Abstract
Periodontal disease (PD) is one of the most common inflammatory oral diseases, affecting approximately 47% of adults aged 30 years or older in the United States. If not treated properly, PD leads to degradation of periodontal tissues, causing tooth movement, and eventually tooth loss.
Conventional clinical therapy for PD aims at eliminating infectious sources, and reducing inflammation to arrest disease progression, which cannot achieve the regeneration of lost periodontal tissues. Over the past two decades, various regenerative periodontal therapies, such as guided tissue regeneration (GTR), enamel matrix derivative, bone grafts, growth factor delivery, and the combination of cells and growth factors with matrix-based scaffolds have been developed to target the restoration of lost tooth-supporting tissues, including periodontal ligament, alveolar bone, and cementum. This review discusses recent progresses of periodontal regeneration using tissue-engineering and regenerative medicine approaches. Specifically, we focus on the advances of biomaterials and controlled drug delivery for periodontal regeneration in recent years. Special attention is given to the development of advanced bio-inspired scaffolding biomaterials and temporospatial control of multi-drug delivery for the regeneration of cementum-periodontal ligament-alveolar bone complex. Challenges and future perspectives are presented to provide inspiration for the design and development of innovative biomaterials and delivery system for new regenerative periodontal therapy.
Graphical abstract
Highlights
-
•
Focus on the recent advances of biomaterials and controlled drug delivery for periodontal regeneration.
-
•
The development of advanced bio-inspired scaffolding biomaterials and multi-drug delivery systems for periodontal regeneration is emphasized.
-
•
Challenges and future perspectives are presented to provide inspiration for new regenerative periodontal therapy.
1. Introduction
Periodontium are tooth-supporting tissues that are composed of gingiva, cementum, periodontal ligament (PDL) and alveolar bone. Periodontitis is an inflammatory disease that leads to degradation of periodontal tissues, causing tooth movement and eventually tooth loss [1]. Currently, clinical treatments for periodontitis focus on plaque removal and local inflammation control, such as scaling and root planing and surgical treatments [[2], [3], [4], [5]]. Those therapies attempt to minimize symptoms and prevent disease progression, but cannot restore the attachment of periodontal tissues to teeth and the original periodontal tissues. Therefore, the functions of teeth and dentition remain impaired after the treatments. Some regenerative approaches, such as guided tissue regeneration (GTR) and bone grafts, were developed to achieve periodontal tissue formation. However, clinical outcomes of those approaches are variable and unpredictable [[6], [7], [8], [9]]. Therefore, it is imperative to develop alternative regenerative strategies to restore the structures and functions of periodontal tissues for periodontitis patients.
During tooth development, niche-resident dental follicle cells differentiate into cementoblasts, fibroblasts, and osteoblasts that form cementum, PDL, and alveolar bone, respectively [10]. The niche (microenvironment) that induces the formation of tooth supporting tissues, however, is not retained after tooth development, leading to the difficulty of restoring damaged/lost periodontium after maturity. As a bioengineering approach, tissue engineering is capable of recapitulating the microenvironment in certain aspects and regenerating functional tissues [11]. When tissue-engineering strategy is adopted for periodontal regeneration, it is pivotal to consider two important elements: scaffold (biomaterials and scaffolding design) and controlled drug delivery (bioactive molecules and methods for controlled delivery) (Fig. 1). Several review articles presented excellent summaries of periodontal regeneration a decade ago [8,[12], [13], [14], [15], [16]]. However, their work mainly focused on stem cells, biological evaluation, and the characteristics of biomaterials or bioactive molecules, but did not pay particular attention to scaffolding design and drug delivery. More importantly, periodontal regeneration is a rapidly expanding research field. A number of new biomaterials, approaches, and technologies have been developed for regenerative periodontal treatment over the past ten years. Therefore, there is a need to summarize recent progresses on periodontal regeneration from a biomaterial perspective.
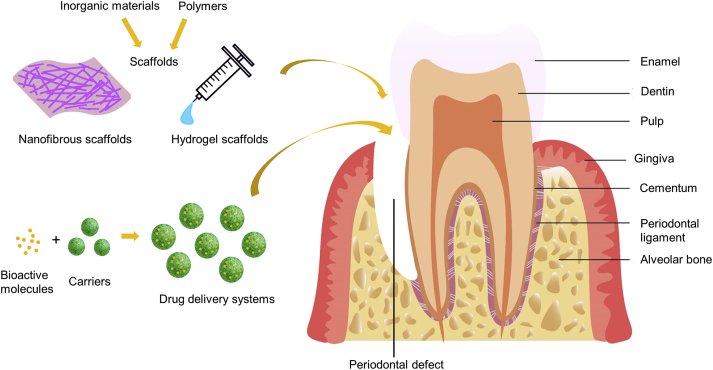
Fig. 1.
Schematic illustration of the anatomy of periodontal tissues, periodontal defect, scaffolds of tissue engineering approach and drug delivery system.
In this paper, we first overview periodontal tissue regeneration strategies, including GTR and tissue engineering. Next, we discuss the biomaterials and drug delivery systems that have been developed for periodontal regeneration. We highlight the development of advanced bio-inspired scaffolding biomaterials and temporospatial control of multi-drug delivery. Furthermore, we summarize the applications of the biomaterials and drug delivery systems for the regeneration of periodontal tissues that include PDL, cementum, and alveolar bone. Special attention is given to the regeneration of PDL-cementum-alveolar bone complex. Finally, our perspectives on the use of bio-inspired materials and drug delivery systems to reconstruct the hierarchical and functional periodontium are provided as signposts for the future advancement of this field.
2. Biomaterials and scaffolding design for periodontal regeneration
2.1. Strategies for periodontal regeneration
There are two strategies for periodontal regeneration: GTR and tissue engineering approaches. GTR has been widely used for periodontium regeneration in clinic for decades. It is a regenerative surgical technique that involves the procedure of raising mucogingival flap around affected teeth, scaling and planing root surfaces and placing barrier membranes temporally under gingiva [17]. The biological basis of the GTR technique is to block the apical growth of epithelium to the space over the denuded root surface by using barrier membrane, therefore facilitating PDL cells and osteoblast to form PDL tissues and alveolar bone [18]. Numerous clinical research have confirmed the benefits of GTR treatments, including greater clinical attainment level (CAL) gain, probing pocket depth (PPD) reduction and bone regeneration compared to open flap debridement (OFD) treatment [7,[19], [20], [21]].
Although GTR has positive treatment outcomes, there are limitations of GTR treatment for periodontal regeneration. First, the regenerative benefits of the GTR treatment vary from case to case [22]. Many factors, such as diabetes, smoking, dental plaque control, tooth anatomy and morphology, affect the results of the GTR treatment [6,7]. Therefore, the outcomes of GTR treatment in clinical practice may not be as successful as those in clinical trials. A quantitative analysis showed that GTR was a predictable method for narrow intrabony defects and class II mandibular furcation defects [6]. The outcomes of GTR treatment for other types of periodontal defects, however, are limited and unpredictable [6]. There are two types of barrier membranes for GTR: non-absorbable and absorbable membranes. For non-absorbable membranes, a second surgery is necessary to remove it from the defect area, which increases the risk of infection as well as surgical burden [22].
Tissue engineering strategy uses stem/progenitor cells, scaffolds and bioactive molecules to build biomimetic systems to induce new tissue formation. Depending on whether biomaterials are used, tissue engineering strategy for periodontal regeneration can be categorized into scaffold-free and scaffold-based approaches. For the scaffold-free approach, cells or cell aggregates are transplanted to a defect area without a cell carrier. Several types of cells, including bone marrow derived mesenchymal stem cells (BMSCs) [[23], [24], [25]], adipose-derived stem cells (ADSCs) [26], periodontal ligament stem cells (PDLSCs) [27,28], and dental pulp stem cells (DPSCs), have been tested for the potential to form periodontal tissues. Direct cell implantation faces the problem of cell diffusion out of the defect area. Cell sheet technique, which entraps cells in the extracellular matrix (ECM) secreted by the cells themselves, is capable of preventing cell migration. It was reported that cell sheet therapy induced more bone formation than cell suspension in swine periodontal defects [29].
Cell sheet technology, however, is capable of regenerating only a layer of tissue with a simple structure. Given the complicated architecture of periodontium which includes two hard tissues (alveolar bone and cementum) and a soft tissue (PDL), the use of scaffold-based approach is the only choice for the regeneration of PDL-cementum-alveolar bone complex. Multiphasic scaffolds with distinctive characteristic in each layer are required to imitate the periodontal structures. Specifically, the architecture, chemical composition, cellular/biochemical composition in each layer need to be tailored to achieve periodontal complex regeneration [[30], [31], [32]]. Details of biomaterials and scaffolding design are discussed in the following sections.
2.2. GTR biomaterials
GTR barrier membranes prevent the ingrowth of epithelial cells and provide space to regenerate PDL and alveolar bone. The barrier membranes should have basic properties, including biocompatibility, cell-occlusiveness, tissue integration, space maintenance, and clinical manageability [[33], [34], [35]]. GTR barrier membranes are classified into non-absorbable and absorbable membranes. Generally, non-absorbable membranes have superior space maintenance compared to absorbable membranes. Gore-tex® is the first GTR barrier membrane that is made of polytetrafluoroethylene (PTFE) with high mechanical properties [17]. The titanium-reinforced PTFE membrane further increased the compressive strength, resulting in better outcomes compared to the PTFE membrane [36]. The ultrathin (0.01 mm) titanium-reinforced PTFE membrane occupied minimal space, therefore, provided more room for new tissue formation [37]. The smoother surface of the titanium-reinforced PTFE also lowered immunological reaction in vivo [37]. However, a second surgery is required for the removal of non-absorbable barrier membranes, which increases the risk of infection, delays wound healing, and impairs the regenerative outcomes.
