Abstract
Objective
To assess cognitive functioning in adolescents (12–17 years old) with schizophrenia during open-label treatment with paliperidone extended-release (pali ER).
Methods
In this exploratory analysis, adolescents treated with pali ER (oral, flexibly dosed, 1.5–12 mg/day) underwent cognitive assessments at baseline and month 6 using a battery of cognitive tests validated in adolescents. Correlation analysis was used to explore the relationship between cognitive assessments and clinical symptoms (Positive and Negative Syndrome Scales [PANSS] and factors) and functionality (Children Global Assessment Scale [CGAS]) at baseline and at 6 months.
Results
A total of 324 of 393 patients had evaluable neurocognitive data. Changes in cognition function tests from baseline to endpoint were generally small to modest, with improvement noted for most cognitive domains (motor speed, attention/working memory, verbal learning and memory, social cognition, speed of processing, executive functioning). No improvement was noted for visual learning and memory. At baseline, there were modest negative correlations between disorganized thoughts and most cognitive domains; these correlations persisted at 6 months. Other significant negative correlations at 6 months were between speed of processing and PANSS total score, positive symptoms, negative symptoms and uncontrolled hostility (p < 0.05). At 6 months, higher CGAS scores (improved functioning) positively correlated with speed of processing and executive functioning, especially among pali ER responders.
Conclusions
In this large sample of adolescents with schizophrenia, frank cognitive deficits across multiple domains were observed. Treatment with pali ER over 6 months did not worsen neurocognitive functioning and was possibly associated with positive improvement in certain domains.
Keywords: Cognitive functioning, Schizophrenia, Paliperidone-extended-release
1. Introduction
Schizophrenia is a serious, lifelong illness with devastating effects on development and functioning. Individuals with early onset of schizophrenia (<18 years of age) often have a worse clinical course and poor achievement in school (Rapoport and Gogtay, 2011). In addition, children and adolescents with schizophrenia have significant cognitive deficits, which are persistent and continue into adulthood (Kremen et al., 2010). Cognitive deficits are also present in children who go on to develop schizophrenia in adulthood, suggesting that these deficits likely pre-date the index diagnosis (Reichenberg et al., 2010). A decline in cognitive performance during adolescent years has been associated with schizophrenia and psychosis more broadly (MacCabe et al., 2013) and may be predictive of risk even when occurring prior to prodromal symptoms (Gur et al., 2014; Kendler et al., 2016). Generally, cognitive deficits are modestly improved with antipsychotic treatment but often persist despite improvements in psychotic symptoms or functioning (Cervellione et al., 2007). Although treatment with atypical antipsychotic medications can significantly and rapidly alleviate positive and disorganized symptoms, neurocognitive symptoms may take longer to recover, and some deficits may remain despite effective antipsychotic treatment (Emsley et al., 2006). As no known treatments exist for cognitive symptoms of schizophrenia, these deficits contribute more to poor overall functioning than psychotic symptoms and may result in chronic disability (e.g. inability to work or live autonomously) underscoring the important unmet need for medicines that stabilize or improve cognition.
Paliperidone extended-release (pali ER) is approved in the United States (US), European Union (EU) and many other countries for treating schizophrenia in adults, and in the US, EU, China, and many other regions for treating schizophrenia in adolescents (12–17 years; EU: 15–17 years) (Invega, product information). Several studies have demonstrated short-term and long-term efficacy and safety of paliperidone in adolescents with schizophrenia (Savitz et al., 2015a; Savitz et al., 2015b; Singh et al., 2011). Although it is known that paliperidone may lead to additional cognitive and social functional improvements in adults and elderly patients (Singh et al., 2011; Suzuki et al., 2014), little information is available on the long-term effect of pali ER on cognitive function in adolescents with schizophrenia. A long-term (2 year), open-label (OL), flexible-dose pali ER safety study (NCT00488319) (Savitz et al., 2015a) demonstrated that the safety profile of pali ER in adolescents was consistent with the known profile of pali ER in adults (Emsley et al., 2008; Marder et al., 2007; Meltzer et al., 2008) and of risperidone in adolescents (Emsley et al., 2008; Findling et al., 2010; Haas et al., 2009; Harrington and English, 2010) as well as its long-term efficacy in treating the symptoms of adolescent schizophrenia. The current exploratory analysis from this OL pali ER study was designed to evaluate the change in cognitive functioning and whether there is a relationship between cognitive deficits and functioning, and to examine correlations between functioning and cognition among patients demonstrating improvement with 6-month pali ER treatment in adolescents with schizophrenia.
2. Methods
The primary long-term safety study with details of study design, inclusion and exclusion criteria is described elsewhere (Savitz et al., 2015a). Briefly, adolescents (12–17 years old) with schizophrenia (diagnosed as per the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [DSM-IV] criteria) were enrolled. Patients could enter the study directly or were eligible after completion of a double-blind, placebo-controlled, 6-week efficacy study of pali ER or after discontinuation of that study due to lack of efficacy but having completed a minimum of 21 days of the study. Patients were excluded if they had a diagnosis of major psychiatric disorder other than schizophrenia or suspected history of substance dependence according to the DSM-IV criteria in the 3-months before screening. Pali ER was flexibly dosed from 1.5–12 mg/day. The study consisted of a screening phase and washout phase of a maximum of 21 days (only for patients who directly enrolled), an open-label treatment phase of up to 2 years, and a post-treatment (follow-up) visit 1 week after the patient's final dose of study drug. Prior to study amendment, the initial study duration was 6 months and some patients had an option to discontinue after receiving 6 months of treatment.
