Highlights
-
•
General health, according to the Likert scale, was considerable affected even in mild hypothyroidism.
-
•
The level of T4 in the brain, expressed as the CSF/serum f-T4 ratio, was associated with decreased general health.
-
•
Depressive symptoms, according to the MADRS scale, correlated with the CSF/serum f-T4 ratio.
-
•
T4 might have a direct effect in the brain, and not only as a storage hormone for the more active T3.
-
•
Further studies on pharmacokinetics of CSF-thyroxine might be of benefit especially in patients not feeling well.
Abbreviations: s-, serum; CSF, cerebrospinal fluid; HYP, hypothyroid subjects in our study; CON, healthy control group in our study; PH, primary hypothyroidism in general; f-T3 and f-T4, free unbound thyroid hormone; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; TPO, thyroid peroxidase antibody; Hb, hemoglobulin; CRP, C reactive protein; BBB, blood brain barrier; M, mean value; NS, non-significant; Md, median value; Q1, first quartile; Q3, third quartile; BSA, body surface area; MCT8, monocarboxylate transporter 8; AHDS, Allan-Herndon-Dudley syndrome; OATP1C1, organic anion transporter polypeptide 1C1; DIO2, type II iodothyronine deiodinase-enzyme; QoL, quality of life; MADRS, Montgomery Asberg Depression Rating Scale; GHLS, General Health Likert Scale
Keywords: Subclinical hypothyroidism, Mild hypothyroidism, Triiodothyronine, Thyroxine, Quality of life, MADRS
Abstract
Background & Objective
Patients with mild hypothyroidism often are depressed and have impaired quality of life despite serum free-T4 and T3 within reference values. Therefore, we investigated whether their symptoms were dependent on the concentrations of free -T4 and T3 in the circulation and cerebrospinal fluid (CSF).
Methods
Twenty-five newly diagnosed, untreated hypothyroid subjects and as many age- and sex-matched healthy controls were investigated. Blood and CSF sampling was performed in the morning after an overnight fast. Quality of life (QoL) was assessed by a Likert scale. In the hypothyroid subjects, the MADRS rating scale was also used to evaluate symptoms of depression. Furthermore, the results obtained by the questionnaires were related to serum and CSF levels of free- T4 and T3 as well as the ratios between them in CSF and in serum.
Results
Self-reported health was considerably lower in hypothyroid subjects. MADRS was considerably higher than the normal range for healthy individuals. Low CSF/serum free-T4 ratio was correlated with an increased depressed state according to MADRS (p < 0.01), and in addition, CSF/serum free-T4 ratio correlated positively with the self-reported general health Likert scale (p < 0.05). Concentrations of TSH, or free-T3 in serum or CSF, were not associated with an increased depressed state or self-reported general health.
Conclusions
Low CSF/serum ratio of free-T4 was correlated with impaired general health and mood, in contrast to serum measurements not showing any correlations. These findings might partly explain why some patients with hypothyroidism suffer from mental symptoms, despite adequate serum levels of free-T4. However, the findings need to be confirmed in further and larger studies.
Introduction
Primary hypothyroidism (PH) is common worldwide with a prevalence in Sweden of 4.5% for individuals over 50 years of age [1], with a female/male ratio of 5:1. The disease develops insidiously and contains many non-specific symptoms as thyroid hormones affect virtually all cells in the body. Impaired cognition, deepened voice, depressive symptoms, dry and coarse skin, chilled sensation, muscle weakness, constipation, and weight gain are observed even in mildly hypothyroid patients [2], [3]. The first symptoms are subtle, slowly worsening over time. Many patients initially believe that they are suffering from normal age-related symptoms such as general fatigue and impaired vitality. An increased level of thyroid stimulating hormone (TSH) in serum is used as a primary indicator for suspected PH [4]. In addition, the diagnostic criteria of pH include typical symptoms [5] as well as the levels of thyroid hormones; free thyroxine (f-T4), and sometimes also total T4, below or in the lower range and normal or reduced levels of serum free triiodothyronine (f-T3), and sometimes total T3 [4]. Thyroxine (T4), which is considered to be a less active pro-hormone, is deiodinated to the metabolically more potent triiodothyronine (T3). The deiodination takes place in virtually all tissues [6]. TSH stimulates the deiodination via the type I and type II iodothyronine deiodinase-enzymes (DIO1 and DIO2) [7]. In the CNS DIO2 is more highly expressed than DIO1 [6]. There are also several thyroid hormone receptors on testicular cells, endothelial cells, erythrocytes and also in CNS with a higher, or exclusive affinity for T4 and with a non-genomic effect [8], [9].
