Abstract
2-acetyl-1-pyrroline (2AP) is a principal aroma compound in scented rice and a mutation in betaine aldehyde dehydrogenase 2 (OsBADH2) is responsible aroma in scented rice. The present study was aimed at inducing 2AP production in non-scented indica rice cultivar IR-64 by silencing OsBADH2 via RNAi technique. A vector pBSK was used for the construction of RNAi cassette and pRI101ON as a binary vector. Agrobacterium (GV3101)-mediated transformation was done using embryogenic calli of IR-64. The resultant transgenic lines showed up to 14-fold reduction in expression of OsBADH2 gene and 50% inhibition in enzyme activity. Gas chromatography (GC–MS) analyses showed a significant amount of 2AP production in RNAi callus, leaves, and seeds of IR-64. A total 39 volatile compounds were identified from the control and RNAi seeds of IR-64. Among them, octanal and 2-pentylfuron were found to be increased (30–40%) in RNAi seeds of IR-64. The content of precursors, proline, and methylglyoxal increased substantially, whereas GABA content reduced up to 25% in transgenic IR-64 lines. The study demonstrated that RNAi approach could be successfully used for imparting pleasant aroma character in non-scented indica rice cultivars.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2131-8) contains supplementary material, which is available to authorized users.
Keywords: OsBADH2, 2AP, RNAi, IR-64, Aroma
Introduction
Aromatic rices are popularly consumed all over the world. As aroma is one of the most important and valued grain quality characters (Sakthivel et al. 2009). India is ethnic museum of basmati, non-basmati aromatic, and non-aromatic rices (Shobha Rani and Singh 2003).
IR-64 is well known non-aromatic rice variety with high yield and quality. It was released by International Rice Research Institute (IRRI) in Philippines in 1985 and became popular in several countries due to its wide adaptability, earliness, good quality, and disease resistance. It was cultivated on more than 10-million hectares within 2 decades of its release. Thus, it is referred as mega variety of rice. Further, it has also been used as a model variety for various scientific studies such as breeding and genetic transformation (Mackill and Khush 2018).
2-acetyl-1-pyrroline (2AP) has been identified as a principle compound contributing to the rice aroma (Buttery 1982). Non functionality of BADH2 gene is responsible for the aroma in the rice and it was reported for the first time by Bradbury et al. (2005). Then, several alleles of BAHD2 gene were reported in diverse aromatic rice varieties and other plants (Wakte et al. 2017).
Direct evidence of involvement of BADH2 in 2AP biosynthesis in aromatic rice was provided in earlier studies by downregulation of BADH2 gene in japonica rice and Chinese non-aromatic rice to induce aroma (Niu et al. 2008; Shan et al. 2015). However, in these reports, they didn’t study the effect of down regulation of BADH2 gene on the expression pattern of other genes and metabolites in the 2AP biosynthetic pathway as well as volatiles in seeds. Recently, we found that there is a variation in mutation pattern in promoter region of indica- and japonica-type rice (Khandagale et al. 2017). On the similar note, we attempted RNAi-mediated downregulation of BADH2 gene in IR-64, an indica-type rice to express 2AP and to study metabolite in 2AP pathway.
Materials and methods
Collection of plant material
Seeds of Oryza sativa cv. IR-64 were procured from Division of Genetics, Indian Agricultural Research Institute (IARI), New Delhi and used in the present study.
Construction of RNAi vector
RNA isolation and cDNA synthesis
The total RNA was extracted from the leaves of IR-64 using Trizol method, and quantity and quality of RNA were checked using Nanodrop (Nanodrop Technologies, USA) and 1% agarose gel electrophoresis. Before proceeding for cDNA synthesis, RNA was treated with DNAase (Promega, USA) to remove traces of DNA. The first strand cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad, USA) as per the manufacturer’s protocol.