An Independent Ethics Committee or Institutional Review Board approved the protocol for each site; ethical standards were followed in accordance with the Declaration of Helsinki and consistent with ICH Good Clinical Practices, along with local regulatory requirements. Informed consent was obtained from parents or legal guardians for each participant, along with the adolescent's assent.
2.1. Assessments
The efficacy endpoint was the change from baseline in the positive and negative symptom scale (PANSS) total score at the end of the OL phase. Other endpoints such as Marder factor scores [positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility and anxiety/depression] and Children Global Assessment Scale (CGAS) were also evaluated. Cognition was an exploratory endpoint in this study. Cognitive data were collected for the first 6 months of this 2-year study to evaluate the impact of treatment with pali ER on cognitive functioning. Cognitive assessments were performed at baseline and 6 months using a battery of cognitive tests validated for use in adolescents with Schizophrenia. This battery assesses multiple cognitive domains, comparable to those assessed in the Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) cognition assessment battery used for assessment in adults. Seven cognitive domains were assessed: speed of processing, motor speed, attention/working memory, visual learning and memory, executive functioning (reasoning and problem solving) and social cognition (Table 1). Language-based tests that were only available in English were either replaced with alternate versions (i.e., Trails A and B with Color Trails Test), or eliminated for non-English speaking patients (i.e., California Verbal Learning Test [CVLT]).
Table 1.
Summary of neurocognitive domains and tests.
| Cognitive domain | Test used | Standard units |
|---|---|---|
| Speed of processing | Phonetic Verbal Fluency, Semantic Verbal Fluency, Trail Making Test- Part A | Z-score (mean = 0, SD = 1) |
| Child Color Trails Test 1 & 2 | T-score (mean = 50, SD = 10) | |
| Motor speed | Finger Tapping Test | Z-score (mean = 0, SD = 1) |
| Attention/working memory | Wechsler Intelligence Scale for Children (WISC-IV)/Wechsler Adult Intelligence Scale (WAIS-III) Digit Span, WISC-IV/WAIS-III Digit Symbol Coding | Scaled score (mean = 10, SD = 3) |
| Visual learning and memory | Rey Complex Figure Test (Immediate Recall, Delayed Recall, and Recognition) | Z-score (mean = 0, SD = 1) |
| Verbal learning and memory | California Verbal Learning Test (CVLT)-C/CVLT-II (Immediate and Short Delay, Delayed Recall and Recognition) | T-score (mean = 50, SD = 10) and Z-score (mean = 0, SD = 1) |
| Wide Ranging Assessment of Memory and Learning (WRAML), Story Memory (Immediate Recall, Delayed Recall, and Recognition) | Scaled score (mean = 10, SD = 3) | |
| Executive functioning (reasoning and problem solving) | Wisconsin Card Sort Test (WCST) | T-score (mean = 50, SD = 10) |
| Trail Making Test-Part B | Z-score (mean = 0, SD = 1) | |
| Social cognition | Theory of Mind Test | Total score (raw score, values not standardized) |
2.2. Statistical analysis
Neurocognitive data were evaluated in all patients of the intent-to-treat (ITT) analysis set (all enrolled patients who received at least one dose of study medication and had both baseline and at least one postbaseline assessment for any of the efficacy variables) and had a baseline neurocognitive assessment score for any test.
Each completed cognitive assessment battery was collected and scored by a centralized group. Raw scores for each relevant variable for each cognitive test were computed and tabulated. Neurocognitive test results (raw scores) were compared with age- and (where available) sex-based normative data and converted to standardized units (z-scores or T-scores). Reference data were not available for the Theory of Mind test, so only raw scores were used to reflect performance on this test. The absolute score cannot be fully interpreted for clinical meaning, however, change from baseline can be used to note improvement. Scaled score data for each neurocognitive test were summarized descriptively, and the change from OL baseline to 6-month endpoint was presented for each of the tests using the last observation carried forward (LOCF) approach.
Correlation analysis was used to explore the relationship between cognitive assessments and clinical symptoms (PANSS total score, factors [positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility and anxiety/depression] and functioning [CGAS]). Correlations among patients treated with pali ER with improved symptom scores (responder) as assessed by at least 20% improvement in PANSS total score at month 6 were also conducted. Differences in mean changes from baseline to month 6 in selected neurocognitive scores were assessed by (1) responder status, and (2) use of anticholinergic medications. No adjustment for multiple comparisons was undertaken.
