Abstract
Background
Bipolar disorder is a disabling disease characterized by the recurrence of mood episodes. Successful strategies for the acute treatment of bipolar depression are still a matter of controversy. Total sleep deprivation (TSD) has shown acute antidepressant effect; however, the prompt relapse of depressive symptoms after sleep recovery has been reported. Taking this into consideration, we aimed to address a twofold research question: what are the acute effects of adding TSD to pharmacological treatment and what are the acute and chronic effects of adding medications to TSD.
Methods
MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases were searched for clinical trials assessing bipolar depression and TSD. Two independent reviewers selected and classified 90 abstracts. The outcomes we assessed were change in Hamilton Depression Rating Scale (HDRS) or Montgomery–Asberg Depression Rating Scale (MADRS), sustained long-term response rate, treatment-emergent mania or hypomania, and tolerability (using dropout rates as a proxy). The compared groups were: TSD alone versus TSD plus medications and medications alone versus medications plus TSD. Data was analyzed using Stata 16.0.
Results
Patients treated with TSD plus medications compared with medications alone showed a significant decrease in depressive symptomatology after one week (SMD −0.584 [95% CI −1.126 to −0.042], p = 0.03. Also, a significant decrease in depressive symptomatology (SMD −0.894 [95% CI −1.388 to −0.399], p < 0.001) was found in the group with TSD plus medications compared with TSD alone, at the 10th day of treatment. We meta-analyzed the long-term effect of the TSD. It showed a sustained antidepressant effect (log OR = 2.365 (95% CI 0.95 to 3.779, p < 0.001) in the group where TSD was combined with medication when compared with patients treated only with TSD. Finally, no differences in tolerability (log OR = 0.234 (95% CI −1.164 to 1.632, p = 0.74) or affective switch were found.
Conclusion
Adding TSD to medications to bipolar depression treatment resulted in an augmentation in acute response. We also found that medications have a positive impact in acute response when added to TSD. Furthermore, this higher response rate was maintained after 3 months while keeping Lithium therapy.
Keywords: sleep deprivation, bipolar depression, meta-analysis, chronotherapy, mood medication, affective switch
Introduction
Bipolar disorder is a major health issue and cause of global disability (1). It is characterized by episodes of mania or hypomania and recurring depressive episodes that account for a considerable morbidity and mortality (2–4).
Depressive episodes, either in its major, dysthymic or mixed forms, are responsible for most of the morbidity in type I and type II bipolar patients at any stage of the disease progression and under any treatment (2, 4). Current treatments are more effective managing manic episodes than in depressive ones. The use of antidepressants reduces symptoms of acute bipolar depression, however, they do not increase clinical response or remission rates and have been associated with a long-term increased risk of treatment-emergent mania or hypomania (5). Cognitive impairment in bipolar depression is associated with dysfunction and disability and a higher rate of premature death when compared with the general population (6). This is caused by higher risk of suicide, accidents (6) and multiple medical causes (7). This highlights the need to explore effective treatment alternatives for acute bipolar depression.
Sleep deprivation (SD) is a treatment technique used for depression developed in the past 50 years (8, 9). Clinical studies have shown rapid antidepressive effects within 24–48 h after SD and even during the same night or following morning (10, 11). However, interest in their clinical use has diluted because it soon became evident that sleep recovery tended to reverse the clinical effect (12, 13). The best documented SD modality used with antidepressant purposes is Total sleep deprivation (TSD), though other modalities have been tested as shown in Table 1 (14, 15).
Table 1.
Sleep deprivation modalities.
| Technique | Sleep Modality |
|---|---|
| Total sleep deprivation (TSD) | In one cycle of TSD patients are told to stay awake for about 36 h, from daytime until next day’s evening. Treatment usually consists in one to six cycles. |
| Partial sleep deprivation (PSD) | Sleep usually restricted to 4 to 5 h, either in the first (PSD-Late) or second half of the night (PSD-Early). |
| Sleep phase advance (SPA) | After one TSD cycle, starting with a scheduled time in bed from 17:00–24:00 h, with daily shift back of time in bed for 1 h, until original nocturnal sleep time is reached (23:00–06:00 h) after seven days. Usually intended to prevent relapse after effective TSD. |
| Sleep phase delay (SPD) | Time in bed from 02:00–07:00 h with a succeeding shift forward (30 minutes per day), until the initial sleep phase (23:00–06:00 h). |
Historically, SD has shown response rates between 50 and 80% (12) in a wide spectrum of depressive disorders, with no differences by gender or age (13, 16). However, these rates are twice as high in so called endogenous depressions than in neurotic depressions or secondary ones (13, 16). Bipolar patients have even better outcomes when compared to endogenous recurrent unipolar ones (17). Other variables associated with SD response are: diurnal variation of mood (13, 14, 18), high level of thyroid function, low peripheral sympathetic activity, an altered Dexamethasone suppression test (11, 12), among others (19–23). Nevertheless, despite the mentioned and well established SD antidepressant effect, the clinical response to SD decreases rapidly (14). Relapse rates to SD are up to 83% after one night of sleep recovery (23); this has limited their clinical use. In order to avoid relapse, different SD schemes have been created, and the use of medication (serotonergic drugs, lithium salts) or other chronotherapeutic therapies has been added (12). Studies demonstrate that a combined treatment can lead to sustained euthymia after several months (12). However, the impact of the acute effect of SD combined with mood medications, as well as the long-term efficacy of TSD have only been evaluated in clinical studies with a limited number of patients. This makes it difficult to obtain conclusive results. In our search, only two previous meta-analysis about SD were found (18, 24), and none of them directly addressed the long-term effect of drug administration in combination with SD.
