Abstract
Chemoradiotherapy (CRT) is extensively used prior to surgery for rectal cancer to provide significantly better local control, but the radiotherapy (RT), as the other component of CRT, has been subject to less interest than the drug component in recent years. With considerable developments in RT, the use of advanced techniques, such as intensity-modulated radiotherapy (IMRT) in rectal cancer, is garnering more attention nowadays. The radiation dose can be better conformed to the target volumes with possibilities for synchronous integrated boost without increased complications in normal tissue. Hopefully, both local recurrence and toxicities can be further reduced. Although those seem to be of interest, many issues remain unresolved. There is no international consensus regarding the radiation schedule for preoperative RT for rectal cancer. Moreover, an enormous disparity exists regarding the RT delivery. With the advent of IMRT, variations will likely increase. Moreover, time to surgery is also quite variable, as it depends upon the indication for RT/CRT in the clinical practices. In this review, we discuss the options and problems related to both the dose–time fractionation schedule and time to surgery; furthermore, it addresses the research questions that need answering in the future.
Keywords: IMRT, preoperative radiotherapy, rectal cancer, surgery, three-dimensional conformal radiotherapy
Introduction
Surgery is the cornerstone of curative therapy for rectal cancer, and combined-modality treatment is the recommended adjuvant, or neoadjuvant therapy. Multimodality therapy is often used for tumor downstaging or downsizing, anal sphincter, or other organ preservation, as well as improvements in local control (LC) or even overall survival (OS). Preoperative chemoradiotherapy (CRT) has been shown comparable or superior to postoperative treatment in terms of various end points,1–3 and preoperative radiation dose and time interval are significant predictors of the pathological complete response (pCR) rate and downstaging.4 However, different viewpoints exist regarding the optimal dose–time fractionation schedule of preoperative radiotherapy (RT) and time to surgery.
Conventionally, long-course RT (i.e. 1.8–2.0 Gy per day; total dose of 45–50.4 Gy), frequently combined with chemotherapy, has been the preferred approach for a majority of patients in most countries, particularly in the United States and in Southern Europe. Short-course RT (i.e. 5 Gy per day; total dose of 25 Gy) and surgery within the following week has been commonly used in Sweden and some other countries in Northern and Western Europe. Recently, short-course RT with delay to surgery has also been demonstrated a useful alternative to these two schedules.5 In fact, treatment differences exist even across institutions within the same country.6
Traditionally, preoperative RT has been delivered via three-dimensional conformal RT (3D-CRT) with three- or four-field techniques for rectal cancer. Nowadays, advanced techniques, such as intensity-modulated radiotherapy (IMRT), are widely and successfully used for prostate, head-and-neck, and other cancers.7–9 The theoretical rationale for using such highly conformal techniques is sound for rectal cancer; however, the potential clinical benefits remain debatable.10 Thus, we performed searches on PubMed, EMBASE, and MEDLINE databases (2000 to May 2019) using the medical subject heading term ‘rectal cancer.’ Additional keywords included ‘preoperative,’ ‘radiotherapy,’ ‘chemoradiotherapy,’ ‘surgery,’ ‘dose,’ and ‘time interval.’ Furthermore, we reviewed reference lists from retrieved articles and textbooks to identify additional articles of interest. We discussed the options of both dose–time fractionation schedule and time to surgery using prior standards (i.e. 3D-CRT), and advanced technologies (i.e. IMRT). Given the growing concerns about precision medicine, understanding the patterns of new technology is particularly important.
The era of 3D-CRT
Local recurrence (LR) is a serious problem because it causes disabling symptoms and successful salvage of pelvic recurrence is rarely possible. Just 34 years ago, the LR risk was greatly reduced from 25% to 16% with the advent of postoperative RT, with anterior and posterior parallel opposed fields.11 In 2004, the German Rectal Cancer Study Group demonstrated improved LC and reduced toxicity when CRT with a three- or four-field technique was delivered preoperatively instead of postoperatively, and LR at 5 years was further reduced from 13% to 6%.12 In 2005, at a median follow up of 13 years, the Swedish rectal cancer trial eventually reported that LR was only 9% after short-course RT with immediate surgery.13 Therefore, preoperative RT/CRT for rectal cancer was found to be beneficial for reducing LR rates.
Conventional as well as hypo- and hyperfractionated RT strategies and time intervals
During the last 2 decades, more modern trials have examined the most appropriate treatment schedule. Polish and Australian trials compared long-course CRT (28 × 1.8 Gy) and surgery 4–6 weeks later with short-course RT (5 × 5 Gy) and surgery within 7 days for cT3/T4 disease.14–16 No significant differences were observed in postoperative complications, LC, late toxicity, recurrence-free survival (RFS), disease-free survival (DFS), or OS; nevertheless, a significantly higher acute radiation toxicity was observed with long-course CRT. In 2017, the Stockholm III trial used three regimens: either short-course RT (5 × 5 Gy) with surgery within 1 week or after 4–8 weeks or 25 × 2 Gy with surgery after 4–8 weeks.5 No significant differences in local and distant recurrences or in RFS and OS were reported among the three different RT regimens. Compared with short-course RT with immediate surgery, postoperative complications were significantly reduced by delaying surgery; however, acute radiation-induced toxicities were seen in ~7% of these patients after much delay. In addition to a hypofractionated RT regimen (5 × 5 Gy), a hypofractionated RT schedule (30 Gy in 10 once-daily fractions) was tested in China to minimize side effects without compromising therapeutic efficacy.17 After a median follow up of 63.8 months, 5-year DFS and OS rates were 64.5% and 75.6% respectively. Moreover, grade ⩾3 acute toxicity rates was only 1.2%, and the total grade ⩾3 late RT toxicity rate was down to 2.7%.18
In order to verify the hypothesis that hyperfractionated accelerated radiotherapy (HART) may provide a favorable long-term outcome compared to hypofractionated RT, the pelvis was irradiated twice daily, with a minimal interfraction interval of 6h, and a total dose of 42 Gy was administered in doses of 1.5 Gy per fraction.19 Surgery was performed 1–2 weeks after RT. The results showed that the physical, emotional, and social functioning of long-term survivors were significantly better with HART; however, when compared with hypofractionated RT, there was no significant difference regarding LC and OS. In order to ensure that the overall treatment time was shorter than the proliferation delay Tk, set to 7 days,20 RT was delivered with a single fraction of 2.5 Gy twice daily (⩾6 h intervals) to a total dose of 25 Gy. Surgery was performed the following week.21 The clinical trial showed that LC was excellent in primarily resectable rectal cancer. Combined with S-1 as a radiosensitizer, this regimen of a 4-week delay in surgery also showed acceptable oncologic outcomes for T3 rectal cancer.22,23
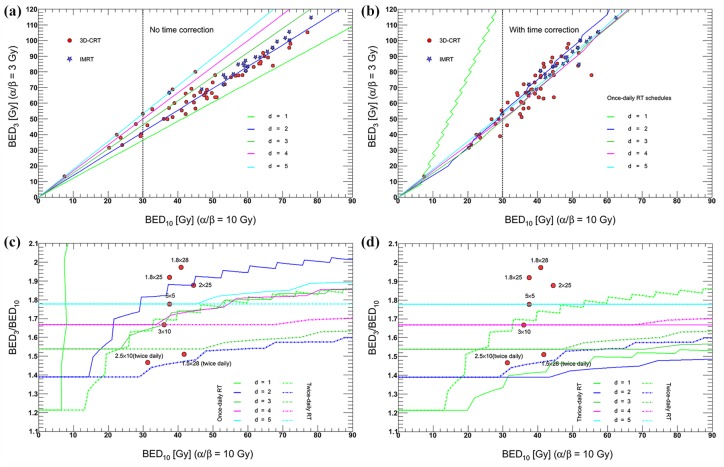
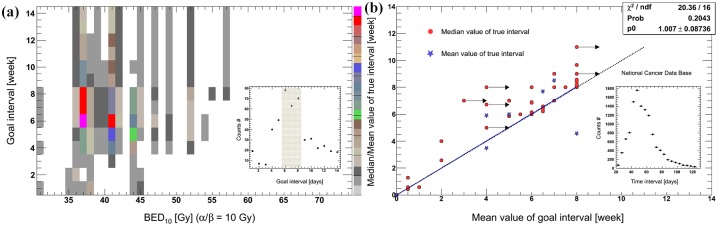
There are many other dose fractionations of preoperative RT in addition to the above schedules. Figure 1(a, b) shows the most commonly used regimens.24–52 Here, a biologically effective dose (BED) was calculated according to a linear–quadratic (LQ) model of radiation effect. BED was evaluated at the isocenter. In this model, α/β ratio of 10 was adopted for tumor tissue.20,53,54 Most of the regimens had larger BEDs of ⩾30 Gy. Meanwhile, the dose curves steepened and became concentrated for d > 1 Gy after the overall treatment time (OTT) was considered. If BED3/BED10 is used to represent the risk/benefit ratio of preoperative RT, 3–4 Gy per fraction using once-daily RT regimen or 2 Gy per fraction using twice/thrice-daily RT regimen might be optimal [Figure 1(c, d)]. In most centers, RT fractionation schedules and time to surgery are based on their clinical practice experiences. Given the different combination schemes of dose fractionation and time to surgery, a goal interval of 6–8 weeks is the most commonly used value in clinical trials [Figure 2(a)].5,15,17,18,21,24,27,28,31,32,34–36,38–42,44,46–48,50,52,55–94 Moreover, because of factors such as acute radiation reaction, there are some discrepancies between the goal intervals and true intervals.95 Despite limited samples, a linear correlation can be observed between them in Figure 2(b). In addition, because of the semi-Poisson distribution of the actual time interval,95 the mean values of goal time gaps are usually smaller than the median true time intervals [Figure 2(b)].5,18,21,24,32,36,38,41,43,44,46,47,52,56,61,70,73,74,79,80,82,90,91,93,96–108 It seems that surgery is usually performed early for most patients within a planned schedule.
