Highlights
-
•
Functional connectivity between amygdala subregions and the brain was studied with resting-state (RS) functional MRI.
-
•
RS functional connectivity was compared between patients with first episode schizophrenia (FES) and healthy controls.
-
•
FES patients showed changes in functional connectivity between amygdala subregions and sensorimotor cortex.
-
•
Altered basolateral amygdala-precentral gyrus connectivity correlated with positive symptoms in FES patients.
Keywords: First-episode schizophrenia, Basolateral amygdala, Centromedial amygdala, Resting–state functional connectivity
Abbreviations: RsFC, resting-state functional connectivity; HC, healthy controls; FES, first-episode schizophrenia; BLA, basolateral amygdala; CMA, centromedial amygdala; SFA, superficial amygdala; FC, functional connectivity; ROI, region of interest; ASD, autism spectrum disorders; DPARSF, Data Processing Assistant for Resting-State fMRI; TR, repetition time; FA, flip angle; FOV, field of view
Abstract
Altered resting-state functional connectivity (rsFC) of the amygdala has been demonstrated to be implicated in schizophrenia neuronal pathophysiology. However, whether rsFC of amygdala subregions is differentially affected in schizophrenia remains unclear. This study compared the functional networks of each amygdala subdivision between healthy controls (HC) and patients with first-episode schizophrenia (FES). In total, 47 HC and 78 patients with FES underwent resting-state functional magnetic resonance imaging. The amygdala was divided into the following three subregions using the Juelich histological atlas: basolateral amygdala (BLA), centromedial amygdala (CMA), and superficial amygdala (SFA). The rsFC of the three amygdala subdivisions was computed and compared between the two groups. Significantly increased rsFC of the right CMA with the right postcentral gyrus and decreased rsFC of the right BLA with the left precentral gyrus were observed in the FES group compared with the HC group. Notably, the right BLA-left precentral gyrus connectivity was negatively correlated with positive symptoms and conceptual disorganization in patients with FES. In conclusion, this study found that patients with FES had abnormal functional connectivity in the amygdala subregions, and the altered rsFC was associated with positive symptoms. The present findings demonstrate the disruptive rsFC patterns of amygdala subregional-sensorimotor networks in FES and may provide new insights into the neuronal pathophysiology of FES.
1. Introduction
Schizophrenia is a common mental disorder that affects brain functions with unknown etiology, with a lifetime prevalence of 1% (Owen et al., 2016). The main clinical manifestations include positive symptoms, negative symptoms, and cognitive impairment (Owen et al., 2016). Positive symptoms, the main clinical manifestations of first-episode schizophrenia (FES) (Insel, 2010), are usually the main reason patients present to clinician (McCutcheon et al., 2019) and one important indicator for predicting relapse (Patel et al., 2014) and have been suggested to be involved in impairment of predicting the sensory consequences of self-generated action and misattributing them from real external events (Shergill et al., 2005). Neuroimaging studies have shown that patients with schizophrenia exhibit functional abnormalities in multiple brain regions (Goghari et al., 2010; Gong et al., 2016), and accumulating evidence supports a leading hypothesis that schizophrenia is a widespread functional dysconnection disorder (Friston et al., 2016; Stephan et al., 2006). However, the underlying neuropathological mechanism is still unclear.
Functional connectivity (FC), which reflects the temporal correlation of activity across brain regions, has been used to investigate the dysfunctional connectivity of brain regions in schizophrenia (Biswal et al., 1995). Because the amygdala plays a significant role in social information processing, particularly in associating emotive salience to sensorial stimuli, through connections with multiple brain regions such as the prefrontal cortex, motor, and sensory regions (LeDoux, 2012), studies have often used the amygdala as a seed region of interest (ROI) to analyze amygdalar FC and its correlation with psychotic symptoms. For instance, the diffusion-weighted MRI study has shown that a direct anatomical connection between the amygdala and motor cortex plays an important role in the context-appropriate behavior expression of individuals in response to environmental cues in healthy individuals (Grèzes et al., 2014). FC study has demonstrated an abnormal association between the limbic and motor system in schizophrenia (Jalbrzikowski et al., 2019; Stegmayer et al., 2018). Specifically, the dysconnection between the limbic and motor cortex has been demonstrated to be correlated with the social behavioral symptoms in patients with schizophrenia and may contribute to the pathophysiology and symptomatology of schizophrenia (Berman et al., 2016; Stegmayer et al., 2018). In addition, studies of people with autism spectrum disorders (ASD) revealed hyper-connectivity between the amygdala and motor area in ASD, suggesting that a disruption of the amygdala-motor network may contribute to impairments in social perception and understanding (Fishman et al., 2018; Gotts et al., 2012). These findings suggest that abnormal FC between the amygdala and sensorimotor cortex might be associated with the corresponding symptoms in these patients with neurodevelopmental disorders, and the dysconnectivity in the amygdala-motor pathway related to impairment in social cognition may underlie the specific psychiatric symptoms in schizophrenia.
