Abstract
The T cell receptor (TCR) is one of the most complicated receptors in mammalian cells, and its triggering mechanism remains mysterious. As an octamer complex, TCR comprises an antigen-binding subunit (TCRαβ) and three CD3 signaling subunits (CD3ζζ, CD3δε, and CD3γε). Engagement of TCRαβ with an antigen peptide presented on the MHC leads to tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in CD3 cytoplasmic domains (CDs), thus translating extracellular binding kinetics to intracellular signaling events. Whether conformational change plays an important role in the transmembrane signal transduction of TCR is under debate. Attracted by the complexity and functional importance of TCR, many groups have been studying TCR structure and triggering for decades using diverse biochemical and biophysical tools. Here, we synthesize these structural studies and discuss the relevance of the conformational change model in TCR triggering.
Keywords: T Cell Receptor, Structure, Conformational change, Triggering
Subject terms: Signal transduction, Structural biology
Introduction
T cells play essential roles in the adaptive immune response against pathogens and cancer cells. T cells specifically recognize peptide antigens loaded on the major histocompatibility complex (peptide-MHC, pMHC) upon T cell receptor (TCR) activation. TCR is an intricate protein complex comprising a disulfide-linked TCRαβ heterodimer noncovalently associated with CD3 signaling subunits, namely, CD3ζζ, CD3δε, and CD3γε (reviewed by Jennifer E. Smith-Garvin et al.1).
A key feature of TCR signaling is specificity, which refers to antigen discrimination.2,3 Another key feature is sensitivity. A very low abundance of agonistic pMHC is sufficient to induce TCR activation.4–6 TCR triggering, the process by which the extracellular pMHC-TCR interaction leads to biochemical changes in the cytoplasmic domains of CD3, is not fully understood.7 Over the years, a variety of models have been proposed to illuminate TCR triggering, including but not limited to kinetic proofreading, kinetic segregation, serial engagement, and conformational change (reviewed by Arthur Weiss et al.8,9). Here, we review TCR structural studies, in particular, antigen-induced conformational changes on the extracellular, transmembrane and intracellular domains. All of studies provide structural insights into the working mechanism of the complicated TCR-CD3 machinery.
TCR structure
Since TCR genes were cloned and sequenced three decades ago,10,11 abundant research has been conducted to study TCR structure. Previous studies have reported high-resolution structural information of the extracellular domain (ECD) of the β chain;12 ECDs of TCRαβ,13 CD3εγ,14 and CD3εδ;15 the transmembrane (TM) domain of the CD3ζζ homodimer;16 and the cytoplasmic domain of CD3ε.17 In addition, the structures of the TCRαβ ECD in complex with pMHC and the coreceptor CD4 have also been reported.18–21 Recently, a significant breakthrough was made in the TCR field. Dong et al. reported a cryo-electron microscopy (cryo-EM) structure of a human TCR–CD3 complex in its unliganded state at 3.7 Å resolution.22 As expected, the TCR-CD3 complex is assembled with a 1:1:1:1 stoichiometry of TCRαβ/CD3γε/CD3δε/CD3ζζ, consistent with early biochemical data.23 Some of these outstanding studies are presented in Fig. 1.
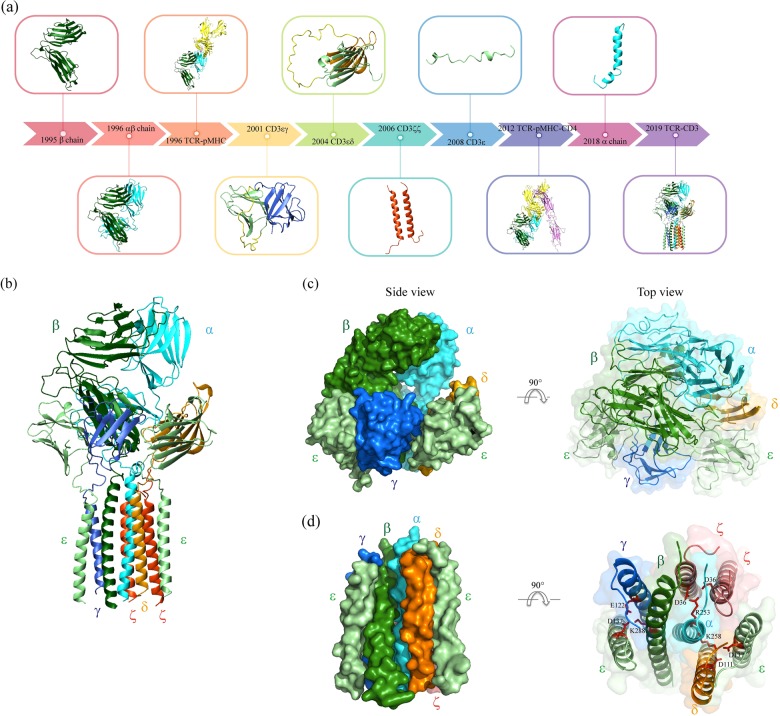
Fig. 1.
Time frame of TCR structure and TCR assembly studies. a A summary of some remarkable studies on TCR structure is presented here (from left to right): crystal structure of the TCRβ chain by X-ray diffraction (Bentley et al.,12 PDB entry 1BEC); crystal structure of TCRαβ by X-ray diffraction (Garcia et al.,20 PDB entry 1TCR); crystal structure of TCRα-pMHC by X-ray diffraction (Garboczi et al.,18 PDB entry 1AO7); solution structure of the CD3εγ ectodomain by NMR (Sun et al.,130 PDB entry 1JBJ); solution structure of CD3εδ ectodomain by NMR (Sun et al.,31 PDB entry 1XMW); solution structure of CD3ζζ transmembrane domain by NMR (Call et al.,16 PDB entry 2HAC); solution structure of membrane-bound CD3ε cytoplasmic tail by NMR (Xu et al.,17 PDB entry 2K4F); crystal structure of TCR-pMHC-CD4 by X-ray diffraction (Yin et al.,19 PDB entry 3T0E); solution structure of TCRα transmembrane domain by NMR (Brazin et al.,109 PDB entry 6MF8); and structure of the intact TCR-CD3 complex by microscopy (Dong De et al.,22 PDB entry 6JXR). The colors and orientations of TCR and the CD3 chains shown are matched to those of the TCR-CD3 complex cryo-EM structure: with TCRα in cyan, TCRβ in dark green, CD3ε in pale green, CD3γ in royal blue, CD3δ in orange, and CD3ζ in red orange. MHC is shown yellow (Garboczi et al.,18 PDB entry 1AO7), the viral peptide is shown in magenta (Garboczi et al.,18 PDB entry 1AO7), CD4 is shown in violet (Yin et al.,19 PDB entry 3T0E), and the linkers between CD3ε and CD3γ (Sun et al.,130 PDB entry 1JBJ) and between CD3ε and CD3δ (Sun et al.,31 PDB entry 1XMW) are shown in yellow. b The structure of an entire T cell receptor complex obtained using single-particle cryogenic electron microscopy (Dong De et al.,22 PDB entry 6JXR). c The space-filling model of the extracellular domain assembly of the TCR-CD3 complex, showing the side and top views. d The space-filling model of the transmembrane domain assembly of the TCR-CD3 complex, showing the side and top views. The nine highly conserved charged residues, including three basic residues in the TM domain of the TCR heterodimer and six acidic residues in the TM domains of CD3 dimers, are shown as red sticks. The basic residue lysine located in the center of the TCRα (K258) and TCRβ (K288) TM domains interacts with two acidic residues in the CD3δε (CD3δ-D111 and CD3ε-D111) and CD3γε (CD3γ-E122 and CD3ε-D137) TM domains, respectively, while another basic residue, arginine, is located in the upper TCRα (R253) TM domain and interacts with two aspartic acids in the CD3ζζ (D36) TM domain
The TCRα and TCRβ chains each contains an extracellular variable (V) domain and a constant (C) domain, a connecting peptide (CP) proximal to the membrane and a transmembrane domain, along with a short cytoplasmic domain.20 TCR diversity is mainly determined by the Vα and Vβ domains located in its N-terminus, with two highly hypervariable loops, complementary determining regions 1 and 2 (CDR1α/β and CDR2α/β), which are positioned toward the α1 and α2 helices of the MHC. Another hypervariable loop, complementary determining region 3 (CDR3α/β), directly interacts with the peptide loaded on the MHC.24 Remarkably, the Vβ and Cβ domains are in close contact in all the TCR structures determined to date in comparison with Vα-Cα and with antibody VL-CL or VH-CH.25 Another feature of the TCRβ chain is a well-structured FG loop extruding from the interface of the Vβ and Cβ chain, which is suggested to be in contact with the top end of the CD3γε heterodimer in the distal membrane26,27 which contributes to negative selection of thymocytes and mature T cell activation.28,29 Moreover, the FG loop is suggested to control the duration of the TCR-pMHC bond, which is essential for antigen recognition.30