Absorbable membranes are gradually degraded in vivo and avoid the drawback of secondary surgery after implantation. Both natural and synthetic biomaterials are tested as absorbable GTR membranes. Generally, natural biomaterials have excellent biocompatibility with cellular binding sites, but have low mechanical strength [38]. On the other side, synthetic biomaterials have tunable degradation rates and mechanical properties, but lack biological recognition (cellular binding motif). The degradation property of membranes affects the ability of space maintenance and new tissue formation. As a rule, the degradation rate should be moderate: fast degradation leads to premature mechanical loss while slow degradation prevents new tissue ingrowth. Generally, compared to non-absorbable membranes, absorbable membranes have a limitation of low mechanical strength.
Recent development of GTR barrier membranes focus on the optimization of mechanical and degradation properties and the incorporation of new functions into GTR membranes. For example, GTR membranes were prepared from composites that combined the advantages of different biomaterials [38]. The combination of natural and synthetic polymers integrated the bioactive recognition of natural materials and improved mechanical properties of synthetic materials [[38], [39], [40]]. GTR membranes were also used as carriers for drug delivery to enhance tissue regeneration [41,42]. Anti-bacteria drugs were loaded into GTR membranes to inhibit local infection and inflammation, therefore, facilitating PDL tissue formation [35]. Multi-layered GTR membranes with different functions in each layer were also developed to enhance periodontal tissue regeneration [40,43,44].
While many GTR membranes have been developed for periodontal regeneration in recent years, most of them are in laboratory stages. To evaluate the biosafety and effectiveness of those GTR membranes, more in vivo studies and clinical trials are required. As discussed above, the outcomes of GTR treatment are unpredictable in several types of periodontal defects. In other words, GTR membranes with improved properties is insufficient to acquire successful treatment outcomes. The combination of GTR membranes with other approaches, such as bone grafts, may provide better regenerative results.
2.3. Tissue engineering biomaterials
Table 1 lists the biomaterials that are used recently for periodontal tissue regeneration. Briefly, polymeric materials that have relative low mechanical strengths (e.g. collagen, gelatin, and chitosan) are the candidate materials for PDL regeneration, while inorganic materials with relative high mechanical strength, such as hydroxyapatite (HA), tricalcium phosphate (TCP), biphasic calcium phosphate (BCP), and bioactive glass (BG) are used for cementum and alveolar bone regeneration. To regenerate cementum-PDL-alveolar bone complex, composite biomaterials that contain polymers and inorganic components have to be used (Table 1).
Table 1.
Summary of biomaterials used for periodontal tissue regeneration.
| Biomaterials | Target tissue | Characteristics | References |
|---|---|---|---|
| Inorganic biomaterials | |||
| Hydroxyapatite (HA) | Alveolar bone; cementum | Similar chemical composition and structure to the inorganic phase of bone Osteoconductive Direct bonding effect to natural bone Slow degradation |
[[45], [46], [47], [48]] |
| Tricalcium phosphate (TCP) | Alveolar bone; cementum | Similar chemical composition to the inorganic phase of bone Bioabsorbable Osteoconductive TCP α and TCP β |
[[49], [50], [51], [52], [53]] |
| Biphasic calcium phosphate (BCP) | Alveolar bone | Mixture of HA and TCP in various ratios to adjust degradation rate and biological activity Similar chemical composition and structure to the inorganic phase of bone |
[[54], [55], [56]] |
| Bioactive glass (BG) | Alveolar bone; cementum | Compositions of bioactive glasses vary Ions dissolved from BG promote angiogenesis, osteogenesis and antibacterial activity Degradation rate vary over a wide rage |
[[57], [58], [59], [60], [61], [62], [63]] |
| Natural polymers | |||
| Collagen | PDL | Most abundant protein in the ECM of alveolar bone, PDL and cementum Biocompatible Low mechanical strength Safety problems: pathogen transmission, immune reaction |
[[64], [65], [66]] |
| Gelatin | PDL; alveolar bone; cementum | Hydrolysis product of collagen No pathogen transmission and immune reaction Easily modified for chemical and light crosslinking |
[[67], [68], [69], [70]] |
| Chitosan | Alveolar bone; PDL; cementum | Derived from chitin Biocompatible Antibacterial property |
[[71], [72], [73], [74]] |
| Synthetic polymers | |||
| Poly (lactic-co-glycolicacid) (PLGA) | Alveolar bone; PDL | Biocompatible Tunable degradation rate No cell recognition motif |
[[75], [76], [77], [78]] |
| Polycaprolactone (PCL) | Alveolar bone; PDL | Biocompatible Slow degradation rate No cell recognition motif |
[[79], [80], [81], [82]] |
| Composite biomaterials | |||
| PLGA + CaP | Alveolar bone | Fabricated into two layers (smooth outer layer and rough microporous inner layer) Designed to support GTR membrane and promote alveolar bone regeneration in dogs |
[83] |
| Collagen + HA | Alveolar bone | Fabricated by freeze-drying of both collagen and HA or precipitating HA to collagen BMSCs seeded into the scaffold to promote alveolar bone formation in a dog’ periodontal defect |
[84] |
| Chitosan+β-TCP | Alveolar bone | Fabricated by freeze-frying HPDLC seeded into the scaffold to recruit host cells and promote osteoblast differentiation |
[85] |
| PLGA + Magnesium | Alveolar bone | Mg in the PLGA increased mechanical strength of composite materials, buffered the acidic by-product of PLGA degradation, and enhanced osteogenic capacity and bone formation in vivo | [86] |
| Gelatin methacrylate + HA | Alveolar bone | Methacrylate was introduced for photo-crosslinkable The composite induced hPDLSCs to differentiate into osteoblast and promoted new bone formation in nude mice |
[67] |
| Gelatin+β-TCP | Alveolar bone | Gelatin and β-TCP were mixed in homogenizer and freeze dried New bone tissue and some fibers parallel to bone surfaces were formed in a dog periodontal defect |
[87] |
| PCL+ β-TCP + CaP coating | PDL; Alveolar bone | PCL electrospun scaffold was fabricated as the PDL layer. A thin layer of CaP was coated on the surface of PCL-β-TCP scaffold to improve the osteogenic capacity CaP coating induced more bone formation |
[88] |
| PGA PCL |
PDL; Alveolar bone | Microchannels in the PDL layer were designed to guide fibers formation Porous structure was fabricated to allow cell proliferation No organized fiber insertion in PDL and bone interface |
[89] |
| PCL + HA | Alveolar bone; PDL; cementum | Three layers of scaffold design was used to mimic the architecture of periodontium No organized fiber insertion in PDL and bone interface |
[90] |
| Chitin + PLGA + BCG | Alveolar bone; cementum; PDL | PLGA was added to increase degradation time and improve mechanical stability. BCG enhanced osteogenic capacity in bone and cementum layers. |
[91] |
In conclusion, most materials used in periodontal regeneration are traditionally used in other regenerative research, such as inorganic materials (HA, tricalcium phosphate, BG ect) and polymers (gelatin, collagen, PCL, PLGA ect). Those traditional biomaterials are modified or combined together into composite materials to make suitable microenvironment and scaffold systems to induce periodontal regeneration in previous research. However, even though these biomaterials can resemble the compositions in certain aspects, they cannot mimic the fine structures of the natural periodontal tissues. Therefore, novel and bio-inspired materials that are designed to closely mimic the architecture of periodontal tissues at micro and nanoscale levels are prerequisite to achieve functional periodontal tissue regeneration.
2.4. Scaffolding design for periodontal regeneration
Several aspects need to be considered when designing a scaffold for periodontal regeneration. These are scaffold compositions, structure, architecture, and ease of use (injectability). Generally, scaffolding materials should mimic the compositions of the ECM of periodontal tissues, therefore, recapitulating the ECM microenvironment of periodontium. As periodontium is composed of cementum, PDL, alveolar bone and gingiva, scaffolding design for each of the components is different. For example, alveolar bone is a hard tissue, and the scaffold for alveolar bone regeneration should induce mineralized tissue formation; while PDL is a fibrous tissue, and the scaffold for PDL regeneration should facilitate soft tissue formation and prevent mineralization. Based on that, inorganic biomaterials, such as hydroxyapatite and calcium phosphate, are the scaffolding components to enhance biomineralization, while polymeric biomaterials are widely used for PDL regeneration [88,90,91].
A scaffold needs to provide structural guidance for the formation of periodontal tissues with proper structures. For example, the ECM of PDL are composed of nanofibrous network, and the nanofibrous scaffold simulates the architecture of the natural ECM [[92], [93], [94], [95], [96], [97], [98], [99]]. Electrospinning is a simple and effective method to prepare nanofibrous matrix, which presents high surface area and porosity to facilitate cell attachment, migration, and proliferation, so it is used to fabricate PDL scaffolds. Besides, PDL has well-organized collagen fibers that are classified into five groups with each group having various locations and directions. The scaffold for PDL regeneration should provide biophysical guidance to regenerate the well-organized PDL fibers. By restoring the proper structure, the regenerated periodontium can perform the functions of supporting tooth and bearing occlusal force, similar to the natural counterparts.
Given PDL is anchored to root cementum and alveolar bone, the regeneration of PDL alone is not sufficient to perform PDL function. Instead, cementum and alveolar bone regeneration should also be included when designing scaffolds for periodontal regeneration. Therefore, a cementum-PDL-alveolar bone multilayer scaffold is often used for periodontal regeneration.
Besides the compositional, structural and functional requirements for periodontal scaffolding design, the shape of scaffolds is another factor to be considered. Hydrogels can be injected into defect areas and be crosslinked in situ, making it desirable for irregular periodontal defects. The drawback of a hydrogel scaffold is the relatively low mechanical strength. The incorporation of components with high mechanical strength, such as calcium phosphate, improved the mechanical property of the hydrogel [100,101]. However, the injectability of the hydrogel was affected as well. In contrast to hydrogels, pre-formed scaffolds have pre-designated size and morphology prior to the implantation [95,96]. Pre-formed scaffolds are fabricated by different methods, such as lyophilization, direct casting, and 3D printing. Among them, 3D printing can precisely present more biophysical cues inside scaffolds to guide tissue regeneration and is the most promising technique. For example, 3D printing was used to fabricate scaffolds with microgroove and microchannel structures to guide fiber orientation [31,75,89,90]. In addition, a 3D printing scaffold can be customized to match the shape of individual patient using cone beam computer tomography (CBCT) images [102]. While 3D printing method has many advantages, it cannot fabricate scaffolds that mimic the ECM architecture at a nanoscale level. To address this issue, the resolution of 3D printing has to be greatly improved.
