Abstract
Driver mutations in oncogenic pathways, rewiring of cellular metabolism and altered ROS homoeostasis are intimately connected hallmarks of cancer. Electrons derived from different metabolic processes are channelled into the mitochondrial electron transport chain (ETC) to fuel the oxidative phosphorylation process. Electrons leaking from the ETC can prematurely react with oxygen, resulting in the generation of reactive oxygen species (ROS). Several signalling pathways are affected by ROS, which act as second messengers controlling cell proliferation and survival. On the other hand, oncogenic pathways hijack the ETC, enhancing its ROS-producing capacity by increasing electron flow or by impinging on the structure and organisation of the ETC. In this review, we focus on the ETC as a source of ROS and its modulation by oncogenic pathways, which generates a vicious cycle that resets ROS levels to a higher homoeostatic set point, sustaining the cancer cell phenotype.
Subject terms: Cancer metabolism, Oncogenes
Background
Altered reactive oxygen species (ROS) homoeostasis is emerging as an important hallmark of the cancer cell phenotype. These alterations are consistent with the “ROS rheostat” theory,1 which states that, depending on their homoeostatic set point, ROS can control different signal transduction pathways, thus acting as either tumour promoters or tumour suppressors. At low/medium levels, ROS enhance mitogenic signalling and cell survival, and may contribute to genetic instability.2 It is thus not surprising that most tumours exhibit a higher ROS set point compared with their healthy counterparts. On the other hand, excessive ROS levels result in extensive macromolecular damage and engage cell death pathways.
It should be noted, however, that ROS-producing pathways are not the only determinants of the ROS rheostat, and cancer cells commonly increase the fuelling of antioxidant pathways and may cope with oxidative damage by upregulating repair systems.3
Mitochondria are an important source of ROS production through the activity of the electron transport chain (ETC).4 Although there are also many other important sources of ROS generation (e.g. NADPH oxidases [NOX]),5 ETC-derived ROS are pivotal regulators of cell fate, given the central role of mitochondria in life and death.
Electron transfer through the ETC is a tight and efficient molecular machine. Nevertheless, it is estimated that in normal conditions, about 0.2–2% of the electrons that pass through the ETC complexes leak from the system and reduce O2, generating superoxide (O2•–),6,7 which is rapidly converted by superoxide dismutases (SOD) into hydrogen peroxide (H2O2), the most important ROS acting as a “second messenger” in signal transduction due to its relatively long half-life and diffusion across membranes via the aquaporin channels.8 The hydroxyl radical (•OH) is a highly damaging ROS with an extremely short half-life that is generated from H2O2 in the presence of iron or copper through the Fenton reaction. O2•– can also interact with nitric oxide (NO), generating the reactive nitrogen species (RNS) peroxynitrite (ONOO−), which controls signalling molecules through the nitration of tyrosine residues.9 For the role of RNS in cancer we refer the reader to recent excellent reviews.10,11
It is important to note that many tumours presenting mutations in ETC components show a strong propensity to produce ROS,12 supporting the crucial role of this machinery in the modulation of the cancer cell phenotype.
Several signal transduction pathways that control cell turnover are known to be ROS-sensitive (reviewed in 13–15). Through the reversible oxidation of cysteine residues to sulfenic acid (SO–)16 and disulfide bonds,17 ROS modulate the activity of redox-sensitive proteins by controlling several aspects of the cancer phenotype. The oxidation of phosphatase and tensin homolog (PTEN) results in AKT-mediated cell survival and proliferation,18 while the oxidation of prolyl hydroxylases leads to hypoxia-inducible factor 1α (HIF-1α) stabilisation,19 resulting in a profound metabolic rewiring of cancer cells. Notably, O2•– may inhibit apoptosis in cancer cells, accounting for resistance to Fas-mediated cell death.20 In this regard, decreasing the levels of O2•– restores apoptosis in cancer cells overexpressing the anti-apoptotic protein BCL-221 (see below).
In addition, increased mitochondrial ROS levels drive “mitohormesis”, a condition of mild mitochondrial stress that favours pro-survival pathways through the activation of mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signalling, an alteration that is frequently associated with poor clinical outcome of cancer patients.22 Notably, the mitochondria-targeted O2•– scavenger mitoTEMPO inhibits tumour cell migration and metastasis in mice,23 corroborating a crucial role for ETC-derived O2•– in tumorigenesis.
In this review, we focus on the ETC as a source of ROS, its alterations in cancer cells and its modulation by oncogenic pathways, highlighting the connection between ETC-derived ROS and the cancer cell phenotype.
Sites of ROS production in the mitochondrial ETC
Functional electron transport provides the bioenergetic fuelling necessary to sustain tumour initiation, growth and dissemination. However, defects in the ETC may also favour tumorigenesis.
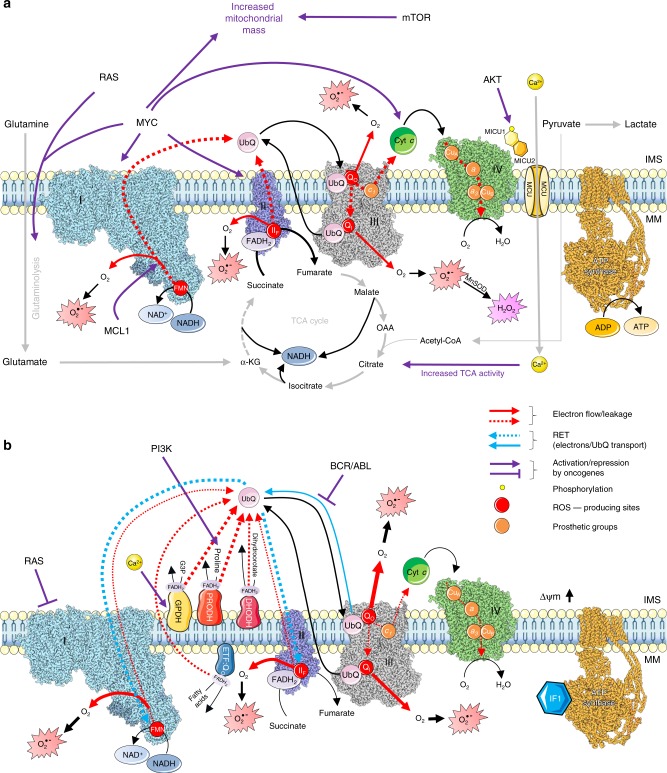
The ETC is responsible for the transfer of electrons from reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), produced in the tricarboxylic acid (TCA) cycle, to oxygen, across complexes I, II, III and IV. This electron flow provides energy for proton translocation, thus generating a transmembrane potential (Δψm) that drives ATP synthesis by the ATP synthase complex (Fig. 1). The proton gradient may be either dissipated or increased by the ATP synthase complex when working in its ATP-producing or ATP-consuming mode, respectively. In mammals, complexes I and III have been identified as the most relevant sites of ROS production within the ETC.24,25
Fig. 1.
Oncogenes regulate ETC-mediated ROS production. Dashed red arrows indicate electron flow through complexes I (blue), II (purple), III (grey) and IV (green). Solid red arrows indicate electron leakage to O2 with consequent generation of O2•–, which can be converted into H2O2 by manganese superoxide dismutase (MnSOD). The arrows thickness represents the intensity of reactions/electron flux. a Enhanced electron flow through the ETC favours ROS production. RAS and MYC induce glutaminolysis, AKT phosphorylates MICU1 leading to calcium influx, and both these mechanisms promote TCA cycle activity and thus NADH production. MCL1 increases electron flow. mTOR and MYC induce mitochondrial biogenesis (purple arrows). b Destabilisation of electron flow across the ETC enhances electron leakage and ROS generation. RAS induces a decrease in complex I activity, thus disrupting the respirasome; PI3K increases proline fuelling through PRODH, bypassing complex II and destabilising electron flow; AKT-driven calcium influx promotes the activity of GPDH, thus inducing RET (light-blue arrows); BCR/ABL impairs electron transfer from complexes I and II to complex III (purple arrow and blunted arrows). IF1 blocks ATP synthase (yellow structure), leading to hyperpolarisation and inducing RET. Both the increase and destabilisation of electron flow drive ROS generation. IMS: intermembrane space. MM: mitochondrial matrix. G3P: glycerol 3-phosphate. Δψm: mitochondrial transmembrane potential. The structures of ETC complexes were obtained from RCSB Protein Data Bank: complex I (5XTD204), complex II (3AEF), complex III (5XTE204), complex IV (5Z62205) and ATP synthase (5ARA206). Structures were prepared with UCSF Chimera.207
Complex I (or NADH-ubiquinone oxidoreductase, Fig. 1) oxidises NADH to NAD+ and transfers electrons to the carrier ubiquinone (UbQ, also known as coenzyme Q10), resulting in its reduction to ubiquinol (UbQH2) and the translocation of protons across the inner mitochondrial membrane (IMM).26 During this process, single-electron reduction of O2 can occur, leading to the generation of O2•–, which is converted into H2O2 by manganese superoxide dismutase (MnSOD). H2O2 can diffuse across the membrane and be reduced to H2O by mitochondrial and cytoplasmic peroxiredoxins, catalases, thioredoxin peroxidases and glutathione peroxidases.27 Mammalian complex I contains 44 subunits, seven of which (ND1, ND2, ND3, ND4, ND4L, ND5 and ND6) are coded by the mitochondrial genome, and the others by the nuclear genome.28 ROS can be generated when the electrons are transferred from NADH to UbQ through the flavin (IF) and the UbQ-reducing (IQ) sites in complex I.
Mutations in complex I components may result in increased production of O2•–, which may sustain ROS-dependent oncogenic pathways and induce damage of mitochondrial DNA.29 In addition to affecting supercomplex assembly (see below), defects in ND2 subunit are known to promote tumorigenesis and metastasis in breast, pancreatic and oral cancers, and head and neck carcinomas.30 Similar phenotypes were also reported for mutations of the ND6 subunit in lung cancer,31 and ND4 in acute myeloid leukaemia32 and in glioblastoma.33 These mutations are probably associated with increased ROS generation, which in turn selects for increased fuelling of scavenging pathways and enhanced production of NADPH by the folate pathway, resulting in the promotion of metastatic dissemination.34 Wheaton et al.35 also demonstrated the importance of complex I in cancer progression by using its inhibitor metformin, which reduced tumorigenesis in vitro and in vivo. Metformin acts upstream of the ROS-producing IF site, inhibiting ROS generation from complex I and reducing electron flow to complex III, thus decreasing the production of ROS from it. In hypoxic conditions, NADH depletion and supercomplex disassembly (see below) hamper complex I activity, and ROS generation occurs mainly from complex III.36 However, during hypoxia, complex I may produce ROS by reverse electron transfer (RET, see below). In contrast, the inhibition of complex I at the IQ site by rotenone increases ROS generation and induces apoptosis of breast cancer cells through the activation of the c-Jun N-terminal kinase (JNK) and p38 pathways.37
Complex III (or ubiquinol–cytochromec oxidoreductase, Fig. 1) transfers the electrons received by UbQH2 from complex I and complex II to cytochrome c and couples this reaction with proton translocation across the IMM.38 Complex III is a dimer containing three highly conserved core subunits: cytochrome b (containing two haem groups—bH and bL), cytochrome c1 and the Rieske iron–sulfur protein (ISP, with an Fe–S cluster). Eight additional subunits in the complex are required for its assembly, stability and regulation.39 The reaction catalysed by complex III, known as the “Q-cycle”,40 involves two different binding sites on cytochrome b, QO for UbQH2 and QI for UbQ.
