Abstract
Measures of handgrip strength can be used to conveniently assess overall muscle strength capacity. Although stand-alone measures of handgrip strength provide robust health information, the clinical meaningfulness to determine prevention and treatment options for weakness remains limited because the etiology of muscle weakness remains unclear. Moreover, clinical outcomes associated with handgrip strength are wide-ranging. Therefore, disentangling how handgrip strength is associated with health conditions that are metabolically or neurologically driven may improve our understanding of the factors linked to handgrip strength. The purpose of this topical review was to highlight and summarize evidence examining the associations of handgrip strength with certain health outcomes that are metabolically and neurologically driven. From this perusal of the literature, we posit that stand-alone handgrip strength be considered an umbrella assessment of the body systems that contribute to strength capacity, and a panoptic measurement of muscle strength that is representative of overall health status, not a specific health condition. Recommendations for future strength capacity–related research are also provided.
Keywords: Geriatric assessment, methods, muscle strength, muscle weakness, sarcopenia
Introduction
Overall muscle strength is conveniently assessed by measuring handgrip strength (HGS) with a handgrip dynamometer.1 Measures of HGS are characterized by completing a maximal isometric grip force task, wherein individuals squeeze a handgrip dynamometer with maximum effort for a short duration (i.e. seconds) and then relax the contracting musculature.2 Given the relatively low cost and ease of assessment, measures of HGS are used in clinical and epidemiological settings for determining strength capacity.3 For example, larger population-based studies such as the National Health and Nutrition Examination Survey,4,5 and UK Biobank have included measures of HGS in their protocols.6 As such, information from HGS measurements have been used in the United States and globally for making several health-related inferences,7 creating HGS percentiles,8,9 and generating weakness cut-points.10,11
Decreased HGS is particularly associated with poor health.12 Specifically, measures of HGS are included in validated criteria for determining frailty,13 and weakness often represents the onset of the frailty phenotype.14 Measures of HGS are also associated with more markers of frailty than age, thereby suggesting that HGS is a superior health marker of frailty relative to chronological age alone.15 Moreover, several investigations have revealed that low HGS is associated with arguably the most impactful health outcome, premature mortality.16–19 Although low HGS is associated with early mortality, the mechanisms connecting HGS with mortality remain relatively unknown. Many have presented a sequence of events that may help to explain the systematic pathways linking HGS with premature mortality.7,20 Within such sequences, decreased HGS has been shown to be associated with a variety of chronic cardiometabolic (e.g. type II diabetes mellitus (diabetes)) and neurodegenerative diseases (e.g. cognitive impairment).7 The widespread evidence that measures of HGS have in predicting future adverse health events demonstrates that HGS is a powerful biomarker of aging and “vital sign” of health.21,22
Muscle weakness, as measured by HGS, is associated with a wide-range of health conditions, which makes it challenging to delineate what body system processes are responsible for weakness. Zamboni et al.23 indicated that muscle weakness is part of sarcopenia, wherein decreases in muscle endurance and increases in muscle fatigability occur primarily at the muscular system level. Others have posited that nervous system dysfunction brings structural and functional changes to skeletal muscle, subsequently contributing to weakness.24 Manini and Clark25 also suggest weakness is a product of age-related physiological deficits in both the muscular and nervous systems. Age-related declines to the muscular and nervous systems each factor into reductions in HGS, explaining why HGS is associated with health outcomes that are metabolically and neurologically driven.
As evidence linking low HGS to poor health status continues to accumulate, measures of HGS will become more commonplace in routine health assessments.26 However, HGS as a stand-alone measure of strength capacity brings a large amount of uncertainty for future health risks because the measure is associated with such varying health conditions. For example, healthcare providers that utilize cut-points for identifying weakness may experience difficulty explaining what weakness is a risk factor exactly to their patients. Such challenges diminish the clinical meaningfulness of HGS and ability for healthcare providers to prescribe appropriate prevention and treatment options for weakness. Therefore, unraveling how HGS is associated with health conditions that are driven by the muscular or nervous systems will help to provide a better understanding of the risk factors for weakness, which in turn, will bolster the interpretation and significance of HGS measurements. The purposes of this topical review were to (1) highlight and summarize evidence examining the associations of HGS with certain health outcomes that tend to be metabolically and neurologically driven and (2) provide some recommendations for future research in this area.
Methods
The PubMed database was used to search for articles. PubMed was selected for this review because articles indexed in PubMed contain several peer-reviewed studies related to HGS and health. Database searches began in August 2019 and concluded in January 2020. The following MeSH and keyword search terms were executed: adult, aged, aged 80 and over, middle aged, humans, hand strength, handgrip strength, grip strength, muscle weakness, muscle strength, sarcopenia, frailty, cardiovascular diseases, blood glucose, diabetes mellitus, falls, gait, dementia, Alzheimer’s disease, Parkinson’s disease, activities of daily living, walking, geriatrics, geriatric assessment, exercise test, prevalence, epidemiology, exercise, physical activity, preventative health. The search terms included for our review were selected based on the purposes of the review. To be considered for inclusion, articles must have been published in English language and analyzed HGS with a health condition that was pertinent to our review. Articles that were not original research and did not assess HGS were not considered.
HGS and chronic cardiometabolic morbidities
Chronic cardiometabolic diseases such as cardiovascular disease and diabetes have a large global disease burden.27 Metabolic syndrome is a strong precursor for both cardiovascular disease and diabetes,28 and HGS is associated with each of the components used to diagnosis metabolic syndrome. For example, in a cross-sectional study of 2677 adults aged 59–73 years, Sayer et al.29 found that every standard deviation decrease of HGS was associated with a 0.05 standard deviation increase in fasting triglycerides (p = 0.006), 1.13 greater odds for high blood pressure (p = 0.004), 0.08 standard deviation unit increase in waist circumference (p < 0.001), 0.07 standard deviation unit increase in 2-h glucose (p = 0.001), and 0.05 standard deviation unit increase in homeostatic model assessment (p = 0.008). This investigation also found that lower HGS was associated with 1.18 greater odds (p < 0.001) for metabolic syndrome when using Adult Treatment Panel III guidelines and 1.11 greater odds (p = 0.03) for metabolic syndrome when utilizing International Diabetes Federation guidelines.29 Another cross-sectional study of adults aged at least 20 years revealed that men (n = 2472) and women (n = 2542) in the strongest HGS quartile had 0.22 (p < 0.0001) and 0.16 decreased odds (p < 0.0001) for metabolic syndrome, respectively.30 Given decreased HGS is associated with metabolic syndrome, and that metabolic syndrome is a precursor for cardiovascular disease,28 it is plausible that HGS is also associated with cardiovascular morbidity.