Three previous clinical studies have measured CSF levels of thyroid hormones in the hypothyroid state [10], [11], [12], but, to our knowledge, no report describes the relationship between thyroid hormones in CSF and quality of life (QoL). In transgenic mice without the ability to convert T4 to T3 in the brain as well as in vitro studies of receptor affinity [13], [14], [15], [16], brain functioning has been found dependent on the function of T4. The levels of f-T3 in the rodent CNS are largely dependent on the local deiodination of f-T4, whereas f-T4 can pass the blood-brain-barrier (BBB) [13], and several studies have shown that T4 is the main thyroid hormone passing the BBB [16], [17], [18], [19], [20]. Therefore, the aim of the present study was to investigate whether T4 levels in CSF are associated with QoL and to elucidate whether the levels of serum TSH, f-T4 and f-T3 reflect those of the CSF in untreated mild primary hypothyroidism.
Materials and Methods
Study population
In this pilot study we investigated the relationships between f-T3 and f-T4 in serum and CSF in newly diagnosed, untreated PH and in age- and sex-matched healthy subjects. In addition, we evaluated if thyroid hormone levels were associated with QoL.
Subjects with hypothyroidism (HYP, n = 25, 20 women) were recruited at the Endocrinology clinic, Halland Central Hospital, Sweden, Table 1. They were asked by their primary physician for interest to be enrolled in the study and was mainly recruited from the same physician. During a certain period, she asked all newly diagnosed PH, and only 2 persons did say no thanks due to lack of time. The subjects displayed at least two pathologically increased TSH (>4.0 mIE/L; reference range: 0.40–4.0 mIE/L) prior to admission. In addition, all subjects had a f-T4 level in the lower-half of the normal range of 11–22 pmol/l (all patients < 15, Md = 11.7 pmol/l).
Table 1.
Basic characteristics of Hypothyroid subjects (HYP) and Healthy controls (CON). Negative and positive TPO Ab́s is divided in two columns for comparison. (BMI = Body mass index, BSA = Body surface area, f-T3 = free component of triiodothyronine, f-T4 = free component of thyroxine, TPO-Ab = Thyroid peroxidase antibody.
| Hypothyroid subjects (HYP) | Healthy controls (CON) | p-value | HYP with TPO < 15 | HYP with TPO > 15 | |
|---|---|---|---|---|---|
| Number | n = 25 | n = 25 | n = 11 | n = 14 | |
| Age, years | Md = 46 (Q1 = 35.4, Q3 = 54.5) | Md = 39 (Q1 = 28, Q3 = 50) | NS | Md = 44 (Q1 = 25, Q3 = 55) | Md = 50 (Q1 = 44, Q3 = 55) |
| Sex, M/F | 5/20 | 5/20 | NS | 1/10 | 4/10 |
| BMI, kg/m2 | M = 26.87 ± 4.48 | M = 25.4 ± 4.36 | NS | M = 27.3 ± 2.7 | M = 26.5 ± 5.3 |
| BSA, m2 (Du Bois formula) | M = 1.90 ± 0.24 | M = 1.85 ± 0.20 | NS | M = 1.90 ± 0.20 | M = 1.90 ± 0.25 |
| Serum TSH (mIE/L) | Md = 4.96 (Q1 = 4.44, Q3 = 6.46) | Md = 1.6 (Q1 = 1.0, Q3 = 2.7) | <0.0001 | Md = 4.8 (Q1 = 4.3, Q3 = 5.5) | Md = 5.5 (Q1 = 4.7, Q3 = 7.7) |
| Serum f-T3 (pmol/L) | Md = 5.75 (Q1 = 4.86, Q3 = 6.20) | Md = 5.90 (Q1 = 5.31, Q3 = 6.40) | NS | Md = 6.04 (Q1 = 4.53, Q3 = 6.37) | Md = 5.54 (Q1 = 5.10, Q3 = 5.90) |
| Serum f-T4 (pmol/L) | Md = 11.7 (Q1 = 11.0, Q3 = 12.9) | Md = 13.5 (Q1 = 11.95, Q3 = 14.7) | <0.005 | Md = 12.5 (Q1 = 11.6, Q3 = 14.2) | Md = 11.3 (Q1 = 10.5, Q3 = 12.2) |
| Zulewski score > 5 points | 25/25 | 0/25 | ∞ | 11/11 | 14/14 |
| Serum TPO-Ab > 15 U/ml | 14/25 | 4/25 | <0.005 | 0/11 | 14/14 |
| Immunological disease | 3/25 | 4/25 | NS | 0/11 | 3/14 |
| Estrogen treatment | 6/25 | 3/25 | NS | 3/11 | 3/14 |
Considering the moderate elevation of TSH, we also used the Zulewski scale [5], >5 points, to ensure that the subjects were clinically hypothyroid. So, the cause of hypothyroidism in the 25 subjects was considered to be primary hypothyroidism, with fourteen of them positive for TPO-antibodies. Patients receiving anti-thyroid hormone or thyroid hormones, or with a recent use of iodine contrast were excluded. Pregnant women were also excluded. Recent onset of fatigue, hypersomnia or lethargy was the main cause for 22 out of the 25 subjects to consult their primary physician. All the 25 subjects had though hypothyroid symptoms. Aside hypothyroidism, one subject was previously diagnosed with type 1 diabetes, one with pernicious anemia and another with rheumatoid arthritis. The proportion of another autoimmune diseases were consistent with previously reported studies [21]. Four subjects were medicated with estrogen hormone replacement (oral estrogen and patch) and two with hormonal contraceptives (drospirenone/etinylestradiol). One subject received glucocorticoid treatment i.e.; Budesonid inhalation td. None of the 25 subjects did fulfill any criteria of a depressive diagnosis.
