Summary
Development of synergistic heterogeneous catalysts with active sites working cooperatively has been a pursuit of chemists. Herein, we report for the first time the fabrication and manipulation of Pt-WO3 dual-active-sites to boost hydrogen generation from ammonia borane. A combination of DFT calculations, structural characterization, and kinetic (isotopic) analysis reveals that Pt and WO3 act as the active sites for ammonia borane and H2O activation, respectively. A trade-off between the promoting effect of WO3 and the negative effect of decreased Pt binding energy contributes to a volcano-shaped activity, and Pt/CNT-5W delivers a 4-fold increased activity of 710.1 molH2·molPt−1·min−1. Moreover, WO3 is suggested to simultaneously act as the sacrificial site that can divert B-containing by-products away from Pt sites against deactivation, yielding an increase from 24% to 68% of the initial activity after five cycles. The strategy demonstrated here could shed a new light on the design and manipulation of dual-active-site catalysts.
Subject Areas: Inorganic Chemistry, Catalysis, Nanomaterials
Graphical Abstract

Highlights
-
•
Mechanism-guided design of Pt-WO3 dual active sites boosts H2 generation
-
•
A trade-off between WO3 loading and Pt B.E. yields a volcano-shaped activity
-
•
WO3 acts as the sacrificial site to divert by-products against deactivation
-
•
Multifunctional Pt-MO/C catalysts achieve enhanced activity and durability
Inorganic Chemistry; Catalysis; Nanomaterials
Introduction
Noble metal catalysts are the workhorses for energy and environment applications, which enable the conversion of feedstock molecules to desired products (Pakhare and Spivey, 2014, Zhou et al., 2010). Nevertheless, their expenses and scarcity limit the viability for large-scale commercialization (Wu et al., 2011, Gong et al., 2012). Continuous efforts have been devoted to engineering the noble metal catalysts by tailoring their sizes, shapes, and compositions to improve the metal utilization efficiency and ultimately the catalytic performance (Aijaz et al., 2012, Zhang et al., 2009, Zecevic et al., 2013). Generally, the supported noble metal catalysts are often chemically and physically complex due to their multi-elemental, porous, and hierarchically structured natures, rendering their rational design and manipulation extremely challenging (Meirer and Weckhuysen, 2018, Yu et al., 2012, Shrestha et al., 2011). Fortunately, with the rapid development of computational chemistry, (micro) kinetics analysis, multiple characterization, isotope experiments, etc., it endows us with great opportunities to explore the reaction mechanism and kinetics with judicious interpretation of their results and then design highly efficient noble metal catalysts (Allian et al., 2011, Aramouni et al., 2018, Chin et al., 2011, Dasgupta et al., 2013, Hmadeh et al., 2014, Norskov et al., 2011, Pan and Bao, 2011, Qiao et al., 2011, Wu et al., 2012, Yamada et al., 2011).
Hydrogen is a well-known ideal energy carrier, and its safe and efficient storage as well as facile release is the key toward a hydrogen economy (Moussa et al., 2013, Turner, 2004, Diwan et al., 2011, Diakov et al., 2007, Demirkan et al., 2019, Sogut et al., 2019, Sen et al., 2018). With high hydrogen content (19.6 wt%), long-term stability, and nontoxicity at room temperature, ammonia borane (NH3BH3, AB) has been regarded as a promising hydrogen storage material (Yan et al., 2008, Valero-Pedraza et al., 2019, Yao et al., 2016, Li et al., 2017, Metin et al., 2010). Although a fast evolution of hydrogen from ammonia borane hydrolysis has been demonstrated by using noble metal catalyst, especially Pt and Ru, optimizing its activity and durability to minimize its usage is still of paramount importance (Zhang et al., 2017, Zhang et al., 2018, Akbayrak and Ozkar, 2012, Mori et al., 2016, Yan et al., 2017). Some studies have identified the interactions between Pt surface and H atom within ammonia borane and its resultant formation of activated complex species as the prerequisite to generate hydrogen (Chen et al., 2017a, Yang et al., 2011). To this end, it is highly desirable to engineer the properties of metal and substrate through two main approaches. One is alloying with other components to integrate multi-components with different properties (Wang et al., 2014, Zhang et al., 2009, Chen et al., 2017b). The other is tailoring the surface chemistry of catalyst support to obtain the targeted properties of supported metal (Chen et al., 2014a, Chen et al., 2015, Chen et al., 2018, Khalily et al., 2016, Lara et al., 2019).
Notably, rational catalyst design relies on understanding of the mechanism by which catalysts operate. Our recent studies on the kinetics and reaction mechanism of Pt-catalyzed ammonia borane hydrolysis (Chen et al., 2017a) indicate that the Pt catalyst displays a good capacity to dissociate the B–H bond within ammonia borane, but it is intrinsically inactive to dissociate the O–H bond within H2O, and the NH3BH2∗ assisted dissociation of O−H bond within H2O∗, i.e., NH3BH2∗+H2O∗→NH3BH2(OH)∗+H∗ as the rate-determining step (RDS). Moreover, DFT calculations reveal that pure Pt metal surface binds H2O too weakly to dissociate H2O, whereas some transition metal oxides exhibit great potentials for facile H2O dissociation (Ishikawa et al., 2002, Yang et al., 2017). Consequently, it is reasonable to assume that fabricating Pt-metal oxide synergistic catalyst with active sites working cooperatively could pave an effective way for hydrogen generation.
In addition to the hydrogen generation activity, the catalyst durability is another important criterion to evaluate the performance of metal catalysts, often more critical for noble metal catalysts. Our previous studies on highly active carbon-supported Pt catalysts have shown that the catalyst deactivation mainly arises from the agglomeration of Pt nanoparticles and the adsorption of poisonous B-containing by-products on the catalyst surfaces during the reaction (Chen et al., 2014b, Chen et al., 2015). Based on the reaction and deactivation mechanisms, tailoring Pt particle sizes and distributions as well as introducing more oxygen-containing groups and defects onto the carbon support surfaces has been demonstrated to effectively suppress the Pt agglomeration and/or to increase the Pt binding energy for the inhibited poison adsorption (Chen et al., 2014b, Chen et al., 2015, Zhang et al., 2017). Considering that introduction of metal oxides could help stabilize noble metal nanoparticles (Cao et al., 2010) and adsorb anions (Sverjensky and Fukushi, 2006), an attempt would be highly desirable to design Pt-metal oxide multi-functional catalyst by employing the metal oxide to act as anchoring and sacrificial sites against deactivation, thus not only enhancing the hydrogen generation activity mentioned above but also improving the catalyst durability.
Herein, we report a strategy to design and fabricate dual-active-site catalysts to boost hydrogen production from ammonia borane hydrolysis. DFT calculations were first carried out to assist the fabrication of Pt-WO3 dual-active-site synergistic catalyst. Along this line, a series of tungsten-incorporated CNT-γW were prepared to immobilize Pt particles with the same loading. Catalytic activity and durability tests were performed to explore the promotion effects of WO3 on the catalytic performance. A combination of comprehensive characterizations, kinetic (isotopic) investigations, and DFT calculations was employed to reveal the structure-performance relationship, and a dual-active-site mechanism was proposed to contribute to the simultaneously enhanced activity and durability. This provides a feasible avenue to design and develop dual-active-site catalysts by combining theoretical and experimental studies in this research area.
