Abstract
Pulmonary artery aneurysm is a rare but important entity in the spectrum of pulmonary vascular diseases. The etiologies can be varied and patients can present with non-specific symptoms with the diagnosis being incidental. There is limited consensus regarding the diagnostic criteria and follow-up imaging for patients diagnosed with this entity. Further the management strategies can be variable depending upon underlying disease, etiology, center dependent expertise, and resources available. We review the etiologies, epidemiology, classification, clinical manifestations, and imaging features of pulmonary artery aneurysm. We also review the current management strategies and suggest an algorithmic approach to these patients.
Keywords: congenital cardiovascular malformations, pulmonary arterial hypertension, vasculitis, pseudoaneurysm
Introduction
Pulmonary artery aneurysm (PAA) is a rare abnormality of pulmonary vasculature. Its incidence has been estimated to be 1 in 14,000 by most studies based on the findings from 109,571 autopsies conducted at the Mayo clinic.1 These aneurysms can arise secondary to various etiologies such as infection, malignancy, congenital heart disease, or vasculitis. Frequently, there is a complex interplay of multiple factors that lead to PAA formation. These aneurysms can frequently be asymptomatic and are incidentally diagnosed on imaging performed for other reasons. But they are also known to cause life-threatening complications such as massive hemoptysis from rupture, coronary artery compression leading to acute coronary syndrome, and pulmonary artery dissection. The objectives of this article are to review current literature regarding the manifestations and management strategies of PAA.
Materials and methods
A PubMed search was conducted for the words “pulmonary artery aneurysm,” and 930 search results were obtained ranging from January 2010 to May 2018. Abstracts of case reports and case series were reviewed for PAA. An online language translation tool (Google translate) was used to translate abstracts and full text of articles to English wherever articles were published in a different language. From 254 articles that were reviewed, we identified 260 cases, of which 6 articles were excluded, as relevant case information was not available. For three cases, no abstract or full text could be obtained, one abstract was in Japanese and English translation could not be extracted from the journal website. In two other cases, further workup was not reported beyond identifying PAA on initial imaging. A total of 248 cases were included in the final literature review. The classification and demographics of the patients based on review of literature is noted in Table 1 and 2.
Table 1.
Classification of PAA.
| Acquired |
||
|---|---|---|
| Congenital | True aneurysms | Pseudoaneurysms |
| Increased pulmonary blood flow (Eisenmenger’s syndrome) | Pulmonary arterial hypertension related | Post infectious |
| Pulmonary valvular abnormalities | Lung conditions other than pulmonary hypertension | Malignancy related |
| Connective tissue abnormalities | Vasculitis | Iatrogenic |
| Idiopathic | Traumatic | |
Table 2.
Classification and demographics of PAA.
| Etiology | Age distribution (years) | Common symptoms | Location | Size (mm) | Treatment |
|---|---|---|---|---|---|
| Congenital | |||||
| Eisenmenger’s | 39.8 (birth to 68) | Shortness of breath Hemoptysis Chest pain Cyanosis | 92% central 8% peripheral | 64 (7.5–140) | Pulmonary artery ligation Pulmonary artery banding Lobectomy |
| Pulmonary valvular abnormalities | 50 (birth to 79) | Asymptomatic Shortness of breath Anginal symptoms | 100% proximal | 51 (21.3–79) | Aneurysm reduction plasty |
| Other connective tissue abnormalities | |||||
| Acquired | |||||
| PAH | 50 (19–83) | Symptoms of progressive PAH Mass effect on surrounding structures | 91% proximal | 69 (34.7–120) | PAH pharmacological therapy Graft repair Lung transplant evaluation |
| Autoimmune disease and vasculitis | 34 (12–81) | Hemoptysis Fever Cough Pleuritic chest pain Shortness of breath | Multiple bilateral | 53 (8–100) | Steroids Immunosuppressants |
| Idiopathic | 54.4 (pediatric age group to 84) | Asymptomatic Hemoptysis Shortness of breath | 64% proximal | 50.6 (10–100) | Surgical resection Graft repair Coiling AMPLATZER plug |
| Iatrogenic | 63 (28–82) | Hemoptysis | 100% distal | 43.2 (9–140) | Coil embolization AMPLATZER plug |
| Infectious | 38.5 (4 month to 66 years) | Hemoptysis Fever Cough | 100% distal | 32.7 (7–96) | Embolization Vascular plug Lobectomy |
| Malignancy | 57.5 (17–80) | Hemoptysis Cough | Mostly distal | 29.2 (14–66) | Coil embolization Surgical repair Localized resection |
PAH: pulmonary arterial hypertension.
Definitions
A true PAA can be described as focal dilatation of all the three layers of pulmonary artery. Pseudoaneurysms, on the contrary, do not involve all the three layers of vessel walls. This differentiation between true and false aneurysms is important, as pseudoaneurysms have a higher risk of bleeding due to the presence of a more friable aneurysmal wall and therefore require more aggressive management.
There have been no standard diameter size parameters that define PAA. Mean diameter for main pulmonary artery in normal healthy adults is considered to be 25 mm ± 3 mm, with 29 mm as upper limit of normal in males and 27 mm as upper limit of normal in females.2 Some authors consider any dilatation of PA beyond this normal limit as PAA, while others consider a higher diameter cutoff in defining PAA. Most commonly, PAA is defined as dilatation greater than 1.5 times upper limit of normal, which is equivalent to 43 mm in males and 40 mm in females. In most of the studies, a gender-based cutoff was not followed. Duijnhouwer et al. considered all cases with PA diameter greater than 40 mm as a PAA.3 Restrepo and Carswell recommended main PA diameter greater than 45 mm and branch PA diameter greater than 30 mm to be considered PAA.4
The location of the aneurysm is also important. Proximal PAA is used to describe aneurysms involving large pulmonary arteries such as main and/or branch pulmonary arteries. Peripheral PAA is used to describe aneurysms involving distal vasculature.
