Abstract
Background:
There are no randomized data to guide treatment decisions for patients with advanced pancreatic adenocarcinoma following first-line FOLFIRINOX. We performed a systematic review and meta-analysis of studies using gemcitabine-based chemotherapy after FOLFIRINOX to assess treatment efficacy and toxicity.
Methods:
We included studies published between 2011 and 2018 that evaluated the efficacy and toxicity of gemcitabine-based chemotherapy after FOLFIRINOX in patients with advanced pancreatic adenocarcinoma. We searched PubMed, Embase, Scopus, and Web of Science. Primary outcomes were objective response rate (ORR), disease control rate (DCR), any grade 3/4 toxicity rate, and progression-free survival (PFS). We used the random-effects model to generate pooled estimates for proportions.
Results:
Sixteen studies met the eligibility criteria. Overall, ORR was 10.8%, DCR was 41.1%, and any grade 3/4 toxicity rate was 28.6%. In subgroup analyses, gemcitabine plus nab-paclitaxel was associated with superior ORR (14.4 versus 8.4%; p = 0.038) and DCR (53.5 versus 30.5%; p < 0.001) compared with single-agent gemcitabine. Median PFS ranged from 1.9 to 6.4 months and numerically favored gemcitabine plus nab-paclitaxel.
Conclusions:
Our study suggests gemcitabine-based chemotherapy likely outperforms best supportive care after FOLFIRINOX in advanced pancreatic cancer. Also, gemcitabine plus nab-paclitaxel seems to be more active than single-agent gemcitabine (CRD42018100421).
Keywords: advanced, cancer, gemcitabine, FOLFIRINOX, pancreatic
Background
Most patients with pancreatic adenocarcinoma present with advanced disease at diagnosis. Roughly 50% of all patients are diagnosed with metastatic disease and approximately 30% of all patients have locally advanced/unresectable disease at presentation.1 Also, disease recurrence occurs in at least 50% of the patients submitted to surgery with curative intent. As a result, the vast majority of patients diagnosed with pancreatic cancer develop metastatic disease at some point of their diseases’ natural history.2
Currently, treatment-naïve patients with advanced pancreatic cancer are best treated using one of two different regimens: FOLFIRINOX or gemcitabine plus nab-paclitaxel.3,4 Both regimens have shown to be superior to single-agent gemcitabine in terms of response rate, progression-free survival (PFS), and overall survival (OS). Nevertheless, complete responses are rare and virtually all patients with advanced pancreatic cancer will experience disease progression.5 Randomized trials in the second-line setting have been undertaken exclusively after gemcitabine-based treatments and not a single randomized trial performed so far has assessed the benefits of additional lines of treatment after progression on FOLFIRINOX.6–9
Owing to differences in the mechanisms of action and its known activity in the first-line setting, the use of gemcitabine-based chemotherapy after disease progression on FOLFIRINOX is sound.10 Despite the dearth of randomized trials, retrospective data support that gemcitabine-based chemotherapy may provide significant clinical benefit in this scenario. Nonetheless, the results of such studies are heterogeneous,11,12 and the real benefit of gemcitabine-based chemotherapy in this setting remains elusive. Moreover, issues related to patient selection and the optimal gemcitabine-based regimen to be used are still sources of contention.
Thus, we conducted a systematic review and meta-analysis to critically assess the available data on gemcitabine-based chemotherapy after FOLFIRINOX for patients with advanced (unresectable or metastatic) pancreatic adenocarcinoma. We aimed to evaluate the response and the toxicity of gemcitabine-based chemotherapy in this setting. As predefined subgroup analyses, we also sought to describe differences in outcomes according to the gemcitabine-based chemotherapy regimen (single-agent gemcitabine versus gemcitabine plus nab-paclitaxel).
Methods
This systematic review and meta-analysis is registered in the PROSPERO database (CRD42018100421) and it was undertaken in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.13 The study protocol can be found at the PROSPERO’s website (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=100421).
Eligibility criteria
Studies were eligible if they were randomized controlled trials, or prospective nonrandomized trials, or observational studies (prospective or retrospective), published from 1 January 2011 through 11 June 2018, undertaken exclusively in humans, and with a sample size of at least 10 patients. Also, patients had to be diagnosed with pancreatic adenocarcinoma (or nonneuroendocrine pancreatic carcinoma) that was either locally advanced/unresectable or metastatic at the start of first-line treatment and had to be treated with FOLFIRINOX in a first-line setting, and gemcitabine-based chemotherapy in second or further lines of treatment.
There were no restrictions based on language or publication status (full text versus meeting abstract). Studies reporting the outcomes of patients treated in first-line with different regimens that included FOLFIRINOX and for whom separate outcomes were not available according to the first-line treatment regimen used were excluded. Likewise, studies using different types of gemcitabine-based chemotherapy in the second-line without proper discrimination of the gemcitabine-based regimens used were excluded. Supplementary Table S1 describes the PICO framework of the systematic review.