The amygdala is an extensively connected structure in the limbic system and can be divided into the centromedial (CMA), basolateral (BLA), and superficial amygdala (SFA) (Amunts et al., 2005). The CMA, which includes the central and medial nuclei, is critical in the expression of emotional responses by receiving inputs from BLA via projections to autonomic and motor centers in the brainstem (Sah et al., 2003). The BLA, including the lateral, basolateral, basomedial, and basoventral nuclei, receives sensorimotor information and plays a core role in the perception and regulation of emotional stimuli via its connection with subcortical circuits (Pessoa and Adolphs, 2010). The complex connections further confirm the core role of the BLA in associative learning, such as threat processing (Adhikari et al., 2015). The SFA associates with the olfactory cortex and is involved in processing socially relevant information and modulating approach-avoidant behavior (Sah et al., 2003).
The roles of the amygdala nuclei and the three subregions have been investigated intensively in animal models, and the findings suggest a strong correlation between the activity of the amygdala subregions and social emotion expression. Different patterns of FC across amygdala subregions have been reported in patients with other neuropsychiatric disorders (Brown et al., 2014; Qin et al., 2014; Rausch et al., 2016). However, only one study has compared amygdala subregional connectivity between healthy people and patients with schizophrenia (Liu et al., 2014), Liu et al. (2014) selected three amygdala subregions (BLA, SFA, CMA) as regions-of-interest (ROIs) and defined the prefrontal cortex (PFC) as mask, then directly compared their rsFC with the PFC mask between the two groups. Yet, it remains unknown whether whole-brain amygdala subregional connectivity differs between the two groups and no studies have examined the correlation between amygdala subregional dysfunction and clinical symptoms in patients with FES.
The current study aimed to examine the integrity of each sub-amygdalar network in FES. We hypothesized that patients with FES have abnormal FC in the subregional amygdala networks compared with healthy controls (HC), and dysfunctional connectivity of amygdala subregions is correlated with positive symptoms in patients with FES. Accordingly, the present study directly compared FC of amygdala subregions between HC and patients with FES using resting-state functional MRI (fMRI) and examined the correlation between FC of amygdala subregions and clinical symptoms in patients with FES.
2. Materials and methods
2.1. Participants
Patients were recruited at the outpatient clinics and from inpatients of Chongqing Three Gorges Central Hospital, and HC were recruited using local advertisements in the same community in China from 2013 to 2015. This study initially enrolled 81 Chinese Han patients with FES and 48 HC. Three FES patients and one control were excluded due to head motion (mean framewise displacement > 0.2 mm) in the image preprocessing. Finally, 78 patients and 47 age-, sex-, and education-matched controls were included in this study.
Patients were diagnosed using the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems diagnostic criteria for schizophrenia (Saxena and Saraceno, 1993), and all patients had FES. The inclusion criteria were as follows: 1) age between 16–45 years; 2) right-handedness; 3) less than 2 years of illness; and 4) consumption of antipsychotic drugs for less than 2 weeks. Medication use was missing in 3 patients. The daily chlorpromazine equivalent dosages were shown in Table 1. The exclusion criteria for patients were as follows: 1) contraindications for fMRI; 2) neurological hard signs (history of severe head trauma, epilepsy, or loss of consciousness); 3) history of major medical illness; 4) drug abuse; 5) mental retardation caused by organic brain disease. The inclusion criteria for HC were: 1) were older than age 16 years and younger than age 45 years; 2) were right-handed; 3) no history of medical or neurological conditions 4) no history of psychiatric disorders. Exclusion criteria for the healthy control subjects were: 1) a family history of psychiatric disorders in a first degree relative; 2) current pregnancy; 3) any fMRI contraindications. 4) an Intelligence Quotient (IQ) < 70. All participants provided written informed consent after a full explanation of the study and were paid for their time.
Table 1.
