Abstract
Alterations in tumour metabolism and acid/base regulation result in the formation of a hostile environment, which fosters tumour growth and metastasis. Acid/base homoeostasis in cancer cells is governed by the concerted interplay between carbonic anhydrases (CAs) and various transport proteins, which either mediate proton extrusion or the shuttling of acid/base equivalents, such as bicarbonate and lactate, across the cell membrane. Accumulating evidence suggests that some of these transporters interact both directly and functionally with CAIX to form a protein complex coined the ‘transport metabolon’. Transport metabolons formed between bicarbonate transporters and CAIX require CA catalytic activity and have a function in cancer cell migration and invasion. Another type of transport metabolon is formed by CAIX and monocarboxylate transporters. In this complex, CAIX functions as a proton antenna for the transporter, which drives the export of lactate and protons from the cell. Since CAIX is almost exclusively expressed in cancer cells, these transport metabolons might serve as promising targets to interfere with tumour pH regulation and energy metabolism. This review provides an overview of the current state of research on the function of CAIX in tumour acid/base transport and discusses how CAIX transport metabolons could be exploited in modern cancer therapy.
Subject terms: Cancer, Cell biology
Background
Solid tumours are highly active tissues that often contain hypoxic regions, and produce vast amounts of metabolic acids. Through the constant release of acid, tumour cells create a hostile environment that fosters tumour growth and simultaneously kills adjacent host cells. Increased production and release of protons, in combination with restricted perfusion and alterations in the pH regulatory mechanisms lead to severe alterations in intracellular and extracellular pH, with significant consequences for tumour development and progression.1–4 While extracellular pH (pHe) can decrease to values as low as pH 6.5,5–7 intracellular pH (pHi) becomes slightly alkaline in cancer cells.3,8 This reversal in the pH gradient has been shown to occur at an early point in malignant transformation and can further increase with proceeding tumour growth.9,10 Extracellular acidification promotes tumour progression via various mechanisms, including pH-dependent modulation of integrin-mediated cell–matrix adhesion, degradation of the extracellular matrix via activation of cathepsins and various matrix metalloproteases and by killing of adjacent host cells.4,11,12 Furthermore, an acidic pHe has been shown to suppress immunity, for example, by inhibition of chemotaxis or blocking of T-cell activation.13,14 Alkaline pHi, on the other hand, fosters cell proliferation15–19 and can limit apoptosis by suppression of caspase activity or alteration of mitochondria-dependent apoptosis.20–22 Furthermore, alkaline pHi supports cancer cell migration by reorganisation of the cytoskeleton via pH-dependent activation of cofilin and talin, as well as membrane blebbing, which facilitates invasion and metastasis.11,23,24 Finally, alkaline pHi triggers glycolytic activity, possibly by pH-mediated alterations in the activity of different glycolytic enzymes, which in turn results in increased acid production and exacerbates extracellular acidification.25–27 Taken together, these alterations in metabolism and pH regulation provide an evolutionary advantage for cancer cells over their surrounding host cells, and thereby contribute to somatic evolution, which selects for more aggressive phenotypes of cancer entities.1,28,29 Nevertheless, even though metabolic alterations and reversed pH gradient can pose an obstacle for conventional cancer therapies, they might also become a tumour’s Achilles’ heel to be exploited for novel therapeutic approaches.3,14,30–34
Acid/base regulation in tumour cells
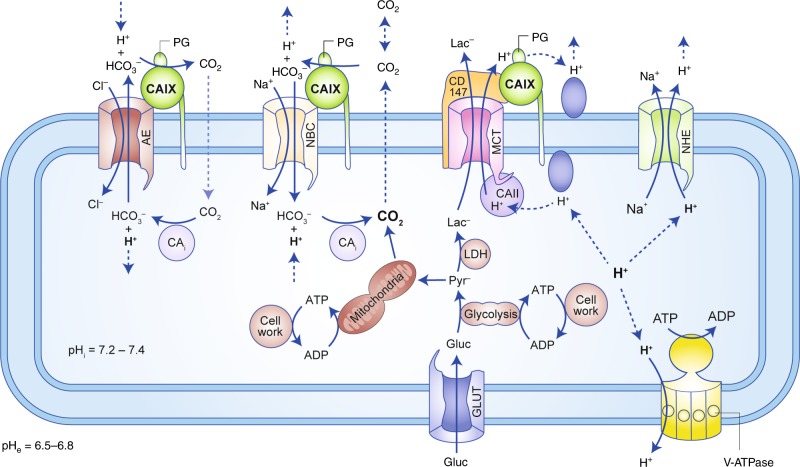
Tumour pH regulation requires the concerted interplay between various acid/base transporters and carbonic anhydrases (CAs), as summarised in Fig. 1. Cancer cells meet their demand for energy mainly by aerobic respiration of glucose or anaerobic glycolysis. Both pathways result in the formation of metabolic acids—either in the form of CO2, which hydrates to HCO3– and H+, or in the form of lactate– (Lac–) and H+ (Fig. 1). CO2 and Lac–/H+ have to be removed from the cell to avoid intracellular acidosis. CO2 can leave the cell by passive diffusion through the plasma membrane or via gas channels, such as aquaporins.35,36 Lactate, as a charged ion, cannot diffuse over the cell membrane, but is removed from the cell via monocarboxylate transporters (MCTs) in cotransport with H+ in a 1:1 stoichiometry37–40 (Fig. 1). Lactate transport in cancer cells is primarily mediated by the two isoforms MCT1 (SLC16A1) and MCT4 (SLC16A3), the expression of which has been shown to be upregulated in various tumour types, including breast cancer, gliomas, colorectal carcinomas and prostate cancer.41–46 An alternative pathway for lactate removal is passive diffusion of the ion through gap junctions, formed by connexins between adjacent tumour cells.47 Lactate is thereby dissipated from glycolytic cancer cells to recipient cells, situated in better perfused regions of the tumour, from where it can either be released to the pericellular space or serves as fuel for oxidative energy production.47 However, removal of metabolic acid by passive diffusion, either through cells or over the plasma membrane alone, is not sufficient for efficient cellular pH regulation, since restricted perfusion in the intracellular and extracellular space will lead to accumulation of the acids in the cell.48,49 Furthermore, sole removal of metabolic acids via MCTs and CO2 diffusion would render cytosolic pH dependent on the cell’s metabolic rate.49 Therefore, additional pH regulatory proteins are required to govern cellular pH. One of the major proton extruders in mammalian cells is the Na+/H+ exchanger NHE1 (SLC9A1). NHE1 is already upregulated in cancer cells at an early stage in tumour development,9 and the oncogene-dependent activation of NHE1, which results in cytosolic alkalinisation and extracellular acidification, has been suggested as a key mechanism in malignant transformation and tumour progression.3,9,50 Accumulating at the leading edge of lamellipodia,51 NHE1 promotes tumour cell migration and invasion of cancer cells by generating a pH gradient along the cell, with an acidic pHe and alkaline pHi at the migrating front.52–55 In addition, NHE1-mediated proton export at the protruding front supports digestion of the extracellular matrix by local acidification.56,57
Fig. 1.
Tumour pH is regulated by the concerted interplay between acid/base transporters and carbonic anhydrase. Metabolic acids are produced by glycolysis and mitochondrial respiration. Anaerobic glycolysis yields lactate and H+ that are excreted from the cell by monocarboxylate transporters (MCTs) in a 1:1 stoichiometry. Mitochondrial respiration produces CO2, which is hydrated in the cell, forming HCO3− and H+. CO2 can leave the cell by passive diffusion over the plasma membrane or through gas channels (not shown). Efficient pH regulation requires the function of additional transporters and enzymes, which either export protons from the cell or mediate the reimport of HCO3−. Additional export of H+ can be mediated by the Na+/H+ exchanger 1 (NHE) and by vacuolar H+-ATPase (V-ATPase). CO2 venting is further supported by the catalytic function of the extracellular carbonic anhydrase (CA) isoforms CAIX and CAXII (the latter one is omitted from this cartoon for clarity). Extracellular CAs catalyse the hydration of CO2 to HCO3− and H+ at the membrane. HCO3− can diffuse away from the cell or can be reimported by Na+/HCO3– cotransporters (NBC) to support intracellular buffering. The extracellular HCO3− can either be formed from ‘endogenous' CO2, which is produced by the cell through mitochondrial respiration or titration of HCO3− and H+, or from extracellular CO2, produced from distant sources. Cl−/HCO3− exchangers (AEs) have been suggested to function either as HCO3− importers for pH buffering or HCO3− exporters that extrude HCO3− to load cellular compartments with Cl– during cell migration. Transport activity of many acid/base transporters is facilitated by interaction with intracellular and extracellular CAs. NBC and AE interact with CAII and CAIX that either provide or remove HCO3− to/from the transporter via their catalytic function. MCTs form a protein complex with CAII and CAIX, in which the CAs function as ‘proton antenna’ for the transporter, which mediates the rapid exchange of H+ between transporter pore and the surrounding protonatable residues.