Information sources
PubMed, Embase, Scopus, and Web of Science databases were searched. Also, abstracts from the American Society of Clinical Oncology (ASCO) annual meeting (2011 to 2017), the European Society of Medical Oncology annual meeting (2011 to 2017), the Gastrointestinal Cancer Symposium (ASCO GI; 2011 to 2018), and the World Congress on Gastrointestinal Cancer (2011 to 2017) were screened (hand-searched).
Search strategy
For PubMed, the following search strategy was used to look for relevant references: (((“fluorouracil”[MeSH Terms] OR “fluorouracil”[All Fields]) AND (“irinotecan”[Supplementary Concept] OR “irinotecan”[All Fields]) AND (“oxaliplatin”[Supplementary Concept] OR “oxaliplatin”[All Fields])) OR “FOLFIRINOX”[All Fields]) AND ((((“pancreatic neoplasm”[MeSH Terms] OR “pancreatic neoplasms”[MeSH Terms]) OR “pancreatic cancer”[MeSH Terms]) OR “pancreatic cancers”[MeSH Terms]) OR ((“pancreatic”[All Fields] OR “pancreas”[All Fields]) AND (“cancer”[All Fields] OR “carcinoma”[All Fields] OR “adenocarcinoma”[All Fields]))) AND (“gemcitabine”[Supplementary Concept] OR “gemcitabine”[All Fields]). Search was limited from 1 January 2011 to 11 June 2018. Supplementary Table S2 describes the strategies used to search the other databases. Abstracts from the aforementioned meetings were searched through the meetings’ official websites to identify relevant citations. Backward reference listing was also performed in the articles selected after the second screening round to look for additional studies.
Study selection
In the first study selection phase, the title and the abstract of all citations were independently screened by two authors (VHFJ and MPGC) in an unblinded manner. In the second phase, the same authors independently examined full-text articles and meeting posters to assess study eligibility. In case of dispute about eligibility, subjects of disagreement were discussed in an attempt to find common ground. In cases in which no consensus could be achieved, a third-part investigator (RPR) decided whether or not to include the study under discussion. In case of different publications of a single study, the most complete source of information was chosen.
Data collection process
Two authors (VHFJ and MPGC) independently collected the data from all the selected studies using a standardized collection form. Again, in case of dispute regarding the extracted data, subjects of disagreement between the two authors were discussed in an attempt to find common ground. In cases in which no consensus could be achieved, an attempt was made to reach the corresponding author and clarify doubts about the data. Whenever that was not possible, a third-part investigator (RPR) decided on the best way to manage the data. We sought to make contact through email with all authors in order to obtain relevant data missing from the original reports.
Data items
Supplementary Table S3 summarizes all the data extracted from the selected studies. The primary outcomes of the systematic review were objective response rate (ORR), disease control rate (DCR), any grade 3/4 toxicity rate, and PFS. ORR was defined as the ratio between the number of patients achieving objective response (complete or partial response) and the total number of patients in the study, regardless of the number of patients that underwent disease response evaluation. Likewise, DCR was defined as the ratio between the number of patients achieving disease control (complete response + partial response + stable disease) and the total number of patients in the study, regardless of the number of patients that underwent disease response evaluation. Any grade 3/4 toxicity rate was defined as the ratio between the number of patients experiencing any grade 3/4 toxicity and the total number of patients.
As secondary outcomes, we assessed OS, biochemical response rate (BRR), and specific grade 3/4 toxicity rates. OS was evaluated from the start of gemcitabine-based chemotherapy (OS-GEM) and from the start of FOLFIRINOX (OS-FFX). BRR was defined as the ratio between the number of patients achieving biochemical response according to CA 19-9 level drop and the total number of patients in the study.
Risk of bias of individual studies
We evaluated the risk of bias within individual studies using a modification of the tool derived from the Newcastle-Ottawa scale by Murad and colleagues.14 It was originally devised to assess the methodological quality of noncomparative case series. Briefly, the tool consists of four domains (selection, ascertainment, causality, and reporting) and eight questions. In this systematic review, questions regarding dose–response, rechallenge, and alternative explanations were excluded.15 Given the importance of treatment toxicity in the scenario of advanced cancer, the reporting domain was divided in two parts (efficacy and toxicity). A binary answer (YES or NO) was given to each of the six questions to assess the bias across the different domains. We considered a case series to be of high-quality when all six questions about methodological quality were positively answered. The quality of the evidence was assessed independently by two authors (VHFJ and RPR). In case of dispute about the risk of bias of individual studies, subjects of disagreement between the two authors were discussed in an attempt to find common ground.
Summary measures and synthesis of results
Data on ORR, DCR, any grade 3/4 toxicity rate, and BRR were summarized as proportions and aggregated (except for BRR) using the inverse variance random-effect method (DerSimonian–Laird estimate) after Freeman–Tukey double arcsine transformation.16 Confidence intervals for the individual studies were calculated based on the Clopper–Pearson interval method.17 Heterogeneity was assessed using Higgin’s I2 statistic and the chi-square test in the random-effects model. As prespecified subgroup analyses, we evaluated whether ORR, DCR, and any grade 3/4 toxicity rate were different according to the type of gemcitabine-based regimen used (gemcitabine single-agent versus gemcitabine plus nab-paclitaxel) using between-subgroups chi-square test.