Figure 1.
Linear–quadratic-model-based BEDs of most commonly used schedules of preoperative RT.
Filled circles and stars: actual used regimens; lines: theoretical calculation values as the ‘standard’ RT protocol, that is, five fractions per week from Monday to Friday. (a) BED without time correction. BED = nd [1 + d/(α/β)], where n = number of fractions, d = dose (Gy) per fraction, α/β = the LQ quotient; (b) time-corrected LQ-formula. BED = nd [1 + d/(α/β)] − γ/α (T − Tk), where γ/α = repair rate (set to 0.6 Gy/day), T = OTT and Tk = proliferation delay (set to 7 days). BED3: BED value when α/β is 3 for late toxicity. BED10: BED value when α/β is 10 for acute (tumor and normal tissue) toxicity; (c) the ratio of BED3 to BED10 as a function of BED10 with OTT correction using once- and twice-daily RT regimens. Filled circles: the mentioned fractionations in this text; lines: theoretical calculation values as the ‘standard’ RT protocol; a >6 h interfraction interval is used for multiple fractions per day; and (d) twice- and thrice-daily RT regimens.
BED, biologically effective dose; IMRT, intensity-modulated radiotherapy; LQ, linear quadratic; OTT, overall treatment time; RT, radiotherapy.
Figure 2.
The most commonly used time intervals including goal and actual values.
(a) The different combination schemes of timing to surgery and BED10 with OTT correction, including distribution of goal time intervals. The upper color in the right means higher frequency of use in clinical practices; and (b) the correlation between goal and actual intervals, including distribution of time to surgery of the National Cancer Database. In most trials, the median values of actual time intervals were presented, but the mean values were seldom reported. Moreover, a linear function was fitted to the mean values of both goal and true time intervals, and the fitting parameter was 1.01 ± 0.09. If the goal interval was represented by a range using the sign of ‘> or ⩾’, the minimum value was used in this figure, and the sign of ‘→’ was attached. Otherwise, we used the mean values of goal interval.
BED, biologically effective dose; OTT, overall treatment time; p0, the fitting parameter; Prob, probobility; ndf, the number of degrees of freedom.
Although the goals of preoperative RT/CRT are to minimize the recurrence risk, optimize survival, and avoid toxicity, different strategies have led to different outcomes. However, it is clinically relevant to wait for the highest degree of pathological response, as this helps to identify the optimal time to surgery and increases the chance of R0 resection. Moreover, patients might exhibit such favorable response that they become candidates for a watch-and-wait approach or local excision.109 Additionally, patients with a pCR might have better DFS and OS.110,111 Therefore, an enhanced radiation response is necessary for a better pathologic response after preoperative RT/CRT.
Early endpoints: pathologic tumor response
Several parameters have been considered to quantify tumor response, such as T (tumor size) and N (number of nearby lymph nodes) status downstaging and pCR. In China, a pCR rate of 4.5% and a downstaging rate of 70.2% were achieved using the 30 Gy protocol and surgery after 2 weeks.18 A high pCR rate of 11.8% was reported in the short-course RT-with-delay arm.112 A Polish trial showed an even higher pCR of 16.1% using conventional long-course CRT and surgery 4–6 weeks later.14 The tumor response could be further increased by the addition of specific chemotherapy regimens in preoperative setting.71
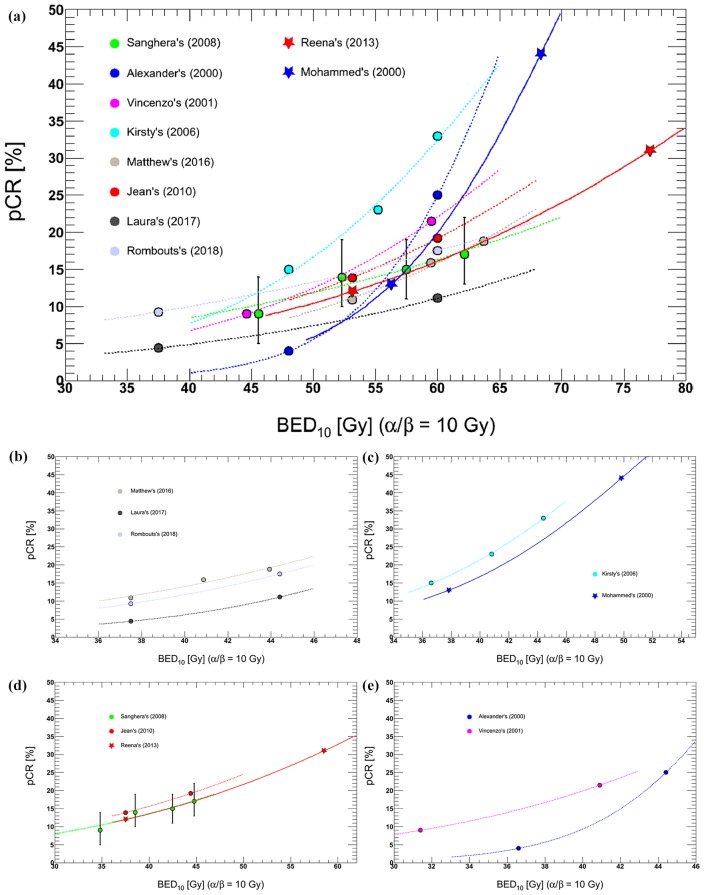
Furthermore, a highly significant dose–response relationship was observed. For example, a trend toward increased pCR with higher doses was reported, with pCR being 15%, 23%, and 33% at 40 Gy, 46 Gy, and 50 Gy, respectively.79 Figure 3(a) also shows that increasing RT doses were associated with tumor response, but the incremental rates were different because of additional chemotherapy, and RT techniques, etc.4,26,74,75,79,113–118 However, if OTT was considered using this LQ model,20 the trends became very similar among some trials [Figure 3(b, c, d)]. Furthermore, improved response could be enhanced with intraoperative RT or with high-dose-rate γ-ray or contact X-ray brachytherapy boost.119–121
Figure 3.
Comparison of reported dose–response relationships between BED10 and pCR.
Studies on preoperative CRT were included if they were conducted to treat rectal cancer, comparing conventional dose with intensified dose. Data from eight comparative studies were analyzed. Logistic response curves fitted for each study. (a) BED10 without OTT correction. D50 (the dose required for 50% response) ranged from 68.3 to 108.0 Gy, and the normalized dose–response gradient G50 at D50 ranged from 0.87 to 2.42 (b, c, d, e) BED10 with OTT correction; (b) three studies conducted by Hall, Kairevičė, and Rombouts4,75,118 showed very close results; D50 was 59.0 ± 13.5 Gy, 58.8 ± 19.0 Gy, and 59.1 ± 13.8Gy, but G50 was 1.40 ± 0.18, 2.12 ± 0.41, and 1.56 ± 0.21, separately; (c) two studies performed by Mohiuddin and Wiltshire79,115 demonstrated that D50 was 49.8 ± 5.5 Gy and 51.6 ± 4.1 Gy, and G50 was 1.65 ± 0.12 and 1.78 ± 0.10; (d) Three groups26,113,114 also got the similar results. D50 was 70.1 ± 15.4 Gy, 70.0 ± 22.3 Gy and 72.6 ± 8.0 Gy, and G50 was 1.08 ± 0.12, 0.98 ± 0.16 and 1.03 ± 0.07, separately; and (e) No similar results were observed.
BED, biologically effective dose; OTT, overall treatment time, pCR, pathological complete response.
Tumor regression takes time (median volume-halving time, 14 days).122 Several studies have previously demonstrated improved pCR after long time intervals (Table 1, longer intervals might not increase pCR in particular cohorts).83,84,96,123–126 The Korean Radiation Oncology Group found that pCR steadily increased after 5–6 weeks, escalated over 10% after 6–7 weeks, and peaked at 9–10 weeks for locally advanced rectal cancer. The downstaging rate increased steadily until 6–7 weeks and declined afterward.127 For patients with cT1-4N0-2M0-1, the highest pCR rates were observed at approximately 10–11 weeks from the end of long-course CRT.128 A waiting time exceeding 11 weeks might be associated with higher morbidity and a more difficult surgical resection because of tissue fibrosis and friability.129 After accounting for well-known confounders, such as comorbidities and tumor characteristics, an optimal threshold of 56 days (8 weeks) was determined after completion of neoadjuvant CRT for minimizing the risk of positive margins and maximizing pathologic downstaging.95
Table 1.