3. Results
3.1. Patient demographics
Overall, 400 patients were enrolled in this OL study conducted in 10 countries (Bulgaria, Estonia, Finland, India, Korea, Poland, Romania, Russia, Ukraine, the US). Of these, 220 patients (55%) completed the study while 180 (45%) withdrew from the study due to patient choice (17%), lack of efficacy (11%), adverse events (AEs, 7%), lost to follow up (6%), or other reasons (4%). A total of 393 patients were included in the ITT analysis set. The mean (SD) age of patients was 15.4 (1.55) years, 61% were boys, and 67% were white. Mean (SD) baseline PANSS score before OL start was 91.0 (12.92) (Table 2).
Table 2.
Baseline and demographic characteristics (intent-to-treat analysis set) for patients with evaluable cognitive scores.
| Total (N = 324) | |
|---|---|
| Agea (years), mean (SD) | 15.4 (1.54) |
| Age category, n (%) | |
| 12–14 years | 83 (25.6) |
| 15–17 years | 238 (73.5) |
| >17 years | 3 (0.9) |
| Sex, n (%) | |
| Boys | 203 (62.7) |
| Girls | 121 (37.3) |
| Race, n (%) | |
| White | 227 (70.1) |
| Black | 21 (6.5) |
| Asian | 75 (23.1) |
| Otherb | 1 (0.3) |
| Weight (kg), mean (SD) | 62.7 (15.03) |
| Baseline body weight category, n (%) | |
| <51 kg | 68 (21.0) |
| ≥51 kg | 256 (79.0) |
| Body mass index (kg/m2), mean (SD) | 22.2 (4.42) |
| Age at diagnosis of schizophrenia, mean (SD) (years) | 13.1 (2.41) |
| Schizophrenia type, n (%) | |
| Paranoid | 248 (76.5) |
| Disorganized | 24 (7.4) |
| Catatonic | 6 (1.9) |
| Undifferentiated | 42 (13.0) |
| Residual | 4 (1.2) |
| Baselinea PANSS total, mean (SD) | 83.2 (19.44) |
| PANSS Factor Scores, mean (SD) | |
| Positive symptoms | 22.9 (6.28) |
| Negative symptoms | 21.5 (6.07) |
| Disorganized thoughts | 19.6 (5.65) |
| Uncontrolled hostility/excitement | 9.8 (3.81) |
| Anxiety/depression | 9.4 (3.25) |
| Baselinea CGAS total, mean (SD) | 50.5 (13.85) |
Patients entered the open-label study with differing exposure to paliperidone ER, depending on whether they entered the open-label trial directly or from having participated in the previous double-blind trial.
Start of open-label study.
Includes one Hispanic patient and one patient classified as multi-racial (Black, Chinese, White, Indian). CGAS, Children Global Assessment Scale; PANSS, Positive and Negative Symptom Score.
A total of 324 patients had evaluable neurocognitive data at baseline and at least one other time point during the 6-month evaluation period; the number of patients with evaluable data varied between tests. Demographic characteristics of patients with evaluable cognitive data were generally similar to those of the overall study population. Seven countries participated in the cognitive testing portion of the study (India, Korea, Russia, Poland, Romania, Ukraine, and the US).
3.2. Cognitive changes with paliperidone treatment
At OL baseline, patients were 0.5 to 2 standard deviations below normative reference scores for same-age peers in cognitive performance across most neurocognitive tests, with the exception of Wisconsin Card Sort Test (WCST) Total Errors, which was in the average range (Table 3). Changes in cognitive test scores from baseline to endpoint were generally small to modest, with improvement noted on most tests and in most cognitive domains. No notable improvement was observed for the visual learning and memory domain. However, small improvements from baseline to endpoint were observed for the domains of motor speed, attention/working memory, verbal learning and memory, social cognition, speed of processing, and executive functioning (reasoning and problem solving) (Table 3). In the executive functioning domain, the Trail Making Test-B-showed slight worsening, while the more extensive and complex WCST-Total Errors showed improvement. There was either a slight improvement or slight worsening in the speed of processing domain: Child Color Trails Test-1 Time, Phonetic Verbal Fluency, and Semantic Verbal Fluency improved slightly, while graphomotor speed worsened slightly (Table 3). Given the potential impact of somnolence and concomitant medications such as anticholinergic drugs on cognition, the data was reviewed. A total of 18.3% of patients experienced somnolence as an AE at any time during the open-label period – somnolence (time-limited). EPS-related AEs were mostly mild to moderate in severity, and only 5 patients in the overall study discontinued due to a potential EPS-related AE. The overall incidence of tremor during the entire open-label period was 11%, although it is unclear whether this accounted for changes in graphomotor speed.
Table 3.
