Abstract
Sleeve gastrectomy is a surgical technique and a leading method in metabolic surgery. Sleeve gastrectomy gained ever-increasing popularity among laparoscopic surgeons involved in bariatric surgery and has proved to be a successful method in achieving considerable weight loss in a short time. There are some disparate effects that patients may experience after sleeve gastrectomy including a reduction in BMI, weight, blood pressure, stroke, and cancer and also a significant remission in obesity-related diseases including type 2 diabetes (T2D), Non-alcoholic fatty liver (NAFLD), cardiovascular disease, obstructive sleep apnea, and craniopharyngioma-related hypothalamic obesity as well as non-obesity-related diseases such as gout, musculoskeletal problems, ovarian disorders and urinary incontinence. The most common complications of sleeve gastrectomy are bleeding, nutrient deficiencies, and leakage. There are several studies on the impact of gender and ethnic disparities on post-operative complications. This study collects state of the art of reports on sleeve gastrectomy. The aim of this study was to analyze recent studies and review the advantages and disadvantages of sleeve gastrectomy.
Keywords: Biochemistry, Obesity, Surgery, Medical education, Laparoscopic sleeve surgery, Sleeve gastrectomy, Post-operative remission, Advantages of surgery
Biochemistry; Obesity; Surgery; Medical education; Laparoscopic sleeve surgery; Sleeve gastrectomy; Post-operative remission; Advantages of surgery.
1. Introduction
Obesity is one of the most critical risk factors of several life-threatening diseases. There are more than 1 billion overweight adults and at least 300 million obese, meaning that their BMI exceeds 30 kg/m [1]. According to recent investigations, the prevalence of obesity in adults has dramatically increased over the past ten years. Cancer death statistics show that one out of seven men, and one out of five women in the United States are obese [2]. Researchers have demonstrated that obese people in the identified classes (I, II, or III) are at the higher risk of obesity-related diseases, co-morbid conditions, lower quality of life (QOL), and increased mortality more than those in the normal range of BMI (18.5–24.9) [3,4]. Although having a healthy lifestyle seems to be an ideal option to lose weight, surgical treatment continues to be the most efficient and scientifically successful method for those with excessive amount of adipose tissue (class II or III). The gastric bypass, sleeve gastrectomy, adjustable gastric band, and biliopancreatic diversion with the duodenal switch are the most popular and common bariatric surgery (BS) procedures [5].
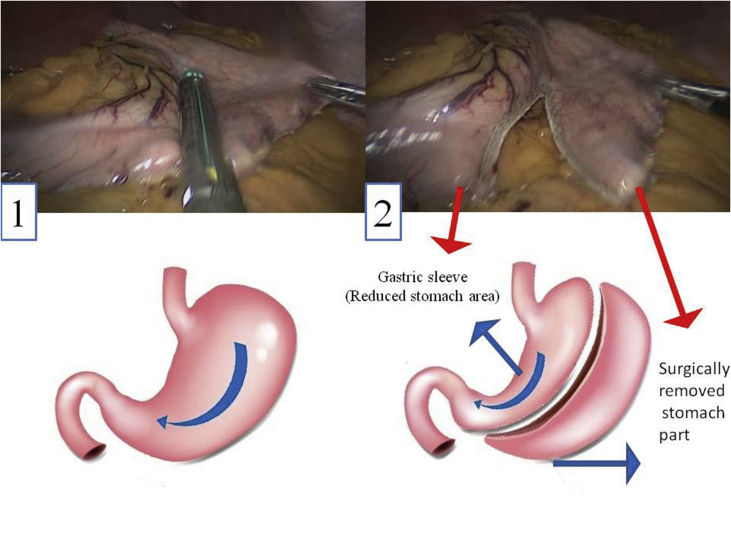
Sleeve gastrectomy is a new, safe, and efficient method for the treatment of obesity with higher survival rates among patients [6]. In this method, a large part of the stomach, which accounts for the regulation of appetite, is resected (Figure 1). Over the last years sleeve gastrectomy has captured remarkable surgical interest mainly because this technique does not require a gastrointestinal anastomosis or intestinal bypass (Figure 2). It is minimally invasive and is considered less technically challenging than laparoscopic Roux-en-Y gastric bypass (LRYGB). Sleeve gastrectomy avoids implantation of an artificial device around the stomach, whereas in laparoscopic adjustable gastric banding technique (LAGB) inflatable silicone device is placed around the top portion of the stomach to decrease food consumption.
Figure 1.
Vast part of stomach is resected in sleeve gastrectomy. 1) Stomach of patient with BMI of 42 before operation. 2) Around 80% of stomach fundus area is resected through laparoscopic technique. The figures were taken through the procedures of sleeve gastrectomy under supervision of Dr. Taha Anbara at Erfan Niayesh Hospital and consent was gathered from the patient.
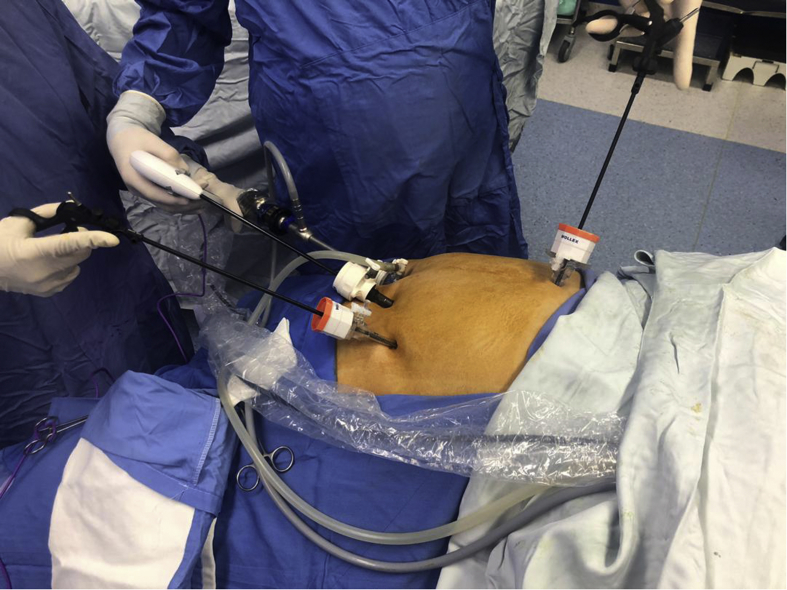
Figure 2.
The laparoscopic technique. This procedure has quickly attracted considerable surgical interest because it does not require a gastrointestinal anastomosis or intestinal bypass and thanks to the laparoscopic technique. The figure was taken through the procedures of sleeve gastrectomy at Erfan Niayesh Hospital and consent was gathered from the patient.
This study centers around the advantages and disadvantages of sleeve gastrectomy in a review of the literature. We tried to present reliable reports about sleeve gastrectomy as a definitive procedure for morbid obesity and to review the positive or negative operational effects of sleeve gastrectomy in different studies from 2014 to 2019.
2. RYGB and sleeve gastrectomy
RYGB and sleeve gastrectomy are currently the most popular bariatric techniques worldwide. While several studies from Switzerland, Finland, and the United States have reported no statistical significance between RYGB and sleeve gastrectomy in regular and excessive weight loss (EWL) [7, 8, 9], a new and multicenter cohort study [10] showed that RYGB led to significant weight loss and further improvement in co-morbidities, especially metabolic disorders such as type 2 diabetes mellitus (T2DM) [11]. Some other studies introduced RYGB as a technically challenging and more complicated method than sleeve gastrectomy, with almost double the rate of complications [10].
3. Sleeve gastrectomy not only a malabsorptive procedure but also, a metabolic procedure
Bariatric surgery initially intended to change anatomy and to alter behavior subsequently, but now we understand that anatomical changes modulate physiology to change behavior [12]. It's no longer considered only mechanically restrictive and/or malabsorptive procedure; instead, is more considered metabolic procedure involving complex physiological changes [13]. Both restriction and hormonal modulation achieve weight loss following sleeve gastrectomy. Reduction in stomach size with sleeve resection restricts distention and increases the patient's satiety (decreasing meal portion size) [14]. This restriction is further facilitated by the natural band effect of the pylorus, which maintains intact during the sleeve gastrectomy. As early evidence suggests, sleeve gastrectomy surgery reduced the hunger feeling of patient. This might be attributed to the decreasing serum levels of ghrelin, a hormone produced mainly by P/D1 cells, which stimulates hunger feeling [6]. Gut Microbiota and its impact on the Gut-Brain axis also may cause a significant decrease in appetite [12].
