Abstract
Statins, the drugs for the treatment of dyslipidemia, have been suggested to impact insulin sensitivity, resulting in pancreatic β-cell dysfunction, and consequently, lead to new onset of diabetes. Taking this as a clue, the present study was designed to evaluate the protective effect of sesamol (a known antioxidant, antidiabetic and antidyslipidemic agent) against the diabetogenic potential of simvastatin. The toxic effects of simvastatin and sesamol on MIN6 insulinoma (Mouse pancreatic β cells) cells were evaluated separately by MTT assay. The protective effect of sesamol was evaluated at the IC50 value of simvastatin at doses ranging from 7.8 to 62.5 micromolar (µM). Further, the reversal of the impact of simvastatin on cell cycle and mitochondrial membrane potential by sesamol pretreatment was studied. The IC50 for simvastatin and sesamol were found to be 70.05 ± 2.34 μM and 2134 ± 8.41 μM, respectively, after 48 h and 72 h of incubation. Sesamol pretreatment protected the MIN6 cells from simvastatin toxicity (70 µM) in a dose-dependent manner from 7.8 to 31.25 µM. Simvastatin induced cell cycle arrest in G0/G1 phase. However, when cells were preincubated with sesamol for 24 h, a reversal in the cell cycle arrest was observed in simvastatin-treated cells (G0/G1). Pretreatment with sesamol also reduced the mitochondrial membrane potential loss compared to simvastatin treatment alone. These in vitro findings indicate that sesamol has a protective effect against simvastatin-induced toxicity on the pancreatic beta cells.
Keywords: Statins, Simvastatin, Sesamol, Cell cycle, Mitochondrial membrane potential, Cytotoxicity, MIN6
Introduction
Around 17.5 million people die each year from cardiovascular diseases (CVD), which is an estimated 31% of all deaths worldwide (WHO). Dyslipidemia is considered to be the crux of most of the cardiovascular problems. Statins are the most effective and first-line agents for the treatment of dyslipidemia in patients with increased risk of CVD. These drugs were initially isolated from the molds Penicillium citrinum (mevastatin) and Aspergillus terreus (lovastatin) which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the key enzyme for the conversion of HMG-CoA to mevalonate in cholesterol biosynthesis pathway (Endo et al. 1976). Though they are the first-line drugs for the management of dyslipidemia, and are considered to be safe, long-term usage produces toxic effects like myopathy, rhabdomyolysis with low incidence of hepatitis, cholestatic jaundice, cirrhosis and hepatic failure.
Recent reports suggested that statins affect glucose metabolism and pancreatic beta cell function (Chogtu et al. 2015). Increasing risk of developing type 2 diabetes mellitus (T2DM) by statin usage is highlighted in the USFDA 2012 report (Food US Drug Administration 2012). From the reports from Heart Protection Study, 335 subjects developed diabetes in the simvastatin group, with outcomes similar to that of the Anglo-Scandinavian Cardiac Outcomes trial, the atorvastatin group-developed diabetes. JUPITER trial also showed a significant increase in the rate of diabetes in patients treated with rosuvastatin (20 mg) due a significant increase in HbA1c (Zhao and Zhao 2015). The reports from other clinical studies suggest that lipophilic statins might cause the induction of diabetes compared to hydrophilic statins (Lee et al. 2016).
The lipophilicity of statins is linked to the inhibition of glucose-induced cytosolic Ca2+ signaling pathway. By inhibiting L-type Ca2+ channels in beta cells, statins inhibit the insulin secretion (Aiman et al. 2014). Besides being the precursor for cholesterol synthesis, mevalonate also plays an important role in other nonsteroidal isoprenoid synthesis. Inhibition of HMG-CoA reductase by statins is responsible for their pleiotropic effects (Stancu and Sima 2001). On long-term treatment, the inhibition of HMG Co-A reductase results in a decrease in intracellular concentration of geranyl-pyrophosphate and CoQ10 levels. CoQ10 acts as a carrier to carry electron from complex I to complex III via complex II, which results in a decrease in ATP production and affects insulin release from beta insulin-secreting cells (Hamilton et al. 2009).