Literature review.
| Study (year) | Study type | RT dose (Gy) | Chemotherapy | TME (yes/no) | Intervals (weeks) | Patients | Preoperative stages | Age (years) | Males (%) | pCR (%) | LR (%) | DFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Francois130 | Prospective | 13 × 3 | No | No | <2 | 99 | T2–4, N0–1 | 66 | 57.6 | 7.1 | 3 years: 9 | NR | 3 years: 78 |
| 6–8 | 102 | T2–3, N0–1 | 64 | 62.7 | 13.7 | 3 years: 9 | NR | 3 years: 73 | |||||
| Stein131 | Prospective | (25–30) × 1.8 | c.i. 5-FU + CPT-11 | Yes | 4–8 | 19 | T2–4, N0–1 | 51 | 74 | 21 | NR | NR | NR |
| 10–14 | 14 | T3–4, N0–1 | 53 | 64 | 14 | NR | NR | NR | |||||
| Moore132 | Retrospective | (26–28) × 1.8 | Bolus 5-FU/LV | No | ⩽44 days | 82 | I–III | 59 | 63.4 | 12 | NR | NR | NR |
| >44 days | 73 | I–III | 62 | 63.0 | 19 | NR | NR | NR | |||||
| Tran133 | Retrospective | (25–30) × 1.8 | 5-FU | Yes | ⩽8 | 16 | II–III | 62 | 62 | 6 | NR | NR | NR |
| >8 | 32 | I–IV | 58 | 56 | 9 | NR | NR | NR | |||||
| Veenhof134 | Retrospective | 5 × 5 | No | Yes | <2 | 57 | T2–3, N0–1* | 67 | 65 | 0* | 5 years: 2 | 5 years: 74.6 | 5 years: 66.4 |
| 6–8 | 51 | T2–4, N0–1* | 63 | 73 | 12* | 5 years: 4 | 5 years: 69.4 | 5 years: 73.3 | |||||
| Lim135 | Retrospective | 28 × 1.8 | FL, CAP, or IC | No | 4–6 | 217 | T2–4 | 55* | 64 | 13.8 | NR | NR | NR |
| 6–8 | 180 | T2–4 | 58* | 69 | 15 | NR | NR | NR | |||||
| Tulchinsky136 | Retrospective | (25–28) × 1.8 | 5-FU | Yes | ⩽7 | 48 | II–III | 59* | 73 | 16.7* | 6 | NR | NR |
| >7 | 84 | II–III | 64* | 64 | 34.5* | 4 | NR | NR | |||||
| Garcia-Aguila137 | Prospective | (25–30) × 1.8 | 5-FU | Yes | 6–8 | 66 | T2–4, NX, 0–2 | 61* | 61 | 18* | NR | NR | NR |
| 5-FU + mFOLFOX-6 | 11–13 | 70 | T2–4, N0–2 | 56* | 54 | 25* | NR | NR | NR | ||||
| de Campos-Lobato101 | Retrospective | 28 × 1.8 | 5-FU | Yes | <8 | 83 | T1–4, N0–2 | 54 | 76 | 16.2* | 3 years: 10.5* | 3 years: 75.3 | 3 years: 85.5 |
| ⩾8 | 94 | T2–4, N0–2 | 57 | 73 | 31.1* | 3 years: 1.2* | 3 years: 84.7 | 3 years: 88.2 | |||||
| Wolthuis138 | Retrospective | 25 × 1.8 | 5-FU | Yes | ⩽7 | 201 | T2–4, N0–2 | 64 | 62 | 15.9* | 5 years: 3 | NR | 5 years: 84 |
| >7 | 155 | T2–4, N0–2 | 62 | 70 | 28.4* | 5 years: 2 | NR | 5 years: 77 | |||||
| Jeong139 | Retrospective | 28 × 1.8 | 5-FU + LV | Yes | <8 | 105 | T1–4, N0–2 | 58 | 75 | 16.2 | 3 years: 7.8 | 3 years: 64.8 | 3 years: 90.2 |
| ⩾8 | 48 | T2–4, N0–2 | 56 | 79 | 18.8 | 3 years: 12.7 | 3 years: 66.7 | 3 years: 87.2 | |||||
| Sloothaak128 | Retrospective | 25 × 2 or 28 × 1.8 | CAP or 5-FU ± oxaliplatin | Yes | <8 | 312 | T1–4, N0–2, M0–1* | 63 | 64 | 10.3* | NR | NR | NR |
| 8–9 | 511 | T1–4, N0–2, M0–1* | 63 | 64 | 13.1* | NR | NR | NR | |||||
| 10–11 | 406 | T1–4, N0–2, M0–1* | 64 | 60 | 18.0* | NR | NR | NR | |||||
| >11 | 364 | T1–4, N0–2, M0–1* | 64 | 63 | 11.8* | NR | NR | NR | |||||
| Zeng96 | Retrospective | 25 × 2 | CAP | Yes | ⩽7 | 111 | T2–4, N0–2 | 59 | 56 | 15.3* | 3 years: 12.9* | 3 years: 72.6 | 3 years: 89.0 |
| >7 | 122 | T2–4, N0–2 | 59 | 56 | 27.1* | 3 years: 4.8* | 3 years: 79.4 | 3 years: 94.5 | |||||
| Calvo83 | Retrospective | 28 × 1.8 + 10–15 Gy (IORT) | Bolus 5-FU + LV | Yes | <6 | 136 | T2–4 | 66 | 67* | 8.8 | 5 years: 9.6 | 5 years: 69.9 | 5 years: 55.9* |
| ⩾6 | 199 | T2–4 | 66 | 55* | 12.1 | 5 years: 5.5 | 5 years: 74.9 | 5 years: 70.4* | |||||
| You140 | Retrospective | 25 × 2 | FOLFOX6 or XELOX | Yes | ⩽7 | 139 | T1–4, N0–2 | 55 | 69 | 27.3* | NR | 5 years: 74.7 | 5 years: 84.4 |
| >7 | 152 | T1–4, N0–2 | 56 | 71 | 29.6* | NR | 5 years: 66.8 | 5 years: 75.3 | |||||
| Mihmanlı141 | Retrospective | (25–28) × (1.8–2) | 5-FU | Yes | <8 | 45 | T2–4 | 54 | 71 | 8.9* | 6 years: 8.9 | 5 years: 55.3* | 5 years: 79.1* |
| ⩾8 | 42 | T2–4 | 58 | 74 | 19.0* | 6 years: 7.1 | 5 years: 85.1* | 5 years: 94.4* | |||||
| Akbar142 | Retrospective | 28 × 1.8 | XELOX | Yes | ⩽8 | 66 | T2–4, N0–2 | NR | 65 | 30 | 12 | 5 years: 66.7* | 5 years: 68.2 |
| >8 | 93 | T2–4, N0–2 | NR | 68 | 33 | 25 | 5 years: 53.8* | 5 years: 54.3 | |||||
| Macchia126 | Retrospective | NR | One or two drugs | Yes | ⩽6 | 300 | II–III | 64* | 60 | 12.6* | NR | NR | NR |
| 7–12 | 1598 | II–III | 65* | 64 | 23.0* | NR | NR | NR | |||||
| ⩾13 | 196 | II–III | 67* | 62 | 31.1* | NR | NR | NR | |||||
| Couwen-berg125 | Retrospective | (25–28) × (1.8–2) | CAP | Yes | 3–6 | 479 | T1–4, N0–2 | 64.4 | 62.0 | 15.7 | NR | NR | NR |
| 7–8 | 1309 | T1–4, N0–2 | 63.5 | 65.8 | 13.9 | NR | NR | NR | |||||
| 9–10 | 1668 | T1–4, N0–2 | 64.1 | 61.2 | 16.9* | NR | NR | NR | |||||
| 11–12 | 1287 | T1–4, N0–2 | 64.3 | 63.0 | 15.6 | NR | NR | NR | |||||
| 13–20 | 945 | T1–4, N0–2 | 64.4 | 63.6 | 16.8* | NR | NR | NR | |||||
| Akgun124 | Prospective | 28 × 1.8 | 5–FU + LV | Yes | ⩽8 | 160 | T3–4, N+ | 60.4 | 59 | 10.0* | NR | NR | NR |
| >8 | 167 | T3–4, N+ | 61.7 | 57 | 18.6* | NR | NR | NR |
p < 0.05.
5-FU, 5-fluorouracil; CAP, capecitabine; c.i., continuous infusion; CPT-11, irinotecan; DFS, disease-free survival; FL, 5-fluorouracil + leucovorin; FOLFOX6, oxaliplatin + leucovorin + 5-FU; IORT, intraoperative radiotherapy; LR, local recurrence; LV, leucovorin; mFOLFOX-6, modified FOLFOX-6; IC, irinotecan + capecitabine; N, number of nearby lymph nodes stage; NR, not reported; OS, overall survival; pCR, pathological complete response; RT, radiotherapy; T, tumor size stage; TME, total mesorectal excision; XELOX, oxaliplatin + capecitabine.
Long-term endpoints: LC and survival
After a median follow up of 11 years, the German CAO/ARO/AIO-94 trial reported that 10-year LR and OS rates were 7.1% and 59.6%, respectively, using 50.4 Gy in 28 fractions.1 After a median follow up of 12 years, the TME trial finally reported that 10-year LR and OS rates were 5% and 48% respectively, with short-course RT.143 The effect of RT on LC persisted, as well as the absence of a survival benefit. Nevertheless, it significantly improved survival in patients with a pCR, downstaging, or a negative circumferential margin.110,111,144,145
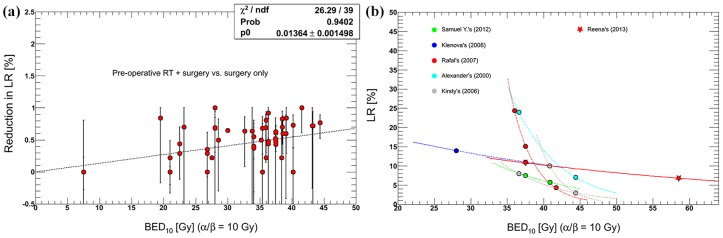
Early systematic reviews concluded that preoperative RT at a BED of >30 Gy reduced LR risk and improved OS,25,146 and that no significant difference was observed in outcomes for different time intervals between conventional neoadjuvant CRT and surgery.147 Moreover, higher doses increased LR reduction. A linear dose–response effect of BED was seen on the risk reduction of LR, and an exponent correlation was detected between LR and BED (Figure 4).15,37,74,78,79,114,148,149 Each 1 Gy increase in BED would reduce LR rates by 1.36–1.72%; hence, it was proposed that a BED of approximately 68.8–73.5 Gy would be needed to achieve 100% LC.29
Figure 4.
Comparison of reported dose–response relationships between BED10 and LR.
(a) The linear regression model fitted for risk reduction of LR as a function of BED10. (b) Exponent curves fitted for LR versus BED. Increasing RT doses was associated with reduction in LR but the rate of decrease was different between trials.