Etiologies
Congenital
Historically congenital causes compromised approximately half of the reported cases of PAA.1 The advent of routine use of computed tomography (CT) of the chest has changed that landscape. In our review, congenital causes compromised 25% (n = 63) of the total cases reported. Two of these cases were diagnosed on prenatal ultrasound, 5 at birth, 5 during infancy, 16 were in the pediatric age group, and 35 were adults. For two cases, age at presentation was not available. Among adults, mean age of presentation was 45 years, with median age of 44 years (range: 19–79 years). For the purpose of this review, congenital etiologies has been further subclassified into three groups – congenital heart diseases with increased blood flow to the pulmonary circulation (Eisenmenger’s syndrome), pulmonary valvular abnormalities, and related to connective tissue abnormalities.
Eisenmenger’s syndrome
Eisenmenger’s syndrome was described by Paul Wood as “pulmonary hypertension at the systemic level due to a high pulmonary vascular resistance, with reversed or bidirectional shunting through a large ventricular septal defect (VSD).”5 Over the years, the definition of this term has been broadened to include other forms of congenital heart diseases with left to right shunting and increased blood flow to pulmonary circulation. Historically, Eisenmenger’s syndrome has been most common cause of PAA. Hemodynamic stress on vessel wall from increased blood flow is postulated to be the mechanism.6
We identified 41 cases of PAA due to Eisenmenger’s syndrome in our study. In our review, the most common cause was patent ductus arteriosus (PDA) (n = 8), followed by tetralogy of Fallot (TOF) (n = 6), atrial septal defect (ASD) (n = 5), Loeys–Dietz syndrome (n = 5), VSD (n = 4), and Transposition of great arteries (TGA) (n = 2). Cor triatriatum sinister caused PAA in one patient.7 In one case report, multiple congenital heart abnormalities were co-existent and were causing severe pulmonary arterial hypertension (PAH).8 In some instances, PAA can develop because of increased blood flow through pulmonary vasculature after surgical correction of congenital heart disease. Pulmonary artery ligation for correction of univentricular heart,9 arterial switch operation for D-TGA,10 Modified Blalock–Taussig Shunt,11,12 pulmonary homograft from truncus arteriosus,13 PDA ligation,14 Senning procedure for TGA,15 and PA banding for VSD16 were found to have resulted in PAA formation. PAA was also seen as a part of symptom complex of genetic disorders such as Loeys–Dietz syndrome and Noonan syndrome.17
Among adult patients, the mean age of presentation was 39.8 years (range: 22–68 years). Information on the presenting manifestations was available for 35 out of 41 of these patients. The most common presentation was shortness of breath in 19 patients, with 2 out of these being ventilator dependent.10,18 Hemoptysis was present in six, chest pain in five, and cyanosis in four patients. On rare occasions, patients presented with symptoms secondary to mechanical compression on surrounding structures. Four patients had mainstem bronchus compression, one had superior vena cava (SVC) compression,19 one had esophageal compression,20 and in one case the patient presented with chest pain related to left main coronary artery compression.21 Two patients were asymptomatic and were incidentally found to have PAA on imaging. Location of the aneurysm was described in 39 out of 41 cases, 36 out of these involved central vasculatures, and 3 involved the peripheral vasculature. Size of the aneurysm was available in 24 cases with a mean of 64 mm (range: 7.5–140 mm).
Management of these patients included various surgical interventions. Twenty-three of these patients underwent definitive surgical procedures for correction of the underlying cardiac anomaly and did not require repeat surgical intervention. AMPLATZER device was used to control hemoptysis in one case.22 In four cases, pulmonary artery ligation or plication was performed. In a neonate with Loeys–Dietz syndrome, bilateral pulmonary banding with clip was attempted for control of right pulmonary artery hemorrhage; however, the patient developed fatal thoracic bleeding 12 days postoperatively.23 In three other cases, pulmonary artery ligation was performed, with two patients developing progression of the aneurysm and required pulmonary artery repair and in one case lobectomy was necessary.
Five of these cases were complicated with pulmonary artery dissection, with dissection being the presenting manifestation in four cases. PA dissection was successfully repaired in two cases of these,24,25 while in the other three, it proved to be fatal.
Pulmonary valvular abnormalities
Pulmonary valve abnormalities related to PAA can include pulmonary valvular and subvalvular stenosis, structural abnormalities of the pulmonary valve, and absent pulmonary valve syndrome. It has been postulated that abnormal blood flow through the pulmonary valve can cause strain on the vessel wall through eccentric right ventricle outflow jet and leads to weakness of vessel wall. Such findings have been demonstrated in bicuspid pulmonic valve patients through four-dimensional cardiac magnetic resonance imaging.26
In our review, we identified 19 patients in which pulmonary valvular abnormalities was the primary etiology of PAA. Patients with pulmonary annulus dilatation with pulmonary valvular regurgitation from PAH were not considered in this category. One patient was diagnosed with absent pulmonary valve syndrome at birth. Other than this patient, there were 4 pediatric cases and 14 cases among adult patients. Median age at presentation was 50 years with cases being reported up to age of 79 years. Gender distribution was equal with 9 males and 10 female patients. Eleven patients were asymptomatic on first presentation, while others most commonly presented with dyspnea (n = 6) or exercise intolerance (n = 2). Some also presented with chest pain or angina-like symptoms. In rare cases, PAA also caused left main and/or left anterior descending coronary artery aneurysm.27,28 All these aneurysms were localized to the main pulmonary artery or proximal branch pulmonary arteries. The average size of PAA that underwent surgical repair was 59 mm (range: 42–70 mm). Among the patients who were symptomatic, average size of aneurysm at the time of surgical repair was 62 mm (range: 51–70 mm). Patients under 18 years of age were not included when evaluating average aneurysm sizes, as there is a different reference range for normal PA diameter in pediatric patients. Two asymptomatic cases were taken for surgical repair at initial presentation. Among the patients where PAA was asymptomatic, nine patients were managed conservatively but four out of these needed surgical repairs due to progression in the size of aneurysm. Three patients had surgical repair on first presentation. Among the cases where PAA was symptomatic (n = 7), six patients had aneurysm repair on initial presentation, while one patient had aneurysm reduction plasty with valve repair nine years after initial diagnosis.29
Connective tissue abnormalities
A review of the current literature revealed three cases of PAA associated with Marfan’s syndrome,30 α1 antitrypsin deficiency,31 and tuberous sclerosis.32 Ehler–Danlos syndrome is also known to cause PAA, but we did not identify any reported cases during our extensive literature review.33
Acquired
True aneurysms
Pulmonary hypertension
PAAs are often associated with PAH. According to Laplace’s law, wall tension is directly related to pressure. Wall tension increases in proportion to pulmonary arterial pressures, leading to the formation of PAA in patients with PAH. In a review of published cases, 35 were identified related to PAH. Mean age at presentation was 50.8 years (range: 19–83 years), 15 of these were males and 18 females. In two cases, gender of patient was not reported. Occasionally, these can be completely asymptomatic and are incidentally discovered on imaging done for some other reason. Most of the cases present with symptoms related to primary pulmonary disease or progressive PAH. Larger aneurysms were again noted to cause airway34–36 or coronary artery compression.37,38 In about 91% of the cases, the aneurysm was localized to main pulmonary artery, and two cases involved the distal vasculature. One patient presented with multiple bilateral pulmonary artery stenosis with post stenotic dilatation.39 Mean aneurysm diameter among 27 cases of proximal aneurysms was 69 mm (range: 34.7–120 mm).