Results
DFT-Assisted Catalyst Design and Fabrication of CNT-γW
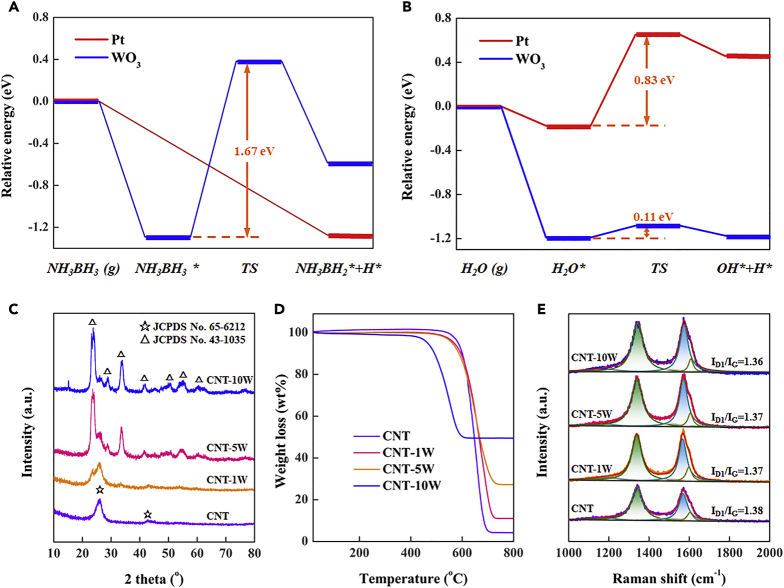
As discussed above, fabricating Pt-metal oxide might be an effective strategy to obtain synergistic catalyst, with dual active sites working cooperatively for the activation of ammonia broane and H2O. Exemplified with WO3, the adsorption and activation of ammonia broane and H2O on the representative WO3(100) and Pt(111) surfaces, as the thermodynamically stable and most exposed facets (Hurtado-Aular et al., 2020, Mahata and Pathak, 2017), were comparatively studied by DFT calculations. The optimized most stable adsorption configurations of the involved species on Pt(111) and WO3(100) surfaces are listed in Figure S1, and the corresponding potential energy profiles are displayed in Figures 1A and 1B, respectively. It can be obviously observed in Figure 1A that the ammonia borane dissociatively adsorbs on the Pt(111) surface, in comparison with the much higher activation barrier of 1.67 eV over the WO3(100) surface. This indicates that the Pt site facilitates the activation of ammonia borane with respect to the WO3 site. In contrary, the WO3 site (i.e., 0.11 eV) shows much lower activation barrier for H2O dissociation than the Pt site (i.e., 0.83 eV) as shown in Figure 1B, in consistent with the larger H-O bond elongation over the WO3(100) surface as shown in Figure S1. Therefore, it can be theoretically predicted that fabricating Pt-WO3 dual sites for acting as a synergistic catalyst can promote ammonia borane hydrolysis.
Figure 1.
DFT Calculations of Ammonia Borane and H2O Activation as well as Structural Characterization of CNT-γW
(A) Potential energy diagrams of ammonia borane activation over Pt(111) and WO3(100) surfaces.
(B) Potential energy diagrams of H2O activation over Pt(111) and WO3(100) surfaces.
(C) XRD patterns of CNT, CNT-1W, CNT-5W, and CNT-10W.
(D) TGA profiles of CNT, CNT-1W, CNT-5W, and CNT-10W.
(E) Raman spectra of CNT, CNT-1W, CNT-5W, and CNT-10W.
Considering that the low specific surface area of WO3 is unfavorable for Pt immobilization (Jin et al., 2017), carbon nanotube (CNT) with high external surface area, close ends, and mesoporous structure (Chen et al., 2014b, Chen et al., 2015) was employed as the catalyst support to enhance the specific surface area of WO3 by incorporating WO3 onto CNT for the following Pt immobilization. Specifically, the tungsten-incorporated supports were prepared by mixing pristine CNT with ammonium tungstate aqueous solutions of different concentrations (i.e., 1, 5 and 10 wt%) at 90°C for 10 h, followed by filtering, washing, drying, and calcination under Ar at 450°C for 2 h. The as-obtained samples were denoted as CNT-γW, in which γ represents the concentration of ammonium tungstate aqueous solution. X-ray diffraction (XRD) spectra in Figure 1C show that, after the incorporation of WO3, the CNT-γW samples exhibit some distinct characteristic diffraction peaks of WO3 (JCPDS No. 43-1035), in addition to the two characteristic diffraction peaks of graphite (JCPDS No. 65-6212) from the CNT. Notably, these diffraction peaks become intensive and sharp with the concentration of ammonium tungstate solution, indicating the increased amount and size of WO3 particles over the CNT-γW.
Thermal gravimetric analysis (TGA) was further carried out to determine the loadings of WO3 over the CNT-γW. As shown in Figure 1D, the residue weight of pristine CNT is estimated around 3.0 wt% originating from the metal catalyst of CNT growth, whereas that of CNT-γW samples increases with the concentration of ammonium tungstate aqueous solution. By excluding the weight of CNT growth catalyst, the loadings of WO3 over the CNT-1W, CNT-5W, and CNT-10W are determined as around 7.0, 24.1, and 47.2 wt%, respectively. It can also be seen that the onset of carbon support decomposition shifts to low temperature with the loading of WO3. This is most likely because the presence of WO3 reduces the thermal stability of CNT in air by catalyzing the low-temperature oxidation of CNT, which has been also observed in previous studies (Chiang et al., 2001, Xin and Li, 2011).
Raman measurements were conducted to probe whether the incorporation of WO3 affects the surface defects of CNT, for which the intensity ratio of D1 band at ∼1340 cm−1 to G band at ∼1570 cm−1 (ID1/IG) is used to quantify the surface defects (Sadezky et al., 2005). As shown in Figure 1E, the ID1/IG values of CNT, CNT-1W, CNT-5W, and CNT-10W are 1.38, 1.37, 1.37, and 1.36, respectively, indicating neglectable influences of the WO3 incorporation on the support surface defects. Low-magnification HAADF-STEM images of CNT-γW in Figures 2A–2C show that the WO3 appears as bright patches against the dark carbon matrix background. It can be seen that, for the CNT-1W with low WO3 loading, the bright patches distribute homogeneously across the CNT surface in small sizes. With the increase of WO3 loading, the density and size of these patches increase to high levels. In the high-magnification image of Figure 2D, some tiny WO3 nanoparticles appear as bright dots on the CNT-1W, which could be due to the low tungsten loading as well as the strong interaction between WO3 and CNT. In comparison, Figures 2E and 2F reveal that the CNT-5W and CNT-10W mainly consist of large strip-shaped particles along the CNT wall. Moreover, the HRTEM images in Figures 2G–2I exhibit continuous ordered lattice fringes, and the lattice spacings of ∼0.36 and ∼0.26 nm correspond to the (200) and (202) facets of WO3, respectively. These results are in good agreement with XRD results that the tungsten oxide species are mainly in the form of WO3, and the amount and size of WO3 particles sharply increases with the WO3 loading.
Figure 2.
