Abstract
Objectives
Anti-citrullinated protein antibodies (ACPAs) are a group of autoantibodies targeted against citrullinated proteins/peptides and are informative rheumatoid arthritis (RA) biomarkers. ACPAs also play a crucial role in RA pathogenesis, and their underlying mechanism merits investigation.
Methods
Immunohistochemical (IHC) assays were carried out to determine IL-1β levels in ACPA+ and ACPA− RA patients. PBMC-derived monocytes were differentiated into macrophages before stimulation with ACPAs purified from RA patients. The localization and interaction of molecules were analyzed by confocal microscopy, co-IP, and surface plasmon resonance.
Results
In our study, we found that IL-1β levels were elevated in ACPA+ RA patients and that ACPAs promoted IL-1β production by PBMC-derived macrophages. ACPAs interacted with CD147 to enhance the interaction between CD147 and integrin β1 and, in turn, activate the Akt/NF-κB signaling pathway. The nuclear localization of p65 promoted the expression of NLRP3 and pro-IL-1β, resulting in priming. Moreover, ACPA stimulation activated pannexin channels, leading to ATP release. The accumulated ATP bound to the P2X7 receptor, leading to NLRP3 inflammasome activation.
Conclusions
Our study suggests a new hypothesis regarding IL-1β production in RA involving ACPAs, which may be a potential therapeutic target in RA treatment.
Keywords: Anti-citrullinated protein antibodies, NLRP3 inflammasome, IL-1β, CD147, Rheumatoid arthritis
Subject terms: Autoimmunity, Innate immunity
Introduction
Rheumatoid arthritis (RA) is one of the most prevalent chronic autoimmune diseases, affecting 0.5–1% of the world’s population.1 RA is characterized by joint pain, stiffness, and swelling as a result of ongoing inflammation of the joint synovia.2 A group of RA-specific autoantibodies identified in the late 1990s has demonstrated exceptional diagnostic specificity and was added to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria.3 These autoantibodies are called anti-citrullinated protein antibodies (ACPAs). ACPAs target proteins/peptides that have undergone a posttranslational modification in which arginine residues have been converted to citrulline by peptidylarginine deiminase. The utilization of ACPAs enables clinicians to identify the onset of RA before its explicit clinical manifestation, which is crucial to the timely control of autoimmune status and disease progression.4 ACPAs also have prognostic value, exhibiting strong associations with radiographic progression and disease activity.5,6
Beyond their obvious clinical utility, recent investigations of the pathogenic role of ACPAs have helped reveal the mechanism by which ACPAs exacerbate inflammation in chronic RA. The main target cells of ACPAs are macrophages. Clavel et al. and Lu et al. reported that ACPAs could stimulate macrophages to produce proinflammatory cytokines such as tumor necrosis factor-α7,8 and that this production could be augmented by immunoglobulin A (IgA) or IgM rheumatoid factor.9,10 In addition, ACPAs can activate and cooperate with the complement system via the classical and alternative complement pathways,11,12 stimulate osteoclasts to enhance bone resorption, and activate sensory neurons via interleukin (IL)-8 production.13,14
IL-1β is a crucial inflammatory mediator of the innate immune response, playing a vital role in the development of pathological conditions that lead to chronic inflammation.15–17 IL-1β expression is elevated in all phases of RA, and IL-1β is abundant at the local level.18 However, whether ACPAs can stimulate macrophages to produce IL-1β has not yet been investigated.
Canonically, the production of IL-1β occurs via an inflammasome-dependent pathway. Inflammasomes are a group of multimeric protein complexes consisting of an inflammasome sensor molecule (for example, NLRP3, NLRC4, etc.), an adaptor protein (ASC) and caspase1. Of the different inflammasomes, the NLRP3 inflammasome is the most commonly studied in RA. Previous studies demonstrated that the expression of NLRP3-inflammasome-related genes (ASC, MEFV, NLRP3-FL, NLRP3-SL, and CASP1) was upregulated in peripheral blood mononuclear cells (PBMCs) from RA patients.19 In addition, NLRP3-mediated IL-1β secretion could be regulated by PTPN22, an important risk factor for the initiation and development of RA. Therefore, we carried out this experiment to investigate whether ACPAs could activate the NLRP3 inflammasome to produce IL-1β in RA and to elucidate the underlying mechanism.
Results
ACPAs induce IL-1β production in an NLRP3-dependent manner
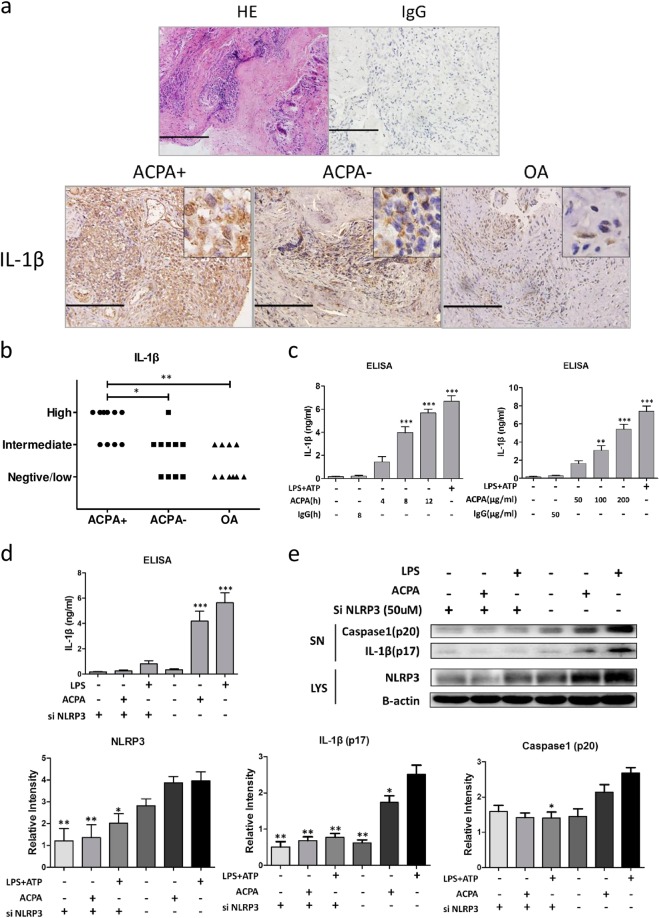
Elevated levels of IL-1β have been observed in RA patients; however, little is known about whether IL-1β levels differ between ACPA-positive and ACPA-negative patients. Therefore, we first analyzed IL-1β expression in RA synovial tissue by immunohistochemical (IHC) methods. Irrelevant rabbit anti-human IgG and rabbit serum were used as the negative controls (Fig. 1a). As shown in Fig. 1a, b, a higher level of IL-1β was observed in ACPA-positive patients than in ACPA-negative patients and osteoarthritis (OA) patients.
Fig. 1.