Demographic and clinical characteristics of patients and healthy controls.
| Variables | Controls (n = 47) | Patients (n = 78) | X2/t | P |
|---|---|---|---|---|
| Sex (male/female) | 26/21 | 42/36 | 0.026 | 0.873 |
| Age (years) | 24.98 ± 5.33 | 24.65 ± 6.22 | 0.140 | 0.889 |
| Education (years) | 10.02 ± 2.98 | 9.68 ± 2.66 | 0.665 | 0.508 |
| IQ | 96.91 ± 13.31 | 72.31 ± 14.20 | 9.604 | < 0.00 |
| Mean FD (mm) | 0.06 ± 0.03 | 0.07 ± 0.04 | −1.714 | 0.089 |
| PANSS total scores | 75.00 ± 14.34 | |||
| P subscores | 19.85 ± 5.08 | |||
| N subscores | 16.86 ± 6.81 | |||
| G subscores | 38.29 ± 7.52 | |||
| CPZ equivalent dose (mg) | 600.53 ± 312.36 |
Data are presented as numbers or mean ± SD. Abbreviations: PANSS = Positive and Negative Syndrome Scale; P = positive symptom; N = negative symptom; G = general psychopathology symptom; IQ = Intelligence Quotient; FD = framewise displacement; CPZ = chlorpromazine.
2.2. Assessment
The Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) was used to assess clinical symptoms of patients by two trained psychiatrists. The Wechsler Adult Intelligence Scale–Fourth Edition (Wechsler, 2008) was used to assess IQ. The medication dosages (chlorpromazine equivalents) were calculated for each patient (Woods, 2003).
2.3. Image acquisition and preprocessing
Imaging was performed on a 3T SIEMENS MRI scanner at Chongqing Three Gorges Central Hospital. Foam pads and earplugs were used to reduce head motion and scanner noise, respectively. All participants were instructed to keep their eyes closed, relax, and not to fall asleep during scanning. The resting-state functional images were obtained with the following scanning parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90º, in-plane resolution = 64 × 64, field of view (FOV) = 200 mm × 200 mm, 32 axial slices, thickness/gap = 4/0 mm, and 210 vol (7 min). Sagittal three-dimensional T1–weighted images were acquired by employing a 3D-magnetization prepared rapid acquisition gradient echo sequence with the following parameters: TE = 2.98 ms, inversion time (TI) = 900 ms, TR = 2300 ms, FA = 9º, FOV = 240 mm × 256 mm, matrix size = 256 × 240, and thickness/gap = 1/0 mm. fMRI data preprocessing was performed using the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox, which is a part of DPABI (Yan et al., 2016) (version 3.2, http://rfmri.org/dpabi) and based on Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm) in MATLAB R2015b. For each participant, the first 10 volumes of functional data were removed to ensure that the magnetic resonance signal reached equilibrium. Preprocessing of the remaining data involved the following steps: 1) Slice timing correction; 2) realignment for head motion correction; 3) registration of individual T1 images to functional images; 4) regressing out the nuisance signals related to head motion (using the Friston-24 approach) and the signals related to the white matter and cerebrospinal fluid signals; 5) spatial normalization (resampled at 3 mm × 3 mm × 3 mm voxels); 6) spatial smoothing with a 4 mm full-width at half-maximum using a Gaussian kernel; 7) linear detrending; and 8) band-pass filtering (0.01–0.08 Hz). The global signal was not regressed out because its removal can introduce artifactual negative correlations (Nalci et al., 2017) and distort results when studying a clinical population (Hahamy et al., 2014) in resting-state FC (rsFC) analysis.
To reduce the effect of motion, we applied the Friston-24 model (Friston et al., 1996) to compute individual-level head motion and calculated framewise displacement (FD) for each participant based on the method proposed by Jenkinson et al. and Yan et al. (Jenkinson et al., 2002; Yan et al., 2013). The exclusion criterion of excessive head motion was a mean frame displacement of > 0.2 mm for all participants (Jenkinson et al., 2002). The mean FD was controlled as a covariate of no interest in the group-level statistical analyses. The mean FD did not differ between the HC and FES groups (P = 0.08).
2.4. Definition of amygdala subregions
Three amygdala subregions of interest (ROIs) for the rsFC analyses were defined in each hemisphere based on stereotaxic probabilistic maps of cytoarchitectonic boundaries using the Juelich histological atlas implemented in FSL (Amunts et al., 2005), as suggested by several previous studies (Etkin et al., 2009; Liu et al., 2014). Accordingly, the human amygdala was divided into three ROIs, i.e., the BLA, CMA, and SFA. The BLA is composed of the lateral nucleus, the basolateral nucleus, the medial nucleus, and the paralaminar nucleus; the CMA includes the medial and central nuclei; and the SFA includes the anterior amygdaloid area, the transition zone of the amygdala piriform cortex, the amygdala-hippocampus area, and the ventral and posterior cortical nucleus (Supplementary Fig. 1). Only voxels with at least a 50% probability of belonging to each subregion were included in an ROI. Each voxel was assigned to only one subregion.
