Abstract
Background
The topic of false memory in schizophrenia has been well documented in earlier research contributions. To date, there is no study exploring the implications of specific neural networks during this phenomenon in patients suffering from schizophrenia.
Methods
We compared 17 patients suffering from psychosis (SCZ) to 33 healthy controls (HC) performing a verbal memory task designed to produce false memories, i.e. the Deese–Roediger–McDermott paradigm (DRM). Electroencephalography was used to specifically analyze the P2 and N400 event-related potentials components.
Results
The SCZ patients showed a reduced ability to distinguish between true and false memories as assessed by the A′ index which was calculated based on the false and true memory rates. The morphology of the P2 differed in frontal electrode region with a lower amplitude in SCZ. In addition, the amplitude of N400 was more pronounced (more negative) in HC than in SCZ in centro-parietal electrode site.
Conclusions
We suggest that the differences found in P2 amplitude are associated with difficulties of SCZ patients to efficiently compare item-specific features of a mnesic elements to incoming stimuli which impair the subsequent verbal memory information processing reflected by the N400 component amplitude decrease. These results are consistent with the idea that SCZ use a different strategy while they perform the DRM paradigm.
Keywords: False memories, Schizophrenia, Event-related potentials (ERPs)
1. Introduction
In addition to the well documented memory deficits in patients suffering from schizophrenia (Aleman et al., 1999; Reichenberg et al., 2009), the phenomenon of false memories is an issue that needs to be addressed because its possible involvement in the pathogenesis of psychotic symptoms is regularly mentioned (Bhatt et al., 2010; Corlett et al., 2009; Elvevag et al., 2004; Laws and Bhatt, 2005; Moritz et al., 2004).
From an experimental perspective, the DRM (Deese–Roediger–McDermott) paradigm allows the study of false memories (Roediger and McDermott, 1995). It consists of a verbal recognition memory test which follows the presentation of a list of words semantically associated to a target word, not presented in the list. The rate of the words truly recognized as being presented in the list (old-yes) and of the words recognized as not being presented (new-no) can be measured. In addition, the lure-yes category refers to the rate of false recollections (false memories, false alarms) of the targets words which are semantically associated to the list but not present in the latter. Usually, a proportion of 60% of the “target” words are falsely identified (lure-yes) as “old” words (Roediger and McDermott, 1995).
The study of DRM performance in patients with schizophrenia brought mixed results (Gallo, 2006). A reduction of true (old-yes) and false memories (lure-yes) were described in schizophrenia patients in the DRM paradigm (Elvevag et al., 2004), whereas impaired true recognition (old-yes) and no difference in the number of false alarms (lure-yes) was reported in another study (Moritz et al., 2004). Moritz et al. (2004) proposed that the production of false alarms in patients suffering from schizophrenia mostly relies on a gist-based strategy when they perform the DRM. In other words, the false alarms are based on the identification of the general topic of the list of words associated to the target word (Brainerd and Reyna, 1998; Gallo, 2010; Straube, 2012). Schizophrenia patients may have difficulties in using the item-specific mnesic information of the words, i.e. their verbatim trace (Straube, 2012) and are therefore more likely to rely on a sense of familiarity (Weiss et al., 2002). In contrast, false recognition in healthy controls is thought to originate from the spreading of activation between conceptual representations of a mental lexicon during the study of words or the recognition phase (Gallo, 2010; Moritz et al., 2004; Roediger III et al., 2001). Altogether, healthy subjects and schizophrenic patients appear to differ in their strategies to fulfill the same memory task. This suggests that they might rely on different patterns of neuronal networks' activation.
Interestingly, event-related potentials (ERPs) analyses from electro-encephalographic (EEG) allow exploring both normal and pathological cognitive processes with an excellent temporal resolution. In healthy controls, the DRM task performances were associated with significant changes in the late ERP components (Cadavid and Beato, 2016; Curran, 2000; Curran et al., 2001, Curran et al., 2006; Duzel et al., 1997; Johnson et al., 1997) including the FN400 component which is a more frontal located form of the N400 (centro-parietal) related to familiarity-based recognition memory (Curran, 2000; Curran et al., 2001, Curran et al., 2006; Yonelinas, 2002). Yet, there are still no neurophysiological studies of the DRM in patients suffering from schizophrenia to our knowledge.
