Abstract
Purpose: This study aimed to determine the epidemiological features of herpes zoster (HZ) in Qatar. Methods: This study was a retrospective review of all reported HZ cases received by the surveillance unit at the Ministry of Public Health, between January 2012 and December 2017. Results: A total of 2815 cases were reported during the study period. The mean incidence of HZ was estimated to be 19/100,000 population, which increased from 9.8/100,000 in 2012 to 36.2/100,000 in 2017. The ratio of male/female was about 4:1. HZ incidence overall was found to be highest in those aged ≥ 50 years. According to nationality, the mean incidence of HZ was estimated to be 79/100,000 among Qataris and 101/100,000 among expatriates. Additionally, more HZ cases were notified during the hot months. Conclusion: Such epidemiological data will contribute to the baseline information, which is necessary for effective preventive and control measures to be implemented in the country.
Keywords: herpes zoster, incidence, Qatar
Introduction
Herpes zoster (HZ), also known as shingles, occurs as a latent reactivation of varicella–zoster virus (VZV) and is characterized by a painful rash of dermatomal distribution.1 VZV is a human DNA virus belonging to the Herpesviridae family and known to be transmissible through the aerosol route and direct skin contact with vesicular lesions of the primary infection (chickenpox). The reactivation of the dormant VZV in the dorsal root ganglia has been associated with the decline in cell-mediated immunity that occurs with aging. In addition, several complications of HZ have been described in the literature such as acute neuritis, postherpetic neuralgia, vasculopathy, myelopathy, retinal necrosis, and cerebellitis.2–3
HZ remains a global public health concern with an incidence ranging from three to five people per 1000 person-years across countries of North America, Europe, Asia, and the Middle East. The incidence of the disease is higher among older than younger people.4 In addition, HZ has a predilection to countries with a temperate climate, occurring mainly in late winter and spring seasons.5 The disease and its complications inflict an increasing economic burden on individual patients, their families, and the entire healthcare system. Moreover, the financial burden can be further stratified into direct and indirect costs. The direct costs refer to expenditures associated with the clinical management of the disease and include clinical consultations (e.g., primary care consultations and emergency visits), medical prescriptions (e.g., antivirals, analgesics, and anti-epileptics), hospital admissions, diagnostic procedures (e.g., blood tests, urine tests, and chest and abdominal X-rays), and surgical procedures. On the other hand, the indirect costs are those incurred due to the loss of productivity and absence from employment.6
In 2002, the State of Qatar became the first in the Middle East region to include the varicella vaccine as part of its national immunization program. Subsequently, most of the children in the country are immunized to VZV and received two doses of the vaccine: the first at 1 year of age and the second between 4–6 years of age.3
Currently, shingles is one of the 78 notifiable infectious diseases in Qatar. The national surveillance system, based at the Ministry of Public Health (MoPH), divides notifiable diseases into two categories according to national and international disease-reporting regulations. Class A denotes all diseases that must be notified immediately (within 24 hours). On the other hand, Class B refers to diseases that should be reported as soon as possible (up to one week) such as shingles.
Due to the limited evidence on the epidemiological characteristics of HZ in Qatar, the current study sought to evaluate the incidence and other epidemiological patterns of HZ. Such evidence will help public health authorities update their current preventive and control measures regarding HZ in Qatar.
Materials And Methods
This study was a retrospective review of all reported HZ cases received by the surveillance unit at the MoPH between January 2012 and December 2017.
Study setting
The national surveillance system in Qatar receives notifications from all healthcare facilities across the country, mainly from Hamad Medical Corporation (HMC) and Primary Health Care Corporation (PHCC). HMC is the main nonprofit healthcare provider that manages 10 highly specialized hospitals. PHCC is the main governmental provider of primary healthcare in the country and operates 27 primary health centers. Other reporting sites include different governmental, semi-governmental, and private health institutions.
Case definition
The diagnosis of HZ is usually based on clinical judgment since the disease is characterized by vesicles in different phases of development on the face, chest, and extremities. Upon latent reactivation of VZV, the disease is known as “shingles” and defined by a sore, unilateral, and vesicular rash with a dermatomal distribution. The latter case definition was used throughout the current study.