4. The advantages of sleeve gastrectomy
4.1. Weight loss
A morbidly obese patient would experience a series of physical changes after sleeve gastrectomy, including a significant long-term weight loss (up to 80% EWL; Around 10 % less than RYGB), maintenance of EWL percentage in a long term, hunger reduction, satiety, food preference changes, and energy expenditure increase [3]. The reduction of BMI percentage is significantly associated with changes in plasma high sensitivity C-reactive protein (hs-CRP) [15].
4.2. Remission of mental problems
Higher preoperative depression, phobic anxiety, interpersonal sensitivity, and binge eating are associated with low postoperative weight loss in patients undergoing sleeve gastrectomy [16, 17]. Several studies have indicated that sleeve gastrectomy in morbidly obese patients has reduced mental problems [16], but further studies are needed to assess the preoperative prevalence of syndromic or subsyndromal atypical depression and its relationship with postoperative weight loss in bariatric surgery candidates [17, 18].
Due to the significant association of depression with obesity, it is one of the common disorders among individuals selected to undergo bariatric surgery. Different studies show that bariatric surgery might be associated with a modest reduction in clinical depression over the initial post-operative years [19]. Researchers found significant improvement in physical, psychosocial, and sexual QOL after bariatric surgery that as a result led to a considerable weight loss, whereas more mediocre improvement in physical, psychosocial, and sexual QOL has been reported in higher preoperative depression [4, 20]. Some other findings indicated significant improvement in psychological dimensions and eating behavior after sleeve gastrectomy. None of the psychological dimensions is associated with the percentage of EWL, which prompts the question of reliable psychological predictors. In clinical contexts, patients with low cognitive restraint would need individual support after bariatric surgery to be able to cope with their new anatomic conditions [16].
4.3. Improvement of clinical markers
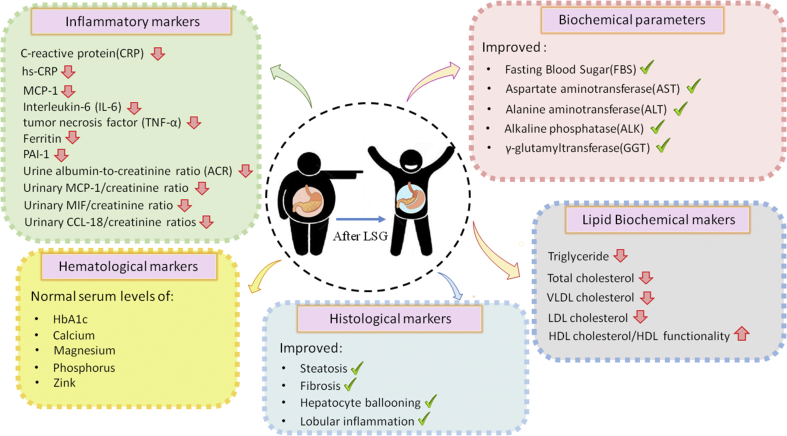
Sleeve surgery has considerable regulatory impacts on a variety of clinical parameters, including serum lipid profile constituents, biochemical, histological, hematological, and inflammatory markers which all of them represent as health indexes. A summary of the sleeve gastrectomy effects on the majority of physiological parameters is presented in Figure 3.
Figure 3.
The details of changes in clinical markers and summary of the sleeve gastrectomy effects on the majority of physiological parameters.
4.3.1. Biochemical parameters
Biochemical parameters clinically represent organs’ health levels. After sleeve gastrectomy, biochemical markers change respectively and some reports have given good news about the improvement of their serum levels. Improvement was found in the serum levels of Fasting Blood Sugar (FBS), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK), and γ-glutamyltransferase [21, 22, 23]. In several studies, mean TSH levels were decreased, whereas low free thyroxine (FT4) levels remained the same after surgery; however, TSH decrease was directly related to baseline TSH levels and not to EWL percentage. There were significant reductions in Urine albumin-to-creatinine ratio (ACR) as well [14]. In another study, one year after surgery normal serum levels of albumin and calcium in the sleeve gastrectomy patients were observed [24]. On the contrary, some researchers indicated that no significant changes in serum levels of glucose, albumin, blood urea nitrogen (BUN), Creatinine and eGFR can be observed six months after sleeve surgery [25]. In another study, a remarkable reduction in the serum levels of creatinine after bariatric surgery was observed, but no significant decrease in cystatin C levels was noted. No correlation was indicated between the UACR and BMI, adiponectin, leptin, resistin, or insulin resistance, while High-Molecular-Weight adiponectin increased and leptin levels reduced significantly [26]. Some studies also reported a sharp drop in the uric acid levels 13 months after bariatric surgery compared to baseline values, which led to a decrease in the incidence of gouty attacks [27]. According to different studies, the rs712221 polymorphism of ESR1 influences the reduction of the uric acid serum levels after bariatric surgery [28]. In fact, patients with the rs712221 genotype showed better glycemic control and more decrease in uric acid levels 12 months after surgery [28]. The general biochemical profile revealed discrepancies involving serum albumin, uric acid, creatinine, AST, and ALT to be higher in men [29].
4.3.2. Lipid disorders
Obese patients are severely involved in hyperlipidemia and other lipid disorders, which could be highly attributed to their unhealthy lifestyle. Sleeve gastrectomy has shown regulatory impacts on lipid markers after the operation (75% remission in lipid disorders). A considerable reduction can be observed in triglyceride [30], total cholesterol, VLDL cholesterol, and LDL cholesterol levels [31]. Although significant growth was observed in serum levels of HDL cholesterol [26, 31] and HDL functionality [32], some other studies showed no significant increase in HDL cholesterol serum levels after sleeve gastrectomy [26]. Surprisingly, in an interesting report in Brazil, HDL cholesterol levels became higher in females [29] and LDL cholesterol and total cholesterol were more tended to be different in men [29]; although the difference wasn't strictly significant. Considering the importance of this matter, more studies are needed to be done to clarify the association of gender with the co-morbidities after sleeve gastrectomy (See Gender and complications section).
4.3.3. Histological markers
Histological improvement, including fibrosis, steatosis, ballooning degeneration, and lobular inflammation was noticed in the non-alcoholic fatty liver activity score of patients after sleeve gastrectomy [33, 34, 35]. Several studies have demonstrated that the histological improvement was more considerable among those who underwent sleeve gastrectomy in comparison to those who underwent RYGB; however, it wasn't statistically significant [23].
4.3.4. Hematological markers
The close relationship between morbid obesity and alterations in the coagulation system was confirmed in several investigations [26, 36, 37]. Reduction in cardiovascular risk leads to a significant decrease in the thrombin generation; the critical process in hemostasis [36]. In a research, it was reported that after one-year post-surgery, patients were experiencing a normal serum levels of hemoglobin and calcium [24], as well as a significant decrease in serum levels of HbA1c and platelet [26]; however, in another study hemoglobin and hematocrit were less than normal for 28.6% and 25% of patients respectively, but ferritin, iron, and total iron-binding capacity remained the same a year after [37]. No complications with calcium, magnesium, phosphorus, and zinc level were observed, although magnesium increased significantly from baseline after a year [29]. HbA1c serum levels also went up in 30% of the samples after a year [37] but, not significantly remarkable, HbA1c tended to be higher in men [29]. Serum levels of ferritin were also considerably different among men [29].
4.3.5. Inflammatory markers
Observations showed more improved systemic and urinary inflammatory marker with a significant decrease in urinary MIF/creatinine, MCP-1/creatinine, and CCL-18/creatinine ratios [38] and also in the blood levels of MCP-1, interleukin-6 (IL-6), CRP, ferritin, and PAI-1 after sleeve gastrectomy [15, 39]. Researchers also indicated a significant reduction in hs-CRP and the urine albumin-to-creatinine ratio (ACR) as well [25]. The reduced levels of CRP and urinary cytokines suggest that bariatric surgery improves systemic and renal inflammatory status [38]. The serum concentrations of IL-6 and TNF-α were seen to decrease following the surgery in both RYGB and sleeve gastrectomy procedures [40]. Both techniques may improve the course of chronic diseases and the state of inflammation associated with obesity [40]. Sleeve gastrectomy has also shown to decrease albuminuria in patients with severe obesity and normal kidney function by affecting the regulation of inflammatory markers and reducing systemic inflammation [25].