Most of these patients were managed conservatively with pharmacological treatment toward PAH and its underlying etiology. Three patients underwent graft repair of PAA, while two patients underwent lung transplant. In four cases of coronary artery compression, stenting of the coronary vessel was performed,37,38,40,41 while in a case with coronary artery dissection42 medical management with beta-blockers and antiplatelets occurred. Percutaneous embolization was performed for a peripheral aneurysm in a patient with scleroderma, wherein PAA was incidentally diagnosed on a CT scan done as a part of pre heart–lung transplant workup.43
Pulmonary arterial dissection is the most dreaded complication of PAA. Five patients had dissection on presentation, while in two cases of dissection developed in patients with known PAA that was being managed medically. Of these patients, four died secondary to pulmonary arterial dissection, whereas in those who survived, one underwent surgical repair and two were managed medically.
Autoimmune disease and vasculitis
Behcet’s disease and Hughes–Stovin syndrome (HSS) contribute to the greatest number of cases of PAA in this category. Of those patients with Behcet’s disease and HSS, the incidence at which they develop PAA is not clearly known. In a single-center study involving 2179 patients, 1.1% patient were diagnosed with PAA.44,45
In our review of the literature, we identified 46 cases related to autoimmune causes. Thirty-one patients carried the diagnosis of Behcet’s, 10 with HSS, and 3 presented with an overlapping picture. Cardiac sarcoidosis46 and giant cell arteritis47 were also associated with one case each of PAA. Mean age at presentation was 34 years old (range: 12–81 years) and is much younger compared to other groups. There was male predominance with a male to female ratio of 3:1. Hemoptysis was most common presentation and occurred in about 76% of the cases. Others presented with fever, cough, pleuritic chest pain, dyspnea, or respiratory failure. One patient had incidental diagnosis of PAA, while undergoing workup for hematuria from internal iliac aneurysm.48 These aneurysms were multiple and in bilateral distribution in most cases. Size of these aneurysms ranged from 8 mm to 10 cm in diameter (mean: 53.31 mm). Aneurysms associated with a vasculitic process almost always involve distal pulmonary circulation.
Patients with vasculitis responded well to steroids and other immunosuppressant treatments. Anti-tumor necrosis factor agents such as infliximab49 and adalimumab50 have been used successfully in cases where patients did not respond to first-line treatment or developed PAA while on immunosuppressant agents. Immunosuppressant treatment alone has been successful in the treatment of large aneurysm.51 In a case of Behcet’s disease with a large 6.4 × 6.7 cm aneurysm, complete thrombosis was achieved within 18 days of starting pulse steroids and cyclophosphamide.52 In people with Behcet’s disease, right ventricular thrombus can also be simultaneously present in rare cases requiring extended periods of anticoagulation in addition to immunosuppressants.53
Rupture of these aneurysms can lead to a life-threatening hemorrhage. In nine cases, embolization was performed, six needed lobectomy, and in two cases, pneumonectomy was done. AMPLATZER plug was also used in two cases for the control of bleeding from ruptured aneurysm. In two cases, hemoptysis was fatal.
Idiopathic
Idiopathic dilatation of pulmonary artery was first reported by Wessler and Jaches in 1923.54 It has been postulated that the etiology is secondary to a congenital weakness of the vessel wall55 and/or cystic medial degeneration56 of vessel wall.
Forty-one idiopathic cases of PAA were identified in our review. Average age at presentation was 54 years, but it has been described in pediatric age group as well. Both genders are fairly equally affected. In current review, there were 21 male and 17 female patients with idiopathic PAA. Idiopathic PAA can often be completely asymptomatic and appear as pulmonary nodule on chest radiograph.57 When symptomatic, hemoptysis, dyspnea, and chest pain are most common symptoms. On rare instances, idiopathic PAA presented with hemothorax58 or recurrent laryngeal nerve palsy (Ortner’s syndrome).59 In one case, PAA was compressing the SVC; however, the patient did not develop signs of SVC syndrome.60 In one patient with chronic dissection, left main coronary artery stenting was performed, as the progressively enlarging PAA was compressing a coronary vessel.61 Out of 41 idiopathic PAA cases in current review, 25 were proximal and 14 were distal aneurysms. Average size of proximal aneurysms was 59 mm (range: 28–95 mm) compared to average size of 38 mm for distal aneurysms (range: 10–100 mm).