HAADF-STEM and HRTEM Images of CNT-γW
(A–C) Low-magnification HAADF-STEM images of (A) CNT-1W, (B) CNT-5W, and (C) CNT-10W.
(D–F) High-magnification HAADF-STEM images of (D) CNT-1W, (E) CNT-5W, and (F) CNT-10W.
(G–I) HRTEM images of (G) CNT-1W, (H) CNT-5W, and (I) CNT-10W.
Synthesis and Structural Characterization of Pt-WO3 Dual Sites
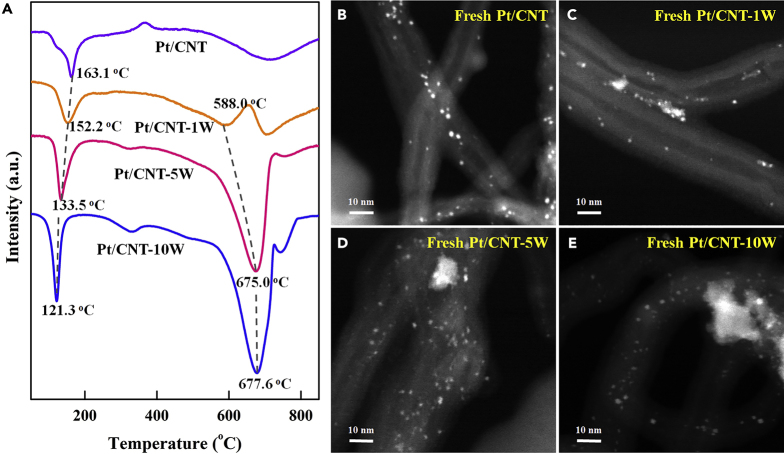
The above fabricated-tungsten-incorporated CNT-γW samples as well as the pristine CNT as a reference were impregnated with H2PtCl6 solutions to prepare the catalysts, with an aim to construct Pt-WO3 dual active sites. H2 temperature-programmed reduction (H2-TPR) measurement was first conducted to explore the interactions among Pt, WO3, and CNT. As shown in Figure 3A, for the pristine-CNT-supported Pt catalyst, two hydrogen consumption peaks could be observed at 163.1 and 709.3°C, which could be due to the reduction of platinum species and methanation of CNT support, respectively. However, for the Pt/CNT-γW catalysts, another new hydrogen consumption peak between the above two peaks is observed and ascribed to the reduction of WO3. Notably, the increasing WO3 loading leads to the upshift of WO3 reduction peak, which eventually overlaps with the methanation peak, possibly due to the formation of larger WO3 particles in Figures 1C and 2. Accordingly, the Pt species reduction peak shifts to low temperature with increasing WO3 loading, resulting from the interaction of Pt with WO3.
Figure 3.
H2-TPR Profiles and HAADF-STEM Images of Catalysts
(A) H2-TPR profiles of Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W.
(B–E) Typical HAADF-STEM images of the fresh (B) Pt/CNT, (C) Pt/CNT-1W, (D) Pt/CNT-5W, and (E) Pt/CNT-10W catalysts.
HAADF-STEM was employed to characterize the Pt particle size and distribution of these catalysts. Figure 3B reveals that the Pt particles on pristine CNT show a relatively homogeneous distribution, and the average Pt particle size based on the measurements of more than 200 random particles is determined to be 1.4 nm. In comparison, the Pt/CNT-1W in Figure 3C exhibits the coexistence of a few small patches, which could be WO3 clusters, with some small spots, which could be Pt and WO3 nanoparticles. Considering that the contrast variations in HAADF-STEM characterization are proportional to the square of the atomic number (Van Aert et al., 2011), the brighter and less bright spots could be ascribed to Pt and WO3 nanoparticles, respectively. Therefore, it can be seen that a majority of Pt nanoparticles remain on the graphitic wall of CNT, whose sizes are similar to those of Pt/CNT, and a few bright Pt spots are observed on the WO3 clusters.
As shown in Figures 3D and 3E, with the increase of WO3 loading, the density of bright spots becomes intensive for Pt/CNT-5W and Pt/CNT-10W, making it difficult to distinguish Pt from WO3 particles. Hence, energy dispersive spectroscopy (EDS) mapping of the selected areas in Figures 4A and 4B was further conducted to analyze the elemental distributions, and the results are shown in Figures 4C–4F. The EDS mapping of oxygen and tungsten in Figures 4D and 4E reveals that the Pt/CNT-5W mainly consists of large WO3 patches as well as a few dispersed particles, consistent with the HAADF-STEM results. Interestingly, as shown in Figure 4F, the EDS mapping of Pt suggests that the Pt particles mainly concentrate on WO3 patches instead of on CNT. Considering that the electron microscopic characterization could only reflect the local information of the sample, another two areas as depicted in Figures S2 and S3 were chosen by the same method to characterize this sample, so as to reduce the errors induced by the selected areas. Obviously, a majority of Pt particles still interact with WO3 patches rather than with CNT, and the corresponding Pt particle sizes still remain in the range of 1–2 nm, which is comparable to that for the pristine CNT. Hence, all the above results indicate that the Pt particles prefer to interact with the WO3 patches, which is consistent with the decreased reduction temperature as shown in Figure 3A.
Figure 4.
Atomic Distribution Characterization of Pt/CNT-5W Catalyst
(A) Typical HAADF-STEM image of Pt/CNT-5W.
(B) Typical HRTEM image of Pt/CNT-5W.
(C–F) The corresponding EDS mappings of (C) C, (D) O, (E) W, and (F) Pt elements.
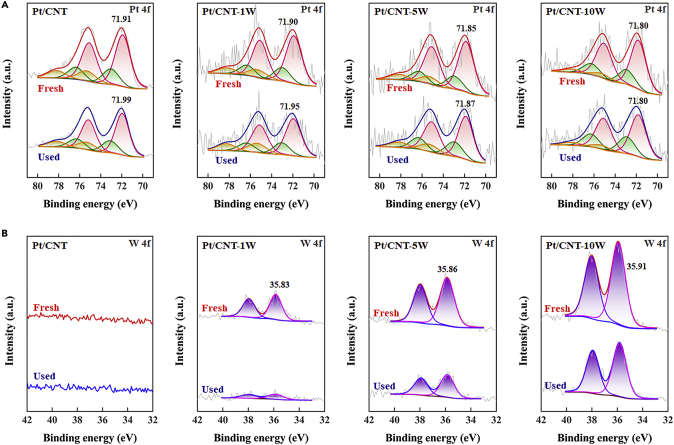
XPS was employed to investigate the electronic properties of these catalysts, and the results are shown in Figure 5. The Pt 4f region of XPS spectra in Figure 5A shows two intense peaks corresponding to Pt 4f7/2 and Pt 4f5/2, which can be deconvoluted into three pairs of doublets, i.e., Pt0, Pt2+, and Pt4+. Table S1 summarizes the binding energy (B.E.) as well as the corresponding percentage of Pt species. It can be seen that all the catalysts exhibit similar percentages of Pt0, which has been identified as the main active species for this reaction (Chandra and Xu, 2006). Interestingly, it is found that the Pt B.E. shifts to lower value with the WO3 loading. Correspondingly, the deconvolution of W 4f region in Figure 5B shows an opposite trend of W B.E. Considering the similar Pt particle sizes for these catalysts, the observed Pt B.E. downshift and W B.E. upshift are mainly ascribed to electron transfer between Pt and WO3. Specifically, WO3 could act as an electron donor and transfer electrons to Pt, giving rise to the electron-rich Pt particles with lower Pt B.E. Hence, with the increase of Pt-WO3 interactions, more and more electrons are transferred to Pt particles, resulting in the continuous decrease of Pt B.E. and increase of W B.E. for the Pt/CNT-γW catalysts.