Vector construction
The primers for sense, antisense, and intronic fragments with respective restriction sites were designed from the BADH2 gene sequence (Table 1). The sense primers were flanked by EcoRI and KpnI sites, antisense was by BamHI and XbaI and intronic by EcoR1 and BamH1. The sense and antisense fragments were 480 bp, whereas intronic fragment was 200 bp in size. The RNAi cassette was constructed in the pBSK vector by sequential cloning of each sense, antisense and spacer fragment with the help of unique restriction sites. This RNAi cassette was then cloned in a pRI101ON binary vector under strong CaMV35S promoter. The cloned RNAi cassette was confirmed by restriction digestion as well as sequencing and transferred to Agrobacterium using tri-parental mating.
Table 1.
List of primers used for construction of RNAi vector and gene expression analyses
| Primer | Sequence | Length |
|---|---|---|
| Badh2SF | 5′AGAATTCGACCAATGGCCAGATTTGCAGT 3′ | 29 |
| Badh2SR | 5′TAGGTACCGCGAGCAGTTCACCCAGATAA 3′ | 29 |
| Badh2AF | 5′CGGATCCGACCAATGGCCAGATTTGCAGT3′ | 29 |
| Badh2AR | 5′ CTCTAGAGCGAGCAGTTCACCCAGATA 3′ | 27 |
| IntF | 5′ AGAATTCCGGAAGTGCGTATCCTCTG 3′ | 26 |
| IntR | 5′CGGATCCTGAGCCATATACACTTGTTG 3′ | 27 |
| badh2 RT_F | TGTGCTAAACATAGTGACTGG | 21 |
| badh2 RT_R | CTTAACCATAGGAGCAGC | 18 |
| P5CS RT_F | GAAGTGGTA ATG GTCTTCT | 19 |
| P5CS RT_R | AGCAAATCTGCGATCTCAT | 19 |
| EF1α RT_F | TTTCACTCTTGGTGTGAAGCAGA | 23 |
| EF1α RT_R | GACTTCCTTCACGATTTCATCGTA | 24 |
Transformation and regeneration
Healthy and mature seeds of IR-64 were selected by physical appearance, dehusked manually, and surface sterilised. Embryonic calli of IR-64 were induced on MS medium (2.5 mg/l 2, 4-D and 500 mg/l proline and 100 mg/l casein hydrolysate) from the mature seeds, and then the transformation and regeneration were done as per Kaikavoosi et al. (2015) with slight modifications. Agrobacterium tumefaciens (GV3101) containing pRI101-RNAi vector was grown on LB medium containing rifampicin and kanamycin (100 mg/l and 50 mg/l, respectively) overnight. The well-grown and friable calli of IR-64 were immersed in bacterial suspension containing 50 μM acetosyringone. The calli were blotted using sterile filter paper and placed on MS medium for cultivation in dark for 3 days. Then, calli were washed with cefotaxime (500 mg/l) and kept on regeneration medium (BAP 2.5 mg/l and NAA 0.1 mg/l) supplemented with 500 mg/l cefotaxime and 100 mg/L kanamycin. After every 10–15 days, calli were sub-cultured on regeneration medium containing reduced amount of cefotaxime. Subsequently, small shoots were transferred on root induction medium (half strength MS with NAA 0.1 mg/l) containing kanamycin. Then, well-grown plantlets were transferred in the greenhouse for hardening.
PCR confirmation of transgenics
Genomic DNA was isolated from the leaves of control and RNAi lines using DNA isolation kit (Promega, USA). Selectable marker (NPTII)-specific primers were used for confirmation of transgene integration in the RNAi lines of IR-64. PCR mixture contained 100 ng DNA, dNTP, 10 × PCR buffer, 1 unit Taq DNA polymerase (NEB) and 10 pmol of each primer and nuclease free water to make the volume 20 µl. Amplification was performed in a thermal cycler (Eppedorf) with initial denaturation for 4 min followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C and extension at 72 °C for 1 min, final extension was done for 10 min at 72 °C. PCR products were resolved on 1.2% agarose gel, Gelred (Thermo scientific, USA) was used as a visualising agent and gel was documented under gel documentation system (Bio-Rad, USA). Further, segregation of transgene in T1 progeny was assessed using above PCR protocol.