3. Controlled delivery systems for periodontal regeneration
3.1. Drugs and growth factors used for periodontal regeneration
A general tissue engineering approach involves in the combination of scaffold, cells and bioactive molecules (drugs and growth factors) to induce tissue regeneration. Among them, bioactive molecules control diseased conditions, stimulate innate regenerative capacity, and provide signals for tissue formation. A number of drugs and growth factors have been tested for periodontal regeneration, and their results are summarized in Table 2.
Table 2.
Summary of drugs and growth factors used for periodontal regeneration.
| Bioactive Molecules | Characteristics | Functions | Applications in periodontal regeneration | References |
|---|---|---|---|---|
| Drugs | ||||
| Statins: Simvastatin (SMV); Atorvastatin (ATV) | Inhibitors of 3-hydroxy-2-methyl-glutaryl coenzyme A (HMG-CoA) reductase, commonly used for arteriosclerosis and hyperlipidemia | Inhibiting osteoclasts activity Increase BMP-2 level Inhibiting HMG-CoA level |
Gels loaded with SMV induced significantly PD reduction, CAL gain and more bone formation ATV exerted better regenerative results than SMV |
[[103], [104], [105], [106], [107], [108]] |
| Metformin | Anti-hyperglycemic biguanide to treat type II diabetes; | Promote osteogenic differentiation and bone formation via LKB1/AMPK pathway | Greater PD reduction and CAL gain when applied to periodontitis patients | [[109], [110], [111]] |
| Growth factors | ||||
| Platelet derived growth factor (PDGF) | Four isomeric composed of dimer of A, B and C chain: PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC Two receptors: α and β receptors |
Chemotaxis Enhance cell proliferation and differentiation Enhance Angiogenesis |
Promoted periodontal bone regeneration in various clinical trials, with the best concentration of 0.3 mg/ml | [[112], [113], [114], [115], [116]] |
| Fibroblasts growth factors (FGF) | 22 subfamily proteins Activate tyrosine kinase activity |
Promote wound healing Enhance cell mitogen Enhance angiogenesis Promote cell differentiation |
Significantly promoted bone regeneration in periodontal defects in patients. | [[117], [118], [119], [120], [121], [122]] |
| Stromal-cell-derived factor-1 (SDF-1) | Also named C-X-C motif ligand 12 (CXCL12) Receptors: C–X–C motif receptor 4 (CXCR4) |
Recruit stem/progenitor cells to defect areas to promote tissue regeneration Promote collagen formation |
Promote bone and fibrous tissues regeneration | [[123], [124], [125], [126], [127], [128]] |
| Bone morphogenic proteins (BMP) family | Belongs to TGF-β super family Contains more than 20 proteins Proteins are homodimeric or heterodimeric to each other BMP-2 and BMP-7 are the most widely used |
Stimulate bone and cartilage formation Recruit bone progenitor cells Promote osteoblasts and the other cells differentiation Wound healing Angiogenesis |
BMP-2: Clinical trials confirm its bone regenerative effects, but may induce root resorption and ankylosis BMP-6: Induce bone, PDL and cementum regeneration in periodontal defects of rats and dogs BMP-7: pleiotropic functions for osteogenic and cementogenesis in Class III furcatioin defects in dogs |
[112,[129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140]] |
3.2. Controlled delivery systems for periodontal regeneration
Most growth factors have short half-lives and narrow therapeutic windows. Therefore, controlling the release patterns of drugs and growth factors from carriers to achieve stable long-term effects and avoid side effects is highly desirable. Particularly, the spatial and temporal controlled delivery of drugs or growth factors is essential for periodontal regeneration. Based on the number of drugs (or growth factors) to be delivered, the release systems can be divided into monodrug and multidrug delivery systems.
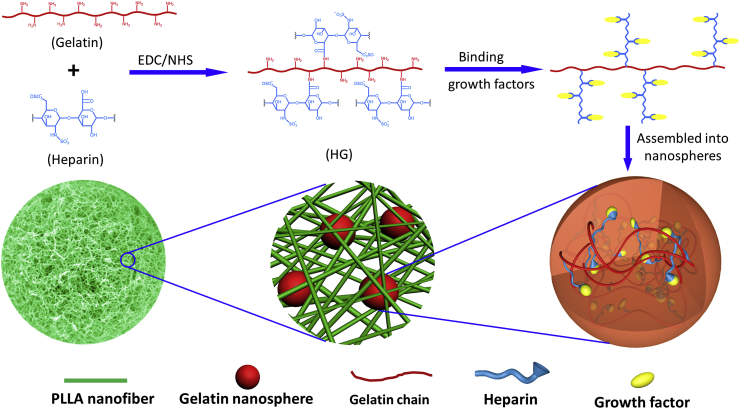
In a monodrug delivery system, a drug or growth factor is applied to fulfill particular functions. For example, stromal cell-derived factor 1 (SDF-1) was used to recruit host mesenchymal stem cells and hematopoietic stem cells to wound areas, and those cells promoted vasculature and new bone formation in rat periodontal defects [127,128]. Bone morphogenetic protein 2 (BMP-2) was used to enhance bone regeneration and accelerate mandibular bone defect repair [141]. The bioactive agents can be incorporated into biomaterials via non-covalent methods, such as physical entrapment, surface adsorption, and ionic complexation [142]. However, these methods cannot control burst release, which is undesirable for drug delivery [142]. In addition, the bioactivity of bioactive agents often reduces after the fabrication processes. Some natural biomaterials, such as heparin and heparan sulfate glycosaminoglycans in ECM have binding domains that exert strong interaction with bioactive molecules. The binding of these glycosaminoglycans to bioactive molecules protects the bioactive molecules (e.g. growth factors) from denaturation and proteolytic degradation, and subsequently prolongs the sustained release. Based on this principle, we recently developed a hierarchical nanosphere-encapsulated microspheres system for bone tissue regeneration [143]. In this system, BMP-2 bound with heparin and was encapsulated into heparin-conjugated gelatin nanospheres, which were further immobilized in nanofibrous microspheres (Fig. 2). This system allows the integration of nanofibrous architecture with controlled growth factor delivery into one injectable microsphere. BMP-2 possesses binding domains with heparin, and the binding of BMP-2 to heparin stabilizes the BMP-2 and controls its sustained release. In addition, the heparin-binding BMP-2 was encapsulated in gelatin nanospheres and entrapped by the microsphere nanofibers, which mimicked the architecture of collagen fibers and had many unique properties including superior surface area, high porosity, low density, and controllable degradation rate. The ECM-mimicking nanofibrous architecture enhanced bone marrow-derived mesenchymal stem cell adhesion, proliferation, differentiation, and new tissue formation [[144], [145], [146], [147]]. The in vivo study showed that the BMP-2-loaded hierarchical nanofibrous microsphere was an excellent carrier and significantly enhanced bone regeneration [143]. The combination of controlled growth factor delivery with an injectable biomimetic scaffold provides new insights into the design of cell-instructive scaffolds.
Fig. 2.
Schematic illustration of the design of hierarchical injectable nanofibrous microspheres for bone regeneration. BMP-2 is encapsulated into heparin-conjugated gelatin nanospheres, which are further immobilized in nanofibrous PLLA microspheres. Adapted with permission from Ref. [143].
Periodontal regeneration is a complicated process that involves multiple aspects including the control of infection and inflammation, recruitment of stem/progenitor cells, promotion of cell proliferation and differentiation, as well as new tissue formation. Besides, periodontium is composed of distinct layers of tissues and different biological cues should be provided to guide PDL, cementum, and alveolar bone formation. Therefore, the delivery of multiple bioactive reagents is necessary for periodontal tissue regeneration. For temporal control of bioactive reagents release in periodontal regeneration, it is essential to understand normal healing process. In terms of periodontitis, the first step is to control local infection and inflammation, and create a healthy and stable environment for new tissue formation. Therefore, antibacterial drugs should be released first to control local bacterium infection in a periodontal release system [148]. Next, drugs/growth factors that regulate local inflammation level, promote cell proliferation and differentiation are applied in sequence [148].
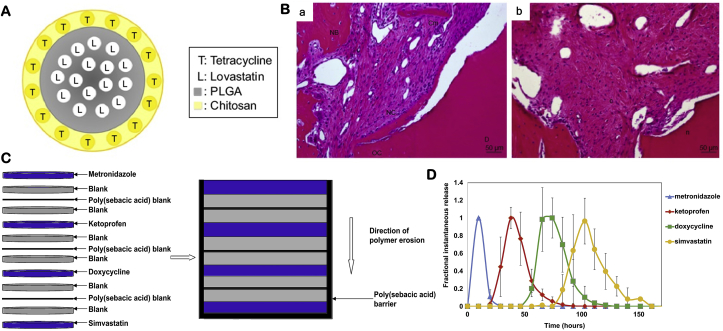
There are several methods to achieve temporal control of multidrug releases: direct presentation, multiphase loading and particulate-based delivery [149]. Direct presentation incorporates two or more bioactive agents into carriers via physical entrapment or absorbance. While this strategy is simple, it has little control of sequential releases of the bioactive agents. Multiphase loading adds each bioactive reagent in different phase of biomaterials to form a multiphased drug delivery carrier. For example, lovastatin was loaded into PLGA microsphere, which was encapsulated in tetracycline-loaded chitosan (Fig. 3A). The core-shell structured microspheres first released tetracycline from the shell to control local infection, followed by the release of lovastatin from the PLGA core to reduce inflammation and oxidation and promote new bone formation (Fig. 3B) [150]. In another multiphase delivery system, four drugs were loaded separately in different layers of a surface-eroding polymeric system composed of cellulose acetate phthalate and Pluronic F-127 (Fig. 3C). The drugs were entrapped from the surface to the deep layers, and blank layers were used to control the lag time between the releases [148]. When the polymer was eroded from the surface to the deep layers, drugs were released in order (Fig. 3D). Particulate-based delivery incorporates bioactive reagents in different particulates, which are further incorporated into a scaffold. In a study, IGF-1 was loaded in alginate microparticles and BMP-6 was loaded in PLGA microparticles, respectively, which were further incorporated into a chitosan scaffold [151]. The fast release of IGF-1 from the alginate microparticles induced osteoblast and cementoblast proliferation and differentiation, while the slow release of BMP-6 from the PLGA microparticles induced periodontal tissue regeneration. Spatial control of drug releases is achieved via the addition of different growth factors in different part of the scaffold. For example, cementum protein 1, FGF-2 and platelet-rich plasma were added to cementum, PDL and bone layer of the chitosan-based scaffold, respectively [91].