BED, biologically effective dose; LR, local recurrence; RT, radiotherapy; ndf, the number of degrees of freedom; p0, the fitting parameter; Prob, probobility.
In contrast to the linear effect of BED on reduction in LR, the effects of BED on DFS and OS were not linear due to considerable heterogeneities. After dose escalation with three dose levels of 40 Gy, 46 Gy and 50 Gy, 2-year actuarial LR-free survival rates were 72%, 90%, and 89%, respectively; DFS rates were 62%, 84%, and 78%, respectively; OS rates were 72%, 94%, and 92%, respectively. A statistically significant increase in survival was seen with doses of ⩾46 Gy, but there was no difference in survival between doses of 46 Gy and 50 Gy.79 However, after a long follow up of 11.9 years, patients with a concomitant dose boost (52.5 Gy) exhibited higher rates of 10-year OS than those for whom a conventional approach was used (45 Gy; 71.6% versus 62.4%).100
As a radiation sensitizer, chemotherapy may augment RT. It may also sterilize circulating micrometastases and impede disease progression and distant organ involvement.150 Prolongation of DFS and OS are ultimately expected. However, previous studies have demonstrated that a combination of preoperative RT and preoperative, with or without postoperative fluorouracil-based chemotherapy, would only further increase LC, without showing any significant differences in DFS and OS.58,151 However, it might benefit patients with a tumor 10–15 cm from the anal verge in terms of DFS.152 Recently, the final results of the German CAO/ARO/AIO-94 trial showed that DFS at 3 years could be improved after adding oxaliplatin to fluorouracil-based neoadjuvant CRT for patients with cT3–4 rectal cancer. However, this trial had serious methodological shortcomings.55 Although the benefits of chemotherapy on DFS are limited, as shown by the published data, current guidelines continue recommending a chemotherapy course because there is no sufficient evidence to conclude there is no absolute benefit of chemotherapy.153,154
Side effects: toxicities and complications
Preoperative CRT can induce serious side effects such as diarrhea, urinary tract infection, sexual dysfunction, and secondary malignancies.155–157 Meanwhile, toxicities and complications related to RT have also increased with the greater use of neoadjuvant CRT.158,159 The impact of RT on sexual, urinary, and anal functions has been documented in many previous trials, although surgery is likely to be the major factor.160–162 However, in 2019, a prospective study demonstrated that neoadjuvant CRT for lower rectal cancer did not affect postoperative urinary function,163 treatments, the timing, and evaluation methods vary largely among these trails. Direct investigations of the effect of RT dose on the anorectal function have been reported recently;164 a higher RT dose to anal sphincter complex tends to worsen the long-term anorectal function.
Furthermore, many trials demonstrated that there were no significant differences in severe late toxicity and quality of life between short-course RT with immediate surgery, and conventionally fractionated CRT with delayed surgery; however, CRT clearly increased the grade 3–4 acute toxicity.5,14,15 A recent retrospective analysis revealed that a dose boost did not increase the grade ⩾ 2 chronic toxicity after neoadjuvant CRT.165 Interestingly, the Radiation Therapy Oncology Group (RTOG) trial 0012 compared hyperfractionated radiation (55–60 Gy) with once-daily radiation (50–55 Gy) and also found the similar acute and late toxicities.57 Regarding the effect of timing intervals on toxicity, although the Stockholm III trial revealed that acute toxicity was only <1% after RT with immediate surgery compared with 5–7% after RT with delay, it is possible that these toxicities were obscured by early postoperative complications.5
The addition of chemotherapy to preoperative RT has a sound radiobiological rationale,58,151 but will simultaneously increase grade III and IV acute toxicities.166,167 In a study, gastrointestinal (GI) toxicity was more frequently observed in the CRT group than in the RT-alone group (28.1% versus 12.9%, respectively);168 a consequence of the increased toxicity was that the patients could not receive the full treatment or they experienced interruptions that could have a negative impact on outcomes. At present, no statistical difference was reported in late toxicity between the preoperative RT and CRT groups.
In summary, moderate RT dose escalation using the 3D-CRT technique and appropriate chemotherapy administration might be effective. The optimal time interval depends on clinical endpoints. There still remains a scope for the optimization of RT/CRT schedules. Although there is conflicting evidence because of various factors, strategies with the potential to improve outcomes, while reducing toxicities, are needed to guide future designs.
The era of IMRT
Early in 1993, MacFarlane and colleagues reported that total mesorectal excision (TME) instead of conventional surgery had led to substantial improvements in morbidity and survival.169 It is hoped that improvements in RT techniques will further reduce LR and adverse events, and increase the survival. As an innovative technique, IMRT allows conformal dose distribution in the target while sparing the bladder and bowels. It is of critical importance for accurate target determination and strict dose–volume constraints. With the integration of image guidance and IMRT, both a more precise definition of target volume and accurate irradiation are allowed. Organ motion with changes in shape, size and position can be observed; a small target margin can be applied, consequently reducing potential toxicity. Using a synchronous integrated boost (SIB) technique, the dose per fraction can be further increased to the primary tumor while shortening the treatment time. However, adequate quality controls of procedures are always required.155
Point: IMRT improves clinical endpoints
Multiple retrospective studies have shown that preoperative IMRT or volumetric-modulated arc therapy (VMAT, arc-based IMRT) is associated with a clinically significant reduction in GI or genitourinary (GU) toxicity, with or without improvement in LC compared with 3D-CRT.106,107,170–172 Furthermore, these modalities can potentially prevent delays in time to surgery and reduce hospitalizations, emergency department visits, and treatment breaks.173 However, no significant differences were noted in tumor responses, DFS and OS.107
Furthermore, several prospective studies have shown encouraging results. Preoperative IMRT with an SIB [46 + 55.2 (Gy) in 23 fractions] was explored. Surgery was performed 6 weeks later.174 The grade ⩾ 3 late GI and GU toxicity was 9% and 4%, respectively; 5-year LC and OS were 97% and 68%, respectively. These values were in line with the results after preoperative CRT.12,151 In order to reach the best loco–regional control and to prevent systemic relapse, RT and chemotherapy are usually integrated. A Turkish study adopted hypofractionated RT (33 Gy/10 fractions), with concurrent oral capecitabine. Surgery was scheduled 6–8 weeks after the end of CRT; 11.5% of patients had pCR, and no grade 3–4 toxicity was observed.175 Another phase II trial studied IMRT (47.5 Gy in 19/20 fractions) in combination with capecitabine and oxaliplatin (CAPOX). TME was scheduled 4–6 weeks after the CRT. A pCR was observed in 13% of patients, and major response in 48%, which seemed to translate into improved outcomes such as LC of 100%, DFS of 84%, and OS of 87%, after a median follow up of 55 months.47
Moreover, preoperative IMRT with an SIB without dose escalation [41.8 + 46.2/48.4 (Gy) in 22 fractions], with concomitant capecitabine, was tested. Surgery was performed 6–8 weeks later. The rate of grade ⩾ 3 acute toxicity was 2.4%. A total of 25.5% patients achieved pCR, with 2-year LC, DFS, and OS rates of 100% for these patients.92 If dose was escalated with an SIB [46 + 57.5 (Gy) in 23 fractions], and concomitant with capecitabine, surgery was planned around 8 weeks. A total of 30.6% of patients could achieve pCR with quite acceptable toxicity profiles.44 At a median follow up of 38.2 months, the similar treatment schemes [45 + 55 (Gy) in 25 fractions, capecitabine, surgery 8 weeks later] resulted in 2-year DFS and OS of 90% and 90%, respectively, with a high pCR rate of 35%.41
To obtain a better tumor response, elevating treatment dose has been considered a feasible method. Preoperative capecitabine and IMRT with an SIB [45 + 55 (Gy) in 25 fractions] were used and TME followed 6 weeks later. The crude pCR rate was up to 38%, with 50% achieving downstaging.91 Recently, a near-total neoadjuvant approach was tested using multiagent chemotherapy, that is, sequential short-course IMRT (5 × 5 Gy) and FOLFOX (fluorouracil, leucovorin calcium, and oxaliplatin) followed by TME. A higher T downstaging of 75% and a superior 3-year DFS rate of 85% were observed compared with conventional neoadjuvant CRT (41% and 68%, respectively).94
Counterpoint on IMRT and corresponding deliberation
Although preoperative IMRT has shown improved oncological outcomes, conflicting results are constantly being published. A retrospective study has demonstrated that IMRT was associated with worse R0 resection rates and sphincter preservation, without any differences in pathologic downstaging, unplanned readmission, or long-term OS.176 A prospective phase II study used VMAT-SIB [45 + 57.5 (Gy) in 25 fractions] and two-drug chemotherapy CAPOX. Radical resection was performed 8 weeks after treatment. Although a very high tumor response was achieved, an acute toxicity rate of 44% was also recorded.50 RTOG 0822 studied IMRT (25 × 1.8 Gy), followed by a boost (3 × 1.8 Gy) using 3D-CRT with concurrent CAPOX. Surgery was performed 4–8 weeks after CRT. A grade ⩾ 2 GI toxicity rate of 51.5% occurred preoperatively, which substantially exceeded the target rate of 28%.177
The role of preoperative IMRT in rectal cancer remains to be determined at this juncture. Moreover, the addition of different chemotherapy and different treatment sequences confound it more. CRT-to-surgery interval also affects these clinical endpoints in the era of IMRT.178 More trials with the prospective aim to further explore the efficiency of preoperative IMRT are expected.
Meanwhile, the limitations and potential difficulties inherent to IMRT, that is, dose inhomogeneity and integral dose, must be considered. Patient selection is of utmost importance. IMRT is also technically challenging, because the oncological outcomes are highly dependent on accurate target determination and dose–volume parameters. Careful quality assurance with regards to target delineation, image guidance, and plan optimization constraints is needed prior to treatment.