In the IIIQO site, electrons may leak and interact with O2, by producing O2•– both in the matrix and the intermembrane space (IMS), where it is converted into H2O2 by SOD.41,42 H2O2 can cross the outer mitochondrial membrane and reach the cytoplasm, where it can act as a signalling molecule. Several studies reported that the inhibition of QI site with antimycin A blocks QO–QI electron transfer, resulting in increased electron leak and consequent ROS generation at the IIIQO site.43,44 Although it is reported that the inhibition of binding of UbQH2 to the QO site by stigmatellin and myxothiazol prevents the production of ROS in complex III,43 it is plausible that a block in electron transfer across complex III could induce RET (see below) and ROS production.
In the context of cancer progression, it was shown that ubiquinol–cytochromec reductase core protein II (UQCR2), an important subunit of complex III, is upregulated in human tumours, including lung adenocarcinoma and breast cancer.45 Interestingly, UQCR2 negatively regulates p53 levels by inducing its degradation through the interaction with PHB, a p53 chaperone. This blunts p53/p21-mediated cell-cycle arrest and senescence, thus promoting tumorigenesis. Although ROS generated from complex III might stabilise p53, the overexpression of UQCR2 can revert this effect, by supporting tumour cell growth and dissemination. The role of UQCR2 in tumorigenesis is supported by a study by Shang et al.,46 who demonstrated a positive correlation between UQCR2 overexpression, tumour progression and poor prognosis in colorectal cancer. Based on these findings, the authors proposed UQCR2 as a prognostic biomarker and therapeutic target.
A similar role is attributable to ubiquinol–cytochromec reductase hinge (UQCRH), another subunit of complex III that is overexpressed in lung adenocarcinoma47 and in hepatocellular carcinoma.48 UQCRH regulates electron transfer from cytochrome c1 to cytochrome c, and its upregulation results in enhanced ROS production.47 In hepatocellular carcinoma, UQCRH overexpression is accompanied by the upregulation of two other subunits of complex III, the previously described UQCR2 and UQCRB. Park et al.48 observed that high expression of these proteins correlates with poor prognosis, suggesting that they may serve as useful cancer outcome biomarkers.
These observations suggest that inhibitors of ETC complexes could present a new opportunity for cancer therapy. Consistent with this notion, complex III inhibitors, such as myxothiazol and antimycin A, block the reoxidation of UbQH2, by impairing the UbQ-dependent processes in breast cancer cells.49 It is thus likely that inhibition of complex III might revert electron flow, thus inducing massive ROS generation from complex I and leading to cell death.
Although complex III was previously considered to play a prominent role in ROS production, Liu et al.50 proposed that the FMN group in complex I is the leading site in the ETC responsible for generating H2O2 from succinate (through RET, see below) and malate/glutamate.
Small amounts of ROS are also produced in the flavin-reducing site (IIF) within complex II (or succinate–ubiquinone oxidoreductase, Fig. 1),51 a heterotetramer composed of two hydrophilic subunits (SDHA, SDHB) and two hydrophobic subunits (SDHC, SDHD).52 The SDHA catalytic domain contains the prosthetic group FAD, which is reduced to FADH2 as a consequence of the oxidation of succinate to fumarate, thus generating electrons that are mobilised to the SDHB subunit. Here, three Fe–S clusters promote electron transfer to the SDHC and SDHD subunits associated with the haem and UbQ prosthetic groups. Haem favours the reduction of UbQ to UbQH2, which shuttles from complex II to complex III, where it is re-oxidised before returning to complex II.53 Although complex II is not involved in the generation of the transmembrane potential, it has an important role in transferring electrons, bypassing complex I.54 Electrons leaked by FADH2 in the IIF site can contribute to ROS generation, although this event can be decreased when the binding of malate or succinate blocks the site’s access to O2.55 Many diseases related to mutations in complex II proteins are characterised by increased ROS production from the IIF site. An example is represented by the loss of complex II function, which occurs in hereditary paraganglioma–pheochromocytoma, renal cell carcinoma and gastrointestinal stromal tumours. These mutations corrupt complex II’s electron transport activity, leading to succinate accumulation, increased ROS generation and decreased ATP production through oxidative phosphorylation (OXPHOS).56,57 The frequent loss of complex II subunit expression in cancer (in particular SDHB) suggests that these subunits might have tumour-suppressor functions; indeed, the accumulation of succinate activates the HIF-1α oncogenic pathway.58
On the other hand, complex II is overexpressed in haematopoietic stem and progenitor cells compared with differentiated cells. In this cell system, a high ratio between complex II and ATP synthase maintains an elevated Δψm, whose dissipation compromises the self-renewal potential of stem cells. Moreover, high expression of complex II in haematopoietic stem cells is associated with low ROS generation.59 The knowledge that cancer stem cells also show decreased levels of ROS60 may suggest that a high complex II/ATP synthase ratio could also preserve the cancer stem-cell reservoir.
The last enzyme of the ETC, complex IV (or cytochromec oxidase, Fig. 1), catalyses the reduction of O2 to H2O. In mammals, it is a 14-subunit complex containing three mitochondrial-encoded subunits (I, II and III) and 11 nuclear-encoded regulatory subunits. Two haem groups, cytochromes a and a3, and two copper centres, CuA and CuB,61 together with a binding site for cytochrome c constitute the electron transport machinery of complex IV. Four electrons from four cytochromec molecules are transferred to the CuA centre and then to the cytochrome a and the a3-CuB binuclear centre, by reducing Fe3+ and Cu2+ to Fe2+ and Cu+. These reactions are accompanied by the passage of four protons across the IMM and the reduction of O2 into two molecules of H2O, in a very rapid reaction that limits the generation of reactive intermediates.62 Although it is not directly involved in ROS production, complex IV activity affects the overall electron flow, with an impact on the electron leakage by previous complexes, and co-operates with oncogenes, such as BCL-2 (see below), to support tumorigenesis. Mutations in subunits I and II of complex IV have been reported in epithelial ovarian cancer, prostate cancers and acute myeloid leukaemia. Interestingly, the expression of complex IV is enhanced by p53, and the frequent co-mutation of complex IV and TP53 in acute myeloid leukaemia patients correlates with worse prognosis,63 probably due to increased damage of mitochondrial DNA and mitochondrial dysfunction.
Organisation of the ETC
Several studies of the organisation of the respiratory complexes have revealed the crucial role of the accessory assembly factors in ETC function and in preventing ROS production resulting from disassembled OXPHOS subunits.64 The “plasticity model”, proposed by Schägger and Pfeiffer,65 describes the coexistence of both independent and associated complexes. In particular, complexes I, III and IV (less frequently, complex II) assemble in supercomplexes.66,67 While complex I is mainly found associated in supercomplexes, 70% of complex III and 15% of complex IV units are associated with supercomplexes.68 In higher eukaryotes, the most frequent supercomplex, known as the respirasome, is composed of one complex I unit, two complex III units and one complex IV unit (I1III2IV1).69 Although the mechanism governing the assembly of the respirasome is not well understood,66,70 there is evidence that complexes III and IV are able to form autonomously, while mature complex I exists only in the respirasome71 and affects its correct assembly. Several studies showed that mitochondrial disorders that reduce the abundance of complex III or IV are often combined with an impairment in complex I expression.72–75 Supercomplex assembly optimises the structural proximity of UbQ with complexes I and III76 and the channelling of electrons to UbQ and cytochrome c, thus enhancing the efficiency of electron transfer among the complexes and reducing ROS generation.
However, mitochondrial remodelling during apoptosis or in hypoxia-induced acidification of the mitochondrial matrix impairs the assembly of supercomplexes.77,78 Supercomplex assembly is also connected to OMA1-dependent remodelling of the mitochondrial cristae.79 The plasticity of ETC organisation can also fine-tune the production of ROS, and consequently, the activity of ROS-sensitive pathways,80 and influences the adaptation of cancer cells to hypoxia.
The balance between supercomplexes and free complexes can influence cellular metabolism. Although assembly of complex I in supercomplexes promotes electron transport through NADH derived from glucose metabolism, free complex I favours electron transport through FAD-linked pathways81 (see below). Indeed, by switching the ETC organisation between free complexes and supercomplexes, cancer cells can tailor their metabolism to different microenvironment conditions.
In several cancer types, loss of supercomplex organisation may favour a metabolic switch towards the Warburg effect phenotype.82 Consistent with the central role of complex I in respirasome assembly, cancer cells with mutations in ND2 exhibit a glycolytic metabolic profile; interestingly, these cancer cells also exhibited increased metastatic potential.30 In addition, the downregulation of NDUFS1, another complex I subunit, is selected by antiangiogenic therapy in ovarian cancer, leading to a stable glycolytic phenotype and increased aggressiveness.83
Although increased ROS levels and metabolic rewiring caused by the loss of supercomplex organisation promote tumorigenesis, some evidence suggests that supercomplex assembly could also be enhanced by oncogenes, thus limiting ROS generation by the ETC. For instance, it has been reported that HER2 can translocate to the inner side of the IMM,84 where it could promote increased assembly of supercomplexes. Interestingly, Rohlenova et al.85 observed that mitochondrial-targeted tamoxifen (MitoTam), which disrupts supercomplex assembly, impairs electron flow from complex I to complex III, thus increasing ROS generation and cell death in HER2-overexpressing breast cancer cells. It is noteworthy that as the electron flow from FAD-linked enzymes to complex III is not affected by MitoTam treatment, hypoxic adaptation could protect cancer cells from its cytotoxic effect. In addition, KRAS contributes to protect against ROS overload through its involvement in the biosynthetic pathway of cardiolipin (see below), an IMM-specific phospholipid. Cardiolipin is sequestered by supercomplexes, being protected from degradation, and in turn, stabilises supercomplexes, thus decreasing the electron leakage.86
ETC-associated enzymes
Metabolic enzymes that link oxidation of their substrates to the reduction of UbQ represent additional ROS-producing sites connected to the ETC (Fig. 1B).