CD3ε, CDδ, and CDγ each comprises an extracellular immunoglobulin-like (Ig-like) domain, a short CP containing a CxxC motif with an intrachain disulfide bond, a TM domain and a CD containing an immunoreceptor tyrosine-based activation motif (ITAM) that can be phosphorylated by Lck to trigger downstream signaling.1 The CD3ε ECD forms heterodimers with CD3δ or the CD3γ ECD through parallel β-strand contacts.31 The CxxC motif in the CD3εδ/εγ CPs also contributes to heterodimer formation. Given that the CPs of TCRαβ are longer than those of CD3εδ and CD3εγ, it is presumed that the TCRαβ ECDs may lean on the CD3εδ/εγ ECDs. A study using complementary small-angle X-ray scattering and cryo-EM technologies indeed shows that CD3 lies underneath TCRαβ instead of alongside TCRαβ.32 In the recently resolved cryo-EM structure,22 this leaning orientation was confirmed. In contrast to other CD3 chains, CD3ζ has only a short nine-amino acid ECD and a longer CD with three phosphorylatable ITAMs. The homodimer formation of CD3ζζ depends on TM contact, including both polar and nonpolar side chains. The two aspartic acids play a critical role in the CD3ζζ dimer formation, and other polar residues strengthen this connection.16 It is worth noting that the CD3ζ CD is a major signaling component of the prevailing chimeric antigen receptors (CARs) used for antitumor immunotherapy.33
Interestingly, both the CPs of TCR and CD3 play important roles in TCR-CD3 assembly and signaling. The connecting peptide α-chain (CPα) motif is a highly conserved domain involved in positive selection but not negative selection.34,35 Thymocytes expressing mutant CPα fail to fully activate Lck, ZAP70, or Erk. Besides, CPα is required for responsiveness to antigenic stimuli. Replacing the TCRα CP with the TCRδ CP causes a deficiency in IL-2 production.36 It was subsequently reported that CPα mediates the interaction of TCR and coreceptor CD8. A TCR lacking CPα can be recruited to the immune synapse (IS), but its ability to interact with CD8 is lost, resulting in deficient signaling initiation.37,38 An evolutionally conserved CxxC motif found in the CPs of CD3ε, CD3δ, and CD3γ is critical for the assembly of the TCR-CD3 complex (discussed below) and outside-in signal transduction.39,40
TCR-CD3 complex assembly
The TCR-CD3 assembly has been studied by many methods, including site-specific or truncation mutagenesis, nuclear magnetic resonance (NMR), small-angle X-ray scattering (SAXS), electron microscopy (EM), and others. TCR-CD3 assembly is mediated by ECD, CP, and TM domains and strictly follows the three-step rule: TCRαβ first binds with CD3δε, then CD3γε joins in the intermediate complex and finally CD3ζζ is incorporated to form the integrated complex.41–43 Without CD3ζζ, the partially assembled complex is subject to quick internalization.44 The long CD of CD3ζ might shield the internalization-inducing di-leucine motif in the CD3γ CD to suppress endocytosis.45
Using a membrane-based in vitro translation system, Kai Wucherpfennig and colleagues showed that TCRαβ is assembled with the CD3 chains mainly through electrostatic interactions in the TM domains.41 A noteworthy feature of the TM helices is the presence of basic residues in the TCRαβ TM domains and the acidic residues in the CD3 TM domains. It has been demonstrated that the lysine residue and the arginine residue in the TCRα TM domain interact with two aspartic acid residues in the CD3δε TM domains and two aspartic acids in the CD3ζζ TM domains, respectively. The lysine residue in the TCRβ TM domain interacts with one aspartic acid and one glutamic acid residue in the CD3εγ TM domains. This 1:2 electrostatic interaction can stably exist in the membrane environment and is found in other receptors.46 In addition, the tetracysteine motif in the CP of the CD3γε and CD3δε dimers has also been implicated as an important structural element for the assembly of the CD3 chains with TCR.40 In the high-resolution cryo-EM structure,22 the TCRα/β heterodimer is further strengthened by the interchain disulfide bond and the tight packing of their CPs. Interestingly, TCRαβ CPs adopt a crossed conformation, while TCRαβ ECDs adopt a parallel conformation. In addition, the TCRα CP forms a hydrogen bond with the CD3δ CP, and the short CD3ζ ECD reinforces this interaction through polar interactions. This finding is consistent with previous data showing that TCRs without CPα have defective interactions with CD3ζ.36 Undoubtedly, CPs play critical roles in TCR-CD3 assembly.22
TCR-CD3 subunit interactions
Since TCRαβ is incapable of intracellular signaling, the interactions between TCRαβ and CD3 subunits are essential for translating TCR-pMHC binding kinetics to CD3 phosphorylation patterns. Extracellular contacts between the TCRαβ and CD3 subunits have been revealed by mutagenesis experiments or other biochemical methods. CD3εγ and CD3εδ interact with the adjacent CC’ loop of Cβ and the DE loop of Cα, respectively.47 Stable CD3εγ-Cβ interaction requires the association between CD3εδ-Cα. The coevolved protein sequence of the Cβ FG loop and CD3γ in Gnathostomata species supports the hypothesis that CD3γε contacts the Cβ FG loop.27 The reported crystal structures of each subunit have greatly helped scientists gain insight into the precise details of the interaction. A special side-to-side hydrophobic interface with combined β sheets of CD3γ and CD3ε has been reported.31 In contrast to their similar TM domains and CDs, the extracellular domains of CD3δ and CD3γ differ. Compared to that of CD3γ, the surface of CD3δ is more negatively charged, and its Ig fold ectodomain is more compact, resulting in a strikingly different surface electrostatic potential of CD3εδ and CD3εγ, though they share a conserved interface.48 These crystal structures also revealed some candidate sites for the TCR-CD3 interaction. A unique feature of human CD3ε, consisting of a sequence of 8 acidic amino acid residues, is suggested to enhance the association with the positively charged regions of TCR.14 Solution NMR also indicated that the docking sites for CD3 on the TCRβ chain are at the lower helices, agreeing with the leaning hypothesis.49 In addition, crystal structures of CD8αα-pMHC50 and CD8αβ-pMHC51 and the complete CD4-pMHC-TCR ternary complex19 greatly contribute to the understanding of the relative position of these molecules and the functions of the coreceptors in TCR triggering. CD8αα makes contact with residues in the MHC I α2, α3, and β2m domains, while CD8αβ binds only with residues at the α3 domain. CD4 is at an angle of ~70° with respect to TCR and an angle of ~50° with respect to MHC II. The ternary structure has an arch shape.
