Abstract
Fragile X–associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that usually begins in the early 60s and affects carriers of premutation expansion (55–200 CGG repeats) of the fragile X mental retardation 1 (FMR1) gene. Additional disorders can co-occur with FXTAS including Alzheimer’s disease (AD). Here we discuss a case report of a male with 67 CGG repeats in FMR1 who had mild late-onset FXTAS symptoms followed by neurocognitive disorder symptoms consistent with AD. The patient has developed tremor and ataxia that are the two characteristic symptoms of FXTAS. In addition, he shows rapid cognitive decline, brain atrophy most substantial in the medial temporal lobe, and decreased metabolism in the brain regions that are the characteristic findings of AD. The purpose of this study is to describe a patient profile with both diseases and review the details of an overlap between these two diseases.
Keywords: FXTAS, Alzheimer’s disease, cognitive decline, neurocognitive disorder, premutation, neurogenetics
Introduction
Fragile X–associated tremor/ataxia syndrome (FXTAS) is a late onset condition in carriers of the premutation in the fragile X mental retardation 1 (FMR1) gene that affects 40% of males and 16% of females.1 The most common features of FXTAS are intention tremor, ataxia, memory and executive function deficits, autonomic dysfunction, and brain atrophy with white matter disease.2 Patients with early FXTAS usually present with autonomic dysfunction such as erectile dysfunction and orthostatic hypotension. Cognitive decline can rarely be the first sign of FXTAS while usually an intention tremor is the first sign.3,4
There are different types of molecular dysfunction that play a role in the pathophysiology of FXTAS, although RNA toxicity caused by elevated levels of the FMR1-mRNA is the most salient. The elevated mRNA can sequester proteins important for neuronal function and form intranuclear inclusions in neurons and astrocytes throughout the brain and in the peripheral nervous system.5,6 In addition, mitochondrial deficits,7 calcium dysregulation,8 iron sequestration,9,10 chronic DNA damage repair,2 microbleeds and the production of a toxic peptide FMRpolyG due to RAN translation11 can occur in the brain. These processes can lead to enhanced cell death of neurons and astrocytes leading to brain atrophy and white matter disease in those with FXTAS.
There are different neurodegenerative disorders which can co-occur with FXTAS including Alzheimer’s disease (AD),12,13 Parkinson’s Disease14 and multiple sclerosis.15 AD is also characterized by cognitive deficits including encoding and recall of new information, agnosia and impaired reasoning, judgement and problem solving.16 The most important difference between FXTAS neurocognitive disorder and AD neurocognitive disorder is that the language performance is lower in AD than FXTAS. On the other hand, executive functions are lower in FXTAS than AD.12 There are several factors that affect the progression of both diseases. The ApoE4 allele is one of the strongest genetic risk factors that predisposes to AD. Although this patient does not have the ApoE4 allele, he has a rapidly progressing neurocognitive disorder which can be suggestive of an overlap between FXTAS and AD.
In this paper, we describe a patient with the premutation and FXTAS who has had a significant progressive cognitive decline over the last 4 years. He developed tremor and ataxia which are characteristic of FXTAS. He also demonstrated rapidly declining IQ and memory testing scores during his follow-up over 10 years. These findings in addition to the neuroimaging showing brain atrophy, white matter disease, and decreased brain metabolism are consistent with a diagnosis of both FXTAS and AD. The purpose of this case study is to demonstrate a profile of a patient with both conditions. We investigated a possible exacerbation in the progression of these two neurodegenerative diseases when there is an overlap between these two disorders.
Methods
Patient has signed a written informed consent for imaging, molecular testing, cognitive testing and publication approved by the institutional IRB. The segmentation of brain structures on the MRI scans was performed automatically using Brain GPS27 followed by machine learning-based error correction28 and manual editing.