Proline dehydrogenase (PRODH) is an IMM enzyme responsible for proline catabolism that transfers electrons through FADH2 to UbQ.87 Proline-derived electrons can leak out to O2, thus generating O2•–. The overexpression of PRODH leads to decreased ETC efficiency and increased ROS generation, due to direct competition for UbQ between PRODH and complex II. This effect may be counteracted by succinate,87 due to its higher efficiency in reducing the UbQ pool. Depending on the cell context, PRODH may act as either a tumour suppressor or an oncogenic factor.88 In cancer cells, by generating ROS, PRODH may trigger apoptosis and suppress mitogenic pathways (e.g. those triggered by the epidermal growth factor receptor [EGFR] and Wnt–β-catenin).89 Moreover, p53 directly upregulates PRODH, which, in turn, induces ROS, DNA damage and promotes cellular senescence.90 Consistent with these findings, many tumour types show downregulation of PRODH expression.89 In prostate cancer, CMYC negatively regulates PRODH expression via miR23b*, thus promoting tumorigenesis and tumour progression.91 All these effects can be reverted by antioxidants.88 However, under specific metabolic conditions, PRODH can take on a pro-survival role; indeed, glucose deprivation and/or hypoxia upregulate PRODH through AMP-activated protein kinase (AMPK), independent of HIF-1α/2α, leading to ROS generation, and consequently, to the activation of autophagy, which, in this context, exerts a pro-survival role. Proline catabolism through PRODH is preferentially used to produce ATP under glucose starvation but not in hypoxia.92 These observations indicate that PRODH could modulate ETC activity based on nutrient availability and oxygen tension. Moreover, PRODH overexpression was observed in breast cancer metastases, compared with primary tumour samples, supporting a role for PRODH in metastasis formation.93
Mitochondrial glycerol-3-phosphate dehydrogenase (GPDH or GPD2), a component of the glycerophosphate shuttle, is associated with the ETC on the outer side of the IMM. Several functions have been described for the glycerophosphate shuttle, the most important being the metabolism of glycerol 3-phosphate, which connects glycolysis, lipogenesis and OXPHOS. GPDH also has an important role in producing ROS by directly leaking electrons and by inducing RET.94 In this regard, a panel of prostate-cancer-derived cell lines was shown to possess upregulated GPDH activity compared with healthy prostate epithelial cells.95 Furthermore, highly proliferating, undifferentiated cancers display higher levels of GPDH activity than more differentiated cancers with low proliferative capacity.96
Mitochondrial dihydro-orotate dehydrogenase (DHODH) is a FAD-linked enzyme that mediates the oxidation of dihydro-orotate to orotate, which fuels electrons in the UbQ pool and thus may contribute to O2•– production at the QO site of complex III.97 As DHODH is a key component of the pyrimidine biosynthesis pathway, which is required for DNA replication, it has an essential role in cancer cell growth.98 Indeed, the oncogenic mitogen-activated protein kinase (MAPK), KRAS and mTOR pathways increase DHODH fuelling by inducing de novo pyrimidine biosynthesis, while MYC increases the expression of DHODH.99 Although no mutations in DHODH have been reported in cancer, malignant cells are dependent on its enzymatic activity to sustain pyrimidine synthesis.100 In fact, inhibition of DHODH through specific antagonists decreases cell growth in many cancer cell lines, in particular acute myeloid leukaemia, and high expression of DHODH correlates with higher-grade gliomas,98 suggesting that DHODH inhibition may be a therapeutic strategy to induce tumour cell differentiation. However, it is important to underline that the catalytic activity of DHODH is strictly connected with a functional ETC, which influences the availability of UbQ. The role of DHODH in ROS production is still controversial, as its inhibition can increase or decrease ROS generation in a context-dependent manner.98
Electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) is an IMM protein that contains FAD and an Fe–S cluster. Together with electron transfer flavoprotein (ETF), ETF:QO catalyses the transfer of electrons derived from β-oxidation of fatty acids to the UbQ pool. However, the impairment of electron transfer from FADH2 to the QO site may lead to O2•– formation.101 ETF:QO activity depends on functional complexes I and III, as they share the acceptor UbQ pool. However, as described above, ETF-mediated electron transfer represents the preferential way to fuel the ETC under chronic hypoxia, as the activity of complexes I and II is impaired. Under low oxygen tension, the activation of HIF-1α induces a metabolic rewiring towards glycolysis and the use of glutamine as a carbon source to synthesise fatty acids,102 which are catabolised through β-oxidation to sustain electron flow through the ETF system, thus maintaining ETC function and Δψm. Indeed, the inhibition of fatty acid β-oxidation by etomoxir proved to be an effective strategy to overcome hypoxia-mediated resistance to radiotherapy in cancer cells,103 suggesting an important role for ETF:QO in cancer survival under hypoxia. Consistent with this hypothesis, a work by Schuetz et al.104 reported that ETF:QO is overexpressed in renal carcinoma and oncocytoma, supporting its putative involvement in tumorigenesis.
Reverse electron transport (RET)
In addition to the canonical “forward” electron flow through the complexes of the ETC described above, electrons may be transferred from UbQH2 back to complex I, with the generation of NADH from NAD+. This process, which is termed RET, produces a significant amount of ROS. Although RET is well characterised in vitro, its relevance in vivo has long been controversial, and only recently has experimental evidence started to accumulate in support of its role in cell physiology and pathologic processes.105
Several studies indicated that the key conditions that lead to RET are accumulation of reduced UbQ and high Δψm. Electrons deriving from complex II, PRODH, GPDH, ETF:QO and DHODH may drive over-reduction of UbQ.106 Furthermore, all conditions inhibiting electron transport downstream of complex III also increase reduction of the UbQ pool. High mitochondrial transmembrane potential may result from increased H+ extrusion by the ETC, by the ATP synthase working in its “reverse” ATP-consuming mode, or through the inhibition of the “forward” depolarising/ATP-synthesising mode of action of the ATP synthase. To this effect, an important role may be played by ATPase inhibitory factor 1 (IF1), which, in its active dimeric form, inhibits both the ATP hydrolase and synthase activities of the complex.107 Acidification of the mitochondrial matrix favours inhibition of the ATP hydrolase function by IF1.108,109 This mechanism plays an important role in hypoxia by preventing ATP depletion by the “reverse” mode of function of the ATP synthase (reviewed by Campanella et al.110).
Most studies investigating the relevance of RET in human pathology have been focused on the tissue damage occurring during ischaemia/reperfusion in heart attack and stroke. Chouchani et al.106 recently showed that ischaemic tissues accumulate succinate, which, during reperfusion, is oxidised by complex II, giving rise to RET that produces high levels of ROS, which cause extensive macromolecular damage and trigger cell death. Consistent with this notion, inhibitors of complex I or complex II (e.g. rotenone and dimethyl-malonate, respectively), as well as antioxidants, protect the heart from ischaemia/reperfusion-mediated tissue damage.106,111
Although the role of RET in cancer has not been directly investigated, it is now clear that oxygen tension in the tumour microenvironment is subjected to ample temporal and spatial fluctuations as a result of the chaotic organisation of the neoangiogenic process.112 The resulting imbalance between oxygen supply and demand suggests that the tumour tissue is constantly subjected to recurrent ischaemia/reperfusion cycles in which RET might play a major role in altering ROS homoeostasis. ROS accumulation in hypoxia/reperfusion could also be attributable to Ca2+ overload and alterations in ETC supercomplex organisation.113
In addition, several studies have provided strong evidence that IF1 is highly overexpressed in primary samples of human colon, lung, breast and ovarian cancer compared with their normal tissue counterparts,114 thus suggesting a potential mechanism controlling RET in cancer cells. Gain-of-function and loss-of-function experiments carried out in different cancer cell lines showed that IF1 enhances glycolytic flux, a finding that is consistent with the inhibition of the ATP synthetic activity of the F0F1 complex by IF1.114–116 Overexpression of IF1 in cancer cell lines also increased the production of O2•– in the mitochondrial compartment,114,115 an effect that is likely to be related to the mitochondrial hyperpolarisation induced by inhibition of the “forward” activity of the ATP synthase. IF1 overexpression also led to the activation of the nuclear factor κB (NF-κB) pathway resulting in a mitogenic or pro-survival effect, depending on the cell type.114,115 The fact that these effects were counteracted by the mitochondrial scavenger MitoQ strongly suggests that they were caused by an increase in ROS in the mitochondrial compartment. The metabolic rewiring and ROS-dependent signalling pathways engaged by IF1 overexpression in cancer cells are consistent with the effects of mitohormesis107 and with the finding that mitochondrial ROS produced via RET increase the lifespan of both Caenorhabditis elegans117 and Drosophila melanogaster.118 However, the final effect of IF1 on the metabolic profile and redox homoeostasis is likely to be more complex and depends on the relative abundance of IF1 versus ATP synthase as well as the phosphorylation of IF1 on S39 by protein kinase A (PKA), which controls its binding to the ATP synthase.119,120 Nevertheless, several studies also suggest that high levels of expression of IF1 are associated with a more aggressive tumour phenotype and poor clinical outcome.121–123 In addition to IF1, inhibition of ATP synthase by the TCA cycle substrate α-ketoglutarate may also increase ROS production.117
Regulation of ETC-mediated ROS production by oncogenes
Since it is known that low/medium levels of ROS promote cell proliferation by activating several pathways, such as MAPKs and the phosphoinositide 3-kinase (PI3K)–AKT pathway,13 it is not surprising that cancer cells are characterised by sustained ROS production that supports their uncontrolled proliferation.124 The activation of oncogenic pathways enhances the production of intracellular ROS, which, in turn, leads to the activation of oncogenes in a vicious circle that boosts cell proliferation and drives aggressiveness of cancer cells, thus affecting the outcome in cancer patients. According to the theory of the ROS rheostat,1 the concerted action of oncogenic pathways both on the production and the elimination of ROS plays a central role in rewiring multiple cellular functions that sustain the different phases of tumorigenesis.
Current knowledge suggests two main oncogene-mediated mechanisms that influence ROS production by the ETC: (i) increased fuelling of carbon sources in the TCA cycle, resulting in increased production of NADH and FADH2, which augments the number of electrons flowing through the ETC (Mechanism A, Fig. 1a); (ii) destabilisation of electron transfer through the ETC, which favours the leakage of electrons at complexes I, II and III (Mechanism B, Fig. 1b).
RAS
The RAS family of small GTPases (KRAS, HRAS and NRAS) transduce external stimuli (e.g. binding of growth factors to their receptors) that promote cell proliferation and survival. Mutations at codon 12, 13 or 61 of RAS lead to the constitutive activation of RAS signalling in cancer cells.
The constitutive activation of KRAS in human cancers125 orchestrates a profound metabolic rewiring that affects mitochondria, leading to ROS generation through Mechanism A (Fig. 1). Oncogenic KRAS signalling promotes the catabolism of glutamine, which fuels the TCA cycle, by enhancing mitochondrial ROS generation, resulting in anchorage-independent growth of colon cancer cells126; interestingly, this effect appeared to be mediated by mitochondrial, but not cytosolic, ROS and a functional ETC was required for KRAS-driven lung tumorigenesis in vivo. Anchorage-independent growth was abolished in ρ0 cells, in which mitochondrial DNA is absent, while cybrids with mutated cytochrome b gene restored the production of O2•– and anchorage-independent growth. These observations suggest that the oncogenic potential of KRAS is mediated by O2•– production from the QO site of complex III. Similarly, Liou et al.127 observed that oncogenic KRAS induces the transformation of pancreatic acinar cells into pancreatic intraepithelial neoplasia through mitochondrial ROS-mediated activation of NF-κB, which drives transcription of the EGFR and its ligands epidermal growth factor (EGF) and transforming growth factor α (TGFα). Interestingly, the authors also demonstrated that the mitochondria-targeted antioxidant MitoQ prevents the development of pancreatic cancer in mice with KRAS mutations, indicating that the oncogenic activity of KRAS requires the generation of mitochondrial ROS. Similar antitumour effects were observed by Weinberg et al.126 by using the mitochondria-targeted O2•– scavengers MCP and MCTPO. The importance of ROS in KRAS-mediated tumorigenesis is further corroborated by the fact that the mitochondria-targeted drugs Mito-CP (carboxy proxyl nitroxide) and Mito-Metformin block the proliferation of colon cancer cells.128
Son et al.129 demonstrated that KRAS-driven pancreatic ductal adenocarcinoma relies on glutamine catabolism to generate aspartate that is fuelled into the aspartate transaminase (GOT1)–malic enzyme 1 (ME1) axis, a major producer of NADPH. In this context, glutamine deprivation results in oxidative stress and decreased tumorigenicity, which is rescued by glutathione and N-acetylcysteine (NAC). It is worth noting that these findings do not contradict observations by Weinberg et al.126 and Liou et al.127 Indeed, the fact that glutamine is required to maintain endogenous antioxidant systems does not exclude that it also fuels the TCA cycle, thus increasing mitochondrial ROS generation. Consistent with these observations, oncogenic KRAS promotes tumorigenesis through the activation of nuclear factor erythroid 2-related factor 2 (NRF2),130 the master regulator of antioxidant responses. In the context of KRAS-driven pancreatic ductal adenocarcinoma with mutant KRAS, glutamine is pivotal both to induce cancer-promoting ROS production and to fuel antioxidant pathways, resulting in an increased homoeostatic ROS set point.