Mean change in neurocognitive test scores from baseline to month 6 (intent-to-treat-analysis set).
| Neurocognitive test | Baseline mean (SD) | Endpoint mean (SD) | Change from baseline mean (SD) |
|---|---|---|---|
| Speed of processing | |||
| Phonetic Verbal Fluency–Total Score (z-score) | n = 217 −2.3 (1.84) |
n = 189 −1.8 (1.80) |
n = 177 0.4 (1.12) |
| Semantic Verbal Fluency–Total Score (z-score) | n = 214 −1.6 (1.17) |
n = 188 −1.5 (1.09) |
n = 173 0.2 (0.91) |
| Trail Making Test- Part A–Total Time (z-score) | n = 55 −0.9 (2.72) |
n = 55 −1.2 (5.29) |
n = 38 0.0 (6.69) |
| Child Color Trails Test 1–Total Time (t-score) | n = 213 27.6 (12.95) |
n = 213 31.7 (14.30) |
n = 180 4.7 (14.62) |
| Child Color Trails Test 2–Total Time (t-score) | n = 213 29.1 (12.31) |
n = 208 31.8 (13.42) |
n = 177 2.5 (13.53) |
| Motor speed | |||
| Finger Tapping Test–Dominant Hand (z-score) | n = 309 −1.1 (1.91) |
n = 311 −0.9 (1.92) |
n = 271 0.2 (1.44) |
| Finger Tapping Test–Non-Dominant Hand (z-score) | n = 309 −0.7 (2.14) |
n = 312 −0.4 (2.08) |
n = 271 0.3 (1.69) |
| Attention/working memory | |||
| WISC-IV/WAIS-III Digit Span (scaled-score) | n = 317 7.6 (3.50) |
n = 312 8.3 (3.52) |
n = 282 0.8 (2.71) |
| WISC-IV/WAIS-III Digit Symbol Coding (scaled-score) | n = 298 5.5 (3.77) |
n = 296 6.0 (3.53) |
n = 260 0.7 (2.75) |
| Visual learning and memory | |||
| Rey Complex Figure Test–Total Score (z-score) | n = 307 −1.4 (2.90) |
n = 306 −1.4 (2.93) |
n = 271 0.0 (2.18) |
| Rey Complex Figure Test–Immediate Score (z-score) | n = 303 −1.4 (1.77) |
n = 301 −0.5 (1.68) |
n = 265 0.8 (1.33) |
| Rey Complex Figure Test–Delayed Score (z-score) | n = 300 −1.5 (1.74) |
n = 296 −0.7 (1.72) |
n = 259 0.8 (1.35) |
| Rey Complex Figure Test–Recognition Score (z-score) | n = 294 −1.6 (1.68) |
n = 291 −1.4 (1.79) |
n = 257 0.3 (1.61) |
| Verbal learning and memory | |||
| CVLT-C/CVLT-II - Total Trials (t-score) | n = 46 35.1 (13.41) |
n = 36 44.5 (15.41) |
n = 30 7.2 (12.70) |
| CVLT-C/CVLT-II-Trial B (z-score) | n = 46 −1.1 (1.22) |
n = 35 −0.9 (1.05) |
n = 29 0.1 (1.36) |
| CVLT-C/CVLT II–Short Delay Free Recall (z-score) | n = 46 −1.3 (1.46) |
n = 35 −0.7 (1.23) |
n = 29 0.2 (1.21) |
| CVLT-C/CVLT-II–Long Delay Free Recall (z-score) | n = 44 −1.2 (1.55) |
n = 34 −0.4 (1.30) |
n = 28 0.5 (1.11) |
| WRAML Story Memory–Total Score (scaled-score) | n = 39 7.6 (3.98) |
n = 35 9.0 (3.92) |
n = 27 1.3 (2.33) |
| WRAML Story Delay–Total Score (scaled score) | n = 39 7.6 (4.22) |
n = 35 9.2 (4.30) |
n = 27 1.5 (3.40) |
| WRAML Story Recognition–Total Score (scaled score) | n = 41 8.2 (3.96) |
n = 34 9.2 (3.69) |
n = 27 1.0 (3.06) |
| Executive functioning (reasoning and problem solving) | |||
| WCST–Total Errors (t-score) | n = 234 50.7 (14.73) |
n = 171 57.9 (14.76) |
n = 190 5.9 (12.38) |
| WCST–Perseverative Errors (scaled score) | n = 234 53.5 (17.32) |
n = 260 60.9 (16.99) |
n = 190 6.0 (15.61) |
| WCST–non-Perseverative Errors: (scaled score) | n = 234 50.7 (13.38) |
n = 259 56.5 (12.04) |
n = 189 4.9 (10.81) |
| Trail Making Test-Part B–Time (z-score) | n = 49 −1.1 (2.64) |
n = 53 −1.6 (2.85) |
n = 33 0.5 (2.57) |
| Social cognition | |||
| Theory of Mind Test–Total Score (raw-score) | n = 305 43.9 (11.70) |
n = 292 48.6 (11.41) |
n = 269 4.7 (8.23) |
WAIS III- Wechsler Adult Intelligence Scale; Under age 16; WISC IV- Wechsler Intelligence Scale for Children; CVLT, California Verbal Learning Test; WCST, Wisconsin Card Sort Test; WRAML, Wide Range Assessment of Memory and Learning; Z scores have a mean = 0, and SD = 1, T-scores have a mean = 50, and SD = 10, and scaled scores have a mean = 10, and SD = 3.