Anti-citrullinated protein antibodies (ACPAs) promote interleukin (IL)-1β production in an NLRP3-dependent manner. a Representative tissue sections from ACPA+ rheumatoid arthritis (RA) patients, ACPA− RA patients and osteoarthritis patients analyzed by immunohistochemical (IHC) methods to determine the expression of IL-1β. In addition, representative images of synovial sections subjected to hematoxylin and eosin staining and IHC staining with irrelevant rabbit immunoglobulin G (IgG) as the isotype control are shown. Scale bar = 100 μm. b Statistical results of IL-1β staining. *p < 0.05 and **p < 0.01. c IL-1β production by peripheral blood mononuclear cell (PBMC)-derived macrophages was measured by enzyme-linked immunosorbent assay after treatment with ACPAs or IgG (purified from serum of healthy individuals) for the indicated durations and at the indicated concentrations. **p < 0.01, ***p < 0.001 (all other groups vs. the first group). d PBMC-derived macrophages were transfected with NLRP3 small interfering RNA (siRNA) or control siRNA for 48 h before ACPA or lipopolysaccharide (LPS)/ATP treatment. After stimulation with ACPAs for 8 h, IL-1β production was measured. ***p < 0.001 (other groups vs. the first group). e Representative western blot results and statistical analysis of protein expression in PBMC-derived macrophages transfected with NLRP3 siRNA or control siRNA and treated with ACPAs or LPS/ATP. Caspase-1 (p20) and IL-1β (p17) levels in the supernatant and NLRP3 levels in the cell lysate were assessed by western blotting. Each experiment was performed at least three times. SN supernatant, LYS cell lysate
Since IL-1β is mainly produced by macrophages, to further study this phenomenon, we directly stimulated PBMC-derived macrophages with ACPAs (Fig. 1c) and measured IL-1β production in the culture medium by enzyme-linked immunosorbent assay (ELISA). The macrophages produced IL-1β in a time- and dose-dependent manner in response to ACPA treatment. Next, the results of an immune assay showed that NLRP3 knockdown inhibited the cleavage of caspase1 and the production of activated IL-1β (Fig. 1d). These data verified that ACPAs stimulate macrophages to produce IL-1β in an NLRP3-dependent manner.
CD147 participates in ACPA-induced NLRP3 activation
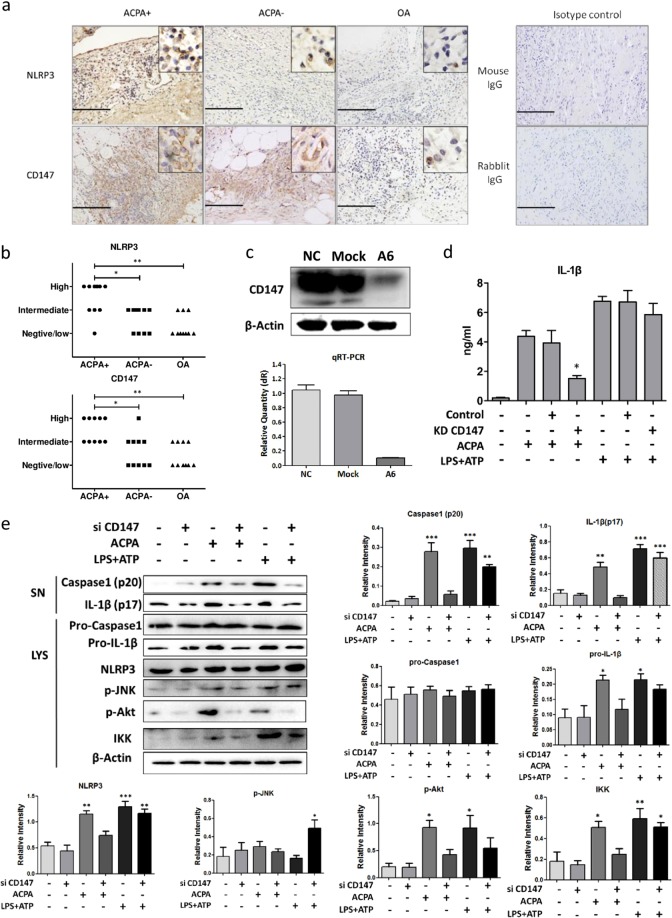
It has been reported that CD147 can enhance the differentiation of M1 macrophages, which exhibit upregulated expression of inflammasome-related molecules,20,21 and that an anti-CD147 antibody can inhibit the production of IL-1β in PBMCs from RA patients. To determine whether CD147 participates in ACPA-induced IL-1β production, we first examined the CD147 and NLRP3 expression levels in synovial tissue from RA patients. The IHC results and analysis showed that CD147 expression was upregulated in ACPA-positive patients, as was the expression of NLRP3 (Fig. 2a, b). In addition, NLRP3 and ACPA positivity were highly correlated in RA patients (rs = 0.728, p < 0.01), indicating that CD147 may induce the expression of NLRP3.
Fig. 2.
CD147 participates in anti-citrullinated protein antibody (ACPA)-induced NLRP3 upregulation and activation. a Representative tissue sections from ACPA+ rheumatoid arthritis (RA) patients, ACPA− RA patients, and osteoarthritis patients analyzed by immunohistochemical (IHC) methods to determine the expression of NLRP3 and CD147. Images of IHC staining with irrelevant rabbit or mouse immunoglobulin G as the isotype control are also shown. Scale bar = 100 μm. b Statistical results of interleukin (IL)-1β staining. *p < 0.05 and **p < 0.01. c The real-time PCR and western blotting results indicated successful knockdown of CD147 mRNA and protein expression (by approximately 90% of the original expression levels) by lentiviral transduction. d After CD147 knockdown, peripheral blood mononuclear cell-derived macrophages were stimulated with ACPAs or lipopolysaccharide/ATP. Cells infected with empty virus served as the control. IL-1β production in the culture medium was measured by enzyme-linked immunosorbent assay. e Representative results and statistical analysis of caspase1 (p20), IL-1β (p17), pro-caspase1, pro-IL-1β, NLRP3, p-JNK, p-Akt, and IKK protein expression as determined by western blotting after the indicated treatments. Each experiment was performed at least three times. *p < 0.05, **p < 0.01, and ***p < 0.001 (other groups vs. the first group). SN supernatant, LYS cell lysate
Therefore, we constructed a CD147 knockdown cell model using lentiviral vectors. Successful CD147 knockdown was verified by both western blotting and quantitative PCR (Fig. 2c). ACPA-induced IL-1β production was significantly diminished after CD147 knockdown, while IL-1β production induced by lipopolysaccharide (LPS)/ATP treatment was not affected (Fig. 2d, e). This result indicated that CD147 may not promote NLRP3 expression by mediating the LPS/Toll-like receptor 4 (TLR4) pathways.
We next determined the pathways that were altered after CD147 knockdown. Since the c-Jun N-terminal kinase (JNK) pathway has been previously reported to be involved in ACPA-induced IL-1β secretion in U937 cells,22 we first examined the phosphorylation of JNK. Surprisingly, the level of p-JNK remained unchanged upon ACPA treatment but was elevated after treatment with LPS or ATP (Fig. 2e). However, the Akt pathway was activated, as p-Akt level increased after ACPA treatment, and CD147 knockdown significantly decreased the p-Akt level (Fig. 2e). Consistent with previous results,23 the nuclear factor (NF)-κB pathway was also activated, which may be responsible for the induction of NLRP3 and pro-IL-1β. In addition, CD147 knockdown reversed ACPA-induced NF-κB activation (Fig. 2e). Thus we hypothesize that CD147 participates in ACPA-induced NLRP3 inflammasome activation by mediating the Akt and NF-κB pathways.