Mutant KRAS (G12V) also translocates to mitochondria and impairs electron transport, thus promoting the production of ROS131 through Mechanism B (Fig. 1). Baracca et al.132 observed that digitonin-permeabilised fibroblasts transformed with KRAS reduced their oxygen consumption rate when supplied with glutamate–malate as the respiratory substrate, suggesting a decrease in complex I activity. Oxygen consumption was not reduced when glutamate–malate was substituted with succinate, suggesting that complex II, III and IV activity was unchanged.132 The defect in complex I resulted in inefficient electron transport, due to the loss of supercomplex assembly, an alteration that may be further potentiated by the general effects of ROS on respirasome assembly.133
In apparent contrast with these observations, Chun et al.134 observed that disruption of oncogenic KRAS led to reduced expression of three genes involved in mitochondrial phospholipid synthesis—ACSL5, PCK2, and AGPAT7. The functional consequence of these changes was a decrease in the synthesis of cardiolipin, a phospholipid that favours supercomplex assembly, thus optimising respiration. By promoting the synthesis of cardiolipin in mitochondria, oncogenic KRAS may thus increase the efficiency of electron transport and decrease the production of ROS by the ETC. It is not clear, however, whether all KRAS-mutated tumours exhibit an increase in cardiolipin levels in mitochondria. These apparently paradoxical effects may be explained by the fact that the studies by Weinberg et al. 126 and Baracca et al. 132 used healthy cells transfected with a construct coding for mutant KRAS, while Chun et al.134 used KRAS-mutated colon cancer cells (HCT116) in which mutated KRAS was removed by knockout. In the latter case, the impact of oncogenic KRAS on ETC activity was studied in the context of a heavily mutated genetic landscape (4,288 mutations are reported in COSMIC for HCT116), in which the acquisition of oncogenic KRAS could have a protective role by promoting the synthesis of cardiolipin and reducing the generation of ROS fuelled by other oncogenes. Instead, introducing mutant KRAS in normal cells permitted a more direct investigation of the effects of mutant KRAS on the ETC. Further investigations will be needed to establish the role of oncogenic KRAS signalling on supercomplex assembly and respiratory efficiency and its possible impact on cancer.
Recent studies suggest that the effects of KRAS on redox homoeostasis are required for maintaining the cancer phenotype and may thus represent attractive therapeutic targets for KRAS-driven cancers, which still represent a clinical challenge due to the lack of effective therapies. In this regard, Shaw et al.135 demonstrated that induction of oxidative stress through the small molecule lanperisone kills mouse embryonic fibroblasts transfected with mutant KRAS and restrains their growth in vivo. Interestingly, Iskandar et al.136 observed that hyperactivation of mutant KRAS with the small molecule C1 leads to the activation of the PI3K–AKT pathway, which enhances ROS generation (see below), leading to mitochondrial dysfunction, cell death and blockade of tumours with mutant KRAS. These effects are blunted by NAC, indicating that the generation of ROS through the KRAS–AKT axis is necessary to mediate C1 cytotoxicity, corroborating the feasibility of a ROS-based anticancer strategy to target KRAS-driven tumours.
MYC
The MYC family of transcription factors (CMYC, LMYC and NMYC) controls cell proliferation and apoptosis by regulating a large number of RNA polymerase I-, II- and III-dependent genes.137,138 MYC amplification is commonly observed in neuroblastoma and in breast, ovary, prostate and uterine cancers, while the CMYC-immunoglobulin translocation is a hallmark of Burkitt’s lymphoma.139
Like RAS, MYC induces complex metabolic rewiring in cancer cells, which is achieved through the stimulation of glycolysis,140 mitochondrial biogenesis141 and glutaminolysis.142 Li et al.141 demonstrated that inducible expression of MYC in the B-cell line P493-6 increases mitochondrial mass and enhances the oxygen consumption rate, an indicator of ETC activity. These effects are in part mediated by the induction of the mitochondrial transcription factor A (TFAM) by MYC,141 thus possibly driving ROS production via enhanced electron flow through the ETC.
Observations by Wise et al.142 in glioma cell lines indicated that MYC controls a transcriptional programme that promotes the catabolism of glutamine as a carbon source to fuel the TCA cycle, thus sustaining ROS production by the ETC. Vafa et al.143 observed that overexpression of CMYC in human fibroblasts induced an increase in ROS levels, which correlated with the formation of foci of DNA damage; the antioxidant NAC reduced both of these effects. As MYC drove cell-cycle entry and proliferation even if DNA was damaged, these observations suggest a mechanism of MYC-induced genomic instability and selection for p53 loss (a frequent alteration in CMYC-driven tumours144), both of which fuel clonal evolution and tumour progression. The role of ROS production following MYC amplification is supported by the fact that exogenous antioxidants (vitamin C and Tiron) inhibited transformation of MYC-overexpressing NIH/3T3 fibroblasts.145 Moreover, blunting ROS through mitochondria-targeted vitamin E blocked the proliferation and induced cell death in osteogenic sarcoma cells.146
In chemotherapy-resistant triple-negative breast cancer, the upregulation of MYC with the anti-apoptotic protein MCL1 selects for a stem-cell phenotype that is dependent on mitochondrial respiration.147 Accumulation of MCL1 in the mitochondrial matrix increases the ability of complexes I, II and IV to transfer electrons. The concerted action of MYC on mitochondrial mass and MCL1 on the ETC resulted in HIF-1α stabilisation, selection of cancer stem cells and resistance to chemotherapy.147 However, as in the case for KRAS, the effects of MYC on ROS homoeostasis are a matter of balance, as MYC may also upregulate mitochondrial peroxiredoxin 3 to protect cells from ROS in hypoxia.148 Moreover, NADPH production through serine and one-carbon metabolism protects hypoxic breast cancer stem cells from oxidative stress.149 This evidence suggests that ROS trigger the selection of cancer stem cells through the upregulation of increased antioxidant defences, which is consistent with the lower ROS set point of cancer stem cells.60
MYC may also act through Mechanism B by upregulating the expression of several mitochondrial nuclear-encoded proteins, resulting in an imbalance between ETC subunits coded by the nuclear and mitochondrial genomes that leads to the generation of misassembled respiratory complexes.150 Interestingly, Herrmann et al.151 observed a strong correlation between the progression from normal prostate epithelium to invasive prostate carcinoma with imbalanced nuclear-encoded versus mitochondrion-encoded subunits of complex IV. This suggests that misassembled respiratory complexes promote tumour progression. MYC also affects ROS production by impinging on cancer cell metabolism. In particular, as mentioned above, MYC promotes the expression of a plethora of genes involved in nucleotide synthesis, comprising DHODH,99 thus triggering the generation of ROS by destabilising the electron flow across the ETC.
MYC overexpression is associated with an increased proliferation rate in breast cancer,152 and MYC amplification in luminal A breast cancer is associated with poor survival and resistance to endocrine therapy.153 Although these studies did not evaluate whether ROS are involved in the aggressiveness of MYC-driven breast cancer, it is known that high ROS levels are associated with resistance to endocrine therapy.154 The impact of MYC-driven ROS production on the clinical outcome of cancer needs to be further studied in the future. Unfortunately, MYC is still an undruggable target. However, the dependency of MYC-driven tumours on NADPH production through serine metabolism and one-carbon metabolism implies that inhibition of these pathways could be an effective anticancer strategy for patients affected by these malignancies.
PI3K–AKT–mTOR
Consistent with their role in apoptosis suppression, cell proliferation, metabolism and anabolic reactions, the interconnected PI3K–AKT and mTOR pathways are hyperactivated in almost 40% of all human cancers.155–157
As the PI3K–AKT–mTOR pathway plays a pivotal role in the induction of the Warburg effect158 and in the inhibition of autophagy,159 cancers with hyperactivation of this pathway accumulate dysfunctional ROS-producing mitochondria that are not eliminated by autophagy.
The metabolism of non-essential amino acids is an important feature of metabolic rewiring in cancer cells and in highly proliferating cells. In fact, cancer cells metabolise non-essential amino acids to obtain nucleotides and lipids, preserve redox homoeostasis and control epigenetic regulation.160 Among the 11 non-essential amino acids, glutamine, serine and proline play important roles in tumorigenesis. Proline synthesis mediated by Δ1-pyrroline-5-carboxylate (P5C) reductases (PYCRs) and proline degradation through PRODH constitute a “proline cycle” between the cytosol and mitochondria.88 In EGFR-mutated non-small-cell lung cancer, constitutive downstream activation of the PI3K pathway drives proline synthesis, which fuels EGFR-regulated proline oxidation.161 The activity of PRODH decreases the efficiency of mitochondrial electron transport, driving the production of ROS from the QO site of complex III through Mechanism B. These findings suggest that proline metabolism could play an important role in non-small-cell lung cancer with EGFR mutations.
Although the PI3K–AKT–mTOR pathway is associated with the induction of aerobic glycolysis, Marchi et al.162 provided a mechanism through which AKT could mediate ROS generation through Mechanism A. These authors observed that mitochondria-localised AKT phosphorylates MICU1, a regulatory subunit of the mitochondrial calcium uniporter (MCU), resulting in the destabilisation of the MICU1–MICU2 heterodimer, thus leading to increased calcium influx in mitochondria.162 Decreased expression of MICU1 following its phosphorylation was associated with increased levels of mitochondrial ROS and enhanced in vivo growth of cancer cells. Mitochondrial calcium overload could account for increased ROS generation through mitochondrial dysfunction, which, however, may trigger permeability-transition-pore-mediated cell death.163 Alternatively, increased calcium levels in mitochondria could drive ROS production by stimulating enzymes of the TCA cycle and OXPHOS, thus accelerating oxygen consumption and generation of ROS by the ETC164 through Mechanism A. Moreover, mitochondrial calcium also increases the activity of GPDH, producing ROS by direct leaking of electrons and by inducing RET towards complex II and complex I94 (Mechanism B). The activity of the PI3K–AKT–mTOR pathway is controlled by the tumour suppressor PTEN, which is inactivated in several human cancer types. Observations by Marchi et al.162 highlight the central role of PTEN status in the AKT-mediated effect on MICU1. Interestingly, ROS inactivate PTEN through oxidation, thus favouring activation of AKT that phosphorylates MICU1 leading to calcium uptake in mitochondria, and calcium drives the production of ROS by the ETC, thus establishing a vicious circle sustaining activation of AKT and boosting tumour progression. In line with this scenario, scavenging mitochondrial O2•– through MitoTEMPO blunted the activation of AKT, reverted the Warburg effect and induced death in melanoma cells.165 Moreover, Variar et al.166 observed that the mitochondria-targeted antioxidant Mito-CP enhances apoptotic cell death in a Burkitt’s lymphoma cell line by decreasing AKT activation and HIF-1α stabilisation under hypoxia, suggesting the possibility to block hypoxic adaptation in cancer cells by decreasing ROS generated by the ETC.
