Abstract
Osteosarcoma (OS) is a rare aggressive bone, presenting low patient survival rate, high metastasis and relapse occurrence, mostly due to multi-drug resistant cells. To surpass that, the use of nanomedicine for the targeted delivery of genetic material, drugs or both have been extensively researched. In this review, we address the current situation of the disorder and some gene therapy options in the nanomedicine field that have been investigated. Among them, polymeric micelles (PM) are an advantageous therapeutic alternative highly explored for OS, as they allow for the targeted transportation of poorly water-soluble drugs to cancer cells. In addition, micelleplexes are PMs with cationic properties with promising features, such as the possibility for a dual therapy, which have made them an attractive research subject. The aim of this review article is to elucidate the application of a micelleplex formulation encapsulating the underexpressed miRNA145 to achieve an active targeting to OS cells and overcome multi-drug resistance, as a new and viable therapeutic strategy.
Keyword: Osteosarcoma, Micelleplexes, miRNA, Poloxamers, Multi-drug resistance, Active targeting
Osteosarcoma: an overview of the disease
Osteosarcoma (OS) is a rare condition, with a yearly worldwide incidence of 3.4 per million people. It is, however, one of the most common cancers in adolescents, behind only to lymphoma and brain tumors. The defining feature that identifies the disease is the observation of osteoid matrix production by cancerous cells (Abarrategi et al. 2016).
In the past two decades, there has been little advancement regarding the prognosis of this disease, despite numerous research attempts. Children and adolescents present the worst prognostic (O’Day and Gorlick 2009). One of the most common problems of OS is the low patient survival rate, which has remained practically unchanged for 15 years, especially those with metastatic tumors or in an advanced stage locally at the time of diagnosis. Additionally, for those patients who experience disease relapse, treatment will depend of whether the tumor is removable, on the prior chemotherapy regimen and the time to relapse (O’Day and Gorlick 2009). As it is assumed that changes in the current chemotherapy scenario will not provide an improvement in the OS landscape, there has been an increasing effort in the discovery of new therapeutic agents (Sampson et al. 2015).
According to the World Health Organization, this primary malignant bone tumor can be classified into seven types (Table 1).
Table 1.
Classification of OS subtypes according to their histological appearance
| Osteosarcoma subtype | Histological characteristics | References |
|---|---|---|
| Low-grade central | Fibroblastic stroma of variable cellularity; osteoids arranged in parallel seams resembling parosteal OS | Malhas et al. (2012) |
| Conventional | Subdivided into osteoblastic, fibroblastic and chondroblastic subtypes; bone or osteoid production by tumor cells | Misaghi et al. (2018) |
| Telangiectatic | Dilated blood-filled cavities; high-grade sarcomatous cells on the septae and peripheral rim | Misaghi et al. (2018) and Yin et al. (2018) |
| Small-cell | Small-cell production; round hypochromatic nuclei with slight nuclear polymorphism | Misaghi et al. (2018) |
| Parosteal | Fibroblastic in appearance; streams of bone trabeculae arranged in a parallel orientation | Hang and Chen (2014) and Misaghi et al. (2018) |
| Periosteal | Chondroblastic in appearance; matrix component mainly cartilaginous | Misaghi et al. (2018) and Patterson et al. (1990) |
| High-grade surface | Malignant spindle cells; high degree of atypical cells | Kumar et al. (2014) |
The pathophysiology of osteosarcoma
The majority of reported cases are sporadic in origin. It develops in rapidly growing bones, preferentially during puberty and in the knee area (Choong et al. 2011). This disease is more prominent in males, with a male to female ratio of 1.5/1. Also, several environmental factors have been connected to the emergence of OS. UV light and ionizing radiation are the best described agents causative of OS. The exposure to radiation is responsible for 2% of the cases observed. Chemical agents including methylcholanthrene, chromium salts, zinc beryllium silicate, beryllium oxide, asbestos, and aniline dyes can induce OS (Cottrell 2018). Additionally, viral infections have been linked to OS. Studies demonstrated the implication of Simian virus 40 (SV40) as a possible tumor agent, however, the virus’ involvement in OS’s development was not proved. Instead, a human polyomavirus with similar properties to SV40 could be behind the obtained immunologic results (Mazzoni et al. 2015).
Genetic alterations
Chromosomal abnormalities, mutations in tumor suppressor genes or protooncogenes are the most common genetic causes of OS. Patients suffering from chromosomal and genetic diseases such as Bloom syndrome, Li-Fraumeni syndrome and hereditary retinoblastoma are at risk of developing OS (Tan et al. 2009). Furthermore, over 90% of documented high-grade cases demonstrate a tendency for aneuploidy, particularly a higher deoxyribonucleic acid, or DNA, content (hyperploidy) (Smeester et al. 2017). Mutations in tumor suppressor genes p53 and Rb (retinoblastoma protein) lead to the impairment of their protective function. Rb1 was the first tumor suppressor described and its loss of heterozygosity occurs in over 40% of cases. An alteration of the p53 loci shows tumorigenic properties with synergistic activity (Lindsey et al. 2017). An amplification of the MDM2 (mouse double minute 2 homolog) gene, which codes for a p53 binding protein, and the c-myc protooncogene have also been reported in OS (Hong et al. 2015).
On the other hand, OS genotype presents a rapidly modifying nature, which makes the study of potential genetic therapeutics a rather difficult task (Martin et al. 2012).
Diagnosis and treatment
OS’ patients often present swelling as well as pain in the metaphyseal bone of the distal femur, the proximal tibia and humerus. Pain is mostly associated with the performance of active tasks and gradually starts appearing at rest. (Cottrell 2018). The pain’s onset is usually in adolescence and is associated with hospitalization, reduced survival and poor quality of life of the patient (Smeester et al. 2017).
Techniques currently used for diagnosis are varied and comprise both invasive and non-invasive methods. Non-invasive options include blood markers, such as alkaline phosphatase and lactose dehydrogenase, and imaging techniques like magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) and bone scintigraphy (Lindsey et al. 2017). MRI is generally performed as the first line, and it is applied to a lesion to ascertain if soft tissues or neovascular structures were harmed, the level of bone marrow that was replaced or if this tissue has extended into bordering joints. CT scans are also employed to determine cortical irregularities, fracture sites, mineralization of the bone and implication of the neurovascular system. Even though it is a less sensitive modality than MRI for tumor location assessment, it is favored for lung metastasis. PET scan, used as a stand-alone or in combination with CT, is useful for determining the outcome of chemotherapy or to predict progression-free survival of the patient. Bone scintigraphy allows detection of osseous metastasis. Invasive methods such as bone biopsy and microscopic evaluation are still a necessary practice to establish a definite diagnosis and determine a subtype classification and the patient’s response to neoadjuvant chemotherapy (Misaghi et al. 2018; Raimondi et al. 2017).
The standard treatment approach typically starts with 10 weeks of neoadjuvant chemotherapy, followed by surgery and 20 weeks of adjuvant chemotherapy. The most utilized antineoplastic agents are MAP (methotrexate, doxorubicin, cisplatin) (O’Day and Gorlick 2009). Immunotherapy as a treatment option for OS is currently being heavily considered, following chemotherapy resistance. Targeted therapies, vaccines, adoptive T-cell immunotherapy, inhibition of immune checkpoints, oncolytic viral therapy and immunomodulation have been a target to research (Cottrell 2018).
In 2005, a randomized controlled trial, EURAMOS-1, was developed from a collaboration of four study groups to assess which patient treatment would exhibit the best results, specifically the addition of ifosfamide and etoposide to cisplatin, doxorubicin and methotrexate chemotherapy (MAPIE) to poor responders or of pegylated interferon α (IFN-α) to MAP in good responders. However, the trial was met with negative results, as MAPIE’s administration increased toxicity without the improving event-free survival, while the addition of pegylated IFN-α did not perform better than MAP alone (Bielack et al. 2015; Marina et al. 2016).
The emergence of nanomedicine in osteosarcoma: recent progress in the area
Nanomedicine refers to the use of materials of nanometric size for therapeutic and imaging purposes, for instance, when it comes to the diagnosis, monitoring and treatment of cancer. Currently, a large number of nano-delivery systems are being studied for cancer treatment, such as liposomes, polymeric micelles (PM), metallic nanoparticles (NPs), solid lipid NPs, dendrimers or albumin NPs (Chow and Ho 2013; Tong and Kohane 2016). These nanocarriers present beneficial characteristics capable of improving the therapeutic efficacy of anticancer drugs. These include their potential to modulate a drug’s pharmacokinetic profile and tissue distribution for a preferred tumor site accumulation, increased in vivo stability leading to an extended blood circulation half-life, and controlled release (Wicki et al. 2015). Furthermore, the coupling of surface ligands to the NPs and the development of stimuli-responsive nanosystems allows for active targeting. These properties lead to a decrease in the toxicity of drugs and thus an improvement of the patient’s safety (Rodríguez-Nogales et al. 2018; Shi et al. 2017).
Since problems including a poor drug pharmacokinetics, high cellular toxicity and drug resistance are still present in the panorama of OS, several nanotechnology studies have been conducted aiming to overcome these challenges (Savvidou et al. 2016).
One recent treatment option subject to heavy research are mesenchymal stromal cells (MSC). One of its studied uses includes inducing bone sarcoma cell death by acting as a delivery vehicle of anti-tumor NPs. Due to MSCs’ homing ability towards tumor stroma and inflammation sites which leads to a targeted local delivery, Duchi et al. (2013) loaded MSCs with fluorescent core–shell poly(methyl methacrylate) (PMMA) NPs (FNPs) and the photosensitizer meso-tetrakis (4-sulfonataphenyl) porphyrin (TPPS). These were co-cultured in vitro with human OS cell line U2OS-RFP-TUBA1B. After being irradiated with light, the production of reactive oxygen species let to controlled OS cell death in a short period of time.