Figure 5.
XPS Characterization of the Fresh and Used Catalysts
(A) Pt 4f spectra of the fresh and used catalysts.
(B) W 4f spectra of the fresh and used catalysts.
Kinetic (Isotopic) and Durability Analyses
Catalytic behaviors of these Pt-WO3 dual sites catalysts together with the reference Pt/CNT catalyst and CNT-5W were explored for ammonia borane hydrolysis, and the results are shown in Figures 6A and 6B. Obviously, the volume of hydrogen generation is proportional to the reaction time at the initial reaction stage (i.e., AB conversion lower than 45 ± 5%), suggesting pseudo-zero order kinetics for the reaction. As a result, the corresponding initial reaction rate (Rinitial) can be calculated based on the slope of linear part for each plot in Figure 6A. As shown in Figure 6C, the Rinitial values of Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W catalysts are determined as 165.2, 439.2, 710.1, and 557.5 molH2·molPt−1·min−1, respectively. By combining previous results that the high Pt B.E. is favorable for ammonia borane hydrolysis (Chen et al., 2014a, Chen et al., 2015), the Pt/CNT-γW catalysts with lower Pt B.E. than the Pt/CNT catalyst should give a lower hydrogen generation rate from the perspective of electronics, which is contradictory to the observation in Figure 6A. This strongly indicates that the fabrication of Pt-WO3 dual sites is favorable for ammonia borane hydrolysis, verifying the above theoretical prediction of Pt-WO3 acting as the dual active sites for this reaction.
Figure 6.
Catalytic Activity and Durability as well as Kinetics Analysis
(A) Hydrogen generation as a function of time for Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W at 30°C.
(B) Hydrogen generation as a function of time for CNT-5W, WO3, and Pt/WO3 at 30°C.
(C) Rinitial and Ea of Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, Pt/CNT-10W, and CNT-5W.
(D) Kinetic isotope effect (KIE) values of Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W.
(E) The relative activities over cycles of Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W.
(F) The ratio of the activity in the fifth cycle to that in the first run, R5th/R1st, for Pt/CNT, Pt/CNT-1W, Pt/CNT-5W, and Pt/CNT-10W.
To gain more insights into the reaction over such Pt-WO3 dual active sites, kinetics analyses of Pt/CNT-γW against Pt/CNT were performed, and the results are shown in Figures S4 and 6C. Clearly, the Pt/CNT-γW catalysts show lower activation energy (Ea) than the Pt/CNT catalyst, and the trend of Ea is consistent with that of Rinitial mentioned above. This suggests that the fabrication of Pt-WO3 dual active sites contributes to the enhanced kinetics. Furthermore, kinetic isotope experiments by replacing H2O with D2O as the reactant were conducted to probe the kinetic isotope effects and further the reaction mechanism (Chen et al., 2000), and the results are shown in Figures S5 and 6D. All the Pt/CNT-γW catalysts exhibit the similar kH/kD values (∼2.6), which are higher than that of the Pt/CNT catalyst (1.9). The change in the kH/kD is most likely ascribed to the change in the active sites, i.e., the single Pt site for the reaction over the Pt/CNT catalyst, but the dual Pt and WO3 sites for the reaction over the Pt/CNT-γW catalysts.
In addition, it was further explored whether the fabricated Pt/CNT-γW catalysts could also improve the catalytic durability. Herein, the catalyst durability was investigated by adding an equivalent ammonia borane solution after the completion of last cycle. As shown in Figure S6, all the catalysts show decreased catalytic activity as a function of catalytic cycle with different extents. To make a clear comparison, the reaction rate in each cycle was normalized to that of the first cycle. It can be seen in Figures 6E and 6F that the incorporation of WO3 remarkably promotes the catalytic durability of Pt/CNT, which increases with the WO3 loading. The pH values of the reaction solutions were firstly measured in the range of 9.1–9.8 for all the catalysts during the reaction. This could be ascribed to the acid-base equilibrium of B(OH)4-⇋B(OH)3+OH− for the B-containing byproducts in the reaction solution (Chen et al., 2017a), considering that the pKa value of acid-base couple of B(OH)3–B(OH)4- is 9.2 (Sanyal et al., 2011). Thus the similar pH values for the catalysts could help exclude their influences as the main reason for the significantly improved durability. To gain more insights, multiple characterizations were carried out to compare the catalyst properties before and after the durability test. HAADF-STEM measurements in Figures S7 and 3 indicate that some Pt agglomerations are observed after the durability test over Pt/CNT and Pt/CNT-1W, whereas no obvious agglomerations for Pt/CNT-5W and Pt/CNT-10W. This is most likely due to the strong interaction of Pt with WO3 to suppress Pt agglomeration, being one factor for the higher catalyst durability of the Pt/CNT-5W and Pt/CNT-10W catalysts.
As mentioned above, the adsorption of B-containing by-products on the Pt surfaces is another main factor leading to the catalyst deactivation (Chen et al., 2014b). The XPS spectra in Figure 5A and their deconvolution results in Table S1 show that the used Pt catalysts exhibit positive shifts in Pt B.E. compared with the fresh ones, mainly ascribed to the electron-deficient nature of the adsorbed B-containing by-products (Chen et al., 2018). Considering that the change in XPS signal intensity is proportional to the concentration of a given element per unit overlayer (Matrab et al., 2007, Precht et al., 2016), it has been previously used in our work (Chen et al., 2015) to compare the amount of species on the Pt surface. As shown in Figure 5, the used catalysts show slightly decreased XPS signal intensities for the Pt spectra but remarkably decreased ones for the W spectra in comparison with the fresh catalysts. This strongly suggests a preferential adsorption of B-containing by-products on the WO3 surface during the reaction. In other words, WO3 acts as the sacrificial site to preferentially adsorb B-containing by-products, which can suppress the adsorption of B-containing by-products on the Pt surfaces. Further combining the Pt/CNT-10W catalyst with the highest WO3 loading, it would provide a rational interpretation for the Pt/CNT-10W catalyst with the highest durability, i.e., retaining 68% of its initial activity at the fifth cycle (R5th/R1st) in comparison with 24% for the Pt/CNT catalyst, which mainly arises from the most sacrificial sites of WO3 for the adsorption of B-containing by-products.
Mechanism of Pt-WO3 Dual Sites for Enhanced Activity and Durability
As described above, DFT calculations show that the activation of ammonia borane reactant proceeds by its dissociative adsorption on the Pt site, and the WO3 site exhibits much lower activation barrier for H2O dissociation than on the Pt site. On a single Pt active site, ammonia borane hydrolysis over the Pt/CNT catalyst has been demonstrated to proceed by the NH3BH2∗-assisted cleavage of O-H bond within H2O (Chen et al., 2017a). Interestingly, on the Pt-WO3 dual active sites, the kinetics (isotope) analysis shows significantly decreased activation energy of the reaction, but an increase in kH/kD ratio from 1.9 to 2.6, which contribute to the enhanced kinetics for the reaction over the Pt/CNT-γW catalysts.