ACPAs enhance the interaction between CD147 and integrin β1
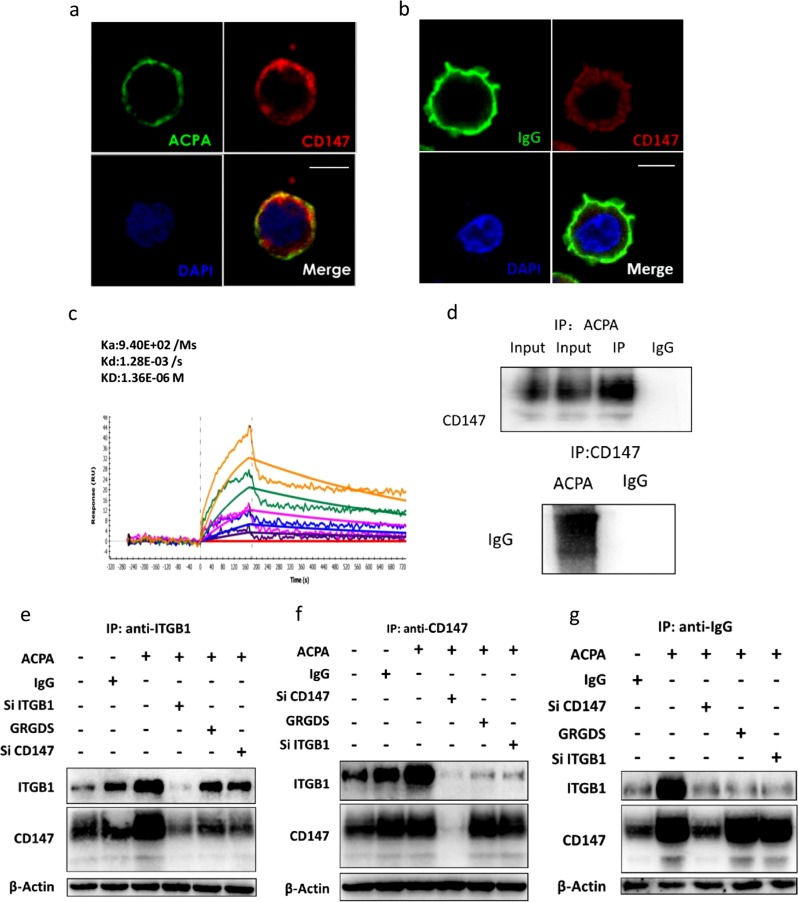
Since CD147 is a transmembrane glycoprotein that can interact with other proteins to induce intracellular signaling alterations,24 we next examined the interaction between CD147 and ACPAs. First, the immunofluorescence staining results showed that CD147 and ACPAs colocalized on the cellular surface (Fig. 3a, b). Second, the results of surface plasmon resonance (SPR) experiments confirmed that ACPAs could interact with CD147 (Fig. 3c). Finally, the results of co-IP experiments also revealed that ACPAs could interact with CD147 (Fig. 3d).
Fig. 3.
Anti-citrullinated protein antibodies (ACPAs) promote the interaction between CD147 and integrin β1. a After ACPA treatment, phorbol-12-myristate-13-acetate (PMA)-differentiated THP-1 cells were probed with an anti-human CD147 antibody, an fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin G (IgG) secondary antibody, and a Cy3-conjugated anti-mouse IgG secondary antibody. The colocalization of ACPAs and CD147 was evaluated by confocal laser scanning microscopy. Scale bars = 10 μM. b After IgG treatment, PMA-differentiated THP-1 cells were probed with an anti-human CD147 antibody, an FITC-conjugated anti-human IgG secondary antibody, and a Cy3-conjugated anti-mouse IgG secondary antibody. The colocalization of control IgG and CD147 was evaluated by confocal laser scanning microscopy. Scale bars = 10 μM. c Biophysical analysis of the CD147/ACPA interaction using surface plasmon resonance. The orange, green, peach, blue, violet purple, and red lines represent different concentrations of CD147 (0, 0.5, 1, 2, 4, and 8 μM, respectively) in the fluid phase. d Lysates of PMA-differentiated THP-1 cells were subjected to immunoprecipitation with a coupling gel prebound to ACPA IgG. Mouse IgG was used as the negative control. CD147 in the precipitate was detected by western blotting (upper image). ACPA IgG and control IgG were subjected to immunoprecipitation with an anti-CD147 antibody. IgG in the precipitate was detected by western blotting (lower image). e–g After preincubation with ACPA IgG or control IgG, lysates of PMA-differentiated THP-1 cells with CD147 or ITGB1 knockdown or GRGDS treatment were subjected to immunoprecipitation with a coupling gel prebound to an anti-CD147 antibody (e), an anti-ITGB1 antibody (f), or an anti-human IgG antibody (g). ITGB1 and CD147 in the precipitate were detected by western blotting
In our previous research, we found that CD147 could interact with and activate ITGB1.25 Accordingly, we next examined the interaction between CD147 and ITGB1 upon ACPA treatment. Phorbol-12-myristate-13-acetate (PMA)-differentiated THP-1 cells were stimulated with either ACPA IgG or control IgG. We first used an anti-CD147 or anti-ITGB1 antibody as bait to capture the interacting proteins. Assays with both antibodies indicated that ACPAs could enhance the interaction between CD147 and ITGB1 (Fig. 3e, lane 3 vs. lanes 1 and 2; Fig. 3f, lane 3 vs. lanes 1 and 2). We then used anti-human IgG as bait for ACPAs. Similar results were observed, as increased levels of CD147 and ITGB1 were detected after ACPA treatment (Fig. 3g; lane 2 vs. lane 1). In the CD147 knockdown group, the amount of ITGB1 precipitated in the experiment was reduced. However, in the ITGB1 knockdown group and the GRGDS treatment group, the amount of CD147 precipitated did not significantly decrease. Therefore, ACPAs may preferentially interact with CD147.