4.4. Obesity-related disease resolution
4.4.1. Type 2 diabetes (T2D)
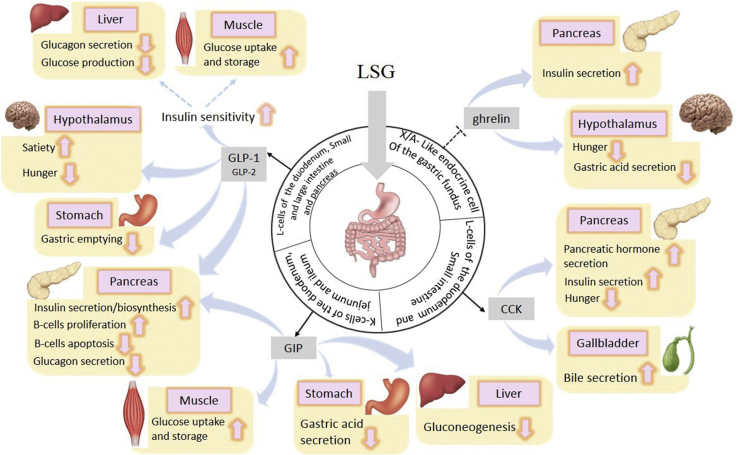
Recent studies showed that among obese patients (BMI from 27 to 43) with T2D, sleeve gastrectomy plus intensive medical therapy was more effective and practical in reducing the hyperglycemia than intensive medical therapy alone [13, 41, 42]. After sleeve gastrectomy, insulin sensitivity increased impressively, along with a significant reduction in FBS and HbA1c levels [24, 37]. This is mainly because of the decrease in the ghrelin serum levels as well as the increase in CCK (a neuropeptide that stimulates insulin secretion), GIP, GLP1, and GLP2 which plays a key role in diabetes resolution and metabolic control (Figure 4) [43,44]. Insulin resistance remission was also associated with serum uric acid decline in severely obese patients undergoing bariatric surgery [28].
Figure 4.
Clinical marker changes after sleeve gastrectomy.
4.4.2. Non-alcoholic fatty liver disease (NAFLD)
Obesity is a risk factor for NAFLD (Non-alcoholic fatty liver disease) and NASH (Nonalcoholic steatohepatitis). 85% of patients with NAFLD were improved after weight loss induced by sleeve gastrectomy and the biochemical improvement was found in serum levels of ALT, γ-glutamyltransferase, and AST [34, 35, 45]. The histological improvement was also noticed in the NAFLD activity score and individual components including steatosis, ballooning degeneration, and lobular inflammation. Fibrosis stage also showed significant improvement [34, 35]. Improvement in NAFLD activity score was various among different ethnics [46].
4.4.3. Cardiovascular disease
Morbid obesity and the coagulation system have a clear relationship [26, 36, 37]. Sleeve surgery for weight loss has proven to remarkably increase life expectancy and reduce cardiovascular risk in morbidly obese patients [36]. Thrombin generation greatly decreased after weight loss but this reduction might be contributed to the reduction of cardiovascular risk that is generally associated with morbid obesity [36]. Even though post-operative death reports might be more than healthy obese subjects after sleeve gastrectomy (2.4% and 1.39%, respectively), the number of cardiovascular diseases, myocardial infarction, stroke, and systolic blood pressure (SBP) significantly reduced about ten years after sleeve gastrectomy [47]. No significant difference was detected in SBP and diastolic blood pressure (DBP) six months after bariatric surgery in some other investigations [22, 25].
4.4.4. Obstructive sleep apnea
Sleeve gastrectomy's direct impacts on obstructive sleep apnea led to improvement of respiratory disturbance which consequently improved sleep quality on morbidly obese patients after the operation [48]. Additionally, minimum oxygen saturation and rapid eye movement latency improved and the requirement for continuous positive airway pressure decreased [3]. For 85.4% of patients the resolution occurred with snoring after sleeve gastrectomy [49].
4.4.5. High blood pressure
Several reports have shown that high blood pressure is resolved after sleeve gastrectomy [23, 39]. Although the resolution of hypertension is the ideal goal, any improvement in hypertension may translate to reduced cardiovascular events or may be considered as a surrogate marker for morbidities like cardiovascular disease [50].
4.4.6. Stroke and heart attack
Obesity is associated with a higher rate of stroke incidence. The data showed that there was a 50 percent lower death rate among participants with bariatric surgery, which considerably reduced the risk of stroke and heart attack among them as well [51].
4.4.7. Craniopharyngioma-related hypothalamic obesity
Craniopharyngioma is a rare type of brain tumor and hypothalamic obesity is considered an adverse consequence of such tumors in the brain. Weight loss after RYGB, but not sleeve gastrectomy, was comparable between patients with craniopharyngioma-related hypothalamic obesity and control subjects [52] (see Table 1).
Table 1.
Reduction in obesity-related comorbidities.
| Author | Year | Type of Disease | Remission (Percent) | Excess weight loss (EWL) | Ref. |
|---|---|---|---|---|---|
| Peterli R | 2018 | morbid obesity | 86.2% after one year | - | [8] |
| Capoccia D | 2018 | Diabetes mellitus | At two months 27% and at six months 63% | - | [79] |
| P. Sieber | 2014 | Type 2 diabetes | 85% after five years LSG | After 1 year: 61.5%After 2 years: 61.1% and after 5 years 57.4% | [80] |
| Ruiz-Tovar J | 2019 | Insulin resistance in 59.2%, dyslipidemia in 23.5%, hepatic steatosis in 16%, and type 2 diabetes mellitus in 3.9% (of 51 patients) | 76% after LSG | At 6 months and 1 and 2 years was 94.6%, 96.2%, and 92.9%, respectively | [81] |
| E. George | 2012 | Diabetes in obese patients | - | After 72, 84, and 96 months LSG:52%, 43%, and 46% | [82] |
| R. Paluszkiewicz | 2012 | morbid obesity and obesity-related comorbidities | -- | At 12 months: 67% | [83] |
| I. Golomb | 2015 | Diabetes in obese patients | -- | After the one year: 76.8% After the three year: 69.7% After the five year: 56.1% |
[79] |
| V. Våge | 2014 | Morbid obesity and obesity-related diseases | 80.7% after two years | --- | [49] |
| W. Lee | 2011 | Type 2 Diabetes Mellitus | 47% after 12 month | --- | [84] |
| M. Milone | 2013 | Diabetes in obese patients | After three months: 62% After six months: 68% After 12 months: 87% |
-- | [85] |
| F. Abbatini | 2012 | Obese diabetic patients | After three months: 29/33 After 12 months: 29/33 After 36 months: 22/26 After 36 months: 10/13 |
-- | [86] |
| A. Algooneh | 2016 | Non-alcoholic fatty liver disease (NAFLD) | 56 % complete resolution of NAFLD after LSG | 55.7% ± 23.0 | [87] |
| J. Ruiz-Tovar | 2017 | Non-alcoholic fatty liver disease (NAFLD) | 90 % complete resolution of NAFLD after LSG | -- | [88] |
| M. Manco | 2017 | Obese Adolescents with Non-alcoholic fatty liver disease (NAFLD) | --- | 21.5% after 1 year | [89] |
| M. Iancu | 2013 | Coronary heart disease (CHD) | --- | 67.3 and 78.3 at six and 12 months | [90] |
| P. Major | 2017 | Cardiovascular disease | --- | 53.18% after one year | [91] |
| D. Gutierrez Blanco | 2017 | Cardiovascular disease | -- | 68.15% after one year | [92] |
| R. Wilhelm | 2014 | Hypertension | Hypertension resulotion:34% of patients | --- | [50] |
| S. Mashaqi | 2018 | Obstructive sleep apnea (OSA) | Apnea-hypopnea index (AHI) resolution: 40 events per hour and seven events per hour after LSG (80%) | --- | [93] |
| A. Christel | 2016 | Obstructive sleep apnea (OSA) | --- | 65.5 % | [94] |
4.5. Non-obesity-related diseases remission
The majority of diseases are not directly associated with obesity but patients might show improvement and remission on these conditions after sleeve gastrectomy (Table 2). Multivariable analysis showed that bariatric surgery was associated with a lower risk of renal failure, pneumonia, sepsis, urinary tract infection, and respiratory failure [53].
Table 2.