Fig. 3.
Development of multi-drugs delivery systems. (A) A PLGA-lovastatin-chitosan-tetracycline release system, in which tetracycline was loaded in chitosan and lovastatin was loaded in PLGA microparticles. (B) The sequential release of tetracycline and lovastatin effectively controlled local infection and promote new bone and cementum regeneration. Adapted with permission from Ref. [150]. (C) The design of four drugs delivery system using a layer-by-layer technique. (D) The release profiles confirmed the sequential releases of the drugs from the system in vitro. Adapted with permission from Ref. [148].
4. Applications of biomaterial and controlled delivery systems for periodontal regeneration
4.1. PDL regeneration
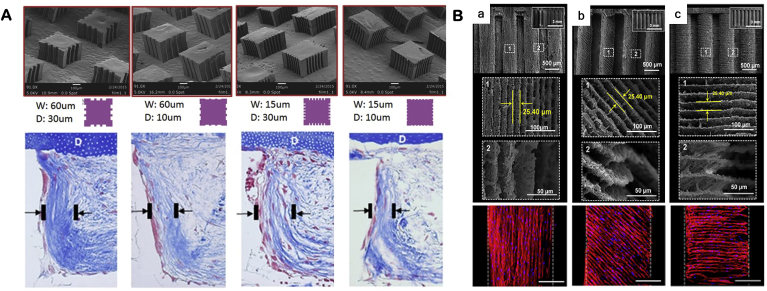
PDL is a fibrous tissue between alveolar bone and cementum. A number of PDL scaffolds were fabricated into nanofibrous fibrous matrices to mimic the architecture of the ECM of PDL. The nanofibrous scaffolds provided ECM-like environment to facilitate fibroblastic differentiation and PDL markers expression [152,153]. The structure of PDL is characterized by the directions of the PDL primary fibers. Thus, imitation of fiber alignment is critical for PDL regeneration. Several methods were developed to induce PDL fiber alignment, such as electrospinning and channel containing scaffold. Electrospinning is a simple method to fabricate oriented matrices, which showed the aligned fibers guided cell elongation and collagen fibers direction on the surface of the matrices [30]. The electrospinning method, however, only controls cell orientation on the surfaces of the matrix. Channel-containing scaffold is an alternative design to guide PDL cells and fiber directions in three-dimension [89]. In another study, 3D printing micro-channel fiber-guiding scaffolds were developed to induce the partial alignment of the fibers [75]. However, in vivo results showed that the fibers were deposited not inside the channels, but parallel to the dentin surface [89]. By introducing microfabrication techniques into scaffolding fabrication, micropatterned scaffolds with various micropatterns and sizes (width and depth) were fabricated and tested for aligned tissue formation (Fig. 4A) [79].
Fig. 4.
Development of micropatterned scaffolds to guide collagen fiber orientation of PDL. (A) The 3D printed micropatterned matrx with various geometry (width, W and depth, D of the grooves) controlled the orientation of collagen deposited on the matrix. (B) The 3D printing technology was used to create angulated microgroove patterns that were used control cell orientations such as parallel (0°), oblique (45°) and perpendicular (90°) angulations. Adapted with permission from Refs. [31,79].
Given the directions of PDL fibers vary in different groups, a freeze-drying scaffold was prepared to mimic fibers directions of apical, horizontal and oblique groups [32]. However, no in vitro or in vivo data showed the effectiveness of the scaffold. Similarly, one study used 3D printing process to produce microgroove structure, which controlled cell orientations in parallel (0°), oblique (45°) or perpendicular (90°) angulations (Fig. 4B) [31]. Similarly, no in vivo results confirmed the guiding function of the 3D printing scaffold.
Besides the reconstruction of PDL structure, functional regeneration of PDL is another issue to be considered. In the body, PDL is continuously subjected to mechanical stress caused by occlusal force. An in vitro study showed that PDL cells sensitively responded to mechanical stress for proliferation and differentiation, and increased the expressions of tendon-related markers such as periostin and tenascin [154]. After the PDL cells were treated with mechanical stress on aligned fibrous scaffolds and transplanted into rat premaxillary periodontal defect, histological results showed more aligned PDL-like fibrous tissue formation, indicating mechanical stimulus influenced fiber regeneration [154]. This ex vivo mechanical stress model, however, cannot mimic the complicated mechanical stress in vivo. A better mechanical stress model that more closely emulates occlusal force in vivo will assist the regeneration of functional PDL.
4.2. Alveolar bone and cementum regeneration
Since alveolar trabecular bone and cementum are both mineralized tissues and have similar compositions, they are discussed together in this section. To induce hard tissue formation, inorganic materials, like calcium phosphate, are included in the scaffold for alveolar bone or cementum regeneration [46,155]. To overcome the limitations of fabrication difficulty and brittleness, natural or synthetic polymers are often incorporated in the scaffold [90,91].
BMP family proteins and amelogenin are widely used to induce alveolar bone regeneration [129,133,156,157]. While these proteins were also tested for cementum regeneration, other bioactive molecules that specifically induce cementum formation were examined. For example, cementum protein 1 (CEMP1) is a specific cementum marker that plays important roles in inducing cementum repair and formation. CEMP1 facilitates PDL cells attachment, proliferation and cementoblast differentiation rather than osteoblast differentiation [158]. In addition, CEMP1 can promote hydroxyapatite crystal and mineralized tissue formation [159,160], making it a potential candidate to induce cementum regeneration. When CEMP1 was loaded into chitin-PLGA/nBCG scaffold for cementum regeneration, a thin layer of mineralized tissue on the surface of root dentin was observed [91]. E-aminocaproic acid (ACA) is another molecule to induce cementum repair. ACA is a proteinase inhibitor that can slow down the fibrinolysis procedure during periodontitis and reduce cementoblast apoptosis. A chitosan particles carrier system loaded with ACA in fibrin matrix promoted cementum regeneration in a dog periodontal defect [161].
Cementum is a thin layer of mineralized tissue with a thickness of a few hundred micrometers. Therefore, scaffolds for bone regeneration are not suitable for cementum regeneration; instead, cell sheet can better fit the requirements. Various cells, such as PDL cells, BMSC and alveolar periosteal cells, were fabricated into cell sheets for cementum regeneration. Results showed that the cell sheets formed a thin layer mineralized cementum-like tissue on the surface of roots with the expression of the CEMP1 marker [[162], [163], [164], [165]]. Because of the regenerative capacity, cell sheet was combined with PDL and bone scaffolds to regenerate cementum-PDL-bone complex [166,167].
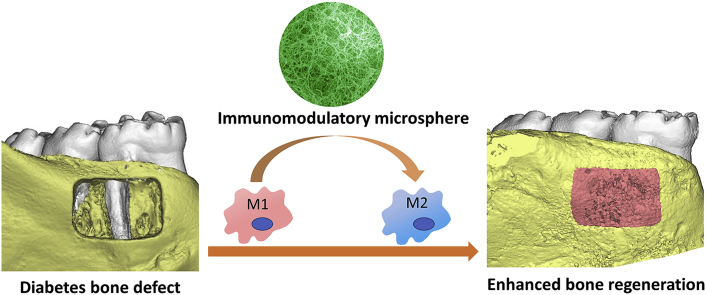
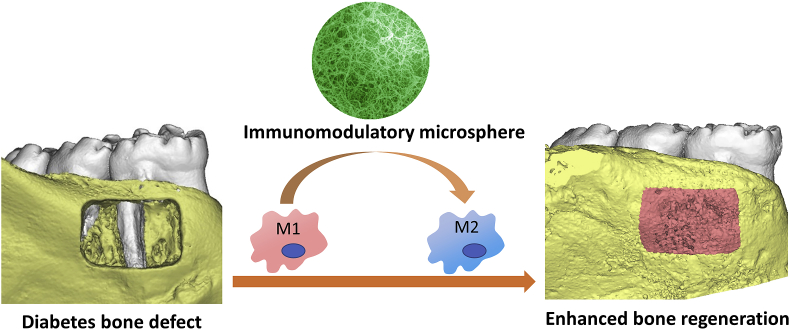
Recently, a new immunomodulatory strategy was developed for alveolar bone regeneration [168]. This strategy targeted on macrophages that play a central role in inflammation activation and resolution. Macrophages can mainly be divided into two phenotypes: classically activated pro-inflammatory M1 macrophages and alternatively activated pro-healing M2 macrophages. Macrophages are capable of dynamically switching from one phenotype to the other depending on the surrounding microenvironment. For example, interleukin 4 (IL-4) is an effective cytokine to change the pro-inflammatory M1 into an anti-inflammatory M2 phenotype. Based on these biological findings, a unique bio-inspired injectable microsphere as an osteoimmunomodulatory scaffolding biomaterial was developed [168]. The microsphere was self-assembled with heparin-modified gelatin nanofibers that mimic the architecture of natural bone ECMs and provided an osteoconductive microenvironment for bone cells. Interleukin 4 (IL4), which has binding domains with heparin, was incorporated into the nanofibrous heparin-modified gelatin microsphere. Binding IL4 to heparin protected the IL4 from denaturation and degradation, and controlled its sustained release. The in vivo study showed the osteoimmunomodulatory microspheres switched the pro-inflammatory M1 macrophage into a pro-healing M2 phenotype, efficiently resolved the inflammation, and subsequently enhanced osteoblastic differentiation and new bone regeneration (Fig. 5). The development of immunomodulatory biomaterials, therefore, is a promising approach for bone healing.