A liposomal dual drug delivery platform has been developed by Caliskan et al. (2019). It incorporates DNA synthesis inhibitors Gemcitabine and Clofazimine, which inhibit Wnt signal transduction pathway. This formulation presented a higher cytotoxicity when compared with the individual treatment with the drugs. Furthermore, a redox-sensitive liposome system targeting both bone and OS cell surface receptor CD44, co-administered with internalizing the tumor-penetrating peptide RGD (Arg-Gly-Asp), and loaded with doxorubicin was investigated. It demonstrated both a significant tumor growth suppression as well as a higher survival time and it reduced lung metastasis and the drug’s cardiotoxicity (Feng et al. 2019). A similar study was conducted where hyaluronic acid-conjugated liposomes carrying a less cardiotoxic H2S-releasing doxorubicin (Sdox) showed a positive drug-release profile and displayed a higher toxicity than normal doxorubicin both in vitro and in vivo, making it an appealing alternative for P-glycoprotein (P-gp) overexpressing patients (Gazzano et al. 2019).
The development of drug-loaded bubbles for concurrent OS treatment, combining the antineoplastic agent doxorubicin and ultrasound sonication proved to be an effective drug delivery system, showing an enhanced tumor microcirculation and, therefore, a faster tumor growth suppression (Kuo et al. 2019).
To target OS initiating cells, a subpopulation that aids tumor progression, recurrence and metastasis, a recent study examined the potential of the drug ATRA, or all-trans retinoic acid, to act in these cells by developing lipid-polymer NPs conjugated with CD133+ aptamers, which are OS stem cell biomarkers. This was the first report on the drug’s use for OS, with positive therapeutic efficacy results, superior when compared to ATRA alone and untargeted NPs (Gui et al. 2019).
Lastly, Zhao et al. (2019) investigated polydopamine-coated NPs for surface modification loaded with paclitaxel and functionalized by alendronate as a ligand for K7M2 wt OS cells targeted treatment. In comparison to non-targeted particles, this stable system presented a higher cytotoxicity to OS cells and tumor accumulation.
Therapeutic targets
Endoplasmic reticulum protein 29 (ERp29), highly regulated in primary OS cells, can be used as a prognostic biomarker and exhibits a protective role given the association between shorter patient survival and low ERp29 expression (Chaiyawat et al. 2019). Additionally, the phosphoinositide 3-kinase (PI3K)-AKT-mTOR pathway and extracellular signal-regulated kinase-mitogen-activated protein kinase (EKR-MAPK) were also found to be upregulated (Moriarity et al. 2015).
As previously mentioned, another biomarker that has been proposed is the antigen CD133+ (Tirino et al. 2011), which is expressed in cancer stem cells of bone sarcomas.
In Zhang et al. (2018), the authors proposed the inhibitor growth family of protein 5 (ING5) as a potential therapeutic target for OS. Identified as a tumor suppressor, its downregulation has been observed in several cancers, such as colorectal and breast cancer. The research group found that the protein was also downregulated in OS tumor cells when compared to normal tissues and identified a negative correlation between ING5 expression and tumor size and metastasis in the lung was established. In that sense, an overexpression of the protein caused apoptosis by activation of the Smad pathway and inhibited OS cell proliferation.
The IL-11 receptor α subunit (IL-11Rα) was shown to be associated with a poor prognosis in patients suffering from OS and was thus suggested as a cell surface marker for tumor progression. It was also showed that the expression of the receptor increases with metastatic progression and that both IL-11Rα and its ligand, IL-11, were upregulated in the human metastatic OS cell lines tested, KRIB, SJSA1 and LM7 (Lewis et al. 2017).
The role of miRNAs in osteosarcoma
miRNAs are small, single-stranded, non-coding endogenous RNA molecules that range between 18 and 22 nucleotides in size. Currently, more than 1000 miRNAs of human origin have been identified that regulate gene expression (Sampson et al. 2015). They specifically recognize and bind to a target mRNA by complete or partial base-pairing at the 3′-UTR (untranslated) region. This results in cleavage or translation repression of the complementary mRNA, depending on the level of complementarity (Murphy and MacFarlane 2010; Zhang et al. 2015).
Regarding human miRNA biogenesis, it involves a nuclear and a subsequent cytoplasmic process (Murphy and MacFarlane 2010). In the nucleus, the miRNA is first transcribed by RNA polymerase II into primary miRNA (pri-miRNA) and subsequently processed by a RNase III enzyme called Drosha into precursor-miRNA (pre-miRNA), a single double-stranded hairpin. Pre-miRNA is translocated to the cytoplasm via exportin 5 where the double-stranded part is cleaved away by another RNase III enzyme, dicer, originating two strands. The passenger strand is degraded and the guided strand becomes the mature miRNA. The latter is further incorporated into RISC (RNA-induced silencing complex), binding imperfectly to the 3′-UTR region of target mRNA (Zhang et al. 2015; Zhou et al. 2013).
miRNAs are involved in numerous biological processes, which include development timing, apoptosis, cell signaling, cell differentiation and proliferation, as well as participating in the immune response (Zhou et al. 2013). It has also been shown that miRNAs could serve as oncogenes or tumor suppressors according to the target gene (Zhang et al. 2015).
In OS cells, several miRNAs have been recorded as overexpressed or underexpressed and are thus implicated in the different stages of disease progression and occurrence. In Hu et al. (2012) studied the human OS cell line MG-63 and identified a total of 268 miRNAs that were significantly dysregulated. 159 were underexpressed, including miR-143, miR-145, miR-335 and miR-539. In contrast, 109 miRNAs were overexpressed, such as miR-9, miR-99a, miR-148a, miR-181a and miR-195.
The subtype miRNA145
The miRNA145 is located on chromosome 5 (5q32-33). As already mentioned, miRNA145 is underexpressed in the OS, as well as in many other types of cancer. In fact, a reduced expression of this RNA has been associated with a worse cancer prognosis. For this reason, it is a promising biomarker/therapeutic target. However, the mechanism behind this downregulation is still not known (Cui et al. 2014).
Currently, miRNA-145 targeting of various oncogenes has been found to inhibit tumor progression and metastasis in OS. Among them, histone deacetylase HDAC4 was identified as a potential target, since miRNA-145-3p (the passenger strand of pre-miR-145) was able to downregulate this protein’s expression level, leading to suppressed OS cell proliferation, as well as increased autophagy and apoptosis (Wu et al. 2018). ROCK1 (rho associated coiled-coil containing protein kinase 1), whose upregulation was associated with a worsened OS prognosis, when downregulated led to OS cancerous cell proliferation and invasion inhibition (Lei et al. 2014). Targeting of a cyclin-dependent kinase, CDK6, is a promising approach, since an inverse correlation between CDK’s and miRNA-145’s expression implies an inhibitory effect of this miRNA upon the kinase, and OS cell growth and mobility (Li et al. 2016a, b). Similarly, VEGF (vascular endothelial growth factor) and MMP16 (matrix metallopeptidase 16) targeting led to a suppression of OS metastasis following their downregulation (Chen et al. 2015; Fan et al. 2012).
Gene therapy
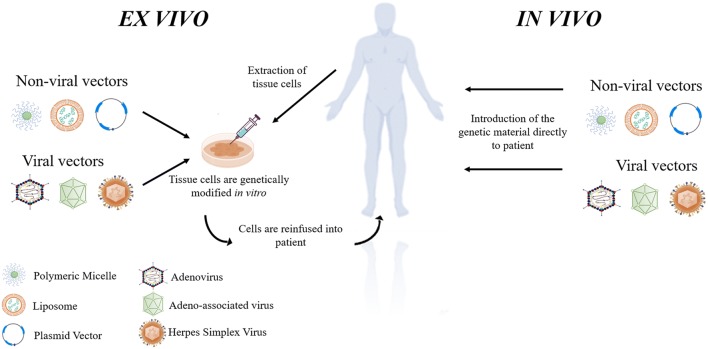
Gene therapy is defined by the introduction of genetic material with the intention of manipulating the expression of the target gene. It can also be applied to living cells to modify their biological characteristics for therapeutic purposes. The foreign genetic material, like RNA or DNA, is transferred into the target cells with the help of a vector, generally of viral or non-viral origin (Sinclair et al. 2018). Furthermore, this transfection can occur in two distinct ways (Fig. 1). Ex vivo, whereby the gene is administered to the target cells in a lab setting and these new genetically modified cells are then reinfused into the patient, or in vivo, with the genetic material being directly introduced into the subject (Worgall and Crystal 2007).
Fig. 1.
Ex vivo vs in vivo gene therapy. In ex vivo gene therapy, the cells are first extracted from the patient’s body and modified/transduced in vitro by a non-viral vector or viral vector. In vivo gene therapy, on the other hand, consists of the direct introduction of the modified genetic material to the patient with the help of a non-viral or viral delivery system. Adapted from (Caffery et al. 2019; Lee et al. 2016; Worgall and Crystal 2007)
This technology is beneficial as it can potentially lead to the cure of treatable but incurable diseases or just simply provide a treatment to a previously untreatable illness, whether of inherited or acquired nature (Kumar et al. 2016; Ramamoorth and Narvekar 2015). In Europe, seven gene therapy products have been approved by the European Medicines Agency (EMA) at the time of writing (“Gene Therapy Products on the Market,” n.d.).
Viral and non-viral vectors
To perform an effective gene transfer, several delivery vehicles have been developed, predominantly divided into viral and non-viral vectors.
Viral vectors are natural viruses that were modified to replace the original genetic makeup of the pathogen with the genes to be transferred to the patient and the sequences that control their expression. It is more efficient due to the maintenance of the virus’ envelope (Sinclair et al. 2018). Depending on the therapeutic objective, these vectors can be designed for transient or permanent expression. Additionally, vectors can be DNA or RNA viruses with single-stranded or double-stranded genomes (Lundstrom 2018). DNA viruses most commonly used include adenoviruses, of transient expression, and adeno-associated virus (AAV) and herpes simplex virus, both of long-term expression. Regarding RNA viruses, these include retroviral and lentiviral vectors, of double-stranded genome, that can establish a permanent expression due to their capacity of host genome integration. However, despite having a higher transfection efficiency than non-viral vectors, the use of viral vehicles induce immunogenicity and cytotoxicity to cells (Lundstrom and Boulikas 2003).