Proximal aneurysms are often conservatively treated. As their size increases, surgical resection or graft repair is considered. In the literature where details of treatment were available, surgical repair was done for 10 out of 20 cases. Among the cases with distal aneurysms, stenting, coiling, AMPLATZER plug, and open surgical repair were all used in managing patients. Four cases underwent lobectomy and video-assisted thoracic surgery wedge resection.
Pseudoaneurysms
Iatrogenic and traumatic
PAA can occur as a complication from various procedures. These are mostly pseudoaneurysms resulting from disruption of the integrity of the vessel wall with extravasation of blood that can be contained within the wall or result in massive hemoptysis. Out of 22 cases in this category, 18 cases of PAA were associated with various procedures, and 4 other others were associated with trauma. Swan–Ganz catheter placement was the most common procedure leading to PAA formation with 10 reported cases. Mean age at presentation was 63 years (range: 28–82 years). Mean aneurysm size among these cases was 43 mm (range: 9–140 mm).
Out of these, four cases were managed with coil embolization, four with AMPLATZER vascular plug, and in one case, hemoptysis was fatal. PAAs were also found to be formed post lung resection procedures,62 cardiac catheterization,63 complicating pulmonary homograft,64 and from embolization of sclerosing material from porto-systemic shunts.65
Among those cases associated with trauma, three cases occurred after gunshot wounds, and one case, which was secondary to multiple rib, fractures after a fall injury.66
Infectious
Both true aneurysms and pseudoaneurysms can occur in relation to infection. More virulent infections like staphylococcus cause damage to all three layers of vessel wall and lead to true aneurysm formation. More commonly, other less virulent infections typically cause pseudoaneurysms.4
In our review, a total of 32 cases were identified to be secondary to infection. Historically, untreated syphilis and tuberculosis have been leading causes for PAA formation.67 With changes in treatment modalities and disease epidemiology, bacterial and fungal infections are now responsible for increasing number of cases. Interestingly, in our review of the literature, none of the cases were attributed to syphilis. Tuberculosis is still responsible for the majority of cases in this category. Pseudoaneurysms associated with tuberculosis are commonly referred to as Rasmussen aneurysms. They usually involve the upper lobes in a setting of reactivation tuberculosis.68 In this review, Rasmussen aneurysms were responsible for more than one-third of cases. Bacterial infections, such as infective endocarditis, bacterial pneumonia, lung abscess, and staphylococcus bacteremia resulted in another 12 cases. Fungal infections like candida,69 mucormycosis,70 aspergillus, and parasitic infections like schistosomiasis71 were responsible for other cases. Intravenous drug abuse also led to PAA formation with some cases related to staphylococcus bacteremia.72 Mean aneurysm size in these patients was 32 mm (range: 7–96 mm).
Infections can either cause direct invasion of vessel wall or embolization of vasa vasorum from bacteremia and septic emboli. Hemoptysis was again the most common presentation. Other presentations included pyrexia, sepsis,73 and hemothorax.74 Rupture of these pseudoaneurysms can often lead to massive hemoptysis, necessitating emergent embolization, vascular plug placement, and lobectomy. Despite these aggressive measures, hemoptysis in this setting can prove fatal.75
Malignancy
PAA related to lung cancer and other malignancies are a rare occurrence. Fifteen cases were identified in the current literature search. Ten of these were male and five were female with a mean age at presentation of 57.5 years (range: 17–80 years). These cases usually involve distal pulmonary vasculature and develop as pseudoaneurysms. The size of these aneurysms ranged from 14 to 66 mm (mean: 29 mm). Squamous cell carcinoma, non-small-cell lung cancer, sarcoma, capillary hemangioma, atrial myxoma, and malignant histiocytoma were found to be associated with PAA. Pathophysiology of PAA in these cases can vary from local invasion, tumor emboli,76 or secondary to treatments for the malignancy itself. In three cases, PAA was found to occur after radio frequency ablation procedures77 and presented with hemothorax. The majority of patients presented with hemoptysis. Based on location of aneurysm and severity of hemoptysis, they were treated with coil embolization, surgical repair, or localized resection of lung tissue. Figures 1-4 demonstrate various cases of PAA with varied etiologies.
Management algorithm
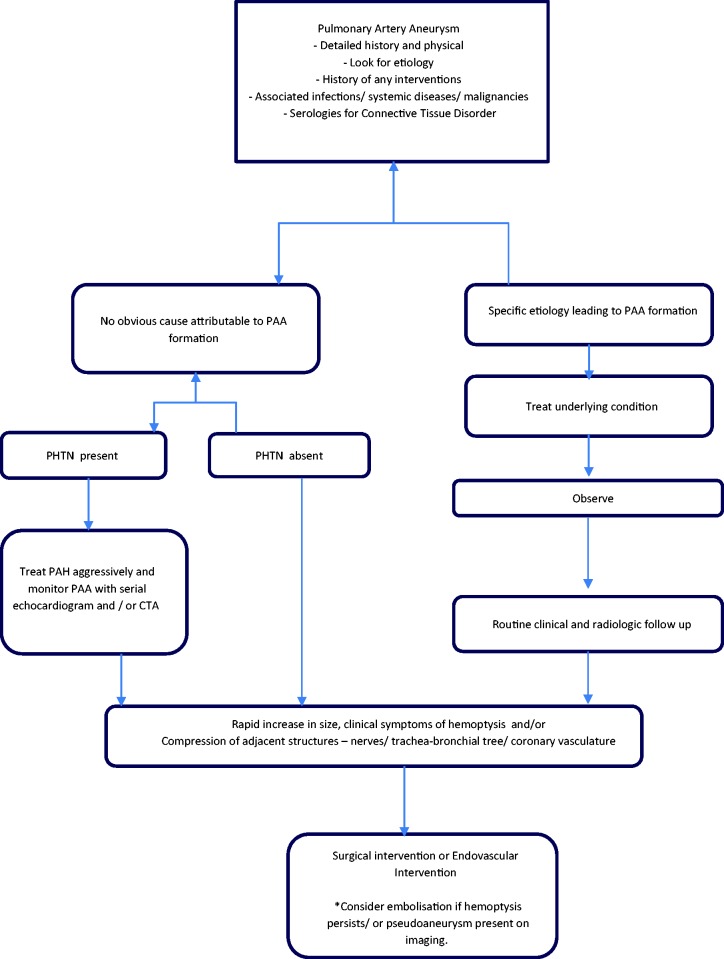
The management strategies for PAA are variable and are based on the underlying etiology, hemodynamics, and associated co-morbidities. The treatment must be tailored to the underlying cause while choosing the least invasive approach with durable results. Conservative treatment options include medical management aimed at the underlying disease, management of PH, as well as routine radiographic follow-up of the PAA. Surgical techniques such as aneurysmorrhaphy, lobectomy, bilobectomy, aneurysmectomy, and pneumonectomy have been described in literature but carry a high morbidity and mortality risk, especially in patients with pulmonary hypertension. The advent of endovascular interventions such as coil embolization and vascular plugs has certainly added to our armamentarium, but lack specific guidelines regarding their use. The experience-based management algorithm used in these patients in our center is described in Fig. 5.
