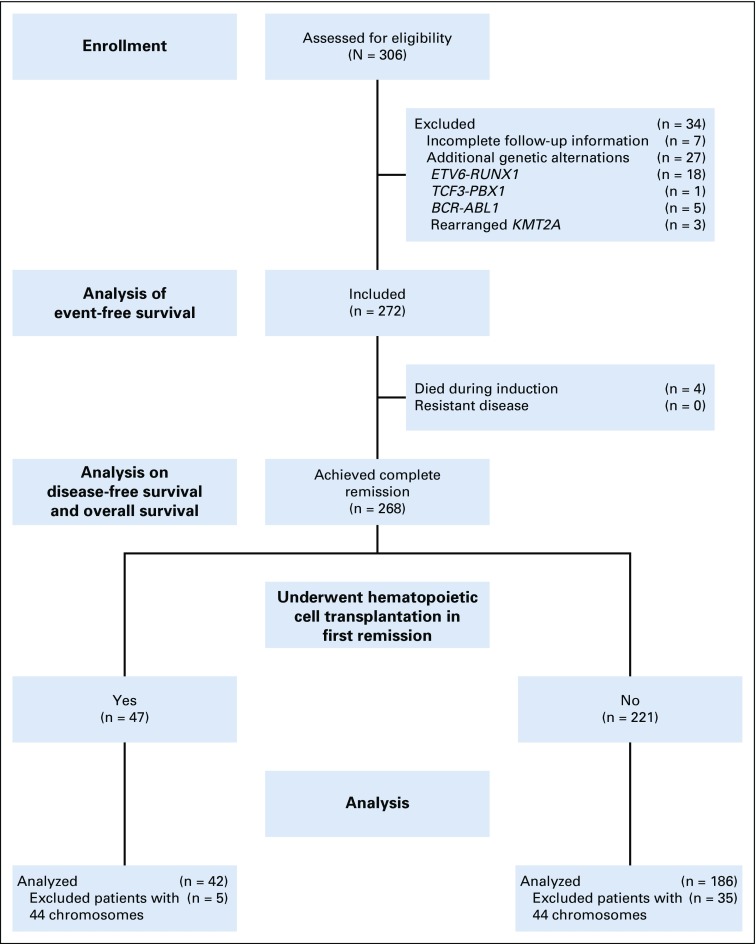
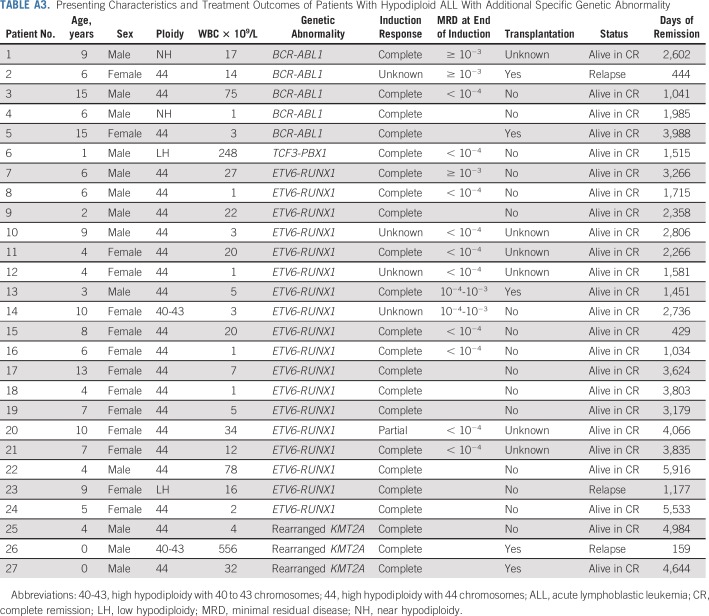
Among 306 patients, 272 were eligible for the study, seven were excluded because of incomplete follow-up, and 27 were excluded because of the concomitant presence of specific genetic alternations with known major prognostic significance (Fig 1; Appendix Table A3, online only).
FIG 1.
Study profile of number of patients assessed and number of patients not analyzed for event-free survival and disease-free survival.
Clinical and Laboratory Characteristics at Diagnosis
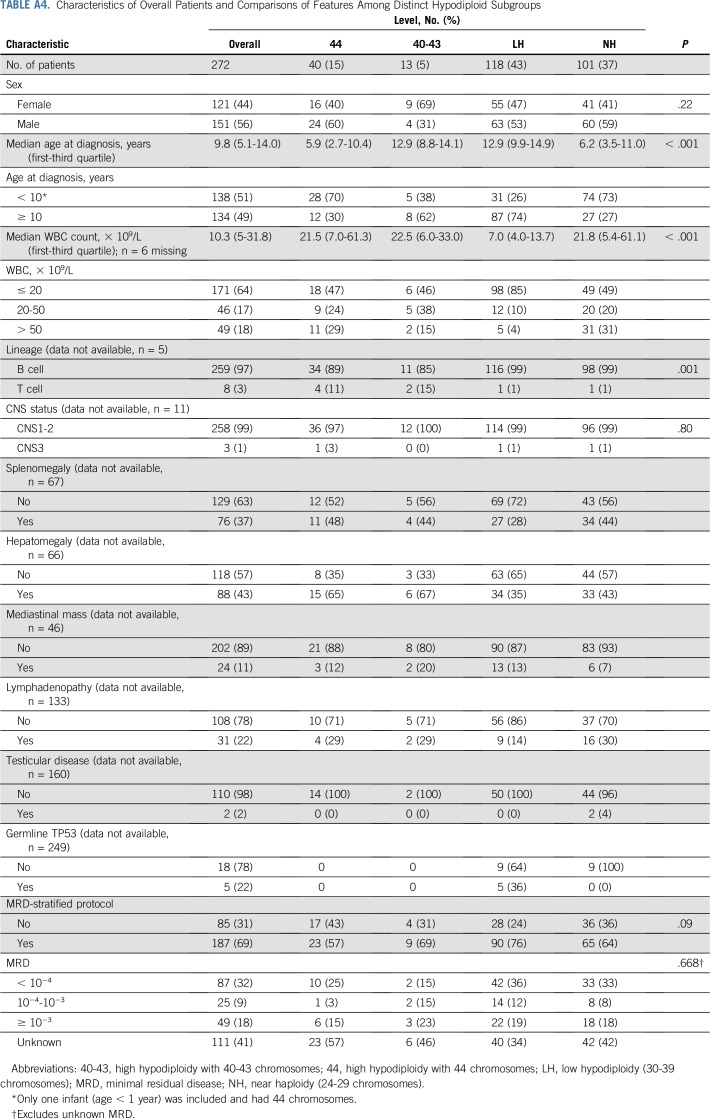
Appendix Table A4 (online only) lists classification of patients into various hypodiploid subgroups on the basis of karyotype and DNA index. One hundred one patients were near haploid, and 118 were low hypodiploid, with 17 and 22 patients, respectively, defined by DNA index only. High hypodiploidy with 44 chromosomes (n = 40) and high hypodiploidy with 40 to 43 chromosomes (n = 13) were less frequent. The cytogenetic characteristics of each ploidy group are listed in Appendix Table A2.