Cardiovascular disease is a leading cause of death worldwide.31 Identifying biomarkers that help in the prevention and treatment for cardiovascular disease may help to mitigate cardiovascular disease mortality. Leong et al.18 revealed that every 5-kg decrease in HGS was associated with a 1.17 higher hazard (p < 0.001) for cardiovascular disease mortality and 1.07 higher hazard (p = 0.002) for myocardial infarction at a median 4-year follow-up in 142,861 adults aged 35–70 years. Interestingly, this investigation also found that HGS was a stronger predictor of cardiovascular mortality than systolic blood pressure.18 Celis-Morales et al.32 found that every 5-kg lower HGS was associated with a 1.11 (p < 0.001) and 1.15 higher hazard (p < 0.001) for cardiovascular disease incidence, and a 1.22 (p < 0.001) and 1.19 higher hazard (p < 0.001) for cardiovascular disease mortality at 7.1-year follow-up in 217,011 men and 260,063 women, respectively. This study also revealed that men who were classified as weak had a 1.36 higher hazard (p < 0.001) for cardiovascular disease incidence and 1.84 higher hazard (p < 0.001) for cardiovascular disease mortality, while women who were considered weak had a 1.30 higher hazard (p < 0.001) for cardiovascular disease incidence and 1.44 higher hazard (p < 0.001) for cardiovascular mortality.32
A secondary data analysis of 452,931 adults from the UK Biobank found that low HGS was associated with cardiovascular disease events (hazard ratios ranged from 1.05 to 1.09) at follow-up.33 Another longitudinal-panel study of 17,431 Americans aged at least 50 years determined that those who were weak had a 1.35 higher hazard (p < 0.05) for developing chronic heart failure compared to persons who were not weak at 4.7 ± 2.7 years of follow-up.34 Although there appears to be an association between HGS and cardiovascular disease, measures of HGS may also help to predict other prevalent chronic morbidities such as diabetes.
Diabetes is a widespread chronic cardiometabolic disease in the United States and worldwide.35,36 Weakness may factor into the incidence of diabetes. A 19-year longitudinal study of adults aged at least 65 years revealed men (n = 801) and women (n = 1102) who were considered weak had a 1.05 (p < 0.001) and 1.38 higher hazard (p < 0.001) for incident diabetes, respectively.37 Another 16-year longitudinal study of 424 adults aged 46.4 ± 2.8 years determined that every 0.1 higher body weight normalized HGS was associated with a 0.81 lower hazard (p = 0.006) for incident diabetes.38 Li et al.39 found that every 5-kg higher HGS was associated with 0.87 decreased odds for incident diabetes in 1632 men aged at least 35 years. While HGS might be useful for predicting diabetes risk, measures of HGS may also help to determine risk for poor health outcomes in persons already living with diabetes.
A retrospective cohort study of 1282 adults aged 63.8 ± 13.9 years with diabetes demonstrated that every 1-kg increase in HGS at baseline was associated with 0.89 decreased odds (p = 0.025) for cardiovascular disease events and 0.96 decreased odds (p < 0.001) for hospitalization at 2.36 ± 0.73 years of follow-up.40 Celis-Morales et al.41 similarly determined that persons with diabetes who also had lower HGS were at greater risk for adverse health outcomes such as cardiovascular disease incidence (hazard ratio: 1.98; p < 0.05), cardiovascular disease mortality (hazard ratio: 2.88; p < 0.05), and all-cause mortality (hazard ratio: 2.05; p < 0.05) compared to those with higher HGS.
Table 1 outlines the studies that were reviewed for the associations between HGS and chronic cardiometabolic health conditions. There is certainly existing evidence for the association of HGS and chronic cardiometabolic diseases such as cardiovascular disease and diabetes. Lower HGS may also be associated with multimorbidity and other chronic diseases that were not reviewed herein.42 Likewise, measures of HGS could be used in those living with a cardiometabolic disease to determine risk for a future adverse health event. Lifestyle behaviors that are associated with cardiometabolic diseases such as sedentary behavior and an unhealthy diet are primary contributors to weakness.43 Changing such poor lifestyle behaviors, especially earlier in life, may help to preserve the muscular system functions that contribute to weakness and cardiometabolic morbidities over the lifespan.
Table 1.
Outline of reviewed studies for the associations between handgrip strength and chronic cardiometabolic health conditions.
| Article | Participants | Health condition | Key findings |
|---|---|---|---|
| Sayer et al.29 | 2677 men aged 59–73 years | Metabolic syndrome | Every standard deviation decrease of HGS was associated with a 0.05 standard deviation increase in fasting triglycerides (p = 0.006), 1.13 greater odds for high blood pressure (p = 0.004), 0.08 standard deviation unit increase in waist circumference (p < 0.001), 0.07 standard deviation unit increase in 2-h glucose (p = 0.001), and 0.05 standard deviation unit increase in homeostatic model assessment (p = 0.008). It was also found that lower HGS was associated with 1.18 greater odds (p < 0.001) for metabolic syndrome when using Adult Treatment Panel III guidelines and 1.11 greater odds (p = 0.03) for metabolic syndrome when utilizing International Diabetes Federation guidelines |
| Yi et al.30 | 2472 men and 2542 women aged ⩾ 20 years | Metabolic syndrome | Men (n = 2472) and women (n = 2542) in the strongest HGS quartile had 0.22 (p < 0.0001) and 0.16 decreased odds (p < 0.0001) for metabolic syndrome, respectively |
| Leong et al.18 | 142,861 adults aged 35–70 years | Cardiovascular disease mortality and myocardial infarction | Every 5-kg decrease in HGS was associated with a 1.17 higher hazard (p < 0.001) for cardiovascular disease mortality and 1.07 higher hazard (p = 0.002) for myocardial infarction |
| Celis-Morales et al.32 | 217,011 men and 260,063 women aged 40–69 years | Cardiovascular disease incidence, cardiovascular disease mortality | Every 5-kg lower HGS was associated with a 1.11 (p < 0.001) and 1.15 higher hazard (p < 0.001) for cardiovascular disease incidence, and a 1.22 (p < 0.001) and 1.19 higher hazard (p < 0.001) for cardiovascular disease mortality in men and women, respectively. Men who were weak had a 1.36 higher hazard (p < 0.001) for cardiovascular disease incidence and 1.84 higher hazard (p < 0.001) for cardiovascular disease mortality, while women who were weak had a 1.30 higher hazard (p < 0.001) for cardiovascular disease incidence and 1.44 higher hazard (p < 0.001) for cardiovascular mortality |
| Farmer et al.33 | 452,931 adults | Cardiovascular disease events | Low HGS was associated with cardiovascular disease events (hazard ratios ranged from 1.05 to 1.09) |
| McGrath et al.34 | 17,431 adults aged ⩾ 50 years | Chronic heart failure | Those who were weak had a 1.35 higher hazard (p < 0.05) for developing chronic heart failure compared to persons who were not weak |
| McGrath et al.37 | 801 men and 1102 women aged ⩾ 65 years | Diabetes | Men and women who were considered weak had a 1.05 (p < 0.001) and 1.38 higher hazard (p < 0.001) for incident diabetes, respectively |
| Karvonen-Gutierrez et al.38 | 424 adults aged 46.4 ± 2.8 years | Diabetes | Every 0.1 higher body weight normalized HGS was associated with a 0.81 lower hazard (p = 0.006) for incident diabetes |
| Li et al.39 | 1632 men aged ⩾ 35 years | Diabetes | Every 5-kg higher HGS was associated with 0.87 decreased odds for incident diabetes |
| Hamasaki et al.40 | 1282 adults aged 63.8 ± 13.9 years with diabetes | Cardiovascular disease events and hospitalization | Every 1-kg increase in HGS at baseline was associated with 0.89 decreased odds (p = 0.025) for cardiovascular disease events and 0.96 decreased odds (p < 0.001) for hospitalization |
| Celis-Morales et al.41 | 347,130 adults aged 55.9 ± 8.1 years | Cardiovascular disease incidence, cardiovascular disease mortality, all-cause mortality | Persons with diabetes who also had low HGS were at greater risk for adverse health outcomes such as cardiovascular disease incidence (hazard ratio: 1.98; p < 0.05), cardiovascular disease mortality (hazard ratio: 2.88; p < 0.05), and all-cause mortality (hazard ratio: 2.05; p < 0.05) compared to those with higher HGS |
HGS: handgrip strength.