Currently, CoQ10 supplementation is considered to minimize the diabetogenic-associated problem of statins (Hamilton et al. 2009). A search for an alternative therapy is also required. Sesamol from sesame seeds is one such moiety, which may be effective in conquering diabetogenic potential of statins due to its hypolipidemic (Kumar et al. 2013), antidiabetic (Topal 2019), and antioxidant potential (Shah et al. 2019). It is also reported to prevent cerulein-mediated oxidative stress-induced pancreatitis both in in vitro and in vivo conditions (Chu et al. 2012) and shows anti-inflammatory effect by inhibiting lipoxygenase (Yashaswini et al. 2017). In addition, sesamol acts peripherally by acting on muscle by regulating mitochondrial lipid metabolism in adipose tissue (3T3L1 cells) and prevents insulin resistance in diet-induced obesity in C57BL/6J mice (Liu et al. 2017). Thus, the present study was designed to evaluate the exact mechanism involved in the diabetogenic potential of simvastatin and protection of the same by sesamol in pancreatic β cells.
Materials and methods
Materials
MIN6 insulinoma (Mouse pancreatic β cells) cells were purchased from NCCS, Pune. Simvastatin was a gift sample from Biocon, Bangalore. Sesamol was purchased from TCI Chemicals, India. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), propidium iodide and JC1 dye were purchased from Sigma Aldrich, USA.
Cell culture maintenance and treatments
Cell lines, procured from NCCS, Pune, were grown in 25 cm2 tissue culture flasks containing suitable media. Cells were maintained in DMEM supplemented with 15% FBS and gentamicin to inhibit microbial growth at 37 ºC in a CO2 incubator (NUAIRE, DHD Auto flow automatic CO2 incubator; NU-5501/E/G) in a humidified atmosphere of 5% CO2 and 95% air. The cells were maintained by routine subculturing in 25 cm2 tissue culture flasks. All drugs for treatment were solubilized in DMSO to make 100 mM stock solution.
Cell viability assay
Exponentially growing cells from the T-25 flasks were harvested and stock suspension of 1 × 106 cells was prepared with the medium. The cells were seeded at a rate of 5000 cells/well in a 96 well plate and incubated for 24 h in a CO2 incubator to allow attachment of cells. Different concentrations of test compounds (3.90–500 µM) were prepared by serial dilution with the medium. The final concentration of DMSO did not exceed 0.2%. After 24 h incubation, the wells were treated with 100 µl of different concentrations of test compounds from the stocks prepared. The control group received only medium, and the vehicle group had vehicle and medium. All the treatments were made in triplicates and the cells allowed to incubate for 48 h and 72 h in simvastatin and sesamol. After incubation, 30 µl of MTT reagent was added and incubated for 3 h. The media was aspirated and 200 µl of DMSO was added and incubated for 3 h to solubilize the formazan crystals. The plate was placed on ELISA plate reader and the optical density was measured at 540 nm (Cheruku et al. 2018).
For in vitro protection study, 5000 cells/well were seeded and incubated for 24 h. One hundred microliters of different concentrations of sesamol were added and incubated for the next 24 h. Simvastatin was added at IC50 value (70 µM) and incubated for 48 h. The MTT was added and absorbance was recorded after dissolving in DMSO as per the cell viability assay protocol. The same treatment protocol was used for cell cycle analysis and mitochondrial membrane potential measurement (Cheruku et al. 2019).
Cell cycle analysis
Cells were treated and harvested to prepare a single cell suspension in wash buffer. Cells were washed twice and resuspended at a rate of 1 × 106 cells/ml. Cold ethanol (70%, −20 °C) was added to 1 ml cells and fixed overnight. Following that the cells were washed with PBS, stained with propidium iodide (1 ml), mixed well and incubated for 20 min in the dark at room temperature. The samples were analyzed by flow cytometry (Pande et al. 2017).