The entire cohort ranged in age from 0.6 to 19.5 years (median, 9.8 years). All patients had B-cell ALL, except six with high hypodiploidy, one with near haploidy, and one with low hypodiploidy. Patients with low hypodiploidy were characterized by older age (median, 12.9 years), low leukocyte count (median, 7.0 × 109/L), and a high incidence of germline TP53 mutations (five [36%] of 14 tested; Appendix Table A4).
Early Treatment Responses
Early response to induction therapy was determined in blood or bone marrow of all 272 patients at various time points by morphologic examination or by MRD assessment (2000 and subsequent years) according to the study design and guidelines (Appendix Table A1). As expected, a high proportion of patients had poor early response by morphologic examination (Appendix).
Of the 272 patients, four died during remission induction, whereas 268 (99%) achieved complete remission, including 12 patients who at the end of induction had greater than or equal to 5% blasts by morphology but reached remission in the following phases according to protocol definition. Altogether, MRD was determined at end of induction in 161 (59%) of the 272 patients by polymerase chain reaction (n = 141) or by flow cytometry (n = 20). MRD was less than 10−4 in 87 patients (54.0%), between 10−4 and 10−3 in 25 patients (15.5%), and greater than or equal to 10−3 in 49 patients (30.4%). More details of MRD are available in the Appendix.
Patterns of Treatment Failure
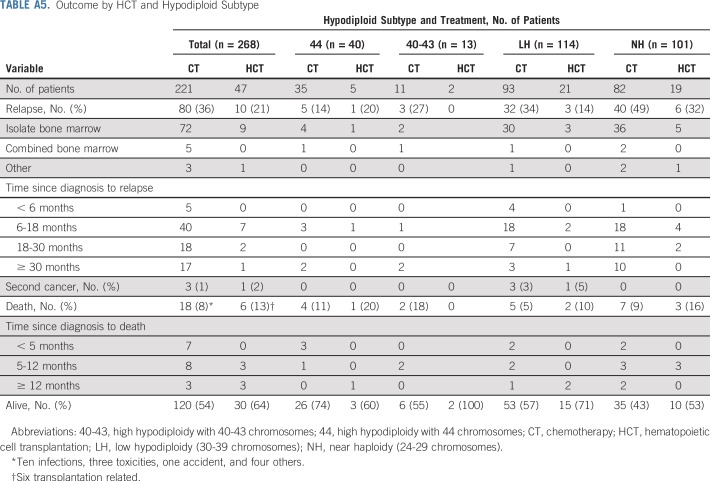
Relapse occurred in 90 patients as follows: isolated bone marrow (n = 81); combined bone marrow and extramedullary sites (n = 5), including CNS (n = 3), testes (n = 1), and parotid gland (n = 1); isolated testes (n = 1); and unknown site (n = 3). In addition, 24 patients died during first remission at a median time of 8 months (range, 2 to 56 months). The cause of death was related to the transplantation (n = 6), infection (n = 10), or other factors (n = 8). Second cancers developed as a first event in four patients, three of whom died as a result. On the date of last evaluation, 150 patients remained in continuous complete remission.
Prognostic Factors for Event-Free Survival and Overall Survival
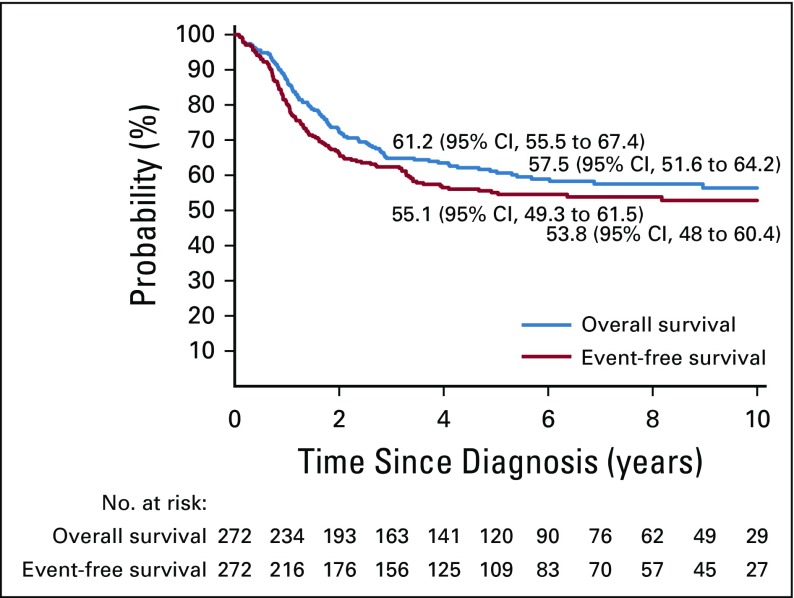
The estimated event-free survival rates were 55.1% (95% CI, 49.3% to 61.5%) and 53.8% (95% CI, 48.0% to 60.4%), whereas overall survival rates were 61.2% (95% CI, 55.5% to 67.4%) and 57.5% (95% CI, 51.6% to 64.2%) at 5 and 8 years, respectively, for the entire cohort of 272 patients (Appendix Fig A1, online only). After relapse, outcome was very poor, with a 2-year survival rate of 12% (95% CI, 6.7% to 21.2%).