Clinical Presentation
This case is an 84 year old male carrier of a FMR1 premutation allele of 67 CGG repeats with approximately 2-fold higher FMR1 mRNA expression levels. He was first suspected to develop FXTAS about 10 years ago. His initial symptoms were a mild intention tremor which began intermittently at age 74 and ataxia that began at age 75. His tremor started after his prostatectomy surgery for prostate cancer that involved inhalant anesthesia. He also noticed mild imbalance with his walking but he denied other symptoms of ataxia. His tremor and ataxia worsened after he developed chronic urinary tract infections. Since that time, he also had a mild but progressive renal failure. At age 76, he started to feel numbness in both feet and right thigh. At age 77, he was diagnosed with severe anemia due to chronic kidney disease, in addition to mitral valve prolapse. He was treated with epoetin alfa, oral iron, and iron injections and his hematocrit increased but his tremor worsened. He began to experience inflammation in his joints particularly his fingers. He was also diagnosed with sleep apnea at age 77 and he started to use a continuous positive airway pressure (CPAP) machine. He tore his right shoulder rotator cuff and he was treated with cortisone injections. He reported that his ongoing stiffness was improved with this treatment.
At age 78, his tremor improved with propranolol treatment. At the age of 79, he reported a decrease in overall stamina and started to nap about 4 times a day. His sense of hearing started to decrease on his left side. His anemia became worse and he started to take iron supplements again. Low energy and fatigue were his primary concerns. At age 82 he reported that he had symptoms of lightheadedness on a regular basis and he had memory problems but he was in the normal range in the cognitive testing (see Table 1). After a year, he started to undergo significant cognitive changes particularly in both long term and short term memory. He had problems with remembering his routines at his temple and with his family. His executive function abilities started to diminish (Table 1). His daughters reported that he was more withdrawn, less verbal, less interactive and less interested in the activities that were going on around him. He also started to overexpress his emotions. He had problems with insomnia for several years even with the CPAP. Currently, he is 84 years old and he has a significant cognitive decline which accelerated 2 years ago. He continues to have problems in word retrieval and focusing his attention. He hardly initiates conversations and he sleeps more during the day. He, however, continues to work out regularly which is a routine that began even before the onset of FXTAS. His wife reports that he sometimes sits on the couch spending hours looking at the wall and playing with his dog. He has high blood pressure which is commonly seen in premutation carriers particularly with FXTAS. He also has an affectionate behavior to all people around him related to his increasing frontal disinhibition. His ataxia was helped by balance therapy administered by his PT. He has a history of major depressive disorder at age 52 which was treated by escitalopram oxalate 20 mg and cognitive behavioral therapy.
Table 1.
The Results of IQ and Memory Testing Over Time
| Time of Testing | Age of Testing | WAIS III/IV VCI | WAIS III/IV PRI | WAIS III/IV WMI | WAIS III/IV PSI | WAIS III/IV FSIQ | BDS-2 | MMSE | WMS III/IV AMI | WMS III/IV VMI | WMS III/IV IMI | WMS III/IV DMI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 84 | 76 | 96 | 92 | 108 | 89 | 9/27 | 22 | - | - | - | - |
| 2017 | 83 | 81 | 102 | - | - | - | 24/27 | 28 | - | - | - | - |
| 2016 | 82 | 91 | 113 | 102 | 122 | 107 | 20/27 | 28 | 100 | 98 | 106 | 91 |
| 2014 | 80 | 102 | 105 | 102 | 124 | 109 | 25/27 | 29 | 95 | 100 | 97 | 96 |
| 2011 | 77 | - | - | - | - | - | 15/27 | 28 | 111 | - | - | 114 |
| 2009 | 75 | - | - | - | - | - | 25/27 | 28 | - | - | - | - |
| 2008 | 74 | 109 | 123 | 109 | 125 | 116 | 19/27 | 29 | 105 | - | - | 102 |
Abbreviations: WAIS, Wechsler Adult Intelligence Test; WMS, Wechsler Memory Scale; VCI, Verbal Comprehension; PRI, Perceptual Reasoning; WMI, Working Memory; PSI, Processing Speed; FSIQ, Full Scale IQ; AMI, Auditory Memory; VMI, Visual Memory; IMI, Immediate Memory; DMI, Delayed Memory; BDS2, Behavioral Dyscontrol Scale2; MMSE, Mini-Mental State Examination.
The family history is significant for the death of his brother from FXTAS in 2016, his daughters are carriers of the FMR1 premutation, and he has a grandson with a full mutation causing fragile X syndrome. The patient reported experiencing the death of his brother as a major life stressor.