Therefore, we aimed to address a twofold research question: What are the acute effects of adding TSD to pharmacological treatment? and, What are the acute and long-term effects of adding medications to TSD?
Methods
Search Strategy and Selection Criteria
In this systematic review and meta-analysis, we searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials databases from their inception until October 28, 2019, using the search term “(sleep AND deprivation AND bipolar AND depression)” with results filters based on study types (clinical trials and randomized controlled trials). We also searched ClinicalTrials.gov using the search term “(sleep AND deprivation AND bipolar AND depression)”. We reviewed references and citations of articles retained in this study for additional unidentified studies.
Inclusion criteria were: clinical trials recruiting patients aged 18–75 years undergoing a depressive episode with a primary diagnosis of bipolar I or bipolar II disorder according to the current versions of the DSM or ICD at the time of the respective trial. Patients must have received combined medication treatment and sleep deprivation therapy (with or without additional chronotherapy, see Tables 2 and 3 ). The existence of a control group was required.
Table 2.
Characteristics of included trials in the first meta-analysis.
| Diagnosis | Medication (MED) | Chrono-therapy (CT) | Mean age (range or SD), years |
Primary outcome instrument |
Duration of treatment | MED | TSD+MED | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | D | AS | n | D | AS | |||||||
| Benedetti et al. (25) Nondouble-blind. Randomized controlled trial. |
Bipolar depression (DSM-III-R) | Fluoxetine | TSD (3 cycles) | 40 ± 12.1, 42 ± 9.7 |
HDRS-17 | 1 month | 5 | 0 | NR | 5 | 0 | NR |
| Wu et al. (11) Nondouble-blind. Randomized controlled trial. |
Bipolar disorder, Major depressive episode (DSM-IV) | Lithium or other mood stabilizer, sertraline or other antidepressant |
TSD (1 cycles), Bright light therapy, SPA (3 cycles) | 39 ± 13.3, 40 ± 14.1 |
HDRS-19 | 7 weeks | 17 | 0 | 0 | 32 | 5 | 2 |
MED, Medication; CT, Chronotherapy; SD, Standard deviation; DSM, Diagnostic and Statistical Manual; TSD, Total sleep deprivation; HDRS, Hamilton depression rating scale; SPA, Sleep phase advance; n, Sample size; D, Dropout; AS, Affective Switch.
Table 3.
Characteristics of included trials in the second and third analysis.
| Diagnosis | Medication (MED) | Chrono-therapy (CT) | Mean age (range or SD), years |
Primary outcome instrument |
Duration of treatment | TSD | TSD+MED | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | D | AS | n | D | AS | |||||||
| Benedetti et al. (26) Nondouble-blind, Nonrandomized controlled trial. |
Bipolar I (DSM-IV) | Lithium | TSD (3 cycles) | 48.2 ± 10.8, 48.3 ± 11.2 | HDRS- 21 | 3 months | 20 | 0 | NR | 20 | 0 | NR |
| Smeraldi et al (27) Double-blind, randomized controlled trial. |
Bipolar I (DSM-IV) HDRS > 18 | Pindolol | TSD (3 cycles) + Placebo |
44.9 ± 11.5, 51.6 ± 12 | HDRS- 21 | 6 months | 20 | 0 | 0 | 20 | 0 | 0 |
| Benedetti et al. (28) Nondouble-blind, Nonrandomized controlled trial. |
Bipolar disorder, Major depressive episode (DSM-IV) | Lithium | TSD (3 cycles), SPA (3 cycles) | 44.6 ± 8.9, 51.9 ± 11.6 |
HDRS-21 | 3 months | 14 | 0 | NR | 16 | 0 | NR |
| Benedetti et al. (29) Double-blind, randomized controlled trial. |
Bipolar disorder, Major depressive episode (DSM-IV) | Amineptine | TSD (3 cycles) + Placebo | 45 ± 10.9, 51.1 ± 10.3 |
MADRS | 3 months | 14 | 0 | 0 | 13 | 1 | 0 |
| Colombo et al. (30) Nonrandomized controlled trial. |
Bipolar disorder, Major depressive episode (DSM-IV) | Lithium | TSD (3 cycles) + Light exposure (intensity: 150–2500 lx) | 45.4 ± 12.1, 46.4 ± 13.3 | VAS | 7 days | 69 | 4 | 4 | 49 | 3 | 3 |
CT, Chronotherapy; MED, Medication; SD, Standard deviation; DSM, Diagnostic and statistical manual; TSD, Total sleep deprivation; HDRS, Hamilton depression rating scale; SPA, Sleep phase advance; n, Sample size; D, Dropout; AS, Affective Switch.