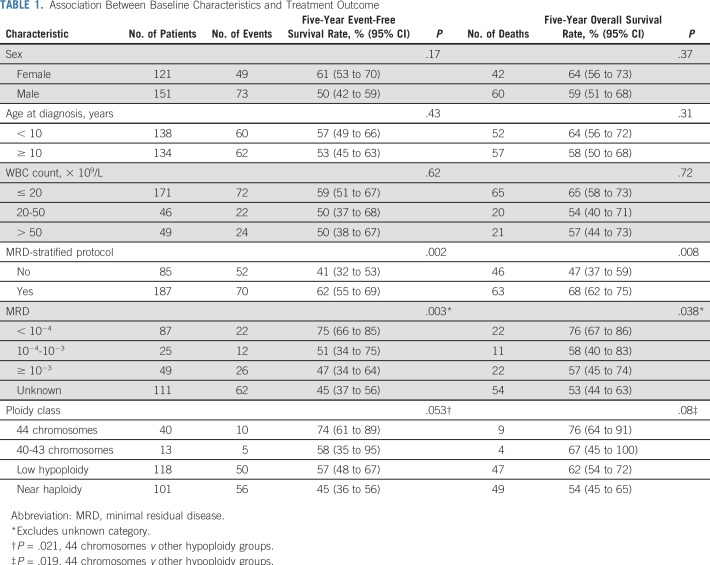
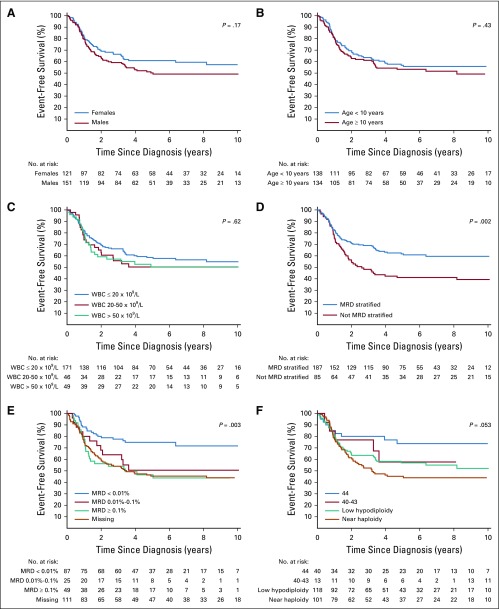
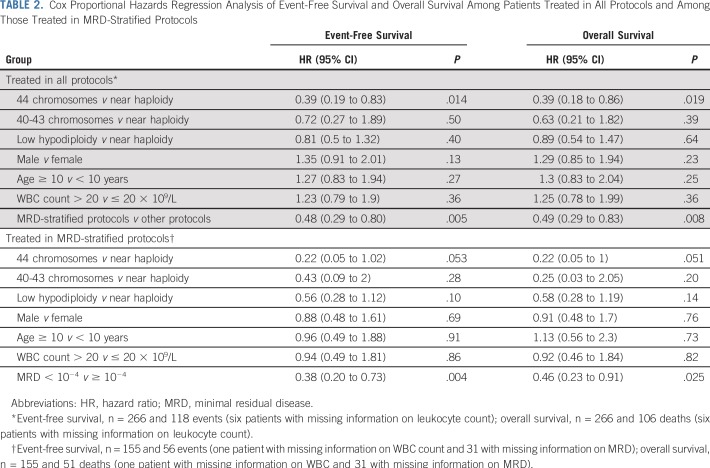
In univariable analysis, negative MRD status at the end of induction, high hypodiploidy with 44 chromosomes, and treatment with MRD-stratified protocols were associated with a favorable outcome as follows: 5-year event-free survival rates of 75% (95% CI, 66% to 85%), 74% (95% CI, 61% to 89%), and 62% (95% CI, 55% to 69%), respectively (Table 1; Fig 2). In the Cox analysis of event-free survival, after adjusting for sex, age, and leukocyte count, high hypodiploidy with 44 chromosomes and treatment with MRD-stratified protocols were associated with a favorable outcome. Similar results were observed in the Cox analysis of overall survival (Table 2). Because treatment with MRD-stratified protocols was mostly used in the recent treatment era, we also analyzed the independent prognostic impact of this factor by including treatment era into the multivariable analysis of event-free survival. Using the cutoff of 2003 for year of diagnosis (when most patients commenced treatment with MRD-stratified protocols), this factor remained significant (hazard ratio, 0.47; 95% CI, 0.24 to 0.92; P = .028), whereas treatment era did not achieve statistical significance (hazard ratio, 1.05; 95% CI, 0.58 to 1.89; P = .88). Similar results were obtained with the cutoff at 2007 (Appendix).
TABLE 1.
Association Between Baseline Characteristics and Treatment Outcome
FIG 2.
Kaplan-Meier estimates of event-free survival by sex (A) age at diagnosis (B) presenting WBC count (C) treatment with or without MRD-stratified protocols (D) MRD result (E) and hypodiploid subtype (F) high hypodiploidy with 44 chromosomes; high hypodiploidy with 40 to 43 chromosomes; low hypodiploidy with 30 to 39 chromosomes; near haploidy with 24 to 29 chromosomes. MRD, minimal residual disease.
TABLE 2.
Cox Proportional Hazards Regression Analysis of Event-Free Survival and Overall Survival Among Patients Treated in All Protocols and Among Those Treated in MRD-Stratified Protocols
In a subgroup analysis of patients treated with MRD-stratified protocols, only negative MRD status was independently associated with both superior event-free survival (hazard ratio, 0.38; 95% CI, 0.20 to 0.73; P = .004) and overall survival (hazard ratio, 0.46; 95% CI, 0.23 to 0.91; P = .025; Table 2). High hypodiploidy with 44 chromosomes showed a lower hazard with borderline significance in this analysis.