Non-viral, or plasmid-based, vectors, on the other hand, are safer than their viral counterparts, can deliver genetic material of larger size, but are less efficient at cellular transfection. Plasmid vectors as a gene delivery therapy option have been widely applied to cancer due to their appealing characteristics. They represent the simplest form of DNA transport vector, as they consist of a double-stranded circular DNA that can be easily found in almost all bacteria strains. These vectors are relatively cheap and simple to produce, remain stable at room temperature for an extended period and can be delivered repeatedly, as opposed to their viral counterparts (Hardee et al. 2017; Williams and Kingston 2011). This plasmid DNA can be delivered to cells by electroporation, ultrasound, direct injection or, alternatively, complexed with liposomes, polymers or peptides (Gill et al. 2009). Lipid-based compositions are widely used as non-viral vectors. Cationic lipids can form a bond with the negatively charged phosphate groups present in nucleic acids to form lipoplexes, which aids in the cellular uptake of DNA, protecting it from enzymatic degradation. They also present a low production cost (Gáscon et al. 2013; Ramamoorth and Narvekar 2015). Polymeric vectors are attractive in the sense that they are chemically diverse and for their functionalization potential. Polymers used can be of natural origin (proteins, peptides and polysaccharides) or synthetic, such as polyethyleinemine (PEI), poly(l-lysine) or PLL, dendrimers and polyphosphoesters. Peptide vectors are beneficial in protecting the genetic material and disrupting endosomal membrane, therefore, leading to the delivery of tightly compact DNA. Moreover, peptides of amphiphilic nature were developed to bind to nucleic acids, destabilizing liposomes and improve cell delivery (Lundstrom and Boulikas 2003; Ramamoorth and Narvekar 2015).
Polymeric micelles
PMs represent a core/shell structure made up of at least two chemically diverse blocks, more precisely a hydrophobic and a hydrophilic segment (Kedar et al. 2010). Through the process of micellization, these blocks will self-assemble into a hydrophobic inner core and an outer hydrophilic shell. This self-assembly happens when the block concentration reaches the critical micellar concentration (CMC). This is defined as the minimal concentration of the polymer necessary for the PM’s formation. Typically, lower CMC values are associated with a higher stability (Aliabadi and Lavasanifar 2006).
PMs typically vary between 10 and 200 nm in size and can present different structural shapes: spheres, tubules, helices, rods and lamellae are some varieties described. These differences occur depending on the length of the hydrophobic/hydrophilic blocks and impact the pharmacokinetic properties PMs (Amin et al. 2017). The spherical shape is the most commonly observed structure. It is also the most stable since its surface energy is the lowest (Deshmukh et al. 2017; Zhang et al. 2014).
As a drug delivery system, PMs are advantageous for their characteristics. The hydrophobic core of the micelle allows for the encapsulation of poorly water-soluble drugs. Due to their small size, passive targeting and tissue penetration is a possibility for solid tumors. Small size NPs also avoid the reticuloendothelial system (RES) and macrophage recognition, allowing for a more prolonged circulation time in the blood (Deshmukh et al. 2017; Kedar et al. 2010). Since PMs can enhance the water solubility of chemotherapeutic agents leading to an increased systemic half-life, PM-bound drugs benefit from the enhanced permeation and retention effect, or simply EPR effect, whereas the drug passively accumulates in the tumor locally due to its high vascular density (Zhang et al. 2014). As their size and shape are controllable, this allows for a higher drug load capacity (Kesharwani et al. 2018). In addition, the hydrophilic block can be tailored by the addition of ligands such as transferrin and peptides, to further improve PM’s efficiency and targeting (Amin et al. 2017; Kedar et al. 2010).
For OS treatment, the use of invertible PMs, formed by altering the environmental polarity, as a means of curcumin delivery has been reviewed. It showed great potential for the controlled delivery of the poorly water soluble drug and for its cell membrane release using macromolecular inversion (Maran et al. 2016; Ramadurai et al. 2019). Additionally, to overcome the poor solubility and tendency for crystallization of zinc phtalocyanine (ZnPc), a powerful second-generation photosensitizer for cancer photodynamic therapy (PDT), a PM was synthesized consisting of the amphiphilic block copolymer poly(ethylene glycol)-poly[2-(methylacryloyl)ethylnicotinate] (PEG-PMAN) for ZnPc loading. This nanosystem was able to surpass those limitations and demonstrated cytotoxicity against OS cells, inhibiting their proliferation both in vivo and in vitro when compared with free ZnPc. It also caused an accumulation of reactive oxygen species (ROS) production and mitochondrial lesion (Yu et al. 2018).
Cationic polymers
Cationic polymers are known to establish electrostatic interactions with proteins, anionic biomolecules and nucleic acids. In fact, a particular interest in the complexation with the latter has risen. Cationic polymers aid transfection by condensing nucleic acids, allowing for their cellular uptake and escape from enzymatic degradation and endolysosomes, the product between the fusion of an endosome and a lysosome, in the endocytosis process.
Different cationic polymer architectures can occur, such as linear, branched, hyperbranched and dendrimer-like. Categorically, these polymers can be further organized into natural or synthetic cationic polymers, depending on their origin. The natural polymers, obtained from renewable sources, generally do not present toxicity or immunogenicity, and are biodegradable and biocompatible. Gelatin, dextran, cyclodextrin, cellulose and chitosan are some of the most common natural cationic polymers utilized. However, a batch to batch variation is a common problem with these polymers. Conversely, the properties of synthetic cationic polymers can be modified in a controlled manner. In this sense, both their therapeutic potential and degradation method can be improved according to their properties. PEI, PLL, poly(amino-co-ester) (PAE), poly(amidoamine) (PAA) and Poly(2-N,N-dimethylaminoethylmethacrylate) (PDMAEMA)are some examples of commonly used synthetic cationic polymers (Samal et al. 2012).
For OS, an osteotropic nanocarrier composed of alendronate sodium trihydrate (ALN) functionalized gelatin, gemini surfactant and DNA was developed for in vitro transfection of the MG-63 OS cell line. The results showed a higher transfection efficiency compared with the positive control (pEGFP plasmid using Lipofectamine 2000), demonstrating the potential of ALN-modified gelatin for bone cell targeting (Mekhail et al. 2016). Also, to deliver astrocyte elevated gene-1 siRNA (siAEG-1) efficiently to OS cells and accomplish the silencing of the target gene, a nanoscale polysaccharide derivative was prepared by click conjugation of azido-modified chitosan with propargyl focal point PLL dendrons and coupling with folic acid. These NPs were capable of delivering siAEG-1 without toxicity to OS cells and perform the knockdown of AEG-1, leading to tumor cell proliferation arrest and invasion inhibition (Wang et al. 2018).
Polyethyleneimine
PEI is the most used cationic polymer, possessing primary, secondary and tertiary amino groups. As for its architecture, this polymer can be synthesized in a linear form, which is solid at room temperature, or the viscous liquid branched form. When in contact with nucleic acids, PEI spontaneously originates electrostatic interactions between its protonable amino groups and the phosphate groups present in the nucleic acids, forming polyelectrolyte complexes. Depending on the molecular weight (Mw), the delivery efficiency and the cellular toxicity of PEI is altered (Nguyen et al. 2000; Samal et al. 2012).
This polycationic polymer has been shown to exhibit high transfection efficiency, both in vitro and in vivo. It also presents an ability to escape from the endosome, releasing the genetic material (Gáscon et al. 2013).
However, the complexes formed are poorly soluble and there is a risk of precipitation when a high concentration of PEI is used. To overcome this drawback and enhance its solubility, several modifications to PEI have been tested, such as the addition of human serum albumin, dextran or PEG [poly(ethylene glycol)] (Liang et al. 2011). Nonetheless, PEI is a non-degradable polymer with low hemocompatibility that demonstrates cytotoxicity due to the high cationic charge. These characteristics largely restrict its therapeutic use (Samal et al. 2012).
For OS, aiming to enhance the anti-tumor activity of the drug berberine (BBR) in the U-2 OS cells, a NP consisting of BBR and heparin was synthesized. The NP was shelled with linear PEI to obtain a reduced release rate of the drug. This nanocomposite led to an increase in the drug’s cellular uptake and apoptosis induction when compared with drug treatment alone (Hsu et al. 2018). Furthermore, to surpass the lack of in vivo tumor-targeted delivery options for CRISPR/Cas9, a genome editing technique, a PEG-PEI-Cholesterol lipopolymer functionalized with a OS cell-specific aptamer (LC09) was developed. LC09 was able to perform a selective distribution of CRISPR/Cas9 encoding vascular endothelial growth factor A (VEGFA) gRNA in orthotopic OS tissue as well as in metastatic lung tissue. Subsequent inhibition of angiogenesis, OS malignancy and lung metastasis was a direct result of effective tumoral VEGFA genome editing and reduced expression and secretion of the protein (Liang et al. 2017).
Chitosan
Chitosan is a positively charged natural polysaccharide of the lineal form obtained from the partial N-deacetylation of chitin. It is similar in structure to cellulose, another natural cationic polymer, and is soluble in aqueous media (Hosseinzadeh et al. 2012; Othayoth et al. 2013). This polymer can vary depending on the degree of N-deacetylation (40–98%) and its Mw, from 50 to 2000 kDa (Hejazi and Amiji 2003). The cationic character given by the free primary amino groups in its structure allows for the interaction with negatively charged biomolecules and cell membranes, therefore, providing an efficient transfection (Samal et al. 2012).