In 2018, on his examination, his blood pressure was 150/80. His weight was 73.9 kg and height was 177.8 cm. His hearth rate was 80. On his eye examination, he had normal eye movements but he had pinpoint pupils that were minimally reactive to light and he had a gray ring at his corneal margin. He had a mildly hypoactive gag and his cranial nerves showed decreased hearing bilaterally at approximately 20% of normal. He had a bifid uvula. His rebound with pressure to arm extension was approximately 6 inches, his muscle strength was normal but his reflexes were absent in all extremities along with an absent vibration sense in both feet. He had normal temperature sensation in all extremities and a negative Babinski reflex bilaterally. He demonstrated a mild fine tremor in the right and left hand during finger to nose testing but no resting tremor. His arm swing was normal with his walking but he had difficulty in tandem walking for greater than 3 steps. He had a positive snout reflex for greater than 4 tabs, and a positive palmomental reflex with stroking the right palm.
At present, he is on losartan 50 mg bid and propranolol 20 mg bid for his high blood pressure. He is on donepezil 10 mg qd and memantine 10 mg bid for his cognitive dysfunction. He takes sertraline 50 mg qd although he denies current depression. He also takes omeprazole 20 mg qd, aspirin 81 mg qd, simvastatin 40 mg qd and iron heme polypeptide for his iron deficiency anemia. He is on several eye drops including bimatoprost, brinzolamide, brimonidine tartrate and timolol maleate for his glaucoma. His supplements include a multiple vitamin without iron, Folic Acid 800 mcg qd, a probiotic, calcium plus Vitamin D and B12 500 mcg per day along with an additional omega-3 tablet.
Results
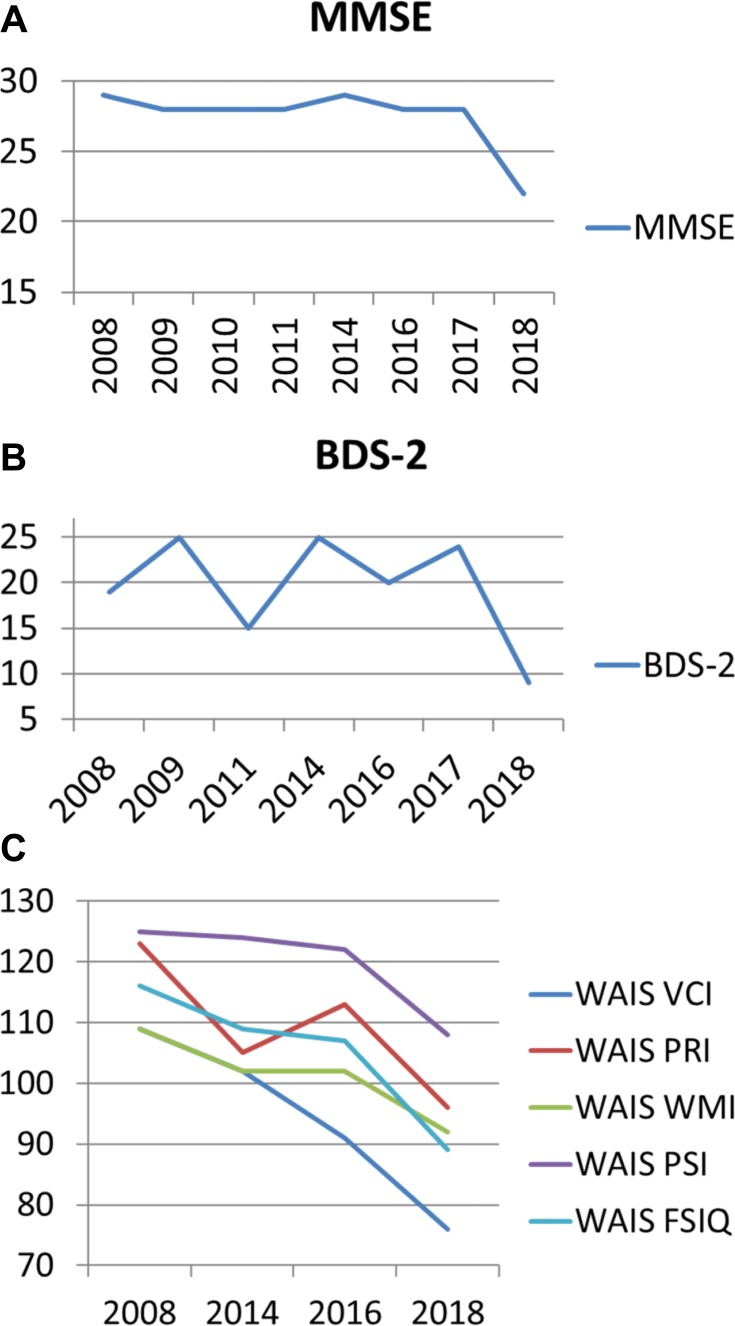
Results of IQ and memory testing indicated a rapid decline during a 10 year follow-up (Table 1, Figure 1A–C). The patient showed a 33 point difference in verbal comprehension, a 27 point difference in perceptual reasoning, a 17 point difference in working memory, a 17 point difference in processing speed and a 27 point difference in his full scale IQ scoring over 10 years. In addition, his behavioral dyscontrol scale showed a 10 point difference and his mini mental state examination demonstrated a 7 point difference. This rapid and significant IQ loss is suggestive of progressive neurodegenerative disorders, such as AD and FXTAS.