Impact of Transplantation on Survival
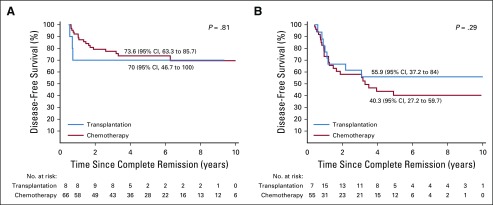
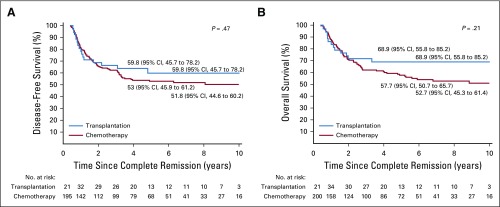
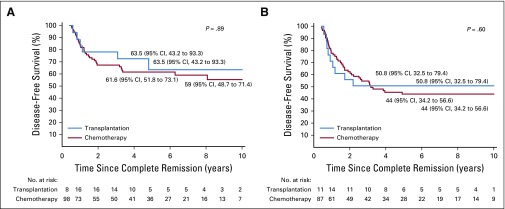
Appendix Table A5 (online only) shows the outcome according to treatment with chemotherapy only or chemotherapy plus transplantation for all patients and for those within various hypodiploid subtypes. Transplantation currently is not offered to patients with high hypodiploidy with 44 chromosomes because of their more favorable outcome. We therefore excluded the 40 patients within this subgroup from analysis of the impact of transplantation. Among the remaining 228 patients, 42 (18%) underwent transplantation. The curves originate at the median time (153 days) from completion remission to transplantation to adjust for bias related to early events. The adjusted 5-year disease-free survival rate was 53.0% (95% CI, 45.9% to 61.2%) for patients who received chemotherapy only and 59.8% (95% CI, 45.7% to 78.2%) for those who underwent transplantation (P = .47; Appendix Fig A2A, online only). No significant difference was found in adjusted 5-year overall survival rates between the two groups (57.7% [95% CI, 50.7% to 65.7%] v 68.9% [95% CI, 55.8% to 85.2%]; P = .21; Appendix Fig A2B). Similar results were obtained when analyses were limited to patients with low hypodiploidy or near haploidy (Appendix Fig A3, online only) or any subgroup according to the baseline characteristics as listed in Table 1 (data not shown). We then examined the impact of transplantation among patients who did or did not achieve MRD-negative status after induction. The 5-year adjusted disease-free survival was not significantly different among patients with MRD-negative status treated with chemotherapy or transplantation (P = .81) or among patients with MRD-positive status (P = .29, Fig 3).
FIG 3.
Comparison of disease-free survival among patients with fewer than 44 chromosomes according to treatment with chemotherapy only or additional transplantation and according to (A) a less than 10−4 or (B) greater than 10−4 minimal residual disease level at the end of remission induction. Curves originate at a landmark of 153 days (median time from complete remission to transplantation) to adjust for bias related to early events and account for delayed entry of patients into the transplantation group. The first set of No. at risk is at 0.42 years (153 days) since complete remission for both panels A and B.
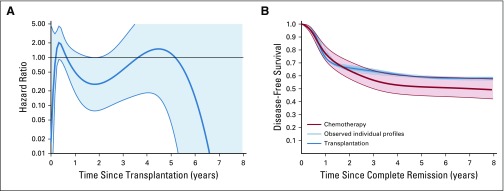
Because the proportional hazard assumption was not retained for the hazard of disease-free survival between patients treated with chemotherapy only and those treated with transplantation, we applied a Poisson model to study the profile of the hazard ratio over time (Appendix Fig A4A, online only). The hazard of failure for patients who underwent transplantation compared with those treated with chemotherapy showed a tendency to be higher only in the first year after transplantation and lower afterward. We derived a model-based estimate of disease-free survival for chemotherapy versus transplantation (Appendix Figure A4B), which does not rely on a landmark yet adjusts for waiting time. The two curves overlapped during the first 6 months of follow-up and then separated as a result of an increased hazard after transplantation (mainly transplantation-related mortality), whereas later (between 2 and 3 years), the transplantation curve plateaued at approximately 60%. The transplantation curve was always within the 95% confidence boundaries of the disease-free survival curve of the chemotherapy-only group. The estimated 5-year rate for the chemotherapy group was 51.5% (95% CI, 44.7% to 59.4%) versus 59% (95% CI, 46.5% to 75.0%) for the transplantation group, and the comparison, adjusted for MRD-stratified protocols and hypodiploid subtypes, was not significant (P = .16).