The CD147/ITGB1/Akt signaling pathway participates in ACPA-induced NLRP3 activation
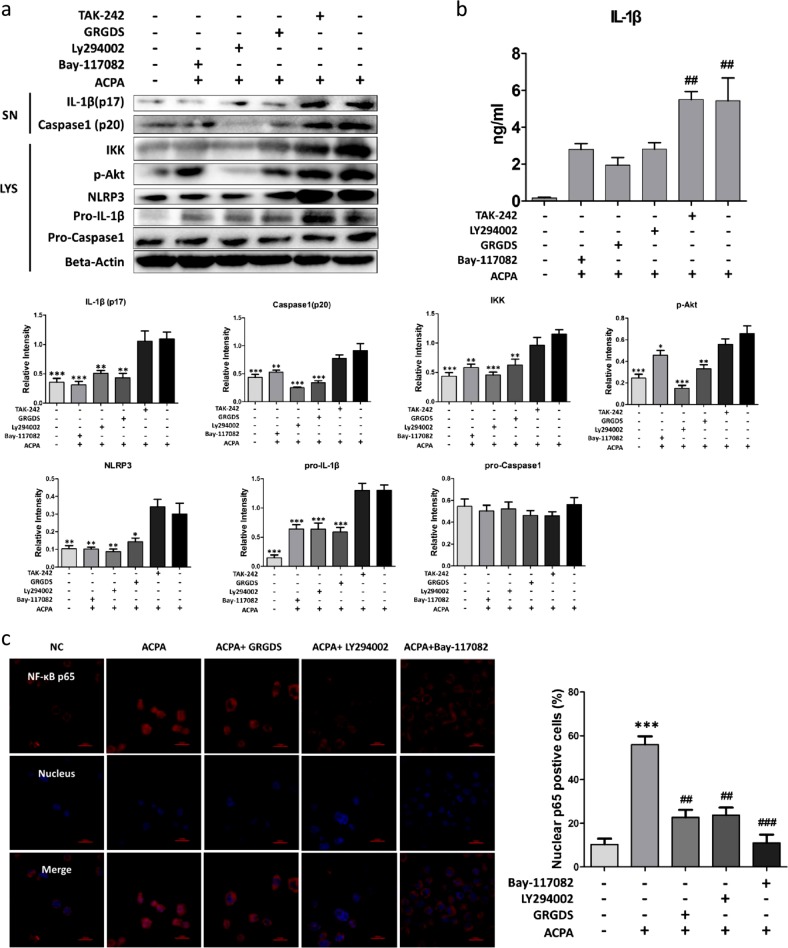
Since the interaction between CD147 and ITGB1 could activate the downstream PI3K/Akt pathway,25 we next investigated whether the Akt pathway was affected by the enhanced interaction between CD147 and ITGB1 after ACPA treatment. As shown in Fig. 4a, blocking the interaction between CD147 and ITGB1 with GRGDS downregulated the expression of p-Akt (Fig. 4a, lane 4 vs. lane 6) and cleaved-IL-1β (Fig. 4a, b). Importantly, the PI3K/Akt inhibitor LY294002 also reversed NLRP3 inflammasome activation and IL-1β production (Fig. 4a, lane 3 vs. lane 6; Fig. 4b), indicating that Akt activation was important in ACPA-induced NLRP3 inflammasome activation. Additionally, TAK-242, a TLR4 inhibitor, did not prevent ACPA-stimulated IL-1β production, indicating that, unlike LPS-induced NLRP3 activation, ACPA-induced NLRP3 activation did not require the activation of the TLR4/MyD88 pathway.
Fig. 4.
The CD147/Integrinβ1/Akt signaling pathway participates in anti-citrullinated protein antibody (ACPA)-induced interleukin (IL)-1β production. a Peripheral blood mononuclear cell (PBMC)-derived macrophages were pretreated with various inhibitors before stimulation with ACPAs for 8 h. IL-1β, pro-IL-1β, and β-actin were detected by immunoblotting. Caspase1 (p20) and IL-1β (p17) in the supernatants and pro-Caspase1, pro-IL-1β, NLRP3, p-Akt, and IKK in the cell lysates were detected by western blotting after the indicated treatments. Each experiment was performed at least three times. *p < 0.05, **p < 0.01, and ***p < 0.001 (other groups compared with group six). SN supernatant, LYS cell lysate. b After pretreatment with various inhibitors, PBMC-derived macrophages were stimulated with ACPAs. IL-1β production in the culture medium was measured by enzyme-linked immunosorbent assay. c After pretreatment with various inhibitors, PBMC-derived macrophages were stimulated with ACPAs and probed with an anti-human nuclear factor (NF)-κB p65 antibody and a Cy3-conjugated secondary antibody. Nuclei were stained with Hoechst 33342. NF-κB p65 nuclear translocation in the cells was observed with a confocal laser scanning microscope. The number of cells positive for nuclear localization of p65 out of at least 500 cells was counted, and the data are presented as the means ± SEMs. Scale bars = 20 μM
It has been previously reported that activated Akt could lead to NF-κB-driven production of NLRP3 inflammasome-related molecules.26 Since we discovered that NF-κB activation was blocked upon CD147 knockdown (Fig. 2e), we hypothesized that Akt pathway activation driven by the enhanced interaction between CD147 and ITGB1 after ACPA treatment could further induce the activation of NF-κB. As shown in Fig. 4a, inhibiting the Akt pathway or interfering with the CD147/ITGB1 interaction reduced NF-κB activation. In addition, the NF-κB inhibitor Bay-117082 reduced NF-κB activation and IL-1β production but did not affect the p-Akt level.
After NF-κB activation, IκB degradation allows the freed p65 (RelA) subunit to enter the nucleus, where it induces the expression of specific genes, such as NLRP3 and pro-IL-1β under these conditions. Nuclear translocation of NF-κB p65 was observed by confocal microscopy. As predicted, the nuclear localization of p65 increased after ACPA treatment and was suppressed after inhibition of the CD147/ITGB1 interaction, inhibition of the Akt pathway, or direct blockade of the NF-κB pathway (Fig. 4c). These results indicated that ACPAs, rather than TLR4 pathway activation, enhanced the interaction between CD147 and ITGB1 and induced Akt phosphorylation and downstream NF-κB activation.
ATP release is required for ACPA-induced NLRP3 activation
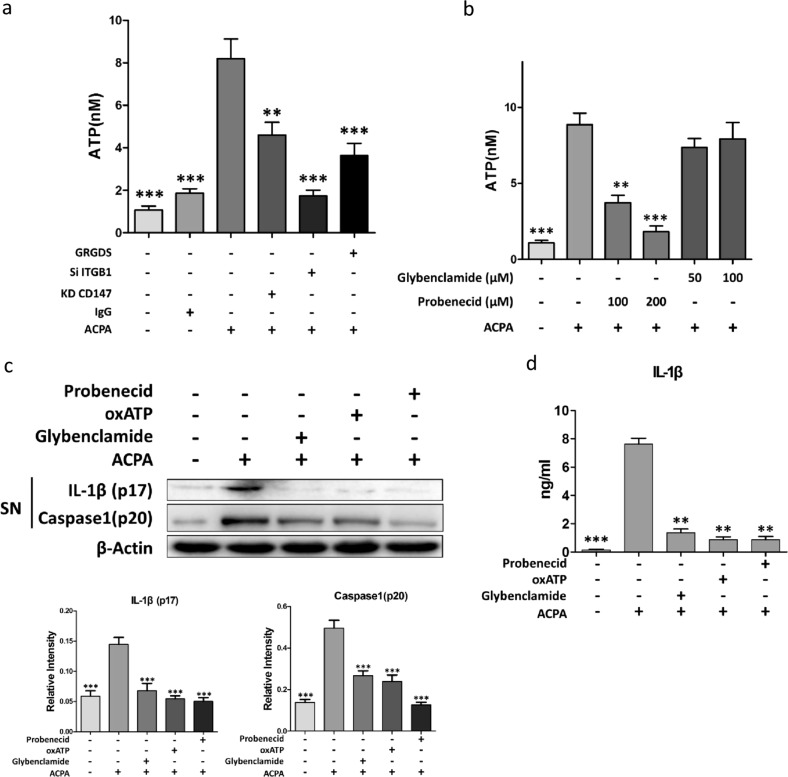
Mechanistically, NLRP3 inflammasome activation requires an activator, such as extracellular ATP, to be released from stressed cells.27 Extracellular ATP can bind to the P2X7 receptor and induce K+ efflux, a common pathway associated with all NLRP3 activators. To examine whether ACPAs caused fluctuations in the level of extracellular ATP, we measured the extracellular levels of ATP produced by PBMC-derived macrophages after ACPA treatment. ACPAs significantly induced ATP release (Fig. 5a). However, this induction was impaired by knockdown of CD147 and ITGB1 or by treatment with GRGDS (Fig. 5a). It has been reported that ATP release can be mediated by the opening of pannexin channels and further binding to the P2X7 receptor, which induces potassium efflux. To test this hypothesis, we used probenecid (a pannexin-specific antagonist) and glibenclamide (a P2X7 channel blocker) in the following experiments. ACPA-induced increases in extracellular ATP levels were attenuated by probenecid but not glibenclamide (Fig. 5b). To determine whether there was a link between ACPA-induced ATP release and NLRP3 inflammasome activation, caspase1 and IL-1β activation was analyzed after treatment with glibenclamide, oxATP (a P2X7 antagonist that blocks the interaction of ATP with the P2X7 receptor), or probenecid. These blockers inhibited the production of activated caspase1 and IL-1β, with probenecid exhibiting the most prominent effect (Fig. 5c, d). These results indicated that ACPAs could activate pannexin channels, inducing ATP release and resulting in NLRP3 inflammasome activation, which was mediated by the enhanced interaction between CD147 and ITGB1.