Contents included in this table may have only captured findings from studies that were relevant for our review.
HGS and neural morbidities
Previous work has demonstrated that the muscle force generated during HGS assessments in older adults is around half of what would be expected if the skeletal musculature itself were fully activated because of age-related neural deficits.44,45 This finding suggests that diminished nervous system functioning plays an important role in strength capacity and may help to explain why HGS is linked to diseases of the nervous system. For example, cognitive impairment without dementia (i.e. mild cognitive impairment) is more prevalent in the United States than dementia,46 and the presence of a mild cognitive impairment often leads to more advanced dementias.47 A cross-sectional study of 1366 men and 1616 women aged at least 65 years found that men and women in the highest HGS quartile had 0.38 (p < 0.001) and 0.51 decreased odds (p < 0.001) for mild cognitive impairment compared to those in the lowest HGS quartile, respectively.48 Another cross-sectional study of 32,715 adults aged 62.0 ± 15.6 years revealed that weakness was associated with 1.41 greater odds (p < 0.05) for mild cognitive impairment.49 Although cross-sectional study designs provide insights into the association of HGS and cognitive impairment, longitudinal study designs will help to reveal temporal inferences.
A mild cognitive impairment is a precursor for Alzheimer’s disease and related dementias,47 and strength capacity is associated with both mild cognitive impairment and Alzheimer’s disease.50 A longitudinal study of 13,828 adults aged at least 50 years that were followed for 8 years determined that every 5-kg lower HGS was associated with 1.10 greater odds (p < 0.05) for any cognitive impairment, 1.18 greater odds (p < 0.05) for severe cognitive impairment, and 1.10 greater odds (p < 0.05) for poorer cognitive functioning.51 Buchman et al.52 revealed that each 1-pound decrease in baseline HGS was associated with a 1.5% increased risk (p < 0.05) for Alzheimer’s disease. Furthermore, Alfaro-Acha et al.53 reported a significant time-by-HGS quartile interaction for cognitive impairment (Q1: β = −0.18 ± 0.06, p < 0.0001; Q2: β = −0.21 ± 0.06, p < 0.001; Q3: β = −0.09 ± 0.05, p > 0.05; Q4 = reference) in a 7-year prospective cohort study of 2160 Mexican Americans aged 71.9 ± 5.9 years. While an association exists between HGS and diseases of the nervous system such as cognitive impairment, HGS may also help to identify the acceleration of cognitive declines.
Decision making after disease diagnosis is critical for the health status of those living with a disease.54 Continuing to utilize measures that detect disease progression may help evaluate treatment strategies. For example, HGS decreased in 220 older women as Alzheimer’s disease advanced from “early Alzheimer’s disease” (HGS: 17.4 ± 3.7 kg; p < 0.01) to “mild Alzheimer’s disease” (HGS: 16.9 ± 3.7 kg; p < 0.0001) to “moderate Alzheimer’s disease” (HGS: 15.8 ± 3.8 kg; p < 0.0001) relative to women with normal cognition (HGS: 20.1 ± 3.3 kg).55 Likewise, a cross-sectional study of 79 adults with Parkinson’s diseases revealed that HGS was correlated with Unified Parkinson Disease Rating Scale scores (r = −0.36; p = 0.006) and Hoehn–Yahr scores (r = −0.37; p = 0.005).56 Therefore, measures of HGS could be a useful indicator for disease progression for certain morbidities that are driven by reduced neural function.
Table 2 outlines the studies that were reviewed for the associations between HGS and neural health conditions. A fair amount of attention has been given to combating muscle weakness at the musculoskeletal level; however, treating weakness at the neural level may show promise for the prevention and treatment of weakness.45 More acknowledgment should be given to the role of motor performance in muscle function and strength capacity, as HGS is also a discriminating measure of neurological functioning.2 For example, the cortical and subcortical portions of the brain that control hand dexterity are also linked to cognitive function, suggesting that factors which represent neurological declines of non-cognitive and cognitive processes may have a shared cause.57 Thus, nervous system deficits that contribute to muscle weakness could be identified by measures of HGS.
Table 2.
Outline of reviewed studies for the associations between handgrip strength and neural health conditions.
| Article | Participants | Health condition | Key findings |
|---|---|---|---|
| Jang and Kim48 | 1366 men and 1616 women aged ⩾ 65 years | Cognitive impairment | Men and women in the highest HGS quartile had 0.38 (p < 0.001) and 0.51 decreased odds (p < 0.001) for mild cognitive impairment compared to those in the lowest HGS quartile, respectively |
| Vancampfort et al.49 | 32,715 adults aged 62.0 ± 15.6 years | Cognitive impairment | Weakness was associated with 1.41 greater odds (p < 0.05) for mild cognitive impairment |
| McGrath et al.51 | 13,828 adults aged ⩾ 50 years | Cognitive impairment | Every 5-kg lower HGS was associated with 1.10 greater odds (p < 0.05) for any cognitive impairment, 1.18 greater odds (p < 0.05) for severe cognitive impairment, and 1.10 greater odds (p < 0.05) for poorer cognitive functioning |
| Buchman et al.52 | 887 older adults | Alzheimer’s disease | Each 1-pound decrease in baseline HGS was associated with a 1.5% increased risk (p < 0.05) for Alzheimer’s disease |
| Alfaro-Acha et al.53 | 2160 adults aged 71.9 ± 5.9 years | Cognitive impairment | A significant time-by-HGS quartile interaction existed for cognitive impairment (Q1: β = −0.18 ± 0.06, p < 0.0001; Q2: β = −0.21 ± 0.06, p < 0.001; Q3: β = −0.09 ± 0.05, p > 0.05; Q4 = reference) |
| Ogawa et al.55 | 352 older adults | Alzheimer’s disease | In women, Alzheimer’s disease advanced from “early Alzheimer’s disease” (HGS: 17.4 ± 3.7 kg; p < 0.01) to “mild Alzheimer’s disease” (HGS: 16.9 ± 3.7 kg; p < 0.0001) to “moderate Alzheimer’s disease” (HGS: 15.8 ± 3.8 kg; p < 0.0001) relative to women with normal cognition (HGS: 20.1 ± 3.3 kg) |
| Roberts et al.56 | 79 adults | Parkinson’s diseases | HGS was correlated with Unified Parkinson Disease Rating Scale scores (r = −0.36; p = 0.006) and Hoehn–Yahr scores (r = −0.37; p = 0.005) |
HGS: handgrip strength; Q: quartile.