Figure 1.
(A–C) Changes in IQ and memory over 10 years follow-up.
Abbreviations: WAIS- Wechsler Adult Intelligence Test; VCI- Verbal Comprehension; PRI- Perceptual Reasoning; WMI- Working Memory; PSI- Processing Speed; FSIQ- Full Scale IQ; BDS-2 Behavioral Dyscontrol Scale-2; MMSE- Mini-Mental State Examination.
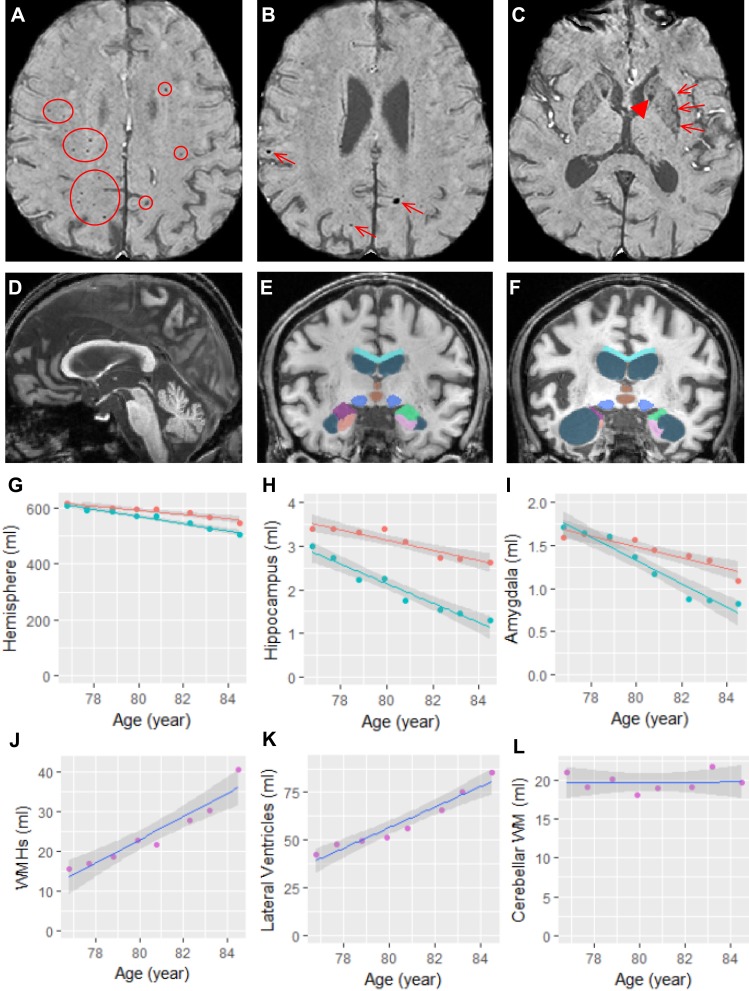
On his MRI, features of cerebral small vessel disease (CSVD)17 including enlarged perivascular spaces, lacune, and white matter hyperintensities (WMHs), are found as early as age 74 before the onset of ataxia. His susceptibility-weighted imaging (SWI) scans acquired at age 78 and 79 years show many cerebral microbleeds in the white matter and all four lobes. Iron depositions are also shown in the caudate nucleus, basal ganglia, substantia nigra, and cerebellar dentate nucleus (Figure 2A–C). Volumetric analysis performed on high-resolution T1-weighted imaging reveals that he has rapid atrophy (left hemisphere 1.24%/year, right hemisphere 2.12%/year) most dramatically in the hippocampus (left 3.35%/year, right 7.82%/year) and amygdala (left 3.73%/year, right 7.73%/year) estimated using mixed-effects modeling. Although he is right handed, his right hemisphere experiences a faster rate of atrophy than the left hemisphere. In contrast, some areas have relatively preserved volumes and annual rate of atrophy including the corpus callosum (0.87%/year), cerebellum white matter (0.08%/year), and brainstem (1.03–1.31%/year) compared with age-matched healthy controls (Table 2, Figure 2D–L).18,19 The WMHs in the cerebral hemispheres and periventricular regions show several confluent areas bilaterally and significant age-related fast expansion (21.7%/year). The white matter disease also involves the right insula and the splenium of the corpus callosum, which maintains normal thickness. He has a moderate degree of ventricular dilation most substantially in the lateral ventricles (13.7%/year for the lateral ventricles versus 4.14%/year for the third ventricle and 0.21%/year for the fourth ventricle) but he does not demonstrate the middle cerebellar peduncle (MCP) sign. His PET scan of metabolism demonstrated mildly decreased Fludeoxyglucose (FDG) uptake in the bilateral parietotemporal lobes (right greater than left), as well as mildly decreased FDG uptake in the bilateral posterior cingulate gyri. This pattern of rapid atrophy of the medial temporal lobe but relatively preserved cerebellum and brainstem would be consistent with early progressive neurodegenerative disorder, such as in AD.