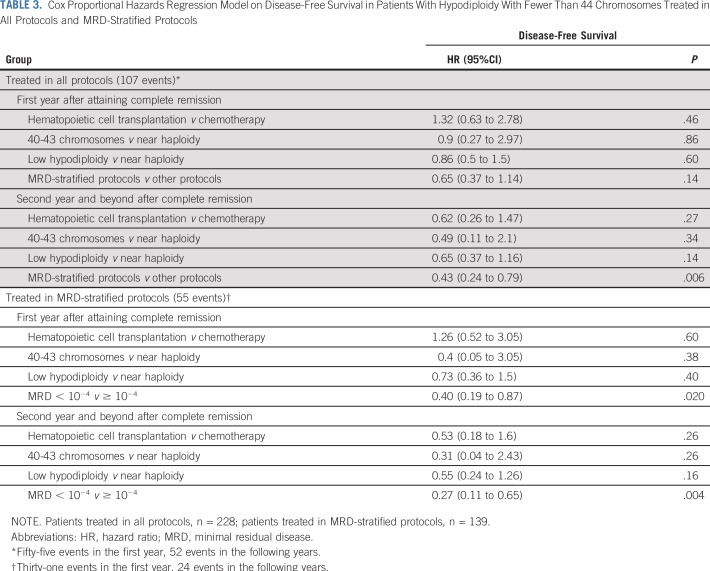
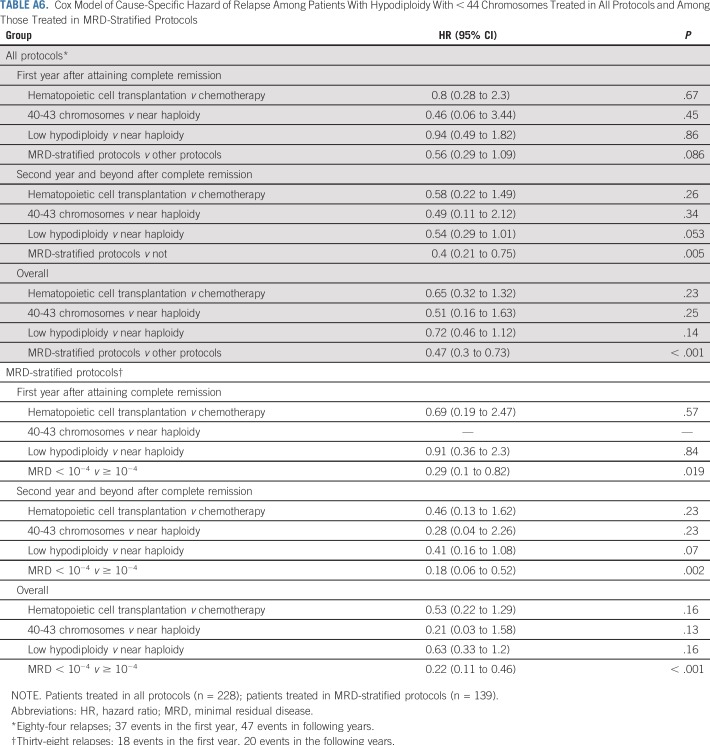
On the basis of Poisson results for the hazard ratio profile over time (Appendix Fig A4A), we applied a Cox model that split time at 1 year since completion of remission. When accounting for MRD-stratified protocols and hypodiploid subtypes (sex, age, and leukocyte count did not change the results in the model when included), transplantation lacked a significant impact on disease-free survival, whereas treatment with MRD-stratified protocols significantly improved disease-free survival in the second time period (after 1 year since transplantation; Table 3). Among patients treated with MRD-stratified protocols, those who attained an MRD-negative status had a significantly improved disease-free survival rate in both time periods (Table 3). Similar results were obtained in multivariable analysis of relapse-free survival (Appendix Table A6, online only).
TABLE 3.
Cox Proportional Hazards Regression Model on Disease-Free Survival in Patients With Hypodiploidy With Fewer Than 44 Chromosomes Treated in All Protocols and MRD-Stratified Protocols
DISCUSSION
In this largest study to our knowledge of childhood hypodiploid ALL to date, we have shown that high hypodiploidy with 44 chromosomes, MRD-negative status after remission induction, and treatment with MRD-stratified regimens were associated with a favorable prognosis and that transplantation failed to improve outcome, regardless of the response to remission induction. Despite a high clinical remission rate (99%), the 8-year event-free survival rate for the entire cohort was only 57.5%, whereas for the 40 patients with high hypodiploidy with 44 chromosomes it was 73.7%. These results were nonetheless superior to the 38.5% and 52.2% reported in our previous study.6 Because no new effective therapies have been developed recently for hypodiploid ALL, increased event-free survival in our current cohort may be partly attributed to MRD-stratified treatments which, by avoiding transplantation in patients with good response to chemotherapy or undertreatment of those with poor response to chemotherapy, has been shown to improve outcome in other subtypes of ALL.11,24,25 If all patients had received MRD-stratified treatment, the overall event-free survival rate likely would have been higher.
Similar to other subtypes of ALL,12,26 substantial heterogeneity existed among patients with hypodiploid ALL. The common finding that links the four disease subtypes is whole chromosome loss. Within the high-hypodiploid groups, whole chromosome loss is reduced, and partial chromosome deletions are greater in number. The high-hypodiploid group with 40 to 43 chromosomes is rare, and the boundary between it and the group with 44 chromosomes is not clear-cut. In any event, we have confirmed previous observations that high hypodiploidy with 44 chromosomes is associated with an improved outcome,6 which reinforces the need for urgent, full cytogenetic analysis to identify such patients.
This study confirmed that patients with near haploidy present at a younger age and that low hypodiploidy is associated with older age, low presenting leukocyte count, and a high rate of germline TP53 mutations.7 The presence of germline TP53 mutations among patients with low hypodiploidy is especially important because it not only conferred an increased risk of relapse but also was associated with the development of secondary cancer.28 In this regard, all four patients who developed secondary cancers in this study had low hypodiploid ALL (Appendix Table A5). Thus, all patients with low hypodiploidy should be tested for the germline TP53 mutation, a finding with important implications not only for the patients but also for their family members in terms of screening and genetic counseling.
This study also showed that hypodiploidy may accompany specific genetic abnormalities, which define known risk groups such as BCR-ABL1, TCF3-PBX1, ETV6-RUNX1, and KMT2A rearrangements (Appendix Table A3). As expected, the treatment outcomes of these patients closely reflected that associated with each specific genetic subtype. For example, all but one of the 18 patients with ETV6-RUNX1 mutation (with only one treated with transplantation) are alive in long-term continuous complete remission (Table A3). Thus, this subgroup of patients should be treated according to their driver mutations and MRD status after remission induction.
The favorable 5-year event-free survival rate (75%) achieved in the 87 patients with negative MRD status after remission induction concurred with the 85.1% reported for 14 patients in a previous study.19 Hence, transplantation is not indicated for patients with hypodiploid ALL with negative MRD status after remission induction. Indeed, no significant differences were found in the outcome among patients who were MRD negative treated with chemotherapy alone or with transplantation (Fig 3A).
Reliable data on the efficacy of transplantation in hypodiploid ALL are scarce. In our previous study, only nine of 30 patients underwent transplantation, and five of them had an adverse post-transplantation event.6 In the 2015 report by the Center for International Blood and Marrow Transplant Research, 78 children with hypodiploid ALL were identified to have undergone transplantation during first (n = 43) or subsequent (n = 35) remission.29 Multivariable analysis showed that transplantation during second remission, chromosome number of 43 or fewer, and an earlier transplantation period were associated with an inferior outcome. After adjustment for disease status and transplantation period, the 5-year leukemia-free survival rate was 37%, with an overall survival of 38% for the 39 patients with 43 or fewer chromosomes. In the current study, results of Cox modeling for the entire group of 228 patients with fewer than 44 chromosomes showed that disease-free survival did not differ significantly between the patients who underwent transplantation and those treated with chemotherapy alone (Table 3). However, although the hazard ratio was 0.62 for the comparison of disease-free survival in the second year or more into remission (Table 3), the wide 95% CI (0.26 to 1.47) shows high variability in outcome and suggests that our study is underpowered. In this regard, our study also was limited by retrospective data collection, heterogeneity in chemotherapy regimens and transplantation procedures, and being nonrandomized.