Fig. 5.
An immunomodulatory approach to enhance alveolar bone healing and regeneration. The nanofibrous microsphere mimic the architecture of bone ECM and switched the transition of macrophages from M1 to M2 phenotypes; therefore, enhanced alveolar bone healing. Adapted with permission from Ref. [168].
It is worth mentioning that the regeneration of periodontal horizontal bone loss has been neglected for a long time. According to a report in 2010, the prevalence of horizontal bone loss is much higher than vertical bone loss in population [169]. However, the majority of alveolar bone regeneration research has been focusing on vertical bone loss regeneration. Guided bone regeneration (GBR) or GTR combined with bone graft materials were examined for horizontal bone augmentation [[169], [170], [171]]. However, as mentioned in previous sections, the outcomes were unpredictable. Currently, there are few studies on horizontal bone regeneration using tissue engineering strategy. One work reported the use of calcium-alginate scaffold for the repair of dog supraalveolar bone defect [172]. The result showed that the buccal-lingual bone thickness remained thinner than normal, and the shape and structure of regenerated bone were not restored as normal alveolar crest [172]. Consequently, tooth function and gingiva appearance were not restored. To date, it is still a major challenge to achieve horizontal bone loss regeneration, which is a direction for future periodontal research.
4.3. Alveolar bone-PDL-cementum complex regeneration
After decades of research on periodontal regeneration, it has been well accepted that alveolar bone, PDL and cementum are a structural and functional entity and the restoration of only one or two portions of the periodontium cannot restore normal periodontal functions [173]. For instance, a biphasic scaffold with only bone and PDL compartments could not form cementum on the root surface of a periodontal defect [75,152]. Without cementum, the connection between PDL and tooth was loose and the periodontium could not play the supporting role. The addition of a thin layer of cell sheet to the biphasic scaffold induced cementum-like tissue formation [88,166]. Since each layer of the periodontium has distinctive characteristics, the scaffold for each part should be designed accordingly to achieve a multi-tissue regeneration.
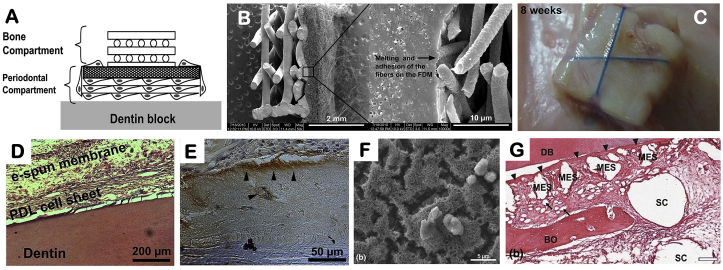
A chitin-PLGA-bioglass composite scaffold was fabricated to regenerate alveolar bone, PDL and cementum [91]. The incorporation of bioactive glass was to enhance mineralized tissue formation on the cementum and bone portion. Bioactive molecules were encapsulated in different layers of the scaffold, separately, to induce bone (platelet-rich plasma derived growth factors), PDL (fibroblast growth factor 2) and cementum (cementum protein 1) regeneration. Another example is the use of cell sheet as the cementum layer combining with bone and PDL compartments (Fig. 6). The multilayer scaffold was hold together with a dentinal slice and subcutaneously transplanted in vivo. PDL-like and cementum-like tissues were observed after 8 weeks [166]. The bone portion of the scaffold was further coated with calcium phosphate to increase osteoconductivity. While cell sheets could be generated from different cells sources, bone marrow mesenchymal stem cell sheet and PDL cell sheet had better results compared to gingival cells in promoting bone, PDL and cementum regeneration [167].
Fig. 6.
Combination of cell sheet technology with bone and PDL scaffolds to regenerate cementum-PDL-alveolar bone. (A) Fabrication scheme showing the combination of PDL and bone compartments with a dentin slice. The bone and PDL layers were fabricated using fused deposition and electrospinning, respectively. (B) SEM image of the biphasic scaffold showing the fusion of the electrospun fibers onto the fused deposition component. (C) Subcutaneously transplanted the construct to induce periodontal regeneration in vivo. (D) PDL-like and (E) cementum-like tissues formation after transplantation for eight weeks. (F) CaP was coated on the PCL fused deposition compartment. (G) The CaP-coated scaffold enhanced bone formation. Adapted with permission from Refs. [88,166].
Although the above scaffolds regenerated periodontal tissues with similar structures to natural periodontal tissues, they failed to regenerate Sharpey's fibers, a pivotal structure of periodontium. Sharpey's fibers are the end part of the PDL collagen fiber bundles embedded in cementum and bone [10]. Sharpey's fibers connect cementum and alveolar bone to PDL, therefore, are essential for the integrity and function of periodontal tissues. Without the formation of Sharpey's fibers, there is no functional connection between cementum/alveolar bone and PDL, and the regenerated periodontium complex has no function. To rebuild the interface between PDL and bone, a study used 3D printing to fabricate PDL and bone scaffolds that provided channels to allow regenerated tissues to penetrate each other [89]. However, this scaffold only guided PDL fiber aligned in the channels of PDL portion, but no Sharpey's fibers inserted into the bone portion. The failure is likely because the large diameter of the guiding structure (0.8 mm in diameter) was ineffective to guide the direction of Sharpe's fibers (several microns in diameter). Multi-layers cell sheets were used and the close connection between the PDL and cementum enabled the fibers from each layer to mingle together to form Sharpey's fiber-like structure [163]. However, the inserted fibers were randomly distributed. To date, there are no feasible and effective ways to regenerate Sharpey's fibers of periodontium complex.
Periodontal mineralization homeostasis is a key factor for the long-term stability of newly formed periodontium complex. Ideally, the regenerated alveolar bone and cementum tissues should be mineralized and PDL be non-mineralized for a long term. However, the regenerated PDL tissue can be influenced by the surrounding hard tissues, leading to gradually mineralization, becoming thinner, and finally disappeared to cause tooth ankyloses. Therefore, maintenance of periodontal mineralization homeostasis is critical for the long-term stability of regenerated tissues. One possible solution is the introduction of moderate mechanical stimulation to the regenerative periodontium, which was believed to prevent dentoalveolar ankylosis and PDL mineralization [174], even though the mechanism is unclear yet. Another way is the controlled delivery of mineralization inhibitors in the PDL portion and mineralization inducers in the alveolar bone and cementum portions of the periodontal scaffold. However, the key mineralization regulators in periodontium warrant to be clarified to fulfill this strategy. In addition, controlled delivery of the regulators for a long period of time is another challenge. Overall, the periodontal mineralization homeostasis is of great significance for long-term stability of regenerated tissues, but how to regulate and achieve the expected results need further exploration.
5. Conclusion and future perspective
Biomaterials used in periodontal regeneration include inorganic materials, polymeric materials and composites. While inorganic biomaterials are components used for bone and cementum regeneration due to their similar compositional and mechanical property, polymeric biomaterials are used for PDL regeneration. The combination of inorganic and polymeric materials is used fabricate biomimetic scaffolds for bone and cementum regeneration. To provide an ECM-mimicking microenvironment, biomimetic nanofibrous and multilayer scaffolds have been developed for periodontal tissue regeneration in recent years. A few studies attempted to regenerate periodontal tissues with the proper structures, such as the oriented PDL fibers, but achieved limited success. Some of the main challenges for regenerating functional periodontal tissues are listed below:
From material perspective, most of the biomaterials used for periodontal regeneration are traditional biomaterials, such as HA, β-TCP, PLLA, PCL, and PLGA. Even though these biomaterials can resemble the compositions in certain aspects, they cannot mimic the fine structures of the natural periodontal tissues, such as the different groups of PDL fibers, cellular and acellular cementum structure. One specific challenge is the regeneration of Sharpey's fibers between cementum-PDL-alveolar bone. Currently, none of the scaffolding systems regenerated functional Sharpey's fibers. Without these fibers, the connections between cementum-PDL-alveolar bone is unstable and cannot support teeth or bear occlusal force. Therefore, novel and bio-inspired materials that are designed to closely mimic the architecture of periodontal tissues at micro and nanoscale levels are prerequisite to achieve functional periodontal tissue regeneration.
To regenerate the hierarchical architecture of periodontal tissues, spatially and temporally controlled delivery of biophysical and biochemical cues is indispensable. For temporal regulation, the time point and sequence for cell migration, proliferation, differentiation and tissue formation need to follow the natural process. As for spatial regulation, inducing the distinguished alveolar bone, PDL, and cementum formation at the same time requires a well-designed regeneration system. While there have been a variety of multidrug delivery systems developed, none of them can achieve precise control to guide periodontal tissue regeneration. There are a number of barriers that hinder optimized tissue regeneration. One essential issue is the lack of complete understanding of basic biology of periodontal tissue repair and healing. Factors that determine periodontal tissue formation have not be clarified, therefore, the selected drugs/growth factors cannot achieve the expected regenerative effects. Another knowledge gap is the proper concentrations of bioactive molecules, because overuse or insufficient drugs/growth factor compromises the outcomes. Therefore, an in-depth understanding of the basic biology is indispensable to provide more detailed information to guide the fabrication of biomimetic materials.
It was reported that mechanical cues plays a role in restoration of functional periodontal tissues [154]. However, the mechanical cues were rarely included in periodontal tissue regeneration. Therefore, integration of mechanical cues to biomaterial design and evaluation of the regenerative effect will be inspiring for future studies.
Other challenges for periodontal regeneration include the restoration of horizontal alveolar bone loss and long-term stability of regenerated periodontal tissues. Addressing these problems will tremendously advance the progresses of periodontal tissue regeneration.
Despite all these challenges, periodontal tissue regeneration is an exciting and rapidly growing field. The advances of this field provide the promising potential to improve the health of dental patients in the near future.