Mitochondrial membrane potential measurement
The treated cell culture was trypsinized. The trypsinized cell suspension was added into 1.5 ml tube with 1 ml media. The cell suspension was shaken and 2.5 μg/ml of JC-1 dye in PBS was added and vortexed for 5 min. Samples were kept in a dark place for 15 min at room temperature. After incubation, cells were centrifuged at 2000 rpm at 4 ºC for 5 min. The medium was removed and cell pellets were washed twice with PBS. Cells were centrifuged at 2000 rpm at 4 ºC for 5 min. PBS was removed and again 300 μl of PBS added. Mitochondrial membrane potential was analyzed by flow cytometry (BD Accuri C6) (Salmataj et al. 2018; Tiwari et al. 2016).
Results
Cell viability and cytoprotection study
The IC50 for the simvastatin and sesamol were found to be 70.05 ± 2.34 μM and 2134 ± 8.41 μM, respectively after 48 h and 72 h of incubation. Sesamol pretreatment significantly (P < 0.05) protected the MIN6 cells from the simvastatin toxicity (70 µM) in a dose-dependent manner from 7.8 µM to 31.25 µM as compared to simvastatin treatment alone. However, at 62.5 µM, the protection was not seen as compared to simvastatin treatment alone (Table 1).
Table 1.
Percentage cell viability of MIN6 cells in the presence of sesamol and simvastatin as obtained from MTT assay
| Treatment drug | Cell viability (%) |
|---|---|
| Simvastatin (70 μM) | 51.55 ± 1.91 |
| Sesamol (7.8 μM) + simvastatin (70 μM) | 77.61 ± 6.24* |
| Sesamol (15.6 μM) + simvastatin (70 μM) | 87.42 ± 5.61** |
| Sesamol (31.25 μM) + simvastatin (70 μM) | 84.76 ± 6.76** |
| Sesamol (62.5 μM) + simvastatin (70 μM) | 54.09 ± 0.41 |
All the values are expressed as mean ± SEM, where *p < 0.05 compared to simvastatin, **p < 0.05 compared to simvastatin. Data were compared with each other by one-way ANOVA followed by Tukey’s post hoc analysis using Prism version 6.01 Demo version (Graph Pad Inc., La Jolla, CA, USA). Statistical values are F (DFn, DFd): F (4, 10) = 12.2, p = 0.0007. IC50 of sesamol is 2134 ± 8.41 μM, IC50 of simvastatin is 70.05 ± 2.34 μM
Cell cycle analysis
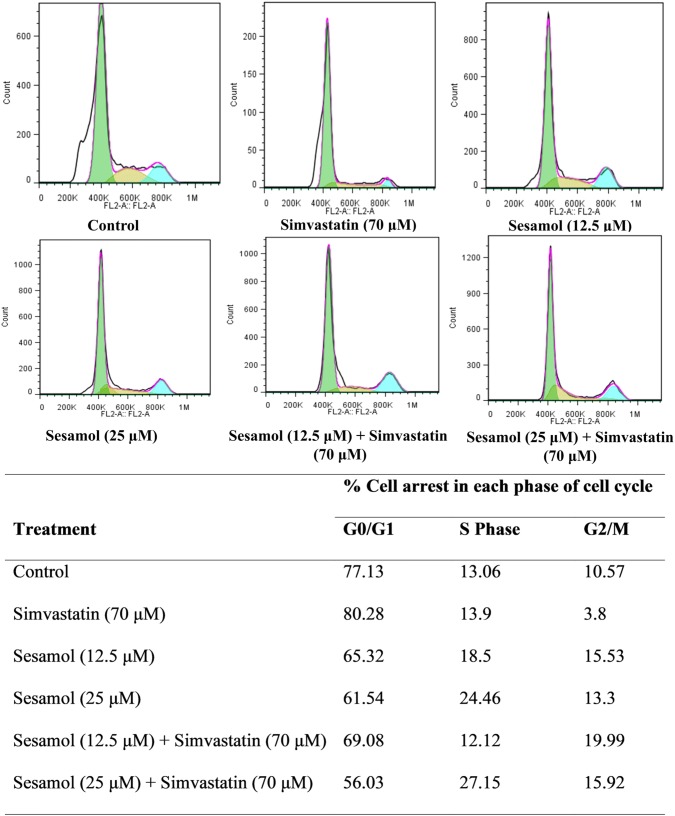
Cell cycle analysis was done to identify the effects of simvastatin and sesamol on the cell cycle in the MIN6 cell line. Sesamol was given as pretreatment 24 h before adding simvastatin to evaluate the cell arrest. Simvastatin produced more cell cycle arrest in the G0/G1 phase when treated alone. However, preincubation of cells with sesamol for 24 h, reduced the simvastatin-induced cell cycle arrest (G0/G1) compared to simvastatin treatment alone (Fig. 1).
