Coronary embolism caused by the thrombus from guiding catheters is a rare and challenging complication, which may result in serious and fatal consequences due to the loss of blood flow of the coronary artery during percutaneous coronary intervention (PCI). Current strategies for the reduction of thrombus burden include pharmacological agents (typically glycoprotein IIb/IIIa platelet inhibitors), embolic protection devices (filters and distal balloon occlusion with aspiration), mechanical thrombectomy and manual or aspiration thrombectomy devices.[1] The above strategies are widely used as the first-line treatments, but have limitations for some refractory thrombus. A few techniques have been invented before to address this issue,[2]–[6] but they are not always effective or unavailable in some medical centers.
Here, we presented two cases of refractory coronary thrombus embolism originated from the guiding catheter. Large intracoronary thrombus poses a challenge during PCI. Following the failure of manual aspiration thrombectomy, the thrombus was finally removed by using a new method named “thrombus aspiration catheter-assisted twisting wire technique”.
Case 1. A 55-year-old male with no risk factors was admitted due to typical angina. The electrocardiogram showed ST-segment depression in V1-V4 leads, and the cardiac troponin TnI was negative. Coronary angiography revealed a severe bifurcation lesion in the proximal left anterior descending (LAD) with Medina 1.1.0 (Figure 1A) and no significant stenosis in the left circumflex artery (LCX) and the right coronary artery (RCA). PCI was performed using a 7Fr BL3.0 guiding catheter (Terumo, Tokyo, Japan) after infusion of 100 U/kg of heparin. Three guidewires (Runthrough NS, Terumo, Tokyo, Japan) were positioned into the LAD, LCX and the first diagonal (D1). After predilation with a 2.0 mm × 20 mm semicompliant balloon (Ryujin, Terumo, Tokyo, Japan), a 3.0 mm × 29 mm sirolimus-eluting stent (Nano, Lepumedical, Beijing, China) was deployed in the proximal LAD crossing D1. However, thrombus was observed at the distal part of the stent (Figure 1B). After postdilatation with a 3.25 mm × 15 mm non-compliant balloon (Hiryu, Terumo, Tokyo, Japan), a large amount of thrombus was found from the proximal segment of the LAD stent to left main (LM) by intravascular ultrasound (IVUS) (Figure 1C). Repeat angiography showed heavy thrombus burden both in LM and proximal LAD with antegrade TIMI III flow (Figure 1D). After careful suction of the guiding catheter, thrombectomy was performed several times using an aspiration catheter (Thrombuster II, Terumo, Tokyo, Japan), and intracoronary administration of Glycoprotein IIb/IIIa inhibitor was initiated as well. However, no visible thrombus was detected in the aspiration catheter, and the LAD was occluded with antegrade TIMI I flow (Figure 1E). Therefore, a Guidezilla Catheter (Boston Scientific, Natick, USA) was advanced to the proximal LAD to aspirate the thrombus (Figure 1F) and the flow in LAD was partially recovered. The thrombus burden was reduced in the proximal-middle LAD, but there was still residual thrombus burden in the distal segment of LAD (Figure 1G). Thrombectomy by a Thrombuster II aspiration catheter was attempted repeatedly, but still failed to remove the thrombus (Figure 1H).
Figure 1. Example use of the “thrombus aspiration catheter-assisted twisting wire technique” in the LAD.
(A): A severe bifurcation lesion in the proximal LAD with Medina 1.1.0; (B): thrombus was observed at the distal part of the stent (arrow); (C): a large amount of thrombus was found from the proximal segment of the LAD stent to LM by intravascular ultrasound; (D): heavy thrombus burden both in LM and proximal LAD with antegrade TIMI III flow (indicated by arrow); (E): LAD was occluded with antegrade TIMI I flow after thrombectomy using an aspiration catheter and intracoronary administration of Glycoprotein IIb/IIIa inhibitor; (F): thrombus aspiration by a Guidezilla Catheter in the proximal LAD (arrow); (G): residual thrombus burden in the distal segment of LAD with TIMI 0 flow; (H): failure to remove the thrombus by the aspiration catheter (arrow); (I): thrombus aspiration catheter-assisted twisting wire technique; (J): a large amount of thrombus was extracted from the guiding catheter; and (K & L): final results. LAD: left anterior descending; LM: left main.