Using the SIB technique, two different doses per fraction are usually delivered in two different target regions, that is, a two-target approach. Neoadjuvant chemotherapy prior to preoperative RT/CRT gives us a chance to induce tumor regression, which allows the dose to the macroscopic postchemotherapy tumor to be increased by several additional Gy using the third targets. Additionally, a dynamic target could be generated within the frame of adaptive RT to accompany dose escalation. Also, it appears promising to harness functional imaging to guide dose to subvolumes of the target with a high tumor load and de-escalate dose to low-risk volumes.
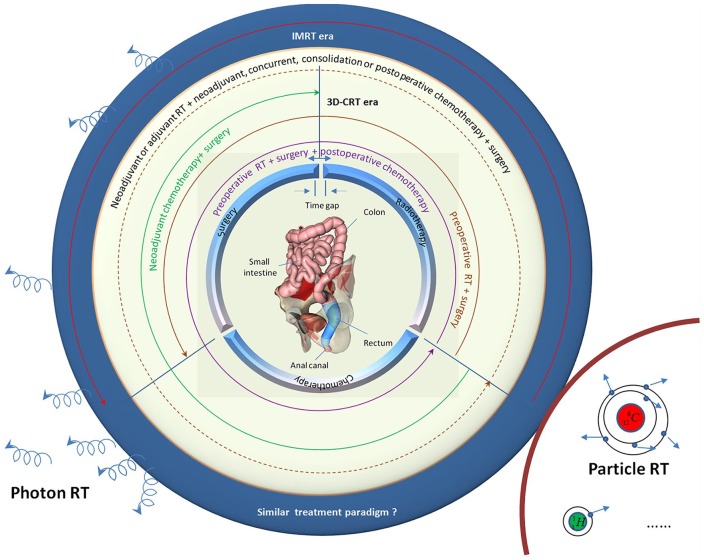
In summary, trimodality therapy for rectal cancer inherently has uncertainties: treatment sequence, timing, and duration of the various modalities. Many treatment paradigms have been tested, such as surgery ± adjuvant RT/CRT, and preoperative RT/CRT/chemotherapy ± surgery ± CRT (Figure 5). However, until now, most fractionation schedules in preoperative RT have been empirical and based on the outcome of clinical trials. Fractionation schedules and the time interval are rather homogeneous across various institutions. Given patient selection and other treatment interventions, one cannot accurately assess whether and to what extent they influence clinical outcomes. If a radiobiological response model for fractionation is established on the basis of previous clinical studies, the controversy regarding dose fractionation schedules and time interval may disappear.
Figure 5.
Treatment schemes for IMRT and CRT.
In the era of photon treatment, the role of RT/CRT as adjunct to surgery has evolved over decades with changes in the timing (preoperative versus postoperative), length (short versus long course), and delivery (3D-CRT versus IMRT). Many treatment paradigms have been tested using 3D-CRT technique. In this figure, different encircling modes of curves represent different treatment schemes, and there are many therapeutic schemes in theory. However, few comparative prospective studies of IMRT exist now. One cannot accurately assess whether the treatment paradigms in the era of IMRT are superior to those in the era of 3D-CRT. Besides photon treatment, particle RT has gained great attention recently, such as carbon ion and proton. In order to provide precision medicine to patients with rectal cancer, the radiobiological response model should be investigated in more depth.
3D-CRT, three-dimensional chemoradiotherapy; IMRT, intensity-modulated radiotherapy; RT, radiotherapy.
In addition, with the widespread standardization of surgery, diversification of drug, and precision of RT, the specific modality will be eliminated or used more sufficiently for a subset of patients, such as a ‘watch and wait’ strategy after preoperative RT/CRT, neoadjuvant chemotherapy only, and multidrug CRT. The priority for future research should be subgroups of patients who might receive relatively greater benefit from innovative treatment techniques. Moreover, with the development of technology and change in people’s understanding, the optimal regimen will also constantly change. These studies will be critical to further implementation of precision medicine through maximizing clinical outcomes, while minimizing associated toxicities.
New era: particle RT such as proton and heavy ions
Particle RT has recently garnered great attention. It can deliver radiation with a highly conformal dose distribution while maintaining minimal excess dose to normal tissues. Additionally, it is coupled with various biological advantages, especially for heavy-ion beam, such as a lack of oxygen effect and less cell cycle-related radiosensitivity. It enables treatment of diseases that are inaccessible with conventional RT, for example, postoperative recurrence of rectal cancer.
A recent report has shown that patients were treated with 73.6 GyE (physical dose multiplied by relative biological effectiveness) in 16 fractions using carbon ion. The 5-year LC rate was 88% and survival was 59%.179 These figures are higher than those with photon RT. Moreover, particle RT might be further optimized by dose escalation or hypofractionation. Given a high rate of distant metastases in most studies, concurrent and adjuvant systemic therapies should also be investigated.
Supplemental Material
Supplemental material, Supplementary_Figure for Dose–time fractionation schedules of preoperative radiotherapy and timing to surgery for rectal cancer by Fu Jin, Huanli Luo, Juan Zhou, Yongzhong Wu, Hao Sun, Hongliang Liu, Xiaodong Zheng and Ying Wang in Therapeutic Advances in Medical Oncology
Acknowledgments
Fu Jin and Huanli Luo contributed equally to this work. The authors thank the following colleagues for their assistance and advice in this study: Shi Li, Xia Tan, Xianfeng Liu, Xia Huang, Qicheng Li, Mingsong Zhong, Han Yang, Chao Li, Yanan He, Xiumei Tian, Da Qiu, Guanglei He, Li Yin, Guang Li, and Bo Li. The interim findings on relationships between biologically effective dose and pathological complete response, and local recurrence were presented at the ESMO as a poster. The poster’s abstract was published in Annals of Oncology (2019) 30 (suppl_9):ix30-ix41.10.1093/annonc/mdz421.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China under grant no. 11575038 and 11805025.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Fu Jin, Department of Radiation Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, People’s Republic of China.
Huanli Luo, Department of Radiation Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, People’s Republic of China.
Juan Zhou, Forensic Identification Center, Southwest University of Political Science and Law, Chongqing, PR China.
Yongzhong Wu, Department of Radiation Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, People’s Republic of China.
Hao Sun, Department of Gynecologic Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, PR China.
Hongliang Liu, Department of Anesthesiology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, PR China.
Xiaodong Zheng, Department of Science Education, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, PR China.
Ying Wang, Department of Radiation Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, 181 Hanyu Road, Shapingba District, Chongqing 400030, China.
References
- 1. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 2. Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009; 373: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song JH, Jeong JU, Lee JH, et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: a meta-analysis of randomized controlled trials. Radiat Oncol J 2017; 35: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall MD, Schultheiss TE, Smith DD, et al. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol 2016; 55: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 5. Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017; 18: 336–346. [DOI] [PubMed] [Google Scholar]
- 6. Morris EJ, Finan PJ, Spencer K, et al. Wide variation in the use of radiotherapy in the management of surgically treated rectal cancer across the English national health service. Clin Oncol (R Coll Radiol) 2016; 28: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 2013; 309: 2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017; 35: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang Y, Feng L, Wang Y, et al. A dosimetric analysis of preoperative intensity-modulated and image-guided radiation therapy with and without simultaneous integrated boost for locally advanced rectal cancer. Technol Cancer Res Treat 2015; 14: 557–563. [DOI] [PubMed] [Google Scholar]
- 11. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 1985; 312: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 12. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 13. Folkesson J, Birgisson H, Pahlman L, et al. Swedish rectal cancer trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23: 5644–5650. [DOI] [PubMed] [Google Scholar]
- 14. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 15. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012; 30: 3827–3833. [DOI] [PubMed] [Google Scholar]
- 16. Ansari N, Solomon MJ, Fisher RJ, et al. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: Trans-Tasman Radiation Oncology Group trial (TROG 01.04). Ann Surg 2017; 265: 882–888. [DOI] [PubMed] [Google Scholar]
- 17. Zhan T, Gu J, Li M, et al. Intermediate-fraction neoadjuvant radiotherapy for rectal cancer. Dis Colon Rectum 2013; 56: 422–432. [DOI] [PubMed] [Google Scholar]
- 18. Zhu XG, Li JL, Li XF, et al. Two-week course of preoperative radiotherapy for locally advanced rectal adenocarcinoma: 8 years’ experience in a single institute. Am J Clin Oncol 2017; 40: 266–273. [DOI] [PubMed] [Google Scholar]
- 19. Wzietek I, Kryj M, Idasiak A, et al. Randomized clinical trial on hyperfractionated versus hypofractionated preoperative radiotherapy for rectal cancer: long term outcomes including quality of life assessment. Int J Radiat Oncol Biol Phys 2014; 90: S21. [Google Scholar]
- 20. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001; 358: 1291–1304. [DOI] [PubMed] [Google Scholar]
- 21. Widder J, Herbst F, Dobrowsky W, et al. Preoperative short-term radiation therapy (25 Gy, 2.5 Gy twice daily) for primary resectable rectal cancer (phase II). Br J Cancer 2005; 92: 1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beppu N, Matsubara N, Kakuno A, et al. Feasibility of modified short-course radiotherapy combined with a chemoradiosensitizer for T3 rectal cancer. Dis Colon Rectum 2015; 58: 479–487. [DOI] [PubMed] [Google Scholar]
- 23. Beppu N, Kimura F, Aihara T, et al. Patterns of local recurrence and oncologic outcomes in T3 low rectal cancer (⩽ 5 cm from the anal verge) treated with short-course radiotherapy with delayed surgery: outcomes in T3 low rectal cancer treated with short-course radiotherapy with delayed surgery. Ann Surg Oncol 2017; 24: 219–226. [DOI] [PubMed] [Google Scholar]
- 24. Derdel J, Mohiuddin M, Kramer S, et al. Is dose/time fractionation important in treating rectal cancer? Int J Radiat Oncol Biol Phys 1985; 11: 579–582. [DOI] [PubMed] [Google Scholar]