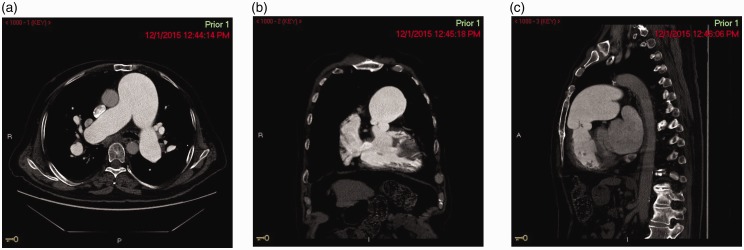
Fig. 1.
(a) CT Angiography of the chest showing pulmonary artery aneurysm – axial cut. (b) CT Angiography of the chest showing pulmonary artery aneurysm – coronal cut. (c) CT Angiography of the chest showing pulmonary artery aneurysm – sagittal cut.
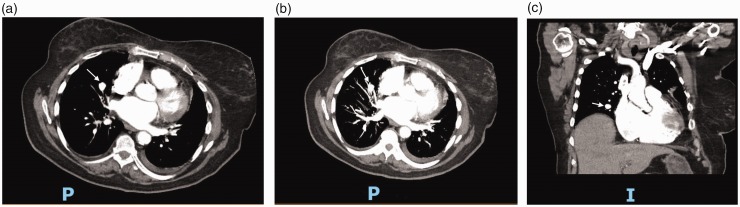
Fig. 2.
(a) CT Angiography of the chest showing an iatrogenic pulmonary artery aneurysm (white arrow) due to Swan–Ganz catheter placement – axial cut. (b) CT Angiography of the chest showing an iatrogenic pulmonary artery aneurysm (white arrow) due to Swan–Ganz catheter placement – axial cut. (c) CT Angiography of the chest showing an iatrogenic pulmonary artery aneurysm (white arrow) due to Swan–Ganz catheter placement – coronal cut.
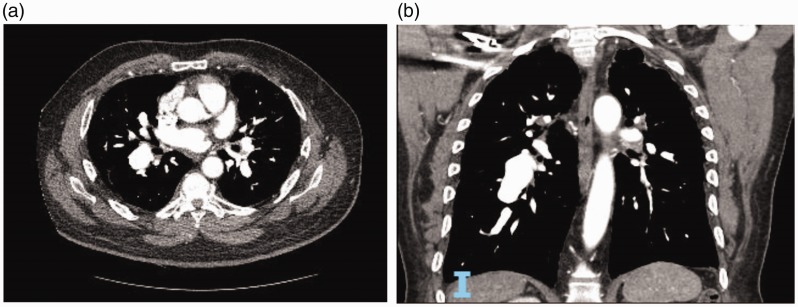
Fig. 3.
(a) CT Angiography of the chest showing pulmonary artery aneurysm on the right side in a patient with pulmonary arterial hypertension – axial cut. (b) CT Angiography of the chest showing pulmonary artery aneurysm on the right side in a patient with pulmonary arterial hypertension – coronal cut.
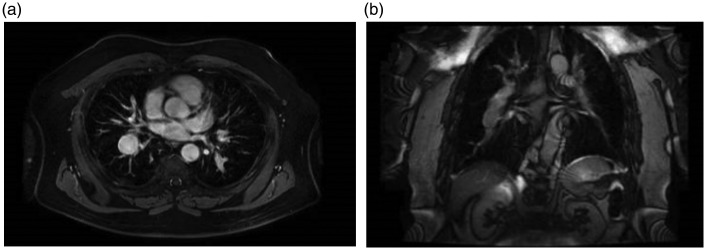
Fig. 4.
(a) Cardiac MRI showing pulmonary artery aneurysm on the right side in a patient with pulmonary arterial hypertension – axial cut. (b) Cardiac MRI showing pulmonary artery aneurysm on the right side in a patient with pulmonary arterial hypertension – coronal cut.
Fig. 5.
An experience-based institutional management algorithm in patients with PAA. PAH: pulmonary arterial hypertension; PAA: pulmonary artery aneurysm; PHTN: pulmonary hypertension; CTA: computed tomography angiography. *indicates a special point to be considered.
Conclusion
PAAs do not present with distinct symptoms and thus are rarely diagnosed based on symptoms. The increased use of CT of the chest has led to an increase in the diagnosis of PAA. To date, there are no guidelines for diagnosis, management, or follow-up on these patients. On the basis of review of literature, we have come up with algorithm as an approach to management of these patients. Further consensus regarding diagnostic criteria, follow-up imaging, and management strategies is warranted.