Fig. 5.
ATP release is required for anti-citrullinated protein antibody (ACPA)-induced NLRP3 activation. a, b ATP release from peripheral blood mononuclear cell (PBMC)-derived macrophages was assessed after CD147 or ITGB1 knockdown or pretreatment with GRGDS (a), glibenclamide, or probenecid (b) before stimulation with ACPAs. **p < 0.05, ***p < 0.001, other groups vs. the group with only ACPA treatment. c, d After pretreatment with various inhibitors, PBMC-derived macrophages were stimulated with ACPAs. c Caspase1 (p20) and interleukin (IL)-1β (p17) levels in the supernatants were assessed by western blotting. d IL-1β production in the culture medium was measured by enzyme-linked immunosorbent assay. **p < 0.05, ***p < 0.001, other groups vs. the group with only ACPA treatment
Discussion
In RA, IL-1β is a pivotal cytokine that participates in disease initiation, neutrophil infiltration, and cartilage destruction.28 Expressing human IL-1β via ex vivo gene transfer in the knee joints of rabbits29 or using an intraarticular injection of recombinant IL-1β resulted in a highly aggressive form of arthritis that recapitulated some features of human RA.30 In RA patients, IL-1β production is strongly induced in monocytes/macrophages, which accumulate at the cartilage/pannus junction after direct cellular contact with stimulated T lymphocytes. ACPAs specifically produced in RA patients have not previously been recognized as stimulators that initiate inflammasome activation. Here we show for the first time that ACPAs promote the interaction between CD147 and ITGB1, which in turn activates the downstream Akt/NF-κB signaling pathway, resulting in the upregulation of NLRP3 and pro-IL-1β expression and further NLRP3 inflammasome activation.
CD147 is a transmembrane glycoprotein and a member of the Ig superfamily of receptors. In our previous research, we found that CD147 participates in RA synovitis and cartilage erosion31 and demonstrated a “CD147-Tm/osteoclast-RA chain” participating in abnormal Tm-cell activation in RA patients.32 CD147 has been found to enhance chemotaxis, matrix metalloproteinase production, synoviocyte invasion,33 and monocyte/macrophage adhesion33,34 and to modulate the differentiation of T helper type17 cells.35 In addition, CD147 enhances the polarization of M1 macrophages and induces the expression of proinflammatory cytokines such as IL-1β, IL-6, and transforming growth factor-β.20 Despite these findings, our experiment is the first to elucidate the pathological mechanism of CD147 in ACPA-positive RA synovial inflammation and provides a possible approach to control excessive IL-1β production in RA.
The interaction between CD147 and ITGB1 has been previously investigated in hepatocellular carcinoma (HCC) cells. The extracellular membrane-proximal domain of CD147 binds to the metal ion-dependent adhesion site in ITGB1, resulting in the activation of focal adhesion kinase and the Akt pathway, which is responsible for the malignant properties of HCC cells, such as proliferation, migration, and invasion.36,37 In our experiments, ACPAs interacted with CD147 and promoted its interaction with ITGB1. Intriguingly, ACPAs may preferentially interact with CD147 rather than ITGB1, as the amount of CD147 captured by anti-human IgG remained unchanged after ITGB1 knockdown or GRGDS treatment, while the amount of captured ITGB1 significantly decreased after CD147 knockdown. However, the ACPA-binding site on CD147 is still undefined and needs to be identified in future experiments.
Moreover, we found that ACPA-induced enhancement of the interaction between CD147 and ITGB1 activated the Akt pathway, which in turn activated the NF-κB pathway. Interestingly, Venkatesan et al. reported that CD147 could activate JNK via MKK7 to induce the production of IL-18 in cardiomyocytes.38 However, in our experiments, the JNK pathway was not affected by ACPA treatment. This discrepancy could be attributed to differences in the cell types (PBMC-derived macrophages vs. cardiomyocytes) and stimulators (ACPAs vs. recombinant CD147) used in the experiments. Since the ACPAs purified in our experiments comprised a repertoire of autoantibodies targeted against different citrullinated proteins, identifying the exact ACPA epitopes that mediate the proinflammatory effects would be of great value.
Classical NLRP3 inflammasome activation requires two steps, priming and activation. In the priming step, the NF-κB pathway is activated, inducing the synthesis of pro-IL-1β and changes in NLRP3 expression at the transcriptional and posttranscriptional levels.39 In our experiment, the priming step could be induced by the Akt pathway, which was activated by the interaction between CD147 and ITGB1. After priming, a broad range of NLRP3-activating stimuli, including pathogen-associated molecular patterns, danger-associated molecular patterns (DAMPs), and irritants (such as uric acid crystals, extracellular ATP, and pore-forming toxins),40 can induce K+ efflux to trigger NLRP3 inflammasome formation. After the protein complexes have formed, the NLRP3 inflammasome activates caspase1, which in turn proteolytically cleaves pro-IL-1β into IL-1β. In our study, we found that ATP, a common stimulator of the NLRP3 inflammasome, accumulated after ACPA treatment. To test whether ACPAs induced ATP release through pannexin channels, as previously reported,26 we utilized probenecid, a pannexin-specific antagonist, to block these channels. A sharp decrease in extracellular ATP was observed after probenecid treatment, indicating the participation of pannexin channels in ACPA-induced ATP release. A possible explanation for pannexin channel activation is that activated ITGB1 could recruit Src family kinases, which may be responsible for the phosphorylation of a conserved region of the intracellular C-terminus of Panx1 and the induction of ATP secretion.26 These hypotheses still need further investigation, since no direct evidence has been obtained.