Fig. 1.
Cell cycle analysis. Table represents comparative % cell arrest in each phase of the cell cycle on treatment with sesamol and simvastatin
Mitochondrial membrane potential
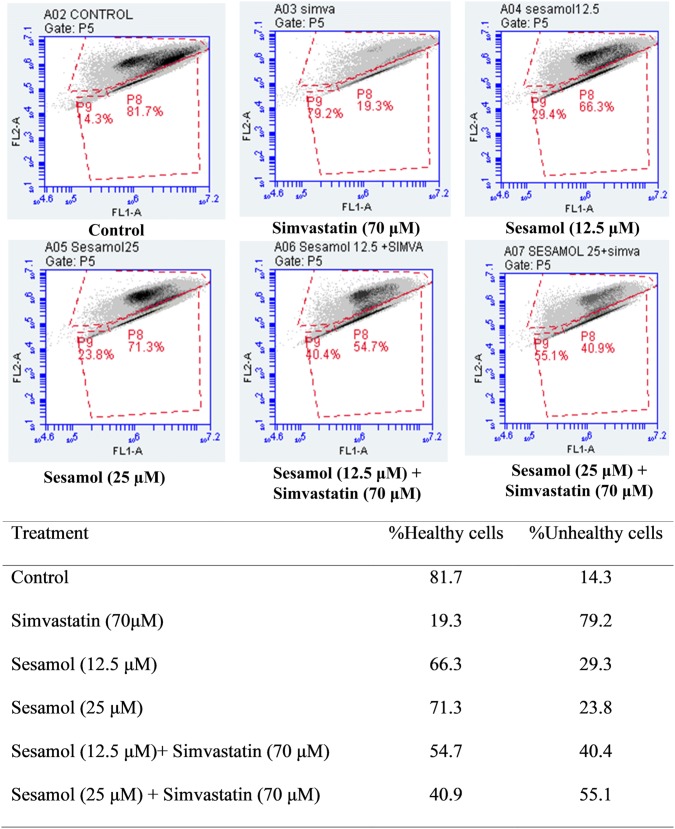
In JC 1 staining, simvastatin caused more mitochondrial membrane potential loss. Pretreatment of cells with sesamol, 24 h before the addition of simvastatin, reduced the mitochondrial membrane potential loss compared to simvastatin-alone treatment. The effects were observed to be dose-dependent (Fig. 2).
Fig. 2.
JC 1 staining showing mitochondrial membrane potential under different treatments. Table represents percentage of healthy cells vs unhealthy cells (in which mitochondrial membrane potential loss) on treatment with sesamol and simvastatin
Discussion
Statins are linked in several clinical studies with the development of new diabetic cases in the dyslipidemic population. The underlying mechanism is linked to their lipophilicity, which induces toxicity on pancreatic beta cells and impairs glucose metabolism (Maki et al. 2017; Goldstein and Mascitelli 2013). Simvastatin is one such diabetogenic lipophilic statin which is associated with higher onset of diabetic cases. In the present study, another antidyslipidemic and antidiabetic agent, sesamol was used to counter the toxic effect of simvastatin on pancreatic beta cells (MIN6).
In vitro studies were performed on MIN6 cells, insulin-secreting pancreatic cells (Hao et al. 2015) using cell viability study, cell cycle analysis and analysis of mitochondrial membrane potential using JC1 stain.
Statins have the pleiotropic actions that might be responsible for unfavorable metabolic effects such as the reduction in insulin secretion and insulin resistance. In the present study, it was found that simvastatin at 70 μM induced 50% of cell death. Sesamol pretreatment produced a dose-dependent rise in cell viability indicating its ability to reverse the damage caused by simvastatin. Based on these results, we selected 12.5 μM and 25 μM concentrations of sesamol for further study.