The healthy controls (CON, n = 25, 20 women) were recruited by posters in the staff rooms at the medical clinic, the ambulance, and at the university of Halmstad. Though considering themselves as healthy one had a mild asthma, one arthrosis, one hypertension, one diverticulitis, one rheumatoid arthritis, and one migraine. Among them, four were taking hormonal contraceptives, one an ACE-antagonist, one a beta-blocker and a statin, and another received bulk-forming laxatives. All had thyroid hormone levels within the normal range, with a Zulewski score of 0–4 points.
There was no significant difference in age or body surface area (BSA) between the groups (Table 1), and the study procedures were identical in both groups. All subjects in both groups were of Caucasian origin. Fourteen subjects in the HYP group, and four subjects in the CON group, had increased levels of thyroid peroxidase (TPO)-antibodies (>15 U/ml). In the HYP, those with TPO-antibodies were similar in all aspects of clinical data compared to those without (see comparison in Table 1), and the prevalence is in accordance with a previous report on patients with this TSH range [22].
Body weight and area
Body weight was measured in the morning to the nearest 0.1 kg, height was measured barefoot to the nearest 0.01 m. Body surface area (BSA) was measured according to the Du Bois formula.
Sampling of blood and CSF
Lumbar puncture was performed at 08:00–08:30 a.m. after a minimum of 8 h fasting. Venous blood sampling was obtained for analyses of TSH, f-T3, f-T4, and TPO antibodies.
The lumbar puncture was performed according to a standardized procedure comprising puncture at the L4-5 interspace in a seated position. CSF was drawn with a disposable needle (Becton-Dickinson, Oxford, UK, quincke: 0.70x75 mm, 22 GA), and collected in polypropylene tubes. A total of 12 mL CSF was obtained and divided in six 2 mL aliquots and in case of bleeding the first volume of CSF was not used for analysis. CSF was immediately transported to the local laboratory for centrifugation at 2000g at +4 °C for 10 min. The supernatant was pipetted off, gently mixed to avoid possible gradient effects, and stored in polypropylene tubes at −70 °C pending biochemical analyses, without being thawed and refrozen.
Biochemical procedures
All samples were analyzed in the same assay run for each specimen to minimize the analytical inter-assay variation. TSH, f-T3 and f-T4 were analyzed by dissociation-enhanced lanthanide fluoro-immunoassays [23] (Auto DELFIA, Wallac Oy, Turkku, Finland). The intra-assay coefficient of variation (CV%) for TSH was <4.4% for all levels down to 0.1 mIE/l, and the intra-assay coefficient for f-T4 and f-T3 were <6.1% for all levels down to 5 and 2 pmol/l, respectively. TPO-antibodies were analyzed by chemiluminescent microparticle immunoassay (CMIA) (Architect i2000, Abbott, Chicago, USA) and the intra-assay coefficient of variation was <10% for all levels down to 5 U/ml; we considered > 15 IU/mL as a positive result.
Quality of life
After the other study procedures had been performed, all subjects completed the self-assessment of general health using a Likert scale (GHLS) [24]. The HYP group also completed used the Montgomery-Åsberg Depression Rating Scale (MADRS) questionnaire to evaluate symptoms of depression [25].
Statistical methods
For statistical analyses SPSS, Version 24.0, and Matlab R2016b were used. The descriptive statistical results are presented as the mean ± SD, or median with 1st (Q1) and 3rd (Q3) quartile. A non-parametric statistical approach was used in all the statistical analyses. Between-group analyses were performed using the Mann-Whitney U test for continuous variables and chi-square tests for categorical variables. Correlations were investigated using the Spearman rank correlation coefficient. A two-tailed P-value < 0.05 was considered significant.
Ethical considerations
The study was approved by the Regional Ethical Committee in Lund, Sweden (2011/1 and 2012/11). Oral and written informed consent was obtained from all participants.
Results
Serum TSH and thyroid hormone levels
Serum TSH was significantly higher in the HYP group (p < 0.001), with median (Md) values of 4.96 mIE/L (Q1: 4.44, Q3: 6.46), in HYP, and 1.60 mIE/L (Q1: 1.00, Q3: 2.7), in CON (Table 1).
Serum f-T4 was significantly lower in HYP (p < 0.01), with Md of 11.7 pmol/L (Q1: 11.0 and Q3: 12.9). In CON 13.5 pmol/L (Q1: 11.95 and Q3: 14.7).
Serum f-T3 (pmol/L) did not differ significantly between the two groups (Table 1).