The strategy demonstrated above has been successfully developed to fabricate high specific surface area of WO3 by incorporating it onto the CNT with larger specific surface area, close ends, and mesoporous structure, which is a promising candidate to immobilize Pt for favorably constructing Pt-WO3 dual active sites and enhancing hydrogen generation activity. If directly using commercial WO3 (usually having relatively low specific surface area) as the catalyst or catalyst support, the catalyst testing results in Figure 6B show that the WO3 is almost inactive for the reaction, and the Pt/WO3 catalyst gives much lower hydrogen generation rate than the Pt/CNT-γW catalysts, mainly due to the larger Pt particle size and undesirable Pt B.E in Figure S8. Therefore, the fabrication of Pt-WO3 immobilized on the CNT is suggested as an effective way to obtain more Pt-WO3 dual active sites for the promoted reaction.
In our previous studies (Chen et al., 2014a, Chen et al., 2017b), it has been found that the electron-deficient Pt particles with the higher Pt B.E. facilitates the adsorption and activation of the reactants toward fast hydrogen evolution. In the present study, the incorporation of WO3 not only leads to the Pt/CNT-γW catalysts with lower Pt B.E. than the Pt/CNT catalyst, unfavorable for the reaction, but also fabricates highly active Pt-WO3 dual active sites, favorable for the reaction. Such trade-off between the promotion effects of WO3 and the negative electronic effects of Pt B.E. can pave an explanation for the observed volcano-shaped activity in Figure 6C.
In addition, the incorporation of WO3 also demonstrates remarkable enhancements in the durability. This is attributed to the preferential adsorption of B-containing by-products over the WO3 sites. As the reaction proceeds, the produced B-containing by-products would transfer and accumulate on the WO3 site instead of the Pt site. Considering that WO3 is in large excess, the accumulation of B-containing by-products has much less influence on the activity. Therefore, the WO3 site is also suggested as the sacrificial site, diverting B-containing by-products away from the Pt site during the reaction. With the increase of WO3 loading, more and more Pt sites in contact with WO3 sites are sterically protected from deactivation.
Based on the discussion of the mechanism of Pt-WO3 dual sites for the enhanced activity and durability, Scheme 1 is proposed to mainly illustrate ammonia borane hydrolysis over Pt-WO3 dual active sites, in which the Pt site and WO3 site contribute to the activation of ammonia borane and H2O, respectively. On the other hand, the WO3 site also acts as the sacrificial site to preferentially adsorb B-containing by-products, which can suppress the adsorption of B-containing by-products on the Pt surfaces. Such cooperativity between Pt and WO3 sites creates a unique synergy and demonstrates robust hydrogen generation from ammonia borane hydrolysis.
Scheme 1.

A Proposed Mechanism for Ammonia Borane Hydrolysis over Pt-WO3 Dual Metal Sites
In addition, Table 1 gives a comparison of the activity and durability of Pt/CNT-W catalysts developed in this study with various monometallic Pt-based catalysts in the literature. Obviously, the Pt/CNT-W catalysts show a superior hydrogen generation activity in addition to relatively high durability. Our previous studies have identified Pt(111) as the dominant active sites for the reaction, whose number reaches the optimal value at the mean Pt particle size of ∼1.8 nm (Chen et al., 2014b); and the high Pt B.E. is favorable for both the hydrogen generation activity and durability (Chen et al., 2014a). Thus, the fabricated Pt-WO3 dual sites enhance not only the hydrogen generation activity and kinetics due to the Pt-WO3 dual active sites but also the durability due to the sacrificial site of WO3 for the preferential adsorption of B-containing byproducts during the reaction. Moreover, it can be seen in Table 1 that the employment of carbon support plays a crucial role in Pt electronic properties to remarkably increase the catalytic activity, and the dual sites of Pt-metal oxides (MO, e.g., CeO2, Fe3O4 and TiO2) endow the catalysts with a significantly enhanced durability. All of these discussions could shed a new light on the rational design and manipulation of highly active and durable Pt-based catalysts for the reaction, e.g., fabricating Pt-MO/C multifunctional catalysts with appropriate transition metal and carbon toward significantly enhanced hydrogen generation activity and durability.
Table 1.
A Comparison of the Activity and Durability over Monometallic Pt-Based Catalysts for Ammonia Borane Hydrolysis
| Catalyst | dPt (nm) | Pt B.E. (eV) | R (molH2·molM−1·min−1) | Ea (kJ·mol−1) | Durability | Ref. |
|---|---|---|---|---|---|---|
| Commercial Pt/C | 2.5 | – | 83.3a,b | – | – | Chandra and Xu, 2007 |
| Pt/C | 1.9 | – | 111a,b | – | – | Chandra and Xu, 2007 |
| Pt(8%)/CCF-500 | 3.4 | 71.80 | 35c | 39.2 | 47% (r5th/r1st)a | Yuan et al., 2017 |
| (Zn’6)Pt/RGO | 1.2 | ∼71.5 | 284b | – | 49% (r5th/r1st)a | Chen et al., 2017c |
| SiO2@Pt@NGO | 1.9 | 71.12 | 324.6c | – | 39% (r6th/r1st) | Ye et al., 2017 |
| Pt20/CNT | 1.9 | 71.5 | 416.5b | 48.3 | 40% (r4th/r1st) | Zhang et al., 2017 |
| Pt/CNT-G | 2.8 | – | 135b | 35.3 | – | Uzundurukan and Devrim, 2019 |
| Pt/CNT-P | 1.4 | 71.9 | 141.7c | – | 24% (r5th/r1st) | Chen et al., 2015 |
| Pt/CNT-O | 1.6 | 71.7 | 54.4c | – | ~43% (r5th/r1st) | Chen et al., 2015 |
| Pt/CNT-D | 1.3 | 72.0 | 416.0c | – | ~62% (r5th/r1st) | Chen et al., 2015 |
| Pt/CNT-5W | ~1.4 | 71.85 | 710c | 27.8 | 45% (r5th/r1st) | This work |
| Pt/CNT-10W | ~1.4 | 71.80 | 558c | 28.7 | 68% (r5th/r1st) | This work |
| Pt-CeO2/rGO | 2.8 | 71.26 | 93.8b | 64.7 | 92% (r10th/r1st) | Yao et al., 2016 |
| Pt/SiO2 | 5.1 | – | 55a | – | – | Chandra and Xu, 2007 |
| Pt@MIL-101 | 1.8 | – | 414b | – | – | Aijaz et al., 2012 |
| Fe3O4@SiO2@Pt@mSiO2 | – | – | 5.5d | 35.4 | 62% (r5th/r1st)a | Xu et al., 2017 |
| Pt-CeO2 | 5.0 | – | 133b | – | 66% (r5th/r1st) | Wang et al., 2012 |
| SEA-Pt/HNTs | 1.5 | – | 321b | 49.2 | 71% (r10th/r1st) | Yin et al., 2019 |
| Pt25@TiO2 | 2.4 | 71.02 | 311b | – | 75% (r3rd/r1st) | Khalily et al., 2016 |
Estimated from the slope of the fitting line.
Measured under 25°C.
Measured under 30°C.
Measured under 35°C.