CRediT authorship contribution statement
Yongxi Liang: Writing - original draft. Xianghong Luan: Conceptualization, Funding acquisition. Xiaohua Liu: Conceptualization, Writing - review & editing, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgement
This work was supported by NIH/NIDCR (DE024979, DE019463).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Health U.S.D.o., Human S. NIH Publication; 2000. Oral Health in America : A Report of the Surgeon General; pp. 155–188. [Google Scholar]
- 2.Deas D.E. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000;71(1):128–139. doi: 10.1111/prd.12114. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Graziani F. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol. 2000;75(1):152–188. doi: 10.1111/prd.12201. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Smiley C.J. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015;146(7):525–535. doi: 10.1016/j.adaj.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 5.John M.T. Network meta-analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. J. Clin. Periodontol. 2017;44(6):603–611. doi: 10.1111/jcpe.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar C.C., Cochran D.L. Regeneration of periodontal tissues: guided tissue regeneration. Dent. Clin. 2010;54(1):73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Kao R.T., Nares S., Reynolds M.A. Periodontal regeneration - intrabony defects: a systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015;86(2 Suppl):S77–S104. doi: 10.1902/jop.2015.130685. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z., Rios H.F., Cochran D.L. Emerging regenerative approaches for periodontal reconstruction: a systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015;86(2 Suppl):S134–S152. doi: 10.1902/jop.2015.130689. [DOI] [PubMed] [Google Scholar]
- 9.Li F. Evaluation of recombinant human FGF-2 and PDGF-BB in periodontal regeneration: a systematic review and meta-analysis. Sci. Rep. 2017;7(1):65. doi: 10.1038/s41598-017-00113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanci A. Elsevier; 2013. Ten Cate's Oral Histology: Development, Structure, and Function. [Google Scholar]
- 11.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 12.Ramseier C.A. Advanced reconstructive technologies for periodontal tissue repair. Periodontol. 2000;59(1):185–202. doi: 10.1111/j.1600-0757.2011.00432.x. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F.M. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Han J. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014;59(Suppl 1):117–130. doi: 10.1111/adj.12100. [DOI] [PubMed] [Google Scholar]
- 15.Sculean A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol. 2000;68(1):182–216. doi: 10.1111/prd.12086. 2015. [DOI] [PubMed] [Google Scholar]
- 16.Larsson L. Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 2016;95(3):255–266. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlow J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984;11(8):494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 18.Karring T. Development of the biological concept of guided tissue regeneration--animal and human studies. Periodontol. 2000;1(1):26–35. 1993. [PubMed] [Google Scholar]
- 19.Needleman I.G. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006;(2) doi: 10.1002/14651858.CD001724.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Rakmanee T. Radiographic outcomes following treatment of intrabony defect with guided tissue regeneration in aggressive periodontitis. Clin. Oral Invest. 2016;20(6):1227–1235. doi: 10.1007/s00784-015-1609-y. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y.C. Comparisons of periodontal regenerative therapies: a meta-analysis on the long-term efficacy. J. Clin. Periodontol. 2017;44(5):511–519. doi: 10.1111/jcpe.12715. [DOI] [PubMed] [Google Scholar]
- 22.Bosshardt D.D., Sculean A. Does periodontal tissue regeneration really work? Periodontol. 2000;51:208–219. doi: 10.1111/j.1600-0757.2009.00317.x. 2009. [DOI] [PubMed] [Google Scholar]
- 23.Du J. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J. Dent. Res. 2014;93(2):183–188. doi: 10.1177/0022034513513026. [DOI] [PubMed] [Google Scholar]
- 24.Li H. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs. 2009;190(2):94–101. doi: 10.1159/000166547. [DOI] [PubMed] [Google Scholar]
- 25.Yu B.H., Zhou Q., Wang Z.L. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J. Biomater. Appl. 2014;29(2):243–253. doi: 10.1177/0885328214521846. [DOI] [PubMed] [Google Scholar]
- 26.Requicha J.F. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng. 2014;20(17–18):2483–2492. doi: 10.1089/ten.tea.2013.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo B.M. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cell. 2008;26(4):1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016;7(1):130. doi: 10.1186/s13287-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang W. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015;25:240–252. doi: 10.1016/j.actbio.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 31.Park C.H. 3D printed, microgroove pattern-driven generation of oriented ligamentous architectures. Int. J. Mol. Sci. 2017;18(9) doi: 10.3390/ijms18091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park C.H. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014;93(12):1304–1312. doi: 10.1177/0022034514550716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh Z. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Biomater. Res. 2017;21:9. doi: 10.1186/s40824-017-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scantlebury T.V. 1982-1992: a decade of technology development for guided tissue regeneration. J. Periodontol. 1993;64(11 Suppl):1129–1137. doi: 10.1902/jop.1993.64.11s.1129. [DOI] [PubMed] [Google Scholar]
- 35.Sam G., Baiju R.M. Evolution of barrier membranes in periodontal Regeneration-“Are the third generation membranes really here? 2014;8 doi: 10.7860/JCDR/2014/9957.5272. ZE14-Z17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortellini P., Pini Prato G., Tonetti M.S. Periodontal regeneration of human intrabony defects with titanium reinforced membranes. A controlled clinical trial. J. Periodontol. 1995;66(9):797–803. doi: 10.1902/jop.1995.66.9.797. [DOI] [PubMed] [Google Scholar]
- 37.Khanna R. Pure titanium membrane (ultra - Ti((R))) in the treatment of periodontal osseous defects: a split-mouth comparative study. J. Clin. Diagn. Res. 2016;10(9) doi: 10.7860/JCDR/2016/18333.8487. ZC47-ZC51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: a review. Polymers. 2016;8(4) doi: 10.3390/polym8040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y. Osteogenic induction of bone marrow mesenchymal cells on electrospun polycaprolactone/chitosan nanofibrous membrane. Dent. Mater. J. 2017;36(3):325–332. doi: 10.4012/dmj.2016-203. [DOI] [PubMed] [Google Scholar]
- 40.Masoudi Rad M. Fabrication and characterization of two-layered nanofibrous membrane for guided bone and tissue regeneration application. Mater Sci Eng C Mater Biol Appl. 2017;80:75–87. doi: 10.1016/j.msec.2017.05.125. [DOI] [PubMed] [Google Scholar]
- 41.Caballe-Serrano J. Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: a systematic review. Arch. Oral Biol. 2019;100:57–68. doi: 10.1016/j.archoralbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Lee B.S. A functional chitosan membrane with grafted epigallocatechin-3-gallate and lovastatin enhances periodontal tissue regeneration in dogs. Carbohydr. Polym. 2016;151:790–802. doi: 10.1016/j.carbpol.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Araujo M.G., Berglundh T., Lindhe J. GTR treatment of degree III furcation defects with 2 different resorbable barriers. An experimental study in dogs. J. Clin. Periodontol. 1998;25(3):253–259. doi: 10.1111/j.1600-051x.1998.tb02436.x. [DOI] [PubMed] [Google Scholar]
- 44.Gentile P. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011;6(10):1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 45.Kawase T. Human periosteum-derived cells combined with superporous hydroxyapatite blocks used as an osteogenic bone substitute for periodontal regenerative therapy: an animal implantation study using nude mice. J. Periodontol. 2010;81(3):420–427. doi: 10.1902/jop.2009.090523. [DOI] [PubMed] [Google Scholar]
- 46.Mao L. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015;10:7031–7044. doi: 10.2147/IJN.S90343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.S. Periodontal tissue reaction to customized nano-hydroxyapatite block scaffold in one-wall intrabony defect: a histologic study in dogs. J Periodontal Implant Sci. 2012;42(2):50–58. doi: 10.5051/jpis.2012.42.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H., Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769–2781. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Matsuura T. Effect of a tunnel-structured beta-tricalcium phosphate graft material on periodontal regeneration: a pilot study in a canine one-wall intrabony defect model. J. Periodontal. Res. 2015;50(3):347–355. doi: 10.1111/jre.12213. [DOI] [PubMed] [Google Scholar]
- 50.Maroo S., Murthy K.R. Treatment of periodontal intrabony defects using beta-TCP alone or in combination with rhPDGF-BB: a randomized controlled clinical and radiographic study. Int. J. Periodontics Restor. Dent. 2014;34(6):841–847. doi: 10.11607/prd.2030. [DOI] [PubMed] [Google Scholar]
- 51.Matsuse K. Periodontal regeneration induced by porous alpha-tricalcium phosphate with immobilized basic fibroblast growth factor in a canine model of 2-wall periodontal defects. Med. Mol. Morphol. 2018;51(1):48–56. doi: 10.1007/s00795-017-0172-9. [DOI] [PubMed] [Google Scholar]
- 52.Lee J.S. Maturation of periodontal tissues following implantation of rhGDF-5/beta-TCP in one-wall intra-bony defects in dogs: 24-week histological observations. J. Clin. Periodontol. 2012;39(5):466–474. doi: 10.1111/j.1600-051X.2012.01862.x. [DOI] [PubMed] [Google Scholar]
- 53.Iwasaki K. The influence of beta-tricalcium phosphate blocks containing extracellular matrix on osteogenic differentiation of rat bone marrow stromal cells. J. Periodontol. 2013;84(10):1484–1492. doi: 10.1902/jop.2012.120490. [DOI] [PubMed] [Google Scholar]
- 54.Santos P.S. Osteoinductive porous biphasic calcium phosphate ceramic as an alternative to autogenous bone grafting in the treatment of mandibular bone critical-size defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(4):1546–1557. doi: 10.1002/jbm.b.33963. [DOI] [PubMed] [Google Scholar]
- 55.Miron R.J. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Invest. 2016;20(8):2259–2265. doi: 10.1007/s00784-016-1724-4. [DOI] [PubMed] [Google Scholar]
- 56.Bansal R. Clinical evaluation of hydroxyapatite and beta-tricalcium phosphate composite graft in the treatment of intrabony periodontal defect: a clinico-radiographic study. J. Indian Soc. Periodontol. 2014;18(5):610–617. doi: 10.4103/0972-124X.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho S.M. Characterization and induction of cementoblast cell proliferation by bioactive glass nanoparticles. J Tissue Eng Regen Med. 2012;6(10):813–821. doi: 10.1002/term.488. [DOI] [PubMed] [Google Scholar]