The P2 ERP component modulation was not particularly addressed in the DRM studies mentioned above. However, we chose to specifically analyzed this component because of its association with the early stages of information processing (Missonnier et al., 2012) which have been shown to be impaired in schizophrenic patients (Boutros et al., 2004; O'Donnell et al., 2004; Salisbury et al., 2010). Importantly, it was also linked to the processing of verbal memory tasks (Evans and Federmeier, 2007; Finnigan et al., 2011) similar to the DRM paradigm. Additionally, we chose to include an analysis of the N400 ERP component because of its involvement in verbal memory tasks (Curran, 2000; Curran et al., 2001, Curran et al., 2006; Finnigan et al., 2002; Yonelinas, 2002) and because specific modulations were previously identified in schizophrenic patients (Guillem et al., 2001; Kim et al., 2004).
In this study, we examined if patients suffering from psychosis (SCZ) are impaired in a false memory task in comparison with healthy controls (HC) and analyzed the neurophysiological correlates of this process. Additionally, we focused on the analysis of specific ERP components (P2 and N400) known to be relevant in the context of schizophrenia and verbal memory. We expected to find a decrease of the P2 and the N400 amplitudes in the SCZ group in comparison with the HC. We also expected a modulation of the N400 component associated with the processing of familiarity during the DRM task.
2. Methods
2.1. Participants
Seventeen French-speaking patients (SCZ) were recruited while being hospitalized at the Mental Health Network Fribourg (RFSM) for a psychotic decompensation. Eleven patients were diagnosed with paranoid schizophrenia (F20.0) and six with acute and transient psychotic disorders (F23). The diagnosis was made according to criteria of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (World Health Organization, 2004) by an experienced psychiatrist who was not part of the research team. Three patients had a history of recreational (mild) cannabis use. All except three patients were receiving antipsychotic medication. Medication dose was converted into chlorpromazine equivalents (Leucht et al., 2014). Thirty-three healthy adult controls closely matched in age were recruited. The presence of neurological disorders and mental retardation (evaluated with a shortened version of the 3rd edition of the Wechsler Adult Intelligence Scale (Ringe et al., 2002) was excluded in HC and SCZ participants. Psychiatric disorders in HC and psychiatric comorbidities in SCZ were excluded by an assessment with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998).
All participants were assessed for symptom severity using the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993) (Table 1) and were administered an extensive neuropsychological Battery, the CogState (see www.cogstate.com for details) (Table 2).
Table 1.
Demographic and clinical data of SCZ patients and HC.
| Characteristics | Patients Mean (SD) |
Controls Mean (SD) |
p-Value |
|---|---|---|---|
| Na | 17 | 33 | |
| Age range | 18–35 | 18–35 | |
| Age (years) | 25.0 (3.8) | 23.5 (4.2) | 0.201 |
| Sex (male: female ratio) | 13/4 | 9/24 | 0.002 |
| Laterality (right: left ratio) | 15:2 | 25:2 | 0.634 |
| BPRSb | 54.1 (9.9) | 24.8 (12.7) | 0.001 |
| - Reality distortion | 12.1 (3.3) | 3.9 (2.3) | 0.001 |
| - Disorganization | 6.0 (3.0) | 2.8 (1.5) | 0.001 |
| - Negative symptoms | 9.9 (2.7) | 2.8 (1.5) | 0.001 |
| CPZ equivalentsc | 542.0 (582.7) | – | 0.003 |
Data are presented as mean (SD). The demographic sex characteristic was included as co-variable in the statistical model. Significant p-values are shown in italics.
Number of participants.
Brief Psychiatric Rating Scale.
Chlorpromazine equivalents.
Table 2.
Cogstate neuropsychological performances for SCZ patients and HC.
| Patients Mean (SD) |
Controls Mean (SD) |
p-Value | |
|---|---|---|---|
| Executive function | |||
| Set-shifting task - ER | 29.5 (20.0) | 16.3 (7.7) | 0.005 |
| Executive function/spatial problem solving | |||
| Groton maze learning test - ER tot | 60.1 (27.4) | 42.3 (16.1) | 0.004 |
| Psychomotor function speed of processing | |||
| Detection task speed, log10(ms) | 2.6 (0.2) | 2.5 (0.1) | 0.001 |
| Visual attention/vigilance | |||
| Identification task speed, log10(ms) | 2.7 (0.1) | 2.7 (0.1) | 0.001 |
| Visual learning and memory | |||
| Groton maze learning test - DRE | 60.1 (27.3) | 42.3 (16.1) | 0.004 |
| Verbal learning and memory | |||
| International shopping list | |||
| - CR tot | 25.9 (4.3) | 30.3 (3.1) | 0.002 |
| - DR | 8.6 (2.0) | 11.2 (0.9) | 0.001 |
| Working memory | |||
| One back task (Acc) | 1.2 (0.2) | 1.3 (0.2) | 0.401 |
| Social cognition | |||
| Social-emotional cognition task (Acc) | 1.0 (0.2) | 1.2 (0.1) | 0.001 |
Notes: Data are presented as mean (SD). AP, accuracy of performance (arcsine transformation of the square root of the proportion of correct responses); ER tot, total number of errors; DR, delayed recall (number of correct responses); CR tot, total number of correct responses.; Acc, accuracy (arcsine transformation of the square root of the proportion of correct responses). Significant p-values are shown in italics.