Discussion
In summary, we report for the first time the fabrication and manipulation of multifunctional Pt-WO3/CNT catalysts with simultaneously enhanced hydrogen generation activity and durability. A combination of DFT calculations and multiple characterizations with kinetic (isotopic) analysis demonstrates that the Pt-WO3 acts as the dual active sites for the activation of ammonia borane and H2O. By incorporating the WO3 onto CNT to immobilize Pt particles, the resultant Pt/CNT-γW catalysts give rise to more Pt-WO3 dual active sites with the desirable Pt B.E. than the commercial WO3-supported Pt catalyst. By increasing WO3 loading, a trade-off between the promotion effect of WO3 and the negative electronic effect of decreased Pt binding energy contributes the volcano-shaped activity, in which the Pt/CNT-5W delivers the highest catalytic activity of 710.1 molH2·molPt−1·min−1, more than four folds higher than that of pristine Pt/CNT. On the other hand, the WO3 site acts as the sacrificial site and diverts B-containing by-products away from the Pt site, thus inhibiting the catalyst deactivation during the reaction and yielding a significant increase from 24% to 68% of the initial catalytic activity after five cycles. This report not only shows a high potential to achieve robust hydrogen generation from ammonia borane hydrolysis but also guides the rational design and manipulation of dual-site catalysts with the multifunctional properties by combining theoretical and experimental studies.
Limitations of the Study
Currently, it is very difficult for this research to precisely characterize the microstructures of the fabricated highly active and durable Pt/CNT-γW catalysts at an atomic level. This would be facilitated by advanced in-situ/ex-situ catalyst characterization, such as Cs-corrected TEM and XAS. Then, more desirable theoretical models are needed for in-depth understanding of the reaction mechanism and kinetics to reveal the underlying dual-active-site mechanism.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (21922803 and 21776077), the Shanghai Natural Science Foundation (17ZR1407300 and 17ZR1407500), the China Postdoctoral Science Foundation (BX20190116), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the Shanghai Rising-Star Program (17QA1401200), the State Key Laboratory of Organic-Inorganic Composites (oic-201801007), and the Open Project of State Key Laboratory of Chemical Engineering (SKLChe-15C03).
Author Contributions
W.C. performed the catalysts preparation and characterization, catalytic measurements, and wrote the manuscript. W.F. and G.Q. contributed to theoretical calculations. B.Z. contributed to the HAADF-STEM characterization. D.C. and X.Z. helped to analyze the data and modify the paper. X.D. conceived the idea, analyzed the data, and wrote the paper. All authors contributed to the preparation of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100922.
Supplemental Information
References
- Aijaz A., Karkamkar A., Choi Y.J., Tsumori N., Ronnebro E., Autrey T., Shioyama H., Xu Q. Immobilizing highly catalytically active Pt nanoparticles inside the pores of metal-organic framework: a double solvents approach. J. Am. Chem. Soc. 2012;134:13926–13929. doi: 10.1021/ja3043905. [DOI] [PubMed] [Google Scholar]
- Akbayrak S., Ozkar S. Ruthenium (0) nanoparticles supported on multiwalled carbon nanotube as highly active catalyst for hydrogen generation from ammonia-borane. ACS Appl. Mater. Interfaces. 2012;4:6302–6310. doi: 10.1021/am3019146. [DOI] [PubMed] [Google Scholar]
- Allian A.D., Takanabe K., Fujdala K.L., Hao X., Truex T.J., Cai J., Buda C., Neurock M., Iglesia E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011;133:4498–4517. doi: 10.1021/ja110073u. [DOI] [PubMed] [Google Scholar]
- Aramouni N.A.K., Touma J.G., Tarboush B.A., Zeaiter J., Ahmad M.N. Catalyst design for dry reforming of methane: analysis review. Renew. Sustain. Energ. Rev. 2018;82:2570–2585. [Google Scholar]
- Cao A.M., Lu R.W., Veser G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010;12:13499–13510. doi: 10.1039/c0cp00729c. [DOI] [PubMed] [Google Scholar]
- Chandra M., Xu Q. A high-performance hydrogen generation system: transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources. 2006;156:190–194. [Google Scholar]
- Chandra M., Xu Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts. J. Power Sources. 2007;168:135–142. [Google Scholar]
- Chen K., Iglesia E., Bell A.T. Kinetic isotopic effects in oxidative dehydrogenation of propane on vanadium oxide catalysts. J. Catal. 2000;192:197–203. [Google Scholar]
- Chen W., Ji J., Duan X., Qian G., Li P., Zhou X., Chen D., Yuan W. Unique reactivity in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane. Chem. Commun. (Camb.) 2014;50:2142–2144. doi: 10.1039/c3cc48027e. [DOI] [PubMed] [Google Scholar]
- Chen W., Ji J., Feng X., Duan X., Qian G., Li P., Zhou X., Chen D., Yuan W. Mechanistic insight into size-dependent activity and durability in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2014;136:16736–16739. doi: 10.1021/ja509778y. [DOI] [PubMed] [Google Scholar]
- Chen W., Duan X., Qian G., Chen D., Zhou X. Carbon nanotubes as support in the platinum-catalyzed hydrolytic dehydrogenation of ammonia borane. ChemSusChem. 2015;8:2927–2931. doi: 10.1002/cssc.201500228. [DOI] [PubMed] [Google Scholar]
- Chen W., Li D., Wang Z., Qian G., Sui Z., Duan X., Zhou X., Yeboah I., Chen D. Reaction mechanism and kinetics for hydrolytic dehydrogenation of ammonia borane on a Pt/CNT Catalyst. AIChE J. 2017;63:60–65. [Google Scholar]
- Chen W., Li D., Peng C., Qian G., Duan X., Chen D., Zhou X. Mechanistic and kinetic insights into the Pt-Ru synergy during hydrogen generation from ammonia borane over PtRu/CNT nanocatalysts. J. Catal. 2017;356:186–196. [Google Scholar]
- Chen Y., Yang X., Kitta M., Xu Q. Monodispersed Pt nanoparticles on reduced graphene oxide by a non-noble metal sacrificial approach for hydrolytic dehydrogenation of ammonia borane. Nano Res. 2017;10:3811–3816. [Google Scholar]
- Chen W., Wang Z., Duan X., Qian G., Chen D., Zhou X. Structural and kinetic insights into Pt/CNT catalysts during hydrogen generation from ammonia borane. Chem. Eng. Sci. 2018;192:1242–1251. [Google Scholar]
- Chiang I.W., Brinson B.E., Smalley R.E., Margrave J.L., Hauge R.H. Purification and characterization of single-wall carbon nanotubes. J. Phys. Chem. B. 2001;105:1157–1161. [Google Scholar]