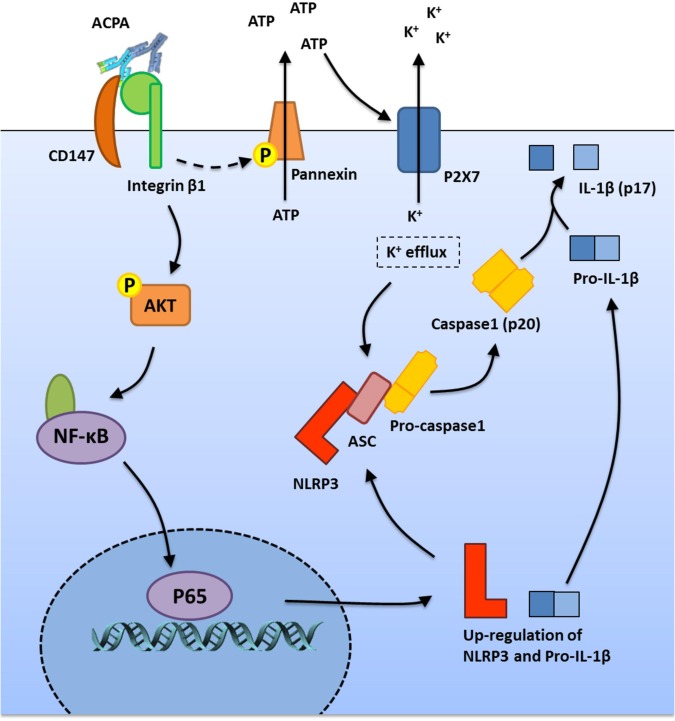
In conclusion, we showed for the first time that ACPAs could activate the NLRP3 inflammasome, which participates in RA pathogenesis. After ACPA treatment, the interaction between CD147 and ITGB1 was enhanced in macrophages, followed by the activation of the downstream Akt/NF-κB signaling pathway and subsequent induction of NLRP3 and pro-IL-1β expression. In addition, ACPAs could activate pannexin channels, resulting in ATP secretion and subsequent NLRP3 inflammasome activation (Fig. 6). Thus, in this study, we proposed a new mechanism of IL-1β production in RA, explored a new role of ACPAs in RA pathogenesis, and provided a potential therapeutic target for RA treatment. However, since blood-derived macrophages may not be completely representative of tissue-derived macrophages, the results should be further verified in tissue macrophages and in vivo models.
Fig. 6.
Schematic model of the mechanism underlying the effect of anti-citrullinated protein antibody (ACPA)-induced interleukin (IL)-1β production
Material and methods
Patients and sample preparation
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethical review board of the Fourth Military Medical University. Ten ACPA-positive RA patients, ten ACPA-negative RA patients (diagnosed according to the American College of Rheumatology 1987 revised criteria41 and pathological examination) and ten OA patients were recruited for this study, and their clinical characteristics are summarized in Supplementary Table 1. Patients with ongoing articular infections or who had received immunosuppressive therapy were excluded. Synovial tissues were obtained from each patient during joint arthroscopy in Xijing Hospital, Fourth Military Medical University. The synovial samples were fixed in 4% paraformaldehyde and embedded in paraffin before staining.
Purification of ACPA IgG and control IgG
ACPA-positive serum samples were pooled from 10 RA patients with high titers of anti-cyclic citrulline peptide (anti-CCP) antibody (>1000 IU/ml, as measured by ELISA) who presented at the Department of Clinical Immunology of Xijing Hospital.
The CCP used for ACPA purification was synthesized according to previously described sequences.42 The CCP was then conjugated to NHS-activated sepharose 4B Fast Flow (GE Healthcare, NJ, USA) according to the manufacturer’s instructions. Prior to affinity purification of ACPAs, IgG was purified from serum by a protein A column (GE Healthcare, NJ, USA). The eluted IgG was immediately neutralized with Tris buffer and dialyzed against phosphate-buffered saline (PBS). The purified IgG was then applied to columns conjugated with CCP and purified using fast protein liquid chromatography with an ÄKTA purifier 10 (GE Healthcare, NJ, USA). The bound fraction was eluted and exchanged against PBS on a desalting column (Zebra Spin, Pierce, Thermo Fisher Scientific, MA, USA). The solution was concentrated by ultrafiltration in a Vivaspin 30 K unit (Merck, Millipore, Darmstadt, Germany). The content of ACPA IgG was verified by SPR. The control IgG was pooled from healthy individuals’ serum using similar methods. Endotoxin was removed with a Toxin EraserTM endotoxin removal kit (GenScript, NJ, USA), and the presence of endotoxin was evaluated with a Toxin SensorTMchromogenic LAL endotoxin assay kit (GenScript, NJ, USA). The LPS titer was determined to be <1 EU/ml.
Reagents and antibodies
Ultra-pure LPS, PMA, and oxATP were obtained from Sigma-Aldrich (MO, USA). TAK242 and Z-YVAD-fmk were purchased from ApexBio (TX, USA). ATP and glibenclamide were purchased from InvivoGen (CA, USA). BAY11-7082, LY294002, and probenecid were obtained from MedChem Express (NJ, USA). GRGDS and GRGES were purchased from Apeptide (Shanghai, China).
The primary antibodies used in the IHC assays were anti-IL-1β (1:200, Proteintech, Hubei, China), anti-NLRP3 (1:200, Abcam, Cambridge, UK), and anti-CD147 (1:200, final concentration 5 μg/ml, produced in our laboratory). The primary antibodies used in the immunoblot and immunoprecipitation assays were anti-cleaved caspase1 (1:1000, Cell Signaling Technology, MA, USA), anti-caspase1 (1:1000, Cell Signaling Technology, MA, USA), anti-IL-1β (1:700, Santa Cruz, CA, USA), anti-cleaved-IL-1β (1:1000, Cell Signaling Technology, MA, USA), anti-NLRP3 (1:1000, Cell Signaling Technology, MA, USA), anti-phospho-SAPK/JNK (Thr183, Tyr185) (1:1000, Cell Signaling Technology, MA, USA), anti-IKK (1:1000, Proteintech, Hubei, China), anti-β-actin (1:1000, HuaBio, Zhejiang, China), anti-integrin β1 (1:1000, Cell Signaling Technology, MA, USA), and anti-phospho-Akt (Ser473) (1:1000, Cell Signaling Technology, MA, USA). The secondary antibodies used in the immunoblot assays were goat anti-rabbit IgG-horseradish peroxidase (HRP) (1:3000, HuaBio, Zhejiang, China) and goat anti-mouse IgG-HRP (1:3,000, HuaBio, Zhejiang, China).
IHC staining and analysis
IHC staining of synovial tissue was carried out with a streptavidin–peroxidase kit (Zymed, CA, USA). Staining was performed according to the kit manual. After heat-induced epitope retrieval and blocking of endogenous peroxidase activity, tissues were incubated with antibodies against NLRP3, IL-1β, and CD147 overnight at 4 °C. Irrelevant mouse/rabbit IgG (BioLegend, San Diego, USA) and rabbit serum were used as the negative controls. Sections were then successively incubated with biotin-labeled goat anti-mouse/rabbit IgG, HRP-labeled streptavidin, and diaminobenzidine. Then nuclei were counterstained with hematoxylin.