How can the outcome of patients with hypodiploid ALL with a poor response to induction therapy be improved? In a previous study, both near-haploid and low-hypodiploid ALL were shown to have activated Ras and phosphatidylinositol 3-kinase (PI3K) signaling, and inhibitors to PI3K and PI3K/mammalian target of rapamycin were demonstrated to inhibit proliferation of both near-haploid and low-hypodiploid cells ex vivo.7 Thus, these inhibitors could be explored as promising new therapeutic strategies for these patients. Recently described treatment options with chimeric antigen receptor–modified T cells and genetically modified antibodies for refractory B-cell ALL also may be useful.30,31
Appendix
Patients and Methods
Study Design and Patients
Patients were registered in clinical trials between January 1997 and December 2013. (Our previous Ponte di Legno study included patients diagnosed between 1986 and 1996.) This enrollment period was chosen specifically to include patients treated with more contemporary risk-directed therapy while allowing for sufficient follow-up. A predefined set of data, including clinical, biologic, and genetic characteristics; protocols, including treatment arm; early treatment responses, including minimal residual disease (MRD) level; and clinical outcomes were collected for each patient. All data were sent to a central coordinating center for review of consistency and completeness. Follow-up observations extended through 2017; 70% of patients without an event (first relapse or death) were followed for more than 5 years, and only seven deaths occurred beyond 5 years. By agreement, in this report, none of the data sets were linked to their participating groups.
Diagnosis and Risk Classification
In relation to karyotypes with 40 to 43 and 44 chromosomes, only patients with a precise chromosome count (rather than a range) were included. Patients were classified into four cytogenetic subgroups—near haploidy (23 to 29 chromosomes), low hypodiploidy (30 to 39 chromosomes), high hypodiploidy with 40 to 43 chromosomes, and high hypodiploidy with 44 chromosomes—on the basis of karyotype and DNA index and supplemented by fluorescence in situ hybridization and median chromosome number.
Statistical Analysis
Baseline characteristics are reported as percentages and compared using the χ2 test for categorical variables or as quartiles and compared with the Wilcoxon rank sum test for continuous variables. The principal end points in the analysis of treatment results were event-free survival, disease-free survival, relapse-free survival, and overall survival. Event-free survival time was calculated from diagnosis to the first failure, such as death during remission induction, induction failure as a result of drug resistance, a leukemia relapse in any site, death during initial remission, or second malignant neoplasm. Disease-free survival was defined as the time from the induction of complete remission until relapse, death during remission, or development of a second malignant neoplasm. Relapse-free survival was calculated from the time of complete remission to relapse in any site. Overall survival was considered the time from diagnosis (or from a complete remission, when stated) to death as a result of any cause. When no events occurred, the observation was censored at the time of last patient contact. The Kaplan-Meier method was used to estimate the probabilities of event-free survival, disease-free survival, relapse-free survival, and overall survival, with associated SEs calculated by the Greenwood method.
To minimize potential bias in the comparison of outcome between patients treated with chemotherapy followed by hematopoietic cell transplantation and those who received intensive chemotherapy only, the Kaplan-Meier curves were adjusted to account for the waiting time to transplantation: The curves originated at a landmark (median time to transplantation) and did not include patients who experienced events or whose data were censored before that time; the curves also were adjusted to account for the delayed entry of patients into the transplantation group, when transplantation occurred after the landmark.21 To deal with the lack of proportional hazards between the two treatment cohorts, univariable comparison between the two curves was performed at a predefined time point of 5 years since remission on the basis of log-log transformation.22
To model the profile of the hazard ratio in time, we applied a piecewise Poisson model on disease-free survival (in intervals of 30 days), with a random effect term denoting the study group to account for within-group correlation. In the model, transplantation was treated as a time-dependent variable: Each patient was included in the chemotherapy group until transplantation. The time since remission was modeled by a flexible B-spline function (6 degrees of freedom), whereas the time dependence of the treatment effect (ie, nonproportional hazards) was accommodated by including a term for interaction between treatment and time since transplantation (modeled as B-spline with one knot at 180 days). Model-based disease-free survival estimates of patients treated with transplantation or chemotherapy only also were derived, which accounted for the observed waiting time until transplantation. Analyses were carried out using R and SAS 9.4 (SAS Institute, Cary, NC) software programs.
Results
Cytogenetic characteristics.
The cytogenetic characteristics of each ploidy group were as follows. The near-haploid and low-hypodiploid subgroups typically were characterized by whole chromosome gains onto the haploid chromosome set. Doubling of the hypodiploid clone was visible at the karyotype level in most of the patients. Both high-hypodiploid subtypes were characterized by structural chromosomal rearrangements, often within highly complex karyotypes. Whole and partial chromosome losses predominantly contributed to the hypodiploid chromosome number in these subtypes. As previously reported,5 chromosomes 9 (37 of 50 patients with full karyotypes), 12 (20 of 50), and 7 (20 of 50) most frequently were involved within their abnormal karyotypes. Although loss of chromosome 7 was recorded in 11 of the 20 patients with chromosome 7 involvement, it could not be confirmed as true monosomy 7 because in most patients, other abnormalities that involved chromosome 7 were seen within the same karyotypes. Four patients with high hypodiploidy with 40 to 43 chromosomes showed a doubled population.
Early response by morphology.
A high proportion of patients had poor early response by morphologic examination. Of the 136 patients with data on blood blast cell count after 1 week (between 6 and 9 days) of glucocorticoid treatment and one dose of intrathecal methotrexate, 42 (31%) had a poor treatment response (ie, a blast cell count greater than 1,000/mm3). An M2 or M3 marrow (greater than or equal to 5% blasts) was seen in 41 (85%) of 48 patients tested after 1 week (between 6 and 9 days) and in 35 (22%) of 156 patients after 2 weeks (between 12 and 19 days) of induction treatment.