Chitosan’s mucoadhesive properties increase the residual time at the site of absorption. It is rather nontoxic, biocompatible, biodegradable and able to regulate drug, protein or peptide release through chitosan NP coating (Ahmad et al. 2018a, b; Hosseinzadeh et al. 2012). Given these attractive properties, the use of chitosan has been employed in diverse areas, including cosmetic, food processing, biomaterials, agriculture and pharmaceutical (Othayoth et al. 2013). For example, NPs composed of biodegradable poly(lactic-co-glycolic acid), or PLGA, have been already approved by the FDA (Food and Drug Administration) as a drug delivery carrier for several clinical applications, but these nanocomposites are incapable of adhering to cells and are thus inapt for in vivo tumor studies. The addition of chitosan to these PLGA-NPs have been found to further improve their potential as an alternative carrier for anticancer drugs such as daunorubicin and irinotecan, as it enhances their oral bioavailability (Ahmad et al. 2018a, 2019a, b). Chitosan has also been applied for other routes of administration. Recently, chitosan was used to increase the mucoadhesive property of a nanoemulsion-(in situ)-gel containing Pluronic®F-127, aiming for an intranasal delivery of naringenin (NRG) to brain cells, having achieved positive results (Ahmad et al. 2019a, b).
The therapeutic use of chitosan as a cationic polymer presents some challenges since it is poorly soluble at physiological pH and can induce a rapid drug release. To surpass such limitations, chemical modifications have been introduced, including quaternization of the amino groups or the grafting of polymer chains or small molecules to the backbone (Samal et al. 2012).
For OS, agraphene oxide functionalized chitosan NP encapsulating siRNA targeting Saos-2 and MG-63 OS cells was developed. The results demonstrated a controlled siRNA release by fickian diffusion, or Fick’s first law of diffusion,1 at an acidic pH with no cytotoxicity detected and viability of normal osteoblast cells matching the control groups. Regarding inflammatory response, these NPs showed insignificant levels of inflammatory cytokines such as TNF-α (tumor necrosis factor α), IL-6 (interleukin-6) and TGF-β (transforming growth factor β) (Saravanabhavan et al. 2019). Moreover, the synthesis of mesoporous ZSM-5, or zeolite socony mobil–5, zeolites/chitosan core–shell nanodisks encapsulating doxorubicin were tested as a drug delivery system. These nanodisks could rapidly release the drug in acidic tumor tissue environment and achieve a higher MG63 cell suppression free of toxicity when compared with doxorubicin alone, therefore, demonstrating their potential as pH-responding drug carrier for a targeted therapy (Yang et al. 2018).
Poloxamers and poloxamines
Poloxamers, or Pluronics®, are non-ionic surfactants, composed of A-B-A triblock copolymers, with A being poly(ethylene oxide) (PEO) and B representing poly(propylene oxide) (PPO). An inverse triblock structure, B-A-B, is also possible. (Moghimi and Hunter 2000). They are amphiphilic in nature with hydrophilic PEO blocks, and PPO blocks presenting an hydrophobic character (Bodratti et al. 2018). During the synthesis procedure, propylene oxide (PO) and unimers of ethylene oxide (EO) are sequentially added to a poly(propylene oxide) oligomer in the presence of sodium or potassium hydroxide, which serve as alkaline catalysts. The latter is subsequently neutralized and removed (Alexandridis and Hatton 1995).
Variation of the copolymer composition (PPO/PEO ratio) and Mw (PEO and PPO block length) leads to the production of molecules with optimum properties that meet the specific requirements in various areas of technological significance. These surfactants have been employed for applications such as emulsification, foaming, lubrication and cosmetic formulation. In the pharmaceutical area, poloxamers were tested for burn wound dressing and the controlled release and solubilization of drugs (Alexandridis and Hatton 1995; Bodratti and Alexandridis 2018).
Poloxamines, or Tetronics®, are also composed of PEO-PPO blocks, however, these amphiphilic block copolymers are organized in an X-form, with four PEO-PPO arms joined to a central ethylene diamine bridge. Because the core is hydrophobic in character, these tetrafunctional copolymers are suitable for the solubilization of poorly water-soluble drugs. Similarly to Poloxamers, several commercial Poloxamines are available in different PEO/PPO block ratios and Mw (Alvarez-Lorenzo et al. 2010; Moghimi and Hunter 2000).
For OS treatment, a Pluronic® F127-trimethyl chitosan NP encapsulating methotrexate was formulated to overcome the drug’s toxicity and improve its therapeutic efficacy. Indeed, these NPs achieved a higher drug accumulation in the cell cytoplasm, a greater toxicity as well as apoptosis effect to MG-63 cells when compared with free methotrexate, proving their potential as a drug carrier for OS chemotherapy treatment (Li et al. 2017).
Pluronic® F68
Pluronic® F68 or Poloxamer 188 (HiMedia Laboratories Pvt Ltd 2019) has an average of 152.73 PEO and 28.97 PPO units and a molar mass of 8400 Daltons. Its HLB is 29 and CMC 4.8 × 10–4 M. The cloud point in 1% aqueous solution is > 100 (Pitto-Barry and Barry 2014).
The polymeric structure of this Pluronic® is composed of a hydrophobic center and hydrophilic tails. The center consists of propylene oxide chains, with ethylene oxide chains at the hydrophilic ends (Fig. 2). The x, y and z values are normally 75, 30 and 75, respectively (Tharmalingam et al. 2008).
Fig. 2.
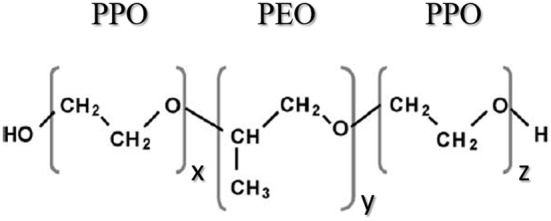
Pluronic® F68 chemical structure. This pluronic is composed of a hydrophobic center of a poly(propylene oxide) (PPO) chain and hydrophilic tails of poly(ethylene oxide) (PEO). The values for x, y and z are 75, 30 and 75, respectively.
Adapted from Santander-Ortega et al. (2006)
It protects cell membranes from shear forces in mechanically agitated gas-sparged bioreactors (Gigout et al. 2008). A capacity to repair cell membrane damage has also been documented (Moloughney and Weisleder 2013). Other potential applications include its use as an antithrombotic agent, stimulation of phagocytosis, production of superoxide anion, or neutrophil degranulation (Moghimi and Hunter 2000). Its use as an additive has been approved for intravenous application, for up to 0.4% w/w (Aliabadi and Lavasanifar 2006).
For OS, a Pluronic® F68 conjugated with curcumin through the acid-responsive cis-aconitic anhydride linkage was developed. The pH-sensitive micelles showed a higher in vitro cytotoxicity and cellular uptake, accompanied by an improved apoptosis effect when compared with free curcumin (Fang et al. 2016). Similarly, a carboxymethyl chitosan-enveloped Pluronic® F68/PLGA NP was formulated to achieve an oral delivery and pH-responsive prolonged release of gefitinib. The results showed that these NPs were physiochemically stable, providing a pH-triggered prolonged release and a high mucoadhesive and muco-penetrating properties, resulting in an easy drug internalization by the intestinal cells (Wang et al. 2019). This is relevant because both curcumin and gefitinib have been appointed as potential therapy options for OS via targeting inositol 1,4,5‑triphosphate receptor type 1 (ITPR1) and by inhibiting receptor-interacting protein kinase 2 (RIPK2), respectively (Luo et al. 2018; Maloney et al. 2017).
Micelleplexes
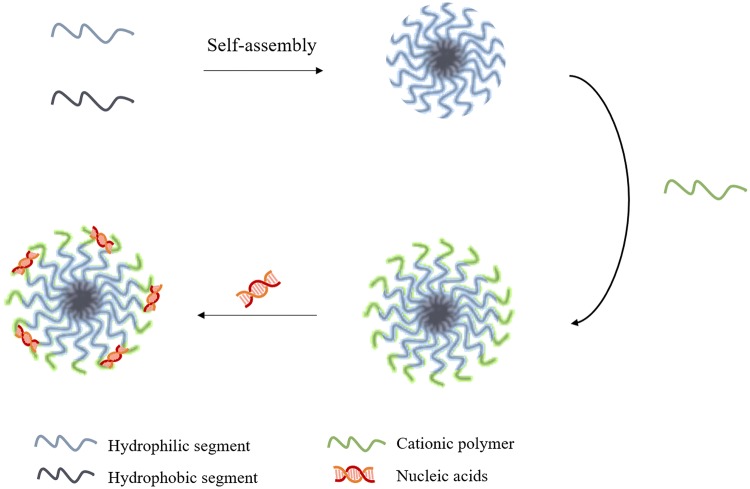
Micelleplex is a novel term in the nanotechnology field that has gained much attention in recent years due to their attractive properties, which include efficient encapsulation, ease of preparation, high stability as well as low toxicity (Lu et al. 2019). The term describes a PM that possesses cationic properties, allowing for the interaction with negatively charged membranes. In other words, micelleplexes are formed by the conjugation of a block of amphiphilic copolymers with a cationic polymer, allowing for the association of nucleic acids to the NP through the establishment of electrostatic interactions (Fig. 3). In order for this association to take place efficiently, it is important to consider the N/P ratio, that is, the ideal ratio established between the positively charged amine, or nitrogen, content in the cationic polymer and the phosphate groups of the genetic material, which carry a negative charge (Almeida et al. 2018).
Fig. 3.
Process of a micelleplex’s formulation. This nanosystem is formed by the conjugation of a block of amphiphilic copolymers, namely a hydrophobic and a hydrophilic segment, with a cationic polymer. The establishment of electrostatic interactions between the positively charged polymer and the negative charge from the nucleic acids allows for the coupling of genetic material
In addition to possessing the characteristics of a PM, namely the capacity to avoid RES recognition and renal clearance due to nanometric size, higher drug loading and blood circulation time, this concept is advantageous as it allows for a dual therapy: the hydrophobic core could encapsulate poorly water-soluble drugs while the cationic hydrophilic shell is effective in transporting genetic material to the target site (Pereira et al. 2017; Zhang et al. 2016).