Figure 2.
Structural changes in the brain. (A–C) The SWI scan acquired at age 76 shows cerebral microbleeds in the white matter and all four lobes (circled in (A) and arrows in (B)) as well as symmetric iron depositions in the caudate nuclei (arrowhead in (C)) and putamen (arrows in (C)). (D) The midsagittal slice of his T1 scan acquired at age 84 shows the relatively reserved the corpus callosum, brainstem, and cerebellum. (E and F) Segmentation of the corpus callosum (cyan), lateral ventricles (steel blue), third ventricle (brown), midbrain (blue), left amygdala (lime green), right amygdala (purple), left hippocampus (pink), and right hippocampus (salmon) on MRI scans acquired at age 76 (E) and age 84 (F). (G-L) Volumetric changes in left (red) and right (turquoise) hemisphere (G), left and right hippocampus (H), left and right amygdala (I), white matter hyperintensities (WMHs) (J), lateral ventricles (K), and cerebellar white matter (WM) (L).
Table 2.
The Results of Mixed-Effects Modeling to Fit MRI Volumetric Data Using Age
| Brain Areas | Intercept (mL) | β (mL) | SE (mL) | Rate (%/Year) | P value |
|---|---|---|---|---|---|
| Left hemisphere | 618.5 | −7.64 | 1.163 | −1.24 | 0.001 |
| Right hemisphere | 612.8 | −12.98 | 0.987 | −2.12 | < 0.001 |
| Corpus callosum | 28.4 | −0.25 | 0.034 | −0.87 | < 0.001 |
| Cerebellar WM | 19.7 | 0.02 | 0.183 | 0.08 | 0.94 |
| Midbrain | 9.76 | −0.11 | 0.018 | −1.1 | 0.001 |
| Pons | 16.89 | −0.22 | 0.031 | −1.31 | < 0.001 |
| Medulla | 5.84 | −0.06 | 0.005 | −1.03 | < 0.001 |
| Left hippocampus | 3.52 | −0.12 | 0.018 | −3.35 | 0.001 |
| Right hippocampus | 2.87 | −0.22 | 0.022 | −7.82 | < 0.001 |
| Left amygdala | 1.69 | −0.06 | 0.010 | −3.73 | 0.001 |
| Right amygdala | 1.76 | −0.14 | 0.013 | −7.73 | < 0.001 |
| Lateral ventricles | 39.3 | 5.36 | 0.578 | 13.65 | < 0.001 |
| Third ventricle | 3.00 | 0.12 | 0.021 | 4.14 | 0.001 |
| Fourth ventricle | 2.85 | 0.01 | 0.020 | 0.21 | 0.76 |
| Whole brain WMHs | 13.5 | 2.93 | 0.392 | 21.71 | < 0.001 |
Notes: The annual rate of change is calculated based on the intercept, the fitted value for the 2010 visit.