Exclusion criteria were: trials including inadequate samples (i.e. not exclusively bipolar patients or not control group) or interventions different from chronotherapeutic plus medication strategies. Studies with nonclinical outcomes (i.e. pharmacokinetics, serum concentrations, neuroimaging, etc.), or focused on clinical outcomes different from improvement, such as safety/tolerability, were also excluded. Studies where data could not be extractible or are not reported in raw form were excluded.
Data Analysis
For each published study, summary estimates were extracted independently by JPRM and ERS, with subsequent verification (by PAV), review, and consensus. The following variables were extracted in a structured manner: patient characteristics (mean age, sex, and primary diagnosis), medication used (dose and titration schedule), chronotherapy technique used, and placebo condition, treatment-emergent hypomania or mania episodes, and dropouts.
The primary outcome was changed in clinician-rated depressive symptom score (Hamilton depression rating scale [HDRS] (31) or Montgomery–Åsberg Depression Rating Scale [MADRS] (32), calculated as standardized mean differences (SMDs) (33). The secondary outcome was clinical response at approximately 3 months, episodes of treatment-emergent mania or hypomania, and tolerability. We used odds ratios (ORs, with 95% CIs). Clinical remission was defined by the study’s authors as HDRS-21 score of 7 or less or MADRS score of 5 or less. We used dropout rates as a proxy for tolerability, although they might also reflect the absence of efficacy.
Since treatment effects are likely to vary between studies due to methodological differences—such as patient selection, primary diagnosis, medication used, and trial duration—we used pooled random-effects models (34). Only intention-to-treat data was analyzed (35). Meta-regression procedures were developed to test the effect of time of treatment on SMDs.
In order to assess heterogeneity between trials (i.e. clinical and methodological diversity), Q statistics and I² were applied, with the threshold for heterogeneity defined as p value for the Q statistic of less than 0,1 or I² greater than 35% (36). Publication bias was evaluated by inspection of funnel plots and Egger’s test. Quality assessment was conducted with Cochrane Risk of Bias Tool for Randomized Controlled Trials (37). All analyses were performed using Stata 16.0 (StataCorp LP, College Station, TX, USA).
Results
We initially identified ninety potentially eligible studies ( Figure 1 ). Removal of duplicates and screening of titles and abstract left ten records, of which three were excluded after full-text review because of inadequate mixed samples (38–40). One included study did not report clinical improvement on previously defined rating scales, however it reported dropout rates and episodes of treatment-emergent mania or hypomania (30).
Figure 1.
Study selection. CENTRAL, Cochrane Central Register of Controlled Trials.
Our meta-analysis included 311 bipolar patients with a depressive episode (80 with bipolar I disorder (DSM IV), 221 for whom the exact diagnosis was unspecified (DSM IV) and 10 with DSM-III-R bipolar disorder, Tables 2 and 3 ). Two trials included only type I bipolar patients. The mean age was 45.7 years (SD 4.2), and the overall sample consisted of 104 men and 170 women (the gender of 37 patients was not specified).
The seven selected trials (11, 25–30) included patients that used TSD as a chronotherapy. Of these seven, four used three cycles of TSD, one used three cycles of TSD plus light exposure (intensity of 150 or 2,500 lx), one used one cycle plus bright light and SPA, and one used three cycles of TSD plus three cycles of SPA ( Tables 2 and 3 ). All seven trials used mood medications. In two studies (11, 25) both intervention and control groups received mood medication. There is no chance for a sleep deprivation placebo condition ( Table 2 ). In the remaining five studies (26–30) both groups underwent TSD, only the intervention group received medication; of these five only two studies administered placebo to the control group ( Table 3 ). The risk of bias assessment was conducted in all included studies, see supplementary material ( Figure S1 and Table S1 ).