In a study of breast cancer cells, Jin et al.167 recently demonstrated that PI3K–AKT-mediated inactivation of glycogen synthase kinase-3β (GSK-3β) through phosphorylation induces an abnormal activity of complexes I and III, thus altering electron flow and enhancing ROS production through Mechanism B. The resulting ROS released in the tumour microenvironment impaired the cytotoxic activity of NK cells by oxidising a serine residue in the initiation factor eIF2B, leading to downregulation of NKG2D and its ligands.167 These results provide evidence for an AKT/ROS-mediated mechanism to inhibit innate immune response in the tumour microenvironment. Interestingly, inactivation of GSK-3β also leads to stabilisation of CMYC,168 which can further enhance generation of ROS by the ETC. It remains to be elucidated whether the inhibition of GSK-3β by AKT requires ROS-mediated PTEN inactivation.
mTOR is a central kinase that integrates energy sensing and anabolic pathways, co-ordinating protein synthesis and cell growth.169 Synthesis of novel cellular components is an energy-consuming process; thus, it is not surprising that mTOR promotes mitochondrial metabolism by indirectly increasing the levels of nuclear-encoded mitochondrial proteins. For instance, mTOR promotes the formation of functional complexes between the transcription factor yin-yang 1 (YY1) and its cofactor peroxisome-proliferator-activated receptor coactivator 1α (PGC-1α), which drives expression of many genes encoding mitochondrial proteins, including cytochrome c,170 resulting in increased mitochondrial respiration and ROS production through Mechanism A. Moreover, mTOR co-operates with oestrogen-related receptor α to promote the transcription of genes involved in OXPHOS and in the TCA cycle.171 Furthermore, through the inactivation of 4E-BP proteins, mTOR upregulates nuclear-encoded subunits of respiratory complex I and ATP synthase, thus increasing mitochondrial respiration.172 Goo et al.173 observed that, in the context of PTEN inactivation, hyperactivated AKT is associated with phosphorylation of 4E-BP1, increased activity of complexes I, III and IV and augmented oxygen consumption. These results suggest that mTOR could enhance mitochondrial respiration, and hence, ROS production through Mechanism A. Hyperactivation of the PI3K–AKT–mTOR pathway could also result in an imbalance between nuclear-encoded and mitochondrial-encoded subunits of the respiratory complexes, as observed for MYC, leading to the production of misassembled complexes, loss of supercomplexes and increased ROS production through Mechanism B.
Given its central role in co-ordinating metabolism, mTOR is at the crossroads between anabolic pathways and mitogenic signalling of cancer cells. mTORC1 phosphorylates S6K1, which activates carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, dihydro-orotase (CAD) through phosphorylation on S1859. CAD is a rate-limiting enzyme as it catalyses the first three steps of de novo pyrimidine synthesis, by generating dihydro-orotate from glutamine,174 thus fuelling DHODH, which produces ROS. Notably, the oxidation of dihydro-orotate to orotate integrates nucleotide synthesis, which is required to sustain the uncontrolled proliferation of cancer cells, with ROS production by the ETC, which drives cancer initiation and progression. Besides mTOR, KRAS, MYC, AKT and other oncogenes also converge on pyrimidine synthesis. Interestingly, Hail et al.175 observed that inhibition of DHODH through terifluonomide decreases mitochondrial ROS levels and has a cytostatic effect in prostatecancer cells. Future studies should be aimed at investigating whether a decreased nucleotide pool or decreased ROS levels may account for the anticancer activity of DHODH inhibitors, as well as the role of DHODH-produced ROS in cancer development.
The hyperactivation of the PI3K–AKT–mTOR pathway is a marker of poor prognosis in several human cancers, such as oesophageal squamous cell carcinoma176 and breast cancer.177 Yu et al.178 recently observed that pterostilbene, an antioxidant compound primarily found in blueberries, slows down the progression of mantle cell lymphoma by targeting the PI3K–AKT–mTOR pathway. However, pterostilbene also directly affects apoptosis and the cell cycle, thus rendering the interpretation of these results more complex; the impact of ROS on cancer in the context of PI3K–AKT–mTOR hyperactivation thus deserves further investigation.
BCR/ABL
The t(9,22) translocation that is pathognomonic of chronic myeloid leukaemia gives rise to the Philadelphia chromosome and to the chimaeric gene BCR/ABL, coding for a constitutively active tyrosine kinase that transduces mitogenic and anti-apoptotic signals to cancer cells.179
The BCR/ABL fusion protein promotes the production of ROS by the ETC partly through the activation of the PI3K–mTOR pathway. Kim et al.180 observed that glucose metabolism is involved in the generation of ROS in BCR/ABL-transformed cells. Treatment with 2-deoxyglucose, the BCR/ABL inhibitor imatinib mesylate or rotenone reduced the production of ROS. The same effect was obtained with wortmannin and rapamycin, which inhibit PI3K and mTORC1, respectively, indicating the tight connections among BCR/ABL, PI3K, mTOR, glucose metabolism and ROS production by the ETC through Mechanism A. The decrease in ROS levels following rotenone treatment suggests the involvement of RET (Mechanism B) in ROS generation by BCR/ABL. Direct action of BCR/ABL on the activity of the ETC has also been observed.181 Nieborowska-Skorska et al.181 observed a sharp decrease in electron flow rates between complexes I and II and between II and III, with an increase in O2•– production through Mechanism B, in BCR/ABL-expressing myeloid precursors. This ROS production was decreased by the mitochondria-targeted antioxidant MitoQ and sustained by complex III, as demonstrated by the rescue effect of the complex III inhibitors myxothiazol, stigmatellin and antimycin A. The small GTPase Rac2 was found to promote ROS generation by complex III, as Rac2 knockout substantially reduced mitochondrial O2•– levels and oxidative stress in BCR/ABL-expressing cells.181 The authors noted that this mechanism underlies ROS production by the ETC in leukaemia cells with a variety of genetic alterations (FLT3–ITD, TEL–ABL1, TEL–JAK2, TEL/PDGFβR, TEL–TRKC, BCR–FGFR1 and mutated JAK2). These observations indicate that different types of leukaemia cells may promote genomic instability and progression through the activation of Rac2, which interferes with electron transport from complexes I to III and II to III, ultimately inducing leakage of electrons from complex III and O2•– generation.
BCL-2
The B-cell lymphoma-2 (BCL-2) family includes several proteins that counteract intrinsic apoptosis by binding pro-apoptotic proteins along the IMM.182 Many tumour types, including breast cancer, prostate cancer, B-cell lymphomas and colorectal adenocarcinomas, display BCL-2 overexpression.182,183 Several lines of evidence suggest that the upregulation of BCL-2, besides directly blocking apoptosis, creates a pro-oxidant state that promotes cell survival. Chen and Pervaiz184,185 showed that the overexpression of BCL-2 in leukaemia cells regulates mitochondrial respiration by affecting ETC activity through direct interaction of BCL-2 with the complex IV subunits Va and Vb, by promoting correct assembly of the complex in the IMM and thus upregulating its activity. Based on these findings, the authors suggested that BCL-2 increases ETC activity and O2•– production, leading to a pro-oxidant milieu that favours cell survival. Interestingly, during hypoxia, in the presence of BCL-2, the relative abundance of the subunit Va is higher than that of subunit Vb, leading to reduced complex IV activity and mitochondrial respiration, which, in this context, maintains the mitochondrial redox state unchanged, thus preventing oxidative stress that would otherwise favour cell death.185 This scenario is supported by the observation that decreasing levels of O2•– trigger apoptosis in BCL-2-overexpressing cancer cells21 and suggests that the balance between O2•– and H2O2 could modulate cancer cell survival through O2•–-mediated inhibition of apoptosis.186 Furthermore, the establishment of a mild pro-oxidant milieu prevents the dephosphorylation of BCL-2 on S70 residue, thus improving BCL-2 binding with BAX and cell survival.187
Concluding remarks
In the 1920s, Otto Warburg postulated defective mitochondrial respiration as the cause of cancer.188 Although the discovery of genetic alterations in oncogenes and tumour-suppressor genes changed our perspective on cancer pathogenesis, many recent studies identified pro-tumorigenic pathways that affect ROS generation by the ETC, thus integrating genetic and bioenergetic alterations of cancer cells in a unified scenario.
The ETC is the principal source of mitochondrial ROS. Activated RAS, MYC, PI3K–AKT–mTOR and BCR/ABL are examples of oncogenic pathways that affect the ETC to enhance ROS production. Oncogenes may control this process through two main mechanisms: increasing TCA cycle fuelling and mitochondrial mass (here defined as Mechanism A); increasing electron leakage from the ETC by either altering its organisation (e.g. disrupting the respirasome) or promoting metabolism through ETC-associated enzymes (defined as Mechanism B).
It is worth noting that different oncogenic pathways co-operate to produce the cancer phenotype. In fact, KRAS, MYC and PI3K–AKT–mTOR constitute an interconnected network that fine-tunes the generation of ROS by the ETC. mTORC2 hyperphosphorylates AKT, thus enhancing ROS production through the above-mentioned mechanisms and MYC protein levels through the inhibition of GSK-3β. MYC, in turn, can blunt the production of ROS triggered by mTORC1, thus curbing ROS overload in cancer cells. In this regard, Hartleben et al.189 observed that MYC-driven upregulation of tuberous sclerosis complex 1 (TSC1) in Burkitt’s lymphoma cells represses the activation of mTORC1, thus limiting the production of ROS by the ETC. In contrast, oncogenic KRAS induces a metabolic shift resulting in enhanced glycolytic flux and lactate production that inhibits the interaction of TSC2 with Rheb, thus leading to mTORC1 activation.190 The observation that oncogenic KRAS promotes MYC protein stability191 further complicates this scenario. This complex network of interactions may act as a rheostat to optimise a ROS set point that exploits the tumour-promoting activity of ROS while negating their anticancer effects.
One of the most intriguing features of cancer cells is their metabolic plasticity. The recently described hybrid metabolic phenotype, in which the Warburg effect and OXPHOS coexist, provides cancer cells with the ability to adapt their metabolism to different microenvironments.192 Mitochondrial ROS play a central role in this process, through the stabilisation of HIF-1α, which promotes glycolysis, and AMPK, which promotes OXPHOS and fatty acid β-oxidation. It is worth noting that the hybrid metabolic phenotype is promoted by MYC and by fatty acid β-oxidation. The importance of β-oxidation suggests a crucial role of the ETC-associated enzyme ETF:QO in tumorigenesis.
Although the mitogenic role of ROS suggests that dietary antioxidants could prove beneficial in cancer prevention,193 experimental and clinical evidence indicates that they favour cancer progression194–196 and impair the efficacy of chemotherapy and radiotherapy.197 Consistent with this notion, non-small-cell lung cancer cells with loss-of-function mutations in the tumour-suppressor liver kinase 1 (LKB1) are more sensitive to oxidative stress.198,199 Indeed, LKB1-mutated patients respond better than wild-type patients to therapies with platinum-based anticancer drugs,200 which are potent inducers of ROS.201 The contrasting effect of untargeted versus mitochondria-targeted antioxidants on tumorigenesis, although not generalisable to all tumour models, further corroborates the concept that tumour progression requires the generation of mitochondrial ROS and the limitation of cytosolic ROS, to maximise mitogenic signalling and avoid oxidative damage. In this regard, the inhibition of endogenous antioxidant systems in order to increase ROS levels above the toxic threshold represents a promising effective strategy to selectively kill cancer cells.202,203
The fact that the described oncogenes are deregulated in a high proportion of extremely aggressive cancers, such as non-small-cell lung cancer, pancreatic ductal adenocarcinoma and triple-negative breast cancer, supports a pivotal role for mitochondrial ROS as determinants of the clinical outcome.
Finally, although well characterised in the context of ischaemia/reperfusion models, the role of RET in cancer is still poorly understood and deserves thorough studies. The fact that two common alterations of cancer cells, i.e. transient intra-tumour hypoxia and IF1 overexpression, are potent inducers of RET provides a strong rationale for a crucial role for RET in the rewiring of redox homoeostasis in cancer.