Abbreviations: WM, white matter; WMHs, white matter hyperintensities.
Discussion
In this case study, we present the neuropsychological, neurobehavioral and motor profile of an initially gifted individual with a progressive cognitive decline and disinhibited behavior in addition to his motor symptoms. He was diagnosed with FXTAS after developing tremor and ataxia in addition to typical white matter disease on his MRI. His IQ, memory and behavioral testing included in this report showed a rapid loss of skills with age. His MRI scans showed features of CSVD (i.e., enlarged perivascular spaces, lacune, WMHs and cerebral microbleeds), iron depositions in the deep nuclei, and a rapid brain atrophy most substantially in the medial temporal lobe but relatively preserved in the cerebellum and brainstem.
Individuals with CGG repeats in the 60s are at lower risk for FXTAS compared to those with higher CGG repeat numbers in the premutation range. The age of onset of FXTAS and the age of death correlate inversely with the CGG repeat number.5 In a review of 40 brains of premutation carriers the only 2 brains without FXTAS inclusions were those with repeats in the 60s.20
Previous reports of rapid cognitive decline in those with FXTAS demonstrated evidence of AD on neuropathology in addition to the findings of FXTAS inclusions.13 This patient showed a 26 point difference between his current VCI and his VCI from 4 years ago which is more typical of AD than FXTAS where verbal abilities are relatively preserved.21 His processing speed and full scale IQ also demonstrated a point difference of 16 and 25 respectively. This IQ loss is not unusual in FXTAS neurocognitive disorder which can also involve psychomotor slowing, retrieval and recall deficits, personality changes and executive deficits.21 However, the pattern of brain atrophy― substantially faster atrophy in the hippocampus and amygdala relative to the rate of atrophy in the corpus callosum, cerebellum, and brainstem―is consistent with AD; and his PET scan for AD demonstrated significantly decreased activity in the parietal regions bilaterally which is also consistent with AD. He had an affectionate behavior to all people around him due to his increasing frontal disinhibition.
There is variability in patients with FXTAS regarding their symptoms. Although many patients with FXTAS remain stable for years, others show rapidly progressive symptoms.22 The rapid decline of these patients may be related to another underlying pathology and in this case, the additional etiology is likely AD. This patient had only mild tremor and ataxia but the cognitive decline was remarkable suggesting that in his later years the AD symptoms were the most significant of his problems.
Since he was at low risk to develop FXTAS because of his low CGG repeat number, the onset and exacerbation of his FXTAS is worthy of discussion. His symptoms of tremor and balance problems started at an older age, greater than 10 years older than the mean age of onset at 62 years.4 His symptoms also started after general surgery for his prostate cancer and the anesthesia and the cancer itself could have precipitated the onset.23 Although his tremor was minimal, his ataxia was more problematic. He also developed anemia and chronic renal failure and these problems can occasionally be seen in those with FXTAS. Interestingly, likely due to RNA toxicity, intranuclear inclusions were identified in the bone marrow and in the kidneys of premutation carriers.6 Alternatively, or additionally, his anemia may have also been related to anemia of chronic disease but the treatment of iron infusions can exacerbate FXTAS and likely lead to cell death (ferroptosis) because impaired iron transportation and iron deposition have been demonstrated in the brain of those with the premutation.9 In addition, endothelial cells with increased iron load may subsequently drop off and facilitate microbleeds.20,24 Indeed, many cerebral microbleeds in the white matter and all four lobes were detected on his SWI scans. The patient was also treated with aspirin, a blood thinner which also predisposes to bleeding and likely progression of white matter disease. Lastly, as he aged into his 80s, the prevalence of AD symptom increases, and his decline of cognition escalated. His PET scan demonstrated dramatically decreased activation bilaterally in the parietal lobes which is typical for AD. His MRI scans also showed preferential degeneration of the medial temporal lobes and relatively preserved cerebellum and brainstem also typical for AD. The rapidity of his decline is more typical for AD combined with FXTAS perhaps the molecular mechanisms leading to neurodegeneration in both disorders are synergistic. His frontal disinhibition is more typical of FXTAS than AD. However, he is in the 40% of males affected by FXTAS who do not show the MCP sign and this is likely why his tremor is so mild. He also exercised daily, took antioxidants and treated his sleep apnea and his hypertension; the latter 2 problems if left untreated can exacerbate FXTAS.25
Further research is needed to understand the anemia in those with FXTAS especially since the treatment of iron supplementation can exacerbate the CNS dysfunction in FXTAS. Iron transportation in the choroid plexus is dysregulated in FXTAS and iron is deposited in the CNS.9,26 His SWI Scans acquired at age 78 and 79 years showed iron depositions in the deep nuclei, including the caudate nuclei, basal ganglia, substantia nigra, and cerebellar dentate nuclei. Perhaps chelation to extract iron from the CNS is needed for those with FXTAS showing iron depositions in the deep nuclei but such therapy has not been studied in FXTAS.