CSF thyroid hormone levels
CSF levels of thyroid hormones are given in Table 2. Neither CSF f-T4 nor CSF f-T3 differed significantly between the HYP and CON groups. For CSF f-T4, the Md was 9.13 pmol/L in the HYP group and 9.78 pmol/L in the CON group.
Table 2.
Selected basic statistical characteristics of results in Hypothyroids subjects (HYP) and Healthy controls (CON). CSF = Cerebrospinal fluid. f-T3 = free component of triiodothyronine, f-T4 = free component of thyroxine.
| Hypothyroid subjects (HYP) | Healthy controls (CON) | p-value | |
|---|---|---|---|
| Number | n = 25 | n = 25 | |
| CSF f-T4 (pmol/L) | Md = 9.13 (Q1 = 8.05, Q3 = 10.12) | Md = 9.78 (Q1 = 9.05, Q3 = 10.50) | NS |
| CSF f-T3 (pmol/L) | Md = 2.50 (Q1 = 2.11, Q3 = 2.87) | Md = 2.25 (Q1 = 1.95, Q3 = 2.72) | NS |
| CSF/serum f-T4 | Md = 0.77 (Q1 = 0.70, Q3 = 0.85) | Md = 0.75 (Q1 = 0.64, Q3 = 0.79) | NS |
| CSF/serum f-T3 | Md = 0.46 (Q1 = 0.35, Q3 = 0.51) | Md = 0.38 (Q1 = 0.35, Q3 = 0.47) | NS |
There was no significant difference between the HYP (Md: 0.77) and CON (Md: 0.75) groups in the CSF/serum f-T4 ratio. CSF/serum f-T3 ratio was also similar in the two groups (Table 2).
Quality of life and depression
Self-assessed health was significantly impaired in HYP group compared to that in the CON group for the Likert scale (median 65 vs 90, p < 0.001) (Fig. 1). In the HYP group, the MADRS score (Md = 10, Q1:5, Q3:16, M = 11.84 ± 7.93) was considerably higher than the normal range for healthy individuals, according to a review article [24] with a mean of 4.0 ± 5.8.
Fig. 1.
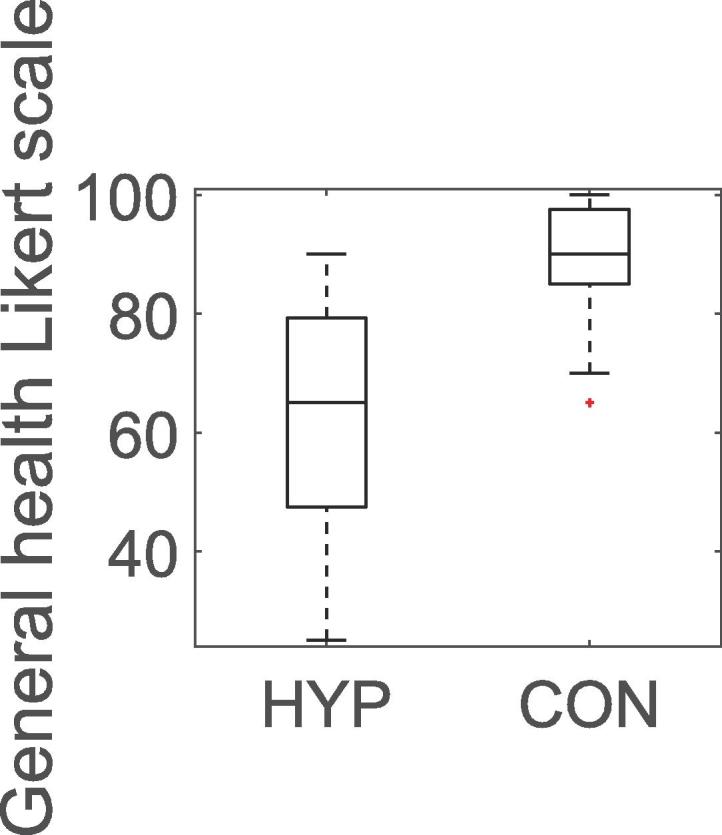
Boxplot of general health (0–100) in hypothyroid subjects (HYP) and healthy controls (CON). The box indicates median (Md) values of 65 vs 90, respectively, p < 0.001, and 25th and 75th percentiles. The whiskers extend to all data, except potential outliers.
Correlations between serum and CSF thyroid hormone levels
As expected, in the HYP group, CSF f-T4 correlated strongly and positively with serum f-T4 (r = 0.72, p < 0.001), and CSF f-T3 correlated positively with serum f-T3 (r = 0.56, p < 0.01). No correlation was found between f-T3 and f-T4 neither in serum nor in CSF.
In the CON group CSF f-T4 correlated with serum f-T4 (r = 0.43, p < 0.05). Serum f-T4 correlated with serum f-T3 (r = 0.44, p < 0.05).