Author contributions
All authors contributed equally to the article.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Abhinav Agrawal https://orcid.org/0000-0003-1080-6449
References
- 1.Deterling RA, Jr, Clagett OT. Aneurysm of the pulmonary artery; review of the literature and report of a case. Am Heart J 1947; 34: 471–499. [DOI] [PubMed] [Google Scholar]
- 2.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 2012; 5: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duijnhouwer AL, Navarese EP, Van Dijk AP, et al. Aneurysm of the pulmonary artery, a systematic review and critical analysis of current literature. Congenit Heart Dis 2016; 11: 102–109. [DOI] [PubMed] [Google Scholar]
- 4.Restrepo CS, Carswell AP. Aneurysms and pseudoaneurysms of the pulmonary vasculature. Semin Ultrasound CT MR 2012; 33: 552–566. [DOI] [PubMed] [Google Scholar]
- 5.Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J 1958; 2: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen ET, Silva CI, Seely JM, et al. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007; 188: W126–W134. [DOI] [PubMed] [Google Scholar]
- 7.Anzouan-Kacou JB, Seka R, N’Guetta R, et al. Giant pulmonary artery aneurysm: etiology and an exceptional 17 years natural course. Ann Cardiol Angeiol (Paris) 2015; 64: 116–120. [DOI] [PubMed] [Google Scholar]
- 8.Derk G, Laks H, Aboulhosn J. Hybrid Melody pulmonary valve replacement in an adult with severe pulmonary hypertension and pulmonary artery aneurysm. Catheter Cardiovasc Interv 2013; 82: 828–832. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury UK, Kapoor PM, Rao K, et al. Bidirectional Glenn with interruption of antegrade pulmonary blood flow: which is the preferred option: ligation or division of the pulmonary artery? Ann Card Anaesth 2016; 19: 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Su W, Li Y, et al. Pulmonary artery aneurysm compressing the tracheobronchial tree following an arterial switch operation. J Card Surg 2016; 31: 106–109. [DOI] [PubMed] [Google Scholar]
- 11.Manuel V, Miguel G, Magalhaes MP, et al. A right upper lobe mass in the thorax after modified Blalock-Taussig shunt. World J Pediatr Congenit Heart Surg 2016; 7: 523–524. [DOI] [PubMed] [Google Scholar]
- 12.Rohit MK, Vadivelu R, Khandelwal N, et al. Post Blalock-Taussig shunt mediastinal mass – a single shadow with two different destinies. Indian Heart J 2014; 66: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert C, Ikemba C, Nugent A. Device closure of a pseudoaneurysm of the right ventricular outflow tract in an infant with right ventricle-to-pulmonary artery homograft. Catheter Cardiovasc Interv 2014; 83: 587–590. [DOI] [PubMed] [Google Scholar]
- 14.Kannan A, Lick S, Teodori MF, et al. Giant pulmonary artery aneurysm in a 40-year-old woman after patent ductus arteriosus ligation at 2 years of age. Tex Heart Inst J 2016; 43: 274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arango Tomas E, Cerezo Madueno F, Salvatierra Velazquez A. Bronchiectasis due to pulmonary artery aneurysm. Interact Cardiovasc Thorac Surg 2013; 17: 176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda M, Okada Y, Saiki Y, et al. Reconstruction of pulmonary artery with donor aorta and autopericardium in lung transplantation. Ann Thorac Surg 2013; 96: e17–e19. [DOI] [PubMed] [Google Scholar]
- 17.Neema PK, Dharan BS, Singha S, et al. Anesthetic implications of aneurysmal main pulmonary artery and left pulmonary artery and right pulmonary artery stenosis in a child undergoing main pulmonary artery and right pulmonary artery plasty and atrial septal defect closure. J Cardiothorac Vasc Anesth 2012; 26: 280–282. [DOI] [PubMed] [Google Scholar]
- 18.Lo Rito M, Kudumula V, Desai T, et al. Early double switch with Lecompte maneuver for life-threatening airway obstruction. Ann Thorac Surg 2013; 96: 695–697. [DOI] [PubMed] [Google Scholar]
- 19.Murakami T, Saito Y, Nakajima H. Compression of the superior caval vein by an aneurysmal right pulmonary artery in a patient with absent pulmonary valve syndrome. Cardiol Young 2015; 25: 191–192. [DOI] [PubMed] [Google Scholar]
- 20.Kayrak M, Erdogan HI, Yildirim O. Dysphagia due compression of right pulmonary artery aneurysm to the esophagus. Turk Kardiyol Dern Ars 2013; 41: 457. [DOI] [PubMed] [Google Scholar]
- 21.Oz F, Emet S, Baykiz D, et al. Left main coronary artery compression by a giant pulmonary artery aneurysm associated with large atrial septal defect and severe pulmonary hypertension. Anadolu Kardiyol Derg 2011; 11: E28–E29. [DOI] [PubMed] [Google Scholar]
- 22.Andrade JG, Leipsic JA. Massive haemoptysis and pulmonary arterial pseudoaneurysm in a patient with unrepaired tetralogy with pulmonary atresia. Cardiol Young 2010; 20: 441. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa H, Kawata H, Iwai S, et al. Pulmonary artery rupture after bilateral pulmonary artery banding in a neonate with Loeys-Dietz syndrome and an interrupted aortic arch complex: report of a case. Surg Today 2015; 45: 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Huang H, Chen W, et al. A case of pulmonary artery dissection. Eur J Echocardiogr 2011; 12: E32. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari N, Ganguly G, Garg A, et al. Pulmonary artery aneurysm with dissection and hemopericardium. Asian Cardiovasc Thorac Ann 2013; 21: 71–73. [DOI] [PubMed] [Google Scholar]