Each sample was analyzed independently by two pathologists. Only clear staining of the membrane (CD147) or cytoplasm (NLRP3 and IL-1β) of synovial lining cells was considered positive. Histochemical scores (H scores) were calculated to indicate the expression of the target molecules. Briefly, the H score was calculated by multiplying the percentage of positive cells per slide (0–100%) by the dominant staining intensity pattern (1, negative or trace; 2, weak; 3, moderate; and 4, intense). Samples with scores of 0–200, 201–300, and 301–400 were classified as having negative or low, intermediate, and high expression, respectively.43
Cell preparation
Cells were cultured in RPMI 1640 medium with 10% fetal bovine serum under standard culture conditions (37 °C and 5% CO2). PBMCs were isolated by density gradient centrifugation over Ficoll-Hypaque (Lymph prep, Oslo, Norway) from informed, healthy volunteers. Primary monocytes were isolated from human PBMCs using an EasySep™ Human Monocyte Enrichment Kit without CD16 Depletion (Stemcell Technologies, Ontario, Canada) and were differentiated by granulocyte macrophages colony-stimulating factor treatment (Pepro Tech, NJ, USA) for 6 days. THP-1 cells were purchased from the American Type Culture Collection (ATCC, VA, USA) and were differentiated overnight with 50 ng/ml PMA. After differentiation, cells were stimulated with 100 μg/ml ACPA for 12 h. For the stimulation and inhibition studies, cells were pretreated in a 37 °C incubator with the following compounds for the indicated time periods and at the indicated concentrations: ACPA IgG (0-200 μg/ml, 0-12 h), control IgG (100 μg/ml, 12 h), LPS (100 ng/ml, 3 h), ATP (5 mM, 1 h), GRGDS (50 μM, 30 min), TAK242 (100 nM, 2 h), Bay-117082 (10 μM, 30 min), glibenclamide (50 μM, 30 min), probenecid (250 μM, 12 h with stimulation), and oxATP (300 μM, 30 min).
Short hairpinRNA- and small interfering RNA (siRNA)-mediated knockdown
To knock down CD147 expression, we used the previously constructed trans-lentiviral pLKO system.44 The plasmids in the trans-lentiviral pLKO system, including psPAX2 (1.125 μg/ml), pMD2G (0.125 μg/ml), and pLKO A6/NC (1.25 μg/ml), were transfected into HEK 293T cells using Lipofectamine 2000 (Invitrogen, Basel, Switzerland) according to the manufacturer’s instructions. The culture medium containing lentivirus was collected and added to PBMC-derived monocytes and THP-1 cells for 48 h. Puromycin at 2 μg/ml (Sigma, Buchs, Switzerland) was added to the medium for further selection.
To knock down NLRP3 and integrin β1 (ITGB1) expression, we purchased siRNA from GenePharma (Shanghai, China). After differentiation in 6-well plates, PBMC-derived monocytes and THP-1 monocytes were transfected with control siRNA (100 nM), NLRP3 siRNA (100 nM), or integrin β1 siRNA (100 nM) for 96 h using Lipofectamine 2000.
Immunoblotting and immunoprecipitation
After different treatments, cell culture medium was collected and centrifuged at 4 °C for 5 min at 2000 × g. The pelleted debris was discarded, and protein was then isolated with Universal Protein Sediment Reagent (Sangon Biotech, Shanghai, China) according to the manufacturer’s protocol. After pH adjustment, the precipitated supernatants were resuspended in 50 µl of Laemmli buffer and boiled for 8 min. Equal amounts of protein were resolved on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride (PVDF) membranes. For cell lysis, RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors (cOmplete, Roche, Basel, Switzerland), phosphatase inhibitors (PhosSTOP, Roche, Basel, Switzerland), and phenylmethanesulfonyl fluoride (Beyotime, Shanghai, China) was added directly to cells grown in a 6-well plate and washed three times with PBS. The cells were lysed on ice for 15 min. After centrifugation at 13,000 × g and 4 °C for 20 min, the supernatant was collected, and protein was quantified with a bicinchoninic acid protein assay (Thermo Scientific, Rockford, USA). Equal amounts of protein were removed and boiled for 8 min in Laemmli buffer before being resolved on 10% or 12.5% polyacrylamide gels and transferred to PVDF membranes. Membranes were blocked in 5% skim milk or 3% bovine serum albumin (BSA; for phosphoproteins) and incubated with the corresponding primary antibodies overnight at 4 °C. After three washes (5 min each) with TBST, membranes were incubated with an HRP-conjugated secondary antibody for 1 h at room temperature (RT). Signals were detected with a ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA, USA) using Pierce™ ECL Western Blotting Substrate (Thermo Fischer Scientific, CA, USA). Each experiment was performed at least three times.
Coimmunoprecipitation was performed using a PierceTM coimmunoprecipitation kit (Thermo Scientific, MA, USA), which utilizes capture antibodies covalently bound directly to beads. After the proteins were eluted with elution buffer, 1 M Tris buffer was added to neutralize the additional acid. Protein quantification was carried out before the immunoblot assay.
Anti-CCP and IL-1β detection
Patient blood samples were collected and stored at 4 °C before detection. Anti-CCP titers were evaluated with an anti-CCP assay (Euroimmun, Lübeck, Germany).
Before measuring IL-1β production in cells, the culture medium was replaced with serum-free medium. After stimulation, the medium was collected and centrifuged at 10,000 × g for 5 min. IL-1β production was measured with a Human IL-1β ELISA Kit (DAKEWE, Guangdong, China) according to the manufacturer’s instructions. The absorbance at 450 nm was read by an ELISA plate reader (Epoch, Biotek, VT, USA).
Surface plasmon resonance
To characterize the kinetic binding parameters, SPR studies were performed on a ProteOn XPR36 system (Bio-Rad, CA, USA) according to the one-shot kinetics protocol.45,46 The experiment was carried out as previously described.47 ProteOn Manager software was used to calculate the equilibrium and rate constants. A local RMax value was calculated, and the data were fitted to a Langmuir model.
Immunofluorescence staining
After differentiation, cells were attached to glass coverslips for 24 h, and the coverslips were then placed in a 6-well plate. After the indicated treatments, the cells were fixed in 4% formaldehyde and permeabilized with 0.2% Triton X-100 for 10 min before blocking with 2% BSA for 1 h. The cells were then incubated with primary antibodies at 4 °C overnight, washed three times with PBS, and incubated with fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies (Thermo Scientific, Rockford, USA, 1:200) and 4,6-diamidino-2-phenylindoleat RT for 1 h. After three washes, the cells were visualized with an A1R-A1 confocal laser microscope system (Nikon, Japan).
ATP measurement
The extracellular ATP concentration was determined with an Enhanced ATP Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions.
Statistical analysis
Statistical analysis was carried out with SPSS. Comparisons between groups were analyzed by one-way analysis of variance and post hoc analysis (Bonferroni test). Data in the graphs are expressed as the mean ± SE. p Values of <0.05 were considered statistically significant.