3.3. Relationship between symptoms, functioning and cognition
At baseline (Table 4), there was modest negative correlation between disorganized thoughts and cognitive domains (speed of processing, attention/working memory, verbal learning & memory, and social cognition) which persisted at month 6. Other negative correlations at month 6 were between speed of processing and PANSS total score, positive symptoms, negative symptoms and uncontrolled hostility. Executive functioning at month 6 also negatively correlated with PANSS total score, positive symptoms, disorganized thought, uncontrolled hostility and anxiety/depression. At 6 months, higher CGAS score (improved functioning) positively correlated with speed of processing and executive functioning and these correlations were more pronounced in patients who improved with treatment (Table 4). In non-responders, CGAS positively correlated at month 6 with visual learning and memory. Changes in cognition were mostly unrelated to symptom or functional improvement at 6 months (Table 5), with modest correlations observed between improved functioning and improved executive function and social cognition.
Table 4.
Correlation between symptom scores, functioning and neurocognitive scores from select tests from each cognitive domain at baseline and month 6.
| Variable | Cog 1 | Cog 2 | Cog 3 | Cog 4 | Cog 5† | Cog 6 | Cog 7 |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| All patients (N = 324) | |||||||
| PANSS total | −0.160⁎ | −0.090 | −0.082 | −0.108 | −0.220 | −0.049 | −0.172 |
| PANSS factors | |||||||
| Positive | −0.118 | 0.070 | 0.015 | −0.093 | −0.239 | −0.029 | −0.097 |
| Negative | −0.184⁎ | −0.134 | −0.140 | −0.061 | −0.015 | −0.058 | −0.185⁎ |
| Disorganized thoughts | −0.189⁎ | −0.119 | −0.193⁎ | −0.116 | −0.376⁎ | −0.147⁎ | −0.237⁎ |
| Uncontrolled hostility | −0.040 | −0.027 | −0.011 | −0.057 | −0.082 | 0.027 | 0.118 |
| Anxiety/depression | −0.005 | 0.045 | 0.090 | −0.076 | −0.214 | 0.091 | −0.052 |
| CGAS | 0.279⁎ | −0.010 | 0.016 | 0.152⁎ | 0.006 | 0.101 | 0.177⁎ |
| Month 6 | |||||||
| All (N = 324) | |||||||
| PANSS total | −0.278⁎ | 0.039 | −0.029 | −0.061 | −0.464⁎ | −0.225⁎ | −0.212⁎ |
| PANSS factors | |||||||
| Positive | −0.164⁎ | 0.159⁎ | 0.070 | −0.062 | −0.441⁎ | −0.210⁎ | −0.111 |
| Negative | −0.309⁎ | −0.090 | −0.135 | −0.044 | −0.243 | −0.200⁎ | −0.236⁎ |
| Disorganized thoughts | −0.321⁎ | −0.035 | −0.150⁎ | −0.116 | −0.557⁎ | −0.264⁎ | −0.282⁎ |
| Uncontrolled hostility | −0.169⁎ | 0.071 | 0.045 | 0.019 | −0.336⁎ | −0.079 | 0.109 |
| Anxiety/depression | −0.019 | 0.070 | 0.141 | 0.014 | −0.345⁎ | −0.040 | −0.015 |
| CGAS | 0.305⁎ | −0.150 | −0.038 | 0.092 | 0.263 | 0.290⁎ | 0.091 |
| Responders (n = 187) | |||||||
| PANSS total | −0.212⁎ | −0.012 | −0.057 | −0.005 | −0.354 | −0.216⁎ | −0.163⁎ |
| PANSS factors | |||||||
| Positive | −0.136 | 0.149 | 0.067 | −0.026 | −0.355 | −0.213⁎ | −0.064 |
| Negative | −0.229⁎ | −0.108 | −0.172⁎ | −0.023 | −0.186 | −0.127 | −0.197⁎ |
| Disorganized thoughts | −0.251⁎ | −0.067 | −0.197⁎ | −0.038 | 0.431 | −0.288⁎ | −0.275⁎ |
| Uncontrolled hostility | −0.145 | −0.035 | −0.025 | 0.081 | −0.223 | −0.101 | −0.072 |
| Anxiety/depression | 0.051 | −0.016 | 0.210⁎ | 0.088 | −0.256 | −0.001 | 0.076 |
| CGAS | 0.303⁎ | −0.146 | −0.034 | 0.099 | 0.307 | 0.322⁎ | 0.103 |
| Non-responders (n = 137) | |||||||
| PANSS total | −0.084 | 0.010 | −0.128 | −0.048 | 0.199 | −0.166 | −0.174 |
| PANSS factors | |||||||
| Positive | −0.001 | −0.041 | −0.109 | −0.157 | −0.135 | −0.188 | −0.151 |
| Negative | −0.068 | 0.137 | −0.026 | 0.101 | 0.175 | −0.008 | −0.023 |
| Disorganized thoughts | −0.097 | −0.086 | −0.076 | −0.016 | 0.239 | −0.103 | −0.051 |
| Uncontrolled hostility | 0.033 | 0.127 | 0.024 | 0.118 | 0.423 | −0.020 | 0.062 |
| Anxiety/depression | −0.157 | −0.108 | −0.206 | 0.178 | 0.018 | −0.077 | −0.209 |
| CGAS | 0.199 | −0.088 | 0.040 | 0.227⁎ | −0.149 | 0.190 | 0.196 |
PANSS: Positive and Negative Syndrome Scale; CGAS: Children Global Assessment Scale.