The most common postoperative complications of SG.
| Complication | Frequency % (Mean ± SD) |
Population (Aggregate) |
Author, Year | Ref. |
|---|---|---|---|---|
| Leakage | 1.27% ± 0.99 | 6242 | Sammour, 2017; Hoogerboord, 2014; Duran, 2019; Alizadeh, 2019; Sakran, 2016; |
[95] [96] [24] [97] [98] |
| Hemorrhage | 1.77% ± 0.32 | 6994 | Hoogerboord, 2014; De Angelis, 2016; Goitein, 2015; Gagner, 2013; Thereaux J, 2019; Sammour, 2010; Sakran, 2016; |
[99] [100] [96] [101] [102] [95] [98] |
| Kidney stones | 1.45 ± 0.35 | 869 | Peterli, 2017; C.lienke, 2015; |
[103] [104] |
| Choleystectomy (For newly acquired gallstones) |
3 ± 0.7 | 868 | Peterli, 2017; Wood, 2019; |
[103] [105] |
| Insufficient weight loss | 2.35 ± 0.35 | 255 | Dang, 2019; Peterli, 2017; |
[10] [103] |
| Splenic injury | 0.30 ± 0.1 | 630 | Gagner, 2013; Gibson, 2015; |
[101] [107] |
| Liver injury | 3.60 ± 3.40 | 583 | Gagner, 2013; Sweeny, 2019; |
[101] [108] |
| Portal vein thrombosis | 0.852 ± 0.76 | 5238 | Gagner, 2013; Salinas, 2014; Duran, 2019; Moy, 2008; Sakran, 2016; |
[101] [109] [24] [110] [98] |
| Venous thromboembolism | 0.16 ± 0.12 | 975 | Gagner, 2013; Genco, 2017; Magee, 2010; |
[101] [111] [112] |
| Respiratory failure | 3.16 ± 1.29 | 239 | Moy, 2008; Duran, 2019; Stroh, 2009; |
[110] [24] [113] |
| Abscess | 0.36 ± 0.33 | 3167 | Thereaux J, 2019; Sakran, 2016; |
[102] [98] |
| Sleeve stricture | 0.40 ± 0.30 | 3167 | Thereaux J, 2019; Sakran, 2016; |
[102] [98] |
| Choledocholithiasis | 5.15 ± 4.45 | 1543 | Thereaux J, 2019; Mishra, 2016; |
[102] [114] |
| Nondysplastic Barrett's esophagus | 15.16 ± 2.04 | 254 | Genco, 2017; Soricelli, 2018; |
[115] [116] |
| Pneumonia | 3.65 ± 2.85 | 257 | Duran, 2019; Cuomo, 2019; |
[24] [117] |
| Sepsis | 0.80 ± 0.08 | 262 | Duran, 2019; Stroh, 2009; |
[24] [113] |
| Infection | 1.33 ± 0.61 | 379 | Moy, 2008; Duran, 2019; Stroh, 2009; |
[110] [24] [113] |
| Minor complications | 7% ± 3 | 196 | Thereaux J, 2019; Hoogerboord, 2014; |
[102] [96] |
| Mortality |
0.33 ± 0.33 |
865 |
Gagner, 2013; Magee, 2010; |
[101] [112] |
| Nutritional Deficiency | ||||
| Vitamin D | 30.5 ± 0.50 | 1064 | Peterli, 2017; M. Koffman 2005; |
[103] [118] |
| Vitamin B12 | 30.5 ± 5.50 | |||
| Iron | 17.85 ± 4.15 | 140 | Peterli, 2017; Sallé, 2010; |
[103] [119] |
| Zink | 7.40 ± 6.59 | 140 | Peterli, 2017; Sallé, 2010; |
[103] [119] |
| Folate | 13.65 ± 4.35 | 1064 | Peterli, 2017; M. Koffman 2005; |
[103] [118] |
4.5.1. Gout
Obesity is a risk factor for the development of several inflammatory and immune-mediated conditions such as psoriasis, lupus, inflammatory bowel disease, and gout [53]. An increased incidence of early gouty attacks after bariatric surgery has been reported, but the data is sparse. The influence of weight loss surgery on the behavior of gout, beyond the immediate postoperative phase remains unclear. In a recent study, the incidence after a month up to a year was decreased significantly [27].
4.5.2. Musculoskeletal pain
Joint pain is a common musculoskeletal complaint of morbidly obese patients that can lead to gait abnormalities, perceived mobility limitations, and declining QOL but it cannot be considered as an obesity-related disease. Improvements in some, but not all, gait parameters, walking speed, QOL, and perceived functional limitations occurred three months after a bariatric procedure [54]. There was a higher frequency of multiple musculoskeletal pain complaints, including non-weight-bearing sites compared to historical controls before surgery which lowered remarkably at most sites following weight loss and physical activity [4]. Rapid and sustained increase of sclerostin and bone turnover markers (CTX and P1NP) also caused an increase in bone metabolism and resulted in more bone mineral density loss at all skeletal sites [55]. 100% resolved joint pain within 12 months after surgery was also observed in another study [4].
4.5.3. Ovarian disorders
Obese women are at higher risk for several pregnancy complications, such as preeclampsia, gestational diabetes, cesarean delivery (particularly for failure to progress), longer stages of labor (first and second), polyhydramnios, and difficulty in spinal and epidural placement. Above that, some studies among obese women revealed that there was a higher risk of neural tube defects and neonatal mortality in their newborn [56]. Several changes in female reproduction including the partial recovery of luteal function [57], enhanced sexual function [58], higher rates of fertility treatments and reduction in the risk of miscarriage, pregnancy complications, and fetal macrosomia [59] were also indicated in a couple of studies. Moreover, amenorrhea was resolved in all premenopausal females after sleeve gastrectomy in several investigations [49].
Polycystic ovary syndrome (PCOS) is the most common cause of female infertility. Visceral obesity and insulin resistance are the fundamental pathophysiological mechanisms behind PCOS [60]. Different researches showed that sleeve gastrectomy improved hirsutism and PCOS but more is required to figure out which technique (RYGB, sleeve gastrectomy, or any other) would be a better option for the young infertile women [60].
4.5.4. Pregnancy and fertility
Despite the increased fertility rate among patients following BS, pregnancy within 18 months is not recommended. it is mainly because of the adverse consequences affecting both mother and the fetus. Ideally, stabilizing the weight after sleeve gastrectomy needs to be considered before pregnancy in patients [59].
4.5.5. Urinary incontinence
Epidemiological studies document obesity as an important risk factor for urinary incontinence. Over the last two decades, the incident urinary incontinence has increased by 30%–60% for each unit in BMI [61]. There might be a stronger association between increasing weight and prevalent stress incontinence than the association of increasing weight with urge incontinence and overactive bladder syndrome [61]. Surgical weight loss is considered the most practical and effective technique to reduce urinary incontinence symptoms (Up to 73% of patients after sleeve gastrectomy) and should be applied as the first-line treatment in these patients [61].
4.5.6. Cancer
Obesity is one of the most influential risk factors for cancer [62]. Sleeve gastrectomy is associated with a significant reduction in cancer incidence and mortality. The cancer-protective role of sleeve surgery is considered the strongest for female obesity-related tumors; however, the underlying mechanisms may involve both weight-dependent and weight-independent effects [63]. In a research among Swedish patients, researchers found an unexpectedly higher prevalence of cancer in female underwent bariatric surgery than obese men [64]. Understanding the precise metabolic mechanisms preventing cancer by metabolic surgery can widen our horizon of how obesity, diabetes, and metabolic syndrome are associated with tumorigenesis and growth [63].
5. The disadvantages of sleeve gastrectomy
5.1. Intra-operative complications
Bleeding, leakage, and gastric fistulae are the most common intraoperative complications and post-operative complications after sleeve gastrectomy [53]. The majority of publications are focused on the post-operative effects rather than intra-operative leaks and bleeds. The methods using for detecting intra-operative staple line bleeds are not standardized but present rather a different challenge in which bleeds are often undocumented or considered as a nuisance and are routinely treated with cauterization, sutures, sealants, and clips or might be self-resolved by the application of pressure along the staple line. Very few studies have addressed the impact of intra-operative leaks and bleeds on other complications or factors such as operative time, cost, and length of stay [65]. A research group reported that while bleeds did not affect operative time in their sleeve gastrectomy operation, they did disrupt the momentum of the operation [66]. Various studies support the premise that intra-operative staple line leaks and bleeds are primarily associated with stapler misfires [65].
5.2. Early complications
A variety of complications can happen in the post-operative period. The most common complications among patients during this time include pulmonary emboli, hemorrhage, chest infections, abscess, incisional hernia, relaparoscopy for retained drain, anatomic leakage, wound infections, gastroesophageal reflux disease (GERD), and rhabdomyolysis in men [5, 49, 67]. Also, dumping usually occurs around an hour after eating and presents with symptoms of bloating, flushing, diarrhea, and light-headedness [67].