2.5. Functional connectivity analysis
After the three ROIs had been normalized into the Montreal Neurological Institute space, we analyzed each rsFC. Briefly, Pearson's correlation coefficients between the time series of each ROI and time series of each voxel in the whole brain were computed based on voxel-based analysis, and a Fisher r-to-Z transform was then calculated to convert the correlation coefficients to z-values to improve the normality on an individual level.
Group-level functional connectivity analyses were conducted using DPABI. All analyses were corrected for multiple comparisons using the AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) in the DPABI, as suggested by Yan et al. (2016). One-sample t-tests were performed to generate group-level correlation maps for each amygdala subregion in the HC and FES groups. The statistical maps were created using a threshold of p < 0.05 in a voxel-level with p < 0.05 in a cluster level (cluster size > 459 voxels), corrected with AlphaSim. Previous studies on schizophrenia have demonstrated the effects of age (Gabard-Durnam et al., 2014), sex (Alarcón et al., 2015; Kilpatrick et al., 2006), and head motion (Van Dijk et al., 2012) on FC. Then, two-sample t-tests were performed to quantitatively compare differences in rsFC of each ROI between the HC and FES groups after controlling for IQ, age, sex, and mean FD. The results were examined at voxel-level p < 0.005 with cluster level p < 0.05 (cluster size >19), as determined by Alphasim correction.
Finally, brain regions with significant between-group rsFC differences were selected as ROIs. To investigate the relationship between the rsFC and symptoms, we extracted rsFC values of these ROIs by REST (http://www.restfmri.net) and calculated Pearson correlation coefficients between the rsFC z-values of brain regions with significant between-group differences and clinical symptoms in FES patients using SPSS 20.0. Follow-up analyses were done to explore the nature of relationships between amygdala subregional networks and symptom domains in PANSS. The statistical level of significance was set at P < 0.05. The PANSS total score and the three components of PANSS, including positive, negative, and general psychopathology symptoms, were used for the correlation analysis.
3. Results
3.1. Demographic and clinical characteristics of participants
Demographical and clinical characteristics of HC and FES patients are presented in Table 1. No significant differences in age, education, or sex (all ps > 0.5) were found between HC and FES patients. In addition, no significant differences in mean frame-wise displacement were observed between the two groups (p > 0.08). However, significant differences in IQ (p < 0.00) were found between the two groups (Table 1).
3.2. Within-group differences in rsFC of subregions
Positive rsFC of amygdala subregions was observed using the nominally significant threshold (p < 0.05) within the two groups. Patients showed similar patterns of rsFC between most of the amygdala subregions and other regions (Supplementary Fig. 2).
3.3. Between-group differences in rsFC of subregions
3.3.1. RsFC analyses of the CMA
Significantly increased positive rsFC between the right CMA and the right postcentral gyrus was found in the FES group compared with the HC group (Fig. 1A and Table 2).
Fig. 1.
Between-group comparison of rsFC of the right CMA and the right BLA. A two-sample t-test was used to assess the differences in rsFC of the right CMA (A) and the right BLA (B) between the two groups. The clusters represent the regions with significant group differences. The results were obtained with a threshold of p < 0.005 and an AlphaSim correction of p < 0.05. Mean functional connectivity intensity of the right CMA and the right BLA with different brain regions is shown in (C). Mean±standard error of the mean functional connectivity for clusters. Abbreviations: rsFC = resting-state functional connectivity; CMA = centromedial amygdala; BLA = basolateral amygdala; HC = healthy controls; FES = first-episode schizophrenia; L = left; R = right.
Table 2.
Brain regions showing significantly between-group differences in rsFC with the three amygdala subregions.
| Amygdala | Peal MNI coordinate |
|||||
|---|---|---|---|---|---|---|
| subregions | Brain regions | Cluster Size | X | Y | Z | T value |
| Right CMA | ||||||
| Right postcentral gyrus | 19 | 57 | −12 | 33 | −3.45 | |
| Right BLA | ||||||
| Left precentral gyrus | 49 | −60 | 0 | 30 | 4.40 | |
Positive T-values represent the regions showing hypoconnectivity in patients with FFS compared with HC. The results were obtained with a threshold of p < 0.005 and an AlphaSim correction of p < 0.05. Abbreviations: MNI = Montreal Neurological Institute; BLA = basolateral amygdala; CMA = centromedial amygdala; HC = healthy controls; FES = first-episode schizophrenia.
3.3.2. RsFC analyses of the BLA
Significantly decreased positive rsFC between the right BLA and the left precentral gyrus was observed in the FES group compared with the HC group (Fig. 1B and Table 2).