Cog 1: Speed of processing: Phonetic Verbal Fluency - Scaled.
Cog 2: Motor speed: Finger Tapping Dominant Hand - Scaled.
Cog 3: Attention/working memory: Digit Span - Scaled.
Cog 4: Visual learning & memory: Rey Complex Figure Test-Delayed Recall: Scaled.
Cog 5: Verbal learning & memory: Wide Range Assessment of Memory and Learning Story-Total: Scaled.
Cog 6: Executive functioning (reasoning & problem solving): Wisconsin Card Sort Test-Total Errors: Scaled.
Cog 7: Social cognition: Theory of Mind-Total.
p < 0.05.
N < 50.
Table 5.
Correlation between change from baseline in symptom scores, functioning and change from baseline in neurocognitive scores to month 6 (N = 324).
| Variable | Cog 1 | Cog 2 | Cog 3 | Cog 4 | Cog 5⁎⁎ | Cog 6 | Cog 7 |
|---|---|---|---|---|---|---|---|
| PANSS total | −0.065 | −0.065 | −0.102 | −0.146⁎ | −0.019 | −0.015 | −0.196⁎ |
| PANSS factors | |||||||
| Positive | −0.065 | −0.044 | −0.099 | 0.181⁎ | −0.145 | −0.055 | −0.164⁎ |
| Negative | −0.102 | 0.009 | −0.073 | −0.057 | −0.096 | −0.036 | −0.172⁎ |
| Disorganized thoughts | −0.061 | −0.014 | −0.117⁎ | −0.090 | −0.077 | 0.061 | −0.115 |
| Uncontrolled hostility | 0.039 | −0.016 | −0.068 | −0.084 | 0.023 | 0.053 | −0.100 |
| Anxiety/depression | −0.035 | 0.114 | 0.151⁎ | −0.150⁎ | −0.012 | −0.006 | −0.200⁎ |
| CGAS | 0.128 | 0.021 | −0.003 | 0.134 | 0.067 | 0.149⁎ | 0.191⁎ |
PANSS: Positive and Negative Syndrome Scale; CGAS: Children Global Assessment Scale.
Cog 1: Speed of processing: Phonetic Verbal Fluency - Scaled.
Cog 2: Motor speed: Finger Tapping Dominant Hand - Scaled.
Cog 3: Attention/working memory: Digit Span - Scaled.
Cog 4: Visual learning & memory: Rey Complex Figure Test-Delayed Recall: Scaled.
Cog 5: Verbal learning & memory: Wide Range Assessment of Memory and Learning Story-Total: Scaled.
Cog 6: Executive functioning (reasoning & problem solving): Wisconsin Card Sort Test-Total Errors: Scaled.
Cog 7: Social cognition: Theory of Mind-Total.
p < 0.05.
N < 50.
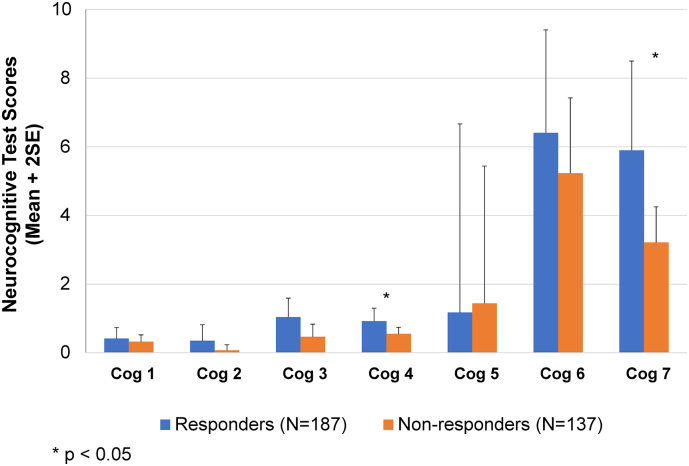
AEs and concomitant medications were closely monitored in the study. Given that certain AEs or medications may have impacted cognitive functioning, observation of cognitive outcomes was conducted for those patients experiencing AEs of potential cognitive relevance. The main AE in this category – somnolence – occurred in a total of 23% of patients overall at any time during the course of the study. These events occurred more frequently in patients previously on placebo in the double-blind study, or started de novo on open-label pali ER than those previously randomized to pali ER in the double-blind study. Events of somnolence were generally time-limited, of mild to moderate intensity, and did not result in study discontinuation. There was no distinct pattern of effects suggesting a greater cognitive deficit in these patients. Additionally, the use of anticholinergic medication was lower during the study than prior to the study – declining from 35% to 25% over the entire study period; no patient discontinued due to cognitive impairment, and the overall rate of reported cognitive AEs was low (<5%). Results based on mean changes from baseline to month 6 in selected neurocognitive scores show numerical improvement being higher among those who also responded to treatment (Fig. 1). However, those receiving anticholinergic medications showed smaller improvements on certain domains than patients not taking anticholinergic medication (Fig. 2).