- 26.Fenster BE, Schroeder JD, Hertzberg JR, et al. 4-Dimensional cardiac magnetic resonance in a patient with bicuspid pulmonic valve: characterization of post-stenotic flow. J Am Coll Cardiol 2012; 59: e49. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Cao J, Miao Q. Surgical treatment of pulmonary artery aneurysm with coronary artery compression. J Card Surg 2016; 31: 161–162. [DOI] [PubMed] [Google Scholar]
- 28.Yeh DD, Ghoshhajra B, Inglessis-Azuaje I, et al. Massive pulmonary artery aneurysm causing left main coronary artery compression in the absence of pulmonary hypertension. Tex Heart Inst J 2015; 42: 465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iosifescu AG, Dorobantu LF, Anca TM, et al. Surgical treatment of a pulmonary artery aneurysm due to a regurgitant quadricuspid pulmonary valve. Interact Cardiovasc Thorac Surg 2012; 14: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu P, Irons M, van de Rijn M, et al. Giant pulmonary artery aneurysm in a patient with Marfan syndrome and pulmonary hypertension. Circulation 2016; 133: 1218–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizarro C, Skowasch D, Nickenig G, et al. Spontaneous rupture of aneurysmatic pulmonary artery in homozygotic alpha-1-antitrypsin deficiency. Dtsch Med Wochenschr 2015; 140: 39–41. [DOI] [PubMed] [Google Scholar]
- 32.Dunet V, Qanadli SD, Lazor R, et al. Multiple pulmonary artery aneurysms in tuberous sclerosis complex. BMJ Case Rep 2013; 2013: bcr2012007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lees MH, Menashe VD, Sunderland CO, et al. Ehlers-Danlos syndrome associated with multiple pulmonary artery stenoses and tortuous systemic arteries. J Pediatr 1969; 75: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita K, Kanna M, Yazawa T, et al. Long-term survivor with pulmonary veno-occlusive disease. Circulation 2012; 125: e503–e506. [DOI] [PubMed] [Google Scholar]
- 35.Shayan H, Sareyyupoglu B, Shigemura N, et al. Lung transplant, double valve repair, and pulmonary artery aneurysm resection. Ann Thorac Surg 2012; 93: e3–e5. [DOI] [PubMed] [Google Scholar]
- 36.Albertson M, Jamous F, Groskreutz D. Expansive pulmonary artery aneurysm in an IV drug user. BMJ Case Rep 2015; 2015: bcr2014208556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demkow M, Kalinczuk L, Kepka C, et al. Left main artery compression by pulmonary artery aneurysm and ostial athero-stenosis of left anterior descending artery in a young female with pulmonary arterial hypertension. Eur Heart J 2012; 33: 2621. [DOI] [PubMed] [Google Scholar]
- 38.Pan HC, Wang KY, Liang KW. Left main coronary artery stenting to relieve extrinsic compression by a giant pulmonary artery aneurysm in a patient with idiopathic pulmonary artery hypertension. Heart Lung Circ 2016; 25: e122–e125. [DOI] [PubMed] [Google Scholar]
- 39.Amano H, Tanabe N, Sakao S, et al. A case of isolated peripheral pulmonary artery branch stenosis associated with multiple pulmonary artery aneurysms. Intern Med 2010; 49: 1895–1899. [DOI] [PubMed] [Google Scholar]
- 40.Nesta M, Cammertoni F, Mangini S, et al. Angina in left main coronary artery occlusion by pulmonary artery aneurysm. Asian Cardiovasc Thorac Ann 2017; 25: 216–218. [DOI] [PubMed] [Google Scholar]
- 41.Jurado-Roman A, Hernandez-Hernandez F, Ruiz-Cano MJ, et al. Compression of the left main coronary artery by a giant pulmonary artery aneurysm. Circulation 2013; 127: 1340–1341. [DOI] [PubMed] [Google Scholar]
- 42.Alkhouli M, Huda N, Bashir R, et al. Left main coronary artery compression syndrome and spontaneous coronary artery dissection: coincidence or pathologic association? Heart Lung 2014; 43: 284–285. [DOI] [PubMed] [Google Scholar]
- 43.Lotan E, Springer J, McWilliams JP, et al. Percutaneous embolization of an incidentally diagnosed pulmonary aneurysm in a scleroderma patient. J Radiol Case Rep 2012; 6: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamuryudan V, Yurdakul S, Moral F, et al. Pulmonary arterial aneurysms in Behcet’s syndrome: a report of 24 cases. Br J Rheumatol 1994; 33: 48–51. [DOI] [PubMed] [Google Scholar]
- 45.Emad Y, Ragab Y, Shawki A, et al. Hughes-Stovin syndrome: is it incomplete Behcet’s? Report of two cases and review of the literature. Clin Rheumatol 2007; 26: 1993–1996. [DOI] [PubMed] [Google Scholar]
- 46.Gerloni R, Merlo M, Vitrella G, et al. Pulmonary artery aneurysm and sarcoidosis. J Cardiovasc Med (Hagerstown) 2015; 16 Suppl 2: S77–S78. [DOI] [PubMed] [Google Scholar]
- 47.Steireif SC, Kocher GJ, Gebhart FT, et al. True aneurysm of the peripheral pulmonary artery due to necrotizing giant cell arteritis. Eur J Cardiothorac Surg 2014; 45: 755–756. [DOI] [PubMed] [Google Scholar]
- 48.Jambeih R, Salem G, Huard DR, et al. Hughes-Stovin syndrome presenting with hematuria. Am J Med Sci 2015; 350: 425–426. [DOI] [PubMed] [Google Scholar]
- 49.Schreiber BE, Noor N, Juli CF, et al. Resolution of Behcet’s syndrome associated pulmonary arterial aneurysms with infliximab. Semin Arthritis Rheum 2011; 41: 482–487. [DOI] [PubMed] [Google Scholar]
- 50.Aamar S, Peleg H, Leibowitz D, et al. Efficacy of adalimumab therapy for life-threatening pulmonary vasculitis in Behcet’s disease. Rheumatol Int 2014; 34: 857–860. [DOI] [PubMed] [Google Scholar]