Clinical Effectiveness of TSD Addition to Mood Medication
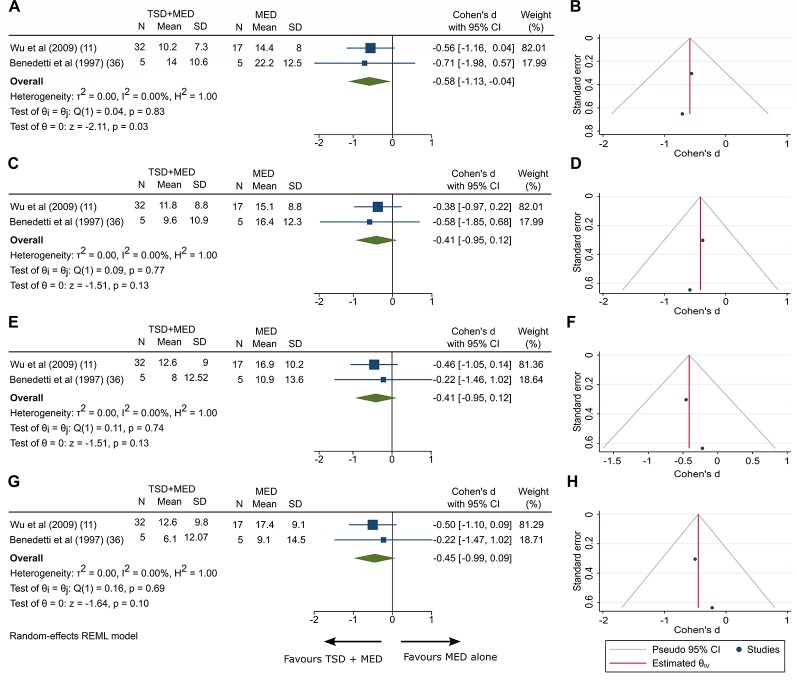
Depressive symptom scores were available for five trials, two of them comparing the addition of TSD to mood medication (11, 25). Symptoms were measured with HDRS-17 and HDRS-19 weekly. Both groups were treated with medications (fluoxetine and antidepressant/mood stabilizer, respectively), and one of the groups received additional three cycles of TSD and one cycle of TSD, bright light and SPA, respectively ( Table 2 ). Compared with medication alone, adding TSD was associated with a significant improvement in clinician-rated depressive symptoms after 1 week (SMD −0.584 [95% CI −1.126 to −0.042], p = 0.03; Figure 2A ). Between-sample heterogeneity was not significant, with a Q statistic of 0.04 (degree of freedom (df) = 1; p = 0.83) and a I2 of 0. Visual inspection of funnel plot showed asymmetry ( Figure 2B ).
Figure 2.
Clinician-rated depressive symptoms adding TSD to mood medication and the funnel plot of included studies at the 1st week (A, B), 2nd week (C, D), 3rd week (E, F) and 4th week (G, H). TSD, Total sleep deprivation; MED, Medication; N; Sample size; CI, Confidence interval; SD, Standard deviation. Tests of heterogeneity, I2 and Q statistic were included. REML, Restricted maximum likelihood.
The following weeks were associated with a nonsignificant tendency towards improvement in clinician-rated depressive symptoms: 2nd week (SMD −0.413 [95% CI −0.949 to 0.124], p = 0.13; Figure 2C , for funnel plot see Figure 2D ), 3rd week (SMD −0.412 [95% CI −0.949 to 0.124], p = 0.13; Figure 2E , for funnel plot see Figure 2F ), and 4th week (SMD −0.45 [95% CI −0.988 to 0.088], p = 0.1; Figure 2G , for funnel plot see Figure 2H ). To test the effect of time as a factor, we performed a meta-regression, that was nonsignificant (Coefficients 0.037 [95% CI −0.204 to 0.278], p = 0.765).
Clinical Effectiveness of Medication Added to TSD at Day 10
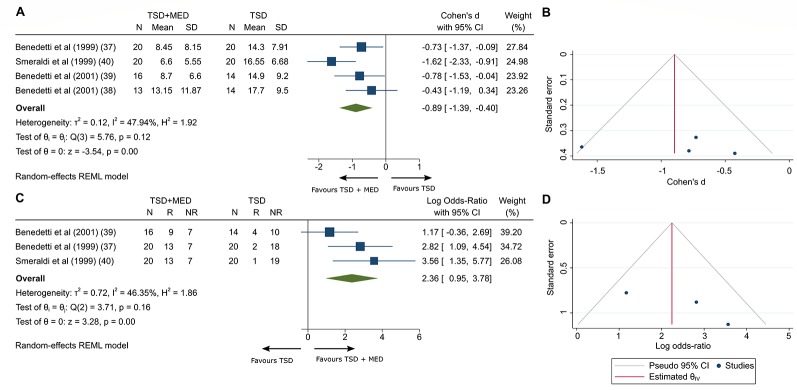
Four trials compared the addition of mood medication to TSD (26–29). Depressive symptoms were measured with HDRS-21 and MADRS at the 10th day. In three studies, patients were treated with three cycles of TSD, and in one of them patients were treated with three cycles of TSD and SPA. Mood medication (lithium, amineptine, and pindolol) was added to one group ( Table 3 ). Compared with TSD alone, adding mood medication was associated with a significant improvement in clinician-rated depressive symptoms after 10 days of treatment (SMD −0.894 [95% CI −1.388 to −0.399], p < 0.001; Figure 3A ). There was evidence of heterogeneity between the studies (I2 = 47.94, Q = 5.76, df = 3, p = 0.12). Visual inspection of funnel plot showed asymmetry ( Figure 3B ), but Egger test did not suggest small-study effects (β 1 = 2.59, SE of β 1 = 12.8, z = 0.2, p = 0.84).