Correlations between serum TSH and thyroid hormone levels
In the HYP group, higher serum TSH level correlated with lower f-T4 in CSF (r = −046, p < 0.05), but only tended to correlate with serum f-T4 (r = −0.39, p < 0.053). Serum TSH did not correlate to f-T3 in serum or in CSF. There was a correlation between serum TSH and the serum fT3/fT4 ratio (r = 0.40, p < 0.05).
In the CON group there was no correlation between serum-TSH and f-T4 or f- T3, but there was a correlation between serum-TSH and the serum fT3/fT4 ratio (r = 0.42, p < 0.05).
Correlations between hormone levels and quality of life
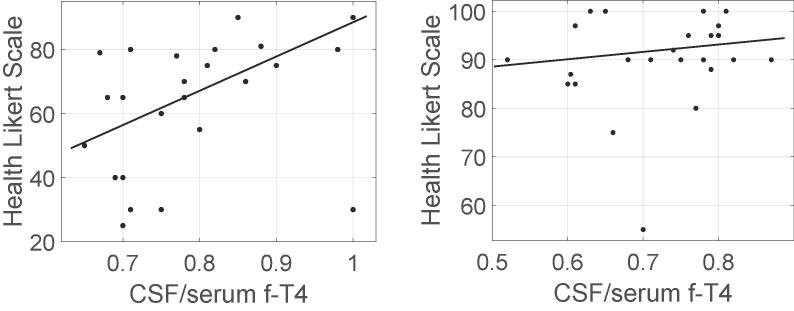
The CSF/serum f-T4 ratio correlated positively with self-assessed general health Likert scale (GHLS), in the HYP group (r = 0.46, p < 0.05), but not in the CON group (Fig. 2a and b). If the three patients in the HYP group with another autoimmune disease were excluded, the positive correlation was even stronger in the remaining 22 subjects (r = 0.71, p < 0.001). The 95% Confidence Interval (CI) for the serum/CSF fT4 ratio, in the HYP group was 0.7856 ± 0.0407. The 95% CI for GHLS, in the HYP group was 62.72 ± 7.82. The 95% CI for the serum/CSF fT4 ratio, in the CON group was 0.7206 ± 0.0349, and the 95% CI for GHLS, in the CON group was 90.24 ± 3.83.
Fig. 2.
Relation between CSF/serum f-T4 ratio and General Health Likert scale (GHLS) in hypothyroid subjects (HYP) (a) and in healthy controls (CON) (b). In hypothyroid patients, there was a significant positive correlation (p < 0.05, r = 0.46), whereas in healthy controls, there was no correlation. Filled line represent a linear regression. The 95% Confidence Interval for the serum/CSF fT4 ratio, in HYP is 0.7856 ± 0.0407. The 95% Confidence Interval for GHLS, in HYP is 62.72 ± 7.82. The 95% Confidence Interval for the serum/CSF fT4 ratio, in CON is 0.7206 ± 0.0349, and the 95% Confidence Interval for GHLS, in CON is 90.24 ± 3.83.
In the CON group, there were no significant correlations between GHLS and any of the thyroid hormone measurements.
Decreased CSF/serum f-T4 ratio correlated with an increased number of depressive symptoms according to MADRS (r = −0.56, p < 0.01) in the HYP group (Fig. 3). If we excluded the three patients in the HYP with another autoimmune disease, the correlation remained (n = 22; r = −0.54, p < 0.01).
Fig. 3.
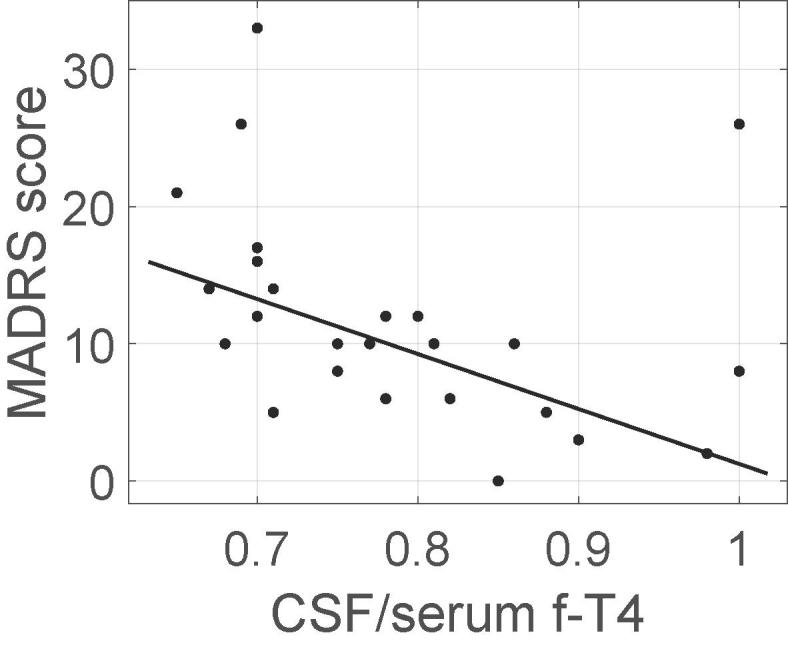
Relation between CSF/serum f-T4 ratio and MADRS score for depression. In hypothyroid subjects, there was a significant negative correlation (p < 0.05, r = −0.56). Filled line represent a linear regression. The 95% Confidence Interval for the serum/CSF fT4 ratio, in HYP is 0.7856 ± 0.0407. The 95% Confidence Interval for MADRS is 11.84 ± 3.11.