Data on time-to-event outcomes (PFS and OS) were summarized as median survival times (in months) and actuarial survival rates (as a percentage) using tables and dot plots. An attempt to aggregate survival data based on the graphical method described by Guyot and colleagues would be made if the number of events and the number of patients at risk at timepoints other than zero were available.18 Whenever possible, when data regarding actuarial survival rates were not given, we performed digital extraction from the available survival curves using the Digitizelt software (version 2.2.2).19 Statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria) along with the meta package.20
Reporting bias
The risk of bias across studies was assessed using objective response data. We designed a funnel plot of study size against ln odds of response as described by Hunter and colleagues and used Peter’s test to evaluate the risk of publication bias.21
Results
Study selection
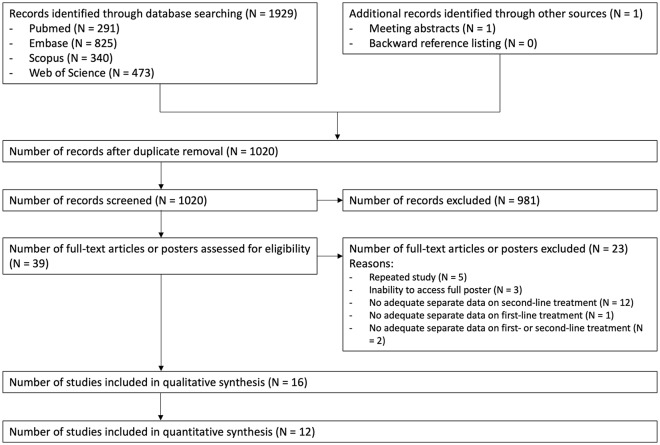
Database searches were undertaken on 11 June and 12 June 2018. Amongst 1019 citations screened in the first phase of study selection, 39 entered the second phase of study selection. A total of 23 of them were excluded for various reasons. Supplementary Table S4 describes the reasons for excluding citations in the second phase of study selection. Finally, 16 studies were selected for qualitative synthesis and 12 studies were used for quantitative synthesis (Figure 1).
Figure 1.
PRISMA flow diagram.
Study characteristics
Supplementary Table S5 describes the characteristics of the selected studies. Except for the study by Portal and colleagues,22 all studies were retrospective. Three studies were two-arm retrospective comparative cohort studies11,23,24 and the remaining 13 studies were case series.12,22,25–35 Six studies were multicentric investigations22,23,26,27,31,34 and seven were available solely as posters or electronic abstracts.11,24,25,29–31,34
Supplementary Table S6 depicts the characteristics of the populations from the selected studies. Sample size ranged from 10–96 patients and median age varied from 55–68 years (14 studies). Men represented 39.2–83.3% of the populations (13 studies) and most patients were Eastern Cooperative Oncology Group performance status (ECOG) 0-1 (45.8–100%; nine studies). At the start of gemcitabine-based chemotherapy, 70.5–100% of patients presented with metastatic disease (13 studies). Prior progression on FOLFIRINOX occurred in 73.3–100% of patients (10 studies). Objective response to FOLFIRINOX in first-line occurred in 3.3–40.0% of patients (six studies).
Supplementary Table S7 illustrates treatment characteristics in the selected studies. Amongst noncomparative studies, five evaluated the activity of single-agent gemcitabine25–27,33,35 and eight assessed the activity of gemcitabine plus nab-paclitaxel.12,22,28–32,34 In the three two-arm comparative studies, treatment arms included single-agent gemcitabine and gemcitabine plus nab-paclitaxel.11,23,24 Median number of gemcitabine-based chemotherapy cycles ranged from 1.5 to 6 (14 studies).
Risk of bias within studies
Supplementary Table S8 describes the risk of bias within individual studies. Four studies were considered high-quality studies.22,23,27,33 Efficacy reporting was consistently well-documented throughout all studies. In contrast, data on toxicity, duration of follow-up, and outcome ascertainment were not systematically described in many studies.
Primary outcomes
Objective response rate (ORR)
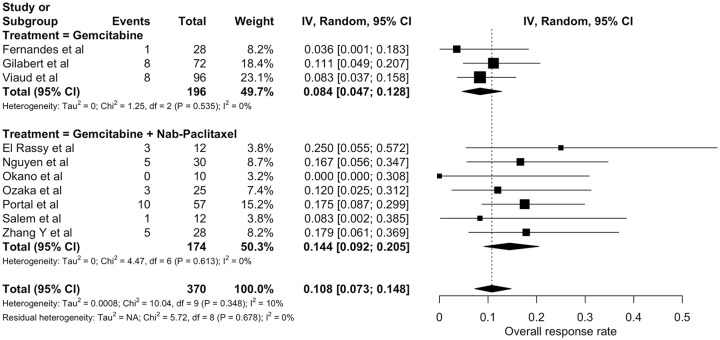
Supplementary Table S9 summarizes objective response data across studies. Ten studies evaluated the ORR of gemcitabine-based chemotherapy (370 patients).12,22,25–32 Seven used RECIST criteria to assess response.22,25–28,31,32 In the remaining studies, the method of response evaluation was not described. Only two studies reported imaging review for purposes of the study.28,32 None of the three comparative studies evaluated ORR. ORR from individual studies ranged from 0.0 to 25.0%. Pooled ORR was 10.8% [95% confidence interval (CI) 7.3–14.8%; Figure 2]. There was no evidence of statistically significant heterogeneity among studies (I2 = 10%; chi-square p = 0.348). Nonetheless, prespecified subgroup analysis pointed to a higher ORR for patients treated with gemcitabine plus nab-paclitaxel (14.4%; 95% CI 9.2–20.5%) when compared with patients treated with single-agent gemcitabine (8.4%; 95% CI 4.7–12.8%) – p = 0.038.