Analysis of transgenic IR-64
Quantitative real-time expression of BADH2 gene
The total RNA was extracted from the calli and leaves using Trizol method, and quantity and quality of RNA were checked using Nanodrop (Nanodrop Technologies, USA) and 1% agarose gel electrophoresis, respectively. Before proceeding for cDNA synthesis, RNA was treated with DNAase (Promega, USA) to remove traces of DNA. The first strand cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad, USA) using 1 µg total RNA. qRT PCR primers for BADH2 and housekeeping gene (EF1α) were designed using the DNA sequences from NCBI database (Table 1). qRT PCR was performed in real-time PCR machine (Real Plex, Eppendorf, Germany). qRT PCR reaction mixture contained 1× fast SYBR® Green qPCR Master Mix (Biorad, USA), 1 μl of primer (10 pmol/μl) and 1.0 μl cDNA in a total volume of 20 μl. Thermal cycler conditions were as follows: initial denaturation at 95 °C for 5 min, 45 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s. EF1α was used as a control and relative expressions of BADH2 was measured following 2-ΔΔCT method (Livak and Schmittgen 2001).
BADH2 enzyme assay
The BADH2 enzyme assay was performed from the calli and leaves in accordance with the method of Weretilnyk and Hanson (1989) and Wakte et al. (2011). The proteins were estimated by Bradford’s method (Bradford 1976) and the enzyme extract (~ 100 μg/reaction) was used for computing enzyme activity. The substrate, GABald, was prepared according to the method of Trossat et al. (1997). The data were recorded in triplicate and activity was calculated following standard formula.
Extraction and quantification of 2AP using HS-SPME-GCMS
2AP was extracted and quantified from the calli, leaves, and seeds of transgenic and non-transgenic IR-64 following optimized conditions of headspace–solid-phase microextraction (HS-SPME) coupled with gas Chromatography–mass spectrometry (GCMS) (Hinge et al. 2016; Kaikavoosi et al. 2015). Three replicates were used for analysis of 2AP content. The quantification of 2AP was done using TMP as internal standard following the method of Hinge et al. (2016).
Estimation of GABA, proline, and MG
GABA was quantified from the callus and leaves of transgenic IR-64 following the method of Karladee and Suriyong (2012). Proline estimation was carried out using the method of Bates et al. (1973). Isolation and quantification of MG were done following the method of Yadav et al. (2005) and Wild et al. (2012), respectively. The quantification of all the biochemicals was performed in triplicate.
Gene expression and enzyme activity analysis of P5CS
The P5CS enzyme activity in the transgenic callus and seedlings was performed by following the protocol of Kavikishore et al. (1995) with some modifications. P5CS gene expression analyses were performed as mentioned earlier Sect. (2.5.2).
Agronomic performance of transgenic IR-64
Agronomic performance was assessed in T1 generation. The plant height was measured from the soil surface to the neck of the highest tiller above ground. Leaf length and leaf width of tallest tiller were measured using centimetre scale. Similarly, panicle length was measured from base of panicle to tip of the last spikelet. Fertility was calculated by measuring the total number of spikelets and number of filled spikelets of the panicle. Further germination percentage of RNAi seed was also estimated. The length and breadth of rice grain were measured by using Vernier caliper. The measurements were recorded for minimum ten grains of each plants. The 100 seed weight was measured using digital balance by randomly selecting 100 seeds in 5 replicates. Similarly, presence of aroma in T1 generation was qualitatively checked following KOH method (Sood and Siddiq 1978).
Results and discussion
Agrobacterium-mediated transformation and regeneration
The RNAi cassette integration into the genome of IR-64 was confirmed by desired amplification of 780 bp amplicon in transformed lines, whereas no amplification was observed in control plants (Fig. 1). A total of 11 RNAi events were generated after transformation with transformation efficiency of 0.9% and all the events were confirmed by PCR. Further, total 110 T1 progeny plants were screened for NPTII and we found that transgene behavior in typical Mendelian pattern.