Multiple linear regression
We evaluated if the Zulewski score, an inclusion criterion, affected our results in the HYP or the CON group. The Zulewski score did not correlate with CSF/serum f-T4 ratio in any of the two groups using bivariate correlation analysis. In multiple regression analysis, using CSF/serum f-T4 ratio as the dependent variable and GHLS and Zulewski score as independent variables, there was also no relationship between the Zulewski score and CSF/serum f-T4 ratio in the HYP or CON groups. Finally, in an additional multiple regression analysis in the HYP group, in which CSF/serum f-T4 ratio was the dependent variable and MADRS and the Zulewski score were the independent variables, the Zulewski score did not impact on the CSF/serum f-T4 ratio. Thus, these analyses suggest that the Zulewski score did not influence the relations between CSF/serum f-T4 ratio and QoL.
Discussion
Theoretical considerations
GHLS showed a significantly lower QoL in the HYP group (p ≤ 0.001; Fig. 1). Furthermore, the MADRS score in this study was much higher than in healthy subjects in previous reports [26], [27], [28]. These results indicate a generally impaired health.
Our results suggest that f-T4 in CSF is important for general health in PH patients, as exhibited by the positive correlation between the CSF/serum f-T4 ratio and the GHLS (r = 0.46, p < 0.05 or r = 0.71, p < 0.001 when excluding other autoimmune diseases). This is also congruent with the strong negative correlation (r = −0.56, p < 0.01) found between this ratio and MADRS. Although f-T3 is considered as a more potent thyroid hormone we did not detect any significant correlations between serum or CSF levels of fT3 and the QoL scales.
The monocarboxylate transporter 8 (MCT8) [16], [29] is a specific transporter for thyroid hormones (TH) into the CNS. Allan-Herndon-Dudleys Syndrome (AHDS) is a disease specifically with a MCT8 mutation [30]. In AHDS, the deficient transport of TH into CNS is considered to be the cause of the mental retardation [17], [19]. In addition, a specific transporter for T4, organic anion transporter polypeptide (OATP1C1) is expressed in capillaries throughout the brain. Transport of TH into the CNS, in particular, T4, is also facilitated by organic anion transporter polypeptide (OATP1C1), which is expressed in capillaries throughout the brain [16]. The primary TH that crosses the BBB is T4 [13], [18], which is due to the higher binding to these transporters. In our study, the correlation between CSF/serum f-T4 ratio and QoL might provide some additional support that the passage of T4 into the CNS is of importance for healthy brain function.
We found an unambiguous correlation (r = 0.72, p < 0.001) between serum and CSF levels of f-T4 and a less pronounced correlation (r = 0.55, p < 0.01) between f-T3 in serum and CSF. The weaker association between the CSF and serum levels of f-T3 might be in line with theory that the main part of T3 in the CNS derives from local production, which to a major extent takes place in the hypothalamus. The hypothalamic astrocytes and tanycytes express DIO2, and hereby convert T4 to T3 [14]. In some mice models with inactivation of DIO2, the animals were grossly physiologically and behaviorally normal without any signs of abnormality and did not show any signs of hypothyroidism in the brain or in the body [31]. However, these results are conflicting with a recent study in mice, which reported that a DIO2 polymorphism affected brain function [32].
Thyroid hormones forward their effect by binding to the thyroid hormone receptors (TR). There are three important isoforms of TR: TRα1, TRβ1 and TRβ2. TRβ2 is expressed in the pituitary, hypothalamus (HPT axis), cone cells of the retina and auditory cells of the cochlear [33] and hence is of little relevance for the other parts of the brain. TRα1 has a much stronger response to T4 than TRβ1 [15], and TRα1 constitutes 70–80% of all TR expression in the adult brain [34]. T4 has a response to TRα1 comparable to that of T3 [15]. This would support our hypothesis of T4 being of greater importance in the brain than merely being a pro-hormone that is converted into T3. Also, thyroid hormones, and particularly T4, has a non-genomic effect on several cell-types including cells in the CNS [8], [9]. Much is known about this regarding testicular function [35], [36], erythrocytes and endothelial cells, but more studies are needed about this non-genomic effect the CNS.