Figure 3.
Clinician-rated depressive symptoms at the 10th day adding medication to TSD (A) and the funnel plot of included studies (B). Clinician remission at the 3rd month (C) and the funnel plot of included studies (D). TSD, Total sleep deprivation; MED, Medication; CI, Confidence interval; SD, Standard deviation; N, Sample size; R, Remission; NR, No remission; Tests of heterogeneity, I2 and Q statistic were included. REML, Restricted maximum likelihood.
Clinical Remission of Medication Added to TSD at 3 Months
Data for response rates were available for three trials that compared the addition of mood medication to TSD (26–28) ( Table 3 ). Clinical remission was defined by the study authors as HDRS-21 score < 8. Pooled log OR was 2.365 (95% CI 0.95 to 3.779, p < 0.001; Figure 3C ), compared with TSD, adding mood medication was associated with a significant clinical remission after 3 months. There was evidence of heterogeneity between the studies (I2 = 46.35, Q = 3.71, df = 2, p = 0.16). Visual inspection of funnel plot showed slight asymmetry ( Figure 3D ), however Egger test did not suggest small-study effects (β 1 = 6.71, SE of β 1 = 3.9, z = 1.72, p = 0.0854).
Tolerability and Treatment-Emergent Mania or Hypomania
The rates of treatment-emergent mania or hypomania were available for four studies (11, 27, 29, 30). At the end of the treatment, five (4.35%) of 115 patients given medication combined with TSD, four (4.17%) of 96 patients given TSD and zero (0%) of 17 patients given mood medication had episodes of treatment-emergent mania or hypomania. Due to different control groups in the studies that reported affective switch (11, 30), it was not possible to pool the data in a meta-analysis ( Tables 2 and 3 ).
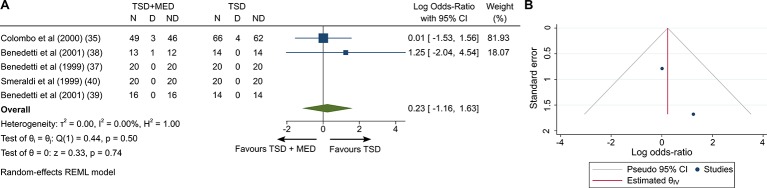
The rates of dropout were available for all studies (11, 26–30). Overall, nine (5.8%) of 155 patients given mood medication combined with TSD, four (2.92%) of 137 given TSD and zero (0%) of 17 given mood medication were lost to follow-up ( Tables 2 and 3 ). Pooled log OR was 0.234 (95% CI −1.164 to 1.632, p = 0.74; Figure 4A ) compared with TSD; adding mood medication was not associated with differences tolerability (dropout rate). Between-sample heterogeneity was not significant (I2 = 0, Q = 0.44, df = 1, p = 0.5). Visual inspection of funnel plot showed slight asymmetry ( Figure 4B ).
Figure 4.
Tolerability at the end of the trial adding medication to TSD (A) and the funnel plot of included studies (B). TSD, Total sleep deprivation; MED, Medication; CI, Confidence interval; SD, Standard deviation; N, Sample size; D, Dropout; NR, No dropout; Tests of heterogeneity, I2 and Q statistic were included; REML, Restricted maximum likelihood.
Discussion
This meta-analysis provides three main results regarding the efficacy of Sleep deprivation (SD) in bipolar depression. Our first result suggests that adding TSD to medication acutely increases the antidepressant effectiveness relative to mood medications alone at the first week, although this effect was not maintained in the following weeks. The second result shows that the addition of medication to TSD increased antidepressant effectiveness at day 10 of treatment relative to TSD alone. The third result yields the conclusion that the use of medication plus TSD increased clinical remission at a 3-month follow-up. Finally, regarding tolerability and treatment-emergent mania or hypomania, our results show no differences between treatments.
