Abstract
Purpose
To describe pertinent imaging studies and clinical features of a torpedo maculopathy presumably associated with congenital Zika syndrome.
Observation
A 23-month-old child, with no prematurity or microcephaly at birth, was examined in the Ophthalmology department of the University Hospital of Fort-de-France (Martinique, French West Indies), as part of a systematic screening of malformations in children suspected of maternal-fetal exposure to Zika virus. Zika infection was confirmed in the mother's serum by Reverse Transcriptase Polymerase Chain Reaction during the third trimester of pregnancy. Fundus examination found a unilateral hypopigmented retinal lesion, temporal to the macula, with an apex pointing to the fovea. Explorations in spectral-domain optical coherence tomography showed a subretinal cleft with broadening and attenuation of the interdigitation zone, elevation of the outer limiting membrane and the ellipsoid zone, without thinning of the outer retinal layers.
Conclusion and importance
There is a proven risk of congenital eye defects after Zika infection during pregnancy. We report here the first case of torpedo maculopathy without microcephaly, in a child suspected of maternal-fetal exposure to Zika.
Keywords: Zika virus, Congenital zika syndrome, Torpedo maculopathy, Spectral-domain optical coherence tomography
1. Introduction
In May 2015, an outbreak of Zika virus infections began in Brazil,1 before spreading to other Latin American countries, including the French West Indies. In Martinique, the first confirmed cases were reported in December 2015.2
This virus, first discovered in Uganda in 1947,3 is transmitted in most cases by mosquito bite (“Aedes Aegypti”). However, transmission can also occur through human-to-human, especially through perinatal or breast milk.4,5 This transmission is even thought to cause birth defects. Indeed during the 2015 epidemic, Zika was found in the amniotic fluid of pregnant women pregnant with microcephaly children, strongly suspecting a link between these two events.6 From this finding, other malformations have been described and incriminated, including numerous ophthalmological anomalies.7, 8, 9, 10 Among them, a case of torpedo maculopathy has already been described in a child with microcephaly.8 In this case, congenital Zika infection was suspected because the mother contracted a viral syndrome during the first trimester of pregnancy, in an endemic area of Zika. However, no biological confirmation of the infection had been made, neither in the mother nor the child.
Today, we describe a case of torpedo maculopathy in a newborn without microcephaly, suspected of congenital Zika infection, in the ophthalmology department of the University Hospital of Fort-de-France (Martinique, French West Indies).
2. Case report
Since 2017, Zika infection was systematically researched in every pregnant woman at the maternity hospital of Fort-de-France (Martinique, French West Indies), even in the absence of viral symptoms. Zika ribonucleic acid (RNA) was detected by Reverse Transcriptase Polymerase Reaction Chain (RT-PCR) in the patient's serum, using the RealStar® Diagnostics (Altona Diagnostics, Germany) diagnostic kit. Each confirmed case of congenital Zika infection was then isolated and the same test was performed in their newborns in the urine and serum, as well as in the placenta and umbilical cord blood. Each of these children was then examined in the ophthalmology department of the University Hospital of Fort-de-France (Martinique, French West Indies), with an examination of the fundus by indirect ophthalmoscopy, photographs (with Retcam and Canon CR-2 AF retinal camera). Zika serology (IgM and IgG antibodies by Elisa Test) was also performed in children in case of a negative RT-PCR test. In case of a proven retinal lesion, a spectral domain optical coherence tomography (ST-OCT) was performed using the Spectralis OCT system (Heidelberg, Germany).