Protons are also removed from the cell against their electrochemical gradient by vacuolar H+-ATPases, which are targeted to the plasma membrane of various cancer cells, including glioma, pancreatic cancer, hepatocellular carcinoma and oral squamous cell carcinoma, where the pumps have been shown to promote cell proliferation, migration and invasion.58–67
Intracellular pH is not only maintained by constant extrusion of protons, but also by import of HCO3− via Na+/HCO3− cotransporters such as NBCe1 (SLC4A4) and NBCn1 (SLC4A7). NBCs were shown to be the predominant net acid extruders in breast cancer tissue, where they facilitate tumour cell proliferation by counteracting metabolic acidosis.50,68–71 Furthermore, NBCs have been demonstrated to play a role in cell migration—NBCe1 is redirected to the leading edge,72 where NBC-mediated import of HCO3− (together with NHE1-mediated H+ extrusion) leads to intracellular alkalinisation, in turn driving cytoskeletal remodelling.11,72 Cancer cell migration is further supported by the functional interaction between NBCe1 and the Cl−/HCO3− exchanger AE2 (SLC4A2).11,72 It was suggested that AE2 imports osmotically active Cl– in exchange for HCO3−, to support osmotic cell swelling.11,72 AE2 and NBCe1 would thereby work together to produce an ‘HCO3− short circuit’, driving Cl− influx into the cell during cell migration. For an in-depth discussion of pH regulation in cancer cells see also refs. 3,4,11,48,49,73–76 In healthy cells, activity of acid/base transporters is tightly regulated in an allosteric way. Transport activity of AE2 increases with alkaline pHi/e and decreases with acidic pHi/e.77–79 Allosteric regulation of AE2 transport activity involves independent H+ sensing of the transporter by amino acid clusters in the cytosolic N-terminal domain and a small region in the transmembrane domain.77–81 Transport activity of NHE1, on the other hand, is allosterically activated by high intracellular proton concentrations and becomes quiescent at alkaline pH.82–84 Activation and inactivation of the transporters ensures tight control of pHi and pHe. In cancer cells, however, these regulatory processes can be disturbed, contributing to dysregulation of pHi and pHe. In cancer cells, transport activity of NHE1 was found to be activated by growth factors, hypoxia, acidic pHe, low serum concentration or by activation of CD44 by hyaluronan.9,85–88 For a detailed discussion on the regulation of acid/base transporters see refs. 10,18,79,89
The role of CAIX in tumour acid/base regulation
Besides the concerted interplay between various acid/base transporters, effective acid venting and pH regulation requires the catalytic activity of intracellular and extracellular CAs, to catalyse the reversible hydration of CO2 to HCO3− and H+.
Out of the six evolutionary distinct classes of CAs (α, β, γ, δ, ζ and η), only the α-class is expressed in mammals.90 The α-class of CAs comprises 16 isoforms, which vary regarding their catalytic activity and subcellular localisation. From the 12 catalytically active isoforms, expressed in humans, five are localised in the cytosol (CAI, CAII, CAIII, CAVII and CAXIII) and four are tethered to the plasma membrane with their catalytic domain facing the extracellular space (CAIV, CAIX, CAXII and CAXIV).91–93 CAVA and CAVB are expressed in the mitochondrial matrix while CAVI is secreted to the saliva.91,92 Three isoforms display no catalytic CA activity (CAVIII, CAX and CAXI). Therefore, these three proteins, which are mainly expressed in the central nervous system, are also termed CA-related proteins (CARPs).94,95
Cancer cells primarily express the plasma-membrane-associated CA isoforms CAIX and CAXII, as well as intracellular CAs such as CAI and CAII.96–104 Amongst the cancer-related CAs, CAIX has gained most attention, since expression of this isoform in healthy tissue is restricted to epithelial cells in the stomach and gut, but is strongly upregulated in many tumour tissues.105–107 The CAIX protein comprises an extracellular-facing catalytic domain tethered to the plasma membrane with a single transmembrane domain, and a short intracellular C-terminal tail. In addition, CAIX features an N-terminal proteoglycan-like domain (PG domain), which is unique to CAIX within the CA family.108–110 The PG domain was shown to contribute to the assembly of focal adhesion contacts during cell migration111,112 and was suggested to function as a proton buffer to support CAIX catalytic activity.113 Furthermore, it might serve as proton antenna for monocarboxylate transporters to facilitate proton-coupled lactate flux.114
CAIX, the expression of which is under control of the hypoxia-inducible factor 1 (HIF-1), is predominantly located in chronically hypoxic tumour regions.110,115 However, CAIX can also be found in mild hypoxic or even normoxic regions, since the expression of CAIX can be activated by components of the mitogen-activated protein kinase (MAPK) pathway.116,117 CAIX is expressed in a wide range of tumour entities, including breast and colorectal cancer, glioblastoma, lung cancer and cervical squamous cell carcinomas, and is usually linked to poor prognosis.98,118–123 In line with the correlation between CAIX expression and poor prognosis, various studies have demonstrated that CAIX can protect tumour cells from intracellular acidosis and functions as a pro-migratory factor that drives migration and invasion by both catalytic and non-catalytic mechanisms, which fosters the formation of metastasis.72,112,123–130
CAIX catalyses the reversible hydration of CO2 at the exofacial site of the plasma membrane. This simple reaction puts CAIX in a central position for the regulation of pHi and pHe.127,131,132 By conversion of cell-derived CO2 into HCO3− and H+, CAIX can maintain a steep outward-directed CO2 gradient over the plasma membrane, thereby facilitating CO2 excretion, which results in extracellular acidification and a more alkaline pHi131,132 (Fig. 1). Furthermore, hydration of CO2 at the extracellular site of the plasma membrane allows the parallel diffusion of CO2, HCO3– and H+ through the periplasm.131,132 This immediate removal of CO2 at the outer site of the plasma membrane is of particular importance for poorly perfused but metabolically active tissue, such as solid tumours, where diffusion distances to the next blood capillary pose a barrier for the diffusive removal of metabolic waste products.131,132 Some HCO3−, produced by CAIX at the cell membrane, is reimported into the cell by adjacent HCO3– transporters, like NBCe1 or NBCn1 (Fig. 1). In the cytosol, HCO3− titrates H+ to generate membrane-permeable CO2, which diffuses out of the cell and contributes to net H+ extrusion. Efficient reimport of CAIX-derived HCO3– requires a close proximity of CAIX to the bicarbonate transporters (Fig. 1). This close proximity could be achieved by direct interaction between the proteins, by forming a complex coined the ‘transport metabolon’, as described in the next section.
Even though an acidic microenvironment fosters tumour progression, pHe must not drop too low in order to avoid over-acidification and necrosis of cancer cells. It was therefore suggested that CAIX can set the pH of the tumour microenvironment to a moderate acidic value, which provides an advantage to cancer cells, while preventing over-acidification-induced cell death.90,131–136 In contrast to other CA isoforms, CAIX is most active at pH 6.8, a value that closely resembles tumour pHe.133,134 At pH values above 6.8, the rate of the hydration reaction (H+ production) is higher than the rate of dehydration, while at pH values below 6.8 the rate of the dehydration reaction (H+ consumption) exceeds the rate of the hydration reaction.133,134 This implies that at a pHe above 6.8, CAIX produces H+ by the hydration of CO2, to acidify the pericellular space. At a pHe below 6.8, CAIX removes H+ by the dehydration reaction, to counteract further acidification. In line with this hypothesis, Lee et al.135 recently showed that CAIX not only contributes to acidification of the extracellular space, but also functions as a ‘pH-stat’ to stabilise pHe at a moderate acidic value in spheroids and tumour xenografts. Since this moderate acidity is well tolerated by cancer cells but could be lethal to normal cells, setting pHe to a constant acidic condition might pose an evolutionary strategy in cancer cells—this creates an environment that fosters tumour growth and invasion in response to microenvironmental selection forces.70,137
The various roles of CAIX in tumour acid/base regulation have been extensively discussed in a variety of excellent reviews.73,90,110,130,138,139 Therefore, the review will not further deepen the discussion of the general functions of CAIX in tumour pH regulation but focus on the role of CAIX in the facilitation of acid/base transporters in tumour cells.
Transport metabolons
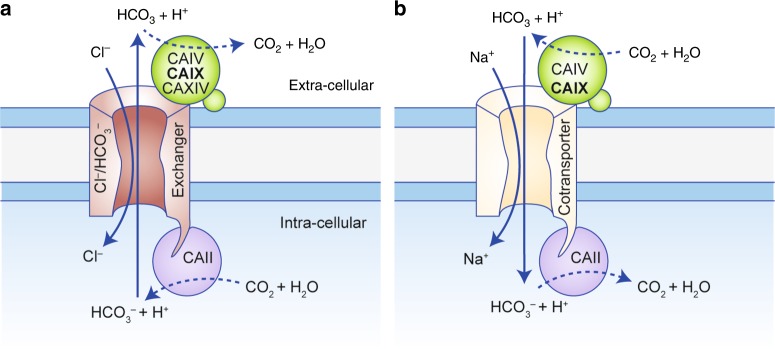
Various acid/base transporters have been shown to both physically and functionally interact with intracellular and extracellular CAs to form a protein complex coined the ‘transport metabolon’. A metabolon has been defined as a ‘temporary, structural-functional, supramolecular complex of sequential metabolic enzymes and cellular structural elements, in which metabolites are passed from one active site to another, without complete equilibration with the bulk cellular fluids (channelling)’.140–142 The most extensively studied CA isoform to interact with acid/base transporters is the intracellular CAII. CAII binds to an acidic cluster in the C-terminal tail of various HCO3− transporters, including the Cl–/HCO3– exchangers AE1, AE2 and AE3,143–147 as well as the Na+/HCO3– cotransporter NBCe1148,149 and NBCn1 (NBC3).150,151 Direct binding of CAII to the transporter’s C-terminal tail was suggested to maximise the local HCO3− concentration in the direct vicinity of the transporter pore.147 Indeed, inhibition of CAII catalytic activity by sulfonamides such as acetazolamide or ethoxyzolamide, as well as co-expression of AEs and NBCs with the catalytically inactive CAII mutant CAII-V143Y, resulted in a loss of the CAII-mediated increase in transport function.147,151–153 Taken together, it can be assumed that CAII, directly bound to the C-terminal tail of AEs and NBCs, provides (or removes) HCO3− to (from) the transporter by the reversible hydration of CO2 (Fig. 2a, b). It was suggested that CAII would maximise HCO3− flux by maximising the transmembrane HCO3− gradients in the immediate vicinity of the transporter.147,154 AE1, for example, has a turnover rate of 5 × 104 s−1 and would therefore rapidly deplete the local substrate pool. The transport metabolon between CAII and AE could increase HCO3− transport by minimising the distance of substrate diffusion between enzyme and transporter, thereby increasing substrate availability for the transporter.147,154
Fig. 2.