We initially proposed a twofold research question. These results suggest that chronotherapeutic strategies such as SD, regardless of their long-term efficacy, may be useful therapeutic tools to treat the symptoms of bipolar depression while the initial effect of antidepressant drugs is achieved. This finding is consistent with the widely documented rapid-onset antidepressant effects of SD (10, 14, 23). It should be considered that, even though drugs’ antidepressant effects increase over a period of weeks, the impact of chronotherapeutic strategies was not statistically significant at week 2 or further on. SD’s use in the medium or long term would not be so well justified if pharmacological therapy is already effective. Furthermore, if mood medication shows a synergistic effect on short-term follow-up, there is no reason to use TSD without drugs. A possible exception would be patients who have an absolute contraindication for mood medication use. Relative to the long-term effects of adding medication to TSD, such strategy suggests a much lower rate of symptomatic relapse after recovery from sleep when TSD is used alone. Our results showed that concomitant use of mood medications and TSD maintained clinical remission on the long-term follow-up phase.
In our search, only two previous meta-analysis related to SD were found. The first (18) studied diurnal variation as a response predictor for SD. Recently another meta-analysis was published including a total of 66 studies with a huge heterogeneity (different SD modalities, different depressive samples, demographics and different definitions of treatment response) and showed an overall response rate of 45% among the six randomized studies and 50% among the remaining nonrandomized included studies (24). Our results differed from this meta-analysis since they did not find a significant effect of adding medications to SD. The fact that they included unipolar, bipolar, and mixed patient samples, and did not make a difference between short- and long-term outcomes might explain our different results. Moreover, the same meta-analysis concluded that response rate was not significantly affected by SD modality or sample type (unipolar, bipolar, or mixed). Unlike what has been reported in previous publications, the results of that meta-analysis indicate a nonsignificant tendency of a lower response in bipolar patients when compared to unipolar patients. This might be explained by the exclusion of studies in which other chronotherapeutic strategies were applied in conjunction with SD that show better response rates. For this reason, the authors propose that bipolar patients could benefit from SD mainly when it is associated with other chronotherapeutic therapies.
Recent clinical guidelines in Major Depression Disorder (41), consider SD as a third-line adjunctive therapy for more severe and refractory forms of MDD in combination with other chronotherapeutic strategies. On the other hand, bipolar disorder clinical guidelines (42), consider chronotherapeutic strategies as novel or experimental options and are therefore seen as the last step among therapeutic alternatives. A recent systematic review claim that sleep deprivation research provides a low level of evidentiary support, mainly from uncontrolled studies, for the acute management of bipolar depression (43). Additionally, some promoters of TSD therapies propose its use even as a first-line strategy for people affected by mood disorders, given its safety (14). On the basis of our results, patients treated with TSD, with the concomitant use of mood medications (Lithium and monoaminergic drugs were used in the included trials) resulted in a rapid and sustained long-term antidepressant effect. TSD seems to be a recommended strategy in bipolar patients who fall into a depressive episode while keeping the previous treatment with mood medications such as lithium.
This study has some limitations and entails further research questions and challenges. The first and probably major limitation is related to the small number of clinical trials available in literature that could meet inclusion criteria. It is important to note that, although there are many studies with available data about response rates and even relapse rates in patients subjected to different modalities of sleep deprivation, many of these have different methodologies and objectives than those analyzed in our study, such as functional brain studies or analysis of biological markers in responder patients versus non-responders to sleep deprivation. However, for the purposes of our initial research questions, clinical trials that directly assessed the impact of pharmacological and chronotherapeutic interventions in comparable terms were necessary. This research feature limited the number of studies that meet the inclusion criteria and showed that this is a research area in which new clinical trials are strongly needed to obtain more conclusive results. This would allow for more informed clinical decision making. Another limitation of this study is related to the small number of patients that each selected clinical trial recruited. The very difficulties that come when implementing these treatment modalities could explain low participant numbers. Recruiting volunteers or patients willing to receive these treatments can be challenging. Moreover, the included trials had differences in dependent and independent variables, such as: differences in prescribed mood medications (different antidepressants and Lithium) and doses, outcome scales (VAS, HDRS, MADRS), and chronotherapeutic modalities (PSD, TSD with or without light therapy or other biological treatments), placebo conditions and diagnosis criteria. These differences must be considered as limitations for the generalization of our findings.
Although a new timeframe criterion for hypomania was added in DSM IV compared to DSM-III-R (44) (the two versions used in the trials included in this meta-analysis), this is probably a comparatively minor limiting factor. However, the evolution of the definition of bipolar disorder type I, type II, and bipolar spectrum disorders is an issue to be considered in the heterogeneity of the samples in future research.
These limitations could not only significantly restrict the generalization of our findings, but they are also a call for researchers to consider lowering levels of heterogeneity in key methodological parameters such as randomization and placebo medication, despite the inherent difficulties of chronotherapy research. Furthermore, trials have not yet systematically addressed other important questions such as the differential effect of TSD regarding bipolar subtypes.
Conclusion
Our results suggest that adding chronotherapeutic interventions to mood medications for depressive episodes in bipolar patients seems to increase the antidepressant effect of the drugs early on of the treatment and maintaining the mood effects for the long-term follow-up.