MRD assessment at the end of induction.
The MRD levels of the 12 patients who at the end of induction had greater than or equal to 5% blasts by morphology but reached remission in the following phases according to protocol definition were as follows: greater than or equal to 10−3 in six, between 10−4 and 10−3 in two, less than 10−4 in one, and missing in three.
Prognostic factors for event-free survival.
The analysis of the prognostic impact of MRD-stratified protocols also was adjusted by treatment era. Using the cutoff of 2007 (for year of diagnosis) when one half of the patients in this study had been enrolled in the protocols, treatment with MRD-stratified protocols remained significant (hazard ratio, 0.53; 95% CI, 0.28 to 0.98; P = .044), whereas treatment era still failed to achieve significance (hazard ratio, 0.88; 95% CI, 0.53 to 1.49; P = .64).
When the model listed in Table 2 was extended to include an interaction term between MRD and cytogenetic class, the interaction was not significant (P = .26), which means that the effect of MRD did not differ substantially among various cytogenetic groups.
FIG A1.
Kaplan-Meier estimates of event-free and overall survival for all 272 patients with hypodiploid acute lymphoblastic leukemia. The 5- and 8-year estimates are shown on the curves.
FIG A2.
(A) Disease-free survival and (B) overall survival of patients with fewer than 44 chromosomes who received chemotherapy only or underwent hematopoietic cell transplantation. Curves originate at a landmark of 153 days (median time from complete remission to transplantation) to adjust for bias related to early events and account for delayed entry of patients into the transplantation group. The first set of No. at risk is at 0.42 years (153 days) since complete remission for both panels A and B.
FIG A3.
Disease-free survival of patients with low hypodiploidy (A) and near haploidy (B) who received chemotherapy only or underwent hematopoietic cell transplantation. The 5- and 8-year estimates are shown on the curves. Curves originate at a landmark of 153 days (median time from complete remission to transplantation) to adjust for bias related to early events and account for delayed entry of patients into the transplantation group. The first set of No. at risk is at 0.42 years (153 days) since complete remission for both panels A and B.
FIG A4.
Poisson model-based estimates of (A) hazard ratio (heavy line) with 95% CIs (thin lines) of hematopoietic cell transplantation versus chemotherapy only by time since transplantation and (B) of disease-free survival between patients treated with chemotherapy only, with 95% CIs (thin lines) or with additional hematopoietic cell transplantation by time since complete remission (blue shade area represents 42 lines, each corresponding to one transplanted patient).
TABLE A1.
Number of Patients Enrolled in the Protocols With or Without MRD Stratification in Each Study Group or Institution
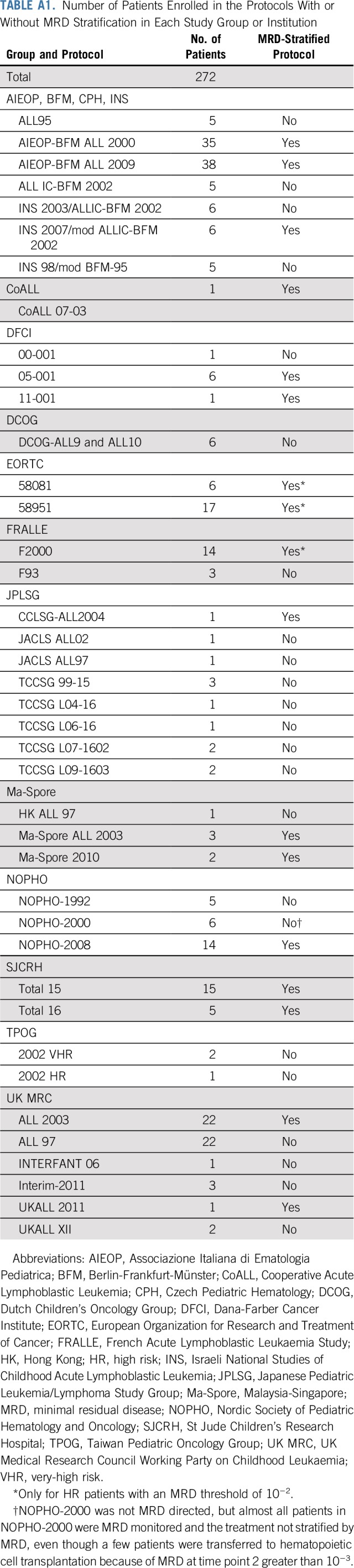
TABLE A2.
Karyotype Data and Genetic Classification of the Patients With Hypodiploidy in the Study
TABLE A3.
Presenting Characteristics and Treatment Outcomes of Patients With Hypodiploid ALL With Additional Specific Genetic Abnormality
TABLE A4.
Characteristics of Overall Patients and Comparisons of Features Among Distinct Hypodiploid Subgroups
TABLE A5.
Outcome by HCT and Hypodiploid Subtype
TABLE A6.
Cox Model of Cause-Specific Hazard of Relapse Among Patients With Hypodiploidy With < 44 Chromosomes Treated in All Protocols and Among Those Treated in MRD-Stratified Protocols
Footnotes
Supported by National Cancer Institute grants CA21765, CA36401, CA176063, and U01 GM92666; Bloodwise; and American Lebanese and Syrian Associated Charities. The funding agencies of individual study groups played no role in the study design, data collection, data analysis, data interpretation, or writing of the article.