- 25. Glimelius B, Grönberg H, Järhult J, et al. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003; 42: 476–492. [DOI] [PubMed] [Google Scholar]
- 26. Sanghera P, Wong DW, McConkey CC, et al. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) 2008; 20: 176–183. [DOI] [PubMed] [Google Scholar]
- 27. Guckenberger M, Saur G, Wehner D, et al. Comparison of preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Strahlenther Onkol 2012; 188: 551–557. [DOI] [PubMed] [Google Scholar]
- 28. Ortholan C, Romestaing P, Chapet O, et al. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int J Radiat Oncol Biol Phys 2012; 83: e165–e171. [DOI] [PubMed] [Google Scholar]
- 29. Viani GA, Stefano EJ, Soares FV, et al. Evaluation of biologic effective dose and schedule of fractionation for preoperative radiotherapy for rectal cancer: meta-analyses and meta-regression. Int J Radiat Oncol Biol Phys 2011; 80: 985–991. [DOI] [PubMed] [Google Scholar]
- 30. Vestermark LW, Jensen HA, Pfeiffer P. High-dose radiotherapy (60 Gy) with oral UFT/folinic acid and escalating doses of oxaliplatin in patients with non-resectable locally advanced rectal cancer (LARC): a phase I trial. Acta Oncol 2012; 51: 311–317. [DOI] [PubMed] [Google Scholar]
- 31. Burbach JP, den Harder AM, Intven M, et al. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol 2014; 113: 1–9. [DOI] [PubMed] [Google Scholar]
- 32. Ceelen W, Boterberg T, Pattyn P, et al. Neoadjuvant chemoradiation versus hyperfractionated accelerated radiotherapy in locally advanced rectal cancer. Ann Surg Oncol 2007; 14: 424–431. [DOI] [PubMed] [Google Scholar]
- 33. Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol 2013; 19: 8489–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartley A, Ho KF, McConkey C, et al. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol 2005; 78: 934–938. [DOI] [PubMed] [Google Scholar]
- 35. Idasiak A, Galwas-Kliber K, Behrendt K, et al. Pre-operative hyperfractionated concurrent radiochemotherapy for locally advanced rectal cancers: a phase II clinical study. Br J Radiol 2017; 90: 20160731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lorchel F, Peignaux K, Créhange G, et al. Preoperative radiotherapy in elderly patients with rectal cancer. Gastroenterol Clin Biol 2007; 31: 436–441. [DOI] [PubMed] [Google Scholar]
- 37. Suwinski R, Taylor JM, Withers HR. Rapid growth of microscopic rectal cancer as a determinant of response to preoperative radiation therapy. Int J Radiat Oncol Biol Phys 1998; 42: 943–951. [DOI] [PubMed] [Google Scholar]
- 38. Brooks S, Glynne-Jones R, Novell R, et al. Short course continuous, hyperfractionated, accelerated radiation therapy (CHART) as preoperative treatment for rectal cancer. Acta Oncol 2006; 45: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 39. Lee JH, Kim JG, Oh ST, et al. Two-week course of preoperative chemoradiotherapy followed by delayed surgery for rectal cancer: a phase II multi-institutional clinical trial (KROG 11-02). Radiother Oncol 2014; 110: 150–154. [DOI] [PubMed] [Google Scholar]
- 40. Zhu J, Gu W, Lian P, et al. A phase II trial of neoadjuvant IMRT-based chemoradiotherapy followed by one cycle of capecitabine for stage II/III rectal adenocarcinoma. Radiat Oncol 2013; 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tey J, Leong CN, Cheong WK, et al. A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer. J Cancer 2017; 8: 3114–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parekh A, Truong MT, Pashtan I, et al. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res 2013; 6: 137–143. [PMC free article] [PubMed] [Google Scholar]
- 43. But-Hadzic J, Anderluh F, Brecelj E, et al. Acute toxicity and tumor response in locally advanced rectal cancer after preoperative chemoradiation therapy with shortening of the overall treatment time using intensity-modulated radiation therapy with simultaneous integrated boost: a phase 2 trial. Int J Radiat Oncol Biol Phys 2016; 96: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 44. Hernando-Requejo O, López M, Cubillo A, et al. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlenther Onkol 2014; 190: 515–520. [DOI] [PubMed] [Google Scholar]
- 45. Wang L, Li ZY, Li ZW, et al. Efficacy and safety of neoadjuvant intensity-modulated radiotherapy with concurrent capecitabine for locally advanced rectal cancer. Dis Colon Rectum 2015; 58: 186–192. [DOI] [PubMed] [Google Scholar]
- 46. Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys 2014; 88: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arbea L, Martínez-Monge R, Díaz-González JA, et al. Four-week neoadjuvant intensity-modulated radiation therapy with concurrent capecitabine and oxaliplatin in locally advanced rectal cancer patients: a validation phase II trial. Int J Radiat Oncol Biol Phys 2012; 83: 587–593. [DOI] [PubMed] [Google Scholar]
- 48. Arbea L, Díaz-González JA, Subtil JC, et al. Patterns of response after preoperative intensity-modulated radiation therapy and capecitabine/oxaliplatin in rectal cancer: is there still a place for ecoendoscopic ultrasound? Int J Radiat Oncol Biol Phys 2011; 81: 439–444. [DOI] [PubMed] [Google Scholar]
- 49. De Ridder M, Tournel K, Van Nieuwenhove Y, et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2008; 70: 728–734. [DOI] [PubMed] [Google Scholar]
- 50. Picardi V, Macchia G, Guido A, et al. Preoperative chemoradiation with VMAT-SIB in rectal cancer: a phase II study. Clin Colorectal Cancer 2017; 16: 16–22. [DOI] [PubMed] [Google Scholar]
- 51. Lupattelli M, Matrone F, Gambacorta MA, et al. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with Capecitabine in locally advanced rectal cancer: short-term results of a multicentric study. Radiat Oncol 2017; 12: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alongi F, Fersino S, Mazzola R, et al. Radiation dose intensification in pre-operative chemo-radiotherapy for locally advanced rectal cancer. Clin Transl Oncol 2017; 19: 189–196. [DOI] [PubMed] [Google Scholar]
- 53. Joo JH, Park JH, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys 2017; 99: 876–883. [DOI] [PubMed] [Google Scholar]
- 54. Kinj R, Bondiau PY, Francois E, et al. Radiosensitivity of colon and rectal lung oligometastasis treated with stereotactic ablative radiotherapy. Clin Colorectal Cancer 2017; 16: e211–e220. [DOI] [PubMed] [Google Scholar]
- 55. Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015; 16: 979–989. [DOI] [PubMed] [Google Scholar]
- 56. Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008; 26: 3687–3694. [DOI] [PubMed] [Google Scholar]
- 57. Mohiuddin M, Paulus R, Mitchell E, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys 2013; 86: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014; 15: 184–190. [DOI] [PubMed] [Google Scholar]
- 59. Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014; 32: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mehta VK, Cho C, Ford JM, et al. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys 2003; 55: 132–137. [DOI] [PubMed] [Google Scholar]
- 61. Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012; 13: 679–687. [DOI] [PubMed] [Google Scholar]
- 62. Zhao L, Bai C, Shao Y, et al. A phase II study of neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for rectal cancer. Cancer Lett 2011; 310: 134–139. [DOI] [PubMed] [Google Scholar]
- 63. Fernández-Martos C, Aparicio J, Bosch C, et al. Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase II multicenter study with 3 years’ follow-up. J Clin Oncol 2004; 22: 3016–3022. [DOI] [PubMed] [Google Scholar]
- 64. Beppu N, Kobayashi M, Matsubara N, et al. Comparison of the pathological response of the mesorectal positive nodes between short-course chemoradiotherapy with delayed surgery and long-course chemoradiotherapy in patients with rectal cancer. Int J Colorectal Dis 2015; 30: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 65. Myerson RJ, Genovesi D, Lockett MA, et al. Five fractions of preoperative radiotherapy for selected cases of rectal carcinoma: long-term tumor control and tolerance to treatment. Int J Radiat Oncol Biol Phys 1999; 43: 537–543. [DOI] [PubMed] [Google Scholar]
- 66. Sato T, Ozawa H, Hatate K, et al. A phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys 2011; 79: 677–683. [DOI] [PubMed] [Google Scholar]
- 67. Read TE, McNevin MS, Gross EK, et al. Neoadjuvant therapy for adenocarcinoma of the rectum: tumor response and acute toxicity. Dis Colon Rectum 2001; 44: 513–522. [DOI] [PubMed] [Google Scholar]
- 68. Panagiotopoulou IG, Parashar D, Qasem E, et al. Neoadjuvant long-course chemoradiotherapy for rectal cancer: does time to surgery matter? Int Surg 2015; 100: 968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fucini C, Pucciani F, Elbetti C, et al. Preoperative radiochemotherapy in T3 operable low rectal cancers: a gold standard? World J Surg 2010; 34: 1609–1614. [DOI] [PubMed] [Google Scholar]
- 70. Guimas V, Boustani J, Schipman B, et al. Preoperative chemoradiotherapy for rectal cancer in patients aged 75 years and older: acute toxicity, compliance with treatment, and early results. Drugs Aging 2016; 33: 419–425. [DOI] [PubMed] [Google Scholar]
- 71. Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015; 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang CM, Huang MY, Tsai HL, et al. An observational study of extending FOLFOX chemotherapy, lengthening the interval between radiotherapy and surgery, and enhancing pathological complete response rates in rectal cancer patients following preoperative chemoradiotherapy. Therap Adv Gastroenterol 2016; 9: 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 1996; 348: 1605–1610. [PubMed] [Google Scholar]
- 74. Chan AK, Wong AO, Langevin J, et al. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: a phase II dose escalation study. Int J Radiat Oncol Biol Phys 2000; 48: 843–856. [DOI] [PubMed] [Google Scholar]
- 75. Kairevičė L, Latkauskas T, Tamelis A, et al. Preoperative long-course chemoradiotherapy plus adjuvant chemotherapy versus short-course radiotherapy without adjuvant chemotherapy both with delayed surgery for stage II-III resectable rectal cancer: 5-year survival data of a randomized controlled trial. Medicina (Kaunas) 2017; 53: 150–158. [DOI] [PubMed] [Google Scholar]