In summary, there is a high degree of variability in the clinical presentation of FXTAS. Additional neurodegenerative diseases can be present and likely exacerbate the progression of the disease, as previously reported.1 Perhaps one disorder may precipitate the onset of another disease because of oxidative stress or mitochondrial dysfunction and further studies are needed to understand synergistic effects of two or more disorders. When significant neurocognitive disorder combined with medial temporal lobe atrophy emerges in those with FXTAS, evaluation for AD and other neurocognitive disorders that can be overlapping with FXTAS should be considered. In the future, neuropathological studies will help us understand the progression of this patient’s symptoms.
Limitations and Further Research
Although the cognitive test and neuroimaging data are suggestive of AD, amyloid imaging is lacking, which can be useful for ruling out AD if amyloid plaques are absent. This case report shows the need for further research to explain the effects of two or more neurodegenerative disorders when they co-occur in a patient.
Acknowledgments
This research was funded by NICHD grant HD036071. In addition, support from the UC Davis MIND Institute Intellectual and Developmental Disabilities Research Center was funded by NICHD U54 HD079125.
Abbreviations
AD, Alzheimer’s Disease; BDS-2, Behavioral Dyscontrol Scale-2; CNS, Central Nervous System; CPAP, Continuous Positive Airway Pressure; CSVD, Cerebral Small Vessel Disease; FDG, Fludeoxyglucose; FMR 1, Fragile X Mental Retardation; FSIQ, Full Scale IQ; FXTAS, Fragile X Associated Tremor Ataxia Syndrome; MMSE, Mini-Mental State Examination; MRI, Magnetic Resonance Imaging; MCP, Middle cerebellar peduncle; PET, Positron Emission Tomography; PRI, Perceptual Reasoning; PSI, Processing Speed; SWI, Susceptibility-weighted imaging; VCI, Verbal Comprehension; WAIS, Wechsler Adult Intelligence Test; WMH, White Matter Hyperintensity; WMI, Working Memory.
Disclosure
Randi J Hagerman has consulted with Fulcrum, Ovid and Zynerba regarding treatment studies in individuals with fragile X syndrome and she is currently funded to carry out treatment studies by Zynerba, Ovid, and the Azrieli Foundation for fragile X syndrome. Dr. Tassone has received funding from Zynerba and Asuragen, Inc. The authors declare no other conflicts of interest in this work.