5.3. Nutritional and metabolic complications
The most common micronutrient deficiencies are of vitamin B12, iron, calcium, and vitamin D [67]. Other micronutrient deficiencies that can lead to severe complications include thiamine, folate, and fat-soluble vitamins [67, 68]. Investigations show that sleeve gastrectomy mostly led to health improvements three years after surgery and at year five, the nutrient levels reverted toward the baseline values [37]. These observations draw attention to the necessary clinical monitoring in the first five years. According to a prospective study, patients experienced fewer nutrient deficiencies after sleeve gastrectomy than the deficiencies they experienced after LRYGB [69].
5.4. Insufficient weight loss
Catheline et al. realized that 77% of patients who had a follow up greater than 18 months showed significant weight loss; however, 23% of patients had insufficient weight loss (defined between 35 to 40 kg/m2 by BMI), progressive weight regain, or persistence of co-morbidities [42, 70]. In insufficient weight loss cases, a second-stage operation like relaparoscopic sleeve gastrectomy or gastric bypass can be proposed [70]. Based on different studies, just a small proportion of patients with insufficient weight loss, about 2.5 %, required a second operation [70, 71, 72].
5.5. Gender and complications
LDL cholesterol and total cholesterol levels were more different in males [29]. Over the last years, some investigations have proven that women are more addicted to sweets than meat products. During reproductive ages, women are naturally susceptible to iron deficiency and anemia [73]. Men, on the other side, tend to be heavier with larger muscle mass that may increase surgical time and general postoperative complications including rhabdomyolysis [74]. More studies are needed to find out the relationship between gender and the co-morbidities of sleeve gastrectomy.
5.6. Ethnic disparities and complications
Although the race and ethnicity are not independently associated with the likelihood of proceeding with bariatric surgery, studies showed that Asians compared with Caucasians are most susceptible to the metabolic complications of obesity at a much lower body mass index [75]. Studies among Indian patients have also demonstrated a higher risk of obesity-related diseases and NAFLD at a much lower body mass index [46]. African-Americans populations showed higher rates of remission compared to European-Americans patients [76]. Coleman et al. indicated that White and Hispanic people experienced more EWL in comparison with Black populations, and Blacks were also more susceptible to post-operative complications compared to White and Hispanics [77]. In another study, the acute renal failure in Hispanic subjects was considerably higher compared to Blacks [78].
6. Conclusion
The surveyed pieces of the literature suggest that sleeve gastrectomy is a safe and efficient technique with no mortality and co-morbidities resolution and less complication. Simple anatomical alterations of the gastrointestinal tract have both intentional and unintentional consequences. The more we learn about these alterations, the more it becomes evident to us that metabolic surgery is more than just a means of weight loss. Whether it can be recommended as a treatment for obesity-related co-morbidities such as NAFLD and cancer remains in controversy. Studying these operations not only helps to improve the effects of surgery, but also gives wider insights into understanding integrated physiology to harness the benefits of surgery without using the scalpel. For further studies, we suggest using rodent models with a series of benefits that make bariatric surgery procedures possible. Rodents are small and breed quickly, making the research possible on large numbers with complex diseases. Applying the knowledge of the gut-brain axis mechanism of action and implementing the data on the physiological bases of food intake regulation in clinical practice may allow for the more functional management of the obesity epidemic.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
The study has been done at: Department of Surgery, Erfan Hospital, Tehran, Iran.
References
- 1.Parikh N.I., Pencina M.J., Wang T.J., Lanier K.J., Fox C.S., D’Agostino R.B. Increasing trends in incidence of overweight and obesity over 5 decades. Am. J. Med. 2007;120(3):242–250. doi: 10.1016/j.amjmed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Marmot M., Atinmo T., Byers T., Chen J., Hirohata T., Jackson A. 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- 3.Sinclair P., Brennan D.J., le Roux C.W. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat. Rev. Gastroenterol. Hepatol. 2018;1 doi: 10.1038/s41575-018-0057-y. [DOI] [PubMed] [Google Scholar]
- 4.Bout-Tabaku S., Gupta R., Jenkins T.M., Ryder J.R., Baughcum A.E., Jackson R.D. Musculoskeletal pain, physical function, and quality of life after bariatric surgery. Pediatrics. 2019 doi: 10.1542/peds.2019-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X., Karmali S., Sharma A.M., Birch D.W. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes. Surg. 2010;20(8):1171–1177. doi: 10.1007/s11695-010-0145-8. [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L., Narbro K., Sjöström C.D., Karason K., Larsson B., Wedel H. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 7.Salminen P., Helmiö M., Ovaska J., Juuti A., Leivonen M., Peromaa-Haavisto P. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterli R., Wölnerhanssen B.K., Peters T., Vetter D., Kröll D., Borbély Y. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullally J.A., Febres G.J., Bessler M., Korner J. Sleeve gastrectomy and Roux-en-Y gastric bypass achieve similar early improvements in beta-cell function in obese patients with type 2 diabetes. Sci. Rep. 2019;9(1):1880. doi: 10.1038/s41598-018-38283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang J.T., Karmali S. Is RYGB more effective than sleeve gastrectomy? Nat. Rev. Endocrinol. 2019;15(3):134–135. doi: 10.1038/s41574-018-0152-8. [Internet] [DOI] [PubMed] [Google Scholar]
- 11.Shoar S., Saber A.A. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg. Obes. Relat. Dis. 2017;13(2):170–180. doi: 10.1016/j.soard.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Tabasi M., Ashrafian F., Khezerloo J.K., Eshghjoo S., Behrouzi A., Javadinia S.A. Changes in gut microbiota and hormones after bariatric surgery: a Bench-To-Bedside Review. Obes. Surg. 2019 Feb doi: 10.1007/s11695-019-03779-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 13.Schauer P.R., Nissen S.E., Kirwan J.P., Pothier C.E., Navaneethan S.D., Aminian A. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Ghanem Y., Inbar R., Tyomkin V., Kent I., Berkovich L., Ghinea R. Effect of sleeve gastrectomy on thyroid hormone levels. Obes. Surg. 2015 doi: 10.1007/s11695-014-1415-7. [DOI] [PubMed] [Google Scholar]
- 15.Wong A.T.Y., Chan D.C., Armstrong J., Watts G.F. Effect of laparoscopic sleeve gastrectomy on elevated C-reactive protein and atherogenic dyslipidemia in morbidly obese patients. Clin. Biochem. 2011 doi: 10.1016/j.clinbiochem.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Rieber N., Giel K.E., Meile T., Enck P., Zipfel S., Teufel M. Psychological dimensions after laparoscopic sleeve gastrectomy: reduced mental burden, improved eating behavior, and ongoing need for cognitive eating control. Surg. Obes. Relat. Dis. 2013 doi: 10.1016/j.soard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Brunault P., Jacobi D., Miknius V., Bourbao-Tournois C., Huten N., Gaillard P. High preoperative depression, phobic anxiety, and binge eating scores and low medium-term weight loss in sleeve gastrectomy obese patients: a preliminary cohort study. Psychosomatics. 2012 doi: 10.1016/j.psym.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Hilgendorf W., Butler A., Timsina L., Choi J., Banerjee A., Selzer D. A behavioral rating system predicts weight loss and quality of life after bariatric surgery. Surg. Obes. Relat. Dis. 2018;14(8):1167–1172. doi: 10.1016/j.soard.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Booth H., Khan O., Prevost A.T., Reddy M., Charlton J., Gulliford M.C. Impact of bariatric surgery on clinical depression. Interrupted time series study with matched controls. J. Affect. Disord. 2015 doi: 10.1016/j.jad.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 20.Brunault P., Frammery J., Couet C., Delbachian I., Bourbao-Tournois C., Objois M. Predictors of changes in physical, psychosocial, sexual quality of life, and comfort with food after obesity surgery: a 12-month follow-up study. Qual. Life Res. 2015 doi: 10.1007/s11136-014-0775-8. [DOI] [PubMed] [Google Scholar]