Author Contributions
JR-M, ER-S, PV, and LR conceived the study. JR-M and ER-S did the literature review, statistical analyses, and drafted the report. JR-M and ER-S extracted the data. All authors interpreted the data and edited and approved the final report.
Funding
Work by JR-M was funded by CONICYT PIA ACT 1414 and CONICYT FONDECYT (Ref: 3190311). PV has received salary support through the Fund for Innovation and Competitiveness (FIC) of the Chilean Ministry of Economy, Development and Tourism, through the Millennium Scientific Initiative (grant number IS130005). The cost of publication was funded by Fundacion Hospital Clínico de la Universidad de Chile.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was carried out at the Psychiatric Clinic of the University of Chile. We acknowledge psychiatrists, psychologists, and residents of the Psychiatric Clinic of the University of Chile for their comments and suggestions. We thank Luz Maria Alliende for his insightful comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00070/full#supplementary-material
References
- 1. Charlson F, van OM, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet (2019) 394(10194):240–8. 10.1016/S0140-6736(19)30934-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldessarini RJ, Salvatore P, Khalsa H-MK, Gebre-Medhin P, Imaz H, González-Pinto A, et al. Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord (2010) 12(3):264–70. 10.1111/j.1399-5618.2010.00812.x [DOI] [PubMed] [Google Scholar]
- 3. Bonnín CDM, Reinares M, Martínez-Arán A, Jiménez E, Sánchez-Moreno J, Solé B, et al. Improving functioning, quality of life, and well-being in patients with bipolar disorder. Int J Neuropsychopharmacol (2019) 22(8):467–77. 10.1093/ijnp/pyz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tondo L, Vázquez GH, Baldessarini RJ. Depression and Mania in bipolar disorder. Curr Neuropharmacol (2017) 15(3):353–8. 10.2174/1570159X14666160606210811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN. Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry (2016) 3(12):1138–46. 10.1016/S2215-0366(16)30264-4 [DOI] [PubMed] [Google Scholar]
- 6. Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry (2010) 18(3):143–57. 10.3109/10673221003747955 [DOI] [PubMed] [Google Scholar]
- 7. Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish National Cohort Study. JAMA Psychiatry (2013) 70(9):931–9. 10.1001/jamapsychiatry.2013.1394 [DOI] [PubMed] [Google Scholar]
- 8. Schulte W. Zum Priblem der provokation und kupierung von Melancholischen Phasen. Schweizer. Arch Neurol Neurochem Psychiatr (1971) 109:427–35. [PubMed] [Google Scholar]
- 9. Steinberg H, Hegerl U. Johann Christian August Heinroth on sleep deprivation as a therapeutic option for depressive disorders. Sleep Med (2014) 15(9):1159–64. 10.1016/j.sleep.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 10. Pflug B. The effect of sleep deprivation on depressed patients. Acta Psychiatr Scand (1976) 53(2):148–58. 10.1111/j.1600-0447.1976.tb00068.x [DOI] [PubMed] [Google Scholar]
- 11. Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry (2009) 66(3):298–301. 10.1016/j.biopsych.2009.02.018 [DOI] [PubMed] [Google Scholar]
- 12. Benedetti F. Antidepressant chronotherapeutics for bipolar depression. Dialog Clin Neurosci (2012) 14(4):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry (1999) 46(4):445–53. 10.1016/S0006-3223(99)00125-0 [DOI] [PubMed] [Google Scholar]
- 14. Dallaspezia S, Benedetti F. Sleep deprivation therapy for depression. Curr Top Behav Neurosci (2015) 25:483–502. 10.1007/7854_2014_363 [DOI] [PubMed] [Google Scholar]
- 15. Riemann D, König A, Hohagen F, Kiemen A, Voderholzer U, Backhaus J, et al. How to preserve the antidepressive effect of sleep deprivation: A comparison of sleep phase advance and sleep phase delay. Eur Arch Psychiatry Clin Neurosci (1999) 249:231–7. 10.1007/s004060050092 [DOI] [PubMed] [Google Scholar]
- 16. Benedetti F, Colombo C. Sleep deprivation in mood disorders. Neuropsychobiology (2011) 64(3):141–51. 10.1159/000328947 [DOI] [PubMed] [Google Scholar]
- 17. Barbini B, Colombo C, Benedetti F, Campori E, Bellodi L, Smeraldi E. The unipolar–bipolar dichotomy and the response to sleep deprivation. Psychiatry Res (1998) 79(1):43–50. 10.1016/S0165-1781(98)00020-1 [DOI] [PubMed] [Google Scholar]
- 18. Bouhuys AL. Towards a model of mood responses to sleep deprivation in depressed patients. Biol Psychiatry (1991) 29(6):600–12. 10.1016/0006-3223(91)90095-4 [DOI] [PubMed] [Google Scholar]