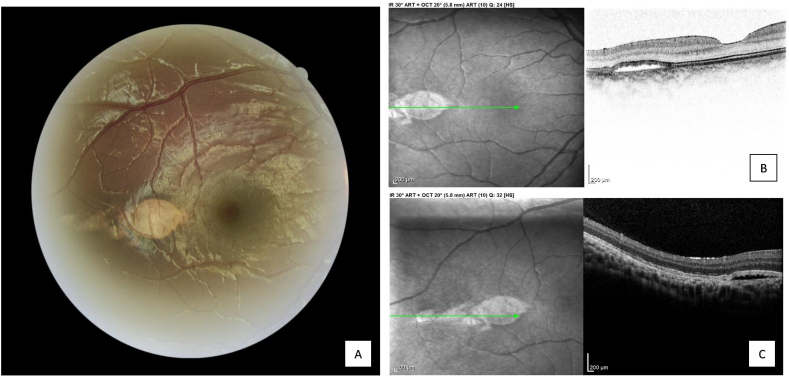
We report the case of a child born at 39 weeks, with a birth weight of 3.5 kg, without microcephaly. Zika RNA was found in the mother at the 8th month of pregnancy. RT-PCR tests in the newborn returned negative, as did those in the umbilical cord blood and placenta. Zika serology was also negative at 20 months old. Child neurological development was normal, particularly without psychomotor abnormalities. No systemic anomalies were found. An ophthalmological consultation was conducted at 23 months old. No strabismus was observed or any signs of amblyopia. Anterior segment was clear in both eyes, without iris coloboma neither congenital cataract. However, examination of the fundus revealed a retinal lesion of the right eye, which did not exist in the contralateral eye (Fig. 1). This flat hypopigmented lesion was oval-shaped, with a large horizontal axis and sharp edges. It was observed temporal to the fovea. Other hypopigmented and less differentiated lesions were present along the temporal median raphe. Optic nerves were normal in both eyes. SD-OCT shown a subretinal cleft in correspondence of the macular lesion with broadening and attenuation of the interdigitation zone, and elevation of the outer limiting membrane and the ellipsoid zone (Fig. 1). Alterations in the retinal pigment epithelium (RPE) and the interdigitation zone were also visible in correspondence of the less differentiated lesions along the median raphe. Choroidal hyper-reflectivity was also present in connection with the RPE alteration, without alterations of choroidal layers. Outer retina thickness seemed to be conserved.
Fig. 1.
Fundus imaging of torpedo maculopathy (Canon CR-2 AF) and Spectral-domain optical coherence tomography (ST-OCT, Spectralis) A, Retinal photograph in the right eye of a child with suspected congenital Zika syndrome showing a hypopigmented lesion with oval-shaped and sharp edges, temporal to the fovea. B, ST-OCT of the right eye showing a subretinal cleft with broadening and attenuation of the interdigitation zone, elevation of the outer limiting membrane and the ellipsoid zone, without thinning of the outer retinal layers. C, ST-OCT of the right eye showing RPE alterations and choroidal hyper-reflectivity.
3. Discussion
In this case, the diagnosis of torpedo maculopathy was made. It represents the first described case of torpedo maculopathy in a non-microcephaly child, highly suspected of congenital Zika infection. However, a Brazilian team described this retinal abnormality in 2016 in a microcephaly child suspected of infected with Zika during pregnancy.8 The mother had developed viral syndrome during the first trimester of her pregnancy, in an area endemic to Zika. However, no biological confirmation of Zika infection had been found neither in the mother nor the child, compared to our case.
Torpedo maculopathy is a rare condition, affecting about two cases per 100,000 patients before sixteen years old.11 Its first description was in 1992 by the Roseman and Gass team, describing hypopigmented nevus of macular pigment epithelium.12 Its origin is unknown but several hypotheses have emerged in recent years. Initially seen as a para-macular coloboma or an abnormality in the development of choroidal vascularization,13,14 Shields proposed the idea that torpedo maculopathy comes from RPE dysgenesis in the fetal temporal bulge.15 This area rich in RPE cells is also observed temporal to the fovea between the 4th and 6th month of gestation, before its involution between the 8th and 9th month.15
In our case, we obtained high quality SD-OCT images showing a subretinal cleft with RPE alterations sparing the outer nuclear layer, external limiting membrane and ellipsoid layer. However, the interdigitation zone (junction between RPE cells and photoreceptor outer segments) seemed to be altered and thickened. These tomographic characteristics have already been described by Wong in 2015, establishing two types of torpedo maculopathy lesions: “type 1” with mild outer retinal disturbance and “type 2” with outer retinal and/or inner choroidal excavation.16 According to his conclusions, these forms represent different stages of torpedo maculopathy. Our case could therefore be classified as “type 2”, according to Wong's classification.
Torpedo maculopathy is not the only ophthalmological lesion described with suspected congenital Zika infection. In 2016, Brazilian teams published successive cases of macular atrophy with or without pigmentary changes, optic atrophy and iris coloboma, in microcephaly children suspected of congenital Zika infection.8,17 Our case did not find other eye abnormalities outside of torpedo maculopathy.
In our description, it is crucial to remember that contact between the mother and Zika was confirmed during pregnancy by RT-PCR, presuming in-utero contact between the virus and the child. However, no evidence of this contact was found in birth RT-PCR tests. In addition, Zika serologies (IgM and IgG) also returned negative at 20 months old, questioning the reality of the congenital Zika infection and the link evoked with torpedo maculopathy. Nevertheless, a case of negative RT-PCR in a newborn infected with Zika virus has already been described.18 Moreover, maternal infection occurred in the third trimester of pregnancy, decreasing the risk of ocular malformation. However, with fetal temporal bulge evolving until the 9th month,15 a link with congenital Zika infection remains possible.