Fig. 1.
a RNAi construct cloned in pRI101ON vector under the CaMV35S promoter and NPTII as a selectable marker. b Co-cultivation of calli with A. tumefaciens for transformation. c Regeneration of transgenic IR-64. d Amplification of NPTII gene in transgenic seedlings (1–5); P, positive control; C, no amplification in wildtype. e Mature control and transformed seedlings of IR-64 grown in greenhouse. f Panicle and g seeds of control and transformed seedlings of IR-64
Analysis of transformed IR-64
Analysis BADH2 gene expression and enzyme activity in transgenic callus and seedlings
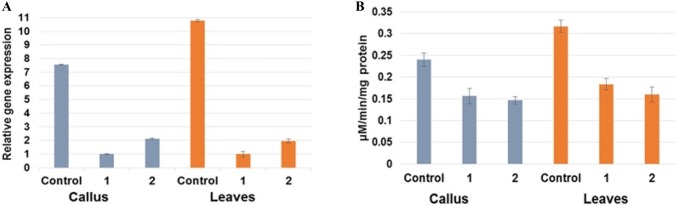
BADH2 gene expression was found to be reduced by up to eight-fold in the RNAi callus and up to 14-fold in the leaves of transgenic IR-64 seedlings (Fig. 2a). The reduction in BADH2 expression level also resulted in the reduction of BADH2 enzyme activity in transgenic IR-64. BADH2 enzyme activity was found to be reduced by 40% in RNAi callus (0.14 ± 0.01 µmol/min/mg protein) compared to control (0.24 ± 0.015 µmol/min/mg protein),whereas there was more than 50% reduction in BADH2 activity in transgenic leaves (0.16 ± 0.012 µmol/min/mg protein) compared to control (0.31 ± 0.014 µmol/min/mg protein) (Fig. 2a). Significant reductions in the transcript levels as well as enzyme activity were the consequences of successful downregulation of BADH2 in IR-64.
Fig. 2.
a Relative BADH2 gene expression in the transformed callus and leaves of IR-64. b Estimation of BADH2 enzyme activity from the transformed callus and leaves of IR-64
The presence of recessive BADH2 gene is a prerequisite for rice to be fragrant (Bradbury et al. 2005). In fragrant rice, transcript levels of BADH2 are always reported to be reduced by many fold compared to non-fragrant rice plants throughout the life cycle (Hinge et al. 2016; Khandagale et al. 2017; Prodhan et al. 2017). Similar pattern of expression was recorded here in transgenic IR-64. According to earlier reports, downregulated BADH2 gene in non-fragrant rice cultivars using antisense RNA and artificial microRNA resulted up to 80% reduction in the expression (Niu et al. 2008; Chen et al. 2012). Similar BADH2 expression scenario was also reported in fragrant variants of sorghum (Zanan et al. 2016) and mung bean (Attar et al. 2017). Subsequently, the reduced expression of BADH2 in IR-64 due to RNAi resulted in the reduction of BADH2 enzyme activity. Fragrant rice also exhibited significant reduction in BADH2 activity (Bradbury et al. 2008; Khandagale et al. 2017). Rice BADH2 enzyme showed greatest activity towards the γ-aminobutyraldehyde (GABald) and showed that the inactivation of this enzyme in fragrant rice caused the provenance of 2AP (Bradbury et al. 2008). BADH2 gene in non-fragrant rice has been downregulated using antisense RNA, microRNA, and TALEN but enzyme activity in transgenic plant was not estimated (Niu et al. 2007; Chen et al. 2012; Shan et al. 2015).