Data analysis
The data were entered in Microsoft Excel and statistical analysis was performed using StataCorp, 2011 (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). The descriptive epidemiological data such as the cumulative yearly incidence, the annual age-specific incidence rates, and the monthly average number of HZ cases by season were calculated based on the study period. Moreover, the age groups were stratified as < 1 year, 1–4 years, 5–9 years, 10–14 years, 15–19 years, 20–29 years, 30–39 years, 40–49 years, and ≥ 50 years. In addition, the nationality variable was categorized into two groups: Qatari and non-Qatari.
This study was approved by the Research Committee at the MoPH in Qatar.
Results
Incidence of HZ
A total of 2815 HZ cases were reported during the six-year study period from 2012 to 2017. Overall, the mean HZ incidence was estimated to be 19/100,000 population with an increasing trend from 9.8/100,000 in 2012 to 36.2/100,000 in 2017. Although a slight gradual decrease was observed between 2012 and 2014, such decline was followed by a significant (p < 0.05) and consistent increase from 2015 to 2017 (Figure 1). Among the notified cases, HZ incidence was highest in men (83%), and the male–female ratio was approximately 4:1. According to nationality, the non-Qatari residents were responsible for the vast majority (88%) of the reported cases than Qatari citizens (12%). Upon accounting for the population representation of each category, the mean incidence of HZ was estimated to be 79/100,000 among Qataris and 101/100,000 among expatriates.
Figure 1.
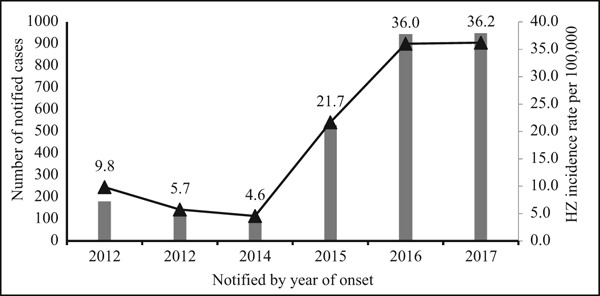
Incidence rate of herpes zoster (HZ) in Qatar, 2012–2017.
Based on age-specific HZ incidence data, there was an overall increase in the incidence of HZ with increasing age. The highest incidence was among those aged 50 years or more (47.7/100,000 populations; Figure 2).
Figure 2.
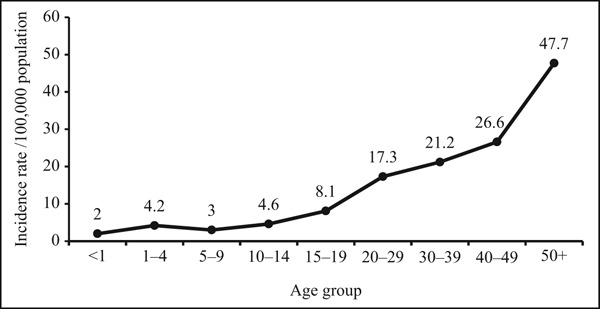
Age-specific incidence rate of herpes zoster in Qatar, 2012–2017.
The total number of notified HZ cases fluctuated across the different seasons with a mean ± standard deviation of 234.6 ± 50.1 (Figure 3). The majority of cases were notified during spring and summer (March to September), whereas fewer cases were reported during the fall and winter (October to February). However, the difference was not statistically significant.
Figure 3.
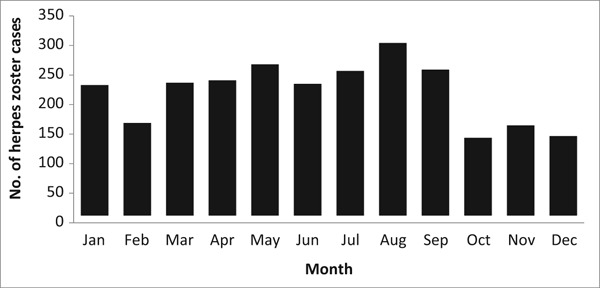
Average notified cases of herpes zoster in Qatar by month, 2012–2017.