Contents included in this table may have only captured findings from studies that were relevant for our review.
HGS and functional disability
Functional capacity is often measured by questionnaires regarding a person’s ability to complete a series of instrumental activities of daily living (IADLs) and basic activities of daily living (BADLs).58 IADLs require higher neuropsychological functioning and are considered necessary for independent living;59 whereas, BADLs are more physically driven and are considered necessary for basic self-care.60 Many working-age and older adults are living with a functional disability,61,62 and the presence of a functional disability increases risk for further disabilities,63 morbidities,64 and premature mortality.65 Given that IADLs are more neural driven and BADLs are more physically driven, it is possible that HGS is associated with functional disability.
A cross-sectional study of 947 adults aged at least 65 years revealed that every 10-kg increase in HGS was associated with 0.61 decreased odds (p < 0.05) for IADL disability.66 Another cross-sectional investigation of 10,149 adults aged 71.8 ± 7.7 years revealed that those in the middle and high HGS tertile had 0.61 (p < 0.05) and 0.47 lower odds (p < 0.05) for an IADL disability compared to individuals in the lowest HGS tertile, respectively.67 A longitudinal investigation of 672 Mexican Americans aged 81.7 ± 4.1 years determined that every 10-kg increase in HGS at baseline was associated with 0.95 decreased odds (p < 0.05) for losses in IADLs at 2-year follow-up.68 When examining individual IADLs, a longitudinal study of 15,336 adults aged at least 50 years who were followed for 8 years uncovered that every 5-kg decrease in HGS was associated with 1.11 greater odds for an impairment in using a map, 1.07 greater odds for an impairment in preparing hot meals, 1.09 greater odds for an impairment in taking medications, 1.06 greater odds for an impairment in managing money, 1.05 greater odds for an impairment in using a telephone, and 1.10 greater odds for an impairment in shopping for groceries (all p < 0.05).69 Impairments in IADLs often precede BADL limitations;59 thus, HGS may also be associated with BADLs.
Al Snih et al.70 revealed that men (n = 1050; age: 72.5 ± 6.2 years) and women (n = 1443; age: 72.3 ± 6.2 years) in the lowest HGS quartiles had a 1.90 and 2.28 higher hazard (p < 0.05) for any BADL limitation over the 7-year study period. Likewise, another longitudinal study of 2270 Mexican Americans aged at least 65 years found that those who were considered weak had a 1.25 higher hazard (p < 0.0001) for incident BADL disability compared to persons who were not weak and did not have diabetes over a 19-year study period.71 Zhang et al.72 examined the association between weakness and BADL disability in 6127 Chinese adults aged at least 45 years and found that those who were considered weak had 2.26 greater odds (p < 0.001) for BADL disability compared to those who were not weak. McGrath et al.73 investigated the association between HGS and individual BADLs in 17,747 Americans aged at least 50 years during an 8-year study period and found every 5-kg decrease in HGS was associated with a 1.20 higher hazard for an eating limitation, 1.14 higher hazard for a walking limitation, 1.14 higher hazard for a bathing limitation, 1.09 higher hazard for a dressing limitation, 1.08 higher hazard for a transferring limitation, and 1.06 higher hazard for a toileting limitation (p < 0.05 for all).
An outline of studies that were reviewed for the associations between HGS and functional health conditions is presented in Table 3. Measures of HGS are unique in that they are associated with performance in functional tasks that are both neuropsychologically (i.e. IADL) and physically driven (i.e. BADL). Clark and Manini74 suggest that muscular and nervous system impairments each contribute to the decreased skeletal muscle activation and force generation that characterizes muscle weakness, which subsequently leads to functional disability. IADL and BADL tasks indirectly reflect neural and muscular system functioning, which are also mechanisms for strength capacity. Therefore, decreased HGS may signify not only impairments of the muscular and neural systems that contribute to muscle weakness but also the impairments of the muscular and neural systems that factor into functional disability.
Table 3.
Outline of reviewed studies for the associations between handgrip strength and functional health conditions.
| Article | Participants | Health condition | Key findings |
|---|---|---|---|
| Gopinath et al.66 | 947 adults aged ⩾ 65 years | IALD disability | Every 10-kg increase in HGS was associated with 0.61 decreased odds (p < 0.05) for IADL disability |
| Germain et al.67 | 10,149 adults aged 71.8 ± 7.7 years | IADL disability | Those in the middle and high HGS tertile had 0.61 (p < 0.05) and 0.47 lower odds (p < 0.05) for an IADL disability compared to individuals in the lowest HGS tertile, respectively |
| McGrath et al.68 | 672 adults aged 81.7 ± 4.1 years | IADL disability | Every 10-kg increase in HGS at baseline was associated with 0.95 decreased odds (p < 0.05) for losses in IADLs |
| McGrath et al.69 | 15,336 adults aged ⩾ 50 years | IADL disability | Every 5-kg decrease in HGS was associated with 1.11 greater odds for an impairment in using a map, 1.07 greater odds for an impairment in preparing hot meals, 1.09 greater odds for an impairment in taking medications, 1.06 greater odds for an impairment in managing money, 1.05 greater odds for an impairment in using a telephone, and 1.10 greater odds for an impairment in shopping for groceries (all p < 0.05) |
| Al Snih et al.70 | 1050 older men and 1443 older women | BADL disability | Men and women in the lowest HGS quartiles had a 1.90 and 2.28 higher hazard (p < 0.05) for any BADL limitation |
| McGrath et al.71 | 2270 older adults aged ⩾ 65 years | BADL disability | Those who were considered weak had a 1.25 higher hazard (p < 0.0001) for incident BADL disability compared to persons who were not weak and did not have diabetes |
| Zhang et al.72 | 6127 adults aged ⩾ 45 years | BADL disability | Those who were considered weak had 2.26 greater odds (p < 0.001) for BADL disability compared to those who were not weak |
| McGrath et al.73 | 17,747 adults aged ⩾ 50 years | BADL disability | Every 5-kg decrease in HGS was associated with a 1.20 higher hazard for an eating limitation, 1.14 higher hazard for a walking limitation, 1.14 higher hazard for a bathing limitation, 1.09 higher hazard for a dressing limitation, 1.08 higher hazard for a transferring limitation, and 1.06 higher hazard for a toileting limitation (p < 0.05 for all) |
BADLs: basic activities of daily living; HGS: handgrip strength; IADLs: instrumental activities of daily living.