- 19. Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog Neuropsychopharmacol Biol Psychiatry (2002) 26(6):1167–70. 10.1016/S0278-5846(02)00255-5 [DOI] [PubMed] [Google Scholar]
- 20. Duncan WC, Zarate CA. Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep (2013) 15(9):394. 10.1007/s11920-013-0394-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuhs H, Tölle R. Sleep deprivation therapy. Biol Psychiatry (1991) 29(11):1129–48. 10.1016/0006-3223(91)90255-K [DOI] [PubMed] [Google Scholar]
- 22. Schmitt K, Holsboer-Trachsler E, Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann Med (2016) 48(1-2):42–51. 10.3109/07853890.2015.1131327 [DOI] [PubMed] [Google Scholar]
- 23. Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry (1990) 147(1):14–21. 10.1176/ajp.147.1.14 [DOI] [PubMed] [Google Scholar]
- 24. Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, et al. Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry (2017) 78(8):e1020–34. 10.4088/JCP.16r11332 [DOI] [PubMed] [Google Scholar]
- 25. Benedetti F, Barbini B, Lucca A, Campori E, Colombo C, Smeraldi E. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci (1997) 247(2):100–3. 10.1007/BF02900200 [DOI] [PubMed] [Google Scholar]
- 26. Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Ongoing lithium treatment prevents relapse after total sleep deprivation. J Clin Psychopharmacol (1999) 19(3):240–5. 10.1097/00004714-199906000-00007 [DOI] [PubMed] [Google Scholar]
- 27. Smeraldi E, Benedetti F, Barbini B, Campori E, Colombo C. Sustained antidepressant effect of sleep deprivation combined with pindolol in bipolar depression. a placebo-controlled trial. Neuropsychopharmacology> (1999) 20(4):380–5. 10.1016/S0893-133X(98)00129-8 [DOI] [PubMed] [Google Scholar]
- 28. Benedetti F, Barbini B, Campori E, Fulgosi MC, Pontiggia A, Colombo C. Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression: new findings supporting the internal coincidence model? J Psychiatr Res (2001) 35(6):323–9. 10.1016/S0022-3956(01)00034-6 [DOI] [PubMed] [Google Scholar]
- 29. Benedetti F, Campori E, Barbini B, Fulgosi MC, Colombo C. Dopaminergic augmentation of sleep deprivation effects in bipolar depression. Psychiatry Res (2001) 104(3):239–46. 10.1016/S0165-1781(01)00332-8 [DOI] [PubMed] [Google Scholar]
- 30. Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res (2000) 95(1):43–53. 10.1016/S0165-1781(00)00164-5 [DOI] [PubMed] [Google Scholar]
- 31. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr (1960) 23:56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry (1979) 134:382–9. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 33. Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med (2002) 21(11):1575–600. 10.1002/sim.1188 [DOI] [PubMed] [Google Scholar]
- 34. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (2011) 342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 35. Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ (2002) 325(7365):652–4. 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borenstein Introduction to Meta-Analysis. Edición: 1. Chichester: John Wiley & Sons; (2009). 456 p. [Google Scholar]
- 37. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baxter LR, Liston EH, Schwartz JM, Altshuler LL, Wilkins JN, Richeimer S, et al. Prolongation of the antidepressant response to partial sleep deprivation by lithium. Psychiatry Res (1986) 19(1):17–23. 10.1016/0165-1781(86)90088-0 [DOI] [PubMed] [Google Scholar]
- 39. Leibenluft E, Moul DE, Schwartz PJ, Madden PA, Wehr TA. A clinical trial of sleep deprivation in combination with antidepressant medication. Psychiatry Res (1993) 46(3):213–27. 10.1016/0165-1781(93)90090-4 [DOI] [PubMed] [Google Scholar]
- 40. Suzuki M, Dallaspezia S, Locatelli C, Uchiyama M, Colombo C, Benedetti F. Does early response predict subsequent remission in bipolar depression treated with repeated sleep deprivation combined with light therapy and lithium? J Affect Disord (2018) 229:371–6. 10.1016/j.jad.2017.12.066 [DOI] [PubMed] [Google Scholar]
- 41. Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. complementary and alternative medicine treatments. Can J Psychiatry (2016) 61(9):576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord (2013) 15(1):1–44. 10.1111/bdi.12025 [DOI] [PubMed] [Google Scholar]
- 43. Gottlieb JF, Benedetti F, Geoffroy PA, Henriksen TEG, Lam RW, Murray G, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord (2019) 21(8):741–73. 10.1111/bdi.12847 [DOI] [PubMed] [Google Scholar]
- 44. Mason BL, Brown ES, Croarkin PE. Historical underpinnings of bipolar disorder diagnostic criteria. Behav Sci (2016) 6(3):14. 10.3390/bs6030014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.