Discussion
The current study describes the epidemiological characteristics of HZ cases in the State of Qatar between 2012 and 2017. The study results showed an increasing trend in the incidence of HZ between 2015 and 2017, a gender predilection for men, a markedly increased occurrence of HZ among those aged ≥ 50 years, and a seasonal variation. The latter finding did not satisfy a level of statistical significance but might denote clinical importance.
The inclining trend of HZ incidence between 2015 and 2017 is in agreement with previous global evidence. Moreover, several factors have been implicated in this regard such as the demographic transition, increased utilization of immune-modulating medications, and expanded childhood vaccination against the VZV.7
The present study showed that HZ predominantly occurred among men, which corroborates previous studies from regional countries that revealed a male–female ratio of 2:1, 3.1:2, and 3:2 in the Kingdom of Saudi Arabia, Yemen, and Iran, respectively.8–10 Recently, a systematic review and meta-analysis on HZ identified the female gender as a risk factor (relative risk = 1.31).11 The majority of HZ cases in the current study were non-Qatari that matches the demographic make-up of the country in which foreign nationals comprise a large portion (88%) of the population.12 The reasons behind such gender disparity could vary between socioeconomic status, health literacy, and occupational background. However, the exact cause could not be determined in the current study because of the scarcity and/or incompleteness of data in the notification forms. To heighten the clinical suspicion of healthcare providers, it is vital to update their knowledge about the gender disparity of HZ incidence in Qatar.
The present study shows a gradual increase with age and was highest in the ≥ 50 years’ age group, which is in agreement with previous local and global data.13–15 This could be due to the fading cell-mediated immunity of the elderly population to the HZ virus and immunosenescence.16 Additionally, based on several studies, HZ disease does not show any specific seasonal variation because it depends on the immunocompetency of each patient. Thus, the time of viral reactivation will vary accordingly between one person and another.16–17 In the present study, seasonal variation was observed in the occurrence of HZ disease but was not statistically significant. The HZ incidence slightly fluctuated between January and September, but it dropped suddenly in October and remained low till December. Our data corroborates a previous study from Bangladesh, where the occurrence of HZ peaked during the rainy season.18 Nevertheless, the literature reveals that the incidence of HZ peaks during the summer season because of a significant association with temperature.19 The higher number of sunny hours during the summer season has been associated with increased exposure to ultraviolet irradiation that might serve as an immunosuppressant and reduce the immunity to VZV.20 Thus, it is suggested that the zoster vaccine be included in the national immunization schedule and administered to the elderly as part of their routine medical care.21
To the best of our knowledge, this is the first epidemiological study to determine the incidence of HZ in the State of Qatar by age, gender, nationality, and seasonality. Unlike earlier hospital-based studies, the present research relies on six years of national data, making it more representative of HZ characteristics in Qatar. However, there are several limitations to the present study. First, the study only included the notified HZ cases, while milder cases might have been missed due to failure to seek healthcare. The lack of an automated electronic notification system in the country resulted in the incomplete reporting of all HZ cases. Therefore, the unreported data elements in some notification forms may have led to an information gap about the epidemiologic characteristics of the disease. Another issue is the categorization of age groups in the notification forms, where the eldest age group category was ≥ 50 years, thus limiting further stratification of risky age groups. In addition, the lack of information about the cases’ comorbidities or complications hindered further analysis to identify more associated factors with HZ in Qatar.
Conclusion
In conclusion, this study aimed to analyze the epidemiological characteristics of HZ in Qatar between 2012 and 2017. Overall, there was an increasing trend of HZ over the six years of the study period. The findings highlighted that HZ infections were more frequent with increasing age, in men, and during hot weather. Therefore, HZ vaccination campaigns during the winter season could be more beneficial. Based on the present study, notification forms should be reviewed and accommodate more variables such as age groups, socioeconomic status, and comorbidities. Such epidemiological data will contribute to the baseline information, which is necessary for effective preventive and control measures to be implemented in the country. In addition, it is vital to raise awareness among healthcare providers regarding the importance of completing the notification forms. Further studies are needed to investigate the possible causes of the increasing trend of the disease in Qatar.
Authors’ Contributions
AA conceived the study; NK and AA analyzed the data; and AA, MC, DB, and EF drafted the manuscript. All authors read and approved the final manuscript.