- Chin Y.H., Buda C., Neurock M., Iglesia E. Reactivity of chemisorbed oxygen atoms and their catalytic consequences during CH4-O2 catalysis on supported Pt clusters. J. Am. Chem. Soc. 2011;133:15958–15978. doi: 10.1021/ja202411v. [DOI] [PubMed] [Google Scholar]
- Dasgupta N.P., Liu C., Andrews S., Prinz F.B., Yang P. Atomic layer deposition of platinum catalysts on nanowire surfaces for photoelectrochemical water reduction. J. Am. Chem. Soc. 2013;135:12932–12935. doi: 10.1021/ja405680p. [DOI] [PubMed] [Google Scholar]
- Demirkan B., Kuyuldar E., Karatas Y., Gulcan M., Sen F. Ex situ synthesis and characterization of a polymer-carbon nanotube-based hybrid nanocatalyst with one of the highest catalytic activities and stabilities for the hydrolytic dehydrogenation of hydrazine-borane at room temperature conditions. J. Colloid Interface Sci. 2019;552:432–438. doi: 10.1016/j.jcis.2019.05.075. [DOI] [PubMed] [Google Scholar]
- Diakov V., Diwan M., Shafirovich E., Varma A. Mechanistic studies of combustion-stimulated hydrogen generation from sodium borohydride. Chem. Eng. Sci. 2007;62:5586–5591. [Google Scholar]
- Diwan M., Hwang H.T., Al-Kukhun A., Varma A. Hydrogen generation from noncatalytic hydrothermolysis of ammonia borane for vehicle applications. AIChE J. 2011;57:259–264. [Google Scholar]
- Gong F., Wang H., Xu X., Zhou G., Wang Z.S. In situ growth of Co0.85Se and Ni0.85Se on conductive substrates as high-performance counter electrodes for dye-sensitized solar cells. J. Am. Chem. Soc. 2012;134:10953–10958. doi: 10.1021/ja303034w. [DOI] [PubMed] [Google Scholar]
- Hmadeh M., Hoepfner V., Larios E., Liao K., Jia J., Jose-Yacaman M., Ozin G.A. New hydrogen-evolution heteronanostructured photocatalysts: Pt-Nb3O7(OH) and Cu-Nb3O7(OH) ChemSusChem. 2014;7:2104–2109. doi: 10.1002/cssc.201402173. [DOI] [PubMed] [Google Scholar]
- Hurtado-Aular O., Vidal A.B., Sierraalta A., Anez R. Periodic DFT study of water adsorption on m-WO3 (001), m-WO3 (100), h-WO3 (001) and h-WO3 (100). Role of hydroxyl groups on the stability of polar hexagonal surfaces. Surf. Sci. 2020;694:121558. [Google Scholar]
- Ishikawa Y., Liao M.S., Cabrera C.R. Energetics of H2O dissociation and COads+OHads reaction on a series of Pt-M mixed metal clusters: a relativistic density-functional study. Surf. Sci. 2002;513:98–110. [Google Scholar]
- Jin Y.X., Han D.M., Jia W.P., Li F., Chen X.Y., Huang G.B., Zhang D. WO3 modified graphene supported Pt electrocatalysts with enhanced performance for oxygen reduction reaction. Int. J. Electrochem. Sci. 2017;12:6535–6544. [Google Scholar]
- Khalily M.A., Eren H., Akbayrak S., Susapto H.H., Biyikli N., Ozkar S., Guler M.O. Facile synthesis of three-dimensional Pt-TiO2 Nano-networks: a highly active catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angew. Chem. Int. Ed. 2016;55:12257–12261. doi: 10.1002/anie.201605577. [DOI] [PubMed] [Google Scholar]
- Lara P., Philippot K., Suarez A. Phosphane-decorated platinum noparticles as efficient catalysts for H2 generation from ammonia borane and methanol. ChemCatChem. 2019;11:766–771. [Google Scholar]
- Li Z., He T., Liu L., Chen W., Zhang M., Wu G., Chen P. Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: from mechanistic study to catalyst design. Chem. Sci. 2017;8:781–788. doi: 10.1039/c6sc02456d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata A., Pathak B. Bimetallic core-based cuboctahedral core-shell nanoclusters for the formation of hydrogen peroxide (2e- reduction) over water (4e- reduction): role of core metals. Nanoscale. 2017;9:9537–9547. doi: 10.1039/c7nr03002a. [DOI] [PubMed] [Google Scholar]
- Matrab T., Save M., Charleux B., Pinson J., Cabet-Deliry E., Adenier A., Chehimi M.M., Delamar M. Grafting densely-packed poly (n-butyl methacrylate) chains from an iron substrate by aryl diazonium surface-initiated ATRP: XPS monitoring. Surf. Sci. 2007;601:2357–2366. [Google Scholar]
- Meirer F., Weckhuysen B.M. Spatial and temporal exploration of heterogeneous catalysts with synchrotron radiation. Nat. Rev. Mater. 2018;3:324–340. [Google Scholar]
- Metin O., Mazumder V., Ozkar S., Sun S. Monodisperse nickel nanoparticles and their catalysis in hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2010;132:1468–1469. doi: 10.1021/ja909243z. [DOI] [PubMed] [Google Scholar]
- Mori K., Miyawaki K., Yamashita H. Ru and Ru-Ni nanoparticles on TiO2 support as extremely active catalysts for hydrogen production from ammonia-borane. ACS Catal. 2016;6:3128–3135. [Google Scholar]
- Moussa G., Moury R., Demirci U.B., Sener T., Miele P. Boron-based hydrides for chemical hydrogen storage. Int. J. Energy Res. 2013;37:825–842. [Google Scholar]
- Norskov J.K., Abild-Pedersen F., Studt F., Bligaard T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. U S A. 2011;108:937–943. doi: 10.1073/pnas.1006652108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhare D., Spivey J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014;43:7813–7837. doi: 10.1039/c3cs60395d. [DOI] [PubMed] [Google Scholar]
- Pan X., Bao X. The effects of confinement inside carbon nanotubes on catalysis. Acc. Chem. Res. 2011;44:553–562. doi: 10.1021/ar100160t. [DOI] [PubMed] [Google Scholar]
- Precht R., Stolz S., Mankel E., Mayer T., Jaegermann W., Hausbrand R. Investigation of sodium insertion into tetracyanoquinodimethane (TCNQ): results for a TCNQ thin film obtained by a surface science approach. Phys. Chem. Chem. Phys. 2016;18:3056–3064. doi: 10.1039/c5cp06659j. [DOI] [PubMed] [Google Scholar]
- Qiao B., Wang A., Yang X., Allard L.F., Jiang Z., Cui Y., Liu J.Y., Li J., Zhang T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- Sadezky A., Muckenhuber H., Grothe H., Niessner R., Poschl U. Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon. 2005;43:1731–1742. [Google Scholar]
- Sanyal U., Demirci U.B., Jagirdar B.R., Miele P. Hydrolysis of ammonia borane as a hydrogen source: fundamental issues and potential solutions towards implementation. ChemSusChem. 2011;4:1731–1739. doi: 10.1002/cssc.201100318. [DOI] [PubMed] [Google Scholar]
- Sen B., Demirkan B., Levent M., Savk A., Sen F. Silica-based monodisperse PdCo nanohybrids as highly efficient and stable nanocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy. 2018;43:20234–20242. [Google Scholar]