Contents included in this table may have only captured findings from studies that were relevant for our review.
HGS and dynamic assessments of function
The muscular and neural systems are important for physical functions that are dynamic in nature such as the timed-up-and-go, 6-min walk, gait speed, and balance tests.75,76 Deficits in such physical functions can carry pronounced health consequences. For example, slower gait speed is strongly associated with mortality.77 Moreover, slow gait speed is a characteristic of individuals with a chronic cardiometabolic disease such as diabetes and neural morbidities such as cognitive impairment.78,79 HGS, which can be considered a measure of muscle function, may also be associated with dynamic, physical functions.80
Cross-sectional evidence from 110 women aged 67.4 ± 5.9 years indicated that HGS on the dominant (r = −0.20; p = 0.03) and non-dominant hand (r = −0.20; p = 0.03) is correlated with the timed-up-and-go test.81 The 6-min walk test is a popular method for assessing physical functioning and fitness.82 Martín-Ponce et al.83 found that adults aged at least 60 years who were in the lower HGS category walked a shorter distance (84.3 ± 12.0 m) on the 6-min walk test than those in the higher HGS category (178.0 ± 14.0 m; p < 0.001). Furthermore, Zhang et al.84 revealed that HGS was correlated with 6-min walk test distance (r = 0.221; p = 0.029) and also predicted 6-min walk distance (β = 3.162; p < 0.001) in cross-sectional study of 106 adults aged 62.0 ± 10.0 years.
HGS is an important factor for gait speed, and both HGS and gait speed are part of frailty assessments.13 Cut-points for determining clinically relevant weakness have also been created from the association between HGS and gait speed.10,11 Harris-Love et al.85 demonstrated that HGS is related to gait speed (r = 0.42; p = 0.021) in a cross-sectional study of 30 men aged 62.5 ± 9.2 years. Balance is important for the prevention of falls, and Arvandi et al.86 examined the association between HGS and fall history in 808 adults aged at least 65 years, revealing that every 1-kg increase in HGS was associated with 0.97 decreased odds (p = 0.26) for falls.
Measures of HGS are associated with deficits in several physical functions. Stevens et al.87 assessed the associations between HGS and components of the Short Physical Performance Battery in 349 men and 280 women aged 63–73 years and determined that every 1-kg increase in HGS was associated with a 0.07-s decrease in the timed-up-and-go test, 0.02-s decrease in 3-m walk time, and 1% decrease in chair rises time for men (all p < 0.001). This study also found that every 1-kg increase in HGS was associated with a 0.13-s decrease in the timed-up-and-go test, 0.03-s decrease in 3-m walking time, and 1% decrease in chair rises time for women (all p < 0.001).87 Table 4 outlines studies that were reviewed for the associations between HGS and dynamic functional assessments. With HGS being associated with mobility and other dynamic measures of physical function, overlap in the muscular and neural system processes that are needed to properly complete measures of HGS and dynamic physical functioning may exist.
Table 4.
Outline of reviewed studies for the associations between handgrip strength and dynamic functional assessments.
| Article | Participants | Assessment | Key findings |
|---|---|---|---|
| Alonso et al.81 | 110 women aged 67.4 ± 5.9 | Timed-up-and-go | HGS on the dominant (r = −0.20; p = 0.03) and non-dominant hand (r = −0.20; p = 0.03) is correlated with the timed-up-and-go test |
| Martin-Ponce et al.83 | 310 adults aged ⩾ 60 years | 6-min walk | Those who were in the lower HGS category walked a shorter distance (84.3 ± 12.0 m) on the 6-min walk test than those in the higher HGS category (178.0 ± 14.0 m; p < 0.001) |
| Zhang et al.84 | 106 adults aged 62.0 ± 10.0 years | 6-min walk | HGS was correlated with 6-min walk test distance (r = 0.221; p = 0.029) and also predicted 6-min walk distance (β = 3.162; p < 0.001) |
| Harris-Love et al.85 | 30 adults aged 62.5 ± 9.2 years | Gait speed | HGS is correlated with gait speed (r = 0.42; p = 0.021) |
| Arvandi et al.86 | 808 adults aged ⩾ 65 years | Fall history | Every 1-kg increase in HGS was associated with 0.97 decreased odds (p = 0.26) for falls |
| Stevens et al.87 | 349 men and 280 women aged 63–73 years | Short physical performance battery | Every 1-kg increase in HGS was associated with a 0.07-s decrease in the timed-up-and-go test, 0.02-s decrease in 3-m walk time, and 1% decrease in chair rises time for men (all p < 0.001). Every 1-kg increase in HGS was associated with a 0.13-s decrease in the timed-up-and-go test, 0.03-s decrease in 3-m walking time, and 1% decrease in chair rises time for women (all p < 0.001) |
HGS: handgrip strength.
Contents included in this table may have only captured findings from studies that were relevant for our review.
Discussion
This review presented and summarized evidence for HGS being associated with a wide-range of health conditions, thereby supporting sentiments that suggest HGS is a powerful biomarker of aging and “vital sign” of health.21,22 Muscular strength, as often measured by HGS, is also an important part of intrinsic capacity.88 Although decreased HGS and being considered clinically weak is a risk factor for morbidity,18 disability,73 and early mortality,16 measures of maximal HGS alone may not provide enough information to determine the specific health risks from low HGS. Therefore, we recommend that measures of HGS be included in routine health assessments (especially for older adults); however, we also suggest that HGS be considered an umbrella assessment of the body system processes that contribute to strength capacity, and a panoptic measurement of muscle strength that is representative of overall health status.
While there is a strong body of evidence that shows HGS is associated with several health conditions,7,22,26 some have posited that HGS alone cannot be assumed a proxy for overall muscle strength89 and that other physical measures such as knee extension strength may provide additional value in assessments of strength capacity.90 Accordingly, HGS has been included, as part, in decision algorithms for defining sarcopenia and dynapenia.25,91 Although HGS alone provides robust health information that is feasible to measure,92 we postulate that HGS could be an incomplete measure of overall strength capacity. The inclusion of HGS as part of decision algorithms and criteria for determining sarcopenia,91 dynapenia,25 and frailty13 support that other measures should be considered for improving precision in the diagnoses of health conditions. Future research should attempt to distinguish the etiology of weakness from the muscular and neural systems, which in turn, may help in determining how weakness is a risk factor for morbidities that are metabolically or neurologically driven. This may include reevaluating HGS measures and methods, the inclusion of additional HGS measures that reflect other aspects of muscle function, and further refining decision algorithms. Avoiding physical measures that share common constructs with HGS, and that are more burdensome to participants such as maximal knee extension strength, may help to avoid overlap in assessing body system processes that contribute to weakness.93 Furthermore, maintaining uniformity in methods for how strength capacity data (including HGS) are collected will help to develop consistencies in protocols, comparisons across studies, and simplicity for healthcare providers and their patients.