Estimation of 2AP in transgenic IR-64
The GC–MS analyses detected the expression of 2AP in RNAi callus, leaves, and seeds, whereas in control 2AP was not detected (Fig. 3a, Supplementary 1). A considerable amount of 2AP was synthesised in the callus, leaves, and seeds of RNAi lines. The seeds Basmati 370 were used for comparison as a positive control and 2AP content in RNAi seeds was found to be 60% of Basmati 370 (Fig. 3b). The amount of 2AP produced in transgenic lines of IR-64 was relatively lower than in Basmati 370, but comparable to other basmati fragrant rice accessions such as Pakistan Basmati, Basmati 6311, Basmati (Dind), Super Basmati, and Pusa basmati 1(0.125 ± 0.032, 0.149 ± 0.026, 0.108 ± 0.022, 0.121 ± 0.009 and 0.161 ± 0.014 mg/kg), respectively (Khandagale et al. 2017) and it is more than some non-basmati fragrant type rice such as Kolam (0.079 ± 0.006), Dubraj (0.124 ± 0.012), and Kalimuch (0.121 ± 0.01) (Mathure et al. 2011). BADH2 downregulation in IR-64 resulted in reduction in transcript level as well enzyme activity and accumulated considerable amount of 2AP. These findings concluded that multiple mutations in BADH2 gene are the sole cause of 2AP production in fragrant rices. Expression of functional BADH2 in fragrant rice made them non-fragrant (Chen et al. 2008). This further established the major role of BADH2 in rice fragrance.
Fig. 3.
a Comparative GC–MS chromatogram showing peak of 2AP in grains of transgenic IR-64 and Basmati 370. b Quantification of 2AP in the transgenic callus, leaves, and seeds of IR-64. c Graphical representation of volatiles detected from transgenic and control seeds of IR-64
The comparative volatile analysis in the seeds of control and transgenic lines detected total 39 volatiles (Fig. 3c, Supplementary 2). These volatiles were grouped under alkane (7), ketone (5), alkene (3), alcohol (9), aliphatic aldehyde (9), aromatic aldehyde (2), N-containing aromatic (1), N-containing aliphatic (1), ester (1), and furans (1) categories. All volatiles except 2AP, were present in both control as well as transgenic seeds, but octanal, an aldehyde compound and 2-pentylfuron, a phenol containing compound, was found to be increased by 30% and 40%, respectively in transgenic seeds. The remaining volatiles were not varied in RNAi and control seeds which indicated that these were the major contributors in the fragrance of transgenic IR-64.
Estimation of precursors and metabolites associated with 2AP biosynthesis
The proline content was significantly increased in the transgenic plants (56.2 ± 0.60 µg/g) compared to control (45.3 ± 0.45 µg/g) at vegetative stage (Fig. 4b). Similarly, methylglyoxal was also increased up to 30% in the RNAi lines of IR-64 (Fig. 4c). Several studies found that proline and methylglyoxal act as the precursors of 2AP in fragrant rice (Yoshihashi et al. 2002; Thimmaraju et al. 2005) and Soybean (Wu et al. 2009). The radio labeling experiments revealed that nitrogen source for 2AP is proline (Yoshihashi et al. 2002; Huang et al. 2008). Similarly, methylglyoxal is the carbon source for 2AP synthesis (Huang et al. 2007, 2008). Non-enzymatic reaction between methylglyoxal and GABAld (Δ1-pyrroline) led to the formation of 2AP (Bradbury et al. 2008; Wakte et al. 2011). Higher amount of proline and methylglyoxal contents has been correlated with presence of 2AP in fragrant rice (Huang et al. 2008; Kaikavoosi et al. 2015; Khandagale et al. 2017) and other plants like sorghum (Zanan et al. 2016), mung bean (Attar et al. 2017), and Pandanus amaryllifolius (Thimmaraju et al. 2005).
Fig. 4.
a Proline content in the transformed callus and leaves of IR-64. b Methylglyoxal content in the transformed callus and leaves of IR-64. c GABA content in the transformed callus and leaves of IR-64. d P5CS enzyme activity in the callus and leaves of transgenic IR-64. e Relative P5CS gene expression in the transformed callus and leaves of IR-64
The quantity of GABA decreased in transformed calli (2.1 µM/g) as well as in the leaves (2.9 µM/g) of RNAi lines compared to control calli (2.7 µM/g) and leaves (3.9 µM/g) (Fig. 4a).The lower amount of GABA content in RNAi lines suggests the successful downregulation of the BADH2 gene as its function is to convert GABald to GABA. Studies in fragrant rice have also reported 30–40% lower level of GABA than non-fragrant rice varieties (Karladee and Suriyong 2012; Khandagale et al. 2017).