Figure 2.
Individual and pooled objective response rates of gemcitabine-based chemotherapy (random-effects model).
Estimates are displayed as proportions with three significance digits.
CI, confidence interval.
Disease control rate (DCR)
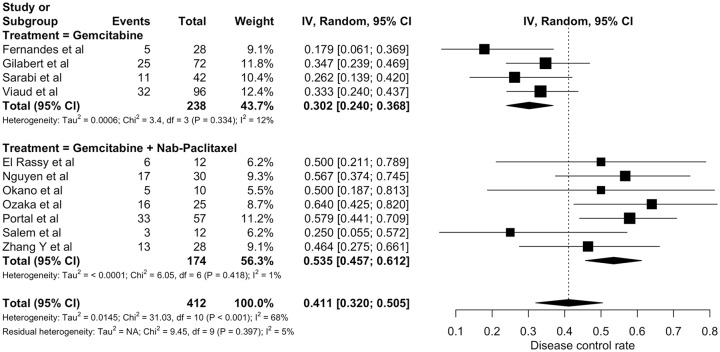
Supplementary Table S9 summarizes disease control data across studies. A total of 11 studies assessed the DCR of gemcitabine-based chemotherapy (412 patient).12,22,25–33 Radiological response evaluation took place every 8 weeks in four studies22,26,27,33 and in one study the median time to first disease response assessment was 8.9 weeks.32 In the remaining investigations, time to first disease response evaluation was not stated. None of the three comparative studies evaluated DCR. DCR from individual studies ranged from 17.8 to 64.0%. Pooled DCR was 41.1% (95% CI 32.0–50.5%; Figure 3). There was evidence of statistically significant heterogeneity among studies (I2 = 68%; chi-square p < 0.001). Prespecified subgroup analysis pointed to a higher DCR for patients treated with gemcitabine plus nab-paclitaxel (53.5%; 95% CI 45.7–61.2%) when compared with patients treated with single-agent gemcitabine (30.2%; 95% CI 24.0–36.8%) – p < 0.001.
Figure 3.
Individual and pooled disease control rates of gemcitabine-based chemotherapy (random-effects model).
Estimates are displayed as proportions with three significance digits.
CI, confidence interval.
Any grade 3/4 toxicity
Supplementary Table S10 summarizes data on any grade 3/4 toxicity. Seven studies reported data on any grade 3/4 toxicity (275 patients).12,22,25,27,29,33,34 Toxicity data was collected according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 in three studies.22,27,33 In the remaining investigations reporting any grade 3/4 toxicity data, the criterium used to classify toxicity was not stated. None of the comparative studies evaluated rates of any grade 3/4 toxicity. Rates of any grade 3/4 toxicity varied from 0.0% to 80.0%. Pooled any grade 3/4 toxicity rate was 28.6% (95% CI 15.9–43.0%; Figure 4). There was evidence of statistically significant heterogeneity among studies (I2 = 81%; chi-square p < 0.001). In a prespecified subgroup analysis, there was no statistically significant difference in any grade 3/4 toxicity rate between gemcitabine monotherapy (22.9%; 95% CI 10.3–38.4%) and gemcitabine plus nab-paclitaxel (34.6%; 95% CI 10.9–62.9%) – p = 0.415.
Figure 4.
Individual and pooled any grade 3/4 toxicity rates of gemcitabine-based chemotherapy (random-effects model).
Estimates are displayed as proportions with three significance digits.
CI, confidence interval.
Progression-free survival (PFS)
Supplementary Table S11 summarizes data on PFS. A total of 13 studies reported data on PFS (541 patients).12,22–31,34,35 In one study, data were available only for time-to-treatment failure.32 Figure 5 depicts median PFS and actuarial PFS rates at three and six months according to the treatment regimen used. Median PFS across studies ranged from 1.9 to 6.4 months. According to the prespecified statistical analysis, the graphical method proposed by Guyot and colleagues was to be used to aggregate data on time-to-event outcomes only if there were enough data on the population at risk at timepoints other than zero and the total number of events. As neither of these data were consistently reported across studies, PFS times were not aggregated. Among comparative studies, gemcitabine plus nab-paclitaxel was associated with improved PFS compared with gemcitabine monotherapy in one study (3.6 versus 2.5 months; p = 0.03)23; in one study there was no significant difference in PFS between the two types of treatment (2.4 versus 1.9 months; p = 0.26)24; and in the last study, no formal comparison of PFS was performed.11
Figure 5.