- 21.Szczuko M., Komorniak N., Hoffmann M., Walczak J., Jaroszek A., Kowalewski B. Body weight reduction and biochemical parameters of the patients after RYGB and SG bariatric procedures in 12-month observation. Obes. Surg. 2017;27(4):940–947. doi: 10.1007/s11695-016-2400-0. https://www.ncbi.nlm.nih.gov/pubmed/27730465 [Internet]. 2016/10/11, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keleidari B., Mahmoudie M., Anaraki A.G., Shahraki M.S., Jamalouee S.D., Gharzi M. Six month-follow up of laparoscopic sleeve gastrectomy. Adv. Biomed. Res. 2016 doi: 10.4103/2277-9175.178786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Praveen Raj P., Gomes R.M., Kumar S., Senthilnathan P., Karthikeyan P., Shankar A. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: “nASHOST” prospective observational trial. Surg. Obes. Relat. Dis. 2015 doi: 10.1016/j.soard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Duran İ.D., Gülçelik N.E., Bulut B., Balcı Z., Berker D., Güler S. Differences in calcium metabolism and thyroid physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Obes. Surg. 2019;29(2):705–712. doi: 10.1007/s11695-018-3595-z. [DOI] [PubMed] [Google Scholar]
- 25.Park S., Kim Y.J., young Choi C., Cho N.J., Gil H.W., Lee E.Y. Bariatric surgery can reduce albuminuria in patients with severe obesity and normal kidney function by reducing systemic inflammation. Obes. Surg. 2018 doi: 10.1007/s11695-017-2940-y. [DOI] [PubMed] [Google Scholar]
- 26.Navaneethan S.D., Kelly K.R., Sabbagh F., Schauer P.R., Kirwan J.P., Kashyap S.R. Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes. Surg. 2010;20(3):308–315. doi: 10.1007/s11695-009-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Talamás H., Daigle C.R., Aminian A., Corcelles R., Brethauer S.A., Schauer P.R. The effect of bariatric surgery on gout: a comparative study. Surg. Obes. Relat. Dis. 2014 doi: 10.1016/j.soard.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Liou T.H., Lee W.J., Hsu C.T., Lee M.F., Chen H.H. ESR1 gene and insulin resistance remission are associated with serum uric acid decline for severely obese patients undergoing bariatric surgery. Surg. Obes. Relat. Dis. 2014 doi: 10.1016/j.soard.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Morais A.A.C., Faintuch J., Leal A.A.D., Noe J.A.B., Bertollo D.M., Morais R.C. Inflammation and biochemical features of bariatric candidates: does gender matter? Obes. Surg. 2011 doi: 10.1007/s11695-010-0080-8. [DOI] [PubMed] [Google Scholar]
- 30.Amin B.K., Hassan K.I. Molecular typing of human Brucella melitensis isolated from patients in Erbil, Iraq. ARO-THE Sci. J. KOYA Univ. 2019;7(1):1–4. [Google Scholar]
- 31.De Vuono S., Ricci M.A., Migliola E.N., Monti M.C., Morretta E., Boni M. Serum bile acid levels before and after sleeve gastrectomy and their correlation with obesity-related comorbidities. Obes. Surg. 2019:1–10. doi: 10.1007/s11695-019-03877-6. [DOI] [PubMed] [Google Scholar]
- 32.Asztalos B.F., Ai M., Stanhope K.L., Swarbrick M.M., Horvath K.V., Havel P.J. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J. Lipid Res. 2009 doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai C.M., Huang C.K., Hwang J.C., Chiang H., Chang C.Y., Lee C.T. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 2012 doi: 10.1007/s11695-011-0579-7. [DOI] [PubMed] [Google Scholar]
- 34.Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Tai C.-M., Huang C.-K., Hwang J.-C., Chiang H., Chang C.-Y., Lee C.-T. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 2012 Jul;22(7):1016–1021. doi: 10.1007/s11695-011-0579-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 36.Ay L., Kopp H.P., Brix J.M., Ay C., Quehenberger P., Schernthaner G.H. Thrombin generation in morbid obesity: significant reduction after weight loss. J. Thromb. Haemostasis. 2010 doi: 10.1111/j.1538-7836.2010.03766.x. [DOI] [PubMed] [Google Scholar]
- 37.Saif T., Strain G.W., Dakin G., Gagner M., Costa R., Pomp A. Evaluation of nutrient status after laparoscopic sleeve gastrectomy 1, 3, and 5 years after surgery. Surg. Obes. Relat. Dis. 2012;8(5):542–547. doi: 10.1016/j.soard.2012.01.013. https://www.ncbi.nlm.nih.gov/pubmed/22398110 [Internet]. 2012/02/01, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bueter M., Dubb S.S., Gill A., Joannou L., Ahmed A., Frankel A.H. Renal cytokines improve early after bariatric surgery. Br. J. Surg. 2010 doi: 10.1002/bjs.7264. [DOI] [PubMed] [Google Scholar]
- 39.Gumbau V., Bruna M., Canelles E., Guaita M., Mulas C., Basés C. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes. Surg. 2014 doi: 10.1007/s11695-014-1186-1. [DOI] [PubMed] [Google Scholar]
- 40.Viana E.C., Araujo-Dasilio K.L., Miguel G.P.S., Bressan J., Lemos E.M., Moyses M.R. Gastric Bypass and Sleeve Gastrectomy: the Same Impact on IL-6 and TNF-$α$. Prospective Clinical Trial. Obes Surg. 2013 Aug;23(8):1252–1261. doi: 10.1007/s11695-013-0894-2. [Internet] [DOI] [PubMed] [Google Scholar]
- 41.Purnell J.Q., Wolfe B.M. Bariatric/Metabolic Surgery for Diabetes: Lessons From the Past and Present. Diabetes Care. 2019;42(2):186–188. doi: 10.2337/dci18-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abd Ellatif M.E., Abdallah E., Askar W., Thabet W., Aboushady M., Abbas A.E. Long term predictors of success after laparoscopic sleeve gastrectomy. Int. J. Surg. 2014 doi: 10.1016/j.ijsu.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Ronveaux C.C., Tomé D., Raybould H.E. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J. Nutr. 2015 Apr;145(4):672–680. doi: 10.3945/jn.114.206029. https://www.ncbi.nlm.nih.gov/pubmed/25833771 [Internet]. 2015/02/04, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adamska E., Ostrowska L., Górska M., Krętowski A. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Przeglad Gastroenterol. 2014;9(2):69–76. doi: 10.5114/pg.2014.42498. https://www.ncbi.nlm.nih.gov/pubmed/25061485 [Internet]. 2014/05/05, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karcz W.K., Krawczykowski D., Kuesters S., Marjanovic G., Kulemann B., Grobe H. Influence of sleeve gastrectomy on NASH and type 2 diabetes mellitus. J Obes. 2011 doi: 10.1155/2011/765473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherla D.V., Rodriguez N.A., Vangoitsenhoven R., Singh T., Mehta N., McCullough A.J. Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg. Endosc. 2019:1–7. doi: 10.1007/s00464-019-07017-0. [DOI] [PubMed] [Google Scholar]
- 47.Blanco D.G., Funes D.R., Giambartolomei G., Lo Menzo E., Szomstein S., Rosenthal R.J. Laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass in cardiovascular risk reduction: a match control study. Surg. Obes. Relat. Dis. 2019;15(1):14–20. doi: 10.1016/j.soard.2018.09.488. [DOI] [PubMed] [Google Scholar]
- 48.Romero-Corral A., Caples S.M., Lopez-Jimenez F., Somers V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest. 2010 doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Våge V., Sande V.A., Mellgren G., Laukeland C., Behme J., Andersen J.R. Changes in obesity-related diseases and biochemical variables after laparoscopic sleeve gastrectomy: A two-year follow-up study. BMC Surg. 2014 doi: 10.1186/1471-2482-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelm S.M., Young J., Kale-Pradhan P.B. Effect of Bariatric Surgery on Hypertension: A Meta-analysis. Ann. Pharmacother. 2014 doi: 10.1177/1060028014529260. [DOI] [PubMed] [Google Scholar]
- 51.Young M.T., Gebhart A., Phelan M.J., Nguyen N.T. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J. Am. Coll. Surg. 2015;220(5):880–885. doi: 10.1016/j.jamcollsurg.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 52.Wijnen M., Olsson D.S., van den Heuvel-Eibrink M.M., Wallenius V., Janssen J.A.M.J.L., Delhanty P.J.D. Efficacy and safety of bariatric surgery for craniopharyngioma-related hypothalamic obesity: a matched case–control study with 2 years of follow-up. Int. J. Obes. 2016 Oct 31;41:210. doi: 10.1038/ijo.2016.195. [Internet] [DOI] [PubMed] [Google Scholar]