The study was approved by the Ethics Committee of the University of Fribourg, Switzerland, and the study protocol was in accord with the Helsinki Declaration.
2.2. Task and procedure
The participants performed a French adapted version of the DRM task (Bonin et al., 2013; Curran et al., 2001; Dehon and Bredart, 2004; Ferrand, 2001; Ferrand and Alario, 1998; Plancher et al., 2008; Thérouanne and Denhière, 2004). They were instructed to memorize an auditory study list that contained 288 words grouped into 24 sets of 12 semantic associates. The semantic sets were common associates of a nonstudied theme word (“target” word). Next, a recognition list containing 288 words with 96 from each of the following three conditions: lure (target word), old (presented in the study list) and new (not present in the study list) was presented visually. The participants were asked to press a button to indicate whether they thought that the words presented on the screen were heard during the study list. Three responses to the DRM were processed for further analyses, i.e. the target words falsely identified as studied words (lure-yes), the truly recognized studied words (old-yes), and the truly rejected new words (new-no).
Stimuli presentation (auditory, visuals), trigger sending and response recording were implemented using the E-Prime software (Psychology Software Tools, Inc., Sharpsburg, USA).
2.3. Electrophysiological recording
EEG was recorded using 128 active surface Ag/AgCl electrodes-ActiveTwo MARK II Biosemi EEG System, BioSemi B.V., Amsterdam, Netherlands - mounted on a head cap (NeuroSpec Quick Cap) and referenced to mastoid channels using the Brain Vision Analyzer 2.0 software (Brain Products GmbH, Munich, Germany). EEG signals were corrected for eye blinks and other eye movement artifacts through an Independent Component Analysis (ICA). In addition, the EEG trials were automatically scanned for contamination by muscular or electrode artifacts. The total analysis window was 2000 ms, starting 495 ms before stimulus onset. The time epochs were band-pass filtered between 0.3 Hz and 30 Hz (−48 dB/octave for a low-pass filter).
2.4. Event-related potential analyses
ERP analyses were performed by averaging the EEG signal over a window of 1200 ms with a 200 ms pre-stimulus onset period. To investigate processes related to recognition memory and pathology, we examined the ERP for trials of words separately (old, new, and lure) in each group. ERPs were averaged with a 200 ms baseline epoch prior to stimulus onset.
The P2 component was analyzed at the frontal (C20, Fz and C22 combined) electrode location where it reached its highest amplitude. The P2 component is mostly described to be maximal at frontal electrodes (Luck and Hillyard, 1994; Ventura-Bort et al., 2016; Zhao et al., 2013). The mean amplitude of the P2 (i.e. area under the curve: AUC) was measured from the pre-stimulus baseline to the maximum peak within a specified window between 195 and 225 ms. This automatic analysis was completed using visual inspection by a trained neurophysiologist.
We measured the N400 component at the mid-parietal (Cz, A2 and CPz combined) electrode location where its amplitude was maximal, as documented in former studies (Kutas and Federmeier, 2011). The N400 component is a large component with no detectable peak. We measured the mean amplitude between two given latencies instead of peak measures (316–416 ms) to minimize the likelihood of confounding real amplitude reduction with amplitude reduction due to latency jitter.
2.5. Statistical analyses
Two-tailed t-tests and Fisher exact tests were used to compare performances (proportion of new-no, old-yes, lure-yes, A′ and B″D values for old/lure (Donaldson, 1992) neuropsychology, psychopathology, age, sex and laterality between HC and SCZ participants (Table 2, Table 3).
Table 3.
Proportion of responses, Accuracy (A′), and response Bias (B″D) of SCZ patients and HC.
| Variable | Patients Mean (SD) |
Controls Mean (SD) |
p-Value |
|---|---|---|---|
| P (lure-yes) | 0.49 (0.2) | 0.48 (0.1) | 0.694 |
| (Old-yes) | 0.57 (0.2) | 0.69 (0.1) | 0.020 |
| (New-no) | 0.76 (0.2) | 0.86 (0.1) | 0.098 |
| A′ old/lure | 0.56 (0.1) | 0.67 (0.1) | 0.022 |
| B″ old/lure | −0.08 (0.6) | −0.30 (0.3) | 0.098 |
Notes: Data are presented as mean (SD). Significant p-values are shown in italics.