Progression-free survival (PFS) across different studies using gemcitabine-based chemotherapy (median, 3-month PFS rate, and 6-month PFS rate).
(*) Refers to time-to-treatment failure. (**) Interval refers to range and not 95% confidence interval (CI).
Secondary outcomes
Overall survival (OS)
Supplementary Table S12 summarizes data on OS-GEM. A total of 14 studies reported data on OS-GEM (552 patients).11,12,22–25,27–30,32–35 Supplementary Figure S1 depicts median OS-GEM and actuarial OS-GEM rates at 6 and 12 months according to the treatment regimen used. Median OS times across studies ranged from 3.1 to 12.4 months. As in the case of PFS, the number of patients at risk at different timepoints and the total number of events were not consistently reported for OS-GEM and OS-FFX. Thus, data on OS were not aggregated. Among comparative studies, gemcitabine plus nab-paclitaxel was associated with improved OS compared with gemcitabine monotherapy in one study (5.7 versus 3.8 months; p = 0.03)23; in one study, there was no statistically significant difference in OS between the two types of treatment (6.1 versus 4.8 months; p = 0.18)24; and in the last study, median OS and 6-month OS rate of patients undergoing treatment with gemcitabine plus nab-paclitaxel were numerically higher (no formal statistical comparison performed).11
Data on OS-FFX are summarized in Supplementary Table S13 and Supplementary Figure S2.
Biochemical response rate (BRR) and specific grade 3/4 toxicities
Supplementary Table S14 describes data on biochemical response to treatment and Supplementary Tables S10 and S15 depict data on specific grade 3/4 toxicities.
Reporting bias
Funnel plot of ln odds (response) against sample size is shown in Supplementary Figure S3. The figure portrays relative asymmetry of the ln odds (response) according to the sample size, denoting possible publication bias. Some degree of publication bias is also suggested by Peters’ test (p = 0.105).
Discussion
To the best of our knowledge, our study represents the most comprehensive evaluation of the activity of gemcitabine-based chemotherapy after FOLFIRINOX in advanced pancreatic cancer. Using the data from the best supportive care (BSC) arm of a randomized controlled trial of second-line treatment,36 ORR and DCR with gemcitabine-based chemotherapy are clearly superior to what would be expected with no active anticancer treatment (Table 1).37 In addition, toxicity seems to be manageable, as the pooled rate of any grade 3/4 toxicity is not numerically different from the one in the BSC arm of the aforementioned study.
Table 1.
Summary of findings.
| Outcomes | No. of participants (No. of studies) | Pooled rate& % (95% CI)# |
Historical control rate for best
supportive care$
% (95% CI)# |
Quality of the
evidence (grade) |
||
|---|---|---|---|---|---|---|
| Gemcitabine plus nab-paclitaxel | Gemcitabine monotherapy | Gemcitabine-based chemotherapy | ||||
| Objective response rate | 370 (10 studies) | 10.8 (7.3–14.8) | 8.4 (4.7–12.8) | 14.4 (9.2–20.5) | 0.6 (0.02–3.5) | ⊕⊕OO (low) |
| Disease control rate | 412 (11 studies) | 41.1 (32.0–50.5) | 30.2 (24.0–36.8) | 53.5 (45.7–61.2) | 19.4 (13.5–26.5) | ⊕OOO (very low) |
| Any grade 3/4 toxicity rate | 275 (7 studies) | 28.6 (15.9–43.0) | 22.9 (10.3–38.4) | 34.6 (10.9–62.9) | 30.3 (22.9–38.5) | ⊕OOO (very low) |
Based on the results of the best supportive care arm from the study by Ciuleanu and colleagues36
Clopper-Pearson method for confidence interval (CI) calculation.
Based on the random-effects model.
Despite the clinically significant data on ORR and DCR, our data must be interpreted in light of the quality of the available evidence. Among 16 studies (one prospective), only 10 provided data on ORR and 11 on DCR. In addition, three studies reporting data on ORR and DCR did not describe the method used to evaluate radiological response. In addition, radiological review was undertaken in only two studies (independent review in one study). Moreover, there was no mention to disease response confirmation in these reports. Finally, analyzing the results of Peter’s test and the disposition of the funnel plot, one might not exclude the possibility of publication bias if we use ORR as the measure of efficacy.