In the absence of biological confirmation, the extremely rare character of torpedo maculopathy suggests, in this case, a link between this abnormality and congenital Zika infection. Indeed, only 3 cases of torpedo maculopathy have been described in Martinique between 1999 and 2019. None of these cases were children.
4. Conclusion
Since the 2015 outbreak, several ocular lesions have been linked to Zika congenital syndrome. In most of these descriptions, microcephaly was found. Congenital Zika infection was then presumed without biological confirmation, neither in the mother nor the child. We report here the first case of torpedo maculopathy in a newborn without microcephaly, with presumed congenital Zika infection.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures.
Acknowledgements
None.
References
- 1.Monitoramento-dos-casos-de-dengue-e-febre-de-chikungunya-20.pdf. http://portalarquivos2.saude.gov.br/images/pdf/2015/junho/30/Monitoramento-dos-casos-de-dengue-e-febre-de-chikungunya-20.pdf [cited 2019 Jul 29]. Available from:
- 2.Emergence of Zika virus in the French West Indies and Guyana - epidemiological situation. http://www.martinique.gouv.fr/content/download/7789/41560/file/PE_Zika_2016-1.pdf [cited 2019 Jul 29]. Available from:
- 3.Dick G.W.A., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952 Sep;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti R.S., Kosoy O.L., Laven J.J. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008 Aug;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnard M., Lastere S., Teissier A., Cao-Lormeau V., Musso D. Evidence of perinatal transmission of Zika virus, French polynesia, december 2013 and february 2014. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014 Apr 3;19(13) [PubMed] [Google Scholar]
- 6.Calvet G., Aguiar R.S., Melo A.S.O. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 Jun;16(6):653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 7.de Paula Freitas B., de Oliveira Dias J.R., Prazeres J. Ocular findings in Infants with microcephaly associated with presumed Zika virus congenital infection in salvador, Brazil. JAMA Ophthalmol. 2016 Feb;134(5):529–535. doi: 10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda HA de, Costa M.C., Frazão M.A.M., Simão N., Franchischini S., Moshfeghi D.M. Expanded spectrum of congenital ocular findings in microcephaly with presumed Zika infection. Ophthalmology. 2016 Aug;123(8):1788–1794. doi: 10.1016/j.ophtha.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Yepez J.B., Murati F.A., Pettito M. Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017 May 1;135(5):440. doi: 10.1001/jamaophthalmol.2017.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventura C.V., Maia M., Dias N., Ventura L.O., Belfort R. Zika: neurological and ocular findings in infant without microcephaly. Lancet. 2016 Jun;387(10037):2502. doi: 10.1016/S0140-6736(16)30776-0. [DOI] [PubMed] [Google Scholar]
- 11.Shirley K., O'Neill M., Gamble R., Ramsey A., McLoone E. Torpedo maculopathy: disease spectrum and associated choroidal neovascularisation in a paediatric population. Eye. 2018 Aug;32(8):1315–1320. doi: 10.1038/s41433-018-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roseman R.L., Gass J.D. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Arch Ophthalmol Chic Ill 1960. 1992 Oct;110(10):1358–1359. doi: 10.1001/archopht.1992.01080220020005. [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum B.A., Hachey D.L., Messner L.V. Torpedo maculopathy. J Am Optom Assoc. 1997 Jun;68(6):373–376. [PubMed] [Google Scholar]
- 14.Pian D., Ferrucci S., Anderson S.F., Wu C. Paramacular coloboma. Optom Vis Sci Off Publ Am Acad Optom. 2003 Aug;80(8):556–563. doi: 10.1097/00006324-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Shields C.L., Guzman J.M., Shapiro M.J., Fogel L.E., Shields J.A. Torpedo maculopathy at the site of the fetal “bulge. Arch Ophthalmol. 2010 Apr 1;128(4):499–501. doi: 10.1001/archophthalmol.2010.29. [DOI] [PubMed] [Google Scholar]
- 16.Wong E.N., Fraser-Bell S., Hunyor A.P., Chen F.K. Novel optical coherence tomography classification of torpedo maculopathy: new OCT classification of torpedo maculopathy. Clin Exp Ophthalmol. 2015 May;43(4):342–348. doi: 10.1111/ceo.12435. [DOI] [PubMed] [Google Scholar]
- 17.de Paula Freitas B., Ventura C.V., Maia M., Belfort R. Zika virus and the eye. Curr Opin Ophthalmol. 2017 Nov;28(6):595–599. doi: 10.1097/ICU.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 18.Vallejo M., Acuña E., Roa J.D. Negative RT-PCR in a newborn infected with Zika virus. A Case Report. 2019;13(6):5. [Google Scholar]