- 58.Han P. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/beta-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials. 2012;33(27):6370–6379. doi: 10.1016/j.biomaterials.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 59.Wu C. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012;8(10):3805–3815. doi: 10.1016/j.actbio.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Chacko N.L. A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas(R): a synthetic, resorbable material in treating periodontal infrabony defects. J. Int. Oral Health. 2014;6(3):20–26. [PMC free article] [PubMed] [Google Scholar]
- 61.Dutra C.E. In vivo evaluation of bioactive glass foams associated with platelet-rich plasma in bone defects. J Tissue Eng Regen Med. 2008;2(4):221–227. doi: 10.1002/term.86. [DOI] [PubMed] [Google Scholar]
- 62.Hoppe A., Guldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Rahaman M.N. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Momose T. Collagen hydrogel scaffold and fibroblast growth factor-2 accelerate periodontal healing of class II furcation defects in dog. Open Dent. J. 2016;10:347–359. doi: 10.2174/1874210601610010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang C. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 66.Berahim Z. Biologic interaction of three-dimensional periodontal fibroblast spheroids with collagen-based and synthetic membranes. J. Periodontol. 2011;82(5):790–797. doi: 10.1902/jop.2010.100533. [DOI] [PubMed] [Google Scholar]
- 67.Chen X. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016;11:4707–4718. doi: 10.2147/IJN.S111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura S., Kubo T., Ijima H. Heparin-conjugated gelatin as a growth factor immobilization scaffold. J. Biosci. Bioeng. 2013;115(5):562–567. doi: 10.1016/j.jbiosc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Li Z. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015;13:88–100. doi: 10.1016/j.actbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Echave M.C. Gelatin as biomaterial for tissue engineering. Curr. Pharmaceut. Des. 2017;23(24):3567–3584. doi: 10.2174/0929867324666170511123101. [DOI] [PubMed] [Google Scholar]
- 71.Varoni E.M. Chitosan-based trilayer scaffold for multitissue periodontal regeneration. J. Dent. Res. 2018;97(3):303–311. doi: 10.1177/0022034517736255. [DOI] [PubMed] [Google Scholar]
- 72.Ignatova M., Manolova N., Rashkov I. Electrospun antibacterial chitosan-based fibers. Macromol. Biosci. 2013;13(7):860–872. doi: 10.1002/mabi.201300058. [DOI] [PubMed] [Google Scholar]
- 73.Li H. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. 2017;105(1):265–273. doi: 10.1002/jbm.a.35900. [DOI] [PubMed] [Google Scholar]
- 74.Zang S. A comparison of physicochemical properties of sterilized chitosan hydrogel and its applicability in a canine model of periodontal regeneration. Carbohydr. Polym. 2014;113:240–248. doi: 10.1016/j.carbpol.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 75.Park C.H. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33(1):137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shang S. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur. Cell. Mater. 2010;19:180–192. doi: 10.22203/ecm.v019a18. [DOI] [PubMed] [Google Scholar]
- 77.Gentile P. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014;15(3):3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campos D.M. Surface entrapment of fibronectin on electrospun PLGA scaffolds for periodontal tissue engineering. Biores Open Access. 2014;3(3):117–126. doi: 10.1089/biores.2014.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pilipchuk S.P. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv Healthc Mater. 2016;5(6):676–687. doi: 10.1002/adhm.201500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Batool F. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: an in vitro andIn vivo study. Materials. 2018;11(4) doi: 10.3390/ma11040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siddiqui N. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018;60:506–532. doi: 10.1007/s12033-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 82.Jo S. Enhanced adhesion of preosteoblasts inside 3D PCL scaffolds by polydopamine coating and mineralization. Macromol. Biosci. 2013;13(10):1389–1395. doi: 10.1002/mabi.201300203. [DOI] [PubMed] [Google Scholar]
- 83.Carlo Reis E.C. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32(35):9244–9253. doi: 10.1016/j.biomaterials.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J. Biomater. Appl. 2016;31(1):121–131. doi: 10.1177/0885328216637978. [DOI] [PubMed] [Google Scholar]
- 85.Liao F. A novel bioactive three-dimensional beta-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J. Mater. Sci. Mater. Med. 2010;21(2):489–496. doi: 10.1007/s10856-009-3931-x. [DOI] [PubMed] [Google Scholar]
- 86.Brown A. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015;11:543–553. doi: 10.1016/j.actbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Shujaa Addin A. Biodegradable gelatin/beta-tricalcium phosphate sponges incorporating recombinant human fibroblast growth factor-2 for treatment of recession-type defects: a split-mouth study in dogs. J. Periodontal. Res. 2017;52(5):863–871. doi: 10.1111/jre.12456. [DOI] [PubMed] [Google Scholar]
- 88.Costa P.F. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014;41(3):283–294. doi: 10.1111/jcpe.12214. [DOI] [PubMed] [Google Scholar]
- 89.Park C.H. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31(23):5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee C.H. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. 2014;20(7–8):1342–1351. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sowmya S. Tri-layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv Healthc Mater. 2017;6(7) doi: 10.1002/adhm.201601251. [DOI] [PubMed] [Google Scholar]
- 92.Liu X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30(12):2252–2258. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X.H., Jin X.B., Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10(5):398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X.H., Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 95.Liu X.H., Ma P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30(25):4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X.H., Ma P.X. The nanofibrous architecture of poly(L-lactic acid)-based functional copolymers. Biomaterials. 2010;31(2):259–269. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X.H. Surface engineering of nano-fibrous poly(L-Lactic Acid) scaffolds via self-assembly technique for bone tissue engineering. J. Biomed. Nanotechnol. 2005;1(1):54–60. [Google Scholar]
- 98.Liu X.H., Won Y.J., Ma P.X. Surface modification of interconnected porous scaffolds. J. Biomed. Mater. Res. 2005;74A(1):84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 99.Liu X.H., Won Y.J., Ma P.X. Porogen-induced surface modification of nano-fibrous poly(L-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27(21):3980–3987. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Struillou X. Treatment of periodontal defects in dogs using an injectable composite hydrogel/biphasic calcium phosphate. J. Mater. Sci. Mater. Med. 2011;22(7):1707–1717. doi: 10.1007/s10856-011-4344-1. [DOI] [PubMed] [Google Scholar]
- 101.Iviglia G. Novel bioceramic-reinforced hydrogel for alveolar bone regeneration. Acta Biomater. 2016;44:97–109. doi: 10.1016/j.actbio.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Rasperini G. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015;94(9 Suppl) doi: 10.1177/0022034515588303. 153S-7S. [DOI] [PubMed] [Google Scholar]
- 103.Elavarasu S., Suthanthiran T.K., Naveen D., Statins A new era in local drug delivery. J. Pharm. BioAllied Sci. 2012;4(Suppl 2):S248–S251. doi: 10.4103/0975-7406.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu S. Effect of simvastatin on the osteogenetic behavior of alveolar osteoblasts and periodontal ligament cells. Hum. Cell. 2012;25(2):29–35. doi: 10.1007/s13577-011-0028-x. [DOI] [PubMed] [Google Scholar]
- 105.Zhao B.J., Liu Y.H. Simvastatin induces the osteogenic differentiation of human periodontal ligament stem cells. Fundam. Clin. Pharmacol. 2014;28(5):583–592. doi: 10.1111/fcp.12050. [DOI] [PubMed] [Google Scholar]
- 106.Pradeep A.R., Thorat M.S. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J. Periodontol. 2010;81(2):214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 107.S S.M. Comparative evaluation of efficacy of subgingivally delivered 1.2% Atorvastatin and 1.2% Simvastatin in the treatment of intrabony defects in chronic periodontitis: a randomized controlled trial. J. Dent. Res. Dent. Clin. Dent. Prospects. 2017;11(1):18–25. doi: 10.15171/joddd.2017.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bertl K. Statins in nonsurgical and surgical periodontal therapy. A systematic review and meta-analysis of preclinical in vivo trials. J. Periodontal. Res. 2018;53(3):267–287. doi: 10.1111/jre.12514. [DOI] [PubMed] [Google Scholar]
- 109.Wang P. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12(2):437–446. doi: 10.1002/term.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pradeep A.R. Efficacy of 1% metformin gel in patients with moderate and severe chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2017;88(10):1023–1029. doi: 10.1902/jop.2017.150096. [DOI] [PubMed] [Google Scholar]
- 111.Pankaj D. Comparative evaluation of subgingivally delivered 1.2% rosuvastatin and 1% metformin gelin treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2018;9:e12324. doi: 10.1002/JPER.17-0434. [DOI] [PubMed] [Google Scholar]
- 112.Sun H.H. Designing biomaterials for in situ periodontal tissue regeneration. Biotechnol. Prog. 2012;28(1):3–20. doi: 10.1002/btpr.698. [DOI] [PubMed] [Google Scholar]
- 113.Javed F. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch. Oral Biol. 2011;56(12):1476–1484. doi: 10.1016/j.archoralbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 114.Boyan L.A. Mitogenic and chemotactic responses of human periodontal ligament cells to the different isoforms of platelet-derived growth factor. J. Dent. Res. 1994;73(10):1593–1600. doi: 10.1177/00220345940730100301. [DOI] [PubMed] [Google Scholar]
- 115.Cooke J.W. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12(6):1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamano S. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials. 2014;35(8):2446–2453. doi: 10.1016/j.biomaterials.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol. 2000;56(1):188–208. doi: 10.1111/j.1600-0757.2010.00365.x. 2011. [DOI] [PubMed] [Google Scholar]
- 118.Shimabukuro Y. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J. Cell. Physiol. 2011;226(3):809–821. doi: 10.1002/jcp.22406. [DOI] [PubMed] [Google Scholar]