A′ provides an estimation of the subjects ability to discriminate between old words and lures. This index is measure of discrimination between healthy controls and patients (adapted from Curran et al., 2001; Gallo, 2006). The formula of the A′ is: = 0.5 + (([old-yes - lure-yes]×[1 + old-yes - lure-yes])/([4×old-yes]×[1 - lure-yes])).
The B″D value reflects the liberal or conservative bias about old/lure discrimination (Donaldson, 1992; Curran et al., 2001). It is obtained by the formula: (([1 - old-yes]× [1 - lure-yes])-[old-yes×lure-yes])/(([1 - old-yes]× [1 - lure-yes] + [old-yes×lure-yes])).
Item conditions (old-yes, lure-yes, new-no) and participant group (HC, SCZ) were included as independent variables in a repeated-measure ANOVA model to analyze their respective influence on each of the EEG dependent variables (P2 latency, P2 and N400 amplitude). Two group comparisons (HC, SCZ) were performed with Fisher exact test, Kruskal-Wallis h test and t-test as appropriate.
The strength of the association between neuropsychological and EEG variables and DRM performance scores were evaluated using Spearman's rank correlation. Statistical analyses were corrected with the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995) as appropriate.
Analyses were performed using the Stata software package, version 14.2. We used the Stata regress command with the “vce(cluster)” option that specifies that the standard errors allow for intragroup correlations. Observations are thereby independent. The statistical threshold for α was set at p < 0.05. All statistical analyses were adjusted for sex and age.
3. Results
3.1. Demographical and cognitive data
The demographic and neuropsychological characteristics of the HC and SCZ groups, including p-values, are summarized in Table 1, Table 2. HC showed better scores than SCZ in most neuropsychological subtests.
3.2. Behavioural data
The proportion of old-yes responses was lower for SCZ than for HC (r = 0.12, p = 0.020). The A′ (old/lure) index which represents the ability to discriminate between old and lure items was also lower in SCZ (r = −0.08, p = 0.022). There were no other significant differences (see Table 3). The A′ index was negatively correlated to the N400 amplitude (r = −0.403, p = 0.004) for all conditions combined.
3.3. Event-related potential analysis
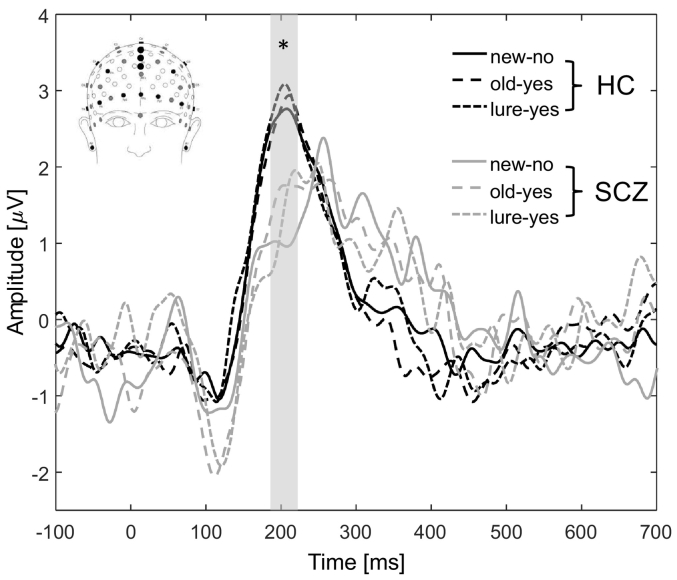
The ANOVA revealed that the amplitude of the P2 in frontal electrode location differed between groups (F(2,46) = 5.95; p = 0.019) with a significantly lower amplitude for SCZ (1.47 ± 1.96) compared to HC (2.86 ± 1.92) (r −1.61, p = 0.006; Fig. 1). There was no significant differences for factors age (F(2,46) = 0.07; p = 0.780), sex (F(2,46) = 0.11; p = 0.745) and conditions (F(2,46) = 0.15; p = 0.859) (Fig. 1).
Fig. 1.
Grand average event-related potentials (ERPs) at frontal (C20, Fz and C22) electrode locations for HC (black line) and SCZ patients (grey line) for the three experimental conditions (new-no: solid line; old-yes: dashed line and lure-yes: dotted line). Note the higher amplitude of P2 ERP in HC for all conditions.
⁎p < 0.05.
For all conditions combined, the P2 amplitude was correlated to the Cogstate verbal and visual memory scores (respectively, r = 0.381, p = 0.012 and r = 0.364, p = 0.018).