Depending on the amphiphilic block copolymer used, the micelleplexes will present a different architecture and physicochemical characteristics that will affect their efficacy as a nanocarrier. One of the most commonly used amphiphilic copolymers is poloxamers. In fact, poloxamers that possess a higher quantity of hydrophobic PPO blocks compared with the hydrophilic PEO blocks, such as Pluronic® F68, will possess a lower HLB and CMC value, which in turn correlates with a more stable NP. As for the cationic polymers, the most popular employed are PEI and chitosan, as their positive charge shields the nucleic acids from nucleases. Higher molecular weight polymers, even though they possess a higher net positive charge and thus greater ability to bind to nucleic acids, often produce higher toxicity (Almeida et al. 2018; Magalhães et al. 2018a, b).
For OS treatment, micelleplexes consisting of Pluronic® L64 and PEI encapsulating miRNA-145 were synthesized to deliver the genetic material to MG-63 cells and assess its therapeutic potential. These nanosystems demonstrated an efficient cellular uptake, a high degree of cell death and showed a reduction in the proliferation and migration capacity of OS cells, further indicating the potential and efficiency of this nanosystem to achieve an OS cell gene-based therapy (Magalhães et al. 2018a, b).
A key factor: multidrug resistance
Multidrug resistance, or MDR, is a cellular occurrence in which cancerous cells are capable of resisting drugs with no structural or mechanical connection/similarities (Namee et al. 2018).
Cancerous cells employ multiple mechanisms to avoid the cytotoxic characteristics of antitumoral agents, such as maintaining a stem-cell-like phenotype and/or dormancy, cell cycle alterations, detoxification increase, increased DNA repair, enhanced drug efflux and resistance to apoptosis (PosthumaDeBoer et al. 2013). This resistance can be of intrinsic or acquired origin. Intrinsic resistance accounts for the ‘poor treatment responders’ and it is where the cells are immune to the drug from the beginning, while acquired resistance represents those cells that gain the capacity to overcome the drug’s effect over time. This eventually leads to a pool of resistant clones and, therefore, the occurrence of metastasis (Namee et al. 2018; PosthumaDeBoer et al. 2013).
Treatment efficiency for OS is limited due to drug resistance. MG-63DXR30, a variant from the human OS cell line MG-63, is known to be doxorubicin resistant. A study (Torreggiani et al. 2016) has shown that exosomes, extracellular vesicles involved in cell-to-cell communication, derived from the doxorubicin-resistant variant are able to enhance MG-63 resistance to the drug by the transmission of P-gp to recipient cells. Conversely, a recent study concluded that exosomes derived from human umbilical cord mesenchymal stromal cells (HUC-MSCs) could be used as drug delivery system and for tumor growth monitorization due to their accumulation in OS cells (Abello et al. 2019). Similarly, liposomes loaded with plant alkaloid voacamine (VOA), which exerts a inhibitory function on P-gp, were developed by Giansanti et al. (2019) for doxorubicin delivery to multi-drug resistant human OS cell line U-2 OS/DX. Depending on the liposome’s composition, the system proved to work more efficiently than free VOA to revert OS cell resistance to doxorubicin.
P-gp or multidrug resistance protein 1 (MDR1) was proven to be a causative factor of doxorubicin resistance. In fact, the protein’s upregulation in tumor cells leads to chemoresistance and drug evasion from the cell and is associated with a worse patient outcome (Xie et al. 2018). A lipid-modified dextran polymeric NP aiming to overcome MDR in OS cell lines KHOSR2 and U-2OSR2 was developed as a delivery system for MDR1 siRNA. Additionally, the efficacy of a combination therapy with doxorubicin was explored. This NP was able to suppress P-gp expression in both cell lines, for a longer period when compared with another commercially available composite for MDR1 siRNA transfection. Its therapeutic use also led to a higher intracellular doxorubicin accumulation, suggesting that a doxorubicin/MDR1 siRNA therapy in low dosages is a promising approach (Susa et al. 2010).
Genetic expression also contributes to treatment resistance. A downregulation of reduced folate carriers (RFC), which are used by methotrexate for cell entrance, is observed in OS. Mutations were identified in the protein sequence, like Leu291Pro, Gly259Trp and Ser4Pro, which inhibit chemotherapy treatment from entering the cells because it promotes changes in the structure of RFC and thereafter methotrexate association. The use of trimetrexate has been appointed as an alternative option for methotrexate, as it does not rely on RFC to enter the tumor cells (Cottrell, 2018).
The use of polymeric systems has been investigated to overcome this limitation. Different drug combinations, P-gp inhibitors or modulators, polymers based on Pluronic®, multifunctional and pH or thermo-sensitive micelles are some approaches examined, with results indicating that the use of polymeric systems to overcome MDR in cancer is a beneficial option (Kesharwani et al. 2018).
Pluronics® are advantageous MDR reversal agents as a result of their ability to block P-gp, multidrug resistance proteins (MRPs) and to induce ATP (adenosine triphosphate) level reduction in MDR cells, among others. To evaluate the MDR reversal capability of Pluronic® P85 unimers in MDR cells, a pH-sensitive mixed micellar delivery complex for doxorubicin was prepared. The mixed PMs were based on PHis-PLA-PEG-PLA-PHis [poly(l-histidine)-poly(d,l-lactide)-polyethyleneglycol-poly(d,l-lactide)-poly(l-histidine)] and Pluronic® F127 modified with a targeting folate ligand (pHendoSM-P85/f). At first, P85 unimers were found inserted in the mixed micelles and later formed triple-component mixed micelles with PHis-PLA-PEG-PLA-PHis and Pluronic® F127 as doxorubicin loading content increased. The results showed that Pluronic P85® unimers inserted in pHendoSM-P85/f achieved a higher intracellular delivery efficiency compared to the triple mixed micelles. Furthermore, it also accomplished a greater antitumor efficiency against the multidrug-resistant human breast cancer cell line MCF-7/ADR, as well as lower cellular ATP levels in the confined tumors, demonstrating the potential of this pH-sensitive mixed micellar delivery system to reverse MDR (Hong et al. 2013).
Another key factor: active targeting
Active targeting refers to the use of biological specific interactions to enhance drug delivery to a target. In this sense, active targeting PMs have been developed so that the selectivity and interaction with the target tumor cells, the intracellular drug delivery as well as its half-life could be increased. When compared to untargeted PMs, these systems are associated with a reduced systemic toxicity and produce fewer adverse side effects (Amin et al. 2017; Oerlemans et al. 2010).
The coupling of ligands to PMs uses receptor-mediated endocytosis as a mode of action for the micelles to be internalized into the cell. This means that a higher drug concentration can be attained (Oerlemans et al. 2010). PMs can be conjugated with mAbs (monoclonal antibodies) or small molecules such as folic acid and tripolyphosphate, oligosaccharides, or peptides (Ayre et al. 2013). Stimuli-responsive PMs have also been developed. Adding an internal or external activator increases targeted drug delivery. These include pH-sensitive, thermo-sensitive and ultrasound-responsive PMs (Mahmud et al. 2007; Oerlemans et al. 2010).
For OS, the coupling of molecules like tetracyclines, diphosphonic acid, oligopeptides and propylene acid, that bind to hydroxyapatite allows for drug selectivity towards bone tissue (Li et al. 2016a, b). Folic acid conjugated micelles have been developed since the overexpression of folic acid receptors is present in OS, resulting in a higher uptake of these systems (Wakaskar 2017). In this regard, a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) NP functionalized with folic acid was developed for the targeted delivery of etoposide to Saos-2 cells to exploit the folate-receptor-mediated endocytosis phenomenon. The results showed an increased cellular uptake, further enhancing the NP’s cytotoxic effect when compared with free etoposide or non-targeted etoposide-encapsulating NPs. This nanocomplex achieved a biphasic drug delivery pattern, starting with a fast drug release followed by a slower discharge (Alp et al. 2017).
The RGD labelled PM was designed containing PEG and poly(trimethylene carbonate) blocks loaded with doxorubicin that demonstrated an increased ability for cellular internalization when compared to non-labelled PMs encapsulating doxorubicin, further demonstrating PM’s potential for active targeting (Fang et al. 2017). Conversely, due to the fact that decorated PMs present a lower stability and that ligands are often expensive, Feng et al. (2018) designed a cationic triblock micelle encapsulating doxorubicin, consisting on a PEG block, a hydrophilic poly(b-benzyl l-aspartate) block and PEI in branched form. This novel system demonstrated a capacity to release the drug in a controlled and rapid manner after successfully entering the cancerous cells.
Tang et al. (2015) synthesized a pH-sensitive micelleplex composed of two amphiphilic polymers, namely polyethyleneimine-block-poly[(1,4-butanediol)-dia-crylate-b-5-hydroxyamylamine] (PEI-PDHA) and polyethylene glycol-block-poly[(1,4-butanediol)-dia-crylate-b-5-hydroxyamylamine] (PEG-PDHA) encapsulating Snail siRNA (siSna) and Twist siRNA (siTwi) as well as paclitaxel, to perform an active targeting against breast cancer cells. This novel nanosystem accomplished a pH-dependent drug release, enhancing its cytotoxic properties and tumor accumulation, resulting in proliferation and metastasis suppression. It also resulted in the induction of cell apoptosis and inhibition of cell migration and invasion by performing a siRNA downregulation of Snail and Twist protein expression.