- 51.Mahfoudhi M, Turki S. Giant aneurysm of the pulmonary artery revealing Behcet syndrome. Pan Afr Med J 2015; 20: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ketari S, Ben Dhaou B, Aydi Z, et al. Pulmonary aneurysms in Behcet disease completely resolved after medical therapy. Tunis Med 2013; 91: 735–736. [PubMed] [Google Scholar]
- 53.Bhandari C, Rathi L, Gupta M, et al. Right ventricular thrombus with pulmonary artery aneurysm in a young male: a rare presentation of Behcet’s disease. Lung India 2015; 32: 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malviya A, Jha PK, Kalita JP, et al. Idiopathic dilatation of pulmonary artery: a review. Indian Heart J 2017; 69: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balboni FA, Lopresti J. Congenital idiopathic dilatation of the pulmonary artery in children. A report of seven cases. Heart Cent Bull (Roslyn) 1961; 17: 1–21. [PubMed] [Google Scholar]
- 56.Deb SJ, Zehr KJ, Shields RC. Idiopathic pulmonary artery aneurysm. Ann Thorac Surg 2005; 80: 1500–1502. [DOI] [PubMed] [Google Scholar]
- 57.Rastogi N, Kabutey NK, Kim D, et al. Percutaneous management of segmental pulmonary artery aneurysm in a patient without pulmonary artery hypertension. Vasc Endovascular Surg 2011; 45: 283–287. [DOI] [PubMed] [Google Scholar]
- 58.Rupprecht H, Ghidau M, Ditterich D. Ruptured pulmonary artery aneurysm mimicking pulmonary embolism. Thorac Cardiovasc Surg 2012; 60: 491–492. [DOI] [PubMed] [Google Scholar]
- 59.Van Melle JP, Meyns B, Budts W. Ortner’s syndrome, presentation of two cases with cardiovocal hoarseness. Acta Cardiol 2010; 65: 703–705. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Li Q, Wu QC, et al. A large, idiopathic, right pulmonary artery aneurysm with superior vena cava compression. Eur J Cardiothorac Surg 2011; 39: 1077. [DOI] [PubMed] [Google Scholar]
- 61.Li CH, Barros AJ, Carreras F, et al. Idiopathic pulmonary artery aneurysm compressing the left main coronary artery. Eur Heart J Cardiovasc Imaging 2012; 13: 696. [DOI] [PubMed] [Google Scholar]
- 62.Matsumura Y, Shiono S, Saito K, et al. Pulmonary artery pseudoaneurysm after lung resection successfully treated by coil embolization. Interact Cardiovasc Thorac Surg 2010; 11: 364–365. [DOI] [PubMed] [Google Scholar]
- 63.Asano M, Gabel G, Allham O, et al. Pulmonary artery pseudoaneurysm in a patient with aortic valve stenosis. Ann Vasc Surg 2013; 27: : 238.e5–238.e7. [DOI] [PubMed] [Google Scholar]
- 64.Bochard-Villanueva B, Estornell-Erill J, de la Espriella R, et al. Giant pulmonary mass complicating pulmonary homograft replacement. Eur Heart J Cardiovasc Imaging 2014; 15: 248. [DOI] [PubMed] [Google Scholar]
- 65.Mourin G, Badia A, Cazes A, et al. An unusual cause of pulmonary artery pseudoaneurysm: acrylate embolism. Interact Cardiovasc Thorac Surg 2012; 15: 1082–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sridhar SK, Sadler D, McFadden SD, et al. Percutaneous embolization of an angiographically inaccessible pulmonary artery pseudoaneurysm after blunt chest trauma: a case report and review of the literature. J Trauma 2010; 69: 729. [DOI] [PubMed] [Google Scholar]
- 67.Bartter T, Irwin RS, Nash G. Aneurysms of the pulmonary arteries. Chest 1988; 94: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 68.Kreibich M, Siepe M, Kroll J, et al. Aneurysms of the pulmonary artery. Circulation 2015; 131: 310–316. [DOI] [PubMed] [Google Scholar]
- 69.Walasangikar V, Dey AK, Sharma R, et al. Pulmonary mycotic pseudo-aneurysm with a prior history of ventricular septal defect. Case report with review of literature. Pneumonol Alergol Pol 2016; 84: 178–180. [DOI] [PubMed] [Google Scholar]
- 70.Lopez-Pastorini A, Koryllos A, Brockmann M, et al. Pseudoaneurysm of the pulmonary artery with massive haemoptysis due to an invasive pulmonary mucormycosis. Thorax 2016; 71: 199–200. [DOI] [PubMed] [Google Scholar]
- 71.Abo-Salem ES, Ramadan MM. A huge thrombosed pulmonary artery aneurysm without pulmonary hypertension in a patient with hepatosplenic schistosomiasis. Am J Case Rep 2015; 16: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papaioannou V, Mikroulis D, Chrysafis I, et al. Hemoptysis due to a mycotic pulmonary artery aneurysm in an injecting drug user. Thorac Cardiovasc Surg 2014; 62: 453–455. [DOI] [PubMed] [Google Scholar]
- 73.Aripoli A, Meek L, Lemons S, et al. A 29-year-old woman with severe sepsis and hemoptysis. Chest 2016; 150: e53–e57. [DOI] [PubMed] [Google Scholar]
- 74.Vaideeswar P, Karande S, Yadav S, et al. Pulmonary artery pseudoaneurysm: a rare cause of hemoptysis in a child. Pediatr Dev Pathol 2016; 19: 146–149. [DOI] [PubMed] [Google Scholar]
- 75.Kim YI, Kang HC, Lee HS, et al. Invasive pulmonary mucormycosis with concomitant lung cancer presented with massive hemoptysis by huge pseudoaneurysm of pulmonary artery. Ann Thorac Surg 2014; 98: 1832–1835. [DOI] [PubMed] [Google Scholar]
- 76.Dong A, Lu J, Zuo C. Multiple peripheral pulmonary artery aneurysms in association with a right atrial myxoma. Circulation 2016; 133: 444–446. [DOI] [PubMed] [Google Scholar]
- 77.Soh J, Toyooka S, Gobara H, et al. A case of delayed massive hemothorax caused by the rupture of a pulmonary artery pseudoaneurysm after radiofrequency ablation of lung tumors. Jpn J Clin Oncol 2012; 42: 646–649. [DOI] [PubMed] [Google Scholar]