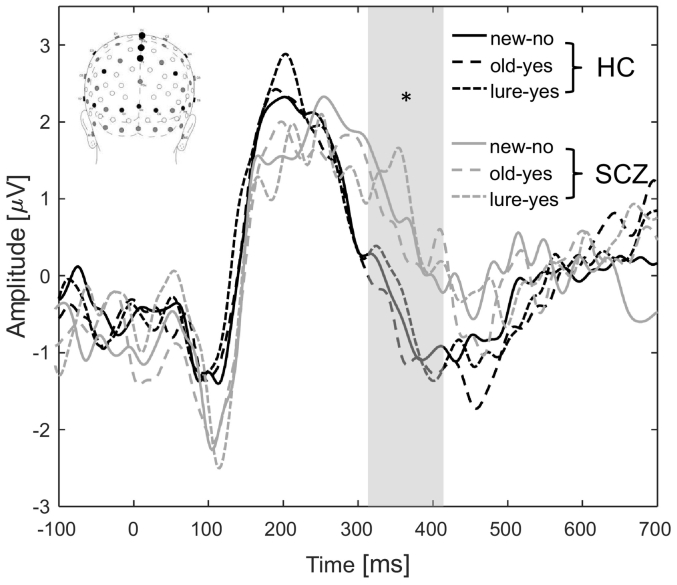
The amplitude of the N400 in centro-parietal electrode location showed a significant group effect (F(2,46) = 4.92; p = 0.032) with a higher amplitude for HC (−0.65 ± 1.37) compared to SCZ (0.66 ± 1.34) (r = 1.04, p = 0.028) (Fig. 2). There was no significant differences for factors age (F(2,46) = 0.40; p = 0.530), sex (F(2,46) = 0.16; p = 0.694) and conditions (F(2,46) = 0.29; p = 0.748). There was a significant correlation between the N400 amplitude and the Cogstate verbal memory score (r = −0.32, p = 0.0369) which failed to resist to multiple correction (p-value threshold: 0.0222).
Fig. 2.
Grand average event-related potentials (ERPs) at centro-parietal (Cz, A2 and CPz) electrode locations for HC (black line) and SCZ patients (grey line) for the three experimental conditions (new-no: solid line; old-yes: dashed line and lure-yes: dotted line). Note the significant decreased amplitude of the N400 component (grey rectangle) in HC for all conditions.
⁎p < 0.05.
4. Discussion
We confirmed that SCZ patients do not produce more false memories (lure-yes) than HC. However, they produced a lower rate of true recollections (old-yes) (Moritz et al., 2004, Moritz et al., 2006). Therefore SCZ patients showed a lower ability to distinguish between true and false memories (as assessed by the A′ index value) which were mainly driven by the differences in true recollection between these two groups. Such differences in verbal memory are well described specific psychosis-related deficits (Aleman et al., 1999; Reichenberg et al., 2009) and were confirmed by our neuropsychological results (Table 2).
Neurophysiological differences between SCZ patients and HC were already apparent at early stages of information processing. Indeed, we found that the P2 amplitude was lower in SCZ patients compared to HC, regardless of the conditions. This suggests difficulties or an inability to efficiently recruit specific neural networks in SCZ patients. Interestingly, it has been proposed that the P2 amplitude might reflect the processing of specific features of a mnesic element (verbatim traces). Indeed, two earlier studies reported that the P2 amplitude was greater when an item was identified among other figures (Luck and Hillyard, 1994) or when a stimulus was expected (Anllo-Vento et al., 1998; Luck and Kappenman, 2012) during visual memory tasks. The modulation of this component was therefore associated with “continued processing of the stimuli containing the attended feature value” (Luck and Kappenman, 2012). In the present study, the higher amplitude of P2 in HC could reflect a better efficiency at processing stored mnesic traces specific to a studied word (auditory study list) and to compare it to perceptual aspects of incoming stimuli (recognition task) (Evans and Federmeier, 2007). However, the correlations we found between the P2 amplitude and both visual and verbal memory tasks' scores suggest that during the DRM task, at this early stage of information processing, the verbatim traces are not exclusively dedicated to verbal information. Besides, the P2 amplitude was not correlated with the A′ index score nor with the true recollection rate, strongly suggesting that this component represents an early activation of multimodal mnesic elements probably not specific to false memory production. This apparent deficit in early information processing might therefore negatively impact subsequent operations reflected by the N400 ERP which is more specifically linked to the DRM performance.