In their recent report of an intact TCR-CD3 complex structure,22 Huang and colleagues indicated some other binding sites, including (1) ECD: CD3δ interacts with TCRαβ, specifically, the AB loop of Cα, through hydrophobic contacts, and the ζ chain contributes to stabilizing this interaction; TCR Cβ is responsible for the TCR interaction with CD3γε. There is also an interface between CD3γε and CD3δε. (2) TMD: the TM domains of TCRα and TCRβ are intimately packed and organized, an observation consistent with a previous prediction.52 Both the TCRα and TCRβ TM helices interact with all three CD3 subunits through both hydrophobic and electrostatic interactions. The TM helices of each CD3ζ interact separately with the TM helices of CD3γε and CD3δε, forming an unclosed α-helical CD3 barrel with two of the TM helices of TCRα/β inserted into the inner space of the barrel. The structure of an intact TCR-CD3 complex can be described by the saying “the overall structure of the TCR-CD3 complex resembles an ice cream cone with the extracellular portion corresponding to the ice cream and the TM region corresponding to the cone”.
However, all the structures determined to date were determined outside the native lipid environment. Lipids are important for shaping the TCR structure and regulating triggered signaling. For example, cholesterol is suggested to directly bind with the TCRβ TM domain, which locks TCR in an inactive conformation.53 Acidic phospholipids can ionically interact with positively charged CD3ε CD and CD3ζ CD to sequester ITAM tyrosines within the membrane. Undoubtedly, it is of great significance to determine the structure within natural membrane, including phospholipids and cholesterol.
Triggering models
Compared with the affinity of antibodies for antigens, TCRs generally exhibit a lower affinity for antigens, typically with a 1–100 μM dissociation constant (KD).54,55 TCR also cross-reacts with antigens with high or low affinity.56,57 The two-step binding mechanism, in which the MHC contact with an antigen initiates binding and the subsequent peptide interaction determines the duration of this binding, might explain the nature of TCR cross-reactivity.58 It is generally believed that CD4 and CD8 enhance TCR signaling in two ways: (1) CD4 and CD8 interact with MHC II and MHC I, respectively, to strengthen the weak TCR-pMHC binding, and (2) CD4 and CD8 are responsible for delivering Lck to the TCR-pMHC complex.59 High-affinity pMHC binding tends to induce a robust immune response, while a weak-affinity pMHC induces weak or negligible response. To explain the triggering mechanism, several models have been proposed, including kinetic segregation, serial engagement, kinetic proofreading, and conformational change (reviewed by Arthur Weiss et al.8,9).
According to the kinetic proofreading model, the half-life (t1/2) of the TCR-pMHC interaction should be maintained long enough to allow for downstream biochemical events.60 Adequate phosphorylation must occur before TCR-pMHC dissociation in order for downstream signaling events, including ZAP-mediated LAT phosphorylation and LAT signalosome formation, to increase.61 In the most recent study, this model was supported by an optogenetic system in which the binding time of pMHC to TCR was controlled by light.62 In addition to t1/2, equilibrium affinity controlled by the on and off rates also contributes to the determination of an antigen response.63,64 In the “coreceptor scanning” model proposed by Ed Palmer and colleagues, only a few coreceptors are coupled with Lck. Therefore, the TCR-pMHC complex scans hundreds of coreceptors to find one that carries Lck.65
Previous experimental data revealed that even a single pMHC could induce digital secretion of TNF-α and IL-2.4 This phenomenon may be better illuminated by the serial engagement model, which suggests that a single pMHC can continuously engage with different TCR molecules such that continuous, cumulative TCR triggering leads to full T cell activation.66,67 Positive feedback regulation of TCR signaling, such as that of the TCR-Ca2+-CD28 loop, Ras, and Erk, also contributes to the digital activation behavior of T cells.68–71
Another model is kinetic segregation, in which TCR triggering is initiated by the exclusion of tyrosine phosphatase from the TCR signalosome, thereby shifting the equilibrium toward Lck-mediated TCR phosphorylation.72,73 The extracellular domain of the tyrosine phosphatase CD45 is rigid and much larger than that of TCR; therefore, CD45 can be excluded from the TCR-pMHC contact site by size. Elongating the pMHC ectodomain leads to an increase in the size of the TCR-pMHC complex, thus allowing CD45 to access SMAC, which significantly abrogates TCR triggering.74 It is worth noting that CD45 is responsible for dephosphorylating Tyr505 in Lck, thus activating Lck in the early phase of TCR triggering; thus, CD45 plays both positive and negative roles in TCR activation.75
Conformational change model
Ligand binding to membrane proteins often results in protein clustering or conformational changes. Since Janeway first proposed that both TCR aggregation and conformational change were required for TCR activation in 1995,76 this model has been controversial. Some contend that TCR-CD3 undergoes conformational changes to preferably interact with pMHCs, while others claim that pMHC binding induces no significant change. It is worth noting that tiny changes may lead to distinct functional impacts since TCR is such a precisely assembled setup. Moreover, all the structures determined to date have been in solution. However, native membrane lipids are known to shape the TCR structure. Kinetic analysis also reveals that the interaction of native membrane-embedded TCRs and pMHCs is different from that of soluble TCRs and pMHCs.77,78 In the following section, we will highlight the conformational changes that occur in the extracellular, transmembrane, and intracellular domains upon TCR triggering.
Extracellular conformational changes and mechanical force
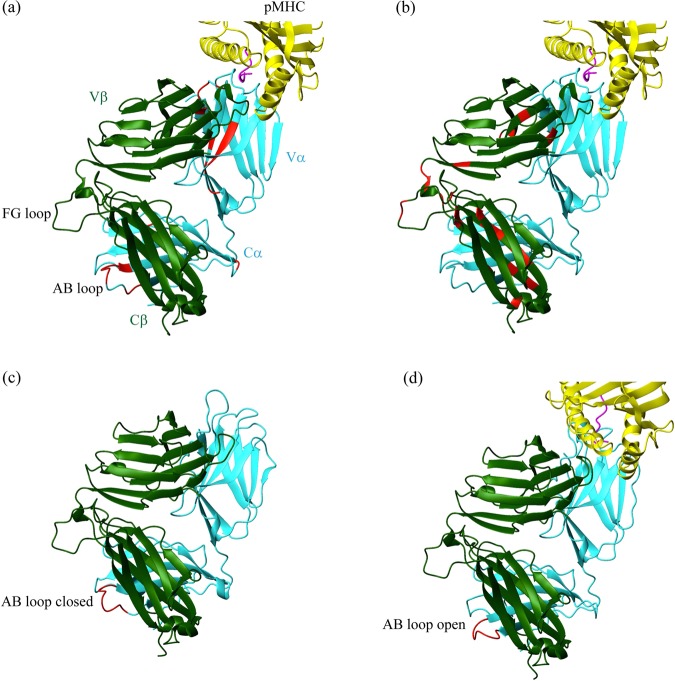
An outstanding advantage of the conformational change model is that it illustrates the way a small number of antigens can trigger T cells. Many studies have shown that pMHC-TCR engagement always leads to some conformational alterations in TCR at the interface, mainly restricted to CDRs, by NMR,79 thermodynamics,80,81 and crystal structures82–84 (reviewed by Markus G. Rudolph et al.85). Nevertheless, conformational changes in sites far from the recognition site have also been reported (Fig. 2a, b). One study revealed that the earliest incident in TCR triggering is the conformational change within the AB loop in Cα, which is reversible when pMHC-TCR dissociates (Fig. 2c, d). This change is specifically induced by agonists but not antagonists.86 This finding was confirmed in different TCRs in another study.87 In TCRβ, CDR3β undergoes a conformational change to fit a complementary interface upon pMHC binding,88 an observation consistent with previous data that shows CDR3β undergoing most conformational adjustments upon pMHC binding (reviewed by Markus G. Rudolph et al.85). The TCR Cβ FG loop, involved in thymic selection and T cell development,28,29 is presumed to regulate T cell signaling. The loop resembles a lever and might transfer information from TCRαβ to CD3εγ. The latest cryo-EM structure confirmed that CD3δ interacts with both TCR Cα and Cβ. TCR Cβ makes contact with both subunits of CD3εγ.22 These interactions enable the relaying of the conformational change from the extracellular domain to the intracellular domain. A recent study indicated that there are long-range effects on the Cβ of TCR, especially near the H3 helix, which is distal to the TCR-pMHC recognition sites and proximal to the membrane.89 In addition, mutagenesis suggests a role for these H3 helix residues in TCR signaling and immune function that is independent of TCR-pMHC binding. Previous studies demonstrated that the αA and αB helices of Cβ are the docking sites for CD3 subunits, making possible the transmission of the conformational change from the recognition site to downstream CD3 signaling.49 Recently, NMR and molecular dynamics (MD) revealed that pMHC binding to a human antiviral TCR induces long-range allosteric communication between pMHC- and CD3-binding sites, which mainly contains residues clustered in three locations: (1) the FG loop connecting Vβ and Cβ; (2) the Vα and Vβ interface; and (3) the Cα and Cβ interface, which are distal to the recognition site but close to the αA and αB helices.87
Fig. 2.