The literature suggests that simvastatin is responsible for cell cycle arrest in the G0/G1 phase (Zhao and Zhao 2015). Our present study also found that simvastatin is responsible for cell cycle arrest in G0/G1 at 70 μM. However, when cells were pretreated with sesamol for 24 h before the challenge with simvastatin, it showed protection against simvastatin-induced cell cycle arrest. Cells were able to travel from one phase to another phase of the cell cycle.
Lipophilic statins may produce mitochondrial damage in pancreatic beta cells due to their permeability through cell membranes thereby binding with either an intramembrane binding site on the electron transport system complexes (Degli Esposti 1998) or by disrupting membrane protein–lipid dynamics (Hwang et al. 2003). In mitochondria, statins inhibit CoQ10 and disturb the mitochondrial electron transport chain (Urbano et al. 2017). This results in depletion of ATP, which affects insulin secretion. Inhibition of CoQ10 results in cytochrome C release, which is responsible for the leakage of cellular contents into the cytosol. This may result in apoptosis of cells. Additionally, simvastatin has also been demonstrated to induce an impairment of mitochondria in pancreatic beta cells in mice and in MIN6 cells by affecting L-type Ca2+ channels in beta cells (Almukhtar et al. 2019). In our study, we found out that simvastatin reduced mitochondrial membrane potential as compared to control cells. Thus, it can be stated that like earlier reports, simvastatin causes disruption of mitochondrial membrane. The pretreatment with sesamol reversed the loss of mitochondrial membrane potential that could have been produced by simvastatin. Thus, it can be inferred that sesamol protects MIN6 from simvastatin-induced loss of mitochondrial membrane potential.
Conclusion
From, the results of in vitro studies, it is concluded that sesamol has a protective effect against simvastatin-induced toxicity. However, further advanced studies are required to understand the molecular mechanism involved in the amelioration of simvastatin-induced toxicity in pancreatic beta cell lines.
Funding
This work was funded by the Science and Engineering Research Board (Grant number: Project File No. SERB/EMR/2017/003834-HS with diary no. SERB/F/7442/2018-2019 dated 24 September, 2018).
Footnotes
Girish A. Ghadge and Karthik Gourishetti contributed equally.
References
- Aiman U, Najmi A, Khan RA. Statin induced diabetes and its clinical implications. J Pharmacol Pharmacother. 2014;5(3):181. doi: 10.4103/0976-500X.136097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almukhtar H, Alahmed J, Curry L, Roberts R, Smith PA. Simvastatin inhibits L-type Ca2+-channel activity through impairment of mitochondrial function. Toxicol Sci. 2019 doi: 10.1093/toxsci/kfz068. [DOI] [PubMed] [Google Scholar]
- Cheruku S, Chamallamudi M, Ramalingayya G, Biswas S, Gourishetti K, Nandakumar K, Devkar R, Mallik S, Nampoothiri M, Kumar N. Neuroprotective potential of methanolic extract of saraca asoca bark against doxorubicin-induced neurotoxicity. Pharmacogn Mag. 2019;15(61):309–316. doi: 10.4103/pm.pm_79_18. [DOI] [Google Scholar]
- Cheruku SP, Ramalingayya GV, Chamallamudi MR, Biswas S, Nandakumar K, Nampoothiri M, Gourishetti K, Kumar N. Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats. Cytotechnology. 2018;70(1):245–259. doi: 10.1007/s10616-017-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chogtu B, Magazine R, Bairy K. Statin use and risk of diabetes mellitus. World J Diabetes. 2015;6(2):352. doi: 10.4239/wjd.v6.i2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PY, Srinivasan P, Deng JF, Liu MY. Sesamol attenuates oxidative stress-mediated experimental acute pancreatitis in rats. Hum Exp Toxicol. 2012;31(4):397–404. doi: 10.1177/0960327111426583. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta. 1998;1364(2):222–235. doi: 10.1016/S0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot. 1976;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Food US Drug Administration . FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. Rockville, MD: US Food and Drug Administration; 2012. [Google Scholar]