We observed a less negative N400 component morphology in SCZ than in HC, regardless of the conditions. The N400 component, which has been shown to be involved in verbal information (Brown and Hagoort, 1993; Kumar and Debruille, 2004; Kutas and Federmeier, 2000, Kutas and Federmeier, 2011; Salisbury, 2008) was found to be reduced in schizophrenic patients during a verbal recognition task (Kim et al., 2004). Therefore, its decrease in SCZ patients suggests difficulties in the processing of verbal information which is of relevance for the well-documented deficits in verbal memory in patients suffering from schizophrenia (Aleman et al., 1999; Reichenberg et al., 2009). Supporting this view, we found a significant correlation between a verbal memory test's score and the amplitude of the N400 although it did not resist multiple correction, possibly because of the limited N. The N400 decrease might reflect a deficit in verbal memory search or in the integration of incoming information with stored verbal information (Kim et al., 2004) which ultimately leads to a worse DRM performance independently of the item status. This was confirmed by a strong negative correlation between N400 amplitude and the A′ index value. Therefore, we propose that the decrease of the amplitude of the N400 component in SCZ patients reflects a deficit in processing (searching or integration) of verbal item-specific memory traces linked specifically to the ability to distinguish between true and false memories.
The present observations should also be interpreted in the light of the familiarity-driven frontal variations of the N400 during the DRM paradigm. Indeed, Curran (2000) showed that the N400 was more negative for the least familiar condition, i.e., the new-no condition, in the frontal electrode location. First, we were not able to find a frontal N400 amplitude variation as described by Curran (2000). In contrast, we identified a typically centro-parietal located N400 (Kutas and Federmeier, 2011). Second, the greater negativity for the new-no condition could not be replicated in a subsequent experiment (Curran et al., 2001), neither in our study. The differences regarding retention time and/or mode of item presentation may explain these discrepancies (Curran et al., 2001). However, it is possible that the lower N400 amplitude in patients, regardless of the conditions, might be associated with a preferential reliance on familiarity reflecting a preference for the gist-strategy (Moritz et al., 2004, Moritz et al., 2006). This could be interpreted as a compensatory mechanism in the deficit in verbal information processing in SCZ patients.
In conclusion, when compared to HC, patients suffering from psychosis showed an impairment in the ability to distinguish between true and false memories while performing the DRM task. Physiologically, key changes were related to the P2 and N400 components regardless of the conditions. We propose that the lower P2 amplitude in SCZ patients represent a deficit in the activation of multimodal mnesic elements not limited to verbal information nor to false memory production. Such deficit might subsequently decrease the efficiency or accentuate the difficulties of verbal information processing during the generation of the N400 component whose amplitude is more specifically linked to the DRM performance. The inability to properly process item-specific mnesic elements, including verbal material, supports the idea that SCZ patients favorize the use of a gist-strategy during the DRM, i.e. the tendency to rely on familiarity instead of item-specific strategy (verbatim), compared to HC.
Author contributions
Grégoire Favre: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Visualization Sibylle K. Horat: Software, Investigation François R. Herrmann: Validation, Formal analysis Isabelle Gothuey: Project administration Joseph Ventura: Writing – Review & Editing Marco C.G. Merlo: Conceptualization, Resources, Writing – Review & Editing, Project administration, Supervision Pascal Missonnier: Methodology, Formal analysis, Investigation, Writing – Review & Editing, Supervision.
Role of the funding source
No funding was received for this work.
Declaration of competing interest
This study has not been submitted elsewhere for publication, in whole or in part, and all the authors listed have approved the manuscript. The authors declare that there are no actual or potential conflicts of interest.
Acknowledgements
The authors gratefully acknowledge colleagues of the Mental Health Network Fribourg (RFSM) for their technical assistance and the recruitment of participants. We thank Dr. J. Lipiec for his support. We thank Prof T. Curran, Prof A. Yonelinas and Dr. G. Plancher for kindly providing information about the DRM design used in their experiments.