Acknowledgements
We are grateful to Donna Mia D’Agostino for paper revising and helpful discussion. We thank Alessandro Grinzato for helping with the figure. Molecular graphics of ETC complexes were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Author contributions
V.R. and F.C. wrote the paper and prepared the figure, V.C. prepared the paragraph on RET and revised the final version of the paper.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Funding information
V.C. is supported by the Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)—IG 17794.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Vittoria Raimondi, Francesco Ciccarese
References
- 1.Maryanovich M, Gross AA. ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Yalcin S, Marinkovic D, Mungamuri SK, Zhang X, Tong W, Sellers R, et al. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(-/-) mice. Embo J. 2010;29:4118–4131. doi: 10.1038/emboj.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott TL, Rangaswamy S, Wicker CA, Izumi T. Repair of oxidative DNA damage and cancer: recent progress in DNA base excision repair. Antioxid. Redox Signal. 2014;20:708–726. doi: 10.1089/ars.2013.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, et al. NADPH oxidases: a perspective on reactive oxygen species production in tumor biology. Antioxid. Redox Signal. 2014;20:2873–2889. doi: 10.1089/ars.2013.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 8.Lennicke C, Rahn J, Lichtenfels R, Wessjohann LA, Seliger B. Hydrogen peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015;13:39. doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaudet, L., Vassalli, G. & Pacher, P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front. Biosci. 14: 4809–4814 (2009). [DOI] [PMC free article] [PubMed]
- 10.Kruk J, Aboul-Enein HY. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med Chem. 2017;17:904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 11.Moldogazieva NT, Lutsenko SV, Terentiev AA. Reactive oxygen and nitrogen species-induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78:6040–6047. doi: 10.1158/0008-5472.CAN-18-0980. [DOI] [PubMed] [Google Scholar]
- 12.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid. Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavallari I, Scattolin G, Silic-Benussi M, Raimondi V, D’Agostino DM, Ciminale V. Mitochondrial proteins coded by human tumor viruses. Front. Microbiol. 2018;9:81. doi: 10.3389/fmicb.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortezaee K, Salehi E, Mirtavoos-Mahyari H, Motevaseli E, Najafi M, Farhood B, et al. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell Physiol. 2019;234:12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 16.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cremers CM, Jakob U. Oxidant Sensing by Reversible Disulfide Bond Formation*. J. Biol. Chem. 2013;288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J. Biol. Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 19.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 20.Clement MV, Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. Embo J. 1996;15:216–225. [PMC free article] [PubMed] [Google Scholar]
- 21.Clement MV, Hirpara JL, Pervaiz S. Decrease in intracellular superoxide sensitizes Bcl-2-overexpressing tumor cells to receptor and drug-induced apoptosis independent of the mitochondria. Cell Death Differ. 2003;10:1273–1285. doi: 10.1038/sj.cdd.4401302. [DOI] [PubMed] [Google Scholar]
- 22.Kenny TC, Craig AJ, Villanueva A, Germain D. MitohormEsis Primes Tumor Invasion And Metastasis. Cell Rep. 2019;27:2292–2303.e2296. doi: 10.1016/j.celrep.2019.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Brand MD. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sazanov LA. The mechanism of coupling between electron transfer and proton translocation in respiratory complex I. J. Bioenerg. Biomembr. 2014;46:247–253. doi: 10.1007/s10863-014-9554-z. [DOI] [PubMed] [Google Scholar]
- 27.Azadmanesh Jahaun, Borgstahl Gloria. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants. 2018;7(2):25. doi: 10.3390/antiox7020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giachin G, Bouverot R, Acajjaoui S, Pantalone S, Soler-Lopez M. Dynamics of human mitochondrial complex I assembly: implications for neurodegenerative diseases. Front Mol. Biosci. 2016;3:43. doi: 10.3389/fmolb.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan AS, Baty JW, Berridge MV. The role of mitochondrial electron transport in tumorigenesis and metastasis. Biochim Biophys. Acta. 2014;1840:1454–1463. doi: 10.1016/j.bbagen.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Marco-Brualla Joaquín, Al-Wasaby Sameer, Soler Ruth, Romanos Eduardo, Conde Blanca, Justo-Méndez Raquel, Enríquez José A., Fernández-Silva Patricio, Martínez-Lostao Luis, Villalba Martín, Moreno-Loshuertos Raquel, Anel Alberto. Mutations in the ND2 Subunit of Mitochondrial Complex I Are Sufficient to Confer Increased Tumorigenic and Metastatic Potential to Cancer Cells. Cancers. 2019;11(7):1027. doi: 10.3390/cancers11071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 32.Qiao C, Zhou C, Zhang S, Guo R, Zhang F, Qian S, et al. [Analysis of ND4 gene mutations in acute myelogenous leukemia] Zhonghua Xue Ye Xue Za Zhi. 2014;35:708–712. doi: 10.3760/cma.j.issn.0253-2727.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Yeung KY, Dickinson A, Donoghue JF, Polekhina G, White SJ, Grammatopoulos DK, et al. The identification of mitochondrial DNA variants in glioblastoma multiforme. Acta Neuropathol. Commun. 2014;2:1. doi: 10.1186/2051-5960-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010;49:141–151. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- 38.Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev. Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 39.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975;59:137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- 41.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 42.Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, et al. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys. Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- 43.Quinlan CL, Gerencser AA, Treberg JR, Brand MD. The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J. Biol. Chem. 2011;286:31361–31372. doi: 10.1074/jbc.M111.267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erecinska M, Wilson DF. The effect of antimycin A on cytochromes b561, b566, and their relationship to ubiquinone and the iron-sulfer centers S-1 (+N-2) and S-3. Arch. Biochem Biophys. 1976;174:143–157. doi: 10.1016/0003-9861(76)90333-7. [DOI] [PubMed] [Google Scholar]
- 45.Han Y, Wu P, Wang Z, Zhang Z, Sun S, Liu J, et al. Ubiquinol-cytochrome C reductase core protein II promotes tumorigenesis by facilitating p53 degradation. EBioMedicine. 2019;40:92–105. doi: 10.1016/j.ebiom.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang Y, Zhang F, Li D, Li C, Li H, Jiang Y, et al. Overexpression of UQCRC2 is correlated with tumor progression and poor prognosis in colorectal cancer. Pathol. Res Pr. 2018;214:1613–1620. doi: 10.1016/j.prp.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Gao Feng, Liu Qicai, Li Guoping, Dong Feng, Qiu Minglian, Lv Xiaoting, Zhang Sheng, Guo Zheng. Identification of ubiquinol cytochrome c reductase hinge (UQCRH) as a potential diagnostic biomarker for lung adenocarcinoma. Open Biology. 2016;6(6):150256. doi: 10.1098/rsob.150256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park ER, Kim SB, Lee JS, Kim YH, Lee DH, Cho EH, et al. The mitochondrial hinge protein, UQCRH, is a novel prognostic factor for hepatocellular carcinoma. Cancer Med. 2017;6:749–760. doi: 10.1002/cam4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncalves RL, Rothschild DE, Quinlan CL, Scott GK, Benz CC, Brand MD. Sources of superoxide/H2O2 during mitochondrial proline oxidation. Redox Biol. 2014;2:901–909. doi: 10.1016/j.redox.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 51.Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion. 2001;1:3–31. doi: 10.1016/s1567-7249(00)00002-7. [DOI] [PubMed] [Google Scholar]
- 52.Kluckova K, Bezawork-Geleta A, Rohlena J, Dong L, Neuzil J. Mitochondrial complex II, a novel target for anti-cancer agents. Biochim. Biophys. Acta. 2013;1827:552–564. doi: 10.1016/j.bbabio.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13:199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 54.Lancaster, C. R. Succinate:quinone oxidoreductases: an overview. Biochim. Biophys. Acta.1553, 1–6 (2002). [DOI] [PubMed]
- 55.Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J. Mitochondrial complex II: at the crossroads. Trends Biochem Sci. 2017;42:312–325. doi: 10.1016/j.tibs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoekstra AS, Bayley JP. The role of complex II in disease. Biochim. Biophys. Acta. 2013;1827:543–551. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Cecchini G. Respiratory complex II: role in cellular physiology and disease. Biochim. Biophys. Acta. 2013;1827:541–542. doi: 10.1016/j.bbabio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Tseng PL, Wu WH, Hu TH, Chen CW, Cheng HC, Li CF, et al. Decreased succinate dehydrogenase B in human hepatocellular carcinoma accelerates tumor malignancy by inducing the Warburg effect. Sci. Rep. 2018;8:3081. doi: 10.1038/s41598-018-21361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morganti C, Bonora M, Ito K. Electron transport chain complex II sustains high mitochondrial membrane potential in hematopoietic stem and progenitor cells. Stem Cell Res. 2019;40:101573. doi: 10.1016/j.scr.2019.101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 62.Alnajjar KS, Hosler J, Prochaska L. Role of the N-terminus of subunit III in proton uptake in cytochrome c oxidase of Rhodobacter sphaeroides. Biochemistry. 2014;53:496–504. doi: 10.1021/bi401535q. [DOI] [PubMed] [Google Scholar]
- 63.Wu S, Akhtari M, Alachkar H. Characterization of mutations in the mitochondrial encoded electron transport chain complexes in acute myeloid leukemia. Sci. Rep. 2018;8:13301. doi: 10.1038/s41598-018-31489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalimonchuk O, Bird A, Winge DR. Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J. Biol. Chem. 2007;282:17442–17449. doi: 10.1074/jbc.M702379200. [DOI] [PubMed] [Google Scholar]
- 65.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. Embo J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Schonfeld P, Wieckowski MR, Lebiedzinska M, Wojtczak L. Mitochondrial fatty acid oxidation and oxidative stress: lack of reverse electron transfer-associated production of reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:929–938. doi: 10.1016/j.bbabio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Schagger H, Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 2001;276:37861–37867. doi: 10.1074/jbc.M106474200. [DOI] [PubMed] [Google Scholar]
- 69.Schafer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, Vonck J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006;281:15370–15375. doi: 10.1074/jbc.M513525200. [DOI] [PubMed] [Google Scholar]
- 70.Guerrero-Castillo S, Baertling F, Kownatzki D, Wessels HJ, Arnold S, Brandt U, et al. The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab. 2017;25:128–139. doi: 10.1016/j.cmet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, et al. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruno C, Santorelli FM, Assereto S, Tonoli E, Tessa A, Traverso M, et al. Progressive exercise intolerance associated with a new muscle-restricted nonsense mutation (G142X) in the mitochondrial cytochrome b gene. Muscle Nerve. 2003;28:508–511. doi: 10.1002/mus.10429. [DOI] [PubMed] [Google Scholar]
- 73.D’Aurelio M, Gajewski CD, Lenaz G, Manfredi G. Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Hum. Mol. Genet. 2006;15:2157–2169. doi: 10.1093/hmg/ddl141. [DOI] [PubMed] [Google Scholar]