Bicarbonate transport metabolons with carbonic anhydrase. (a) Cl–/HCO3− exchangers (AEs) and (b) Na+/HCO3− cotransporters (NBCs) form bicarbonate transport metabolons with intracellular and extracellular carbonic anhydrases (CAs). Cytosolic CAII binds to the transporter’s C-terminal tail. Extracellular CAs, which are tethered to the plasma membrane by a transmembrane domain (CAIX, CAXIV) or GPI anchor (CAIV), bind to the transporter’s fourth extracellular loop. By catalysing the reversible hydration of CO2 to HCO3− and H+ in the immediate vicinity of the transporter, intracellular and extracellular CAs either provide or remove HCO3− to/from the transporter. Through this mechanism, they suppress the depletion of HCO3− at the cis-side of the transporter and HCO3− accumulation at the trans-side, which, in turn, drives HCO3− flux across the cell membrane.
HCO3− transporters do not only interact with intracellular CAII, but also with extracellular isoforms, such as CAIV, CAIX and CAXIV155–157 (Fig. 2a, b). A physical and functional interaction between HCO3− transporters and CAIX was demonstrated in 2007 by Patricio Morgan et al. in vitro and in HEK293 cells.157 By applying co-immunoprecipitation experiments with AEs and CAIX in HEK293 cells and pull-down assays with GST-fusion proteins, the authors could demonstrate that the catalytic domain of CAIX directly binds to AE1, AE2 and AE3. The authors could further show that CAIX increases transport activity of AE1–3 when the proteins were co-expressed in HEK293 cells.157 Interestingly, CAIX did not alter transport activity of the putative Cl–/HCO3− exchanger SLC26A7. Furthermore, SLC26A7 could not be co-precipitated with CAIX, indicating no physical interaction between the two proteins. These results suggest that the direct interaction between CAIX and AEs is indeed a prerequisite for the CAIX-mediated facilitation of AE transport activity.
CAs can also from transport metabolons with NHEs. CAII was shown to bind to and enhance transport activity of NHE1 and NHE3.158–161 Furthermore, it was suggested that extracellular CAIV can facilitate NHE transport activity in rabbit non-pigmented ciliary epithelium cells.162 In both cases, facilitation of NHE transport function required CA catalytic activity. These results suggest that CAII and CAIV facilitate NHE transport activity by a similar mechanism as demonstrated for bicarbonate transporters. First evidence that CAIX might form a transport metabolon with NHE1 in cancer cells was recently provided by Liskova et al.163 The authors could co-precipitate NHE1 with CAIX and the Na+/Ca2+ exchanger NCX1 from lysates of hypoxic SiHa cells. From these and other data, the authors suggested that the three proteins, which are colocalised in hypoxic SiHa cells (as shown by an in situ proximity ligation assay), can form a transport metabolon that contributes to tumour pH regulation.163
Despite the constantly increasing number of publications in favour of the concept of the transport metabolon, the existence of these protein complexes has also been questioned by several studies.164–167 One major point of criticism relates to the direct interaction between transporter and enzyme. Most binding studies were performed by using GST-fusion proteins of the transporters’ C-terminal tails,147,150,151,155,158,159 which were criticised to result in false-positive binding.165 Piermarini et al.165 could reproduce previous findings that CAII binds to a GST-fusion protein of the C-terminal tails of AE1, NBCe1 and NDCBE. However, when performing the assay with pure peptides, no binding to CAII could be observed.165 The concept of a bicarbonate transport metabolon was also fundamentally questioned by a study from Al-Samir et al.167, who examined the interaction between AE1 and CAII in native human red blood cells and the tsA201 human embryonic kidney cell line. The authors did not find convincing evidence for a direct interaction between AE1 and CAII. Furthermore, their mathematical models favoured equal distribution of CAII throughout the cytosol over accumulation of the enzyme at the cell membrane for efficient HCO3– transport.167 Based on these results, it was suggested that CAs could improve bicarbonate supply to membrane transporters without the necessity to form a physical protein complex.167 An in-depth discussion of the various types of interactions between acid/base transporters and CAs, including the controversies on transport metabolons, is provided in a number of recommendable reviews.142,154,168,169
Role of bicarbonate transport metabolons with CAIX in cancer cell motility
Transport metabolons have been suggested to play a role in different physiological processes, including gas exchange in erythrocytes,144 acid/base regulation in the heart,170,171 brain and retina,160,172,173 gastric acid secretion157,174 and the reabsorption of salt and water in kidney and intestine.161
First evidence for a bicarbonate transport metabolon in cancer cells was provided by the laboratory of Silvia Pastorekova and Jaromir Pastorek in hypoxic squamous cell carcinoma and lung carcinoma cells.72,175 The authors demonstrated that CAIX accumulates in the lamellipodia of hypoxic A549 lung carcinoma cells, where it colocalised with the Na+/HCO3− cotransporter NBCe1.72,175 CAIX also colocalised with the Cl–/HCO3– exchanger AE2 in the leading edge of SiHa squamous cell carcinoma cells, which migrated from hypoxic spheroids. An in situ proximity ligation assay, which detects colocalisation of proteins with a maximum distance of 40 nm between the epitopes in native cells, suggested that CAIX directly interacts with NBCe1 and AE2 in the lamellipodia of migrating A549 and SiHa cells.72
Both NBCe1 and AE2 have been attributed a central function for cell migration. NBCe1 contributes to the reversal of the pH gradient, which is required for intracellular remodelling of the actin cytoskeleton and extracellular detachment from the matrix. By catalysing the hydration of CO2 at the cell membrane, CAIX ensures local availability of extracellular HCO3− for direct import via the NBCe1 to increase intracellular buffer capacity. The remaining protons contribute to the acidification of the pericellular space and drive tumour cell invasiveness.175 The exact role of AE2 in cell migration, however, is still under debate. AE2 might extrude HCO3− in exchange to osmotically active Cl– to support cell swelling.11,72 CAIX, which directly interacts with NBCe1 and AE2 at the leading edge of migrating cancer cells, could provide (or remove) HCO3− to (from) the transporters to support their transport activity. Indeed, inhibition of CAIX activity—either by application of acetazolamide or overexpression of a CAIX mutant—lacking the catalytic domain, resulted in a significant reduction of migratory activity in hypoxic HeLa cells.72 Based on these results, it appears plausible that the transport metabolons, formed between NBCe1, AE2 and CAIX in the lamellipodia of migrating cells, can contribute to the reversal of the pH gradient and Cl−-mediated swelling at the protruding front of the cell to drive cell migration.
Non-catalytic transport metabolons with CAIX and MCTs
Tumour cells, especially those that reside in a hypoxic environment, display a substantial increase in glycolytic activity, resulting in increased production of lactate and protons. Lactate and protons are removed from the cell via the monocarboxylate transporters MCT1 and MCT4, which contributes to the formation of an acidic microenvironment. Lactate transport was found to be increased in hypoxic breast cancer cells.176 Knockdown of CAIX, however, abolished the hypoxia-induced increase in lactate flux.176 Surprisingly, inhibition of CAIX enzymatic activity with ethoxyzolamide had no effect on MCT transport activity, indicating that CAIX facilitates MCT transport activity by a mechanism that is independent from the enzyme’s catalytic function.176 In line with these results, Crispr-mediated knockout of CAIX decreases proton excretion rates from glycolysis (GlycoPER) in the triple-negative breast cancer cell line UFH-001.136 Isoform-specific inhibition of CAIX with three different ureido-substituted benzene sulfonamides (USBs), however, did not change GlycoPER, suggesting a non-catalytic function of CAIX in glycolysis-derived acid secretion.136 Such a non-catalytic facilitation of MCT transport activity was previously observed in Xenopus laevis oocytes, heterologously expressing MCT1/4 and intracellular CAII.177–185 CAII binds to an acidic cluster in the C-terminal tail of MCT1 (E489EE) and MCT4 (E431EE), but not to MCT2.182,185 Since CAII catalytic activity is not required to facilitate MCT1/4 transport activity, it was suggested that CAII could function as a ‘proton antenna’ for the transporter180 (Fig. 3). The physiological need for such a proton antenna results from the slow diffusion of highly buffered protons within the cytosol. Martinez et al.186 calculated that the maximum supply rate of H+ via diffusion through the cytosol is significantly lower than the apparent turnover rate of MCT1. In other words, MCTs extract H+ from their surrounding at a rate that exceeds the capacity for simple diffusion to supply H+ to the transporter. To solve this paradox, the authors suggested that the transporters extract H+ from surrounding ‘proton harvesting compartments’ and not directly from the cytosol.186 Like other CAs, CAII facilitates an intramolecular proton shuttle to move H+ between the catalytic centre and the surrounding bulk solution. Proton transfer is mediated by His64, which shuttles H+ between the bulk solvent and a well-ordered water wire in the enzyme’s active site cavity.187 Modelling studies further suggested that the active site proton pathway exits to the protein surface, leading to the two acid residues Glu69 and Asp72.188 Indeed, investigations in Xenopus oocytes showed that these two residues mediate proton transfer between MCT1/4 and CAII, while CAII–His64 is not involved in proton shuttling between enzyme and transporter but mediates binding of CAII to the MCT1/4 C-terminal tail.184 In analogy to the findings on CAII, it was suggested that CAIX could serve as an extracellular proton antenna for MCTs in cancer cells.114,176,189 The catalytic domain of CAIX seems to lack a homologue cluster to CAII–Glu69 and Asp72. However, the CAIX–PG domain features eight aspartate and 18 glutamate residues, which have been suggested to function as an intramolecular proton buffer for the enzyme.113 Indeed, co-expression of MCT1/4 with a truncation mutant of CAIX, missing the PG domain (CAIX–ΔPG), in Xenopus oocytes did not facilitate MCT transport activity.114 Furthermore, application of an antibody, mapping against the CAIX–PG domain, supressed CAIX-mediated facilitation of lactate transport in Xenopus oocytes and hypoxic breast cancer cells.99 Based on these results, it was suggested that the CAIX–PG domain functions as proton antenna, by mediating the rapid exchange of H+ between transporter and surrounding protonatable residues114 (Fig. 3). Interestingly, CAII and CAIX can facilitate MCT-mediated H+/lactate transport both in the inward and outward direction.114,180 During H+-coupled lactate efflux, as observed in lactate-producing cancer cells, intracellular CAII would collect H+ from surrounding protonatable residues near the inner face of the cell membrane and donate them to the transporter. On the extracellular site, CAIX would remove H+ from the transporter pore and transfer it to surrounding protonatable residues near the extracellular face of the plasma membrane (Fig. 3). During H+-coupled lactate influx (as found in lactate-consuming cell types) the shuttle would operate in the opposite direction.