References
- Aleman A., Hijman R., de Haan E.H., Kahn R.S. Memory impairment in schizophrenia: a meta-analysis. Am. J. Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Anllo-Vento L., Luck S.J., Hillyard S.A. Spatio-temporal dynamics of attention to color: evidence from human electrophysiology. Hum. Brain Mapp. 1998;6(4):216–238. doi: 10.1002/(SICI)1097-0193(1998)6:4<216::AID-HBM3>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal. Stat. Soc. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bhatt R., Laws K.R., McKenna P.J. False memory in schizophrenia patients with and without delusions. Psychiatry Res. 2010;178(2):260–265. doi: 10.1016/j.psychres.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Bonin P., Méot A., Ferrand L., Bugaïska A. Normes d’associations verbales pour 520 mots concrets et étude de leurs relations avec d’autres variables psycholinguistiques. Annee Psychol. 2013;113(1):63–92. [Google Scholar]
- Boutros N.N., Korzyuko O., Oliwa G., Feingold A., Campbell D., McClain-Furmanski D., Struve F., Jansen B.H. Morphological and latency abnormalities of the mid-latency auditory evoked responses in schizophrenia: a preliminary report. Schizophr. Res. 2004;70(2–3):303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Brainerd C., Reyna V. When things that were never experienced are easier to "remember" than things that were. Psychol. Sci. 1998;9(6):484–489. [Google Scholar]
- Brown C., Hagoort P. The processing nature of the n400: evidence from masked priming. J. Cogn. Neurosci. 1993;5(1):34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Cadavid S., Beato M.S. Memory distortion and its avoidance: an event-related potentials study on false recognition and correct rejection. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0164024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett P.R., Simons J.S., Pigott J.S., Gardner J.M., Murray G.K., Krystal J.H., Fletcher P.C. Illusions and delusions: relating experimentally-induced false memories to anomalous experiences and ideas. Front. Behav. Neurosci. 2009;3:53. doi: 10.3389/neuro.08.053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Mem. Cogn. 2000;28(6):923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Curran T., Schacter D.L., Johnson M.K., Spinks R. Brain potentials reflect behavioral differences in true and false recognition. J. Cogn. Neurosci. 2001;13(2):201–216. doi: 10.1162/089892901564261. [DOI] [PubMed] [Google Scholar]
- Curran T., Tepe K., Piatt C. Event-related potential explorations of dual processes in recognition memory. In: Zimmer H.D., Mecklinger A., Lindenberger U., editors. Binding in Human Memory: A Neurocognitive Approach. Oxford University Press; Oxford: 2006. pp. 467–492. [Google Scholar]
- Dehon H., Bredart S. False memories: young and older adults think of semantic associates at the same rate, but young adults are more successful at source monitoring. Psychol. Aging. 2004;19(1):191–197. doi: 10.1037/0882-7974.19.1.191. [DOI] [PubMed] [Google Scholar]
- Donaldson W. Measuring recognition memory. J. Exp. Psychol. Gen. 1992;121(3):275–277. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- Duzel E., Yonelinas A.P., Mangun G.R., Heinze H.J., Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc. Natl. Acad. Sci. U. S. A. 1997;94(11):5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B., Fisher J.E., Weickert T.W., Weinberger D.R., Goldberg T.E. Lack of false recognition in schizophrenia: a consequence of poor memory. Neuropsychologia. 2004;42(4):546–554. doi: 10.1016/j.neuropsychologia.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Evans K.M., Federmeier K.D. The memory that's right and the memory that's left: event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45(8):1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand L. Normes d'associations verbales pour 260 mots “abstraits”. Annee Psychol. 2001;101(4):683–721. [Google Scholar]
- Ferrand L., Alario F. Normes d'associations verbales pour 366 noms d'objets concrets. Annee Psychol. 1998;98(4):659–709. [Google Scholar]
- Finnigan S., Humphreys M.S., Dennis S., Geffen G. ERP 'old/new' effects: memory strength and decisional factor(s) Neuropsychologia. 2002;40(13):2288–2304. doi: 10.1016/s0028-3932(02)00113-6. [DOI] [PubMed] [Google Scholar]
- Finnigan S., O'Connell R.G., Cummins T.D., Broughton M., Robertson I.H. ERP measures indicate both attention and working memory encoding decrements in aging. Psychophysiology. 2011;48(5):601–611. doi: 10.1111/j.1469-8986.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- Gallo D.A. Psychology Press; 2006. Associative Illusions of Memory: False Memory Research in DRM and Related Tasks. [Google Scholar]
- Gallo D.A. False memories and fantastic beliefs: 15 years of the DRM illusion. Mem. Cogn. 2010;38(7):833–848. doi: 10.3758/MC.38.7.833. [DOI] [PubMed] [Google Scholar]
- Guillem F., Bicu M., Hooper R., Bloom D., Wolf M.A., Messier J., Desautels R., Debruille J.B. Memory impairment in schizophrenia: a study using event-related potentials in implicit and explicit tasks. Psychiatry Res. 2001;104(2):157–173. doi: 10.1016/s0165-1781(01)00305-5. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Nolde S.F., Mather M., Kounios J., Schacter D.L., Curran T. The similarity of brain activity associated with true and false recognition memory depends on test format. Psychol. Sci. 1997;8(3):250–257. [Google Scholar]