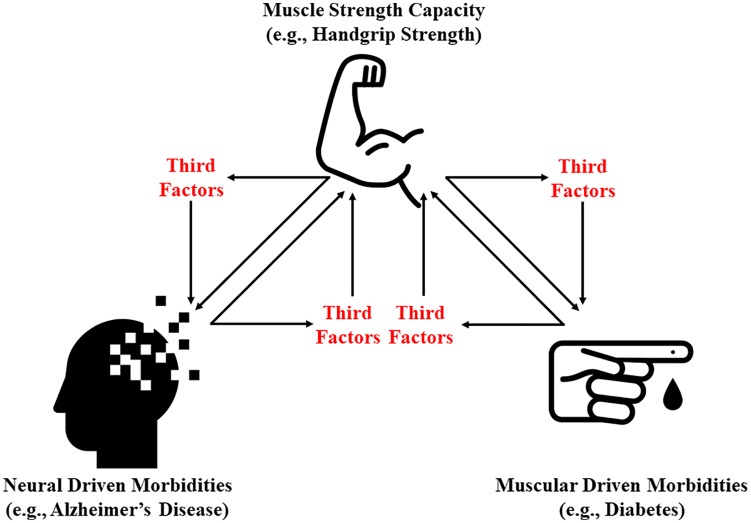
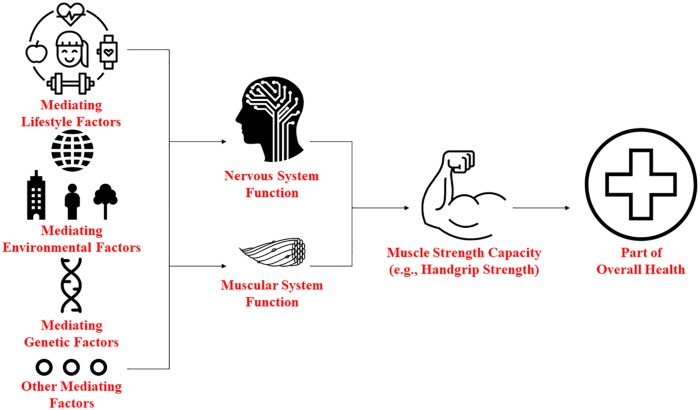
To determine the role of the muscular and neural systems within the etiology of weakness, examining potential third factors (mediators) may help to reveal new insights into the associations of strength capacity on metabolic and neural diseases. This might be especially helpful because it is challenging trying to separate the contribution of the muscular and neural systems in strength capacity. For example, previous research has revealed that a temporal, bidirectional association exists for HGS and cognitive function.94,95 Similarly, a longitudinal, bidirectional association may exist between HGS and metabolic diseases such as diabetes.96,97 Such findings indicate that when paralleling associations exist, losses of function in one factor could lead to losses in the other. Figure 1 provides a conceptual model for identifying third factors for paralleling associations between HGS and diseases that are metabolically or neurologically driven, while Figure 2 postulates how such mediators may influence strength capacity and health through the muscular and nervous systems. Identifying these mediating factors may help not only in distinguishing how the muscular and neural systems contribute to weakness but also in uncovering new prevention and treatment strategies for weakness.
Figure 1.
Conceptual model for identifying third factors for paralleling associations between muscle strength capacity and morbidities that are metabolically or neural driven.
Figure 2.
Conceptual model for evaluating how strength capacity and health could be influenced through the muscular and nervous systems by different categories of mediating factors.
Some limitations of this review should be acknowledged. While this topical review highlighted evidence for the association between HGS and several clinically relevant health conditions, this work is not intended to be an all-encompassing review that used systematic methods. Thus, the articles included in the narrative portion of this topical review share common limitations with other similar types of reviews.98 Articles that did not meet our criteria for the narrative portion of this review could have been used to support relevant text and directions for future research in HGS. Nonetheless, all the included articles were germane to the overarching premise of this review, thereby allowing for a detailed description of published articles on HGS and health, and rational for future HGS research.99
Conclusion
This review highlighted evidence for the associations of HGS and outcomes that are metabolically and neurologically driven. There were several studies that found low HGS was associated with chronic cardiometabolic diseases, neural morbidities, functional declines, and mobility limitations. The wide-range of conditions linked to low HGS suggests that the muscular and neural systems may both factor into strength declines. Although stand-alone measures of HGS are feasible to complete and provide robust health information, the clinical meaningfulness, interpretability, and treatment options for low HGS are currently limited because the mechanistic processes that may contribute to weakness remains unclear and associated outcomes are broad. As such, we suggest that HGS as a stand-alone measure be considered an umbrella assessment of the body system processes that contribute to strength capacity, and a measurement of muscle strength that is representative of overall health status, not a specific health condition. Nevertheless, measures of HGS should be included in routine health assessments in clinical and epidemiological settings. Continuing to distinguish the etiology of lower HGS from the muscular and neural systems may provide insights into how deficiencies in these systems contribute to weakness, which subsequently can help in providing specificity in future risk, prevention, and treatment for health conditions associated with low HGS.
Acknowledgments
N.J., L.K., S.M., and K.T. are all co-second authors. The icons included in this review were permitted for use from the Noun Project (https://thenounproject.com/), wherein R.M. is a member.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ryan McGrath  https://orcid.org/0000-0002-0644-5524
https://orcid.org/0000-0002-0644-5524
References
- 1. Lu ZL, Wang TR, Qiao YQ, et al. Handgrip strength index predicts nutritional status as a complement to body mass index in Crohn’s disease. J Crohns Colitis 2016; 10(12): 1395–1400. [DOI] [PubMed] [Google Scholar]
- 2. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging 2018; 71: 189–222. [DOI] [PubMed] [Google Scholar]
- 3. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011; 40(4): 423–429. [DOI] [PubMed] [Google Scholar]
- 4. National Health Nutrition Examination Survey (NHANES). Muscle strength procedures manual, April 2011, https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/Muscle_Strength_Proc_Manual.pdf (accessed 19 September 2019).
- 5. National Health Nutrition Examination Survey (NHANES). Muscle strength procedures manual, January 2013, https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/Muscle_Strength_2013.pdf (accessed 19 September 2019).
- 6. UK Biobank. Grip Strength Measurement. Version 1.0, https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/Gripstrength.pdf (accessed 19 September 2019).