Then, the aspiration catheter was advanced over the original wire distal to the thrombus. Another shaped guidewire (Sion, Asahi, Tokyo, Japan) as demonstrated in Figure 2 was advanced to the distal LAD through the central lumen of the aspiration catheter. The aspiration catheter was then pulled back into the guiding catheter. The second guidewire was rotated continuously in one direction until it was difficult to further rotate under fluoroscopy (Figure 1I), and the two guidewires were twisted to form a double helix. Subsequently, the twisted guidewires entrapped the entire thrombus and then the guidewires with the thrombus were carefully withdrawn into the guiding catheter. As shown in Figure 1J, a large amount of thrombus was successfully removed. After engaging a new guiding catheter, angiography revealed the final result was satisfactory (Figures 1K & 1L). Demonstration of the “thrombus aspiration catheter-assisted twisting wire technique” was shown in Figure 3. The patient was discharged after five days. No adverse events occurred during an 8-month follow-up period.
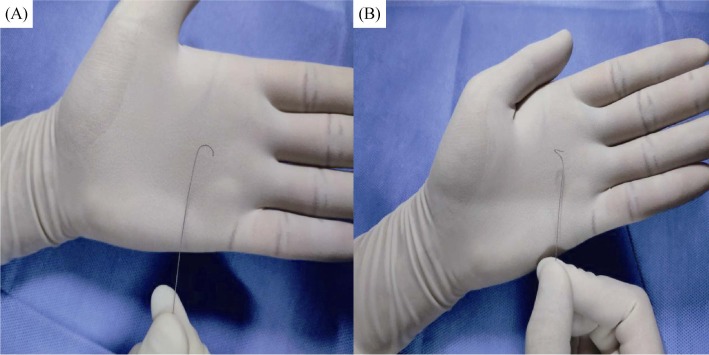
Figure 2. Demonstration of the “3D-shaped” tip of the second guidewire.
(A): The first U-shaped tip; and (B): the second U curve at a location perpendicular to the first one to form a “3D-shaped” tip.
Figure 3. Description of the “thrombus aspiration catheter-assisted twisting wire technique”.
(A): Advance the first guidewire (black) through the embolus; (B): advance the aspiration catheter over the wire and shape the head of the second guidewire (blue) into a “3D” pattern. Advance the second guidewire through the embolus as far as possible with the assistance of the thrombus aspiration catheter; (C): pull back the aspiration catheter into the guiding catheter; (D): rotate the second guidewire continuously in one direction; (E): withdraw the twisted guidewires with the retrieved embolus into the guiding catheter and pull the guiding catheter out; and (F): final results.
Case 2. A 60-year-old female was admitted for exertional angina. There was a severe stenosis in the distal LCX (Figure 4A). A 7Fr BL3.0 guiding catheter (Terumo, Tokyo, Japan) was engaged, and both LCX and obtuse marginal (OM) were crossed by two guidewires. After predilation with a 2.0 mm × 20 mm balloon (Ryujin, Terumo, Japan) in the LCX and jailing a 1.5 mm × 15 mm balloon (Ryujin, Terumo, Japan) in the OM, a 2.25 mm × 23 mm (Firehawk, MicroPort Medical Co, Shanghai, China) and a 2.75 mm × 26 mm (Resolute, Minneapolis, MN, USA) drug-eluting stents were deployed in the LCX. Kissing balloon dilatation was performed with a 2.5 mm × 15 mm non-compliant balloon and a 2.0 mm × 15 mm semicompliant balloon (Figure 4B). Thrombus was present in LM to LCX ostium (Figure 4C) and unfortunately, the thrombus was pushed into LCX while performing the angiography, causing the complete occlusion of LCX (Figure 4D). Thrombectomy using a Thrombuster II aspiration catheter and intracoronary administration of tirofiban were ineffective. Therefore, we performed “thrombus aspiration catheter-assisted twisting wire technique” as described in Case 1 (Figure 4E), and the thrombus was successfully removed (Figure 4F). Thrombus burden was significantly reduced, but the OM ostium was still occluded (Figure 4G). After rewiring and kissing balloon dilatation (Figure 4H), TIMI III flow was achieved in the OM and only a small amount of thrombus was left in the LM to ostial LCX (Figure 4I). A Guidezilla Catheter (Boston Scientific, Natick, USA) was advanced to the LM for aspiration (Figure 4J), and the thrombus was successfully removed (Figures 4K & 4L). The patient was discharged after five days. No adverse events occurred during a 2-month follow-up period.