Conformational changes in TCR extracellular domains. a, b Conformational changes in human antiviral TCR A6 induced by Tax–HLA-A2 binding (Ding et al.,131 PDB entry 1QRN). The TCR α chain is colored cyan, TCR β chain is colored dark green, the antigenic peptide is colored magenta, and part of the MHC molecule is colored yellow. The TCR A6 α chain (a) and β chain (b) residues with experimentally significant changes in chemical shifts and peak intensities upon addition of Tax–HLA-A2 are highlighted in red. c, d Structures of the ligated and free LC13 TCR. c The conformation of free LC13 TCR (Kjer-Nielsen et al.,132 PDB entry 1KGC). In this “closed” conformation, the AB loop projects toward the Cβ domain. d The conformation of LC13 TCR in complex with HLA-B8 (Kjer-Nielsen et al.,133 PDB entry 1MI5). The TCR α chain is colored cyan, the TCR β chain is colored dark green, the antigenic peptide is colored magenta, and part of the MHC molecule is colored yellow. The variable and constant domains of TCRαβ are labeled Vα, Vβ, and Cα, Cβ, respectively. The position of the AB loop is indicated and highlighted in red. In this “open” conformation, the A-B loop is projected away from the Cβ domain
The mechanism of how pMHC binding results in such conformational alterations may be partially explained by the existence of mechanical force. Mechanical effects are involved in nearly all cell types and many cellular functions.90 Abundant studies have reported that force exertion has a great effect on lymphocytes; for example, by regulating pseudopodia localization in T cells, promoting endocytosis activity,91 potentiating the cytotoxicity of cytotoxic T lymphocytes (CTLs) by enhancing perforin activity,92 and determining the functions of T cells by regulating antigen-binding kinetics.93 Mechanical force is presumed to be generated upon TCR engagement and plays a critical role in TCR triggering. A series of artificial APCs expressing certain TCR ligands was used to show direct evidence that the physical force exerted on TCRs, but not on other T cell surface receptors, can initiate TCR triggering.94 In this regard, TCR acts as a mechano-sensor, transforming mechanical force to biochemical signals. Results from NMR experiments indicate that agonistic anti-CD3 monoclonal antibody (mAb) binding is centered in the CD3ε chain, while non-agonistic mAbs vertically bind to the crevice between CD3ε and CD3γ.95 However, non-agonistic mAbs can become stimulatory when an external tangential, but not perpendicular, force of 50 pN is applied by optical tweezers. These findings indicate that TCR is an anisotropic mechanosensor that can be activated only by specific antigens that can generate direction-specific physical force. External torque applied with force can lead to quaternary structural changes and provide energy for signaling. In addition, crystal structures show that agonist pMHC and nonagonist pMHC adopt different docking geometries with TCR.96,97 These results provide an explanation for TCR discrimination. As biophysics technologies develop, there is increasing evidence of mechanical force at the TCR-pMHC interface. Bond formation between adhesion molecules is regulated by force, which is important for lymphocyte tethering and rolling on the vascular wall.98,99 Similarly, catch bonds are formed by shear force upon agonistic pMHC binding to enhance the pMHC-TCR interaction, while slip bonds are formed between antagonistic pMHC and TCR.100,101 Single-molecule optical tweezer assays indicate that both the sensitivity and specificity of the T cell response are dependent on force-based interactions.102 It has been reported that force prolongs the duration of catch bonds but shortens those of slip bonds. The Ca2+ response requires rapid, cumulative and long-lasting bond formation in the early stage for agonistic pMHC to form catch bonds with TCR to trigger TCR signaling, while antagonistic pMHC fails to form these bonds.103,104 Using steered molecular dynamics (SMD) simulation and single-molecule biophysical approaches, a recent study demonstrated that cognate pMHCs maintained hydrogen bonds with the TCR CDR loops through their hotspot residues.105 When force is applied, the pMHC-TCR interface gradually twists to become almost parallel to the force direction, and the MHC β2m domain may dissociate from its α domain, with TCR remaining stable during the process. The pre-existing hydrogen bonds are strengthened, and additional hydrogen bonds are generated, which contribute to the formation of TCR-pMHC catch bonds. Such force-induced MHC conformational changes are consistent with the evidence from previous studies.106 Membrane-distal sites of the CD3γε and CD3δε ectodomains are presumed to contact TCRαβ, as described above, enabling the transmission of force to the transmembrane domain and cytoplasmic domain.
Extracellular conformational change was found in some studies but not in others.22,107 The different TCR/pMHC constructs used in these studies might be a reason for these discrepancies. Additionally, these studies were performed with purified soluble TCR and MHC extracellular domains and thus lacked the influence of the membrane environment and mechanical force. Nevertheless, tiny changes might be functionally meaningful in the precisely organized TCR-CD3 complex.
Transmembrane conformational change
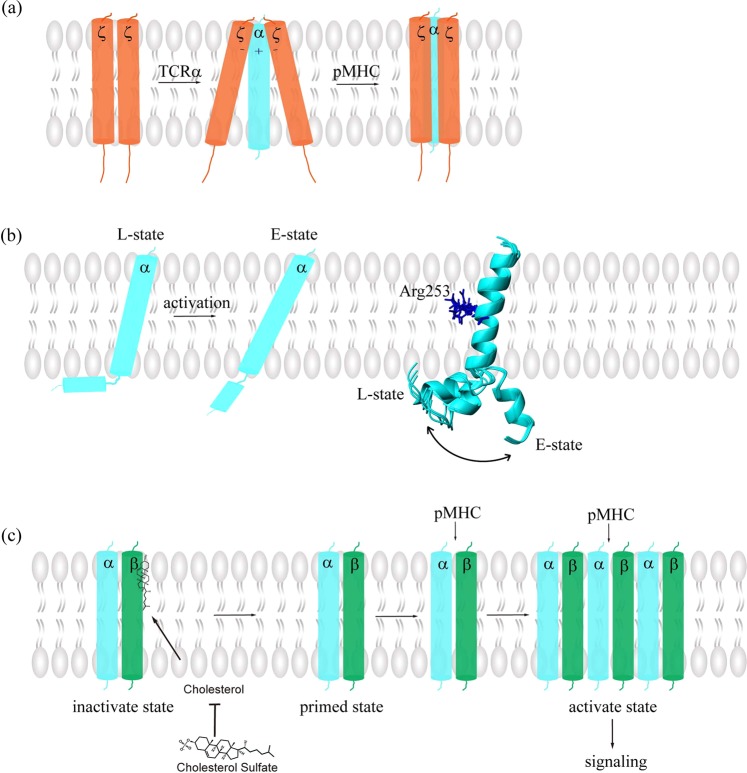
Despite an in-depth study of external recognition and internal signaling, there is less knowledge on the mechanism by which information is relayed across the membrane. Conformational changes in the ECD and biochemical signaling in CD imply that TM domains may undergo conformational alterations. With respect to this view, Kuhns and colleagues proposed a mechanical switch that coupled TCR triggering to the cytoplasmic juxtamembrane regions of CD3ζζ.108 C-terminal CD3ζζ TM regions stand in apposition, similar to two legs, when CD3ζζ is unassembled with the TCR-CD3 complex (Fig. 3a). Upon assembly with the TCR-CD3 complex, the C-termini of CD3ζζ TM domains are separated in a manner similar to the splitting of firewood. The CD3ζζ C-terminal domains are again in parallel when TCR encounters the cognate pMHC. This model supports the assumption that the negatively charged TMD of CD3ζζ is associated with the positively charged TMD of TCRα, forming a responsive hinge.41 A more recent study depicts an L-shaped transmembrane structure of TCRα with bipartite helixes with a hinge between them (Fig. 3b). TCRα loosely associates with the CD3ζζ TM domains, partially through its basic residue Arg253 when in an inactive state.109 Such helical tilt is altered by force transduced through the membrane, resulting in a straightened configuration and a new Arg253 position. Then, CD3ζζ dissociates from TCRα and initiates intracellular signaling. While these studies all emphasize the importance of TCRα and the ζζ chains in transmembrane conformational change and assembly, the