- 76. Taher AN, El-Baradie MM, Nasr AM, et al. Locally advanced rectal carcinoma: preoperative radiotherapy versus postoperative chemoradiation, 10-year follow-up results of a randomized clinical study. J Egypt Natl Canc Inst 2006; 18: 233–243. [PubMed] [Google Scholar]
- 77. Duncan W, Smith AN, Freedman LS, et al. The evaluation of low dose pre-operative X-ray therapy in the management of operable rectal cancer; results of a randomly controlled trial. Br J Surg 1984; 71: 21–25. [DOI] [PubMed] [Google Scholar]
- 78. Klenova A, Parvanova V, Georgiev R, et al. Preoperative radiotherapy in rectal cancer: treatment results of three different dose regimens. J BUON 2006; 11: 161–166. [PubMed] [Google Scholar]
- 79. Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys 2006; 64: 709–716. [DOI] [PubMed] [Google Scholar]
- 80. Boulis-Wassif S, Gerard A, Loygue J, et al. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone or in combination with 5-fluorouracil, followed by radical surgery. Trial of the European organization on research and treatment of cancer gastrointestinal tract cancer cooperative group. Cancer 1984; 53: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 81. Ciammella P, Ruggieri MP, Galeandro M, et al. Short-course preoperative radiotherapy combined with chemotherapy in resectable locally advanced rectal cancer: local control and quality of life. Radiol Med 2013; 118: 1397–1411. [DOI] [PubMed] [Google Scholar]
- 82. Pettersson D, Holm T, Iversen H, et al. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg 2012; 99: 577–583. [DOI] [PubMed] [Google Scholar]
- 83. Calvo FA, Morillo V, Santos M, et al. Interval between neoadjuvant treatment and definitive surgery in locally advanced rectal cancer: impact on response and oncologic outcomes. J Cancer Res Clin Oncol 2014; 140: 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Du D, Su Z, Wang D, et al. Optimal interval to surgery after neoadjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 2018; 17: 13–24. [DOI] [PubMed] [Google Scholar]
- 85. Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012; 99: 918–928. [DOI] [PubMed] [Google Scholar]
- 86. Foster JD, Jones EL, Falk S, et al. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum 2013; 56: 921–930. [DOI] [PubMed] [Google Scholar]
- 87. Stuyck C, Wegge M, Bulens P. Moderate dose escalation with volumetric modulated arc therapy improves outcome in rectal cancer. Acta Oncol 2017; 56: 1501–1506. [DOI] [PubMed] [Google Scholar]
- 88. Nguyen NP, Ceizyk M, Vock J, et al. Feasibility of image-guided radiotherapy for elderly patients with locally advanced rectal cancer. PLoS One 2013; 8: e71250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Freedman GM, Meropol NJ, Sigurdson ER, et al. Phase I trial of preoperative hypofractionated intensity-modulated radiotherapy with incorporated boost and oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007; 67: 1389–1393. [DOI] [PubMed] [Google Scholar]
- 90. Engels B, Tournel K, Everaert H, et al. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int J Radiat Oncol Biol Phys 2012; 83: 142–148. [DOI] [PubMed] [Google Scholar]
- 91. Ballonoff A, Kavanagh B, McCarter M, et al. Preoperative capecitabine and accelerated intensity-modulated radiotherapy in locally advanced rectal cancer: a phase II trial. Am J Clin Oncol 2008; 31: 264–270. [DOI] [PubMed] [Google Scholar]
- 92. But-Hadzic J, Velenik V. Preoperative intensity-modulated chemoradiation therapy with simultaneous integrated boost in rectal cancer: 2-year follow-up results of phase II study. Radiol Oncol 2018; 52: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yamashita H, Ishihara S, Nozawa H, et al. Comparison of volumetric-modulated arc therapy using simultaneous integrated boosts (SIB-VMAT) of 45 Gy/55 Gy in 25 fractions with conventional radiotherapy in preoperative chemoradiation for rectal cancers: a propensity score case-matched analysis. Radiat Oncol 2017; 12: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Markovina S, Youssef F, Roy A, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys 2017; 99: 417–426. [DOI] [PubMed] [Google Scholar]
- 95. Sun Z, Adam MA, Kim J, et al. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Am Coll Surg 2016; 222: 367–374. [DOI] [PubMed] [Google Scholar]
- 96. Zeng WG, Zhou ZX, Liang JW, et al. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol 2014; 110: 463–467. [DOI] [PubMed] [Google Scholar]
- 97. Shivnani AT, Small W, Jr, Stryker SJ, et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg 2007; 193: 389–393; discussion 393–394. [DOI] [PubMed] [Google Scholar]
- 98. Kim SY, Hong YS, Kim DY, et al. Preoperative chemoradiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: a multicenter phase II study. Int J Radiat Oncol Biol Phys 2011; 81: 677–683. [DOI] [PubMed] [Google Scholar]
- 99. Avallone A, Delrio P, Pecori B, et al. Oxaliplatin plus dual inhibition of thymidilate synthase during preoperative pelvic radiotherapy for locally advanced rectal carcinoma: long-term outcome. Int J Radiat Oncol Biol Phys 2011; 79: 670–676. [DOI] [PubMed] [Google Scholar]
- 100. Gunther JR, Chadha AS, Shin US, et al. Preoperative radiation dose escalation for rectal cancer using a concomitant boost strategy improves tumor downstaging without increasing toxicity: a matched-pair analysis. Adv Radiat Oncol 2017; 2: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Campos-Lobato LF, Geisler DP, Da Luz, Moreira A, et al. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011; 15: 444–450. [DOI] [PubMed] [Google Scholar]
- 102. Guckenberger M, Wulf J, Thalheimer A, et al. Prospective phase II study of preoperative short-course radiotherapy for rectal cancer with twice daily fractions of 2.9 Gy to a total dose of 29 Gy–long-term results. Radiat Oncol 2009; 4: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beppu N, Matsubara N, Noda M, et al. The timing of surgery after preoperative short-course S-1 chemoradiotherapy with delayed surgery for T3 lower rectal cancer. Int J Colorectal Dis 2014; 29: 1459–1466. [DOI] [PubMed] [Google Scholar]
- 104. Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017; 390: 469–479. [DOI] [PubMed] [Google Scholar]
- 105. Kaytan-Saglam E, Balik E, Saglam S. Delayed versus immediate surgery following short-course neoadjuvant radiotherapy in resectable (T3N0/N+) rectal cancer. J Cancer Res Clin Oncol 2017; 143: 1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bae BK, Kang MK, Kim JC, et al. Simultaneous integrated boost intensity-modulated radiotherapy versus 3-dimensional conformal radiotherapy in preoperative concurrent chemoradiotherapy for locally advanced rectal cancer. Radiat Oncol J 2017; 35: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang CM, Huang MY, Tsai HL, et al. A retrospective comparison of outcome and toxicity of preoperative image-guided intensity-modulated radiotherapy versus conventional pelvic radiotherapy for locally advanced rectal carcinoma. J Radiat Res 2017; 58: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Passoni P, Fiorino C, Slim N, et al. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys 2013; 87: 67–72. [DOI] [PubMed] [Google Scholar]
- 109. Marijnen CA. Organ preservation in rectal cancer: have all questions been answered? Lancet Oncol 2015; 16: e13–e22. [DOI] [PubMed] [Google Scholar]
- 110. Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844. [DOI] [PubMed] [Google Scholar]
- 111. Abdel-Rahman O, Kumar A, Kennecke HF, et al. Impact of duration of neoadjuvant radiation on rectal cancer survival: a real world multi-center retrospective cohort study. Clin Colorectal Cancer 2018; 17: e21–e28. [DOI] [PubMed] [Google Scholar]
- 112. Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III trial of radiotherapy regimens for rectal cancer. Br J Surg 2015; 102: 972–978; discussion 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010; 28: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 114. Engineer R, Mohandas KM, Shukla PJ, et al. Escalated radiation dose alone vs. concurrent chemoradiation for locally advanced and unresectable rectal cancers: results from phase II randomized study. Int J Colorectal Dis 2013; 28: 959–966. [DOI] [PubMed] [Google Scholar]
- 115. Mohiuddin M, Regine WF, John WJ, et al. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys 2000; 46: 883–888. [DOI] [PubMed] [Google Scholar]
- 116. Valentini V, Coco C, Cellini N, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys 2001; 51: 371–383. [DOI] [PubMed] [Google Scholar]
- 117. Appelt AL, Pløen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013; 85: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rombouts AJM, Hugen N, Verhoeven RHA, et al. Tumor response after long interval comparing 5x5Gy radiation therapy with chemoradiation therapy in rectal cancer patients. Eur J Surg Oncol 2018; 44: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 119. Díaz-González JA, Calvo FA, Cortés J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys 2006; 64: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 120. Chuong MD, Fernandez DC, Shridhar R, et al. High-dose-rate endorectal brachytherapy for locally advanced rectal cancer in previously irradiated patients. Brachytherapy 2013; 12: 457–462. [DOI] [PubMed] [Google Scholar]
- 121. Buckley H, Wilson C, Ajithkumar T. High-dose-rate brachytherapy in the management of operable rectal cancer: a systematic review. Int J Radiat Oncol Biol Phys 2017; 99: 111–127. [DOI] [PubMed] [Google Scholar]
- 122. Dhadda AS, Zaitoun AM, Bessell EM. Regression of rectal cancer with radiotherapy with or without concurrent capecitabine–optimising the timing of surgical resection. Clin Oncol (R Coll Radiol) 2009; 21: 23–31. [DOI] [PubMed] [Google Scholar]
- 123. Kuan FC, Lai CH, Ku HY, et al. The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer 2017; 140: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 124. Akgun E, Caliskan C, Bozbiyik O, et al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2018; 105: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 125. Couwenberg AM, Intven MPW, Hoendervangers S, et al. The effect of time interval from chemoradiation to surgery on postoperative complications in patients with rectal cancer. Eur J Surg Oncol 2019; 45: 1584–1591. [DOI] [PubMed] [Google Scholar]