- 7. McGrath RP, Kraemer WJ, Snih SA, et al. Handgrip strength and health in aging adults. Sports Med 2018; 48(9): 1993–2000. [DOI] [PubMed] [Google Scholar]
- 8. Dodds RM, Syddall HE, Cooper R, et al. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing 2016; 45(2): 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGrath R, Hackney KJ, Ratamess NA, et al. Absolute and body mass index normalized handgrip strength percentiles by gender, ethnicity, and hand dominance in Americans. Adv Geriatr Med Res 2020; 2(1): e200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014; 69(5): 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duchowny KA, Peterson MD, Clarke PJ. Cut points for clinical muscle weakness among older Americans. Am J Prev Med 2017; 53(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musalek C, Kirchengast S. Grip strength as an indicator of health-related quality of life in old age—a pilot study. Int J Environ Res Public Health 2017; 14(12): E1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3): M146–M156. [DOI] [PubMed] [Google Scholar]
- 14. Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Syddall H, Cooper C, Martin F, et al. Is grip strength a useful single marker of frailty? Age Ageing 2003; 32(6): 650–656. [DOI] [PubMed] [Google Scholar]
- 16. Duchowny K. Do nationally representative cutpoints for clinical muscle weakness predict mortality? Results From 9 Years of Follow-up in the health and retirement study. J Gerontol A Biol Sci Med Sci 2019; 74(7): 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al Snih S, Markides KS, Ray L, et al. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc 2002; 50(7): 1250–1256. [DOI] [PubMed] [Google Scholar]
- 18. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet 2015; 386(9990): 266–273. [DOI] [PubMed] [Google Scholar]
- 19. McGrath R, Vincent BM, Peterson MD, et al. Weakness may have a causal association with early mortality in older Americans: a matched cohort analysis. J Am Med Dir Assoc. Epub ahead of print 27 November 2019. DOI: 10.1016/j.jamda.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckner SL, Dankel S, Bell ZW, et al. The association of hand grip strength and mortality: what does it tell us and what can we do with it? Rejuvenation Res 2018; 22(3): 230–234. [DOI] [PubMed] [Google Scholar]
- 21. Sayer AA, Kirkwood TB. Grip strength and mortality: a biomarker of ageing. Lancet 2015; 386(9990): 226–227. [DOI] [PubMed] [Google Scholar]
- 22. Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 2008; 31(1): 3–10. [DOI] [PubMed] [Google Scholar]
- 23. Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 2008; 18(5): 388–395. [DOI] [PubMed] [Google Scholar]
- 24. Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron’s perspective. Curr Opin Clin Nutr Metab Care 2013; 16(1): 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 2011; 67(1): 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care 2015; 18(5): 465–470. [DOI] [PubMed] [Google Scholar]
- 27. Hay SI. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390(10100): 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson PW, D‘Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112(20): 3066–3072. [DOI] [PubMed] [Google Scholar]
- 29. Sayer AA, Syddall H, Dennison E, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. QJM 2007; 100(11): 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yi D, Khang AR, Lee HW, et al. Relative handgrip strength as a marker of metabolic syndrome: the Korea national health and nutrition examination survey (KNHANES) VI (2014–2015). Diabetes Metab Syndr Obes 2018; 11: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved. Integr Pharm Res Pract 2019; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Celis-Morales CA, Welsh P, Lyall DM, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018; 361: k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farmer RE, Mathur R, Schmidt AF, et al. Associations between measures of sarcopenic obesity and risk of cardiovascular disease and mortality: a cohort study and Mendelian randomization analysis using the UK Biobank. J Am Heart Assoc 2019; 8(13): e011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGrath R, Lee D-C, Kraemer WJ, et al. Weakness is associated with time to incident chronic heart failure in aging Americans. J Nutr Health Aging 2020; 24(1): 16–19. [DOI] [PubMed] [Google Scholar]
- 35. Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67(12): 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 37. McGrath R, Vincent BM, Al Snih S, et al. The association between muscle weakness and incident diabetes in older Mexican Americans. J Am Med Dir Assoc 2017; 18(5): 452.e7–452.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karvonen-Gutierrez CA, Peng Q, Peterson M, et al. Low grip strength predicts incident diabetes among mid-life women: the Michigan study of women’s health across the Nation. Age Ageing 2018; 47(5): 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li JJ, Wittert GA, Vincent A, et al. Muscle grip strength predicts incident type 2 diabetes: population-based cohort study. Metabolism 2016; 65(6): 883–892. [DOI] [PubMed] [Google Scholar]
- 40. Hamasaki H, Kawashima Y, Katsuyama H, et al. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci Rep 2017; 7(1): 7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Celis-Morales CA, Petermann F, Hui L, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: evidence from UK Biobank, a prospective population-based cohort study. Diabetes Care 2017; 40(12): 1710–1718. [DOI] [PubMed] [Google Scholar]
- 42. Cheung C-L, Nguyen Au E, Tan KC, et al. Association of handgrip strength with chronic diseases and multimorbidity. Age 2013; 35(3): 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rom O, Kaisari S, Aizenbud D, et al. Lifestyle and sarcopenia—etiology, prevention, and treatment. Rambam Maimonides Med J 2012; 3(4): e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shinohara M, Li S, Kang N, et al. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol 2003; 94(1): 259–270. [DOI] [PubMed] [Google Scholar]
- 45. Clark BC. Neuromuscular changes with aging and Sarcopenia. J Frailty Aging 2019; 8(1): 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29(1–2): 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campbell NL, Unverzagt F, LaMantia MA, et al. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med 2013; 29(4): 873–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jang JY, Kim J. Association between handgrip strength and cognitive impairment in elderly Koreans: a population-based cross-sectional study. J Phys Ther Sci 2015; 27(12): 3911–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vancampfort D, Stubbs B, Firth J, et al. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low-and middle-income countries. Int J Geriatr Psychiatry 2019; 34(4): 609–616. [DOI] [PubMed] [Google Scholar]
- 50. Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 2009; 66(11): 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McGrath R, Robinson-Lane SG, Cook S, et al. Handgrip strength is associated with poorer cognitive functioning in aging Americans. J Alzheimers Dis 2019; 70(4): 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buchman AS, Wilson RS, Boyle PA, et al. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology 2007; 29(1–2): 66–73. [DOI] [PubMed] [Google Scholar]
- 53. Alfaro-Acha A, Al Snih S, Raji MA, et al. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci 2006; 61(8): 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Croft P, Altman DG, Deeks JJ, et al. The science of clinical practice: disease diagnosis or patient prognosis? Evidence about “what is likely to happen” should shape clinical practice. BMC Med 2015; 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ogawa Y, Kaneko Y, Sato T, et al. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front Neurol 2018; 9: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roberts HC, Syddall HE, Butchart JW, et al. The association of grip strength with severity and duration of Parkinson’s: a cross-sectional study. Neurorehabil Neural Repair 2015; 29(9): 889–896. [DOI] [PubMed] [Google Scholar]