Fig. 3.
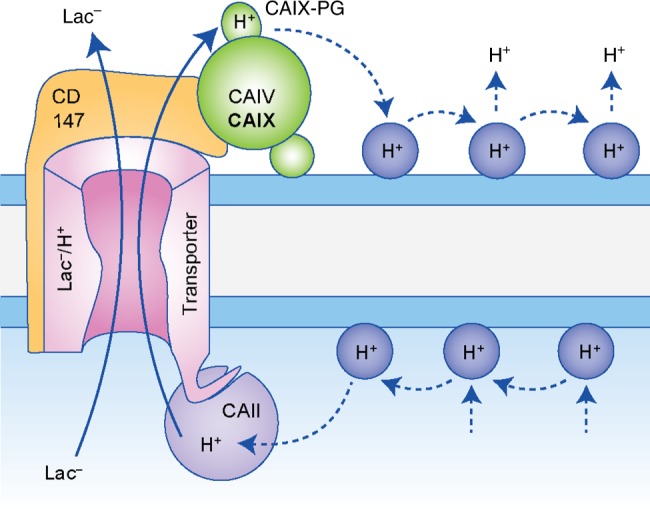
Carbonic anhydrases function as proton antenna for monocarboxylate transporters. The monocarboxylate transporter isoforms 1 and 4 (MCT1/4) form a non-catalytic transport metabolon with CAII, CAIV and CAIX. This type of interaction is independent from carbonic anhydrase (CA) catalytic activity. Intracellular CAII, which is bound to the MCT1/4 C-terminal tail, functions as proton antenna for the transporter, which mediates the rapid exchange of H+ between transporter pore and surrounding protonatable residues (blue–grey circles) at the inner face of the plasma membrane. On the extracellular site, CAIV and CAIX, which are bound to the Ig1 domain of the MCT chaperone CD147, mediate shuttling of protons between the transporter and protonatable residues at the extracellular face of the plasma membrane. In CAIX, proton shuttling is mediated by the CAIX–PG domain. The proton shuttle in CAIV is yet unidentified.
While CAII directly binds to the transporter, physical interaction between MCT1/4 and CAIX is mediated by the transporters’ chaperone CD147.189 Pull-down assays demonstrated that CAIX binds to the Ig1 domain of CD147 by forming a hydrogen bond between CD147–Glu73 and CAIX–His200, the residue analogue to CAII–His64 and the central residue of the CAIX intramolecular proton shuttle.189 CAIX does not only interact with MCT1 and MCT4, but also with the high-affinity lactate transporter, MCT2,189 found in various tumour tissues, including breast carcinoma, colon adenocarcinoma, lung cancer, ovarian adenocarcinoma and prostate cancer.190–192 The chaperone of MCT2, GP70,193 features a positively charged lysine instead of a negatively charged glutamate at the CA-binding site.189,194 By combining pull-down experiments with electrophysiological studies in Xenopus oocytes, it was demonstrated that CAIV–His88 (the analogue residue to CAII–His64 and CAIX–His200) either serves as hydrogen donor or hydrogen acceptor, depending on the properties of its binding partner.194 Based on these findings, it was suggested that CAIX–His200 could also serve as hydrogen donor and acceptor to mediate binding of CAIX to different MCT chaperones.189 Binding of CAIX to the transporter’s chaperone is mandatory for the CAIX-mediated facilitation of MCT activity. This was shown by application of an antibody against the CD147–Ig1 domain that displaced CAIX from the transporter and decreased MCT-mediated lactate flux in Xenopus oocytes and breast cancer cells.189
The MCT1/4–CAIX transport metabolon was not only observed in cultivated breast cancer cells, but also in human tumour tissue. By use of an in situ proximity ligation assay (PLA), Ames et al. could recently demonstrate a direct interaction between MCT1, MCT4 and CAIX in tissue samples of human breast cancer patients.189 Interestingly, the number of PLA signals increased with higher tumour grade, indicating that the number of MCT1/4–CAIX transport metabolons increases during tumour progression.189
Not only is lactate transport in cancer cells facilitated by extracellular CAIX, but also by intracellular CAII.184 CAII was found to physically interact with MCT1 in MCF-7 breast cancer cells, as shown by PLA.184 Knockdown of CAII, but not inhibition of catalysis, decreased lactate transport in normoxic and hypoxic MCF-7 breast cancer cells and reduced cell proliferation.176,184 These results indicate that efficient lactate efflux from cancer cells requires both intracellular and extracellular CAs. That intracellular and extracellular CAs can work in concert to drive MCT-mediated lactate transport was demonstrated for CAII and CAIV in Xenopus oocytes.181 CAII and CAIV together increased MCT1 activity by a factor of up to 3.5, while each isoform alone increased MCT1 activity by a factor of 1.5–2.7.181 Since diffusion of H+ to/from the transporter pore is slow, H+-coupled lactate transport via MCTs would lead to a local depletion/accumulation of H+ in the immediate vicinity of the transporter pore (termed proton microdomain), which would impair MCT transport activity.180,186 A proton antenna on only one site of the membrane could not prevent formation of the proton microdomain on the other side. This proton microdomain prevents a further increase in transport function. Combination of an intracellular and an extracellular proton antenna would prevent formation of proton microdomains on both sides of the membrane and allow maximum transport activity. Therefore, it was suggested that intracellular and extracellular CAs co-operate by a ‘push and pull’ principle—pushing protons towards the transporter pore on one side of the membrane and pulling them away from the transporter on the other side181 (Fig. 3). In cancer cells, efficient efflux of lactate and protons would therefore require the concerted action of both extracellular CAIX and intracellular CAII. This assumption is supported by a mathematical model of proton-coupled lactate transport in cancer cells.195 The model suggested the existence of local H+ pools near the cell membrane, which influence MCT-mediated lactate transport. By functioning as proton antenna, CAII and CAIX control these proton pools to provide a stable proton gradient for the transporter and drive proton-coupled lactate flux across the membrane of hypoxic cancer cells.195
Taken together, these findings demonstrate that MCT1 and MCT4 form a transport metabolon with CAII and CAIX in cancer cells (Fig. 3). Intracellular CAII binds to an acidic cluster in the transporters’ C-terminal tail, while extracellular CAIX binds to the Ig1 domain of the transporters’ chaperone CD147. Binding brings the CAs close enough to the transporter to establish an efficient proton shuttle between transporter pore and surrounding protonatable residues at the cell membrane. This proton shuttling counteracts the formation of proton microdomains around the transport and drives the export of glycolysis-derived lactate and protons from the cancer cell (Fig. 1).