- Goldstein MR, Mascitelli L. Do statins cause diabetes? Curr Diabetes Rep. 2013;13(3):381–390. doi: 10.1007/s11892-013-0368-x. [DOI] [PubMed] [Google Scholar]
- Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F, Kang J, Cao Y, Fan S, Yang H, An Y, Pan Y, Tie L, Li X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic beta-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis. 2015;20(11):1420–1432. doi: 10.1007/s10495-015-1150-0. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42(46):13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- Kumar N, Mudgal J, Parihar VK, Nayak PG, Kutty NG, Rao CM. Sesamol treatment reduces plasma cholesterol and triacylglycerol levels in mouse models of acute and chronic hyperlipidemia. Lipids. 2013;48(6):633–638. doi: 10.1007/s11745-013-3778-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Noh Y, Shin S, Lim H-S, Park RW, Bae SK, Oh E, Kim GJ, Kim JH, Lee S. Impact of statins on risk of new onset diabetes mellitus: a population-based cohort study using the Korean National Health Insurance claims database. Ther Clin Risk Manag. 2016;12:1533–1543. doi: 10.2147/TCRM.S117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Qiao Q, Sun Y, Chen Y, Ren B, Liu X. Sesamol ameliorates diet-induced obesity in C57BL/6J mice and suppresses adipogenesis in 3T3-L1 cells via regulating mitochondria-lipid metabolism. Mol Nutr Food Res. 2017;61(8):1600717. doi: 10.1002/mnfr.201600717. [DOI] [PubMed] [Google Scholar]
- Maki KC, Diwadkar-Navsariwala V, Kramer MW. Statin use and risk for type 2 diabetes: what clinicians should know. Postgrad Med. 2017 doi: 10.1080/00325481.2018.1402658. [DOI] [PubMed] [Google Scholar]
- Pande AN, Biswas S, Reddy ND, Jayashree BS, Kumar N, Rao CM. In vitro and in vivo anticancer studies of 2'-hydroxy chalcone derivatives exhibit apoptosis in colon cancer cells by HDAC inhibition and cell cycle arrest. EXCLI J. 2017;16:448–463. doi: 10.17179/excli2016-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmataj SA, Kamath SU, Murty VR, Pai SR. Amelioration of arsenic-induced oxidative stress in CHO cells by Ixora coccinea flower extract. 3 Biotech. 2018;8(10):446. doi: 10.1007/s13205-018-1446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Lobo R, Krishnadas N, Surubhotla R. Sesamol and health—a comprehensive review. Indian J Pharm Edu Res. 2019;53(2):S28–S42. doi: 10.5530/ijper.53.2s.46. [DOI] [Google Scholar]
- Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Gopalan Kutty N, Kumar N, Chaudhary A, Vasanth Raj P, Shenoy R, Mallikarjuna Rao C. Synthesis and evaluation of selected 1,3,4-oxadiazole derivatives for in vitro cytotoxicity and in vivo anti-tumor activity. Cytotechnology. 2016;68(6):2553–2565. doi: 10.1007/s10616-016-9979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M. The inhibition profile of sesamol against α-glycosidase and acetylcholinesterase enzymes. Int J Food Prop. 2019;22(1):1527–1535. doi: 10.1080/10942912.2019.1656234. [DOI] [Google Scholar]
- Urbano F, Bugliani M, Filippello A, Scamporrino A, Di Mauro S, Di Pino A, Scicali R, Noto D, Rabuazzo AM, Averna M, Marchetti P, Purrello F, Piro S. Atorvastatin but not pravastatin impairs mitochondrial function in human pancreatic islets and rat β cells direct effect of oxidative stress. Sci Rep. 2017;7(1):11863–11863. doi: 10.1038/s41598-017-11070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashaswini PS, Rao AG, Singh SA. Inhibition of lipoxygenase by sesamol corroborates its potential anti-inflammatory activity. Int J Biol Macromol. 2017;94(Pt B):781–787. doi: 10.1016/j.ijbiomac.2016.06.048. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhao SP. Different effects of statins on induction of diabetes mellitus: an experimental study drug design. Dev Ther. 2015;9:6211–6223. doi: 10.2147/dddt.s87979. [DOI] [PMC free article] [PubMed] [Google Scholar]