3.4. Correlation analysis
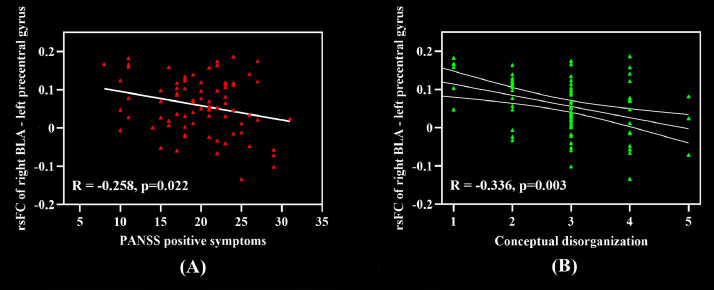
The rsFC z-values of brain regions showing significant differences between the two groups were used for correlation analyses with the clinical symptoms. Pearson correlation analyses revealed a significant negative correlation between right BLA-left precentral gyrus connectivity and PANSS positive symptom subscores in the FES group. No correlations between the other subregions and the clinical measures are observed in the FES group. Higher positive symptom scores were associated with decreased positive connectivity between the right BLA and left precentral gyrus (r = −0.258, p = 0.022). (Fig. 2A).
Fig. 2.
Correlation between altered rsFC of amygdala subregions and clinical symptoms in patients with FES. (A) Correlation between the right BLA connectivity and positive symptoms in patients with FES. In particular, patients with more severe positive symptoms showed decreased functional connectivity between the right BLA and the left precentral gyrus. (B) Correlation between the right BLA connectivity and conceptual disorganization in patients with FES. Abbreviations: rsFC = resting–state functional connectivity; BLA = basolateral amygdala.
To further investigate the nature of significant relationships between BLA Connectivity and positive symptom, we tested correlations between right BLA-left precentral gyrus connectivity that are significantly correlated with PANSS positive symptom scores and the global rating for each of the positive symptom domains from the PANSS. Connectivity between the right BLA and left precentral gyrus was negatively correlated with conceptual disorganization symptoms (PANSS-P2) (r = −0.336, p = 0.003) (Fig. 2B).
4. Discussion
The FES-related changes in the spontaneous brain activity were examined by assessing the FC of three amygdala subregions using resting-state fMRI. To our knowledge, this is the first study to systematically investigate rsFC changes of the amygdala subregions in patients with FES. We also investigated the relationship between the rsFC of amygdala subregions and positive symptoms. Our results demonstrated widespread disruptions of rsFC of amygdala subregions with regions of the motor cortex, including the left precentral gyrus and the right postcentral gyrus, at the early stage of schizophrenia. Moreover, the decreased BLA-precentral gyrus connectivity was negatively correlated with total positive symptoms in patients with FES patients and specifically with the positive symptom domains of conceptual disorganization.
This study found that patients with FES exhibited decreased rsFC between the BLA and precentral gyrus. The precentral gyrus has been demonstrated to be positively correlated with the BLA in HC (Roy et al., 2009), and our findings support this speculation. The precentral gyrus, a key region of the mirror neuron system, has been shown to play an important role in social cognition, including facial emotion expressing (Jáni and Kašpárek, 2018). As a sensory-motor cortical region of the amygdala-sensorimotor pathway, the precentral gyrus is involved in emotion processing (Schürmann et al., 2011). Functional and structural connections between the amygdala and precentral gyrus, particularly between the BLA and precentral gyrus, have been demonstrated in previous studies (Roy et al., 2009; Sripanidkulchai et al., 1984). Anatomically, the motor networks, including the precentral gyrus, can receive social-emotional stimuli inputs from the amygdala, specifically the BLA (Grèzes et al., 2014). Previous DTI studies (Grèzes et al., 2014; Rizzo et al., 2018) have confirmed that the amygdala and motor-related areas including precentral gyrus are connected via direct structural tracts traveling in the external capsule. Studies in patients with first episode schizophrenia have also reported FA reductions in the external capsule (Lee et al., 2013; Lyu et al., 2015). These suggests that disconnection between the BLA and precentral gyrus may exit due to the white matter deficits in the external capsule at the early stage of schizophrenia. In additional, distributed volume reduction in the precentral gyrus has been reported to be associated with deficits in sensory integration, which can lead to abnormal self-experience, such as conceptual disorganization (Dazzan et al., 2004). Dysfunction in motor networks involving the precentral gyrus has also been demonstrated to be associated with positive symptoms in schizophrenia (Berman et al., 2016; Bernard et al., 2017; Zhao et al., 2019). The disconnection of the limbic-motor network in schizophrenia may underlie the symptomatology and pathophysiology of schizophrenia (Berman et al., 2016). Together, these findings suggest that the BLA may modulate the activation of the precentral gyrus by inputting social stimuli, and the decreased BLA-precentral network may induce misconnection in social emotion information received by the precentral gyrus in schizophrenia, thus leading to impairments of motor and social function. For example, when processing social-emotional information, abnormal connections between the BLA and sensorimotor processing cortex may lead to deficits in predicting the consequences of actions or misunderstanding of others’ behavior (Shergill et al., 2005), resulting in positive symptoms in schizophrenia (Bernard et al., 2017). Further, white matter deficits in the external capsule was also reported in first-degree relatives of patients with schizophrenia (Lyu et al., 2015). In additional, recent fMRI study on motor-related networks also suggested that abnormal activation of sensory-motor cortex, especially motor cortex, also exits in people at clinical high-risk (CHR) of schizophrenia. And the abnormal activation in motor cortex was correlated with symptom severity in CHR individuals (Bernard et al., 2017). All these suggest that decreased connection between BLA and precentral gyrus may prior to the onset of psychosis during the clinical high risk period. However, it is unclear whether the decreased BLA-precentral network is a cause or an influencing factor based on our data alone. If the BLA-precentral network impairment would occur before the onset of schizophrenia, the assessment of BLA-precentral network might indeed provide a useful early biomarker to study the prevention and treatment in high-risk individuals before they develop schizophrenia.