Competing Financial Interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public.
References
- 1.Yawn B, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller N, Gilden D, Cohrs R, Mahalingam R, Nagel M. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008;26(3):675–697. doi: 10.1016/j.ncl.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Turab M, Chehadeh W. Varicella infection in the Middle East: prevalence, complications, and vaccination. J Res Med Sci. 2018;23:19. doi: 10.4103/jrms.JRMS_979_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai K, Gebremeskel B, Acosta C. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields B, Knipe D, Howley P. 6th Ed. Philadelphia: Wolters Kluwer; Lippincott Williams & Wilkins; 2013. Fields virology. [Google Scholar]
- 6.Panatto D, Bragazzi N, Rizzitelli E, Bonanni P, Boccalini S, Icardi G et al. Evaluation of the economic burden of Herpes Zoster (HZ) infection. Hum Vaccin Immunother. 2015;11(1):245–262. doi: 10.4161/hv.36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehrhahn M, Dwyer D. Herpes zoster: epidemiology, clinical features, treatment and prevention. Aust Prescr. 2012;35(5):143–147. [Google Scholar]
- 8.Alakloby O, Al Jabre S, Randhawa M, Alzahrani A, Al Wunais K, Bukhari I. Herpes zoster in eastern Saudi Arabia: clinical presentation and management. J Drugs Dermatol. 2008;7(5):457–462. [PubMed] [Google Scholar]
- 9.Al-Shami M. Herpes zoster in Al-Kuwait university hospital in Sana'a city Yemen: clinical presentation and complications. Alandalus Journal for Social and Applied Sciences. 2014;6:17–38. [Google Scholar]
- 10.Babamahmoodi F, Alikhani A, Ahangarkani F, Delavarian L, Barani H, Babamahmoodi A. Clinical manifestations of herpes zoster, its comorbidities, and its complications in North of Iran from 2007 to 2013. Neurol Res Int. 2015;2015:896098. doi: 10.1155/2015/896098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai K, Yawn B. Risk factors for herpes zoster: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4(1):S313–S314. [Google Scholar]
- 12.Chehab M, Bala M, Al-Dahshan A. Travel medicine in Qatar: strategic action for better public health outcome. Int J Travel Med Glob Health. 2018;6(3):137–138. [Google Scholar]
- 13.Lu W, Lin C, Wang C, Chen L, Hsiao F. Epidemiology and long-term disease burden of herpes zoster and postherpetic neuralgia in Taiwan: a population-based, propensity score-matched cohort study. BMC Public Health. 2018;18(1):369. doi: 10.1186/s12889-018-5247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marziano V, Poletti P, Guzzetta G, Ajelli M, Manfredi P, Merler S. The impact of demographic changes on the epidemiology of herpes zoster: Spain as a case study. Proc Biol Sci. 2015;282(1804):20142509. doi: 10.1098/rspb.2014.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Zheng H, Qu H, Deng H, Zhang J, Ma W et al. Epidemiology of herpes zoster among adults aged 50 and above in Guangdong. China. Hum Vaccin Immunother. 2015;11(8):2113–2118. doi: 10.1080/21645515.2015.1016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershon A, Gershon M, Breuer J, Levin M, Oaklander A, Griffiths P. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Lee C, Lim C, Jeon W, Park Y. Population-based study of the epidemiology of herpes zoster in Korea. J Korean Med Sci. 2014;29(12):1706–1710. doi: 10.3346/jkms.2014.29.12.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman M, Karim M, Tauhid S, Das A, Sarkar S. Is there any seasonal influence of herpes zoster? Faridpur Med Coll J. 2017;12(1):14–17. [Google Scholar]
- 19.Wu P, Wu H, Chou T, Sung F. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One. 2013;8(10):e77709. doi: 10.1371/journal.pone.0077709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zak-Prelich M, Borkowski J, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect. 2002;129(3):593–597. doi: 10.1017/s0950268802007793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di-Pietro A, Facciolà A, Visalli G. Herpes zoster vaccine: a protection for the elderly. Ann Ig. 2018;30(4):23–27. doi: 10.7416/ai.2018.2230. [DOI] [PubMed] [Google Scholar]