RNAi-mediated BADH2 downregulation in IR-64 increased P5CS transcript levels and enzyme activity up to 17% and 35%, respectively over control (Fig. 4d, e). The P5CS is one of the key enzymes in 2AP biosynthetic pathway. It was found that more P5CS enzyme activity and transcript levels in fragrant rice genotypes, higher the proline contents and ultimately 2AP levels (Huang et al., 2008; Hinge et al., 2016) and in fragrant soybean (Wu et al. 2009). Chen et al. (2012) downregulated BADH2 gene in nipponbare variety of japonica rice and found that there was also higher accumulation of proline in transgenic lines. This indicated the coexistence of proline with 2AP biosynthesis. The overexpression of P5CS led to increase the proline content in fragrant rice Indrayani and Ambemohar which doubled 2AP contents in these varieties (Kaikavoosi et al. 2015).
Agronomic performance of IR-64 transgenic lines
The overall performance of transgenic IR-64 lines in T1 generation was found to be reduced as compared to control lines. The pollen viability, panicle length, filled spikelets per panicle, and spikelet fertility were found to be significantly reduced in RNAi lines (Table 2). Other characters were at par with the control. The silencing of BADH2 gene in japonica rice variety also resulted in slowed down of growth under salinity stress compared to wild type (Niu et al. 2008). BADH2 is involved in GABA gradient panicle formation which may decreased the yield in transgenic plants (Bradbury et al. 2008). In the present study, downregulation of BADH2 led in the reduction of plant growth and yield than control plants. This is in agreement with the previous reports. Out of 110 plants screed for aroma, 78 plants were having typical basmati-like aroma.
Table 2.
Phenotypic analysis in transgenic and control IR-64 lines
| Sr. No | Phenotype | Control IR-64 line | Transgenic IR-64 line |
|---|---|---|---|
| 1 | Pollen viability (%) | 96.3 ± 1.3* | 86.2 ± 1.8* |
| 2 | Plant height (cm) | 73 ± 2.3 | 69.1 ± 1 |
| 3 | Panicle length (cm) | 19.1 ± 0.52* | 17.1 ± 0.51* |
| 4 | Leaf length (cm) | 35.9 ± 1.13 | 34.53 ± 1.15 |
| 5 | Leaf width (cm) | 0.95 ± 0.07 | 0.86 ± 0.02 |
| 6 | Total spikelets per panicle | 100.6 ± 5.2 | 89.1 ± 6.1 |
| 7 | Filled spikelets per panicle | 81.6 ± 3.1* | 64.6 ± 3.2* |
| 8 | Spikelet fertility (%) | 81.2 ± 1.1* | 72.7 ± 1.2* |
| 9 | 100 seed weight (gm) | 2.28 ± 0.03 | 2.2 ± 0.01 |
| 10 | Grain length (mm) | 8.23 ± 0.12 | 8.13 ± 0.12 |
| 11 | Grain breadth (mm) | 2.3 ± 0.15 | 2.2 ± 0.1 |
| 12 | Germination (%) | 95.6 ± 3.3 | 96.6 ± 2.8 |
*Indicate significant values at p = 0.05, ± standard error
Conclusions
The study confirmed the role of badh2 in imparting pleasant odour in terms of 2AP in indica rice. Aromatic rice varieties in general, are low yielding. The similar observation here in transgenic IR-64 aromatic line supports the role of GABA in yield. The induction of 2AP elevates the other aroma volatiles like octanal and 2-pentylfuran suggesting coexistence of their pathways with 2AP biosynthesis. Similarly, the down regulation of BADH2 also elevated the 2AP precursors, confirming their role in 2AP synthesis. The study demonstrated that RNAi approach could be successfully used in expressing pleasant aroma character (2AP) in non-fragrant indica rice IR-64, thus could be successfully applied to impart aroma as a quality trait in elite non-fragrant indica rice varieties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
First author acknowledges Council of Scientific and Industrial Research (CSIR), India (Sanction No. 09/137/(0541)/2012-EMR-1) for the award of SRF.