- Kim M.S., Kwon J.S., Kang S.S., Youn T., Kang K.W. Impairment of recognition memory in schizophrenia: event-related potential study using a continuous recognition task. Psychiatry Clin. Neurosci. 2004;58(5):465–472. doi: 10.1111/j.1440-1819.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- Kumar N., Debruille J.B. Semantics and N400: insights for schizophrenia. J. Psychiatry Neurosci. 2004;29(2):89–98. [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn. Sci. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu. Rev. Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K.R., Bhatt R. False memories and delusional ideation in normal healthy subjects. Pers. Individ. Differ. 2005;39(4):775–781. [Google Scholar]
- Leucht S., Samara M., Heres S., Patel M.X., Woods S.W., Davis J.M. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr. Bull. 2014;40(2):314–326. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J., Hillyard S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31(3):291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Kappenman E.S. Oxford University Press; USA: 2012. The Oxford Handbook of Event-related Potential Components. [Google Scholar]
- Missonnier P., Herrmann F.R., Zanello A., Badan Ba M., Curtis L., Canovas D., Chantraine F., Richiardi J., Giannakopoulos P., Merlo M.C. Event-related potentials and changes of brain rhythm oscillations during working memory activation in patients with first-episode psychosis. J. Psychiatry Neurosci. 2012;37(2):95–105. doi: 10.1503/jpn.110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S., Woodward T.S., Cuttler C., Whitman J.C., Watson J.M. False memories in schizophrenia. Neuropsychology. 2004;18(2):276–283. doi: 10.1037/0894-4105.18.2.276. [DOI] [PubMed] [Google Scholar]
- Moritz S., Woodward T.S., Rodriguez-Raecke R. Patients with schizophrenia do not produce more false memories than controls but are more confident in them. Psychol. Med. 2006;36(5):659–667. doi: 10.1017/S0033291706007252. [DOI] [PubMed] [Google Scholar]
- O'Donnell B.F., Vohs J.L., Hetrick W.P., Carroll C.A., Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int. J. Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Plancher G., Nicolas S., Piolino P. Influence of suggestion in the DRM paradigm: what state of consciousness is associated with false memory? Conscious. Cogn. 2008;17(4):1114–1122. doi: 10.1016/j.concog.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Harvey P.D., Bowie C.R., Mojtabai R., Rabinowitz J., Heaton R.K., Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009;35(5):1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe W.K., Saine K.C., Lacritz L.H., Hynan L.S., Cullum C.M. Dyadic short forms of the Wechsler Adult Intelligence Scale-III. Assessment. 2002;9(3):254–260. doi: 10.1177/1073191102009003004. [DOI] [PubMed] [Google Scholar]
- Roediger H.L., McDermott K.B. Creating false memories: remembering words not presented in lists. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21(4):803. [Google Scholar]
- Roediger H.L., III, Balota D.A., Watson J.M. 2001. Spreading Activation and Arousal of False Memories. The Nature of Remembering: Essays in Honor of Robert G. Crowder; pp. 95–115. [Google Scholar]
- Salisbury D.F. Semantic activation and verbal working memory maintenance in schizophrenic thought disorder: insights from electrophysiology and lexical ambiguity. Clin. EEG Neurosci. 2008;39(2):103–107. doi: 10.1177/155005940803900217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury D.F., Collins K.C., McCarley R.W. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr. Bull. 2010;36(5):991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34-57) [PubMed] [Google Scholar]
- Straube B. An overview of the neuro-cognitive processes involved in the encoding, consolidation, and retrieval of true and false memories. Behav. Brain Funct. 2012;8:35. doi: 10.1186/1744-9081-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thérouanne P., Denhière G. Normes d'association libre et fréquences relatives des acceptions pour 162 mots homonymes. Annee Psychol. 2004;104(3):537–595. [Google Scholar]
- Ventura J., Green M.F., Shaner A., Liberman R.P. Training and quality assurance with the Brief Psychiatric Rating Scale: “the drift busters”. Int. J. Methods Psychiatr. Res. 1993;3(4):221–244. [Google Scholar]
- Ventura-Bort C., Low A., Wendt J., Molto J., Poy R., Dolcos F., Hamm A.O., Weymar M. Binding neutral information to emotional contexts: brain dynamics of long-term recognition memory. Cogn. Affect. Behav. Neurosci. 2016;16(2):234–247. doi: 10.3758/s13415-015-0385-0. [DOI] [PubMed] [Google Scholar]
- Weiss A.P., Dodson C.S., Goff D.C., Schacter D.L., Heckers S. Intact suppression of increased false recognition in schizophrenia. Am. J. Psychiatry. 2002;159(9):1506–1513. doi: 10.1176/appi.ajp.159.9.1506. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2nd ed. World Health Organization; 2004. ICD-10 : international statistical classification of diseases and related health problems : tenth revision. [Google Scholar]
- Yonelinas A.P. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46(3):441–517. [Google Scholar]
- Zhao X., Zhou R., Fu L. Working memory updating function training influenced brain activity. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071063. [DOI] [PMC free article] [PubMed] [Google Scholar]