Fig. 1.
Mean change in neurocognitive scores from baseline to month 6 using selected tests from each cognitive domain by responder status.
Fig. 2.
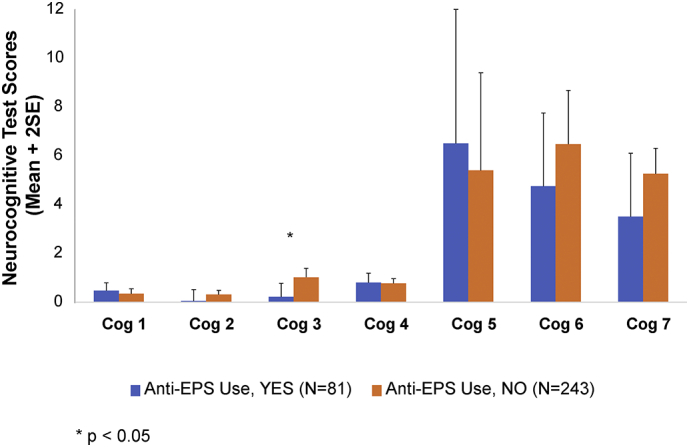
Mean change in neurocognitive scores from baseline to month 6 using selected tests from each cognitive domain by use of anticholinergic medications. Cog 1: Speed of processing: Phonetic Verbal Fluency - Scaled; Cog 2: Motor speed: Finger Tapping Dominant Hand - Scaled; Cog 3: Attention/working memory: Digit Span - Scaled; Cog 4: Visual learning & memory: Rey Complex Figure Test-Delayed Recall: Scaled; Cog 5: Verbal learning & memory: Wide Range Assessment of Memory and Learning Story-Total: Scaled; Cog 6: Executive functioning (reasoning & problem solving): Wisconsin Card Sort Test-Total Errors: Scaled; Cog 7: Social cognition: Theory of Mind-Total.
4. Discussion
In this long-term 2-year study, adolescents with schizophrenia with clinically relevant impairment when treated with pali ER did not report worsening on cognitive measures at 6 months for several domains in a large sample of over 300 adolescents. Clinical symptoms were modestly associated with select neurocognitive deficits at baseline. Improvements in cognition appeared to be most notable in those patients who demonstrated a response to antipsychotic treatment. Following pali ER treatment, the observed changes in neurocognition were weakly associated with the small changes in positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility, and anxiety symptoms (Savitz et al., 2015a). The PANSS scores negatively correlated with cognition in overall patients as well as in patients who responded to pali ER, both at baseline and 6 months. However, the CGAS positively correlated with speed of processing and functioning, especially in patients who improved with pali ER treatment (Table 4).
In the present study, the degree of baseline impairment can be considered mild to moderate in the domains of verbal learning, working memory, sustained attention, processing speed, and verbal productivity. These baseline impairments in cognition are consistent with those from published studies of early-onset schizophrenia: other samples of adolescents with early-onset schizophrenia have shown similar cognitive deficits (Juuhl-Langseth et al., 2014; Kravariti et al., 2003).
A pattern of cognitive decline may also be a good predictor of later onset of schizophrenia in adolescence. Similar deficits are observed in children and adolescents on the psychosis spectrum who may not yet have developed a full-blown episode of schizophrenia (Gur et al., 2014) and also those who may be at ultra-high risk for development of psychosis (Niendam et al., 2006). In contrast, adults with chronic schizophrenia often have more pervasive and severe cognitive deficits.
Our results are consistent with the findings of the earlier Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, which demonstrated that all of the antipsychotic treatment groups (olanzapine, perphenazine, quetiapine fumarate, risperidone, and ziprasidone hydrochloride) showed a small improvement in neurocognition as measured by change in a composite score derived from 11 neurocognitive tests after 2 months of treatment. However, there was no significant difference between the groups. Neurocognitive improvement was found to contribute to the overall effectiveness of some of the antipsychotic treatments as measured by time to discontinuation (Keefe et al., 2007).
There have been few treatment studies of early-onset schizophrenia (EOS) that also assessed cognitive functioning over time. In one such study, the Treatment of Early-Onset Schizophrenia Spectrum Disorders or TEOSS (Clinicaltrial.gov NCT00053703), a similar small positive change in cognition, though these changes were not statistically significant when controlling for multiple comparisons (Frazier et al., 2012). The current data support a modest relationship between neurocognitive improvement and treatment adherence. However, changes in cognition were mostly unrelated to symptom or functional improvement. Overall, there was a modest positive change in cognitive measures although change could be expected given the normal temporal cognitive development of adolescents. However, there is insufficient evidence whether adolescents with schizophrenia have the same level of cognitive improvement as typically developing adolescents in the brief time period examined.