Figure 4. Example use of the “thrombus aspiration catheter-assisted twisting wire technique” in the LCX.
(A): A severe stenosis in the distal LCX; (B): kissing balloon dilatation; (C): blurring thrombus in the LM (arrow); (D): large thrombus burden was pushed into the LCX with the injection of contrast, causing complete occlusion of the vessel, and the subsequent thrombectomy was ineffective; (E): thrombus aspiration catheter assist twisting wire technique; (F): large organized thrombus was extracted; (G): significantly decreased in the thrombus burden and complete occlusion of the OM ostium; (H): kissing balloon was performed; (I): TIMI III flow in OM and reduced thrombus in the LM to the ostial LCX (arrow); (J): aspiration by the Guidezilla Catheter (arrow); and (K & L): final results. LCX: left circumflex artery; LM: left main; OM: obtuse marginal.
In previous studies, catheter-related thrombosis was considered as a rare complication of PCI, only occurred in 0.4%–0.9% of the PCI procedures.[7],[8] However, a recent study showed that the prevalence of thrombus in the guiding catheter was about 60% if detected by optical coherence tomography, which might be attributed to the use of the imaging modality for thrombus detection. The occurrence of thrombus in the guiding catheter is associated with gender, acute coronary syndrome, frequency of the second dose of heparin, total procedural time, and time lag between each dose of heparin administered.[9]
In our cases, the reason for thrombus formation was not very clear. We did not measure activated coagulation time (ACT) due to the lack of equipment, but a large amount of thrombus was found by IVUS and the thrombus was pushed into coronary while performing the angiography. Furthermore, a large amount of thrombus was extracted from the guiding catheter in both cases. Thrombus formation could probably be associated with the insufficient anticoagulation. A possible explanation of this problem might be the individual variability of heparin clearance from the bloodstream.[10] This raises the importance of performing ACT during PCI, particularly in patients at higher thrombosis risks.[11]
The primary treatment of coronary embolism is to recover the coronary flow as quickly as possible, and thrombectomy with manual or mechanical aspiration devices and intracoronary administration of Glycoprotein IIb/IIIa inhibitors are commonly used.[1] In addition, we reported the use of 5F child catheter for successful retrieval of coronary refractory thrombus.[12] The 5F child catheter is suitable for proximal coronary thrombus, but it is difficult to advance the catheter into the distal coronary due to the larger diameter.
In the above described cases, both the aspiration catheter and the 5F child catheter failed to reduce the thrombus burden. One main reason is that the thrombus formed in the guiding catheter is partially organized and is not easy to be extracted by conventional aspiration catheters. Moreover, the 5F child catheter could not be delivered to the distal coronary artery. To solve the problem, we invented the “thrombus aspiration catheter-assisted twisting wire technique” to remove the refractory coronary embolism in small, inaccessible vessels. The details of this method are described in Figure 2 & 3.
The underlying mechanism of this technique is to entrap the thrombus with the helix of twisted guidewires. However, there are two major differences in comparison to the original “twisting wire technique”. To fully capture the partially organized thrombus, the second guidewire must be specially shaped to form a tight hinge, which is more effective than the conventional “twisting wire technique” in fixing the thrombus. Due to the special form, the second guidewire can only be advanced through the over-the-wire chamber of an aspiration catheter or a double lumen microcatheter. There are a number of advantages with this technique, such as easy operation, short learning curve, less procedure time, low cost, suitable for promotion at all levels of medical centers and etc.