Clinical implications
The results of the present study suggest that in primary hypothyroidism, a low f-T4 CSF/serum ratio, is of importance for some of the most plaguing symptoms, supposed to be of central nervous origin. Therefore, simultaneously measuring f-T4 in serum and in CSF, to get the ratio of individuals with a persistent severe psychiatric disturbance, despite a normalized serum fT4, may give us a pathophysiological explanation. However, larger studies are required to confirm our results. In addition, the correlations between thyroid hormones in CSF and QoL need to be investigated in more detail before the clinical relevance of our findings can be evaluated. Unfortunately, there are no other therapeutic options available today.
We found that high serum TSH in the HYP group correlated with low CSF f-T4 (r = −0.46, p < 0.05), and only tended to correlate with low serum f-T4 (r = −0.39, p = 0.053). These findings seem to be plausible as the concentration of f-T4 in the CSF might influence the thyroid releasing hormone (TRH) production in the hypothalamic nuclei (in parvocellular cells in the wall of the third ventricle belonging mostly to the periventricular hypothalamic area (PVH) and arcuate nucleus (ARC)), which controls the pituitary production of TSH to a greater extent than the circulating level of f-T4. However, this must be confirmed in a larger study.
Limitations
MADRS was not assessed in CON subjects because of their reported high index of perceived QoL according to SF-36 and general health scales. Thus, they were assumed to be healthy, and there was no reason to believe that our healthy controls would be different from those in other studies. A review article by Zimmerman [26] presented a mean MADRS score in healthy controls of 4.0 ± 5.8, which was considerably different from our HYP-group (Md = 10, Q1:5, Q3:16).
Funding and support
This work was granted by Södra Sjukvårdsregionen and Region Hallands forskningsfond.
Contribution
Anders Funkquist is a primary investigator, data collection, data analysis incl statistical analysis, primary writer and planning of the study.
Anders Bengtsson was involved in data collection and planning of the study.
PM Johansson was involved in setting up the study, involved in the planning.
Johan Svensson was involved in setting up the study, involved in the writing of the article.
Per Bjellerup did the analysis of thyroid hormones.
Kaj Blennow did additional analysis that mostly is part of next studies.
Birger Wandt was involved in setting up the study. Involved in the writing of the article.
Stefan Sjöberg is guarantor for the study. Involved in all processes from setting up the study to data analysis and writing the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Mrs. Christine Palm, Mrs. Maria Winterqvist for excellent technical assistance throughout the study. MSC Anders Holmén and statistician Amir Baigi for valuable statistical evaluation. We are most grateful for the excellent proofreading by PhD Maura Heverin. MD Elisabeth Larsson, Ekens familjeläkarmottagning AB for providing us with several patients.
Contributor Information
Anders Funkquist, Email: anders.funkquist@regionhalland.se.
Anders Bengtsson, Email: anders.bengtsson@regionhalland.se.
P.M. Johansson, Email: pmj@gu.se.
Johan Svensson, Email: johan.svensson@medic.gu.se.
Per Bjellerup, Email: per.bjellerup@ltv.se.
Kaj Blennow, Email: kaj.blennow@neuro.gu.se.
Stefan Sjöberg, Email: Stefan.sjoberg@ki.se.
References
- 1.The National Board of Health and Welfare statistical database on pharmaceutical products http://www.socialstyrelsen.se/statistik/statistikdatabas/lakemedel.
- 2.Chaker L., Bianco A.C., Jonklaas J., Peeters R.P. Hypothyroidism. Lancet. 2017 Jan 6;390(10101):1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canaris G.J., Manowitz N.R., Mayor G., Ridgway E.C. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 4.Pearce S.H., Brabant G., Duntas L.H., Monzani F., Peeters R.P., Razvi S. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013 Dec;2(4):215–228. doi: 10.1159/000356507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zulewski H., Müller B., Exer P., Miserez A.R., Staub J. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls 1. J Clin Endocrinol Metab. 1997;82(3):771–776. doi: 10.1210/jcem.82.3.3810. [DOI] [PubMed] [Google Scholar]
- 6.Bianco A.C., Salvatore D., Gereben B., Berry M.J., Larsen P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 7.Williams G.R., Bassett J.H. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. J Endocrinol. 2011 Jun;209(3):261–272. doi: 10.1530/JOE-10-0448. [DOI] [PubMed] [Google Scholar]
- 8.Davis P.J., Leonard J.L., Davis F.B. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29(2):211–218. doi: 10.1016/j.yfrne.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Davis P.J., Goglia F., Leonard J.L. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 10.Sjöberg S., Eriksson M., Werner S., Bjellerup P., Nordin C. L-thyroxine treatment in primary hypothyroidism does not increase the content of free triiodothyronine in cerebrospinal fluid: a pilot study. Scand J Clin Lab Invest. 2011;71(1):63–67. doi: 10.3109/00365513.2010.541931. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J.M., Siersbaek-Nielsen K. Cerebrospinal fluid thyroxine. J Clin Endocrinol Metab. 1969;29(8):1023–1026. doi: 10.1210/jcem-29-8-1023. [DOI] [PubMed] [Google Scholar]