- 126. Macchia G, Gambacorta MA, Masciocchi C, et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: a population study on 2094 patients. Clin Transl Radiat Oncol 2017; 4: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kwak YK, Kim K, Lee JH, et al. Timely tumor response analysis after preoperative chemoradiotherapy and curative surgery in locally advanced rectal cancer: a multi-institutional study for optimal surgical timing in rectal cancer. Radiother Oncol 2016; 119: 512–518. [DOI] [PubMed] [Google Scholar]
- 128. Sloothaak DA, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013; 100: 933–939. [DOI] [PubMed] [Google Scholar]
- 129. Lefevre JH, Mineur L, Kotti S, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 2016; 34: 3773–3780. [DOI] [PubMed] [Google Scholar]
- 130. Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999; 17: 2396–2402. [DOI] [PubMed] [Google Scholar]
- 131. Stein DE, Mahmoud NN, Anné PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum 2003; 46: 448–453. [DOI] [PubMed] [Google Scholar]
- 132. Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004; 47: 279–286. [DOI] [PubMed] [Google Scholar]
- 133. Tran CL, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006; 192: 873–877. [DOI] [PubMed] [Google Scholar]
- 134. Veenhof AA, Kropman RH, Engel AF, et al. Preoperative radiation therapy for locally advanced rectal cancer: a comparison between two different time intervals to surgery. Int J Colorectal Dis 2007; 22: 507–513. [DOI] [PubMed] [Google Scholar]
- 135. Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008; 248: 243–251. [DOI] [PubMed] [Google Scholar]
- 136. Tulchinsky H, Shmueli E, Figer A, et al. An interval > 7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008; 15: 2661–2667 [DOI] [PubMed] [Google Scholar]
- 137. Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011; 254 :97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012; 19: 2833–2841. [DOI] [PubMed] [Google Scholar]
- 139. Jeong DH, Lee HB, Hur H, et al. Optimal timing of surgery after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Korean Surg Soc 2013; 84:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. You KY, Huang R, Zhang LN, et al. Tailored selection of the interval between neoadjuvant chemoradiotherapy and Surgery for locally advanced rectal cancer: analysis based on the pathologic stage or chemoradiation response. J Cancer Res Clin Oncol 2015; 141:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mihmanli M, Kabul Gurbulak E, Akgun IE, et al. Delaying surgery after neoadjuvant chemoradiotherapy improves prognosis of rectal cancer. World J Gastrointest Oncol 2016; 8: 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Akbar A, Bhatti AB, Niazi SK, et al. Impact of time interval between chemoradiation and surgery on pathological complete response and survival in rectal cancer. Asian Pac J Cancer Prev 2016; 17: 89–93. [DOI] [PubMed] [Google Scholar]
- 143. Van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12: 575–582. [DOI] [PubMed] [Google Scholar]
- 144. Keskin M, Bayraktar A, Sivirikoz E, et al. Sparing sphincters and laparoscopic resection improve survival by optimizing the circumferential resection margin in rectal cancer patients. Medicine (Baltimore) 2016; 95: e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Patel A, Green N, Sarmah P, et al. The clinical significance of a pathologically positive lymph node at the circumferential resection margin in rectal cancer. Tech Coloproctol 2019; 23: 151–159. [DOI] [PubMed] [Google Scholar]
- 146. Wong RK, Tandan V, De Silva S, et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev 2007; 2: CD002102. [DOI] [PubMed] [Google Scholar]
- 147. Petrelli F, Sgroi G, Sarti E, et al. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg 2016; 263: 458–464. [DOI] [PubMed] [Google Scholar]
- 148. Glimelius B, Isacsson U, Jung B, et al. Radiotherapy in addition to radical surgery in rectal cancer: evidence for a dose-response effect favoring preoperative treatment. Int J Radiat Oncol Biol Phys 1997; 37: 281–287. [DOI] [PubMed] [Google Scholar]
- 149. Suwinski R, Wzietek I, Tarnawski R, et al. Moderately low alpha/beta ratio for rectal cancer may best explain the outcome of three fractionation schedules of preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2007; 69: 793–799. [DOI] [PubMed] [Google Scholar]
- 150. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. Epub ahead of print 15 July 2019. DOI: 10.1097/SLA.0000000000003471. [DOI] [PubMed] [Google Scholar]
- 151. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 152. Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015; 16: 200–207. [DOI] [PubMed] [Google Scholar]
- 153. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 154. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer. Version 3, https://nccn.org. (2017, accessed 1 June 2017). [DOI] [PubMed]
- 155. Jin F, Luo HL, Zhou J, et al. Cancer risk assessment in modern radiotherapy workflow with medical big data. Cancer Manag Res 2018; 10: 1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tiv M, Puyraveau M, Mineur L, et al. Long-term quality of life in patients with rectal cancer treated with preoperative (chemo)-radiotherapy within a randomized trial. Cancer Radiother 2010; 14: 530–534. [DOI] [PubMed] [Google Scholar]
- 157. Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg 2011; 253: 71–77. [DOI] [PubMed] [Google Scholar]
- 158. Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol 2005; 23: 6199–6206. [DOI] [PubMed] [Google Scholar]
- 159. Herman JM, Narang AK, Griffith KA, et al. The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2013; 85: e15–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Huang M, Lin J, Yu X, et al. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int J Colorectal Dis 2016; 31: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 161. Bruheim K, Guren MG, Dahl AA, et al. Sexual function in males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010; 76: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 162. Contin P, Kulu Y, Bruckner T, et al. Comparative analysis of late functional outcome following preoperative radiation therapy or chemoradiotherapy and surgery or surgery alone in rectal cancer. Int J Colorectal Dis 2014; 29: 165–175. [DOI] [PubMed] [Google Scholar]
- 163. Hirata Y, Nozawa H, Kawai K, et al. The influence of neoadjuvant chemoradiation for lower rectal cancer on urinary function. Asian J Surg 2019; 42: 731–739. [DOI] [PubMed] [Google Scholar]
- 164. Van der Sande ME, Hupkens BJP, Berbee M, et al. Impact of radiotherapy on anorectal function in patients with rectal cancer following a watch and wait programme. Radiother Oncol 2019; 132: 79–84. [DOI] [PubMed] [Google Scholar]
- 165. Badakhshi H, Ismail M, Boskos C, et al. The role of concomitant radiation boost in neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Anticancer Res 2017; 37: 3201–3205. [DOI] [PubMed] [Google Scholar]
- 166. Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009; 2: CD006041. [DOI] [PubMed] [Google Scholar]
- 167. De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2013; 2: CD006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Fiorica F, Cartei F, Licata A, et al. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat Rev 2010; 36: 539–549. [DOI] [PubMed] [Google Scholar]
- 169. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341: 457–460. [DOI] [PubMed] [Google Scholar]
- 170. Ng SY, Colborn KL, Cambridge L, et al. Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer. Radiother Oncol 2016; 121: 252–257. [DOI] [PubMed] [Google Scholar]
- 171. Dröge LH, Weber HE, Guhlich M, et al. Reduced toxicity in the treatment of locally advanced rectal cancer: a comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer 2015; 15: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. De Bari B, Franzetti-Pellanda A, Saidi A, et al. Neoadjuvant chemoradiotherapy delivered with helical tomotherapy under daily image guidance for rectal cancer patients: efficacy and safety in a large, multi-institutional series. J Cancer Res Clin Oncol 2019; 145: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jabbour SK, Patel S, Herman JM, et al. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol 2012; 2012: 891067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Engels B, Platteaux N, Van den Begin R, et al. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol 2014; 110: 155–159. [DOI] [PubMed] [Google Scholar]
- 175. Kaplan SO, Akbörü H, Tabak SD, et al. Hypofractionated preoperative chemoradiotherapy in locally advanced rectal cancer: preliminary results. Turk J Oncol 2019; 34: 21–26. [Google Scholar]
- 176. Sun Z, Adam MA, Kim J, et al. Intensity-modulated radiation therapy is not associated with perioperative or survival benefit over 3D-conformal radiotherapy for rectal cancer. J Gastrointest Surg 2017; 21: 106–111. [DOI] [PubMed] [Google Scholar]
- 177. Hong TS, Moughan J, Garofalo MC, et al. NRG oncology radiation therapy oncology group 0822: a phase 2 study of preoperative chemoradiation therapy using intensity modulated radiation therapy in combination with capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2015; 93: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chang H, Jiang W, Ye WJ, et al. Is long interval from neoadjuvant chemoradiotherapy to surgery optimal for rectal cancer in the era of intensity-modulated radiotherapy? A prospective observational study. Onco Targets Ther 2018; 11: 6129–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yamada S, Kamada T, Ebner DK, et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int J Radiat Oncol Biol Phys 2016; 96: 93–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure for Dose–time fractionation schedules of preoperative radiotherapy and timing to surgery for rectal cancer by Fu Jin, Huanli Luo, Juan Zhou, Yongzhong Wu, Hao Sun, Hongliang Liu, Xiaodong Zheng and Ying Wang in Therapeutic Advances in Medical Oncology