- 74.Vempati UD, Han X, Moraes CT. Lack of cytochrome c in mouse fibroblasts disrupts assembly/stability of respiratory complexes I and IV. J. Biol. Chem. 2009;284:4383–4391. doi: 10.1074/jbc.M805972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz F, Fukui H, Garcia S, Moraes CT. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol. Cell Biol. 2006;26:4872–4881. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. Embo J. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cogliati S, Lorenzi I, Rigoni G, Caicci F, Soriano ME. Regulation of mitochondrial electron transport chain assembly. J. Mol. Biol. 2018;430:4849–4873. doi: 10.1016/j.jmb.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Ramirez-Aguilar SJ, Keuthe M, Rocha M, Fedyaev VV, Kramp K, Gupta KJ, et al. The composition of plant mitochondrial supercomplexes changes with oxygen availability. J. Biol. Chem. 2011;286:43045–43053. doi: 10.1074/jbc.M111.252544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bohovych I, Fernandez MR, Rahn JJ, Stackley KD, Bestman JE, Anandhan A, et al. Metalloprotease OMA1 fine-tunes mitochondrial bioenergetic function and respiratory supercomplex stability. Sci. Rep. 2015;5:13989. doi: 10.1038/srep13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whelan SP, Zuckerbraun BS. Mitochondrial signaling: forwards, backwards, and in between. Oxid. Med. Cell Longev. 2013;2013:351613. doi: 10.1155/2013/351613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lenaz G, Tioli G, Falasca AI, Genova ML. Complex I function in mitochondrial supercomplexes. Biochim Biophys. Acta. 2016;1857:991–1000. doi: 10.1016/j.bbabio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 82.Sun Dayan, Li Bin, Qiu Ruyi, Fang Hezhi, Lyu Jianxin. Cell Type-Specific Modulation of Respiratory Chain Supercomplex Organization. International Journal of Molecular Sciences. 2016;17(6):926. doi: 10.3390/ijms17060926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curtarello M, Zulato E, Nardo G, Valtorta S, Guzzo G, Rossi E, et al. VEGF-targeted therapy stably modulates the glycolytic phenotype of tumor cells. Cancer Res. 2015;75:120–133. doi: 10.1158/0008-5472.CAN-13-2037. [DOI] [PubMed] [Google Scholar]
- 84.Ding Y, Liu Z, Desai S, Zhao Y, Liu H, Pannell LK, et al. Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat. Commun. 2012;3:1271. doi: 10.1038/ncomms2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rohlenova K, Sachaphibulkij K, Stursa J, Bezawork-Geleta A, Blecha J, Endaya B, et al. Selective disruption of respiratory supercomplexes as a new strategy to suppress Her2high breast cancer. Antioxid. Redox Signal. 2017;26:84–103. doi: 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milenkovic D, Blaza JN, Larsson NG, Hirst J. The enigma of the respiratory chain supercomplex. Cell Metab. 2017;25:765–776. doi: 10.1016/j.cmet.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 87.Hancock CN, Liu W, Alvord WG, Phang JM. Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids. 2016;48:859–872. doi: 10.1007/s00726-015-2134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Phang JM. Proline dehydrogenase (oxidase) in cancer. Biofactors. 2012;38:398–406. doi: 10.1002/biof.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Borchert GL, Donald SP, Diwan BA, Anver M, Phang JM. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res. 2009;69:6414–6422. doi: 10.1158/0008-5472.CAN-09-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagano T, Nakashima A, Onishi K, Kawai K, Awai Y, Kinugasa M, et al. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. J. Cell Sci. 2017;130:1413–1420. doi: 10.1242/jcs.196469. [DOI] [PubMed] [Google Scholar]
- 91.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl Acad. Sci. USA. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu W, Glunde K, Bhujwalla ZM, Raman V, Sharma A, Phang JM. Proline oxidase promotes tumor cell survival in hypoxic tumor microenvironments. Cancer Res. 2012;72:3677–3686. doi: 10.1158/0008-5472.CAN-12-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 2017;8:15267. doi: 10.1038/ncomms15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mracek T, Drahota Z, Houstek J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys. Acta. 2013;1827:401–410. doi: 10.1016/j.bbabio.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 95.Singh G. Mitochondrial FAD-linked glycerol-3-phosphate dehydrogenase: a target for cancer therapeutics. Pharmaceuticals (Basel) 2014;7:192–206. doi: 10.3390/ph7020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Disco. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 97.Hey-Mogensen M, Goncalves RL, Orr AL, Brand MD. Production of superoxide/H2O2 by dihydroorotate dehydrogenase in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2014;72:149–155. doi: 10.1016/j.freeradbiomed.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Madak JT, Bankhead A, 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharm. Ther. 2019;195:111–131. doi: 10.1016/j.pharmthera.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Liu YC, Li F, Handler J, Huang CR, Xiang Y, Neretti N, et al. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS ONE. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sykes DB. The emergence of dihydroorotate dehydrogenase (DHODH) as a therapeutic target in acute myeloid leukemia. Expert Opin. Ther. Targets. 2018;22:893–898. doi: 10.1080/14728222.2018.1536748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watmough NJ, Frerman FE. The electron transfer flavoprotein: ubiquinone oxidoreductases. Biochim Biophys. Acta. 2010;1797:1910–1916. doi: 10.1016/j.bbabio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Metallo Christian M., Gameiro Paulo A., Bell Eric L., Mattaini Katherine R., Yang Juanjuan, Hiller Karsten, Jewell Christopher M., Johnson Zachary R., Irvine Darrell J., Guarente Leonard, Kelleher Joanne K., Vander Heiden Matthew G., Iliopoulos Othon, Stephanopoulos Gregory. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dheeraj A, Agarwal C, Schlaepfer IR, Raben D, Singh R, Agarwal R, et al. A novel approach to target hypoxic cancer cells via combining beta-oxidation inhibitor etomoxir with radiation. Hypoxia (Auckl.) 2018;6:23–33. doi: 10.2147/HP.S163115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuetz AN, Yin-Goen Q, Amin MB, Moreno CS, Cohen C, Hornsby CD, et al. Molecular classification of renal tumors by gene expression profiling. J. Mol. Diagn. 2005;7:206–218. doi: 10.1016/S1525-1578(10)60547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scialo F, Fernandez-Ayala DJ, Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Esparza-Molto PB, Nuevo-Tapioles C, Cuezva JM. Regulation of the H(+)-ATP synthase by IF1: a role in mitohormesis. Cell Mol. Life Sci. 2017;74:2151–2166. doi: 10.1007/s00018-017-2462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia-Bermudez J, Cuezva JM. The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival. Biochim. Biophys. Acta. 2016;1857:1167–1182. doi: 10.1016/j.bbabio.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Cabezon E, Butler PJ, Runswick MJ, Walker JE. Modulation of the oligomerization state of the bovine F1-ATPase inhibitor protein, IF1, by pH. J. Biol. Chem. 2000;275:25460–25464. doi: 10.1074/jbc.M003859200. [DOI] [PubMed] [Google Scholar]
- 110.Campanella M, Parker N, Tan CH, Hall AM, Duchen MR. IF(1): setting the pace of the F(1)F(o)-ATP synthase. Trends Biochem Sci. 2009;34:343–350. doi: 10.1016/j.tibs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Brand MD, Goncalves RL, Orr AL, Vargas L, Gerencser AA, Borch Jensen M, et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 2016;24:582–592. doi: 10.1016/j.cmet.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hardee ME, Dewhirst MW, Agarwal N, Sorg BS. Novel imaging provides new insights into mechanisms of oxygen transport in tumors. Curr. Mol. Med. 2009;9:435–441. doi: 10.2174/156652409788167122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jang S, Javadov S. Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch. Biochem. Biophys. 2017;630:1–8. doi: 10.1016/j.abb.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez-Arago M, Formentini L, Martinez-Reyes I, Garcia-Bermudez J, Santacatterina F, Sanchez-Cenizo L, et al. Expression, regulation and clinical relevance of the ATPase inhibitory factor 1 in human cancers. Oncogenesis. 2013;2:e46. doi: 10.1038/oncsis.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Formentini L, Sanchez-Arago M, Sanchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol. Cell. 2012;45:731–742. doi: 10.1016/j.molcel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 116.Formentini L, Martinez-Reyes I, Cuezva JM. The mitochondrial bioenergetic capacity of carcinomas. IUBMB Life. 2010;62:554–560. doi: 10.1002/iub.352. [DOI] [PubMed] [Google Scholar]
- 117.Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scialo F, Sriram A, Fernandez-Ayala D, Gubina N, Lohmus M, Nelson G, et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Esparza-Molto PB, Nuevo-Tapioles C, Chamorro M, Najera L, Torresano L, Santacatterina F, et al. Tissue-specific expression and post-transcriptional regulation of the ATPase inhibitory factor 1 (IF1) in human and mouse tissues. Faseb j. 2019;33:1836–1851. doi: 10.1096/fj.201800756R. [DOI] [PubMed] [Google Scholar]
- 120.Garcia-Bermudez J, Sanchez-Arago M, Soldevilla B, Del Arco A, Nuevo-Tapioles C, Cuezva JM. PKA phosphorylates the ATPase inhibitory factor 1 and inactivates its capacity to bind and inhibit the mitochondrial H(+)-ATP synthase. Cell Rep. 2015;12:2143–2155. doi: 10.1016/j.celrep.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 121.Song Ruipeng, Song Huiwen, Liang Yingjian, Yin Dalong, Zhang Heng, Zheng Tongsen, Wang Jiabei, Lu Zhaoyang, Song Xuan, Pei Tiemin, Qin Youyou, Li Yuejin, Xie Changming, Sun Boshi, Shi Huawen, Li Shuai, Meng Xianzhi, Yang Guangchao, Pan Shangha, Zhu Jiyuan, Qi Shuyi, Jiang Hongchi, Zhang Zhiyong, Liu Lianxin. Reciprocal activation between ATPase inhibitory factor 1 and NF-κB drives hepatocellular carcinoma angiogenesis and metastasis. Hepatology. 2014;60(5):1659–1673. doi: 10.1002/hep.27312. [DOI] [PubMed] [Google Scholar]
- 122.Yin T, Lu L, Xiong Z, Wei S, Cui D. ATPase inhibitory factor 1 is a prognostic marker and contributes to proliferation and invasion of human gastric cancer cells. Biomed. Pharmacother. 2015;70:90–96. doi: 10.1016/j.biopha.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 123.Wu J, Shan Q, Li P, Wu Y, Xie J, Wang X. ATPase inhibitory factor 1 is a potential prognostic marker for the migration and invasion of glioma. Oncol. Lett. 2015;10:2075–2080. doi: 10.3892/ol.2015.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liou GY, Doppler H, DelGiorno KE, Zhang L, Leitges M, Crawford HC, et al. Mutant KRas-Induced mitochondrial oxidative stress in acinar cells upregulates EGFR Signaling to Drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boyle KA, Van Wickle J, Hill RB, Marchese A, Kalyanaraman B, Dwinell MB. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J. Biol. Chem. 2018;293:14891–14904. doi: 10.1074/jbc.RA117.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y, et al. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012;22:399–412. doi: 10.1038/cr.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G. Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim. Biophys. Acta. 2010;1797:314–323. doi: 10.1016/j.bbabio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 133.Lenaz G, Genova ML. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int J. Biochem Cell Biol. 2009;41:1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 134.Chun SY, Johnson C, Washburn JG, Cruz-Correa MR, Dang DT, Dang LH. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1alpha and HIF-2alpha target genes. Mol. Cancer. 2010;9:293. doi: 10.1186/1476-4598-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl Acad. Sci. USA. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iskandar K, Rezlan M, Yadav SK, Foo CH, Sethi G, Qiang Y, et al. Synthetic lethality of a novel small molecule against mutant KRAS-expressing cancer cells involves AKT-dependent ROS production. Antioxid. Redox Signal. 2016;24:781–794. doi: 10.1089/ars.2015.6362. [DOI] [PubMed] [Google Scholar]