Author contributions
KK performed experiment and wrote a manuscript. ABN is a senior author, who conceived the idea and proofread the manuscript. RC provided facility for the GCMS analyses.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Contributor Information
Kiran S. Khandagale, Email: kkhandagale253@gmail.com
Altafhusain B. Nadaf, Email: abnadaf@unipune.ac.in
References
- Attar U, Hinge V, Zanan R, Adhav R, Nadaf A. Identification of aroma volatiles and understanding 2-acetyl-1-pyrroline biosynthetic mechanism in aromatic mung bean (Vigna radiata (L.) Wilczek) Physiol Mol Biol Plants. 2017;23(2):443–451. doi: 10.1007/s12298-017-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradbury LM, Fitzgerald TL, Henry RJ, Jin Q, Waters DL. The gene for fragrance in rice. Plant Biotechnol J. 2005;3(3):363–370. doi: 10.1111/j.1467-7652.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- Bradbury LM, Gillies SA, Brushett DJ, Waters DL, Henry RJ. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Biol. 2008;68(4–5):439–449. doi: 10.1007/s11103-008-9381-x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buttery RG. 2-Acetyl-1-pyrroline: an important aroma component of cooked rice. Chem Ind. 1982;23:958–959. [Google Scholar]
- Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, et al. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20(7):1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wei X, Shao G, Tang S, Luo J, Hu P. Fragrance of the rice grain achieved via artificial microRNA-induced down-regulation of OsBADH2. Plant Breed. 2012;131(5):584–590. doi: 10.1111/j.1439-0523.2012.01989.x. [DOI] [Google Scholar]
- Hinge VR, Patil HB, Nadaf AB. Aroma volatile analyses and 2AP characterization at various developmental stages in basmati and non-basmati scented rice (Oryza sativa L.) cultivars. Rice. 2016;9(1):38. doi: 10.1186/s12284-016-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TC, Huang YW, Hung HJ, Ho CT, Wu ML. Δ1-Pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J Agric Food Chem. 2007;55(13):5097–5102. doi: 10.1021/jf0700576. [DOI] [PubMed] [Google Scholar]
- Huang TC, Teng CS, Chang JL, Chuang HS, Ho CT, Wu ML. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J Agric Food Chem. 2008;56(16):7399–7404. doi: 10.1021/jf8011739. [DOI] [PubMed] [Google Scholar]
- Kaikavoosi K, Kad TD, Zanan RL, Nadaf AB. 2-Acetyl-1-pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through Δ 1-pyrroline-5-carboxylate synthetase (P5CS) gene transformation. Appl Biochem Biotechnol. 2015;177(7):1466–1479. doi: 10.1007/s12010-015-1827-4. [DOI] [PubMed] [Google Scholar]
- Karladee D, Suriyong S. γ-Aminobutyric acid (GABA) content in different varieties of brown rice during germination. Sci Asia. 2012;38(13):13–17. doi: 10.2306/scienceasia1513-1874.2012.38.013. [DOI] [Google Scholar]
- Kavi Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS. Overexpression of delta 1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108(4):1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandagale KS, Zanan RL, Mathure SV, Nadaf AB. Haplotype variation of Badh2 gene, unearthing of a new fragrance allele and marker development for non-basmati fragrant rice ‘Velchi’ (Oryza sativa L.) Agric Gene. 2017;6:40–46. doi: 10.1016/j.aggene.2017.09.003. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mackill DJ, Khush GS. IR64: a high-quality and high-yielding mega variety. Rice. 2018;11(1):18. doi: 10.1186/s12284-018-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathure SV, Wakte KV, Jawali N, Nadaf AB. Quantification of 2-acetyl-1-pyrroline and other rice aroma volatiles among Indian scented rice cultivars by HS-SPME/GC-FID. Food Anal Methods. 2011;4(3):326–333. doi: 10.1007/s12161-010-9171-3. [DOI] [Google Scholar]