Transport metabolons as potential therapeutic targets in cancer therapy
A variety of preclinical studies have demonstrated that inhibition of CAIX catalytic activity can decrease proliferation and metastatic potential of various types of tumour cells. The potential use of CAIX inhibitors for cancer therapy has been intensively discussed in various reviews139,196–198 and should therefore not be discussed again here. However, bicarbonate transport metabolons with CAIX, per se, have not been subject to preclinical investigations as drug targets in cancer cells. Nevertheless, since CAIX-mediated facilitation of bicarbonate transporters requires CAIX catalytic activity, it can be assumed that inhibition of CAIX activity by small-molecule inhibitors or antibodies also inhibits CAIX-mediated facilitation of acid/base flux via NBCs and AEs. Therefore, it appears plausible that the effects of CAIX inhibitors on tumour progression can be partly attributed to the interference with bicarbonate transport metabolons. Targeting acid/base transporters in cancer cells via CAIX might even be advantageous over direct inhibition of the acid/base transporters, since these proteins are usually expressed in a variety of healthy tissue, rendering cancer cell-specific targeting difficult. For example, the NHE1 inhibitor cariporide, which was a promising agent for treatment of myocardial infarction, failed in Phase 3 clinical trial due to severe side effects. These side effects have been attributed to the widespread expression of NHE1.199,200
Since CAIX-mediated facilitation of MCT transport activity is independent from the enzyme’s catalytic function, classical sulfonamide-based CAIX inhibitors will most likely not target the MCT–CAIX transport metabolon. Indeed, inhibition of total CA activity with ethoxyzolamide had no effect on lactate flux in hypoxic MCF-7 breast cancer cells.176 In line with that, a recent study demonstrated that inhibition of CAIX catalytic activity with ureido-substituted benzene sulfonamides, which selectively inhibit CAIX activity in breast cancer cells,201 does not suppress the CAIX-mediated facilitation of proton secretion in UFH-001 cancer cells.136 However, the application of antibodies directed against the CD147–Ig1 domain (anti-CD147) or the CAIX–PG domain (anti-PG) resulted in a significant decrease in lactate transport and reduced cell proliferation in hypoxic tumour cells.114,189 Anti-CD147 displaces CAIX from the transporter–chaperone complex, thereby acting as a ‘metabolon disruptor',189 while anti-PG was suggested to interfere with the shuttling function of CAIX.114 These findings provide a proof of concept that the MCT1/4–CD147–CAIX transport metabolon is a potential target that could be exploited to interfere with cancer cell metabolism to reduce cell proliferation and thereby inhibit tumour progression. Lactate transport in cancer cells could also be targeted by direct inhibition of MCTs.202–204 Indeed, the MCT1-specific inhibitor AZD3965 is currently in Phase 1 clinical trial (clinicaltrials.gov identifier: NCT01791595). Since MCT1 is ubiquitously expressed throughout the body, systemic MCT1 inhibition might lead to side effects. Therefore, inhibition of MCT1/4 transport activity by ‘disruption’ of the MCT1/4–CAIX transport metabolon might provide a more targeted approach than systemic blocking of the transporter via MCT1/4 inhibitors. However, without further investigations these thoughts remain purely speculative.
Conclusion
Alterations in energy metabolism and acid/base homoeostasis are emerging hallmarks of cancer cells.205,206 Tumour pH regulation is governed by the concerted interplay between various acid/base transporters and CAs, some of which form a structural and functional complex, coined the ‘transport metabolon’. Transport metabolons with CAIX have been suggested to play fundamental roles in tumour metabolism and pH regulation. CAIX can directly interact with the HCO3− transporters NBCe1 and AE2 in the leading edge of migrating cancer cells to facilitate HCO3− flux across the plasma membrane and support the generation of a pH gradient at the cell’s protruding front, which drives cancer cell migration and thereby formation of metastasis.72 CAIX was further shown to function as a ‘proton antenna’ for the lactate transporters MCT1 and MCT4, which mediate the rapid exchange of H+ between transporter pore and surrounding protonatable residues to drive proton-coupled lactate transport across the cell membrane and allow tumour cells to keep up a high glycolytic rate under hypoxia.114,176,189 Based on these findings, transport metabolons might serve as promising targets to interfere with tumour pH regulation and energy metabolism. Even though numerous studies investigated the therapeutic benefit of CAIX inhibitors for cancer treatment,196–198 transport metabolons have not been studied as therapeutic targets until now. Indeed, it could be speculated that targeting acid/base transporters via their interaction with CAIX might even provide an advantage over a direct targeting, since these transporters are expressed in a wide range of tissue, posing the risk of severe side effects by direct inhibition. CAIX, however, is almost exclusively expressed in tumour cells. CAIX transport metabolons might therefore be more specific targets than the transporters themselves. Since CAIX-mediated facilitating of HCO3− transport requires CAIX catalytic activity, conventional CAIX inhibitors can be expected to also inhibit bicarbonate transport metabolons in cancer cells. CAIX-mediated facilitation of proton-coupled lactate transport, however, appears independent from CAIX catalytic activity.114,176,189 New types of CAIX inhibitors have to be designed to target these transport metabolons, in order to suppress the direct interaction between enzyme and transporter, to interfere with lactate flux and thereby interfere with energy metabolism in tumour cells.
Acknowledgments
Author contributions
H.M.B. wrote the paper.
Competing interests
The author declares no competing interests.
Ethics approval and consent to participate
Not applicable.
Funding
The author’s own contributions to the field were funded by the Deutsche Forschungsgemeinschaft (BE 4310/6-1), the International Research Training Group (IRTG 1830/1), the Research Initiative BioComp, the Stiftung Rheinland-Pfalz für Innovation (961-386261/957) and the Landesschwerpunkt Membrantransport.
Consent to publish
Not applicable.
Data availability
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 2.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 3.Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 4.White KA, Grillo-Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017;130:663–669. doi: 10.1242/jcs.195297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Kallinowski F, Okunieff P. Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 6.Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. Physiol. 1994;267:195–203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 7.van Sluis R, Raghunand N, Bhujwalla ZM, Cerdán S, Galons J, Ballesteros P, et al. In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 2002;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths JR, Stevens AN, Iles RA, Gordon RE, Shaw D. 31P-NMR investigation of solid tumours in the living rat. Biosci. Rep. 1981;1:319–325. doi: 10.1007/BF01114871. [DOI] [PubMed] [Google Scholar]
- 9.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- 10.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 11.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers. Arch. 2009;458:981–992. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 12.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 13.Lardner A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001;69:522–530. [PubMed] [Google Scholar]
- 14.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76:1381–1390. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouyssegur J, Sardet C, Franchi A, L’Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH (H+-suicide selection/cytoplasmic pH/Na+ influx/growth control/somatic cell genetics) Cell Biol. 1984;81:4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouyssegur J, Franchi A, L’Allemain G, Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985;190:115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- 17.Putney LK, Barber DL. Na-H Exchange-dependent Increase in Intracellular pH Times G2/M Entry and Transition. J. Biol. Chem. 2003;278:44645–44649. doi: 10.1074/jbc.M308099200. [DOI] [PubMed] [Google Scholar]
- 18.Reshkin SJ, Greco MR, Cardone RA. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. R Soc. B Biol. Sci. 2014;369:20130100–20130100. doi: 10.1098/rstb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillo-Hill BK, Choi C, Jimenez-Vidal M, Barber DL. Increased H.+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife. 2015;2015:1–31. doi: 10.7554/eLife.03270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama S, Reed JC. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000;7:1155–1165. doi: 10.1038/sj.cdd.4400779. [DOI] [PubMed] [Google Scholar]
- 21.Huc L, Rissel M, Solhaug A, Tekpli X, Gorria M, Torriglia A, et al. Multiple apoptotic pathways induced by p53-dependent acidification in benzo[a]pyrene-exposed hepatic F258 cells. J. Cell Physiol. 2006;208:527–537. doi: 10.1002/jcp.20686. [DOI] [PubMed] [Google Scholar]
- 22.Hardonnière K, Huc L, Sergent O, Holme JA, Lagadic-Gossmann D. Environmental carcinogenesis and pH homeostasis: not only a matter of dysregulated metabolism. Semin. Cancer Biol. 2017;43:49–65. doi: 10.1016/j.semcancer.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Frantz C, Barreiro G, Dominguez L, Chen X, Eddy R, Condeelis J, et al. Cofilin is a pH sensor for actin free barbed end formation: role of phosphoinositide binding. J. Cell Biol. 2008;183:865–879. doi: 10.1083/jcb.200804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J. Biol. Chem. 1966;241:4110–4112. [PubMed] [Google Scholar]