latest cryo-EM structure shows that both TM helices of TCRαβ interact with all three CD3 subunits.22 All three studies were consistent in the finding that the charged residues in the TM domains of the TCR-CD3 complex play important roles in the assembly of the TM segments. However, it is noteworthy that the three studies have different views of the TM conformations. Neither the C-terminal splitting conformation of the CD3ζζ TM domains nor the L-shaped conformation of the TCRα TM domain were observed in the cyro-EM structure, which was presumed to be in a resting state. It is of great importance to study the TM conformational change in the context of the whole TCR-CD3 complex in a native membrane environment. Therefore, integrating all the information is helpful for obtaining reasonable insights into the conformational change model. Other studies have focused on the interaction of the TCR-CD3 TMD and surrounding lipids, among which cholesterol occupies a central position. Based on the classic Monod-Wyman-Changeux model, Schamel et al. proposed an allosteric model of TCR in which TCR can switch from a resting state to a primed state spontaneously.110 Cholesterol acts as a naturally existing negative regulator of TCR by binding to TCRβ TM, preventing TCR from autoactivation in the absence of its ligand. Cholesterol can dissociate from TCRβ spontaneously, which causes TCR to transit to the primed state. pMHC binds only to primed TCRs and stabilizes their activated conformation, which leads to signal transduction53 (Fig. 3c). Biochemical experiments show that TCR nanoclustering is also mediated by the specific binding of the TCRβ TM to cholesterol, as well as some other saturated lipids, such as sphingomyelin, through which TCRs can much more strongly respond to cognate pMHC, according to the positive cooperation model and homotropic model.111 Elevating the PM cholesterol level in CD8 T cells by inhibiting cholesterol esterification leads to increased TCR nanoclustering and signaling, thus enhancing killing function.112 On the other hand, cholesterol sulfate, a negatively charged cholesterol derivative, inhibits TCR nanoclustering, likely by displacing cholesterol from TCR,113 verifying the critical role of cholesterol in transmembrane signaling.
Fig. 3.
Conformational changes in TCR transmembrane domains. Several models are proposed to illustrate how TCR signaling is transduced through the membrane. a In the proposed mechanical switch coupling model, in the unassembled CD3ζζ, the C-termini of the TM domains are in apposition, but when CD3ζζ is assembled with the TCR-CD3 complex, the C-termini of the TM domains are split apart.108 When TCR encounters pMHC, the CD3ζζ domains become parallel again. b L-shaped and extended conformations of the TCRα TM helix. The TCRα TM domain is loosely associated with the CD3ζζ TM domains, partially through its basic residue Arg253 in an L-shaped inactive state. This helical tilt is altered by forced transduction through the membrane, resulting in a more extended configuration.109 Ribbon representation of an assemblage of TCRα TM helix conformers depicting the dynamic region undergoing conformational exchange. c Specific interaction between cholesterol and TCRβ prevents autoactivation of TCR in the resting state. TCR can spontaneously switch to a primed state to which pMHC is able to bind; then, TCR is engaged, and signaling is initiated. Cholesterol sulfate can displace cholesterol from TCR and thus inhibit TCR signaling53
In summary, antigen-induced conformational changes in the TCR-CD3 transmembrane domains are not yet fully understood, and further studies need to be carried out to determine their roles in TCR triggering.
Intracellular conformational change
Regarding the CD3 cytoplasmic domains, the CD3ε CD contains three functional motifs: juxtamembrane basic residue-rich sequence (BRS) followed by a proline residue-rich sequence (PRS) and a C-terminal ITAM. CD3ζ CD has three ITAMs with BRSs in between each of them. The CD3δ CD and CD3γ CD each possess one ITAM. The earliest biochemical event in TCR triggering is phosphorylation of the tyrosine residues in the CD3 ITAMs by Lck tyrosine kinase. As much as ~40% of Lck is constitutively active in resting T cells, as previously reported,114 though another study claims a much lower percentage, at ~2%.115 It is unclear why there is no spontaneous phosphorylation in the presence of constitutively active Lck. The ITAM sequestration model may explain this confusing result. In resting T cells, CD3ε and CD3ζ are buried in the membrane, which protects the pivotal tyrosine sites from phosphorylation due to the interaction between the positively charged CDs and the negatively charged phospholipids in the membrane. CD3δ and CD3γ carry no charges and thus are exposed to the cytosol17,116–119 (Fig. 4a). Specifically, the BRS region interacts with the headgroups of acidic phospholipids, and the peptide backbone is embedded in the interface of hydrophilic headgroups and hydrophobic acyl chains of lipids, which guarantees the deep insertion of the key residues of ITAM (YxxI and YxxL) into the lipid bilayer.17 A recent study found that association of CD3ε-BRS at the membrane is necessary for thymocyte development and peripheral T cell function.120 Intriguingly, CD3ε is the only CD3 chain that can efficiently recruit Lck through its BRS by ionically interacting with the unique domain (UD) domain of Lck.121 CD3ε dissociates from the plasma membrane upon pMHC binding, leading to the recruitment of Lck and phosphorylation of Tyr in the ITAM of CD3 subunits. CD3δ CD and CD3γ CD cannot independently recruit Lck, which explains why they are exposed in the cytosol but not spontaneously phosphorylated in resting T cells. Recently, using an atomic force microscopy (AFM) method, we identified a secondary lipid binding site in the CD3ε CD, in addition to that in the N-terminal BRS region.122 The secondary site encompasses the PRS region plus the proximal half ITAM. The distal C-terminal region and the linker region between the two lipid-binding regions seem only to enhance the interaction but do not bind directly. NMR experiments suggest that the distal C-terminal region and the linker region are much more dynamic. CD3ε dissociation is thought to be initiated at the distal C-terminal region and terminate at the most stable membrane-proximal BRS region. We suggest that there is a stable half-open state between the known closed state and the open state where both the C-terminal region and the secondary site have dissociated from the membrane while the major site and the linker are still in the membrane (Fig. 4b). There is expected to be lipid-dependent conformational intermediates among these three stable states, but they vary too extensively to be detected by the current AFM technique, and further experiments need to be done. These studies underline the importance of membrane lipids in TCR signaling.
Fig. 4.