- Niu X, Tang W, Huang W, Ren G, Wang Q, Luo D, et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.) BMC Plant Biol. 2008;8(1):100. doi: 10.1186/1471-2229-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodhan ZH, Faruq G, Taha RM, Rashid KA. Agronomic, transcriptomic and metabolomic expression analysis of aroma gene (badh2) under different temperature regimes in rice. Int J Agric Biol. 2017;19:569–576. doi: 10.17957/IJAB/15.0340. [DOI] [Google Scholar]
- Sakthivel K, Sundaram RM, Rani NS, Balachandran SM, Neeraja CN. Genetic and molecular basis of fragrance in rice. Biotech Adv. 2009;27(4):468–473. doi: 10.1016/j.biotechadv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Shan Q, Zhang Y, Chen K, Zhang K, Gao C. Creation of fragrant rice by targeted knockout of the Os BADH 2 gene using TALEN technology. Plant Biotechnol J. 2015;13(6):791–800. doi: 10.1111/pbi.12312. [DOI] [PubMed] [Google Scholar]
- Shobha Rani N, Singh RK. Efforts on aromatic rice improvement in India. A treatise on the scented rices of India. New Delhi: Kalyani Publishers; 2003. pp. 23–72. [Google Scholar]
- Sood BG, Siddiq EA. A rapid technique for scent determination in rice. Indian J Genet Plant Breed. 1978;38:268–271. [Google Scholar]
- Thimmaraju R, Bhagyalakshmi N, Narayan MS, Venkatachalam L, Ravishankar GA. In vitro culture of Pandanus amaryllifolius and enhancement of 2-acetyl-1-pyrroline, the major flavouring compound of aromatic rice, by precursor feeding of L-proline. J Sci Food Agric. 2005;85(15):2527–2534. doi: 10.1002/jsfa.2286. [DOI] [Google Scholar]
- Trossat C, Rathinasabapathi B, Hanson AD. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and [omega]-aminoaldehydes. Plant Physiol. 1997;113(4):1457–1461. doi: 10.1104/pp.113.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakte KV, Kad TD, Zanan RL, Nadaf AB. Mechanism of 2-acetyl-1-pyrroline biosynthesis in Bassia latifolia Roxb. flowers. Physiol Mol Biol Plants. 2011;17(3):231–237. doi: 10.1007/s12298-011-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakte K, Zanan R, Hinge V, Khandagale K, Nadaf A, Henry R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): a status review. J Sci Food Agric. 2017;97(2):384–395. doi: 10.1002/jsfa.7875. [DOI] [PubMed] [Google Scholar]
- Weretilnyk EA, Hanson AD. Betaine aldehyde dehydrogenase from spinach leaves: purification, in vitro translation of the mRNA, and regulation by salinity. Arch Biochem Biophys. 1989;271(1):56–63. doi: 10.1016/0003-9861(89)90255-5. [DOI] [PubMed] [Google Scholar]
- Wild R, Ooi L, Srikanth V, Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-l-cysteine assay. Anal Bioanal Chem. 2012;403(9):2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- Wu ML, Chou KL, Wu CR, Chen JK, Huang TC. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L.) J Food Sci. 2009;74(5):S192–S197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun. 2005;337(1):61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Yoshihashi T, Huong NTT, Inatomi H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J Agric Food Chem. 2002;50(7):2001–2004. doi: 10.1021/jf011268s. [DOI] [PubMed] [Google Scholar]
- Zanan R, Khandagale K, Hinge V, Elangovan M, Henry RJ, Nadaf A. Characterization of fragrance in sorghum (Sorghum bicolor (L.) Moench) grain and development of a gene-based marker for selection in breeding. Mol Breed. 2016;36(11):146. doi: 10.1007/s11032-016-0582-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.