- 26.Peak M, al-Habori M, Agius L. Regulation of glycogen synthesis and glycolysis by insulin, pH and cell volume. Interactions between swelling and alkalinization in mediating the effects of insulin. Biochem. J. 1992;282:797–805. doi: 10.1042/bj2820797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read JA, Winter VJ, Eszes CM, Sessions RB, Brady RL. Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins. 2001;43:175–185. doi: 10.1002/1097-0134(20010501)43:2<175::aid-prot1029>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 29.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber V, De Milito A, Harguindey S, Reshkin SJ, Wahl ML, Rauch C, et al. Proton dynamics in cancer. J. Transl. Med. 2010;8:2–5. doi: 10.1186/1479-5876-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Chao M, Wu H. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal Transduct. Target Ther. 2017;2:16047. doi: 10.1038/sigtrans.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persi E, Duran-Frigola M, Damaghi M, Roush WR, Aloy P, Cleveland JL, et al. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018;9:2997. doi: 10.1038/s41467-018-05261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itel F, Al-Samir S, Öberg F, Chami M, Kumar M, Supuran CT, et al. CO2 permeability of cell membranes is regulated by membrane cholesterol and protein gas channels. FASEB J. 2012;26:5182–5191. doi: 10.1096/fj.12-209916. [DOI] [PubMed] [Google Scholar]
- 36.Arias-Hidalgo M, Al-Samir S, Gros G, Endeward V. Cholesterol is the main regulator of the carbon dioxide permeability of biological membranes. Am. J. Physiol. Physiol. 2018;315:C137–C140. doi: 10.1152/ajpcell.00139.2018. [DOI] [PubMed] [Google Scholar]
- 37.Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 38.Bröer S, Schneider HP, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- 40.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br. J. Cancer. 2002;86:1262–1269. doi: 10.1038/sj.bjc.6600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. [DOI] [PubMed] [Google Scholar]
- 43.Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows. Arch. 2008;452:139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 45.Hao J, Chen H, Madigan MC, Cozzi PJ, Beretov J, Xiao W, et al. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br. J. Cancer. 2010;103:1008–1018. doi: 10.1038/sj.bjc.6605839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J. Bioenerg. Biomembr. 2012;44:127–139. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 47.Dovmark TH, Saccomano M, Hulikova A, Alves F, Swietach P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene. 2017;36:4538–4550. doi: 10.1038/onc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R Soc. B Biol. Sci. 2014;369:20130099–20130099. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swietach P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Rev. 2019;38:5–15. doi: 10.1007/s10555-018-09778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen Anne Poder, Samsøe-Petersen Jacob, Oernbo Eva Kjer, Boedtkjer Ebbe, Moreira José M.A., Kveiborg Marie, Pedersen Stine Falsig. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. International Journal of Cancer. 2018;142(12):2529–2542. doi: 10.1002/ijc.31276. [DOI] [PubMed] [Google Scholar]
- 51.Grinstein S, Woodside M, Waddell TK, Downey GP, Orlowski J, Pouyssegur J, et al. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: assessment of effects on intracellular pH. EMBO J. 1993;12:5209–5218. doi: 10.1002/j.1460-2075.1993.tb06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stock C, Mueller M, Kraehling H, Mally S, Noël J, Eder C, et al. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol. Biochem. 2007;20:679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- 53.Stüwe L, Müller M, Fabian A, Waning J, Mally S, Noël J, et al. pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 2007;585:351–360. doi: 10.1113/jphysiol.2007.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin C, Pedersen SF, Schwab A, Stock C. Intracellular pH gradients in migrating cells. Am. J. Physiol. Cell Physiol. 2011;300:490–495. doi: 10.1152/ajpcell.00280.2010. [DOI] [PubMed] [Google Scholar]
- 55.Ludwig FT, Schwab A, Stock C. The Na+/H+-exchanger (NHE1) generates pH nanodomains at focal adhesions. J. Cell Physiol. 2013;228:1351–1358. doi: 10.1002/jcp.24293. [DOI] [PubMed] [Google Scholar]
- 56.Stock C, Cardone RA, Busco G, Krähling H, Schwab A, Reshkin SJ. Protons extruded by NHE1: digestive or glue? Eur. J. Cell Biol. 2008;87:591–599. doi: 10.1016/j.ejcb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Vahle A-K, Domikowsky B, Schwöppe C, Krähling H, Mally S, Schäfers M, et al. Extracellular matrix composition and interstitial pH modulate NHE1-mediated melanoma cell motility. Int. J. Oncol. 2014;44:78–90. doi: 10.3892/ijo.2013.2158. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am. J. Physiol. Cell Physiol. 2013;265:1015–1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 59.Ohta T, Numata M, Yagishita H, Futagami F, Tsukioka Y, Kitagawa H, et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer. Br. J. Cancer. 1996;73:1511–1517. doi: 10.1038/bjc.1996.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu X, Qin W, Li J, Tan N, Pan D, Zhang H, et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 2005;65:6843–6849. doi: 10.1158/0008-5472.CAN-04-3822. [DOI] [PubMed] [Google Scholar]
- 61.Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG, et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J. Biol. Chem. 2009;284:16400–16408. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capecci J, Forgac M. The function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J. Biol. Chem. 2013;288:32731–32741. doi: 10.1074/jbc.M113.503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cotter K, Capecci J, Sennoune S, Huss M, Maier M, Martinez-Zaguilan R, et al. Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J. Biol. Chem. 2015;290:3680–3692. doi: 10.1074/jbc.M114.611210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulshrestha A, Katara GK, Ibrahim S, Pamarthy S, Jaiswal MK, Gilman Sachs A, et al. Vacuolar ATPase ‘a2’ isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget. 2015;6:3797–3810. doi: 10.18632/oncotarget.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Xie R, Liu X, Wen G, Jin H, Yu Z, et al. Expression and functional role of vacuolar H(+)-ATPase in human hepatocellular carcinoma. Carcinogenesis. 2012;33:2432–2440. doi: 10.1093/carcin/bgs277. [DOI] [PubMed] [Google Scholar]
- 66.Stransky L, Cotter K, Forgac M. The function of V-ATPases in cancer. Physiol. Rev. 2016;96:1071–1091. doi: 10.1152/physrev.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGuire C, Stransky L, Cotter K, Forgac M. Regulation of V-ATPase activity. Front. Biosci. (Landmark Ed) 2017;22:609–622. doi: 10.2741/4506. [DOI] [PubMed] [Google Scholar]
- 68.Boedtkjer E, Moreira JMA, Mele M, Vahl P, Wielenga VT, Christiansen PM, et al. Contribution of Na+,HCO3–cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7) Int. J. Cancer. 2013;132:1288–1299. doi: 10.1002/ijc.27782. [DOI] [PubMed] [Google Scholar]
- 69.Parks SK, Pouyssegur J. The Na+/HCO3− co-transporter SLC4A4 plays a role in growth and migration of colon and breast cancer cells. J. Cell Physiol. 2015;230:1954–1963. doi: 10.1002/jcp.24930. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, Axelsen TV, Jessen N, Pedersen SF, Vahl P, Boedtkjer E. Na+,HCO3– cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene. 2018;37:5569–5584. doi: 10.1038/s41388-018-0353-6. [DOI] [PubMed] [Google Scholar]
- 71.Boedtkjer Ebbe. Na+,HCO3− cotransporter NBCn1 accelerates breast carcinogenesis. Cancer and Metastasis Reviews. 2019;38(1-2):165–178. doi: 10.1007/s10555-019-09784-7. [DOI] [PubMed] [Google Scholar]
- 72.Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012;287:3392–3402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J. Cell Physiol. 2011;226:299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 74.Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. Regulation and roles of bicarbonate transporters in cancer. Front. Physiol. 2014;5:130. doi: 10.3389/fphys.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parks SK, Cormerais Y, Marchiq I, Pouyssegur J. Hypoxia optimises tumour growth by controlling nutrient import and acidic metabolite export. Mol. Aspects Med. 2016;47–48:3–14. doi: 10.1016/j.mam.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Parks SK, Cormerais Y, Pouysségur J. Hypoxia and cellular metabolism in tumour pathophysiology. J. Physiol. 2017;595:2439–2450. doi: 10.1113/JP273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am. J. Physiol. Physiol. 1994;267:C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- 78.Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. Am. J. Physiol. Physiol. 2001;281:C1344–C1354. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- 79.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J. Gen. Physiol. 2002;120:707–722. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J. Biol. Chem. 2009;284:6126–6139. doi: 10.1074/jbc.M802051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aronson PS, Nee J, Suhm MA. Modifier role of internal H+ in activating the Na+–H+ exchanger in renal microvillus membrane vesicles. Nature. 1982;299:161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- 83.Wakabayashi S, Hisamitsu T, Pang T, Shigekawa M. Mutations of Arg440 and Gly455/Gly456 oppositely change pH sensing of Na+/H+ exchanger 1. J. Biol. Chem. 2003;278:11828–11835. doi: 10.1074/jbc.M213243200. [DOI] [PubMed] [Google Scholar]
- 84.Lacroix J, Poët M, Maehrel C, Counillon L. A mechanism for the activation of the Na/H exchanger NHE-1 by cytoplasmic acidification and mitogens. EMBO Rep. 2004;5:91–96. doi: 10.1038/sj.embor.7400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paris S, Pouyssegur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+ J. Biol. Chem. 1984;259:10989–10994. [PubMed] [Google Scholar]
- 86.Bourguignon LYW, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J. Biol. Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 87.Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010;24:3903–3915. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 88.Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal s6 kinase (p90RSK) PLoS One. 2011;6:e28851. doi: 10.1371/journal.pone.0028851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amith SR, Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 2013;73:1259–1264. doi: 10.1158/0008-5472.CAN-12-4031. [DOI] [PubMed] [Google Scholar]
- 90.Mboge MY, Mahon BP, McKenna R, Frost SC. Carbonic anhydrases: role in pH control and cancer. Metabolites. 2018;8:19. doi: 10.3390/metabo8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pastorekova S, Zavada J. Carbonic anhydrase IX (CA IX) as a potential target for cancer therapy. Caner Ther. 2004;2:245–262. [Google Scholar]
- 92.Frost, S. C. & McKenna, R. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications (Springer Netherlands, Dordrecht 2014).