Conformational changes in TCR intracellular domains. a In the resting state, CD3ε and CD3ζ are inserted into the membrane through electrostatic interactions, preventing their phosphorylation by Lck. b In the two-step activation model, in a stable half-open state, between the known closed state and open state, both the C-terminal region and PRS dissociate from the membrane while BRS and the linker are still in the membrane. The dissociation can be triggered by antigen ligation or Ca2+. Ca2+ interacts with negatively charged phospholipids and displaces positively charged CD3 tails. It is worth noting that there may be more intermediate states with complex dynamics that cannot be detected by conventional methods. c In the open state, ITAMs fully dissociate from the plasma membrane. The Tyr sites in ITAM are phosphorylated by Lck and recruit the downstream signaling molecule Zap70. The PRS region recruits Nck, and the cytoskeleton is remodeled to fit T cell activation
In addition to antigen ligation, CD3 CD conformational change can also be induced by Ca2+. TCR activation induces Ca2+ influx via the CRAC channel. The TCR-proximal Ca2+ concentration is dramatically increased in activated T cells because of the colocalization of TCR and CRAC in the immunological synapse. Ca2+ can directly bind to the lipid phosphate group, neutralizing its charge and therefore disrupting the ionic interaction between the lipid phosphate group and the CD3 CD, which exposes ITAM tyrosines for phosphorylation123 (Fig. 4c). This mechanism likely facilitates the activation of bystander TCRs that have not engaged antigens; thus, this feedback regulation amplifies and sustains TCR signaling. In this regard, strongly bound antigens induce a higher concentration of Ca2+, leading to higher levels of CD3 CD exposure and, consequently, a higher phosphorylation level and stronger T cell response. Such a Ca2+-mediated conformational change of the CD3 CD was confirmed by a recent single-molecule study.124 It is worth mentioning that reciprocal lipid/Ca2+ regulation also applies to CD28, and TCR-Ca2+-CD28 forms a dual feedback loop to amplify the initial antigen signal, thus providing a signaling basis for T cell sensitivity.68,125
Another crucial motif in CD3ε, i.e., PRS, also contributes to TCR triggering. PRS is known to recruit the noncatalytic region of tyrosine kinase (Nck). It has been previously reported that Nck interacts through its first SH3 domain (SH3.1), recruits SLP76 through its SH2 domain, and interacts with other downstream signaling molecules through a second or third SH3 domain, thus being involved in a later period signaling.126 However, a recent study of full-length Nck interacting with CD3ε showed that the SH3.1 domain and SH2 domain of Nck both participate in binding to partially phosphorylated CD3ε in a cooperative manner.127 According to the model, Nck binding to CD3ε depends mainly on the SH3.1 domain, while its SH2 domain likely enhances the binding by interacting with Tyr177 in the ITAM region. The function of the CD3-Nck interaction is not fully understood. However, it is certainly involved in cytoskeleton recruitment, IS formation and T cell activation, including the phosphorylation of CD3 ITAM and ZAP70, as well as downstream Akt and Erk1/2.128 Since the Nck-PRS interaction is required for weak antigens, not strong antigens, drugs targeted to the Nck-PRS interaction offer a promising approach to mitigate autoimmune disease without impairing the immune response to pathogen-derived antigens. Using computational modeling and small-molecular-weight compound libraries, researchers have screened a satisfactory drug named AX-024, which apparently inhibits Nck recruitment to PRS, thus hindering TCR crossing-induced actin polymerization.129 Phosphorylation of ZAP70 is also inhibited by AX-024, while T cell proliferation triggered by IL-2 or PMA+ionomycin is not affected, indicating that AX-024 specifically impedes TCR signaling.
Concluding remarks
Here, we review some remarkable studies that deconstructed the TCR-CD3 complex through various techniques and have greatly shaped our understanding of TCR. The conformational change model, considered to preferentially illustrate cross-membrane signal transduction, is also summarized. Most results corroborate each other and are useful for revealing the mechanism of TCR triggering. Since lipids play essential roles in regulating TCR structure and function, studying the TCR-CD3 complex in a native membrane environment is warranted in future research. Moreover, T cell-based immunotherapies such as TCR-T and CAR-T can be further developed and applied in the clinic based on a better understanding of TCR triggering.
Acknowledgements
We thank Wei Wu and Chengsong Yan for thoughtful discussions. C.X. is funded by CAS grants (Strategic Priority Research Program XDB29000000, Facility-based Open Research Program QYZDB-SSW-SMC048, Fountain-Valley Life Sciences Fund of University of Chinese Academy of Sciences Education Foundation), NSFC grant (31861133009, 31621003), MOST Grant (2018YFA0800700) and the Ten Thousand Talent Program “Leading Talent” of China. H.L. is funded by an NSFC grant (31670751).
Author contributions
C.X. designed the framework. X.X. wrote the manuscript. H.L. and C.X. revised it. H.L. and X.X. made the figures.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xinyi Xu, Hua Li
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Boehmer H, Kisielow P. Self-nonself discrimination by T cells. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- 3.Pielak RM, et al. Early T cell receptor signals globally modulate ligand:receptor affinities during antigen discrimination. Proc. Natl Acad. Sci. USA. 2017;114:12190–12195. doi: 10.1073/pnas.1613140114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39:846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 6.Schamel WW, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat. Rev. Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 8.Courtney AH, Lo WL, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 2018;43:108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat. Immunol. 2014;15:798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 11.Yanagi Y, et al. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984;308:145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- 12.Bentley GA, Boulot G, Karjalainen K, Mariuzza RA. Crystal structure of the beta chain of a T cell antigen receptor. Science. 1995;267:1984–1987. doi: 10.1126/science.7701320. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Atomic structure of an alphabeta T cell receptor (TCR) heterodimer in complex with an anti-TCR fab fragment derived from a mitogenic antibody. EMBO J. 1998;17:10–26. doi: 10.1093/emboj/17.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjer-Nielsen L, et al. Crystal structure of the human T cell receptor CD3 epsilon gamma heterodimer complexed to the therapeutic mAb OKT3. Proc. Natl Acad. Sci. USA. 2004;101:7675–7680. doi: 10.1073/pnas.0402295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett KL, Harrison SC, Wiley DC. Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl Acad. Sci. USA. 2004;101:16268–16273. doi: 10.1073/pnas.0407359101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Call ME, et al. The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell. 2006;127:355–368. doi: 10.1016/j.cell.2006.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garboczi DN, et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 19.Yin Y, Wang XX, Mariuzza RA. Crystal structure of a complete ternary complex of T-cell receptor, peptide-MHC, and CD4. Proc. Natl Acad. Sci. USA. 2012;109:5405–5410. doi: 10.1073/pnas.1118801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia KC, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 21.Rossjohn J, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015;33:169–200. doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 22.Dong De, et al. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019;573:546–552. doi: 10.1038/s41586-019-1537-0. [DOI] [PubMed] [Google Scholar]
- 23.Punt JA, Roberts JL, Kearse KP, Singer A. Stoichiometry of the T cell antigen receptor (TCR) complex: each TCR/CD3 complex contains one TCR alpha, one TCR beta, and two CD3 epsilon chains. J. Exp. Med. 1994;180:587–593. doi: 10.1084/jem.180.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104:1–4. doi: 10.1016/S0092-8674(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 25.Alcover A, Alarcon B, Di Bartolo V. Cell biology of T cell receptor expression and regulation. Annu. Rev. Immunol. 2018;36:103–125. doi: 10.1146/annurev-immunol-042617-053429. [DOI] [PubMed] [Google Scholar]
- 26.Ghendler Y, Smolyar A, Chang HC, Reinherz EL. One of the CD3epsilon subunits within a T cell receptor complex lies in close proximity to the Cbeta FG loop. J. Exp. Med. 1998;187:1529–1536. doi: 10.1084/jem.187.9.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim ST, et al. Distinctive CD3 heterodimeric ectodomain topologies maximize antigen-triggered activation of alpha beta T cell receptors. J. Immunol. 2010;185:2951–2959. doi: 10.4049/jimmunol.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasada T, et al. Involvement of the TCR Cbeta FG loop in thymic selection and T cell function. J. Exp. Med. 2002;195:1419–1431. doi: 10.1084/jem.20020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touma M, et al. The TCR C beta FG loop regulates alpha beta T cell development. J. Immunol. 2006;176:6812–6823. doi: 10.4049/jimmunol.176.11.6812. [DOI] [PubMed] [Google Scholar]