- 137.Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim. Biophys. Acta. 2002;1602:61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 138.Oskarsson T, Trumpp A. The Myc trilogy: lord of RNA polymerases. Nat. Cell Biol. 2005;7:215–217. doi: 10.1038/ncb0305-215. [DOI] [PubMed] [Google Scholar]
- 139.Kalkat Manpreet, De Melo Jason, Hickman Katherine, Lourenco Corey, Redel Cornelia, Resetca Diana, Tamachi Aaliya, Tu William, Penn Linda. MYC Deregulation in Primary Human Cancers. Genes. 2017;8(6):151. doi: 10.3390/genes8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol. Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 144.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.CS K, Carcamo JM, Golde DW. Antioxidants prevent oxidative DNA damage and cellular transformation elicited by the over-expression of c-MYC. Mutat. Res. 2006;593:64–79. doi: 10.1016/j.mrfmmm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 146.Anso E, Mullen AR, Felsher DW, Matés JM, DeBerardinis RJ, Chandel NS. Metabolic changes in cancer cells upon suppression of MYC. Cancer Metab. 2013;1:7. doi: 10.1186/2049-3002-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017;26:633–647.e637. doi: 10.1016/j.cmet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wonsey DR, Zeller KI, Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc. Natl Acad. Sci. USA. 2002;99:6649–6654. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Samanta D, Semenza GL. Serine synthesis helps hypoxic cancer stem cells regulate redox. Cancer Res. 2016;76:6458–6462. doi: 10.1158/0008-5472.CAN-16-1730. [DOI] [PubMed] [Google Scholar]
- 150.Morrish F, Giedt C, Hockenbery D. c-MYC apoptotic function is mediated by NRF-1 target genes. Genes Dev. 2003;17:240–255. doi: 10.1101/gad.1032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herrmann PC, Gillespie JW, Charboneau L, Bichsel VE, Paweletz CP, Calvert VS, et al. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics. 2003;3:1801–1810. doi: 10.1002/pmic.200300461. [DOI] [PubMed] [Google Scholar]
- 152.Dueck AC, Reinholz MM, Geiger XJ, Tenner K, Ballman K, Jenkins RB, et al. Impact of c-MYC protein expression on outcome of patients with early-stage HER2+ breast cancer treated with adjuvant trastuzumab NCCTG (alliance) N9831. Clin. Cancer Res. 2013;19:5798–5807. doi: 10.1158/1078-0432.CCR-13-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Green AR, Aleskandarany MA, Agarwal D, Elsheikh S, Nolan CC, Diez-Rodriguez M, et al. MYC functions are specific in biological subtypes of breast cancer and confers resistance to endocrine therapy in luminal tumours. Br. J. Cancer. 2016;114:917–928. doi: 10.1038/bjc.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Penney RB, Roy D. Thioredoxin-mediated redox regulation of resistance to endocrine therapy in breast cancer. Biochim Biophys. Acta. 2013;1836:60–79. doi: 10.1016/j.bbcan.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 155.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 156.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 157.Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2016;2:1565–1573. doi: 10.1001/jamaoncol.2016.0891. [DOI] [PubMed] [Google Scholar]
- 158.Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol. Biol. Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 159.Li X, Hu X, Wang J, Xu W, Yi C, Ma R, et al. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int J. Mol. Med. 2018;42:1917–1924. doi: 10.3892/ijmm.2018.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Choi Bo-Hyun, Coloff Jonathan L. The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers. 2019;11(5):675. doi: 10.3390/cancers11050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Angulo B, Suarez-Gauthier A, Lopez-Rios F, Medina PP, Conde E, Tang M, et al. Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J. Pathol. 2008;214:347–356. doi: 10.1002/path.2267. [DOI] [PubMed] [Google Scholar]
- 162.Marchi, S., Corricelli, M., Branchini, A., Vitto, V. A. M., Missiroli, S., Morciano, G. et al. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca(2+) levels and tumor growth. Embo J. 10.15252/embj.201899435 (2019) [DOI] [PMC free article] [PubMed]
- 163.Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Roy SS, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 165.Nazarewicz RR, Dikalova A, Bikineyeva A, Ivanov S, Kirilyuk IA, Grigor’ev IA, et al. Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid. Redox Signal. 2013;19:344–349. doi: 10.1089/ars.2013.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Variar G, Pant T, Singh A, Ravichandran A, Swami S, Kalyanaraman B, et al. Effect of mitochondrially targeted carboxy proxyl nitroxide on Akt-mediated survival in Daudi cells: Significance of a dual mode of action. PLoS ONE. 2017;12:e0174546. doi: 10.1371/journal.pone.0174546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jin F, Wu Z, Hu X, Zhang J, Gao Z, Han X, et al. The PI3K/Akt/GSK-3beta/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility. Cancer Biol. Med. 2019;16:38–54. doi: 10.20892/j.issn.2095-3941.2018.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J. Biol. Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 169.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 171.Chaveroux C, Eichner LJ, Dufour CR, Shatnawi A, Khoutorsky A, Bourque G, et al. Molecular and genetic crosstalks between mTOR and ERRalpha are key determinants of rapamycin-induced nonalcoholic fatty liver. Cell Metab. 2013;17:586–598. doi: 10.1016/j.cmet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 172.Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 173.Goo, C. K., Lim, H. Y., Ho, Q. S., Too, H. P., Clement, M. V. & Wong, K. P. PTEN/Akt signaling controls mitochondrial respiratory capacity through 4E-BP1. PLoS ONE7, e45806 (2012). [DOI] [PMC free article] [PubMed]
- 174.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hail N, Jr., Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia. 2010;12:464–475. doi: 10.1593/neo.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu, N., Du, Z., Zhu, Y., Song, Y., Pang, L. & Chen, Z. The expression and prognostic impact of the PI3K/AKT/mTOR signaling pathway in advanced esophageal squamous cell carcinoma. Technol. Cancer. Res. Treat. 17, 1533033818758772 (2018). [DOI] [PMC free article] [PubMed]
- 177.Sobhani N, Roviello G, Corona S, Scaltriti M, Ianza A, Bortul M, et al. The prognostic value of PI3K mutational status in breast cancer: a meta-analysis. J. Cell Biochem. 2018;119:4287–4292. doi: 10.1002/jcb.26687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu D, Zhang Y, Chen G, Xie Y, Xu Z, Chang S, et al. Targeting the PI3K/Akt/mTOR signaling pathway by pterostilbene attenuates mantle cell lymphoma progression. Acta Biochim Biophys Sin (Shanghai) 2018;50:782–792. doi: 10.1093/abbs/gmy070. [DOI] [PubMed] [Google Scholar]
- 179.Salesse S, Verfaillie CM. BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia. Oncogene. 2002;21:8547–8559. doi: 10.1038/sj.onc.1206082. [DOI] [PubMed] [Google Scholar]
- 180.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 181.Nieborowska-Skorska M, Kopinski PK, Ray R, Hoser G, Ngaba D, Flis S, et al. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood. 2012;119:4253–4263. doi: 10.1182/blood-2011-10-385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Campbell Kirsteen J., Tait Stephen W. G. Targeting BCL-2 regulated apoptosis in cancer. Open Biology. 2018;8(5):180002. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suvarna V, Singh V, Murahari M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur. J. Pharm. 2019;862:172655. doi: 10.1016/j.ejphar.2019.172655. [DOI] [PubMed] [Google Scholar]
- 184.Chen ZX, Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007;14:1617–1627. doi: 10.1038/sj.cdd.4402165. [DOI] [PubMed] [Google Scholar]
- 185.Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408–420. doi: 10.1038/cdd.2009.132. [DOI] [PubMed] [Google Scholar]
- 186.Pervaiz S, Clement MV. Superoxide anion: oncogenic reactive oxygen species? Int J. Biochem Cell Biol. 2007;39:1297–1304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 187.Low IC, Loh T, Huang Y, Virshup DM, Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood. 2014;124:2223–2234. doi: 10.1182/blood-2014-03-563296. [DOI] [PubMed] [Google Scholar]
- 188.Brand RA. Biographical sketch: Otto Heinrich Warburg, PhD, MD. Clin. Orthop. Relat. Res. 2010;468:2831–2832. doi: 10.1007/s11999-010-1533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hartleben, G., Muller, C., Kramer, A., Schimmel, H., Zidek, L. M., Dornblut, C. et al. Tuberous sclerosis complex is required for tumor maintenance in MYC-driven Burkitt’s lymphoma. Embo J. 10.15252/embj.201798589 (2018) [DOI] [PMC free article] [PubMed]
- 190.Byun HO, Jung HJ, Seo YH, Lee YK, Hwang SC, Hwang ES, et al. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor (TGF) beta1-induced senescence. Exp. Cell Res. 2012;318:1808–1819. doi: 10.1016/j.yexcr.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 191.Vaseva AV, Blake DR, Gilbert TSK, Ng S, Hostetter G, Azam SH, et al. KRAS Suppression-Induced Degradation of MYC Is Antagonized by a MEK5-ERK5 Compensatory Mechanism. Cancer Cell. 2018;34:807–822.e807. doi: 10.1016/j.ccell.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc. Natl. Acad. Sci. USA. 2019;116:3909–3918. doi: 10.1073/pnas.1816391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ma H, Das T, Pereira S, Yang Z, Zhao M, Mukerji P, et al. Efficacy of dietary antioxidants combined with a chemotherapeutic agent on human colon cancer progression in a fluorescent orthotopic mouse model. Anticancer Res. 2009;29:2421–2426. [PubMed] [Google Scholar]
- 194.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 195.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 196.Sotgia F, Martinez-Outschoorn UE, Lisanti MP. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011;9:62. doi: 10.1186/1741-7015-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jung AY, Cai X, Thoene K, Obi N, Jaskulski S, Behrens S, et al. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am. J. Clin. Nutr. 2019;109:69–78. doi: 10.1093/ajcn/nqy223. [DOI] [PubMed] [Google Scholar]
- 198.Zulato E, Ciccarese F, Agnusdei V, Pinazza M, Nardo G, Iorio E, et al. LKB1 loss is associated with glutathione deficiency under oxidative stress and sensitivity of cancer cells to cytotoxic drugs and gamma-irradiation. Biochem Pharm. 2018;156:479–490. doi: 10.1016/j.bcp.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 199.Zulato E, Ciccarese F, Nardo G, Pinazza M, Agnusdei V, Silic-Benussi M, et al. Involvement of NADPH Oxidase 1 in Liver Kinase B1-Mediated Effects on Tumor Angiogenesis and Growth. Front Oncol. 2018;8:195. doi: 10.3389/fonc.2018.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bonanno L, De Paoli A, Zulato E, Esposito G, Calabrese F, Favaretto A, et al. LKB1 expression correlates with increased survival in patients with advanced non-small cell lung cancer treated with chemotherapy and bevacizumab. Clin. Cancer Res. 2017;23:3316–3324. doi: 10.1158/1078-0432.CCR-16-2410. [DOI] [PubMed] [Google Scholar]
- 201.Casares C, Ramirez-Camacho R, Trinidad A, Roldan A, Jorge E, Garcia-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur. Arch. Otorhinolaryngol. 2012;269:2455–2459. doi: 10.1007/s00405-012-2029-0. [DOI] [PubMed] [Google Scholar]
- 202.Ciccarese F, Ciminale V. Escaping death: mitochondrial redox homeostasis in cancer cells. Front Oncol. 2017;7:117. doi: 10.3389/fonc.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Silic-Benussi M, Scattolin G, Cavallari I, Minuzzo S, Del Bianco P, Francescato S, et al. Selective killing of human T-ALL cells: an integrated approach targeting redox homeostasis and the OMA1/OPA1 axis. Cell Death Dis. 2018;9:822. doi: 10.1038/s41419-018-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guo R, Zong S, Wu M, Gu J, Yang M. Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell. 2017;170:1247–1257.e1212. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 205.Zong S, Wu M, Gu J, Liu T, Guo R, Yang M. Structure of the intact 14-subunit human cytochrome c oxidase. Cell Res. 2018;28:1026–1034. doi: 10.1038/s41422-018-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. Elife. 2015;4:e10180. doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.