- 93.Tolvanen MEE, Ortutay C, Barker HR, Aspatwar A, Patrikainen M, Parkkila S. Analysis of evolution of carbonic anhydrases IV and XV reveals a rich history of gene duplications and a new group of isozymes. Bioorg. Med. Chem. 2013;21:1503–1510. doi: 10.1016/j.bmc.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 94.Aspatwar A, Tolvanen ME, Parkkila S. Phylogeny and expression of carbonic anhydrase-related proteins. BMC Mol. Biol. 2010;11:25. doi: 10.1186/1471-2199-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aspatwar A, Tolvanen MEE, Ortutay C, Parkkila S. Carbonic anhydrase related proteins: molecular biology and evolution. Subcell Biochem. 2014;75:135–156. doi: 10.1007/978-94-007-7359-2_8. [DOI] [PubMed] [Google Scholar]
- 96.Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelník V, Opavský R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 97.Parkkila AK, Herva R, Parkkila S, Rajaniemi H. Immunohistochemical demonstration of human carbonic anhydrase isoenzyme II in brain tumours. Histochem. J. 1995;27:974–982. [PubMed] [Google Scholar]
- 98.Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastorekova S, Pastorek J, et al. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am. J. Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karhumaa P, Kaunisto K, Parkkila S, Waheed A, Pastorekova S, Pastorek J, et al. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol. Hum. Reprod. 2001;7:611–616. doi: 10.1093/molehr/7.7.611. [DOI] [PubMed] [Google Scholar]
- 100.Yoo CW, Nam BH, Kim JY, Shin HJ, Lim H, Lee S, et al. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat. Oncol. 2010;5:1–10. doi: 10.1186/1748-717X-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng Y, Xu B, Zhao Y, Gu H, Li C, Wang Y, et al. CA1 contributes to microcalcification and tumourigenesis in breast cancer. BMC Cancer. 2015;15:1–15. doi: 10.1186/s12885-015-1707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Y, Mokhtari RB, Pan J, Cutz E, Yeger H. Carbonic anhydrase II mediates malignant behavior of pulmonary neuroendocrine tumors. Am. J. Respir Cell Mol. Biol. 2015;52:183–192. doi: 10.1165/rcmb.2014-0054OC. [DOI] [PubMed] [Google Scholar]
- 103.Wang D-B, Lu X-K, Zhang X, Li Z-G, Li C-X. Carbonic anhydrase 1 is a promising biomarker for early detection of non-small cell lung cancer. Tumour Biol. 2016;37:553–559. doi: 10.1007/s13277-015-3834-z. [DOI] [PubMed] [Google Scholar]
- 104.Parkkila Seppo, Lasota Jerzy, Fletcher Jonathan A, Ou Wen-bin, Kivelä Antti J, Nuorva Kyösti, Parkkila Anna-Kaisa, Ollikainen Jyrki, Sly William S, Waheed Abdul, Pastorekova Silvia, Pastorek Jaromir, Isola Jorma, Miettinen Markku. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Modern Pathology. 2010;23(5):743–750. doi: 10.1038/modpathol.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pastorekova S, Parkkila S, Parkkila AK, Opavský R, Zelník V, Saarnio J, et al. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 106.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, et al. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J. Histochem. Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 107.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Opavský R, Pastorekova S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, et al. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 109.Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl Acad. Sci. USA. 2009;106:16233–16238. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015;31:52–64. doi: 10.1016/j.semcancer.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Závada J, Závadová Z, Pastorek J, Biesová Z, Jez J, Jezek J, et al. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br. J. Cancer. 2000;82:1808–1813. doi: 10.1054/bjoc.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Csaderova L, Debreova M, Radvak P, Stano M, Vrestiakova M, Kopacek J, et al. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Front. Physiol. 2013;4:271. doi: 10.3389/fphys.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Innocenti A, Pastorekova S, Pastorek J, Scozzafava A, De Simone G, Supuran CT. The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg. Med. Chem. Lett. 2009;19:5825–5828. doi: 10.1016/j.bmcl.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 114.Ames S, Pastorekova S, Becker HM. The proteoglycan-like domain of carbonic anhydrase IX mediates non-catalytic facilitation of lactate transport in cancer cells. Oncotarget. 2018;9:27940–27957. doi: 10.18632/oncotarget.25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 116.Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastorekova S, Pastorek J, et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1α stabilization: a role for phosphatidylinositol 3′-kinase. Cancer Res. 2002;62:4469–4477. [PubMed] [Google Scholar]
- 117.Kopacek J, Barathova M, Dequiedt F, Sepelakova J, Kettmann R, Pastorek J, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim. Biophys. Acta - Gene Struct. Expr. 1729;2005:41–49. doi: 10.1016/j.bbaexp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Liao SY, Brewer C, Závada J, Pastorek J, Pastorekova S, Manetta A, et al. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am. J. Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- 119.Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 120.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 121.Potter CPS, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br. J. Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Proescholdt Ma, Merrill MJ, Stoerr E-M, Lohmeier A, Pohl F, Brawanski A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro. Oncol. 2012;14:1357–1366. doi: 10.1093/neuonc/nos216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 124.Švastová E, Žilka N, Zat’ovičová M, Gibadulinová A, Čiampor F, Pastorek J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Exp. Cell Res. 2003;290:332–345. doi: 10.1016/s0014-4827(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 125.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160–6165. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y, Wang X-Y, Subjeck JR, Kim HL. Carbonic anhydrase IX has chaperone-like functions and is an immunoadjuvant. Mol. Cancer Ther. 2008;7:3867–3877. doi: 10.1158/1535-7163.MCT-08-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 128.McIntyre A, Patiar S, Wigfield S, Li J-L, Ledaki I, Turley H, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 2012;18:3100–3111. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 130.Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014;4:400. doi: 10.3389/fphys.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008;283:20473–20483. doi: 10.1074/jbc.M801330200. [DOI] [PubMed] [Google Scholar]
- 132.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009;284:20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Y, Tu C, Wang H, Silverman DN, Frost SC. Catalysis and pH control by membrane-associated carbonic anhydrase IX in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2011;286:15789–15796. doi: 10.1074/jbc.M110.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen Z, Ai L, Mboge MY, Tu C, McKenna R, Brown KD, et al. Differential expression and function of CAIX and CAXII in breast cancer: a comparison between tumorgraft models and cells. PLoS ONE. 2018;13:1–25. doi: 10.1371/journal.pone.0199476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee Shen-Han, McIntyre Dominick, Honess Davina, Hulikova Alzbeta, Pacheco-Torres Jesús, Cerdán Sebastián, Swietach Pawel, Harris Adrian L., Griffiths John R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. British Journal of Cancer. 2018;119(5):622–630. doi: 10.1038/s41416-018-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mboge Mam Y., Chen Zhijuan, Khokhar Daniel, Wolff Alyssa, Ai Lingbao, Heldermon Coy D., Bozdag Murat, Carta Fabrizio, Supuran Claudiu T., Brown Kevin D., McKenna Robert, Frost Christopher J., Frost Susan C. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochemical Journal. 2019;476(10):1497–1513. doi: 10.1042/BCJ20190177. [DOI] [PubMed] [Google Scholar]
- 137.Lloyd MC, Cunningham JJ, Bui MM, Gillies RJ, Brown JS, Gatenby RA. Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer Res. 2016;76:3136–3144. doi: 10.1158/0008-5472.CAN-15-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 139.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites. 2017;7:48. doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Srere PA. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 141.Srere PA. The metabolon. Trends Biochem. Sci. 1985;10:109–110. [Google Scholar]
- 142.Deitmer JW, Becker HM. Transport metabolons with carbonic anhydrases. Front. Physiol. 2013;4:291. doi: 10.3389/fphys.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kifor G, Toon MR, Janoshazi A, Solomon AK. Interaction between red cell membrane band 3 and cytosolic carbonic anhydrase. J. Membr. Biol. 1993;134:169–179. doi: 10.1007/BF00234498. [DOI] [PubMed] [Google Scholar]
- 144.Vince JW, Reithmeier RAF. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J. Biol. Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 145.Vince JW, Reithmeier RAF. Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1. Biochemistry. 2000;39:5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- 146.Vince JW, Carlsson U, Reithmeier RAF. Localization of the Cl-/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry. 2000;39:13344–13349. doi: 10.1021/bi0015111. [DOI] [PubMed] [Google Scholar]
- 147.Sterling D, Reithmeier RAF, Casey JR. A transport metabolon: functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 148.Gross E, Pushkin A, Abuladze N, Fedotoff O, Kurtz I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: role of Asp986, Asp988 and kNBC1-carbonic anhydrase II binding. J. Physiol. 2002;544:679–685. doi: 10.1113/jphysiol.2002.029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, et al. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J. Physiol. 2004;559:55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loiselle FB, Jaschke P, Casey JR. Structural and functional characterization of the human NBC3 sodium/bicarbonate co-transporter carboxyl-terminal cytoplasmic domain. Mol. Membr. Biol. 2003;20:307–317. doi: 10.1080/0968768031000122520. [DOI] [PubMed] [Google Scholar]
- 151.Loiselle FB, Morgan PE, Alvarez BV, Casey JR. Regulation of the human NBC3 Na+/HCO3- cotransporter by carbonic anhydrase II and PKA. Am. J. Physiol. Cell Physiol. 2004;286:1423–1433. doi: 10.1152/ajpcell.00382.2003. [DOI] [PubMed] [Google Scholar]
- 152.Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3- cotransporter. J. Biol. Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 153.Schueler C, Becker HM, McKenna R, Deitmer JW. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS ONE. 2011;6:e27167. doi: 10.1371/journal.pone.0027167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson, D. E. & Casey, J. R. Bicarbonate transport metabolons. In Drug Design of Zinc-Enzyme Inhibitors (eds Claudiu T. Supuran & Jean-Yves Winum)415–437 (John Wiley & Sons, Inc., Hoboken, NJ, 2014).