- 30.Das DK, et al. Pre-T cell receptors (Pre-TCRs) leverage vbeta complementarity determining regions (CDRs) and hydrophobic patch in mechanosensing thymic self-ligands. J. Biol. Chem. 2016;291:25292–25305. doi: 10.1074/jbc.M116.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun ZY, et al. Solution structure of the CD3epsilondelta ectodomain and comparison with CD3epsilongamma as a basis for modeling T cell receptor topology and signaling. Proc. Natl Acad. Sci. USA. 2004;101:16867–16872. doi: 10.1073/pnas.0407576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birnbaum ME, et al. Molecular architecture of the alphabeta T cell receptor-CD3 complex. Proc. Natl Acad. Sci. USA. 2014;111:17576–17581. doi: 10.1073/pnas.1420936111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backstrom BT, Muller U, Hausmann B, Palmer E. Positive selection through a motif in the alphabeta T cell receptor. Science. 1998;281:835–838. doi: 10.1126/science.281.5378.835. [DOI] [PubMed] [Google Scholar]
- 35.Werlen G, Hausmann B, Palmer E. A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 36.Backstrom BT, et al. A motif within the T cell receptor alpha chain constant region connecting peptide domain controls antigen responsiveness. Immunity. 1996;5:437–447. doi: 10.1016/S1074-7613(00)80500-2. [DOI] [PubMed] [Google Scholar]
- 37.Mallaun M, et al. The T cell receptor’s alpha-chain connecting peptide motif promotes close approximation of the CD8 coreceptor allowing efficient signal initiation. J. Immunol. 2008;180:8211–8221. doi: 10.4049/jimmunol.180.12.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naeher D, Luescher IF, Palmer E. A role for the alpha-chain connecting peptide motif in mediating TCR-CD8 cooperation. J. Immunol. 2002;169:2964–2970. doi: 10.4049/jimmunol.169.6.2964. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, et al. A conserved CXXC motif in CD3epsilon is critical for T cell development and TCR signaling. PLoS Biol. 2009;7:e1000253. doi: 10.1371/journal.pbio.1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Call ME, Wucherpfennig KW. A membrane-proximal tetracysteine motif contributes to assembly of CD3deltaepsilon and CD3gammaepsilon dimers with the T cell receptor. J. Biol. Chem. 2006;281:36977–36984. doi: 10.1074/jbc.M607164200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/S0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisler C. Failure to synthesize the CD3-gamma chain. Consequences for T cell antigen receptor assembly, processing, and expression. J. Immunol. 1992;148:2437–2445. [PubMed] [Google Scholar]
- 43.Call ME, Wucherpfennig KW. Molecular mechanisms for the assembly of the T cell receptor-CD3 complex. Mol. Immunol. 2004;40:1295–1305. doi: 10.1016/j.molimm.2003.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Oro U, et al. Regulation of constitutive TCR internalization by the zeta-chain. J. Immunol. 2002;169:6269–6278. doi: 10.4049/jimmunol.169.11.6269. [DOI] [PubMed] [Google Scholar]
- 45.Lauritsen JP, et al. Masking of the CD3 gamma di-leucine-based motif by zeta is required for efficient T-cell receptor expression. Traffic. 2004;5:672–684. doi: 10.1111/j.1600-0854.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 46.Stenkamp RE, Teller DC, Palczewski K. Crystal structure of rhodopsin: a G-protein-coupled receptor. Chembiochem. 2002;3:963–967. doi: 10.1002/1439-7633(20021004)3:10<963::AID-CBIC963>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Kuhns MS, Davis MM. Disruption of extracellular interactions impairs T cell receptor-CD3 complex stability and signaling. Immunity. 2007;26:357–369. doi: 10.1016/j.immuni.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Kelly ,LA, Stephen ,CH, Wiley ,DC. Crystal structure of a human CD3ed dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl Acad. Sci. USA. 2004;101:16268–16273. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y, et al. Identification of the docking site for CD3 on the T cell receptor beta chain by solution NMR. J. Biol. Chem. 2015;290:19796–19805. doi: 10.1074/jbc.M115.663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao GF, et al. Crystal structure of the complex between human CD8alpha(alpha) and HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Natarajan K, Margulies DH. Structural basis of the CD8 alpha beta/MHC class I interaction: focused recognition orients CD8 beta to a T cell proximal position. J. Immunol. 2009;183:2554–2564. doi: 10.4049/jimmunol.0901276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krshnan L, Park S, Im W, Call MJ, Call ME. A conserved alphabeta transmembrane interface forms the core of a compact T-cell receptor-CD3 structure within the membrane. Proc. Natl Acad. Sci. USA. 2016;113:E6649–E6658. doi: 10.1073/pnas.1611445113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swamy M, et al. A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity. 2016;44:1091–1101. doi: 10.1016/j.immuni.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Matsui K, et al. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991;254:1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- 55.Gee MH, et al. Stress-testing the relationship between T cell receptor/peptide-MHC affinity and cross-reactivity using peptide velcro. Proc. Natl Acad. Sci. USA. 2018;115:E7369–E7378. doi: 10.1073/pnas.1802746115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin Y, Mariuzza RA. The multiple mechanisms of T cell receptor cross-reactivity. Immunity. 2009;31:849–851. doi: 10.1016/j.immuni.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Birnbaum ME, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature. 2002;418:552–556. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 59.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc. Natl Acad. Sci. USA. 2010;107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl Acad. Sci. USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thill PA, Weiss A, Chakraborty AK. Phosphorylation of a tyrosine residue on Zap70 by Lck and its subsequent binding via an SH2 domain may be a key gatekeeper of T cell receptor signaling in vivo. Mol. Cell Biol. 2016;36:2396–2402. doi: 10.1128/MCB.00165-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yousefi O. S., et al. Optogenetic control shows that kinetic proofreading regulates the activity of the T cell receptor. Elife8, 42475 (2019). [DOI] [PMC free article] [PubMed]
- 63.Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc. Natl Acad. Sci. USA. 2010;107:8724–8729. doi: 10.1073/pnas.1000966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aleksic M, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity. 2010;32:163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stepanek O, et al. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell. 2014;159:333–345. doi: 10.1016/j.cell.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valitutti S. The serial engagement model 17 years after: from TCR triggering to immunotherapy. Front. Immunol. 2012;3:272. doi: 10.3389/fimmu.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 68.Yang W, et al. Dynamic regulation of CD28 conformation and signaling by charged lipids and ions. Nat. Struct. Mol. Biol. 2017;24:1081–1092. doi: 10.1038/nsmb.3489. [DOI] [PubMed] [Google Scholar]
- 69.Das J, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stefanova I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 71.Wertek F, Xu C. Digital response in T cells: to be or not to be. Cell Res. 2014;24:265–266. doi: 10.1038/cr.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 73.Chang VT, et al. Initiation of T cell signaling by CD45 segregation at ‘close contacts’. Nat. Immunol. 2016;17:574–582. doi: 10.1038/ni.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 75.Courtney AH, et al. A Phosphosite within the SH2 domain of Lck regulates its activation by CD45. Mol. Cell. 2017;67:498–511. doi: 10.1016/j.molcel.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janeway CA., Jr. Ligands for the T-cell receptor: hard times for avidity models. Immunol. Today. 1995;16:223–225. doi: 10.1016/0167-5699(95)80163-4. [DOI] [PubMed] [Google Scholar]
- 77.Liu B, et al. 2D TCR-pMHC-CD8 kinetics determines T-cell responses in a self-antigen-specific TCR system. Eur. J. Immunol. 2014;44:239–250. doi: 10.1002/eji.201343774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hawse WF, et al. TCR scanning of peptide/MHC through complementary matching of receptor and ligand molecular flexibility. J. Immunol. 2014;192:2885–2891. doi: 10.4049/jimmunol.1302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol. Cell. 2003;12:1367–1378. doi: 10.1016/S1097-2765(03)00474-X. [DOI] [PubMed] [Google Scholar]
- 81.Schon A, Freire E. Thermodynamics of intersubunit interactions in cholera toxin upon binding to the oligosaccharide portion of its cell surface receptor, ganglioside GM1. Biochemistry. 1989;28:5019–5024. doi: 10.1021/bi00438a017. [DOI] [PubMed] [Google Scholar]