However, despite these drawbacks, we found no evidence of statistical heterogeneity in the ORR analysis or in the DCR analysis (within-subgroup analysis). Furthermore, these data are in line with the activity of gemcitabine-based chemotherapy in the first-line setting. Moreover, the pooled objective response and DCRs of gemcitabine plus nab-paclitaxel in our study are similar to the ones found in a recently published phase II trial that evaluated the role of this chemotherapy regimen after FOLFIRINOX in patients with unresectable pancreatic cancer.38 That said, given the fact that spontaneous regression of pancreatic adenocarcinoma is a rare phenomenon,39 that most patients were treated with gemcitabine-based chemotherapy after progression on FOLFIRINOX (decreasing the possibility of a carryover effect),40 and that the pooled ORR is clearly superior to that of BSC,36 we believe that this constitutes the best available evidence that Gemcitabine-based chemotherapy is superior to BSC in terms of ORR in patients with good performance status. Regarding DCR, despite the fact that disease control is numerically more common in those treated with gemcitabine-based chemotherapy, and that there seems to be a dose–response effect in the sense that subgroup analyses showed that patients treated with gemcitabine plus nab-paclitaxel fared better than those given gemcitabine monotherapy, one cannot exclude the possibility that other variables, such as the biological behavior of the disease, played part in these results. For that, we believe that these results constitute inferior quality evidence of improved DCR in favor of gemcitabine-based regimens over no active anticancer treatment.
Data on toxicity are a bit more puzzling. It is known that retrospective evaluation of toxicities is associated with a great deal of bias.41 We tried to reduce this bias by selecting only grade 3/4 toxicities. However, rates of any grade 3/4 toxicity ranged from 0% to 80% and there was evidence of statistically significant heterogeneity (even within subgroups). The most important specific grade 3/4 toxicities were fatigue and myelosuppression. The subjectivity of fatigue assessment could have driven the bias for the assessment of this toxicity.42 In addition, the duration of the previous treatment with FOLFIRINOX and the timing of blood cell count analysis during the chemotherapy cycle might have impacted the myelotoxicity analysis. Also, one cannot exclude geographical variations in treatment tolerability, as the highest rate of any grade 3/4 toxicity was seen in an Asian study. However, gemcitabine plus nab-paclitaxel has been evaluated in Asian patients in the first-line setting and the toxicity profile seems to be similar to that of Western patients.43 Finally, the number of patients with data on grade 3/4 toxicity is relatively small, and studies with extreme any grade 3/4 toxicity rates (on both sides) had very limited sample sizes (12 and 10 patients). For that, we believe the pooled data on grade 3/4 toxicity should be interpreted very carefully.
In subgroup analyses, we showed that both ORR and DCR were higher in patients treated with gemcitabine plus nab-paclitaxel compared with those treated with gemcitabine monotherapy. While the difference in ORR seems rather small, the difference in effect size in terms of DCR is substantial. Despite the fact that no statistical test was performed, graphical analyses of PFS and OS-GEM suggest that gemcitabine plus nab-paclitaxel is associated with improved survival. These data are in consonance with the results of the MPACT trial, which demonstrated improved ORR, PFS and OS for patients treated with gemcitabine plus nab-paclitaxel (versus single-agent gemcitabine) in first-line.4 In addition, among three studies that compared survival outcomes of patients treated with gemcitabine plus nab-paclitaxel and single-agent gemcitabine, one study suggested superior PFS and OS for patients with ECOG performance status 0 that underwent combined treatment (not statistically significant)24 and another study found improved PFS and OS for patients treated with gemcitabine plus nab-paclitaxel in the overall population.23
We acknowledge these data must be interpreted with caution given the lack of randomization and differences in the distribution of known prognostic factors among the different study populations, as patients treated with gemcitabine monotherapy more often had ECOG performance status ⩾ 2 than those treated with gemcitabine plus nab-paclitaxel. It has been previously shown that poor ECOG performance status is independently associated with worse PFS and OS in the second-line setting10,27,44; thus, performance status should be considered a confounder in this analysis. However, for those patients with adequate performance status, we believe that the combination of gemcitabine plus nab-paclitaxel is likely associated with superior outcomes.
While our study suggests gemcitabine plus nab-paclitaxel offers better outcomes after FOLFIRINOX, the overall results are still poor and chemotherapy has reached a plateau in this disease. It is imperative to improve our understanding of the molecular mechanisms underpinning disease onset and progression. In this sense, a molecularly tailored treatment approach has already started to yield results. Responses to checkpoint inhibitor45 and NTRK inhibitors46,47 have been reported in patients with microsatellite instable and NTRK fusion-positive pancreatic cancer, respectively. Moreover, patients with germline BRCA-1/-2 mutations seems to derive significant benefit from treatment with PARP inhibitors, as shown in the recent POLO trial.48 Therefore, further work is needed to identify subgroup of patients with other molecular abnormalities suitable for targeted treatments.
Our study has limitations. Searches on some databases were not possible. Despite attempts to get in touch with authors to clarify some doubts and ask for additional details, important data could not be collected. Moreover, we acknowledge that the graphical extraction method used to estimate actuarial survival rates is subject to bias. In addition, besides patients with metastatic disease, many of the individual studies also included patients with locally advanced/unresectbale pancreatic cancer. That probably increased heterogeneity and might have affected the results, as different molecular mechanisms are likely to underpin progression in different disease stages. In addition, the included studies did not report data on quality of life or any other patient reported outcomes, which we believe are important endpoints in this setting. Finally, we were not able to aggregate time-to-event outcomes. Nevertheless, this systematic review and meta-analysis is the largest assessment of efficacy of gemcitabine-based chemotherapy after progression on FOLFIRINOX in pancreatic cancer so far. Despite the methodological quality of most of the included studies, we performed a rigorous evaluation of the available evidence, with detailed information on studies’ and patients’ characteristics, treatments, and outcomes. Moreover, we were able to report data on the toxicity of such treatments.