- 53.Sharma P., McCarty T.R., Lange A., Ngu J.N., Njei B. Impact of bariatric surgery on outcomes of patients with celiac disease: a Nationwide Inpatient Sample Analysis, 2004-2014. Ann. Gastroenterol. 2019;32(1):73–80. doi: 10.20524/aog.2018.0323. https://www.ncbi.nlm.nih.gov/pubmed/30598595 [Internet]. 2018/11/02, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent H.K., Ben-David K., Conrad B.P., Lamb K.M., Seay A.N., Vincent K.R. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg. Obes. Relat. Dis. 2012;8(3):346–354. doi: 10.1016/j.soard.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Muschitz C., Kocijan R., Marterer C., Nia A.R., Muschitz G.K., Resch H. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015 doi: 10.1210/jc.2014-3367. [DOI] [PubMed] [Google Scholar]
- 56.Slopien R., Horst N., Jaremek J.D., Chinniah D., Spaczynski R. The impact of surgical treatment of obesity on the female fertility. Gynecol. Endocrinol. 2019;35(2):100–102. doi: 10.1080/09513590.2018.1500536. [DOI] [PubMed] [Google Scholar]
- 57.Rochester D., Jain A., Polotsky A.J., Polotsky H., Gibbs K., Isaac B. Partial recovery of luteal function after bariatric surgery in obese women. Fertil. Steril. 2009 doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Z., Liang Y., Deng W., Qiu P., Li M., Zhou Z. Impact of Bariatric Surgery on Female Sexual Function in Obese Patients: a Meta-Analysis. Obes. Surg. 2019:1–13. doi: 10.1007/s11695-019-04240-5. [DOI] [PubMed] [Google Scholar]
- 59.Basbug A., Ellibeş Kaya A., Dogan S., Pehlivan M., Goynumer G. Does pregnancy interval after laparoscopic sleeve gastrectomy affect maternal and perinatal outcomes? J. Matern Neonatal. Med. 2019;32(22):3764–3770. doi: 10.1080/14767058.2018.1471678. [DOI] [PubMed] [Google Scholar]
- 60.Butterworth J., Deguara J., Borg C.-M. Bariatric Surgery, Polycystic Ovary Syndrome, and Infertility. J Obes. 2016 doi: 10.1155/2016/1871594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subak L.L., Richter H.E., Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J. Urol. 2009 Dec;182(6 Suppl):S2–7. doi: 10.1016/j.juro.2009.08.071. https://www.ncbi.nlm.nih.gov/pubmed/19846133 [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacKintosh M.L., Derbyshire A.E., McVey R.J., Bolton J., Nickkho-Amiry M., Higgins C.L. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int. J. Canc. 2019;144(3):641–650. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashrafian H., Ahmed K., Rowland S.P., Patel V.M., Gooderham N.J., Holmes E. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117(9):1788–1799. doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 64.Sjöström L., Gummesson A., Sjöström C.D., Narbro K., Peltonen M., Wedel H. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009 doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh S.K., Roy S., Chekan E., Fegelman E.J. A Narrative of Intraoperative Staple Line Leaks and Bleeds During Bariatric Surgery. Obes. Surg. 2016;26(7):1601–1606. doi: 10.1007/s11695-016-2177-1. [Internet]. 2016/04/19. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27094877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al Hajj G.N., Haddad J. Preventing staple-line leak in sleeve gastrectomy: Reinforcement with bovine pericardium vs oversewing. Obes. Surg. 2013 doi: 10.1007/s11695-013-1062-4. [DOI] [PubMed] [Google Scholar]
- 67.Sarker A., Meek C.L., Park A. Biochemical consequences of bariatric surgery for extreme clinical obesity. Ann. Clin. Biochem. 2016 doi: 10.1177/0004563215588116. [DOI] [PubMed] [Google Scholar]
- 68.Malinowski S.S. Nutritional and metabolic complications of bariatric surgery. Am. J. Med. Sci. 2006 doi: 10.1097/00000441-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Gehrer S., Kern B., Peters T., Christofiel-Courtin C., Peterli R. Fewer nutrient Deficiencies after laparoscopic sleeve gastrectomy (LSG) than after Laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes. Surg. 2010 doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 70.Catheline J.-M., Fysekidis M., Dbouk R., Boschetto A., Bihan H., Reach G. Weight loss after sleeve gastrectomy in super superobesity. J. Obes. 2012:959260. doi: 10.1155/2012/959260. https://www.ncbi.nlm.nih.gov/pubmed/22888410 [Internet]. 2012/07/22. 2012, Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ser K.H., Lee W.J., Lee Y.C., Chen J.C., Su Y.H., Chen S.C. Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: Staple-line reinforcement is important for preventing leakage. Surg. Endosc. 2010 doi: 10.1007/s00464-010-0945-x. [DOI] [PubMed] [Google Scholar]
- 72.Baltasar A., Serra C., Pérez N., Bou R., Bengochea M., Ferri L. Laparoscopic sleeve gastrectomy: A multi-purpose bariatric operation. Obes. Surg. 2005 doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 73.Dalcanale L., Oliveira C.P.M.S., Faintuch J., Nogueira M.A., Rondó P., Lima V.M.R. Long-term nutritional outcome after gastric bypass. Obes. Surg. 2010 doi: 10.1007/s11695-009-9916-5. [DOI] [PubMed] [Google Scholar]
- 74.Kochkodan J., Telem D.A., Ghaferi A.A. Physiologic and psychological gender differences in bariatric surgery. Surg. Endosc. 2018;32(3):1382–1388. doi: 10.1007/s00464-017-5819-z. [DOI] [PubMed] [Google Scholar]
- 75.Panel C.D.C. NIH conference: gastrointestinal surgery for severe obesity. Ann. Intern. Med. 1991 [PubMed] [Google Scholar]
- 76.Sheka A.C., Kizy S., Leslie D.B., Jahansouz C., Wirth K., Grams J. Racial Disparities in Bariatric Surgery Patients. J. Am. Coll. Surg. 2017 [Google Scholar]
- 77.Coleman K.J., Huang Y.C., Hendee F., Watson H.L., Casillas R.A., Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg. Obes. Relat. Dis. 2014 doi: 10.1016/j.soard.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 78.Turner P.L., Oyetunji T.A., Gantt G., Chang D.C., Cornwell E.E., Fullum T.M. Demographically associated variations in outcomes after bariatric surgery. Am. J. Surg. 2011 doi: 10.1016/j.amjsurg.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 79.Capoccia D., Coccia F., Guarisco G., Testa M., Rendina R., Abbatini F. Long-term Metabolic Effects of Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2018 doi: 10.1007/s11695-018-3153-8. [DOI] [PubMed] [Google Scholar]
- 80.Sieber P., Gass M., Kern B., Peters T., Slawik M., Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2014 doi: 10.1016/j.soard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 81.Ruiz-Tovar J., Zubiaga L. Validation of biochemical scores for liver steatosis before and 1 year after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2019 doi: 10.1016/j.soard.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Eid G.M., Brethauer S., Mattar S.G., Titchner R.L., Gourash W., Schauer P.R. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann. Surg. 2012;256(2):262–265. doi: 10.1097/SLA.0b013e31825fe905. [DOI] [PubMed] [Google Scholar]
- 83.Paluszkiewicz R., Kalinowski P., Wróblewski T., Bartoszewicz Z., Biaołbrzeska-Paluszkiewicz J., Ziarkiewicz-Wróblewska B. Prospective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux-en-Y gastric bypass for the management of patients with morbid obesity. Wideochirurgia I Inne Tech Maloinwazyjne. 2012 doi: 10.5114/wiitm.2012.32384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee W.J., Chong K., Ser K.H., Lee Y.C., Chen S.C., Chen J.C. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: A randomized controlled trial. Arch. Surg. 2011 doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 85.Milone M., Di Minno M.N.D., Leongito M., Maietta P., Bianco P., Taffuri C. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J. Gastroenterol. WJG. 2013;19(39):6590. doi: 10.3748/wjg.v19.i39.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbatini F., Capoccia D., Casella G., Soricelli E., Leonetti F., Basso N. Long-term remission of type 2 diabetes in morbidly obese patients after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2013 doi: 10.1016/j.soard.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Algooneh A., Almazeedi S., Al-Sabah S., Ahmed M., Othman F. Non-alcoholic fatty liver disease resolution following sleeve gastrectomy. Surg. Endosc. 2016 doi: 10.1007/s00464-015-4426-0. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Tovar J., Alsina M.E., Alpera M.R. Improvement of nonalcoholic fatty liver disease in morbidly obese patients after sleeve gastrectomy: association of ultrasonographic findings with lipid profile and liver enzymes. Acta Chir. Belg. 2017 doi: 10.1080/00015458.2017.1334858. [DOI] [PubMed] [Google Scholar]