Conclusion and future perspectives
OS is a rare malignancy associated with a high metastasis and recurrence rate that affects primarily children and adolescents. Due to multi-drug resistance, treatment options are still limited and the need for an effective solution is evident. Amid the emerging therapeutic alternatives is the use of gene therapy, more precisely employing nanosystems to specifically deliver a drug, genetic material or both to the tissue cells. miRNAs participate in the development, progression and metastasis of several tumors, including OS. miRNA145 is underexpressed in this disorder and has been proved to be beneficial in reducing tumor progression. Gene therapy refers to the introduction of foreign genetic material to the target cells by resorting to a vector of viral or non-viral origin. Non-viral vectors, although less efficient at cellular transfection, are less immunogenic and oncogenic than viral vectors and have been largely studied for cancer treatment. Non-viral polymeric vectors, such as micelleplexes, are polymeric micelles that are formed by functionalization of amphiphilic copolymer(s) with a cationic polymer, such as chitosan or polyethyleneimine (PEI). This cationic property makes possible the interaction with negatively charged molecules, such as nucleic acids. Due to their structural diversity, micelleplexes allow for a dual therapy: incorporation of a poorly water soluble drug in the hydrophobic inner core and the encapsulation of genetic material by the establishment of electrostatic interactions with the cationic polymer. Thus, micelleplexes could be a beneficial option to overcome multidrug resistance and be able to perform an active targeting to the cancerous cells. However, despite its promising features, research involving the use of micelleplexes for OS treatment remains scarce. In this sense, we propose the use of micelleplexes for the transportation of genetic material as well as poorly-water soluble drugs (dual therapy), carrying miRNA145 as a therapeutic option to surpass MDR and achieve active targeting towards OS cells. Furthermore, we expect that this approach might increase patient’s survival rate and halt tumor progression and metastasis, thereby improving OS’ outcome. In the future we aim to assess the potential of a micelleplex formulation consisting on the amphiphilic copolymer Pluronic® F68 and a cationic polymer, either PEI or chitosan, encapsulating miRNA145 for a targeted therapy against OS cells.
Acknowledgements
The present work was financially supported by National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência) through project UID/QUI/50006/2013 and co-financed by the European Union (FEDER under the Partnership Agreement PT2020). Additionally, it was financed by the FCT PTDC/BTM-MAT/30255/2017 grant (POCI-01-0145-FEDER-030255) from the Portuguese Foundation for Science and Technology (FCT) as well as the European Community Fund (FEDER) by the COMPETE2020 program.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this review.
Footnotes
A chemical law stating that the rate of diffusion observed for a compound in an aqueous solution rises according to the difference in concentration between the two adjoining locations. This difference potentiates the particle movement toward the region with the lowest concentration (Barrera 2005).
References
- Abarrategi A, Tornin J, Lucia MC, Hamilton A, Enrique MC, Rodrigo JP, González MV, Baldini N, Garcia-Castro J, Rodriguez R. Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Int. 2016 doi: 10.1155/2016/3631764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abello J, Nguyen TDT, Marasini R, Aryal S, Weiss ML. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics. 2019;9(8):2325–2345. doi: 10.7150/thno.30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Alam MA, Ahmad R, Naqvi AA, Ahmad FJ. Preparation and characterization of surface-modified PLGA-polymeric nanoparticles used to target treatment of intestinal cancer. Artif Cells Nanomed Biotechnol. 2018;46(2):432–446. doi: 10.1080/21691401.2017.1324466. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Alam MA, Ahmad R, Umar S, Ahmad FJ. Improvement of oral efficacy of Irinotecan through biodegradable polymeric nanoparticles through in vitro and in vivo investigations. J Microencapsul. 2018;35(4):327–343. doi: 10.1080/02652048.2018.1485755. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Ahmad R, Ahmad FJ, Ahmad W, Alam MA, Amir M, Ali A. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J Biol Sci. 2019 doi: 10.1016/j.sjbs.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Ahmad R, Alam MA, et al. Daunorubicin oral bioavailability enhancement by surface coated natural biodegradable macromolecule chitosan based polymeric nanoparticles. Int J Biol Macromol. 2019;128:825–838. doi: 10.1016/j.ijbiomac.2019.01.142. [DOI] [PubMed] [Google Scholar]
- Alexandridis P, Hatton TA. Poly(ethylene oxide)poly(propylene oxide)poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids Surf A Physicochem Eng Aspects. 1995;96(1–2):1–46. doi: 10.1016/0927-7757(94)03028-X. [DOI] [Google Scholar]
- Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv. 2006;3(1):139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- Almeida M, Magalhães M, Veiga F, Figueiras A. Poloxamers, poloxamines and polymeric micelles: definition, structure and therapeutic applications in cancer. J Polym Res. 2018 doi: 10.1007/s10965-017-1426-x. [DOI] [Google Scholar]
- Alp E, Çirak T, Demirbilek M, Mustafa T, Güven E. Targeted delivery of etoposide to osteosarcoma cells using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanoparticles. Turk J Biol. 2017;41(5):719–733. doi: 10.3906/biy-1612-17. [DOI] [Google Scholar]
- Alvarez-Lorenzo C, Rey-Rico A, Sosnik A, Taboada P, Concheiro A. Poloxamine-based nanomaterials for drug delivery. Front Biosci Elite. 2010;2E(2):424–440. doi: 10.2741/e102. [DOI] [PubMed] [Google Scholar]
- Amin MCIM, Butt AM, Amjad MW, Kesharwani P (2017) Polymeric micelles for drug targeting and delivery. Nanotechnology-based approaches for targeting and delivery of drugs and genes. Elsevier Inc., Oxford. https://doi.org/10.1016/B978-0-12-809717-5.00006-3
- Ayre A, Kadam V, Dand N, Patel P. Polymeric micelles as a drug carrier for tumor targeting. Chron Young Sci. 2013;4(2):94. doi: 10.4103/2229-5186.115544. [DOI] [Google Scholar]
- Barrera E. On the sesquicentennial of Fick’s laws of diffusion. Nat Struct Mol Biol. 2005;12(4):280. doi: 10.1038/nsmb0405-280. [DOI] [PubMed] [Google Scholar]
- Bielack SS, Werner M, Tunn PU, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33(20):2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodratti AM, Alexandridis P. Formulation of poloxamers for drug delivery. J Funct Biomater. 2018 doi: 10.3390/jfb9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffery B, Lee JS, Alexander-Bryant AA. Vectors for glioblastoma gene therapy: viral & non-viral delivery strategies. Nanomaterials. 2019 doi: 10.3390/nano9010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliskan Y, Dalgic AD, Gerekci S, Gulec EA, Tezcaner A, Ozen C, Keskin D. A new therapeutic combination for osteosarcoma: Gemcitabine and Clofazimine co-loaded liposomal formulation. Int J Pharm. 2019;557:97–104. doi: 10.1016/j.ijpharm.2018.12.041. [DOI] [PubMed] [Google Scholar]
- Chaiyawat P, Pruksakorn D, Pipatwattana P, Phanphaisarn A, Teeyakasem P, Klangjorhor J, Settakorn J. Endoplasmic reticulum protein 29 (ERp29) as a novel prognostic marker and tumor suppressor in osteosarcoma. J Bone Oncol. 2019 doi: 10.1016/j.jbo.2019.100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Huang Z, Zhang Y, Chen Y, Li Z. MicroRNA-145 suppresses osteosarcoma metastasis via targeting MMP16. Cell Physiol Biochem. 2015;37(6):2183–2193. doi: 10.1159/000438575. [DOI] [PubMed] [Google Scholar]
- Choong PFM, Broadhead ML, Clark JCM, Myers DE, Dass CR. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011 doi: 10.1155/2011/959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EKH, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5(216):1–12. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- Cottrell J. A review of osteosarcoma therapeutics. J Cancer Treat Diagn. 2018;2(2):21–29. doi: 10.29245/2578-2967/2018/2.1127. [DOI] [Google Scholar]
- Cui SY, Wang R, Chen LB. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med. 2014;18(10):1913–1926. doi: 10.1111/jcmm.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AS, Chauhan PN, Noolvi MN, et al. Polymeric micelles: basic research to clinical practice. Int J Pharm. 2017;532(1):249–268. doi: 10.1016/j.ijpharm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Duchi S, Sotgiu G, Lucarelli E, et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. J Control Rel. 2013;168(2):225–237. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Fan L, Wu Q, Xing X, Wei Y, Shao Z. MicroRNA-145 targets vascular endothelial growth factor and inhibits invasion and metastasis of osteosarcoma cells. Acta Biochimica et Biophysica Sinica. 2012;44(5):407–414. doi: 10.1093/abbs/gms019. [DOI] [PubMed] [Google Scholar]
- Fang X, Bin Zhang JM, Xie X, Liu D, He CW, Wan JB, Chen MW. pH-sensitive micelles based on acid-labile pluronic F68-curcumin conjugates for improved tumor intracellular drug delivery. Int J Pharm. 2016;502(1–2):28–37. doi: 10.1016/j.ijpharm.2016.01.029. [DOI] [PubMed] [Google Scholar]
- Fang Z, Sun Y, Xiao H, et al. Targeted osteosarcoma chemotherapy using RGD peptide-installed doxorubicin-loaded biodegradable polymeric micelle. Biomed Pharmacother. 2017;85:160–168. doi: 10.1016/j.biopha.2016.11.132. [DOI] [PubMed] [Google Scholar]