To our knowledge, this is the first study to demonstrate that the dysfunctional connectivity of the BLA with the precentral gyrus is related to the severity of positive symptoms in schizophrenia. Decreased rsFC of the BLA with the precentral gyrus in our findings may provide new insights into the limbic-motor network underlying positive symptoms in schizophrenia.
In addition, we detected increased FC between the right CMA and the right postcentral gyrus in patients with FES. The postcentral gyrus, located in the somatosensory cortex, plays an important role in somatosensory processing, especially in the sensory-discrimination of external stimuli (Nelson and Chen, 2008). As the output station of the amygdala, the CMA can modulate and produce the corresponding physiological and behavioral responses after receiving the stimulus information (Roy et al., 2009). Evidence from animal studies has indicated that somatosensory areas can receive direct inputs from the pontine parabrachial (pPB) and are thought to send somatosensory information related to nociceptive information through the pPB–CMA pathway to the CMA (Bernard et al., 1993; Sah et al., 2003). Robust abnormalities in the postcentral gyrus have been demonstrated in schizophrenia in a meta-analytic study (Xiao et al., 2017), and postcentral gyrus dysfunction has been found to be correlated with clinical symptoms in FES (Zhao et al., 2019). The conjoint activation of the postcentral gyrus and amygdala has also been demonstrated to contribute to the rapid modulation of external stimuli, thereby promoting the production of adaptive behavioral responses (Grèzes et al., 2014). Numerous studies have shown that people with schizophrenia have sensory predictive dysfunction involving discrimination of their actions more than external stimuli, which is thought to underlie positive symptoms (Shergill et al., 2005, 2014). Together, the increased CMA-precentral gyrus connectivity in our study may represent a function compensation in the dysfunctional sensory predictive processing in FES. However, the strength of positive connectivity between the two regions was not significantly strong in the two groups, and individual variation of functional connectivity strength in samples may give a possible explanation.
In this study, we did not observe significantly decreased rsFC between the amygdala subregions and PFC region in schizophrenia when compared to the HC, which has been reported by Liu et al. (2014). Differences in connectivity patterns between studies may be related to differences in methodology of fMRI data processing, medication, and the course of disorder. Future studies are necessary to examine the dynamic relationship of amygdala subregions-PFC functional connectivity and individual differences in schizophrenia across wide course ranges, to explore whether the dysconnectivity of the amygdala subregions with PFC region in schizophrenia is a state-dependent phenomenon.
5. Conclusion
Resting-state fMRI was used to observe the rsFC of three amygdala subregions in patients with FES. We found that patients with FES had abnormal patterns of amygdala subregion-based functional networks compared with HC, suggesting a dysconnectivity of functional amygdala networks in schizophrenia. The decreased BLA-precentral gyrus network was negatively associated with the severity of positive symptoms in patients with FES, indicating that disruptive amygdala–motor networks may be associated with positive symptoms in patients with FES. This study suggests that disruptions in the amygdala subregion-based functional networks may be a neuropathological mechanism underlying the symptomatology of schizophrenia. Our findings provide some insights into the understanding of the neural mechanisms associated with positive symptoms in schizophrenia.