- Sogut E.G., Acidereli H., Kuyuldar E., Karatas Y., Gulcan M., Sen F. Single-walled carbon nanotube supported Pt-Ru bimetallic superb nanocatalyst for the hydrogen generation from the methanolysis of methylamine-borane at mild conditions. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-52182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Liu Y., Mustain W.E. Electrocatalytic activity and stability of Pt clusters on state-of-the-art supports: a review. Catal. Rev. 2011;53:256–336. [Google Scholar]
- Sverjensky D.A., Fukushi K. Anion adsorption on oxide surfaces: inclusion of the water dipole in modeling the electrostatics of ligand exchange. Environ. Sci. Technol. 2006;40:263–271. doi: 10.1021/es051521v. [DOI] [PubMed] [Google Scholar]
- Turner J.A. Sustainable hydrogen production. Science. 2004;305:972–974. doi: 10.1126/science.1103197. [DOI] [PubMed] [Google Scholar]
- Uzundurukan A., Devrim Y. Carbon nanotube-graphene hybrid supported platinum as an effective catalyst for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy. 2019;44:26773–26782. [Google Scholar]
- Valero-Pedraza M.J., Cot D., Petit E., Aguey-Zinsou K.F., Alauzun J.G., Demirci U.B. Ammonia borane nanospheres for hydrogen storage. ACS Appl. Nano Mater. 2019;2:1129–1138. [Google Scholar]
- Van Aert S., Batenburg K.J., Rossell M.D., Erni R., Van Tendeloo G. Three-dimensional atomic imaging of crystalline nanoparticles. Nature. 2011;470:374–377. doi: 10.1038/nature09741. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu D., Song S., Zhang H. Synthesis of highly active Pt-CeO2 hybrids with tunable secondary nanostructures for the catalytic hydrolysis of ammonia borane. Chem. Commun. (Camb.) 2012;48:10207–10209. doi: 10.1039/c2cc33363e. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang D., Ma Y., Zhang H., Gao J., Nie Y., Sun X. Aqueous solution synthesis of Pt-M (M= Fe, Co, Ni) bimetallic nanoparticles and their catalysis for the hydrolytic dehydrogenation of ammonia borane. ACS Appl. Mater. Interfaces. 2014;6:12429–12435. doi: 10.1021/am502335j. [DOI] [PubMed] [Google Scholar]
- Wu G., More K.L., Johnston C.M., Zelenay P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science. 2011;332:443–447. doi: 10.1126/science.1200832. [DOI] [PubMed] [Google Scholar]
- Wu Y., Cai S., Wang D., He W., Li Y. Syntheses of water-soluble octahedral, truncated octahedral, and cubic Pt-Ni nanocrystals and their structure-activity study in model hydrogenation reactions. J. Am. Chem. Soc. 2012;134:8975–8981. doi: 10.1021/ja302606d. [DOI] [PubMed] [Google Scholar]
- Xin F., Li L. Decoration of carbon nanotubes with silver nanoparticles for advanced CNT/polymer nanocomposites. Compos.Part A Appl. Sci. Manuf. 2011;42:961–967. [Google Scholar]
- Xu D., Cui Z., Yang J., Yuan M., Cui X., Zhang X., Dong Z. Pt nanoparticles immobilized in mesoporous silica-coated magnetic nanocapsules: a non-leaching catalyst for hydrogen generation from hydrolysis of ammonia borane. Int. J. HydrogenEnergy. 2017;42:27034–27042. [Google Scholar]
- Yamada Y., Tsung C.K., Huang W., Huo Z., Habas S.E., Soejima T., Aliaga C.E., Somorjai G.A., Yang P. Nanocrystal bilayer for tandem catalysis. Nat. Chem. 2011;3:372–376. doi: 10.1038/nchem.1018. [DOI] [PubMed] [Google Scholar]
- Yan J.M., Zhang X.B., Han S., Shioyama H., Xu Q. Iron-nanoparticle-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Angew. Chem. Int. Ed. 2008;47:2287–2289. doi: 10.1002/anie.200704943. [DOI] [PubMed] [Google Scholar]
- Yan H., Lin Y., Wu H., Zhang W., Sun Z., Cheng H., Liu W., Wang C.L., Li J.J., Huang X.H. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 2017;8:1070. doi: 10.1038/s41467-017-01259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Cheng F., Tao Z., Chen J. Hydrolytic dehydrogenation of ammonia borane catalyzed by carbon supported Co core-Pt shell nanoparticles. J. Power Sources. 2011;196:2785–2789. [Google Scholar]
- Yang Y., Niu S., Han D., Liu T., Wang G., Li Y. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 2017;7:1700555. [Google Scholar]
- Yao Q., Shi Y., Zhang X., Chen X., Lu Z.H. Facile synthesis of platinum-cerium (IV) oxide hybrids arched on reduced graphene oxide catalyst in reverse micelles with high activity and durability for hydrolysis of ammonia borane. Chem. Asian J. 2016;11:3251–3257. doi: 10.1002/asia.201601147. [DOI] [PubMed] [Google Scholar]
- Ye W., Ge Y., Gao Z., Lu R., Zhang S. Enhanced catalytic activity and stability of Pt nanoparticles by surface coating of nanosized graphene oxide for hydrogen production from hydrolysis of ammonia-borane. Sustain. Energy Fuels. 2017;1:2128–2133. [Google Scholar]
- Yin L., Feng Y., Zhou X., Dai K., Gao X., Zhao Y., Zhang B. Synthesis of Pt nanocatalyst supported on halloysite nanotubes via strong electronic adsorption for hydrolytic dehydrogenation of ammonia borane. Chem. Lett. 2019;48:1084–1087. [Google Scholar]
- Yu W., Porosoff M.D., Chen J.G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem. Rev. 2012;112:5780–5817. doi: 10.1021/cr300096b. [DOI] [PubMed] [Google Scholar]
- Yuan M., Cui Z., Yang J., Cui X., Tian M., Xu D., Ma J., Dong Z. Ultrafine platinum nanoparticles modified on cotton derived carbon fibers as a highly efficient catalyst for hydrogen evolution from ammonia borane. Int. J. Hydrogen Energy. 2017;42:29244–29253. [Google Scholar]
- Zecevic J., van der Eerden A.M., Friedrich H., de Jong P.E., de Jong K.P. Heterogeneities of the nanostructure of platinum/zeolite Y catalysts revealed by electron tomography. ACS Nano. 2013;7:3698–3705. doi: 10.1021/nn400707p. [DOI] [PubMed] [Google Scholar]
- Zhang X.B., Yan J.M., Han S., Shioyama H., Xu Q. Magnetically recyclableFe@Pt core-shell nanoparticles and their use as electrocatalysts for ammonia borane oxidation: the role of crystallinity of the core. J. Am. Chem. Soc. 2009;131:2778–2779. doi: 10.1021/ja808830a. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen C., Chen S., Hu Q., Gao Z., Li Y., Qin Y. Highly dispersed Pt nanoparticles supported on carbon nanotubes produced by atomic layer deposition for hydrogen generation from hydrolysis of ammonia borane. Catal. Sci. Technol. 2017;7:322–329. [Google Scholar]
- Zhang J., Chen W., Ge H., Chen C., Yan W., Gao Z., Gan J., Zhang B.Y., Duan X.Z., Qin Y. Synergistic effects in atomic-layer-deposited PtCox/CNTs catalysts enhancing hydrolytic dehydrogenation of ammonia borane. Appl. Catal. B Environ. 2018;235:256–263. [Google Scholar]
- Zhou Y., Neyerlin K., Olson T.S., Pylypenko S., Bult J., Dinh H.N., Gennett T., Shao Z.P., O'Hayre R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energ. Environ. Sci. 2010;3:1437–1446. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.