See accompanying Editorial on page 763
AUTHOR CONTRIBUTIONS
Conception and design: Ching-Hon Pui, Martin Schrappe, Rob Pieters, Christine J. Harrison, Maria Grazia Valsecchi
Financial support: Ching-Hon Pui
Administrative support: Ching-Hon Pui
Provision of study material or patients: All authors
Collection and assembly of data: Ching-Hon Pui, Paola Rebora, Martin Schrappe, Andishe Attarbaschi, Andre Baruchel, Hélène Cavé, Sarah Elitzur, Katsuyoshi Koh, Hsi-Che Liu, Kajsa Paulsson, Rob Pieters, Lewis Silverman, Jan Stary, Allen Yeoh, Christine J. Harrison, Maria Grazia Valsecchi
Data analysis and interpretation: Ching-Hon Pui, Paola Rebora, Martin Schrappe, Andre Baruchel, Giuseppe Basso, Katsuyoshi Koh, Rob Pieters, Ajay Vora, Christine J. Harrison, Maria Grazia Valsecchi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcome of Children With Hypodiploid Acute Lymphoblastic Leukemia: A Retrospective Multinational Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ching-Hon Pui
Honoraria: Amgen, Bristol-Myers Squibb
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Amgen, Sanofi
Martin Schrappe
Research Funding: Shire (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Andre Baruchel
Leadership: DBV Technologies (I), Lysogene (I), MedDay Pharmaceuticals (I)
Honoraria: Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, Servier, Celgene
Research Funding: Shire (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Giuseppe Basso
Consulting or Advisory Role: Beckman Coulter, Becton Dickinson
Speakers’ Bureau: Beckman Coulter, Becton Dickinson
Rob Pieters
Honoraria: Erytech Pharma, Jazz Pharmaceuticals, Kite Pharma
Consulting or Advisory Role: Erytech Pharma, Kite Pharma, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Erytech Pharma, Kite Pharma, Jazz Pharmaceuticals, Servier
Lewis Silverman
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: Baxalta (Inst), Shire (Inst), Servier (Inst)
Ajay Vora
Consulting or Advisory Role: Jazz Pharmaceuticals, Pfizer, Medac Pharma, Amgen, Janssen Pharmaceuticals, Novartis
Travel, Accommodations, Expenses: Medac Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pui CH, Williams DL, Raimondi SC, et al. : Hypodiploidy is associated with a poor prognosis in childhood acute lymphoblastic leukemia. Blood 70:247-253, 1987 [PubMed] [Google Scholar]
- 2.Pui CH, Carroll AJ, Raimondi SC, et al. : Clinical presentation, karyotypic characterization, and treatment outcome of childhood acute lymphoblastic leukemia with a near-haploid or hypodiploid less than 45 line. Blood 75:1170-1177, 1990 [PubMed] [Google Scholar]
- 3.Heerema NA, Nachman JB, Sather HN, et al. : Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: A report from the Children’s Cancer Group. Blood 94:4036-4045, 1999 [PubMed] [Google Scholar]
- 4.Raimondi SC, Zhou Y, Mathew S, et al. : Reassessment of the prognostic significance of hypodiploidy in pediatric patients with acute lymphoblastic leukemia. Cancer 98:2715-2722, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Harrison CJ, Moorman AV, Broadfield ZJ, et al. : Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol 125:552-559, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Nachman JB, Heerema NA, Sather H, et al. : Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 110:1112-1115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmfeldt L, Wei L, Diaz-Flores E, et al. : The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45:242-252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pui CH, Yang JJ, Hunger SP, et al. : Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol 33:2938-2948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz KR, Devidas M, Bowman WP, et al. : Philadelphia chromosome-negative very high-risk acute lymphoblastic leukemia in children and adolescents: Results from Children’s Oncology Group Study AALL0031. Leukemia 28:964-967, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliansky DM, Camitta B, Gaynon P, et al. : Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: Update of the 2005 evidence-based review. Biol Blood Marrow Transplant 18:505-522, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Pui CH, Pei D, Coustan-Smith E, et al. : Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: A prospective study. Lancet Oncol 16:465-474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pui CH, Pei D, Raimondi SC, et al. : Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia 31:333-339, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor D, Enshaei A, Bartram J, et al. : Genotype-specific minimal residual disease interpretation improves stratification in pediatric acute lymphoblastic leukemia. J Clin Oncol 36:34-43, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrappe M, Bleckmann K, Zimmermann M, et al. : Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol 36:244-253, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. : Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol 34:2591-2601, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Roberts KG, Li Y, Payne-Turner D, et al. : Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 371:1005-1015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pui CH, Roberts KG, Yang JJ, et al. : Philadelphia chromosome-like acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 17:464-470, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts KG, Pei D, Campana D, et al. : Outcomes of children with BCR-ABL1–like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol 32:3012-3020, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullighan CG, Jeha S, Pei D, et al. : Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood 126:2896-2899, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaffer LG, Slovak ML and Campbell LJ (eds): An International System for Human Cytogenetic Nomenclature. Basel, Switzerland, Karger, 2009. [Google Scholar]
- 21.Simon R, Makuch RW: A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Stat Med 3:35-44, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Klein JP, Logan B, Harhoff M, et al. : Analyzing survival curves at a fixed point in time. Stat Med 26:4505-4519, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Rebora P, Galimberti S, Valsecchi MG: Using multiple timescale models for the evaluation of a time-dependent treatment. Stat Med 34:3648-3660, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Vora A, Goulden N, Mitchell C, et al. : Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): A randomised controlled trial. Lancet Oncol 15:809-818, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Attarbaschi A, Panzer-Grümayer R, Mann G, et al. : Minimal residual disease-based treatment is adequate for relapse-prone childhood acute lymphoblastic leukemia with an intrachromosomal amplification of chromosome 21: The experience of the ALL-BFM 2000 trial. Klin Padiatr 226:338-343, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Stanulla M, Dagdan E, Zaliova M, et al. : IKZF1plus defines a new minimal residual disease-dependent very-poor prognostic profile in pediatric B-cell precursor acute lymphoblastic leukemia. J Clin Oncol 36:1240-1249, 2018 [DOI] [PubMed] [Google Scholar]
- 27. Reference deleted.
- 28.Qian M, Cao X, Devidas M, et al. : TP53 germline variations influence the predisposition and prognosis of B-cell acute lymphoblastic leukemia in children. J Clin Oncol 36:591-599, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta PA, Zhang MJ, Eapen M, et al. : Transplantation outcomes for children with hypodiploid acute lymphoblastic leukemia. Biol Blood Marrow Transplant 21:1273-1277, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gökbuget N, Dombret H, Bonifacio M, et al. : Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 131:1522-1531, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]