- Feng H, Chu D, Li Z, et al. A DOX-loaded polymer micelle for effectively inhibiting cancer cells. RSC Adv. 2018;8(46):25949–25954. doi: 10.1039/c8ra04089c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Wu ZX, Zhao Z, et al. Engineering of bone- and CD44-dual-targeting redox-sensitive liposomes for the treatment of orthotopic osteosarcoma. ACS Appl Mater Interfaces. 2019;11(7):7357–7368. doi: 10.1021/acsami.8b18820. [DOI] [PubMed] [Google Scholar]
- Gáscon AR, del Pozo-Rogríguez A, Solinís MÁ. Non-viral delivery systems in gene therapy. Intech. 2013 doi: 10.5772/52704. [DOI] [Google Scholar]
- Gazzano E, Buondonno I, Marengo A, et al. Hyaluronated liposomes containing H2S-releasing doxorubicin are effective against P-glycoprotein-positive/doxorubicin-resistant osteosarcoma cells and xenografts. Cancer Lett. 2019;456(April):29–39. doi: 10.1016/j.canlet.2019.04.029. [DOI] [PubMed] [Google Scholar]
- Giansanti L, Condello M, Altieri B, Galantini L, Meschini S, Mancini G. Influence of lipid composition on the ability of liposome loaded voacamine to improve the reversion of doxorubicin resistant osteosarcoma cells. Chem Phys Lipids. 2019 doi: 10.1016/j.chemphyslip.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Gigout A, Buschmann MD, Jolicoeur M. The fate of Pluronic F-68 in chondrocytes and CHO clls. Biotechnol Bioeng. 2008;100(5):975–987. doi: 10.1002/bit.21840. [DOI] [PubMed] [Google Scholar]
- Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16(2):165–171. doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- Gui K, Zhang X, Chen F, et al. Lipid-polymer nanoparticles with CD133 aptamers for targeted delivery of all-trans retinoic acid to osteosarcoma initiating cells. Biomed Pharmacother. 2019;111:751–764. doi: 10.1016/j.biopha.2018.11.118. [DOI] [PubMed] [Google Scholar]
- Hang JF, Chen PCH. Parosteal osteosarcoma. Arch Pathol Lab Med. 2014;138(5):694–699. doi: 10.5858/arpa.2013-0030-RS. [DOI] [PubMed] [Google Scholar]
- Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. 2017 doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Rel. 2003;89(2):151–165. doi: 10.1016/S0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- HiMedia Laboratories Pvt Ltd. (2019) Pluronic (R) F-68: product information, 400086. https://himedialabs.com/TD/TC222.pdf. Accessed 25 Sep 2019
- Hong W, Chen D, Zhang X, Zeng J, Hu H, Zhao X, Qiao M. Reversing multidrug resistance by intracellular delivery of Pluronic® P85 unimers. Biomaterials. 2013;34(37):9602–9614. doi: 10.1016/j.biomaterials.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Hong ES, Burkett SS, Morrow J, et al. Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget. 2015;6(30):29469–29481. doi: 10.18632/oncotarget.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomed. 2012;7:1851–1863. doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HK, Hsu KH, Cheng YM, Suen HY, Peng SF. Development and in vitro evaluation of linear PEI-shelled heparin/berberine nanoparticles in human osteosarcoma U-2 OS cells. Molecules (Basel, Switzerland) 2018 doi: 10.3390/molecules23123121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zhang Y, Cai XH, Huang JF, Cai L. Changes in microRNA expression in the MG-63 osteosarcoma cell line compared with osteoblasts. Oncol Lett. 2012;4(5):1037–1042. doi: 10.3892/ol.2012.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed Nanotechnol Biol Med. 2010;6(6):714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kesharwani SS, Kaur S, Tummala H, Sangamwar AT. Overcoming multiple drug resistance in cancer using polymeric micelles. Expert Opin Drug Deliv. 2018;15(11):1127–1142. doi: 10.1080/17425247.2018.1537261. [DOI] [PubMed] [Google Scholar]
- Kumar VS, Barwar N, Khan SA. Surface osteosarcomas: diagnosis, treatment and outcome. Indian J Orthop. 2014;48(3):255–261. doi: 10.4103/0019-5413.132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SR, Markusic DM, Biswas M, High KA, Herzog RW. Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev. 2016;3:16034. doi: 10.1038/mtm.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TT, Wang CH, Wang JY, Chiou HJ, Fan CH, Yeh CK. Concurrent osteosarcoma theranostic strategy using contrast-enhanced ultrasound and drug-loaded bubbles. Pharmaceutics. 2019 doi: 10.3390/pharmaceutics11050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Mohammed KA, Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am J Cancer Res. 2016;6(5):1118–1134. [PMC free article] [PubMed] [Google Scholar]
- Lei P, Xie J, Wang L, Yang X, Dai Z, Hu Y. microRNA-145 inhibits osteosarcoma cell proliferation and invasion by targeting ROCK1. Mol Med Rep. 2014;10(1):155–160. doi: 10.3892/mmr.2014.2195. [DOI] [PubMed] [Google Scholar]
- Lewis VO, Devarajan E, Cardó-Vila M, et al. BMTP-11 is active in preclinical models of human osteosarcoma and a candidate targeted drug for clinical translation. Proc Natl Acad Sci USA. 2017;114(30):8065–8070. doi: 10.1073/pnas.1704173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Liu XZ, Zhang L, Chen LB, Shi X, Wu SJ, Zhao JN. Advances in bone-targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma. Orthop Surg. 2016;8(2):105–110. doi: 10.1111/os.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu J, Liu ZZ, Wei WB. MicroRNA-145 inhibits tumour growth and metastasis in osteosarcoma by targeting cyclin-dependent kinase, CDK6. Eur Rev Med Pharmacol Sci. 2016;20(24):5117–5125. [PubMed] [Google Scholar]
- Li S, Xiong Y, Zhang X. Poloxamer surface modified trimethyl chitosan nanoparticles for the effective delivery of methotrexate in osteosarcoma. Biomed Pharmacother. 2017;90:872–879. doi: 10.1016/j.biopha.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Liang W, Gong H, Yin D, Lu S, Fu Q. High-molecular-weight polyethyleneimine conjuncted pluronic for gene transfer agents. Chem Pharm Bull. 2011;59(9):1094–1101. doi: 10.1248/cpb.59.1094. [DOI] [PubMed] [Google Scholar]
- Liang C, Li F, Wang L, et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials. 2017;147:68–85. doi: 10.1016/j.biomaterials.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. 2017;4(1):25–43. doi: 10.1007/s40744-016-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HH, Huang CH, Shiue TY, Wang FS, Chang KK, Chen Y, Peng CH. Erratum: highly efficient gene release in spatiotemporal precision approached by light and pH dual responsive copolymers. Chem Sci. 2019;10(1):284–292. doi: 10.1039/C8SC01494A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K. Viral vectors in gene therapy. Diseases. 2018 doi: 10.3390/diseases6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K, Boulikas T. Viral and non-viral vectors in gene therapy: technology development and clinical trials. Technol Cancer Res Treat. 2003;2(5):471–485. doi: 10.1177/153303460300200513. [DOI] [PubMed] [Google Scholar]
- Luo Z, Li D, Luo X, Li L, Gu S, Yu L, Ma Y. Curcumin may serve an anticancer role in human osteosarcoma cell line U-2 OS by targeting ITPR1. Oncol Lett. 2018;15(4):5593–5601. doi: 10.3892/ol.2018.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães M, Almeida M, Tavares-da-Silva E, et al. miR-145-loaded micelleplexes as a novel therapeutic strategy to inhibit proliferation and migration of osteosarcoma cells. Eur J Pharm Sci. 2018;123(July):28–42. doi: 10.1016/j.ejps.2018.07.021. [DOI] [PubMed] [Google Scholar]
- Magalhães M, Figueiras A, Veiga F. Smart micelleplexes: an overview of a promising and potential nanocarrier for alternative therapies. Des Dev N Nanocarr. 2018 doi: 10.1016/b978-0-12-813627-0.00007-7. [DOI] [Google Scholar]
- Mahmud A, Xiong XB, Aliabadi HM, Lavasanifar A. Polymeric micelles for drug targeting. J Drug Targ. 2007;15(9):553–584. doi: 10.1080/10611860701538586. [DOI] [PubMed] [Google Scholar]
- Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: a difficult condition to diagnose. Sarcoma. 2012 doi: 10.1155/2012/764796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney CW, Edelman M, Symons M, Steinberg BM, Soffer SZ. Gefitinib blocks macrophage-promoted invasion of osteosarcoma via inhibition of receptor-interacting protein kinase 2 (RIPK2) and prevents progression of pulmonary micrometastases. J Am Coll Surg. 2017;225(4):S150. doi: 10.1016/j.jamcollsurg.2017.07.337. [DOI] [Google Scholar]
- Maran A, Yaszemski MJ, Kohut A, Voronov A. Curcumin and osteosarcoma: can invertible polymeric micelles help? Materials. 2016;9(7):10–13. doi: 10.3390/ma9070520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JW, Squire JA, Zielenska M. The genetics of osteosarcoma. Sarcoma. 2012;2012:11. doi: 10.1155/2012/627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni E, Benassi MS, Corallini A, et al. Significant association between human osteosarcoma and Simian virus 40. Cancer. 2015;121(5):708–715. doi: 10.1002/cncr.29137. [DOI] [PubMed] [Google Scholar]
- Mekhail GM, Kamel AO, Awad GAS, Mortada ND, Rodrigo RL, Spagnuolo PA, Wettig SD. Synthesis and evaluation of alendronate-modified gelatin biopolymer as a novel osteotropic nanocarrier for gene therapy. Nanomedicine. 2016;11(17):2251–2273. doi: 10.2217/nnm-2016-0151. [DOI] [PubMed] [Google Scholar]
- Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. Sicot-J. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC. Poloxamers and poloxamines in nanoparticleengineering and experimental medicine. Science. 2000;18(October):2958–2964. doi: 10.1016/s0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- Moloughney JG, Weisleder N. Poloxamer 188 (P188) as a membrane resealing reagent in biomedical applications. Recent Patents Biotechnol. 2013;6(3):200–211. doi: 10.2174/1872208311206030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity BS, Otto GM, Rahrmann EP, et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet. 2015;47(6):615–624. doi: 10.1038/ng.3293.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, MacFarlane LA. MicroRNA: biogenesis, function and role in cancer. Curr Genom. 2010;11(7):537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namee NM. O’Driscoll L (2018) Extracellular vesicles and anti-cancer drug resistance. Biochimi Biophys Acta Rev Cancer. 1870;2:123–136. doi: 10.1016/j.bbcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Nguyen HK, Lemieux P, Vinogradov SV, et al. Evaluation of polyether-polyethyleneimine graft copolymers as gene transfer agents. Gene Ther. 2000;7(2):126–138. doi: 10.1038/sj.gt.3301052. [DOI] [PubMed] [Google Scholar]
- O’Day K, Gorlick R. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther. 2009;9(4):511–523. doi: 10.1586/ERA.09.7. [DOI] [PubMed] [Google Scholar]
- Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othayoth R, Kumar KS, Karthik V. Development and characterization of chitosan-pluronic polymeric nanoparticles for the breast cancer treatment. Int J Mech Eng Robot. 2013;1(1):71–79. [Google Scholar]
- Patterson LA, Greer RO, Howard D. Periosteal osteosarcoma of the maxilla: a case report and review of literature. J Oral Maxillofac Surg. 1990;48(5):522–526. doi: 10.1016/0278-2391(90)90246-X. [DOI] [PubMed] [Google Scholar]
- Pereira P, Barreira M, Queiroz JA, Veiga F, Sousa F, Figueiras A. Smart micelleplexes as a new therapeutic approach for RNA delivery. Expert Opin Drug Deliv. 2017;14(3):353–371. doi: 10.1080/17425247.2016.1214567. [DOI] [PubMed] [Google Scholar]
- Pitto-Barry A, Barry NPE. Pluronic® block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advances. Polym Chem. 2014;5(10):3291–3297. doi: 10.1039/c4py00039k. [DOI] [Google Scholar]
- PosthumaDeBoer J, van Royen B, Helder M. Mechanisms of therapy resistance in osteosarcoma: a review. Oncol Discov. 2013;1(1):8. doi: 10.7243/2052-6199-1-8. [DOI] [Google Scholar]
- Raimondi L, De Luca A, Costa V, et al. Circulating biomarkers in osteosarcoma: new translational tools for diagnosis and treatment. Oncotarget. 2017;8(59):100831–100851. doi: 10.18632/oncotarget.19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadurai S, Kohut A, Sarangi NK, Zholobko O, Baulin VA, Voronov A, Keyes TE. Macromolecular inversion-driven polymer insertion into model lipid bilayer membranes. J Colloid Interface Sci. 2019;542:483–494. doi: 10.1016/j.jcis.2019.01.093. [DOI] [PubMed] [Google Scholar]
- Ramamoorth M, Narvekar A. Non viral vectors in gene therapy—an overview. J Clin Diagn Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reghupaty SC, Sarkar D. Current status of gene therapy in hepatocellular carcinoma. Cancers. 2019 doi: 10.3390/cancers11091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nogales C, González-Fernández Y, Aldaz A, Couvreur P, Blanco-Prieto MJ. Nanomedicines for pediatric cancers. ACS Nano. 2018;12(8):7482–7496. doi: 10.1021/acsnano.8b03684. [DOI] [PubMed] [Google Scholar]
- Samal SK, Dash M, Van Vlierberghe S, et al. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41(21):7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA, Yustein J. MicroRNAs and potential targets in osteosarcoma: review. Front Pediatr. 2015;3(August):1–13. doi: 10.3389/fped.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santander-Ortega MJ, Jódar-Reyes AB, Csaba N, Bastos-González D, Ortega-Vinuesa JL. Colloidal stability of Pluronic F68-coated PLGA nanoparticles: a variety of stabilisation mechanisms. J Colloid Interface Sci. 2006;302(2):522–529. doi: 10.1016/j.jcis.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Saravanabhavan SS, Rethinasabapathy M, Zsolt S, et al. Graphene oxide functionalized with chitosan based nanoparticles as a carrier of siRNA in regulating Bcl-2 expression on Saos-2 & MG-63 cancer cells and its inflammatory response on bone marrow derived cells from mice. Mater Sci Eng C. 2019;99:1459–1468. doi: 10.1016/j.msec.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Savvidou OD, Bolia IK, Chloros GD, Goumenos SD, Sakellariou VI, Galanis EC, Papagelopoulos PJ. Applied nanotechnology and nanoscience in orthopedic oncology. Orthopedics. 2016;39(5):280–286. doi: 10.3928/01477447-20160823-03. [DOI] [PubMed] [Google Scholar]
- Shi J, Kantoff P, Wooster R, Farokhzad O. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A, Islam S, Jones S. Gene therapy: an overview of approved and pipeline technologies. Cadth Issues Emerg Health Technol. 2018;171:1–23. [PubMed] [Google Scholar]
- Smeester BA, Moriarity BS, Beitz AJ. Osteosarcomagenesis: biology, development, metastasis, and mechanisms of pain. Osteosarcoma Biol Behav Mech. 2017;i:21. doi: 10.5772/67070. [DOI] [Google Scholar]
- Susa M, Iyer AK, Ryu K, et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010 doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ML, Choong PFM, Dass CR. Osteosarcoma: conventional treatment vs. gene therapy. Cancer Biol Ther. 2009;8(2):106–117. doi: 10.4161/cbt.8.2.7385. [DOI] [PubMed] [Google Scholar]
- Tang S, Yin Q, Su J, et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials. 2015;48:1–15. doi: 10.1016/j.biomaterials.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Tharmalingam T, Ghebeh H, Wuerz T, Butler M. Pluronic enhances the robustness and reduces the cell attachment of mammalian cells. Mol Biotechnol. 2008;39(2):167–177. doi: 10.1007/s12033-008-9045-8. [DOI] [PubMed] [Google Scholar]
- Gene Therapy Products on the Market. (n.d.) Gene therapy net. https://www.genetherapynet.com/gene-therapy-products-on-the-market.html. Accessed 24 Sep 2019
- Tirino V, Desiderio V, Paino F, et al. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25(6):2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- Tong R, Kohane DS. New strategies in cancer nanomedicine. Annu Rev Pharmacol Toxicol. 2016;56(1):41–57. doi: 10.1146/annurev-pharmtox-010715-103456. [DOI] [PubMed] [Google Scholar]
- Torreggiani E, Roncuzzi L, Perut F, Zini N, Baldini N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol. 2016;49(1):189–196. doi: 10.3892/ijo.2016.3509. [DOI] [PubMed] [Google Scholar]
- Wakaskar RR. Passive and active targeting in tumor microenvironment. Int J Drug Dev Res. 2017;9(2):37–41. [Google Scholar]
- Wang F, Pang JD, Huang LL, et al. Nanoscale polysaccharide derivative as an AEG-1 siRNA carrier for effective osteosarcoma therapy. Int J Nanomed. 2018;13:857–875. doi: 10.2147/IJN.S147747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang F, Li X, Zhou Y, Wang H, Zhang Y. Uniform carboxymethyl chitosan-enveloped Pluronic F68/poly(lactic-co-glycolic acid) nano-vehicles for facilitated oral delivery of gefitinib, a poorly soluble antitumor compound. Colloids Surf B Biointerfaces. 2019;177:425–432. doi: 10.1016/j.colsurfb.2019.02.028. [DOI] [PubMed] [Google Scholar]
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Controll Rel. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Williams PD, Kingston PA. Plasmid-mediated gene therapy for cardiovascular disease. Cardiovasc Res. 2011;91(4):565–576. doi: 10.1093/cvr/cvr197. [DOI] [PubMed] [Google Scholar]
- Worgall S, Crystal RG (2007) Gene therapy. Oncology nursing, 4th edn. Elsevier, Oxford. https://doi.org/10.5005/jp/books/10569_34
- Wu G, Yu W, Zhang M, Yin R, Wu Y, Liu Q. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells Nanomed Biotechnol. 2018;46(sup2):579–586. doi: 10.1080/21691401.2018.1464459. [DOI] [PubMed] [Google Scholar]
- Xie B, Li Y, Zhao R, et al. Identification of key genes and mirnas in osteosarcoma patients with chemoresistance by bioinformatics analysis. BioMed Res Int. 2018;2018:10. doi: 10.1155/2018/4761064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wen X, Ke QF, Xie XT, Guo YP. pH-responsive mesoporous ZSM-5 zeolites/chitosan core-shell nanodisks loaded with doxorubicin against osteosarcoma. Mater Sci Eng C. 2018;85:142–153. doi: 10.1016/j.msec.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Yin JQ, Fu YW, Xie XB, et al. Telangiectatic osteosarcoma: outcome analyses and a diagnostic model for differentiation from aneurysmal bone cyst. J Bone Oncol. 2018;11:10–16. doi: 10.1016/j.jbo.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ye M, Zhu J, et al. Zinc phthalocyanine encapsulated in polymer micelles as a potent photosensitizer for the photodynamic therapy of osteosarcoma. Nanomed Nanotechnol Biol Med. 2018;14(4):1099–1110. doi: 10.1016/j.nano.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang Y, Li S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech. 2014;15(4):862–871. doi: 10.1208/s12249-014-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH, Wang WJ. MicroRNAs in osteosarcoma. Clin Chim Acta. 2015;444:9–17. doi: 10.1016/j.cca.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Buhrman JS, Liu Y, Rayahin JE, Gemeinhart RA. Reducible micelleplexes are stable systems for anti-miRNA delivery in cerebrospinal fluid. Mol Pharm. 2016;13(6):1791–1799. doi: 10.1021/acs.molpharmaceut.5b00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu ZH, Xie H, Sun YW, Liu J, Zhao YB. ING5 is a potential target for osteosarcoma therapy. Technol Cancer Res Treat. 2018;17:1–5. doi: 10.1177/1533033818762680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bi D, Qi X, Guo Y, Yue F, Wang X, Han M. Polydopamine-based surface modification of paclitaxel nanoparticles for osteosarcoma targeted therapy. Nanotechnology. 2019;30(25):255101. doi: 10.1088/1361-6528/ab055f. [DOI] [PubMed] [Google Scholar]
- Zhou G, Shi X, Zhang J, Wu S, Zhao J. MicroRNAs in osteosarcoma: from biological players to clinical contributors, a review. J Int Med Res. 2013;41(1):1–12. doi: 10.1177/0300060513475959. [DOI] [PubMed] [Google Scholar]