To conclude, a subset of patients with pancreatic adenocarcinoma previously treated with FOLFIRINOX derive benefit from second-line gemcitabine-based chemotherapy. Moreover, gemcitabine plus nab-paclitaxel seems to be associated with improved outcomes compared with gemcitabine monotherapy. The use of information on patients’ clinical condition is paramount to aid treatment selection in this setting. Also, a sincere and honest conversation with the patient is necessary to align expectations and realistic treatment results, as many patients treated in this setting do not experience any benefit from chemotherapy. Despite these data, novel treatments guided by the characteristic molecular alterations of pancreatic cancer are needed to further improve survival.
Supplemental Material
Supplemental material, Supplementary_Material_TO_SEND_TAMO for Systematic review and meta-analysis of gemcitabine-based chemotherapy after FOLFIRINOX in advanced pancreatic cancer by Victor H. F. de Jesus, Marcos P. G. Camandaroba, Vinicius F. Calsavara and Rachel P. Riechelmann in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Ines Vendrell and Jennifer Knox for providing us with additional information regarding their groups’ studies on this topic.
V.H.F.d.J.: conceptualization, methodology, data curation, data analyses, writing, and visualization; M.P.G.C.: data curation, writing, and visualization; V.F.C.: methodology, data analyses, and visualization; R.P.R.: methodology, data analyses, writing, and visualization.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: V.H.F.d.J. received honoraria from United Medical and had travel expenses payed by United Medical in the past 12 months. United Medical is the company responsible for the distribution and sale of nab-paclitaxel in Brazil. R.P.R. received consultancy fees from Astra Zeneca in the past 12 months.
ORCID iD: Victor H. F. de Jesus  https://orcid.org/0000-0003-4702-116X
https://orcid.org/0000-0003-4702-116X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Victor H. F. de Jesus, Medical Oncology Department, A.C. Camargo Cancer Center, Rua Prof. Antônio Prudente, 211, São Paulo, 01509-010, Brazil.
Marcos P. G. Camandaroba, Medical Oncology Department, A.C. Camargo Cancer Center, São Paulo, Brazil
Vinicius F. Calsavara, Department of Epidemiology and Statistics – International Research Center (CIPE), A.C. Camargo Cancer Center, São Paulo, Brazil
Rachel P. Riechelmann, Medical Oncology Department, A.C. Camargo Cancer Center, São Paulo, Brazil
References
- 1. Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362: 1605–1617. [DOI] [PubMed] [Google Scholar]
- 2. Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016; 388: 73–85. [DOI] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus Gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Jesus VHF, Camandaroba MPG, Donadio MDS, et al. Retrospective comparison of the efficacy and the toxicity of standard and modified FOLFIRINOX regimens in patients with metastatic pancreatic adenocarcinoma. J Gastrointest Oncol 2018; 9: 694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011; 47: 1676–1681. [DOI] [PubMed] [Google Scholar]
- 7. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous Gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 8. Gill S, Ko YJ, Cripps C, et al. PANCREOX: A randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received Gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 9. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for Gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 10. de Jesus VHF, Camandaroba MPG, Donadio MDS, et al. Retrospective analysis of efficacy and safety of Gemcitabine-based chemotherapy in patients with metastatic pancreatic adenocarcinoma experiencing disease progression on FOLFIRINOX. J Gastrointest Oncol 2018; 9: 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aung KL, Creighton S, Fraser A, et al. Overall survival (OS) achieved with Gemcitabine (G) or Gemcitabine-abraxane (GA) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) who received first line modified FOLFIRINOX (m-FFX) palliative chemotherapy. J Clin Oncol 2017; 35(Suppl.): abstract e15715. [Google Scholar]
- 12. El Rassy E, Assi T, El Karak F, et al. Could the combination of nab-paclitaxel plus Gemcitabine salvage metastatic pancreatic adenocarcinoma after folfirinox failure? A single institutional retrospective analysis. Clin Res Hepatol Gastroenterol 2017; 41: e26–e28. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–W94. [DOI] [PubMed] [Google Scholar]
- 14. Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bazerbachi F, Sawas T, Vargas EJ, et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc 2018; 87: 30–42. [DOI] [PubMed] [Google Scholar]
- 16. Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67: 974–978. [DOI] [PubMed] [Google Scholar]
- 17. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857–872. [DOI] [PubMed] [Google Scholar]
- 18. Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Rich B, Hanley JA. Recovering the raw data behind a non-parametric survival curve. Syst Rev 2014; 3: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwarzer G. Meta: an R package for meta-analysis. R News 2007; 7: 40–45. [Google Scholar]
- 21. Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014; 67: 897–903. [DOI] [PubMed] [Google Scholar]