- 119.Murakami S. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J. Periodontal. Res. 2003;38(1):97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 120.Kitamura M. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PloS One. 2008;3(7) doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kitamura M. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J. Dent. Res. 2011;90(1):35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- 122.Khoshkam V. Outcomes of regenerative treatment with rhPDGF-BB and rhFGF-2 for periodontal intra-bony defects: a systematic review and meta-analysis. J. Clin. Periodontol. 2015;42(3):272–280. doi: 10.1111/jcpe.12354. [DOI] [PubMed] [Google Scholar]
- 123.Kollet O., Dar A., Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu. Rev. Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 124.Kimura Y., Tabata Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010;21(1):37–51. doi: 10.1163/156856209X410193. [DOI] [PubMed] [Google Scholar]
- 125.Ji W. Incorporation of stromal cell-derived factor-1alpha in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials. 2013;34(3):735–745. doi: 10.1016/j.biomaterials.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 126.Du L., Yang P., Ge S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J. Periodontol. 2012;83(3):379–388. doi: 10.1902/jop.2011.110201. [DOI] [PubMed] [Google Scholar]
- 127.Liu H. Local administration of stromal cell-derived factor-1 promotes stem cell recruitment and bone regeneration in a rat periodontal bone defect model. Mater Sci Eng C Mater Biol Appl. 2015;53:83–94. doi: 10.1016/j.msec.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Cai X. Periodontal regeneration via chemoattractive constructs. J. Clin. Periodontol. 2018;45(7):851–860. doi: 10.1111/jcpe.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Anusuya G.S. Bone morphogenetic proteins: signaling periodontal bone regeneration and repair. J. Pharm. BioAllied Sci. 2016;8(Suppl 1):S39–s41. doi: 10.4103/0975-7406.191964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.King G.N. Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J. Dent. Res. 1997;76(8):1460–1470. doi: 10.1177/00220345970760080801. [DOI] [PubMed] [Google Scholar]
- 131.Oortgiesen D.A. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med. 2014;8(3):202–209. doi: 10.1002/term.1514. [DOI] [PubMed] [Google Scholar]
- 132.Hakki S.S. Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102(1):119–130. doi: 10.1002/jbm.b.32988. [DOI] [PubMed] [Google Scholar]
- 133.Chiu H.C. Effects of bone morphogenetic protein-6 on periodontal wound healing/regeneration in supraalveolar periodontal defects in dogs. J. Clin. Periodontol. 2013;40(6):624–630. doi: 10.1111/jcpe.12075. [DOI] [PubMed] [Google Scholar]
- 134.Ern C. Differentiation of hMSC and hPDLSC induced by PGE2 or BMP-7 in 3D models. Prostaglandins Leukot. Essent. Fatty Acids. 2017;122:30–37. doi: 10.1016/j.plefa.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 135.Kitagawa M. F-spondin regulates the differentiation of human cementoblast-like (HCEM) cells via BMP7 expression. Biochem. Biophys. Res. Commun. 2012;418(2):229–233. doi: 10.1016/j.bbrc.2011.12.155. [DOI] [PubMed] [Google Scholar]
- 136.Hakki S.S. Bone morphogenetic protein-7 enhances cementoblast function in vitro. J. Periodontol. 2010;81(11):1663–1674. doi: 10.1902/jop.2010.100074. [DOI] [PubMed] [Google Scholar]
- 137.Giannobile W.V. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J. Periodontol. 1998;69(2):129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 138.Yin X. Growth/differentiation factor-5 promotes in vitro/vivo periodontal specific differentiation of induced pluripotent stem cell-derived mesenchymal stem cells. Exp Ther Med. 2017;14(5):4111–4117. doi: 10.3892/etm.2017.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee J., Wikesjo U.M. Growth/differentiation factor-5: pre-clinical and clinical evaluations of periodontal regeneration and alveolar augmentation--review. J. Clin. Periodontol. 2014;41(8):797–805. doi: 10.1111/jcpe.12260. [DOI] [PubMed] [Google Scholar]
- 140.Stavropoulos A. A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF-5/beta-TCP for periodontal regeneration. J. Clin. Periodontol. 2011;38(11):1044–1054. doi: 10.1111/j.1600-051X.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- 141.Song W.-Y. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Eng. Regen. Med. 2016;13(6):750–761. doi: 10.1007/s13770-016-9123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen F.M. Localized delivery of growth factors for periodontal tissue regeneration: role, strategies, and perspectives. Med. Res. Rev. 2009;29(3):472–513. doi: 10.1002/med.20144. [DOI] [PubMed] [Google Scholar]
- 143.Ma C. Hierarchical nanofibrous microspheres with controlled growth factor delivery for bone regeneration. Adv Healthc Mater. 2015;4(17):2699–2708. doi: 10.1002/adhm.201500531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang B., Ma C., Liu X. Nanofibers regulate single bone marrow stem cell osteogenesis via FAK/RhoA/YAP1 pathway. ACS Appl. Mater. Interfaces. 2018;10(39):33022–33031. doi: 10.1021/acsami.8b11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X., Holzwarth J.M., Ma P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012;12(7):911–919. doi: 10.1002/mabi.201100466. [DOI] [PubMed] [Google Scholar]
- 146.Ma C., Liu X. formation of nanofibrous matrices, three-dimensional scaffolds, and microspheres: from theory to practice. Tissue Eng. C Methods. 2017;23(1):50–59. doi: 10.1089/ten.tec.2016.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun Y. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng. 2013;19(15–16):1754–1763. doi: 10.1089/ten.tea.2012.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sundararaj S.C. Design of a multiple drug delivery system directed at periodontitis. Biomaterials. 2013;34(34):8835–8842. doi: 10.1016/j.biomaterials.2013.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen F.M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J. Contr. Release. 2011;149(2):92–110. doi: 10.1016/j.jconrel.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 150.Lee B.S. Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016;11:285–297. doi: 10.2147/IJN.S94270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Duruel T. Sequential IGF-1 and BMP-6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int. J. Biol. Macromol. 2017;104(Pt A):232–241. doi: 10.1016/j.ijbiomac.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 152.Nivedhitha Sundaram M. Bilayered construct for simultaneous regeneration of alveolar bone and periodontal ligament. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(4):761–770. doi: 10.1002/jbm.b.33480. [DOI] [PubMed] [Google Scholar]
- 153.Yang F. Electrospun zein/gelatin scaffold-enhanced cell attachment and growth of human periodontal ligament stem cells. Materials. 2017;10(10) doi: 10.3390/ma10101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J.H. Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Li S., Wu C. The in vitro and in vivo cementogenesis of CaMgSi(2)O(6) bioceramic scaffolds. J. Biomed. Mater. Res. 2014;102(1):105–116. doi: 10.1002/jbm.a.34679. [DOI] [PubMed] [Google Scholar]
- 156.Bhakta G. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33(26):6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanimoto K. Differential effects of amelogenin on mineralization of cementoblasts and periodontal ligament cells. J. Periodontol. 2012;83(5):672–679. doi: 10.1902/jop.2011.110408. [DOI] [PubMed] [Google Scholar]
- 158.Komaki M. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J. Cell. Physiol. 2012;227(2):649–657. doi: 10.1002/jcp.22770. [DOI] [PubMed] [Google Scholar]
- 159.Chen X. The synthesis of hydroxyapatite with different crystallinities by controlling the concentration of recombinant CEMP1 for biological application. Mater Sci Eng C Mater Biol Appl. 2016;59:384–389. doi: 10.1016/j.msec.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 160.Villarreal-Ramirez E. Characterization of recombinant human cementum protein 1 (hrCEMP1): primary role in biomineralization. Biochem. Biophys. Res. Commun. 2009;384(1):49–54. doi: 10.1016/j.bbrc.2009.04.072. [DOI] [PubMed] [Google Scholar]
- 161.Park C.H. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017;61:134–143. doi: 10.1016/j.actbio.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 162.Xie H., Liu H. A novel mixed-type stem cell pellet for cementum/periodontal ligament-like complex. J. Periodontol. 2012;83(6):805–815. doi: 10.1902/jop.2011.110267. [DOI] [PubMed] [Google Scholar]
- 163.Flores M.G. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J. Periodontal. Res. 2008;43(3):364–371. doi: 10.1111/j.1600-0765.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 164.Flores M.G. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008;35(12):1066–1072. doi: 10.1111/j.1600-051X.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 165.Tsumanuma Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32(25):5819–5825. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 166.Vaquette C. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33(22):5560–5573. doi: 10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 167.Vaquette C. Periodontal tissue engineering with a multiphasic construct and cell sheets. J. Dent. Res. 2019;98(6):673–681. doi: 10.1177/0022034519837967. [DOI] [PubMed] [Google Scholar]
- 168.Hu Z. Immunomodulatory ECM-like microspheres for accelerated bone regeneration in diabetes mellitus. ACS Appl. Mater. Interfaces. 2018;10(3):2377–2390. doi: 10.1021/acsami.7b18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jayakumar A. Horizontal alveolar bone loss: a periodontal orphan. J. Indian Soc. Periodontol. 2010;14(3):181–185. doi: 10.4103/0972-124X.75914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Merli M. Comparing membranes and bone substitutes in a one-stage procedure for horizontal bone augmentation. Three-year post-loading results of a double-blind randomised controlled trial. Eur. J. Oral Implant. 2018;11(4):441–452. [PubMed] [Google Scholar]
- 171.Chiapasco M., Casentini P. Horizontal bone-augmentation procedures in implant dentistry: prosthetically guided regeneration. Periodontol. 2000;77(1):213–240. doi: 10.1111/prd.12219. 2018. [DOI] [PubMed] [Google Scholar]
- 172.Weng Y. Repair of experimental alveolar bone defects by tissue-engineered bone. Tissue Eng. 2006;12(6):1503–1513. doi: 10.1089/ten.2006.12.1503. [DOI] [PubMed] [Google Scholar]
- 173.Ivanovski S. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014;93(12):1212–1221. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mine K. Occlusal forces promote periodontal healing of transplanted teeth and prevent dentoalveolar ankylosis: an experimental study in rats. Angle Orthod. 2005;75(4):637–644. doi: 10.1043/0003-3219(2005)75[637:OFPPHO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]