References
- 1.Hagerman RJ, Hagerman P. Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol. 2016;12(7):403–412. doi: 10.1038/nrneurol.2016.82 [DOI] [PubMed] [Google Scholar]
- 2.Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome. Ann N Y Acad Sci. 2015;1338:58–70. doi: 10.1111/nyas.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sévin M, Kutalik Z, Bergman S, et al. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. J Med Genet. 2009;46(12):818–824. doi: 10.1136/jmg.2008.065953 [DOI] [PubMed] [Google Scholar]
- 4.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22(2):203–206. doi: 10.1002/mds.21252 [DOI] [PubMed] [Google Scholar]
- 5.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. 2006;129(Pt 1):243–255. doi: 10.1093/brain/awh683 [DOI] [PubMed] [Google Scholar]
- 6.Hunsaker MR, Greco CM, Spath MA, et al. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011;122(4):467–479. doi: 10.1007/s00401-011-0860-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napoli E, Ross-Inta C, Wong S, et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20(15):3079–3092. doi: 10.1093/hmg/ddr211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robin G, Lopez JR, Espinal GM, Hulsizer S, Hagerman PJ, Pessah IN. Calcium dysregulation and Cdk5-ATM pathway involved in a mouse model of fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2017;26(14):2649–2666. doi: 10.1093/hmg/ddx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariza J, Steward C, Rueckert F, et al. Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain Res. 2015;1598:88–96. doi: 10.1016/j.brainres.2014.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers H, Ariza J, Monterrubio A, Hagerman P, Martinez-Cerdeno V. Cerebellar mild iron accumulation in a subset of FMR1 premutation carriers with FXTAS. Cerebellum. 2016;15(5):641–644. doi: 10.1007/s12311-016-0798-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd PK, Oh SY, Krans A, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013;78(3):440–455. doi: 10.1016/j.neuron.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seritan AL, Nguyen DV, Farias ST, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer’s disease. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147b(7):1138–1144. doi: 10.1002/ajmg.b.30732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassone F, Greco CM, Hunsaker MR, et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11(5):577–585. doi: 10.1111/j.1601-183X.2012.00779.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu YQ, Yang JC, Hall DA, et al. Parkinsonism in fragile X-associated tremor/ataxia syndrome (FXTAS): revisited. Parkinsonism Relat Disord. 2014;20(4):456–459. doi: 10.1016/j.parkreldis.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco CM, Tassone F, Garcia-Arocena D, et al. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008;65(8):1114–1116. doi: 10.1001/archneur.65.8.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1(3):83–92. doi: 10.1136/svn-2016-000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JY, Hessl D, Hagerman RJ, et al. Abnormal trajectories in cerebellum and brainstem volumes in carriers of the fragile X premutation. Neurobiol Aging. 2017;55:11–19. doi: 10.1016/j.neurobiolaging.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JY, Trivedi AM, Carrillo NR, et al. Open-label allopregnanolone treatment of men with fragile X-associated tremor/ataxia syndrome. Neurotherapeutics. 2017;14(4):1073–1083. doi: 10.1007/s13311-017-0555-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Cerdeno V, Lechpammer M, Hagerman PJ, Hagerman R. Two FMR1 premutation cases without nuclear inclusions. Mov Disord. 2017;32(9):1328–1329. doi: 10.1002/mds.27060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seritan A, Cogswell J, Grigsby J. Cognitive dysfunction In FMR1 premutation carriers. Curr Psychiatry Rev. 2013;9(1):78–84. doi: 10.2174/157340013805289635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano R, Saito N, Reed D, et al. Aging in Fragile X premutation carriers. Cerebellum. 2016;15(5):587–594. doi: 10.1007/s12311-016-0805-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligsay A, El-Deeb M, Salcedo-Arellano MJ, Schloemerkemper N, Grayson JS, Hagerman R. General anesthetic use in Fragile X spectrum disorders. J Neurosurg Anesthesiol. 2018;31(3):285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariza J, Rogers H, Hartvigsen A, et al. Iron accumulation and dysregulation in the putamen in fragile X-associated tremor/ataxia syndrome. Mov Disord. 2017;32(4):585–591. doi: 10.1002/mds.26902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polussa J, Schneider A, Hagerman R. Molecular advances leading to treatment implications for Fragile X premutation carriers. Brain Disord Ther. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JY, Hagerman RJ, Rivera SM. A multimodal imaging analysis of subcortical gray matter in fragile X premutation carriers. Mov Disord. 2013;28(9):1278–1284. doi: 10.1002/mds.v28.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori S, Wu D, Ceritoglu C, et al. MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput Sci Eng. 2016;18(5):21–35. doi: 10.1109/MCSE.2016.93 [DOI] [Google Scholar]
- 28.Wang H, Das SR, Suh JW, et al. A learning-based wrapper method to correct systematic errors in automatic image segmentation: consistently improved performance in hippocampus, cortex and brain segmentation. NeuroImage. 2011;55(3):968–985. doi: 10.1016/j.neuroimage.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]