- 89.Manco M., Mosca A., De Peppo F., Caccamo R., Cutrera R., Giordano U. The Benefit of Sleeve Gastrectomy in Obese Adolescents on Nonalcoholic Steatohepatitis and Hepatic Fibrosis. J. Pediatr. 2017 doi: 10.1016/j.jpeds.2016.08.101. [DOI] [PubMed] [Google Scholar]
- 90.Iancu M., Copaescu C., Serban M., Ginghina C. Laparoscopic sleeve gastrectomy reduces the predicted coronary heart disease risk and the vascular age in obese subjects. Chir. 2013;108(5):659–665. [PubMed] [Google Scholar]
- 91.Major P., Kowalczuk A., Wysocki M., Osadnik S., Pędziwiatr M., Głuszewska A. Effects of bariatric surgery on cardiovascular risk factors among morbidly obese patients. Pol. J. Surg. 2018 doi: 10.5604/01.3001.0009.7176. [DOI] [PubMed] [Google Scholar]
- 92.Blanco D.G., Funes D.R., Lo Menzo E., Szomstein S., Rosenthal R. Cardiovascular Risk Reduction after Laparoscopic Sleeve Gastrectomy and Laparoscopic Gastric Bypass: A Match Control Study. Surg. Obes. Relat. Dis. 2017;13(10):S7. doi: 10.1016/j.soard.2018.09.488. [DOI] [PubMed] [Google Scholar]
- 93.Mashaqi S., Steffen K., Crosby R., Garcia L. The Impact of Bariatric Surgery on Sleep Disordered Breathing Parameters From Overnight Polysomnography and Home Sleep Apnea Test. Cureus. 2018 doi: 10.7759/cureus.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Raaff C.A.L., Coblijn U.K., de Vries N., Heymans M.W., van den Berg B.T.J., van Tets W.F. Predictive Factors for Insufficient Weight Loss After Bariatric Surgery: Does Obstructive Sleep Apnea Influence Weight Loss? Obes. Surg. 2016 doi: 10.1007/s11695-015-1830-4. [DOI] [PubMed] [Google Scholar]
- 95.Sammour T., Hill A.G., Singh P., Ranasinghe A., Babor R., Rahman H. Laparoscopic sleeve gastrectomy as a single-stage bariatric procedure. Obes. Surg. 2010 doi: 10.1007/s11695-009-0038-x. [DOI] [PubMed] [Google Scholar]
- 96.Hoogerboord M., Wiebe S., Klassen D., Ransom T., Lawlor D., Ellsmere J. Laparoscopic sleeve gastrectomy: Perioperative outcomes, weight loss and impact on type 2 diabetes mellitus over 2 years. Can. J. Surg. 2014 doi: 10.1503/cjs.024212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alizadeh R.F., Li S., Inaba C., Penalosa P., Hinojosa M.W., Smith B.R. Risk Factors for Gastrointestinal Leak after Bariatric Surgery: MBASQIP Analysis. J. Am. Coll. Surg. 2018 doi: 10.1016/j.jamcollsurg.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 98.Sakran N., Raziel A., Goitein O., Szold A., Goitein D. Laparoscopic Sleeve Gastrectomy for Morbid Obesity in 3003 Patients: Results at a High-Volume Bariatric Center. Obes. Surg. 2016 doi: 10.1007/s11695-016-2063-x. [DOI] [PubMed] [Google Scholar]
- 99.Abdelgawad M., De Angelis F., Iossa A., Rizzello M., Cavallaro G., Silecchia G. Management of Complications and Outcomes After Revisional Bariatric Surgery: 3-Year Experience at a Bariatric Center of Excellence. Obes. Surg. 2016 doi: 10.1007/s11695-016-2071-x. [DOI] [PubMed] [Google Scholar]
- 100.Goitein D., Raziel A., Szold A., Sakran N. Assessment of perioperative complications following primary bariatric surgery according to the Clavien–Dindo classification: comparison of sleeve gastrectomy and Roux-Y gastric bypass. Surg. Endosc. 2016 doi: 10.1007/s00464-015-4205-y. [DOI] [PubMed] [Google Scholar]
- 101.Gagner M., Deitel M., Erickson A.L., Crosby R.D. Survey on laparoscopic sleeve gastrectomy (LSG) at the fourth international consensus summit on sleeve gastrectomy. Obes. Surg. 2013 doi: 10.1007/s11695-013-1040-x. [DOI] [PubMed] [Google Scholar]
- 102.Thereaux J., Lesuffleur T., Czernichow S., Basdevant A., Msika S., Nocca D. Long-term adverse events after sleeve gastrectomy or gastric bypass: a 7-year nationwide, observational, population-based, cohort study. Lancet Diabetes Endocrinol. 2019;7(10):786–795. doi: 10.1016/S2213-8587(19)30191-3. [DOI] [PubMed] [Google Scholar]
- 103.Peterli R., Wölnerhanssen B.K., Vetter D., Nett P., Gass M., Borbély Y. Laparoscopic sleeve gastrectomy versus Roux-Y-Gastric bypass for morbid obesity - 3-year outcomes of the prospective randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS) Ann. Surg. 2017 doi: 10.1097/SLA.0000000000001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lieske J.C., Mehta R.A., Milliner D.S., Rule A.D., Bergstralh E.J., Sarr M.G. Kidney stones are common after bariatric surgery. Kidney Int. 2015 doi: 10.1038/ki.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wood S.G., Kumar S.B., Dewey E., Lin M.Y., Carter J.T. Safety of concomitant cholecystectomy with laparoscopic sleeve gastrectomy and gastric bypass: a MBSAQIP analysis. Surg. Obes. Relat. Dis. 2019 doi: 10.1016/j.soard.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Gibson S.C., Le Page P.A., Taylor C.J. Laparoscopic sleeve gastrectomy: Review of 500 cases in single surgeon Australian practice. ANZ J. Surg. 2015 doi: 10.1111/ans.12483. [DOI] [PubMed] [Google Scholar]
- 108.Sweeny A., Buglino L., La Vella E., Yarbrough D. Comparison of a Novel, Trocar-Free Internal Liver Retractor to Standard Liver Retraction in Bariatric Surgery. Obes. Surg. 2019;29(9):3071–3075. doi: 10.1007/s11695-019-04049-2. [DOI] [PubMed] [Google Scholar]
- 109.Salinas J., Barros D., Salgado N., Viscido G., Funke R., Pérez G. Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy. Surg. Endosc. 2014 doi: 10.1007/s00464-013-3055-8. [DOI] [PubMed] [Google Scholar]
- 110.Moy J., Pomp A., Dakin G., Parikh M., Gagner M. Laparoscopic sleeve gastrectomy for morbid obesity. Am. J. Surg. 2008;196(5):e56–e59. doi: 10.1016/j.amjsurg.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Lalor P.F., Tucker O.N., Szomstein S., Rosenthal R.J. Complications after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2008 doi: 10.1016/j.soard.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 112.Magee C.J., Barry J., Javed S., MacAdam R., Kerrigan D. Extended thromboprophylaxis reduces incidence of postoperative venous thromboembolism in laparoscopic bariatric surgery. Surg. Obes. Relat. Dis. 2010 doi: 10.1016/j.soard.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 113.Stroh C., Birk D., Flade- Kuthe R., Frenken M., Herbig B., Höhne S. Results of sleeve gastrectomy-data from a nationwide survey on bariatric surgery in Germany. Obes. Surg. 2009 doi: 10.1007/s11695-009-9801-2. [DOI] [PubMed] [Google Scholar]
- 114.Mishra T., Lakshmi K.K., Peddi K.K. Prevalence of Cholelithiasis and Choledocholithiasis in Morbidly Obese South Indian Patients and the Further Development of Biliary Calculus Disease After Sleeve Gastrectomy, Gastric Bypass and Mini Gastric Bypass. Obes. Surg. 2016 doi: 10.1007/s11695-016-2113-4. [DOI] [PubMed] [Google Scholar]
- 115.Genco A., Soricelli E., Casella G., Maselli R., Castagneto-Gissey L., Di Lorenzo N. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg. Obes. Relat. Dis. 2017 doi: 10.1016/j.soard.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 116.Soricelli E., Casella G., Baglio G., Maselli R., Ernesti I., Genco A. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2018 doi: 10.1016/j.soard.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Cuomo R., Giardino F.R., Nisi G., Brandi C., Zerini I., Voglino C. Aspiration Pneumonia: a Shadow in Post-Bariatric Patient. Obes. Surg. 2019;1–4 doi: 10.1007/s11695-019-04081-2. [DOI] [PubMed] [Google Scholar]
- 118.Koffman B.M., Greenfield L.J., Ali, Pirzada N.A. Neurologic complications after surgery for obesity. Muscle Nerve. 2006 doi: 10.1002/mus.20394. [DOI] [PubMed] [Google Scholar]
- 119.Sallé A., Demarsy D., Poirier A.L., Lelièvre B., Topart P., Guilloteau G. Zinc deficiency: A frequent and underestimated complication after bariatric surgery. Obes. Surg. 2010 doi: 10.1007/s11695-010-0237-5. [DOI] [PubMed] [Google Scholar]