6. Limitations
Some limitations of the present study should be noted. First, the sample size was relatively small due to difficulties in recruiting patients with FES; therefore, further studies with a large sample size are needed to validate the present findings. Second, the cross-sectional comparison in the study prevented the observation of the dynamic rsFC patterns of the amygdala subregions in patients with schizophrenia. We found a significant correlation between rsFC of the amygdala subregions and positive symptoms; however, the relationship between them could not be determined. Therefore, further studies with a longitudinal design are needed to examine the changes in amygdala subregional rsFC and explore their effects on positive symptoms in schizophrenia. Third, the specific illness duration was not clear in patients; thus, duration effects on the results cannot be excluded. Therefore, whether changes in rsFC of the amygdala subregions are related to illness duration remains to be investigated. Fourth, the resolution of defining amygdala subregions is limited by the resolution of the functional images in this study. Our study would benefit from high functional image resolution by supporting more precisely defined amygdala subregions in the future. Acquisition with high spatial resolution can alleviate the effect of localization errors and distortions. Thus, a comparably high resolution fMRI combined with probabilistic anatomical maps may help to imagine amygdala subregions and to confirm our findings of functional connections between the amygdala subregions and sensorimotor regions in the future.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the ethics committee of the Beijing Huilongguan Hospital and with the Helsinki Declaration of 1975, as revised in 2008. All participants provided written informed consent after a full explanation of the study and were paid for their time.
Declaration of Competing Interest
All authors have no conflicts of interest to declare.
Acknowledgments
M.Z., F.D.Y., S.P.T., and L.E.H. wrote and revised the manuscript. M.Z. and F.M.F. analyzed the data. Z.R.W., F.M.F., and X.H. contributed to data collection and management. F.D.Y. and T.Y.L. provided essential suggestions and revised the manuscript. All authors have approved the final manuscript. This study was supported by grants from the National Natural Science Foundation of China (31671145) (SPT), the Beijing Municipal Natural Science Foundation (7162087) (SPT), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Grant number, XMLX201609) (SPT), the Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20182001) (FDY), and the Beijing Municipal Science & Technology Commission grant (D171100007017002) (F.D.Y). We thank all the participants in our study for their hard work and significant contributions to the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102218.
Contributor Information
Fude Yang, Email: yangfd2002@163.com.
Shuping Tan, Email: shupingtan@126.com.
Appendix. Supplementary materials
References
- Adhikari A., Lerner T.N., Finkelstein J., Pak S., Jennings J.H., Davidson T.J., Ferenczi E., Gunaydin L.A., Mirzabekov J.J., Ye L. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527:179–185. doi: 10.1038/nature15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Berman R.A., Gotts S.J., McAdams H.M., Greenstein D., Lalonde F., Clasen L., Watsky R.E., Shora L., Ordonez A.E., Raznahan A., Martin A., Gogtay N., Rapoport J. Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain. 2016;139:276–291. doi: 10.1093/brain/awv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., JRM G., Maldonado T. A case for motor network contributions to schizophrenia symptoms: evidence from resting-state connectivity. Hum Brain Mapp. 2017;38:4535–4545. doi: 10.1002/hbm.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.F., Alden M., Besson J.M. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J. Comp. Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin Zerrin, F. Haughton, V.M. Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brown V.M., Labar K.S., Haswell C.C., Gold A.L., Workgroup M.A.M., Mccarthy G., Morey R.A. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzan P., Morgan K.D., Orr K.G., Hutchinson G., Chitnis X., Suckling J., Fearon P., Salvo J., McGuire P.K., Mallett R.M., Jones P.B., Leff J., Murray R.M. The structural brain correlates of neurological soft signs in AESOP first‐episode psychoses study. Brain. 2004;127:143–153. doi: 10.1093/brain/awh015. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry. 2009;47:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I., Linke A.C., Hau J., Carper R.A., Müller R.A. Atypical functional connectivity of amygdala related to reduced symptom severity in children with autism. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57 doi: 10.1016/j.jaac.2018.06.015. 764-774.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K., Brown H.R., Siemerkus J., Stephan K.E. The dysconnection hypothesis (2016) Schizophr. Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari V.M., Sponheim S.R., MacDonald III A.W. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Lui S., Sweeney J.A. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am. J. Psychiatry. 2016;173:232–243. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- Gotts S.J., Simmons W.K., Milbury L.A., Wallace G.L., Cox R.W., Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J., Valabrègue R., Gholipour B., Chevallier C. A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Hum. Brain Mapp. 2014;35:5974–5983. doi: 10.1002/hbm.