- 12.Burman P., Hetta J., Wide L., Månsson J., Ekman R., Karlsson F. Growth hormone treatment affects brain neurotransmitters and thyroxine. Clin Endocrinol (Oxf) 1996;44(3):319–324. doi: 10.1046/j.1365-2265.1996.617439.x. [DOI] [PubMed] [Google Scholar]
- 13.Crantz F., Silva J., Larsen P. An analysis of the sources and quantity of 3, 5, 3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110(2):367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- 14.Guadano-Ferraz A., Escamez M.J., Rausell E., Bernal J. Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci. 1999 May 1;19(9):3430–3439. doi: 10.1523/JNEUROSCI.19-09-03430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder A.C., Privalsky M.L. Thyroid hormones, t3 and t4, in the brain. Front Endocrinol. 2014;5:40. doi: 10.3389/fendo.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki T., Abe T. Thyroid hormone transporters in the brain. Cerebellum. 2008;7(1):75–83. doi: 10.1007/s12311-008-0029-9. [DOI] [PubMed] [Google Scholar]
- 17.Friesema E.C., Grueters A., Biebermann H., Krude H., von Moers A., Reeser M. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364(9443):1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 18.Heuer H. The importance of thyroid hormone transporters for brain development and function. Best Practice Res Clin Endocrinol Metab. 2007;21(2):265–276. doi: 10.1016/j.beem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kakinuma H., Itoh M., Takahashi H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T 4 levels in cerebrospinal fluid. J Pediatr. 2005;147(4):552–554. doi: 10.1016/j.jpeds.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 20.van der Deure Wendy M, Hansen P.S., Peeters R.P., Kyvik K.O., Friesema E.C., Hegedüs L. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology. 2008;149(10):5307–5314. doi: 10.1210/en.2008-0430. [DOI] [PubMed] [Google Scholar]
- 21.Bjoro T., Holmen J., Kruger O., Midthjell K., Hunstad K., Schreiner T. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT) Eur J Endocrinol. 2000 Nov;143(5):639–647. doi: 10.1530/eje.0.1430639. [DOI] [PubMed] [Google Scholar]
- 22.Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 2010;123(2):183.e1–183.e9. [DOI] [PubMed]
- 23.Beever K., Bradbury J., Phillips D., McLachlan S.M., Pegg C., Goral A. Highly sensitive assays of autoantibodies to thyroglobulin and to thyroid peroxidase. Clin Chem. 1989 Sep;35(9):1949–1954. [PubMed] [Google Scholar]
- 24.Gudex C., Dolan P., Kind P., Williams A. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996;5(6):521–531. doi: 10.1007/BF00439226. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979 Apr;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman M., Chelminski I., Posternak M. A review of studies of the Montgomery-Asberg Depression Rating Scale in controls: implications for the definition of remission in treatment studies of depression. Int Clin Psychopharmacol. 2004;19(1):1–7. doi: 10.1097/00004850-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Razvi S., McMillan C.V., Weaver J.U. Instruments used in measuring symptoms, health status and quality of life in hypothyroidism: a systematic qualitative review. Clin Endocrinol (Oxf) 2005;63(6):617–624. doi: 10.1111/j.1365-2265.2005.02381.x. [DOI] [PubMed] [Google Scholar]
- 28.Demartini B., Ranieri R., Masu A., Selle V., Scarone S., Gambini O. Depressive symptoms and major depressive disorder in patients affected by subclinical hypothyroidism: a cross-sectional study. J Nerv Ment Dis. 2014 Aug;202(8):603–607. doi: 10.1097/NMD.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 29.Verge C.F., Konrad D., Cohen M., Di Cosmo C., Dumitrescu A.M., Marcinkowski T. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97(12):4515–4523. doi: 10.1210/jc.2012-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allan W., Herndon C., Dudley F.C. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 31.Galton V.A., Schneider M.J., Clark A.S., St. Germain D.L. Life without thyroxine to 3, 5, 3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150(6):2957–2963. doi: 10.1210/en.2008-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo S., Fonseca T.L., Bocco B.M., Fernandes G.W., McAninch E.A., Bolin A.P. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest. 2018;129(1) doi: 10.1172/JCI123176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan I.H., Privalsky M.L. Isoform-specific transcriptional activity of overlapping target genes that respond to thyroid hormone receptors α1 and β1. Mol Endocrinol. 2009;23(11):1758–1775. doi: 10.1210/me.2009-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallis K., Dudazy S., Hogerlinden Mv, Nordström K., Mittag J., Vennström B. The thyroid hormone receptor α1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24(10):1904–1916. doi: 10.1210/me.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Vignera S., Vita R., Condorelli R.A., Mongioì L.M., Presti S., Benvenga S. Impact of thyroid disease on testicular function. Endocrine. 2017;58(3):397–407. doi: 10.1007/s12020-017-1303-8. [DOI] [PubMed] [Google Scholar]
- 36.La Vignera S, Vita R. Thyroid dysfunction and semen quality 2018. [DOI] [PMC free article] [PubMed]