Supplementary information
Acknowledgements
This work was supported by the National Basic Research Program “973 Grants” (2015CB553704), the National Basic Research Program of China grant (2014ZX09508002-002), and the National Key Research and Development Program of China grant (2017YFC0909000). We would also like to express our gratitude to Professor Zhinan Chen, who helped us design the experiments and provided us with essential experimental equipment.
Author contributions
X.D. and Z.Z. wrote the article. P.L. and X.F. assisted in carrying out the experiments and analyzing the data. F.L. helped to proofread the article. J.J. and P.Z. designed the experiments.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiwen Dong, Zhaohui Zheng, Peng Lin.
Contributor Information
Jianli Jiang, Phone: +86-29-84774547, Email: jiangjl@fmmu.edu.cn.
Ping Zhu, Phone: +86-29-84775359, Email: zhuping@fmmu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0201-9) contains supplementary material.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 4.Dekkers J, Toes RE, Huizinga TW, van der Woude D. The role of anticitrullinated protein antibodies in the early stages of rheumatoid arthritis. Curr. Opin. Rheumatol. 2016;28:275–281. doi: 10.1097/BOR.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, et al. Determinants of mortality among postmenopausal women in the women’s health initiative who report rheumatoid arthritis. Arthritis Rheumatol. 2014;66:497–507. doi: 10.1002/art.38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jilani, A. A. & Mackworth-Young, C. G. The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int. J. Rheumatol. 2015, 728610 (2015). [DOI] [PMC free article] [PubMed]
- 7.Lu MC, et al. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 8.Clavel C, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 9.Sokolove J, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent L, et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann. Rheum. Dis. 2015;74:1425–1431. doi: 10.1136/annrheumdis-2013-204543. [DOI] [PubMed] [Google Scholar]
- 11.Van Steendam K, et al. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010;12:R132. doi: 10.1186/ar3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwannalai P, et al. Low-avidity anticitrullinated protein antibodies (ACPA) are associated with a higher rate of joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2014;73:270–276. doi: 10.1136/annrheumdis-2012-202615. [DOI] [PubMed] [Google Scholar]
- 13.Roux C. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:79–86. doi: 10.1038/nrrheum.2016.200. [DOI] [PubMed] [Google Scholar]
- 15.Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best. Pract. Res. Clin. Rheumatol. 2006;20:879–896. doi: 10.1016/j.berh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 18.Dayer JM, Bresnihan B. Targeting interleukin-1 in the treatment of rheumatoid arthritis. Arthritis Rheum. 2002;46:574–578. doi: 10.1002/art.10168. [DOI] [PubMed] [Google Scholar]
- 19.Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Biologics in Rheumatoid Arthritis Genetics. et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann. Rheum. Dis. 2014;73:1202–1210. doi: 10.1136/annrheumdis-2013-203276. [DOI] [PubMed] [Google Scholar]
- 20.Geng JJ, et al. Enhancement of CD147 on M1 macrophages induces differentiation of Th17 cells in the lung interstitial fibrosis. Biochim. Biophys. Acta. 2014;1842:1770–1782. doi: 10.1016/j.bbadis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Awad F, et al. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS ONE. 2017;12:e0175336. doi: 10.1371/journal.pone.0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai NS, et al. Anti-citrullinated protein antibodies suppress let-7a expression in monocytes from patients with rheumatoid arthritis and facilitate the inflammatory responses in rheumatoid arthritis. Immunobiology. 2015;220:1351–1358. doi: 10.1016/j.imbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai Y, et al. CD147 promotes IKK/IkappaB/NF-kappaB pathway to resist TNF-induced apoptosis in rheumatoid arthritis synovial fibroblasts. J. Mol. Med. 2016;94:71–82. doi: 10.1007/s00109-015-1334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai JY, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer. 2009;9:337. doi: 10.1186/1471-2407-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortimer L, Moreau F, Cornick S, Chadee K. The NLRP3 inflammasome is a pathogen sensor for invasive Entamoeba histolytica via activation of alpha5beta1 integrin at the macrophage-amebae intercellular junction. PLoS Pathog. 2015;11:e1004887. doi: 10.1371/journal.ppat.1004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walle LV, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512:69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghivizzani SC, et al. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997;4:977–982. doi: 10.1038/sj.gt.3300486. [DOI] [PubMed] [Google Scholar]
- 30.Ghivizzani SC, et al. Constitutive intra-articular expression of human IL-1 beta following gene transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. J. Immunol. 1997;159:3604–3612. [PubMed] [Google Scholar]
- 31.Jia J, et al. Inhibitory effect of CD147/HAb18 monoclonal antibody on cartilage erosion and synovitis in the SCID mouse model for rheumatoid arthritis. Rheumatology. 2009;48:721–726. doi: 10.1093/rheumatology/kep099. [DOI] [PubMed] [Google Scholar]
- 32.Guo, N. et al. A critical epitope in CD147 facilitates memory CD4(+) T-cell hyper-activation in rheumatoid arthritis. Cell. Mol. Immunol. 10.1038/s41423-018-0012-4. (2018). [DOI] [PMC free article] [PubMed]
- 33.Wang CH, et al. Expression of CD147 (EMMPRIN) on neutrophils in rheumatoid arthritis enhances chemotaxis, matrix metalloproteinase production and invasiveness of synoviocytes. J. Cell. Mol. Med. 2011;15:850–860. doi: 10.1111/j.1582-4934.2010.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu P, et al. CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res. Ther. 2006;8:R44. doi: 10.1186/ar1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, et al. CD147 modulates the differentiation of T-helper 17 cells in patients with rheumatoid arthritis. APMIS. 2017;125:24–31. doi: 10.1111/apm.12629. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, et al. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin beta1 to modulate malignant properties of hepatoma cells. J. Biol. Chem. 2012;287:4759–4772. doi: 10.1074/jbc.M111.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, et al. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: a potential role for integrin beta1 signaling. Mol. Cancer Ther. 2015;14:553–563. doi: 10.1158/1535-7163.MCT-14-0618. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesan B, et al. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J. Mol. Cell. Cardiol. 2010;49:655–663. doi: 10.1016/j.yjmcc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaidt MM, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 41.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 42.Schellekens GA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch FR, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 44.Guo N, et al. CD147 and CD98 complex-mediated homotypic aggregation attenuates the CypA-induced chemotactic effect on Jurkat T cells. Mol. Immunol. 2015;63:253–263. doi: 10.1016/j.molimm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Nahshol O, et al. Parallel kinetic analysis and affinity determination of hundreds of monoclonal antibodies using the ProteOn XPR36. Anal. Biochem. 2008;383:52–60. doi: 10.1016/j.ab.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Bravman T, et al. Exploring “one-shot” kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal. Biochem. 2006;358:281–288. doi: 10.1016/j.ab.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Cui HY, et al. CD147 regulates cancer migration via direct interaction with Annexin A2 and DOCK3-beta-catenin-WAVE2 signaling. Oncotarget. 2016;7:5613–5629. doi: 10.18632/oncotarget.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.