“Thrombus aspiration catheter-assisted twisting wire technique” is an effective and safe method to remove the thrombus from the guiding catheter in distal coronary arteries when conventional treatments fail. It is highly deliverable via the thrombus aspiration catheter and is quite safe with no intraprocedural or post-procedural complications. Given these promising merits, further studies are needed to assess clinical and safety outcomes of this method of thrombectomy in PCI.
Acknowledgments
All authors had no conflicts of interest to disclose.
References
- 1.Boztosun B, Acar RD. Treating thrombus in the coronary arteries. Herz. 2015;40:60–65. doi: 10.1007/s00059-013-3920-1. [DOI] [PubMed] [Google Scholar]
- 2.Khoo DZL, Lee JH, Watson TJ, et al. The Solitaire device-on the cards for retrieval of recalcitrant thrombus in acute coronary syndrome. EuroIntervention. 2019;14:e1834–e1835. doi: 10.4244/EIJ-D-18-00234. [DOI] [PubMed] [Google Scholar]
- 3.Crimi G, Moramarco L, Mandurino-Mirizzi A, et al. The combined use of stent retriever and neuro-aspiration as successful bail-out reperfusion strategy in a patient with embolic myocardial infarction. Catheter Cardiovasc Interv. 2019;94:E78–E81. doi: 10.1002/ccd.28167. [DOI] [PubMed] [Google Scholar]
- 4.Dauvergne C, Araya M, Uriarte P, et al. ‘Mother-in-child’ thrombectomy technique: a novel and effective approach to decrease intracoronary thrombus burden in acute myocardial infarction. Cardiovasc Revasc Med. 2013;14:14–17. doi: 10.1016/j.carrev.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 5.van de Hoef TP, Woudstra P, Sprengers ME, et al. First-in-man intracoronary use of the Trevo® Pro 4 mechanical thrombectomy device for the retrieval of large intracoronary thrombus in patients with acute coronary syndromes. EuroIntervention. 2013;9:505–509. doi: 10.4244/EIJV9I4A81. [DOI] [PubMed] [Google Scholar]
- 6.Gracida M, Romaguera R, Jacobi F, et al. The MGuard coronary stent: safety, efficacy, and clinical utility. Vasc Health Risk Manag. 2015;11:533–539. doi: 10.2147/VHRM.S68007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–1476. doi: 10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Mehta SR, Chrolavicius S, et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519–1530. doi: 10.1001/jama.295.13.joc60038. [DOI] [PubMed] [Google Scholar]
- 9.Scalone G, Brugaletta S, Garcia-Garcia HM, et al. Frequency and predictors of thrombus inside the guiding catheter during interventional procedures: an optical coherence tomography study. Int J Cardiovasc Imaging. 2015;31:239–246. doi: 10.1007/s10554-014-0544-3. [DOI] [PubMed] [Google Scholar]
- 10.Uprichard J, Manning RA, Laffan MA. Monitoring heparin anticoagulation in the acute phase response. Br J Haematol. 2010;149:613–619. doi: 10.1111/j.1365-2141.2010.08129.x. [DOI] [PubMed] [Google Scholar]
- 11.Mottillo S, Filion KB, Joseph L, et al. Defining optimal activated clotting time for percutaneous coronary intervention: a systematic review and Bayesian meta-regression. Catheter Cardiovasc Interv. 2017;89:351–366. doi: 10.1002/ccd.26652. [DOI] [PubMed] [Google Scholar]
- 12.Sheng L, Li JQ, Sun DH, et al. Removal of refractory thrombus by 5F child catheter in patients with subacute myocardial infarction. J Geriatr Cardiol. 2019;16:168–172. doi: 10.11909/j.issn.1671-5411.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]