- 155.Sterling D, Brown NJD, Supuran CT, Casey JR. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am. J. Physiol. Cell Physiol. 2002;283:1522–1529. doi: 10.1152/ajpcell.00115.2002. [DOI] [PubMed] [Google Scholar]
- 156.Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 2003;42:12321–12329. doi: 10.1021/bi0353124. [DOI] [PubMed] [Google Scholar]
- 157.Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. AJP Cell Physiol. 2007;293:738–748. doi: 10.1152/ajpcell.00157.2007. [DOI] [PubMed] [Google Scholar]
- 158.Li X, Alvarez BV, Casey JR, Reithmeier RAF, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 159.Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry. 2006;45:2414–2424. doi: 10.1021/bi051132d. [DOI] [PubMed] [Google Scholar]
- 160.Ro H, Carson JH. pH microdomains in oligodendrocytes. J. Biol. Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- 161.Krishnan D, Liu L, Wiebe SA, Casey JR, Cordat E, Alexander RT. Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3. Am. J. Physiol. - Ren. Physiol. 2015;309:383–392. doi: 10.1152/ajprenal.00464.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu Q, Pierce WM, Delamere NA. Cytoplasmic pH responses to carbonic anhydrase inhibitors in cultured rabbit nonpigmented ciliary epithelium. J. Membr. Biol. 1998;162:31–38. doi: 10.1007/s002329900339. [DOI] [PubMed] [Google Scholar]
- 163.Liskova V, Hudecova S, Lencesova L, Iuliano F, Sirova M, Ondrias K, et al. Cancers. 2019. Type 1 sodium calcium exchanger forms a complex with carbonic anhydrase IX and via reverse mode activity contributes to pH control in hypoxic tumors; p. 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, et al. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J. Biol. Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- 165.Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J. Biol. Chem. 2007;282:1409–1421. doi: 10.1074/jbc.M608261200. [DOI] [PubMed] [Google Scholar]
- 166.Yamada H, Horita S, Suzuki M, Fujita T, Seki G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO3− cotransporter NBCe1 expressed in Xenopus oocytes. Channels. 2011;5:106–109. doi: 10.4161/chan.5.2.14341. [DOI] [PubMed] [Google Scholar]
- 167.Al-Samir S, Papadopoulos S, Scheibe RJ, Meißner JD, Cartron J-P, Sly WS, et al. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J. Physiol. 2013;591:4963–4982. doi: 10.1113/jphysiol.2013.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, et al. The bicarbonate transport metabolon. J. Enzyme Inhib. Med. Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 169.Becker, H. M., Klier, M. & Deitmer, J. W. Carbonic anhydrases and their interplay with acid/base-coupled membrane transporters. In Sub-Cellular Biochemistry (eds Frost, S. C. & McKenna, R.) 105–134 (Dordrecht: Springer Netherlands, Dordrecht, 2014). [DOI] [PubMed]
- 170.Alvarez BV, Johnson DE, Sowah D, Soliman D, Light PE, Xia Y, et al. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J. Physiol. 2007;579:127–145. doi: 10.1113/jphysiol.2006.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Villafuerte FC, Swietach P, Youm J-B, Ford K, Cardenas R, Supuran CT, et al. Facilitation by intracellular carbonic anhydrase of Na+-HCO3− co-transport but not Na+/H+ exchange activity in the mammalian ventricular myocyte. J. Physiol. 2014;592:991–1007. doi: 10.1113/jphysiol.2013.265439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Svichar N, Waheed A, Sly WS, Hennings JC, Hubner CA, Chesler M. Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl–HCO3− exchange in hippocampal neurons. J. Neurosci. 2009;29:3252–3258. doi: 10.1523/JNEUROSCI.0036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alvarez BV, Gilmour GS, Mema SC, Martin BT, Shull GE, Casey JR, et al. Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PLoS ONE. 2007;2:e839. doi: 10.1371/journal.pone.0000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stuart-Tilley A, Sardet C, Pouyssegur J, Schwartz MA, Brown D, Alper SL. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am. J. Physiol. 1994;266:559–568. doi: 10.1152/ajpcell.1994.266.2.C559. [DOI] [PubMed] [Google Scholar]
- 175.Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, et al. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res. 2011;71:7558–7567. doi: 10.1158/0008-5472.CAN-11-2520. [DOI] [PubMed] [Google Scholar]
- 176.Jamali S, Klier M, Ames S, Barros LF, McKenna R, Deitmer JW, et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 2015;5:13605. doi: 10.1038/srep13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Becker HM, Hirnet D, Fecher-Trost C, Sültemeyer D, Deitmer JW. Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J. Biol. Chem. 2005;280:39882–39889. doi: 10.1074/jbc.M503081200. [DOI] [PubMed] [Google Scholar]
- 178.Becker HM, Deitmer JW. Nonenzymatic proton handling by carbonic anhydrase II during H+-lactate cotransport via monocarboxylate transporter 1. J. Biol. Chem. 2008;283:21655–21667. doi: 10.1074/jbc.M802134200. [DOI] [PubMed] [Google Scholar]
- 179.Becker HM, Klier M, Deitmer JW. Nonenzymatic augmentation of lactate transport via monocarboxylate transporter isoform 4 by carbonic anhydrase II. J. Membr. Biol. 2010;234:125–135. doi: 10.1007/s00232-010-9240-y. [DOI] [PubMed] [Google Scholar]
- 180.Becker HM, Klier M, Schüler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc. Natl Acad. Sci. USA. 2011;108:3071–3076. doi: 10.1073/pnas.1014293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klier M, Andes FT, Deitmer JW, Becker HM. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J. Biol. Chem. 2014;289:2765–2775. doi: 10.1074/jbc.M113.537043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Noor SI, Dietz S, Heidtmann H, Boone CD, McKenna R, Deitmer JW, et al. Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II. J. Biol. Chem. 2015;290:4476–4486. doi: 10.1074/jbc.M114.624577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Noor SI, Pouyssegur J, Deitmer JW, Becker HM. Integration of a ‘proton antenna’ facilitates transport activity of the monocarboxylate transporter MCT4. FEBS J. 2017;284:149–162. doi: 10.1111/febs.13964. [DOI] [PubMed] [Google Scholar]
- 184.Noor SI, Jamali S, Ames S, Langer S, Deitmer JW, Becker HM. A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. eLife. 2018;7:1–31. doi: 10.7554/eLife.35176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stridh MH, Alt MD, Wittmann S, Heidtmann H, Aggarwal M, Riederer B, et al. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J. Physiol. 2012;590:2333–2351. doi: 10.1113/jphysiol.2011.220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martínez C, Kalise D, Barros LF. General requirement for harvesting antennae at Ca2+ and H+ channels and transporters. Front. Neuroenergetics. 2010;2:1–8. doi: 10.3389/fnene.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fisher SZ, Maupin CM, Budayova-Spano M, Govindasamy L, Tu C, Agbandje-McKenna M, et al. Atomic crystal and molecular dynamics simulation structures of human carbonic anhydrase II: Insights into the proton transfer mechanism. Biochemistry. 2007;46:2930–2937. doi: 10.1021/bi062066y. [DOI] [PubMed] [Google Scholar]
- 188.Shinobu A, Agmon N. Mapping proton wires in proteins: carbonic anhydrase and GFP chromophore biosynthesis. J. Phys. Chem. A. 2009;113:7253–7266. doi: 10.1021/jp8102047. [DOI] [PubMed] [Google Scholar]
- 189.Ames, S., Andring, J. T., McKenna, R. & Becker, H. M. CAIX forms a transport metabolon with monocarboxylate transporters in human breast cancer cells. Oncogene; 10.1038/s41388-019-1098-6 (2019). [DOI] [PubMed]
- 190.Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010;2010:427694. doi: 10.1155/2010/427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pertega-Gomes N, Felisbino S, Massie CE, Vizcaino JR, Coelho R, Sandi C, et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: a role for monocarboxylate transporters as metabolic targets for therapy. J. Pathol. 2015;236:517–530. doi: 10.1002/path.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eilertsen M, Andersen S, Al-Saad S, Kiselev Y, Donnem T, Stenvold H, et al. Monocarboxylate transporters 1-4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS ONE. 2014;9:e105038. doi: 10.1371/journal.pone.0105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wilson MC, Meredith D, Manning Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is embigin (gp70) J. Biol. Chem. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 194.Forero-Quintero LS, Ames S, Schneider H-P, Thyssen A, Boone CD, Andring JT, et al. Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70. J. Biol. Chem. 2018;294:593–607. doi: 10.1074/jbc.RA118.005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hiremath SA, Surulescu C, Jamali S, Ames S, Deitmer JW, Becker HM. Modeling of pH regulation in tumor cells: direct interaction between proton-coupled lactate transporters and cancer-associated carbonic anhydrase. Math. Biosci. Eng. 2019;16:320–337. doi: 10.3934/mbe.2019016. [DOI] [PubMed] [Google Scholar]
- 196.McDonald PC, Winum J, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3:84–97. doi: 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs. 2018;27:963–970. doi: 10.1080/13543784.2018.1548608. [DOI] [PubMed] [Google Scholar]
- 198.Supuran CT, Alterio V, Di Fiore A. D’ Ambrosio K, Carta F, Monti SM et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med. Res. Rev. 2018;38:1799–1836. doi: 10.1002/med.21497. [DOI] [PubMed] [Google Scholar]
- 199.Avkiran M, Cook AR, Cuello F. Targeting Na+/H+ exchanger regulation for cardiac protection: a RSKy approach? Curr. Opin. Pharmacol. 2008;8:133–140. doi: 10.1016/j.coph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 200.Mentzer RM, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann. Thorac. Surg. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 201.Mboge MY, Brown KD, Bozdag M, Chen Z, Carta F, Mathias JV, et al. Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: disconnect between activity and growth inhibition. PLoS ONE. 2018;13:e0207417. doi: 10.1371/journal.pone.0207417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 202.Polański R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 2014;20:926–937. doi: 10.1158/1078-0432.CCR-13-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guan X, Bryniarski MA, Morris ME. In vitro and in vivo efficacy of the monocarboxylate transporter 1 inhibitor AR-C155858 in the murine 4T1 breast cancer tumor model. AAPS J. 2019;21:3. doi: 10.1208/s12248-018-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guan X, Rodriguez-Cruz V, Morris ME. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 2019;21:13. doi: 10.1208/s12248-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 206.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat. Cell Biol. 2002;4:97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.