- 82.Garcia KC, et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 83.Holland CJ, et al. In silico and structural analyses demonstrate that intrinsic protein motions guide T cell receptor complementarity determining region loop flexibility. Front. Immunol. 2018;9:674. doi: 10.3389/fimmu.2018.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reiser JB, et al. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat. Immunol. 2003;4:241–247. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 85.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 86.Beddoe T, et al. Antigen ligation triggers a conformational change within the constant domain of the alphabeta T cell receptor. Immunity. 2009;30:777–788. doi: 10.1016/j.immuni.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 87.Rangarajan S, et al. Peptide-MHC (pMHC) binding to a human antiviral T cell receptor induces long-range allosteric communication between pMHC- and CD3-binding sites. J. Biol. Chem. 2018;293:15991–16005. doi: 10.1074/jbc.RA118.003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reiser JB, et al. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/S1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 89.Natarajan K, et al. An allosteric site in the T-cell receptor Cbeta domain plays a critical signalling role. Nat. Commun. 2017;8:15260. doi: 10.1038/ncomms15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Ju L, Rushdi M, Ge C, Zhu C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell. 2017;28:3134–3155. doi: 10.1091/mbc.e17-04-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anvari B, Torres JH, McIntyre BW. Regulation of pseudopodia localization in lymphocytes through application of mechanical forces by optical tweezers. J. Biomed. Opt. 2004;9:865–872. doi: 10.1117/1.1778178. [DOI] [PubMed] [Google Scholar]
- 92.Basu R, et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell. 2016;165:100–110. doi: 10.1016/j.cell.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong J, et al. Force-regulated in situ TCR–peptide-bound MHC class II kinetics determine functions of CD4+ T cells. J. Immunol. 2015;195:3557–3564. doi: 10.4049/jimmunol.1501407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim ST, et al. The alphabeta T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adams JJ, et al. T cell receptor signaling is limited by docking geometry to peptide-major histocompatibility complex. Immunity. 2011;35:681–693. doi: 10.1016/j.immuni.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction ‘codon’. Nat. Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- 98.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki T, et al. Mechanical force effect on the two-state equilibrium of the hyaluronan-binding domain of CD44 in cell rolling. Proc. Natl Acad. Sci. USA. 2015;112:6991–6996. doi: 10.1073/pnas.1423520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sibener LV, et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell. 2018;174:672–687. doi: 10.1016/j.cell.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, et al. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl Acad. Sci. USA. 2016;113:5610–5615. doi: 10.1073/pnas.1600163113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das DK, et al. Force-dependent transition in the T-cell receptor beta-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl Acad. Sci. USA. 2015;112:1517–1522. doi: 10.1073/pnas.1424829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pryshchep S, Zarnitsyna VI, Hong J, Evavold BD, Zhu C. Accumulation of serial forces on TCR and CD8 frequently applied by agonist antigenic peptides embedded in MHC molecules triggers calcium in T cells. J. Immunol. 2014;193:68–76. doi: 10.4049/jimmunol.1303436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu B, Chen W, Evavold BD, Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu P, et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol. Cell. 2019;73:1015–1027. doi: 10.1016/j.molcel.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wieczorek M, et al. Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernandes RA, et al. T cell receptors are structures capable of initiating signaling in the absence of large conformational rearrangements. J. Biol. Chem. 2012;287:13324–13335. doi: 10.1074/jbc.M111.332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee MS, et al. A mechanical switch couples T cell receptor triggering to the cytoplasmic juxtamembrane regions of CD3zetazeta. Immunity. 2015;43:227–239. doi: 10.1016/j.immuni.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brazin KN, et al. The T cell antigen receptor alpha transmembrane domain coordinates triggering through regulation of bilayer immersion and CD3 subunit associations. Immunity. 2018;49:829–841. doi: 10.1016/j.immuni.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schamel WW, Alarcon B, Hofer T, Minguet S. The allostery model of TCR regulation. J. Immunol. 2017;198:47–52. doi: 10.4049/jimmunol.1601661. [DOI] [PubMed] [Google Scholar]
- 111.Beck-Garcia K, et al. Nanoclusters of the resting T cell antigen receptor (TCR) localize to non-raft domains. Biochim. Biophys. Acta. 2015;1853:802–809. doi: 10.1016/j.bbamcr.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 112.Yang W, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang F, Beck-Garcia K, Zorzin C, Schamel WW, Davis MM. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016;17:844–850. doi: 10.1038/ni.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nika K, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ballek O, Valecka J, Manning J, Filipp D. The pool of preactivated Lck in the initiation of T-cell signaling: a critical re-evaluation of the Lck standby model. Immunol. Cell Biol. 2015;93:384–395. doi: 10.1038/icb.2014.100. [DOI] [PubMed] [Google Scholar]
- 116.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat. Struct. Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 117.Deford-Watts LM, et al. The cytoplasmic tail of the T cell receptor CD3 epsilon subunit contains a phospholipid-binding motif that regulates T cell functions. J. Immunol. 2009;183:1055–1064. doi: 10.4049/jimmunol.0900404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H, Cordoba SP, Dushek O, van der Merwe PA. Basic residues in the T-cell receptor zeta cytoplasmic domain mediate membrane association and modulate signaling. Proc. Natl Acad. Sci. USA. 2011;108:19323–19328. doi: 10.1073/pnas.1108052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.DeFord-Watts LM, et al. The CD3 zeta subunit contains a phosphoinositide-binding motif that is required for the stable accumulation of TCR-CD3 complex at the immunological synapse. J. Immunol. 2011;186:6839–6847. doi: 10.4049/jimmunol.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bettini ML, et al. Membrane association of the CD3epsilon signaling domain is required for optimal T cell development and function. J. Immunol. 2014;193:258–267. doi: 10.4049/jimmunol.1400322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li L, et al. Ionic CD3-Lck interaction regulates the initiation of T-cell receptor signaling. Proc. Natl Acad. Sci. USA. 2017;114:E5891–E5899. doi: 10.1073/pnas.1701990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo X, et al. Lipid-dependent conformational dynamics underlie the functional versatility of T-cell receptor. Cell Res. 2017;27:505–525. doi: 10.1038/cr.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi X, et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493:111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 124.Sasmal, D. K. et al. TCR-pMHC bond conformation controls TCR ligand discrimination. Cell. Mol. Immunol. (2019). 10.1038/s41423-019-0273-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 125.Dobbins J, et al. Binding of the cytoplasmic domain of CD28 to the plasma membrane inhibits Lck recruitment and signaling. Sci. Signal. 2016;9:ra75. doi: 10.1126/scisignal.aaf0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/S0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 127.Paensuwan P, et al. Nck binds to the T cell antigen receptor using its SH3.1 and SH2 domains in a cooperative manner, promoting TCR functioning. J. Immunol. 2016;196:448–458. doi: 10.4049/jimmunol.1500958. [DOI] [PubMed] [Google Scholar]
- 128.Hem CD, et al. T cell specific adaptor protein (TSAd) promotes interaction of Nck with Lck and SLP-76 in T cells. Cell Commun. Signal. 2015;13:31. doi: 10.1186/s12964-015-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borroto A, et al. First-in-class inhibitor of the T cell receptor for the treatment of autoimmune diseases. Sci. Transl. Med. 2016;8:370ra184. doi: 10.1126/scitranslmed.aaf2140. [DOI] [PubMed] [Google Scholar]
- 130.Sun ZJ, Kim KS, Wagner G, Reinherz EL. Mechanisms contributing to T cell receptor signaling and assembly revealed by the solution structure of an ectodomain fragment of the CD3 epsilon gamma heterodimer. Cell. 2001;105:913–923. doi: 10.1016/S0092-8674(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 131.Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/S1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 132.Kjer-Nielsen L, et al. The 1.5 A crystal structure of a highly selected antiviral T cell receptor provides evidence for a structural basis of immunodominance. Structure. 2002;10:1521–1532. doi: 10.1016/S0969-2126(02)00878-X. [DOI] [PubMed] [Google Scholar]
- 133.Kjer-Nielsen L, et al. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/S1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]