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A., Calhoun V., Pearlson G., Harel M., Stern N., Attar F., Malach R., Salomon R. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 2014;4:395–403. doi: 10.1089/brain.2014.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M., Murty V.P., Tervo-Clemmens B., Foran W., Luna B. Age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders: relevance to psychotic symptoms. Am. J. Psychiatry. 2019;176:196–207. doi: 10.1176/appi.ajp.2018.18040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jáni M., Kašpárek T. Emotion recognition and theory of mind in schizophrenia: a meta-analysis of neuroimaging studies. World J. Biol. Psychiatry. 2018;19:S86–86S96. doi: 10.1080/15622975.2017.1324176. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L.A., Zald D.H., Pardo J.V., Cahill L.F. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kubicki M., Asami T., Seidman L.J., Goldstein J.M., Mesholam-Gately R.I., McCarley R.W., Shenton M.E. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Iimaging (DTI) study. Schizophr. Res. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Tang Y., Womer F., Fan G., Lu T., Driesen N., Ren L., Wang Y., He Y., Blumberg H.P., Xu K., Wang F. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40:469–477. doi: 10.1093/schbul/sbt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu H., Hu M., Eyler L.T., Jin H., Wang J., Ou J., Guo X., He Z., Liu F., Zhao J., Guo W. Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Aust. N. Z. J. Psychiatry. 2015;49:246–254. doi: 10.1177/0004867414554268. [DOI] [PubMed] [Google Scholar]
- McCutcheon R.A., Reis Marques T., Howes O.D. Schizophrenia-An overview. JAMA Psychiatry. 2019:1–10. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- Nalci A., Rao B.D., Liu T.T. Global signal regression acts as a temporal downweighting process in resting-state fMRI. Neuroimage. 2017;152:602–618. doi: 10.1016/j.neuroimage.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Nelson A.J., Chen R. Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cereb. Cortex. 2008;18:2341–2351. doi: 10.1093/cercor/bhm257. [DOI] [PubMed] [Google Scholar]
- Owen M.J., Sawa A., Mortensen P.B. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.R., Jessica C., Kunj G., Dylan A. Schizophrenia: overview and treatment options. Peer-Rev. J. Formulary Manag. 2014;39:638–645. [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. Emotion processing and the amygdala: from a 'low road' to 'many roads' of evaluating biological significance. Nat. Rev. Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch A., Zhang W., Haak K.V., Mennes M., Hermans E.J., Oort E.V., Wingen G.V., Beckmann C.F., Buitelaar J.K., Groen W.B. Altered functional connectivity of the amygdaloid input nuclei in adolescents and young adults with autism spectrum disorder: a resting state fMRI study. Mol. Autism. 2016;7:13. doi: 10.1186/s13229-015-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G., Milardi D., Bertino S., Basile G.A., Di Mauro D., Calamuneri A., Chillemi G., Silvestri G., Anastasi G., Bramanti A., Cacciola A. The limbic and sensorimotor pathways of the human amygdala: a structural connectivity study. Neuroscience. 2018;385:166–180. doi: 10.1016/j.neuroscience.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E.S., De Armentia M L., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Saxena S., Saraceno B. WHO; 1993. The ICD-10 Classification of Mental and Behavioural Disorders; pp. 705–709. [Google Scholar]
- Schürmann M., Hlushchuk Y., Hari R. Embodied visual perception of distorted finger postures. Hum. Brain Mapp. 2011;32:612–623. doi: 10.1002/hbm.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill S.S., Samson G., Bays P.M., Frith C.D., Wolpert D.M. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., White T.P., Joyce D.W., Bays P.M., Wolpert D.M., Frith C.D. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry. 2014;71:28–35. doi: 10.1001/jamapsychiatry.2013.2974. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K., Sripanidkulchai B., Wyss J.M. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. J. Comp. Neurol. 1984;229:419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Bohlhalter S., Vanbellingen T., Federspiel A., Wiest R., Müri R.M., Strik W., Walther S. Limbic interference during social action planning in schizophrenia. Schizophr. Bull. 2018;44:359–368. doi: 10.1093/schbul/sbx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Baldeweg T., Friston K.J. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Pearson; San Antonio. TX: 2008. WAIS-Ⅳ Technical and Interpretive Manual. [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Xiao B., Wang S., Liu J., Meng T., He Y., Luo X. Abnormalities of localized connectivity in schizophrenia patients and their unaffected relatives: a meta-analysis of resting-state functional magnetic resonance imaging studies. Neuropsychiatr. Dis. Treat. 2017;13:467–475. doi: 10.2147/NDT.S126678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Wang X., Zuo X., Zang Y. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di M.A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yao J., Lv Y., Zhang X., Han C., Chen L., Ren F., Jin Z., Li Y., Sui Y. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging. Behav. 2019;13:503–513. doi: 10.1007/s11682-018-9882-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.