- 57. Christensen H, Mackinnon AJ, Korten A, et al. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging 2001; 16(4): 588–599. [DOI] [PubMed] [Google Scholar]
- 58. Pavela G. Functional status and social contact among older adults. Res Aging 2015; 37(8): 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liang Y, Welmer AK, Moller J, et al. Trends in disability of instrumental activities of daily living among older Chinese adults, 1997-2006: population based study. BMJ Open 2017; 7(8): e016996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mlinac ME, Feng MC. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol 2016; 31(6): 506–516. [DOI] [PubMed] [Google Scholar]
- 61. Seeman TE, Merkin SS, Crimmins EM, et al. Disability trends among older Americans: national health and nutrition examination surveys, 1988–1994 and 1999–2004. Am J Public Health 2010; 100(1): 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stevens AC, Carroll DD, Courtney-Long EA, et al. Adults with one or more functional disabilities—United States, 2011–2014. MMWR Morb Mortal Wkly Rep 2016; 65(38): 1021–1025. [DOI] [PubMed] [Google Scholar]
- 63. McGrath RP, Clark BC, Erlandson KM, et al. Impairments in individual autonomous living tasks and time to self-care disability in middle-aged and older adults. J Am Med Dir Assoc 2018; 20(6): 730–735.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee J, Phillips D, Wilkens J, et al. Cross-country comparisons of disability and morbidity: evidence from the gateway to global aging data. J Gerontol A Biol Sci Med Sci 2017; 73(11): 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Majer IM, Nusselder WJ, Mackenbach JP, et al. Mortality risk associated with disability: a population-based record linkage study. Am J Public Health 2011; 101(12): e9–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gopinath B, Kifley A, Liew G, et al. Handgrip strength and its association with functional independence, depressive symptoms and quality of life in older adults. Maturitas 2017; 106: 92–94. [DOI] [PubMed] [Google Scholar]
- 67. Germain CM, Vasquez E, Batsis JA, et al. Sex, race and age differences in muscle strength and limitations in community dwelling older adults: data from the health and retirement survey (HRS). Arch Gerontol Geriatr 2016; 65: 98–103. [DOI] [PubMed] [Google Scholar]
- 68. McGrath R, Robinson-Lane SG, Peterson MD, et al. Muscle strength and functional limitations: preserving function in older Mexican Americans. J Am Med Dir Assoc 2018; 19(5): 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGrath R, Erlandson K, Vincent B, et al. Decreased handgrip strength is associated with impairments in each autonomous living task for aging adults in the United States. J Frailty Aging 2019; 8(3): 141–145. [DOI] [PubMed] [Google Scholar]
- 70. Al Snih S, Markides KS, Ottenbacher KJ, et al. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res 2004; 16(6): 481–486. [DOI] [PubMed] [Google Scholar]
- 71. McGrath RP, Vincent BM, Snih SA, et al. The association between handgrip strength and diabetes on activities of daily living disability in older Mexican Americans. J Aging Health 2018; 30(8): 1305–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang L, Guo L, Wu H, et al. Role of physical performance measures for identifying functional disability among Chinese older adults: data from the China health and retirement longitudinal study. PLoS ONE 2019; 14(4): e0215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McGrath RP, Vincent BM, Lee I-M, et al. Handgrip strength, function, and mortality in older adults: a time-varying approach. Med Sci Sports Exerc 2018; 50(11): 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clark BC, Manini TM. What is dynapenia? Nutrition 2012; 28(5): 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008; 28(1): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord 2017; 10(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305(1): 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 2008; 56(7): 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brach JS, Talkowski JB, Strotmeyer ES, et al. Diabetes mellitus and gait dysfunction: possible explanatory factors. Phys Ther 2008; 88(11): 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bohannon RW. Considerations and practical options for measuring muscle strength: a narrative review. Biomed Res Int 2019; 2019: 8194537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Alonso AC, Ribeiro SM, Luna NMS, et al. Association between handgrip strength, balance, and knee flexion/extension strength in older adults. PLoS ONE 2018; 13(6): e0198185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 83. Martín-Ponce E, Hernandez-Betancor I, Gonzalez-Reimers E, et al. Prognostic value of physical function tests: hand grip strength and six-minute walking test in elderly hospitalized patients. Sci Rep 2014; 4: 7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Q, Lu H, Pan S, et al. 6MWT performance and its correlations with VO2 and handgrip strength in home-dwelling mid-aged and older Chinese. Int J Environ Res Public Health 2017; 14(5): E473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Harris-Love M, Benson K, Leasure E, et al. The influence of upper and lower extremity strength on performance-based sarcopenia assessment tests. J Funct Morphol Kinesiol 2018; 3(4): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arvandi M, Strasser B, Volaklis K, et al. Mediator effect of balance problems on association between grip strength and falls in older adults: results from the KORA-Age Study. Gerontol Geriatr Med 2018; 4: 2333721418760122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stevens P, Syddall H, Patel H, et al. Is grip strength a good marker of physical performance among community-dwelling older people. J Nutr Health Aging 2012; 16(9): 769–774. [DOI] [PubMed] [Google Scholar]
- 88. Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci 2018; 73(12): 1653–1660. [DOI] [PubMed] [Google Scholar]
- 89. Yeung SSY, Reijnierse EM, Trappenburg MC, et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc 2018; 19(8): 703–709. [DOI] [PubMed] [Google Scholar]
- 90. Yeung SS, Reijnierse EM, Trappenburg MC, et al. Knee extension strength measurements should be considered as part of the comprehensive geriatric assessment. BMC Geriatr 2018; 18(1): 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019; 393(10191): 2636–2646. [DOI] [PubMed] [Google Scholar]
- 92. McGrath RP. Understanding the feasibility and validity of muscle strength measurements in aging adults. J Am Med Dir Assoc 2019; 20(1): 99–100. [DOI] [PubMed] [Google Scholar]
- 93. Bohannon RW. Are hand-grip and knee extension strength reflective of a common construct? Percept Mot Skills 2012; 114(2): 514–518. [DOI] [PubMed] [Google Scholar]
- 94. Kim GR, Sun J, Han M, et al. Evaluation of the directional relationship between handgrip strength and cognitive function: the Korean longitudinal study of ageing. Age Ageing 2019; 48(3): 426–432. [DOI] [PubMed] [Google Scholar]
- 95. McGrath R, Vincent BM, Hackney KJ, et al. The longitudinal associations of handgrip strength and cognitive function in aging Americans. J Am Med Dir Assoc. Epub ahead of print 6 November 2019. DOI: 10.1016/j.jamda.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mesinovic J, Zengin A, De Courten B, et al. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes 2019; 12: 1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yeung CHC, Au Yeung SL, Fong SSM, et al. Lean mass, grip strength and risk of type 2 diabetes: a bi-directional Mendelian randomisation study. Diabetologia 2019; 62(5): 789–799. [DOI] [PubMed] [Google Scholar]
- 98. Delfini. Primer: problems with narrative reviews (aka overviews) 2002–2013, http://www.delfini.org/Delfini_Primer_NarrativeReviewProbs.pdf (accessed 15 January 2020).
- 99. Ferrari R. Writing narrative style literature reviews. Medical Writing 2015; 24: 230–235. [Google Scholar]