- 22. Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus Gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015; 113: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang H, Kellett C, Lambert P, et al. Efficacy and tolerability of second-line Nab-paclitaxel and Gemcitabine after failure of first-line FOLFIRINOX for advanced pancreas cancer: a single-institution experience. Clin Colorectal Cancer 2018; 17: e451–e456. [DOI] [PubMed] [Google Scholar]
- 24. Chan EM, Hong TS, Clark JW, et al. Gemcitabine (G) + nab-paclitaxel (nab-P) versus G in patients (pts) with advanced pancreatic cancer (PDAC) after FOLFIRINOX: A single center, retrospective review. J Clin Oncol 2016; 34(Suppl. 4): abstract 348. [Google Scholar]
- 25. Fernandes BM, Bitton RC, Sabbaga J, et al. Is Gemcitabine effective for pancreatic cancer after progression to FOLFIRINOX? J Clin Oncol 2017; 35(Suppl. 4): abstract 489. [Google Scholar]
- 26. Gilabert M, Chanez B, Rho YS, et al. Evaluation of Gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine (United States) 2017; 96: e6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viaud J, Brac C, Artru P, et al. Gemcitabine as second-line chemotherapy after Folfirinox failure in advanced pancreatic adenocarcinoma: A retrospective study. Dig Liver Dis 2017; 49: 692–696. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen KT, Kalyan A, Beasley HS, et al. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer-retrospective analysis of response. J Gastrointest Oncol 2017; 8: 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okano N, Kawai K, Naruge D, et al. Second-line treatments after FOLFIRINOX or Gemcitabine plus nabpaclitaxel failure for unresectable pancreatic cancer under real-life clinical conditions: Experience at a single institute. Pancreatology 2016; 16(Suppl.): S172–S173. [Google Scholar]
- 30. Ozaka M, Sasaki T, Yamada I, et al. Second-line treatment of modified FOLFIRINOX or nab-paclitaxel plus Gemcitabine for metastatic pancreatic adenocarcinoma. J Clin Oncol 2018; 36(Suppl. 4): abstract 458. [Google Scholar]
- 31. Salem ME, Alistar AT, Dyson G, et al. Albumin-bound paclitaxel plus Gemcitabine after first-line FOLFIRINOX therapy in patients with pancreatic cancer. J Clin Oncol 2014; 32(Suppl.): abstract e15252. [Google Scholar]
- 32. Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: Single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarabi M, Mais L, Oussaid N, et al. Use of Gemcitabine as a second-line treatment following chemotherapy with folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett 2017; 13: 4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vendrell I, Ferreira AR, Pulido C, et al. Retrospective multicenter cohort of nab-paclitaxel plus Gemcitabine (NG) after FOLFIRINOX failure in advanced pancreatic cancer (APC): Effectiveness, tolerability, and response markers. J Clin Oncol 2017; 35(Suppl.): abstract e15743. [Google Scholar]
- 35. Lino AD, Abrahao CM, Brandao RM, et al. Role of Gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis. J Gastrointest Oncol 2015; 6: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciuleanu TE, Pavlovsky AV, Bodoky G, et al. A randomised Phase III trial of glufosfamide compared with best supportive care in metastatic pancreatic adenocarcinoma previously treated with Gemcitabine. Eur J Cancer 2009; 45: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 37. Schünemann H, Brozek J, Guyatt G, et al. GRADE Handbook [Internet]. GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html (2013, accessed 10 September 2019).
- 38. Mita N, Iwashita T, Uemura S, et al. Second-line Gemcitabine plus Nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J Clin Med 2018; 8(6): 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chin KM, Chan CY, Lee SY. Spontaneous regression of pancreatic cancer: A case report and literature review. Int J Surg Case Rep 2018; 42: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cleophas TJ. Carryover bias in clinical investigations. J Clin Pharmacol 1993; 33, 799–804. [DOI] [PubMed] [Google Scholar]
- 41. Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol (Phila) 2007; 45: 943–945. [DOI] [PubMed] [Google Scholar]
- 42. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007; 12(Suppl. 1): 11–21. [DOI] [PubMed] [Google Scholar]
- 43. Xu R, Yu X, Hao J, et al. Efficacy and safety of weekly nab-paclitaxel plus Gemcitabine in Chinese patients with metastatic adenocarcinoma of the pancreas: a phase II study. BMC Cancer 2017; 17: 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vienot A, Beinse G, Louvet C, et al. Overall survival prediction and usefulness of second-line chemotherapy in advanced pancreatic adenocarcinoma. J Natl Cancer Inst 2017; 109(10): djx037. [DOI] [PubMed] [Google Scholar]
- 45. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018; 378: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pishvaian MJ, Rolfo CD, Liu SV, et al. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. J Clin Oncol 2018; 36(Suppl. 4): abstract 521. [Google Scholar]
- 48. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019; 381: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material_TO_SEND_TAMO for Systematic review and meta-analysis of gemcitabine-based chemotherapy after FOLFIRINOX in advanced pancreatic cancer by Victor H. F. de Jesus, Marcos P. G. Camandaroba, Vinicius F. Calsavara and Rachel P. Riechelmann in Therapeutic Advances in Medical Oncology