This analysis was limited by the exploratory nature and inadequate power to detect differences between time points. Additionally, the tests were not completed at all time points for all patients, and hence may not be classified as a true ITT analysis. The verbal learning and the memory tests were conducted only in the English language, with some of them being performed only in the US, further restricting the sample size and the conclusions drawn from the study. At the time this study was conducted, validated cognitive batteries were not available to evaluate cognitive effects of new treatments in adolescents with schizophrenia. A modified neuropsychological battery with tasks mirroring those available in the MATRICS battery was selected in this study based on the relationship of the measures to critical functional outcomes (Suzuki et al., 2014). These outcomes are also known to be impaired in those at high risk for developing a psychotic disorder and across the lifespan for those with a schizophrenia spectrum illness. The current study adds to the historical data in our understanding of cognitive functioning in adolescents with schizophrenia, and the potential impact of antipsychotic treatment.
There are some limitations to the study. The original study duration was only planned for a 6-month duration at the time of study design, and thus cognitive and other evaluations were planned for the 6-month study end. The protocol was later amended to increase the study duration to 2 years, and it was decided not to include cognitive endpoints in the remaining subset of patients that would complete 2 years. This was an open-label treatment study and therefore no reference group was available for comparison aside from normative controls. Changes in cognitive functioning over time could potentially have been influenced by practice effects, though these may have been mitigated by the long intervals between assessments. Only a small number of patients were evaluated for verbal memory and learning as there were insufficient parallel validated versions of these tests available outside of the English language, and to reduce potential complexity associated with implementation of multiple test versions and different scoring conventions. Pre-morbid IQ was not available for all patients, and as such may have had some impact on cognitive performance and could potentially change cognitive performance with pali ER treatment. Anticholinergic medications which can negatively impact cognition were not controlled for.
4.1. Conclusions
Adolescent patients with schizophrenia demonstrated significant cognitive deficits on a comprehensive neuropsychological battery. Treatment with pali ER in this OL study did not result in worsening of neurocognitive functioning and was possibly associated with positive improvements in certain domains of cognitive functioning in adolescents with schizophrenia, some of which were associated with treatment response.
Author contribution
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors provided direction and comments on the manuscript, made the final decision about where to publish these data, and approved submission to this journal.
Declaration of competing interest
This study was sponsored by Janssen Research & Development, LLC, USA. Dr. Petersen was a consultant for Janssen Research & Development for this investigation. Drs Pandina, Singh, Savitz, Nuamah, and Hough are employees of Janssen Research & Development, LLC and hold stocks in the company.
Data sharing statement
The data sharing policy of the study sponsor, Janssen Pharmaceutical Companies of Johnson & Johnson, is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Funding
The study was sponsored by Janssen Research & Development, LLC, USA. The sponsor also provided support for development of this manuscript.
Acknowledgments
Shruti Shah, PhD and Ramji Narayanan, MPharm, ISMPP CMPP (both SIRO Clinpharm Pvt. Ltd.) provided writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) provided additional editorial support for this manuscript. The authors thank the study participants, without whom this study would not have been accomplished, as well as the following investigators for their participation in this study: Belgium: Croonenberghs, J, MD; Bulgaria: Slavchev, A, MD, PhD; Estonia: Aavik, P, MD; Madisson, R, MD; Finland: Moilanen, K, MD; India: Kammammettu, C, MD; Jhanwar, VG, MD; Kombettu, K, MD; Sathianathan, R, MD; Thunga, R, MD; Korea: Cheon, KA, MD, PhD; Cho, S, MD, PhD; Hong, S, MD, PhD; Lee, S, MD, PhD; Lim, M, MD, PhD; Song, D, MD, PhD; Poland: Dabkowski, M, MD, PhD; Gmitrowicz, A, MD, PhD; Janas-kozik, M, MD, PhD; Namyslowska, I, MD; Rajewski, A, MD; Wolanczyk, T, MD; Romania: Dobrescu, I, MD, PhD; Russia: Agarkov, A, MD, PhD; Bardenstain, L, MD; Bylim, I, MD, PhD; Dobrovolskaya, N, MD, PhD; Grigoryeva, E, MD; Kozlova, I, MD, PhD; Popov, Y, MD, PhD; Reshetko, O, MD, PhD; Serdyuk, O, MD; Suchkov, Y, MD; Vaulin, S, MD, PhD; Yakhin, K, MD, PhD; Ukraine: Bitenskyy, V, MD, PhD; Demchenko, V, MD; Maruta, N, MD, PhD; Mishyyev, V, MD, PhD; Verbenko, V, MD, PhD; Yur'yeva, L, MD, PhD; Zilberblat, G, MD; United States of America: Childress, A, MD; Chueh, D, MD; Delbello, M, MD; Findling, R, MD; Gonzalez-Heydrich, J, MD; Grewal, H, MD; Holloway, W, M.D.; Kablinger, A, MD; Lowy, A, MD; Lucka, I, MD, PhD; Pathak, A, MD; Plopper, M, MD; Pugliese, R, MD; Robb, A, MD; Sonnenberg, J, MD; Unis, A, MD; Valencerina, M, MD; Yadalam, K, MD.
Footnotes
Previous Presentation: Posters presented at: 58th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) Toronto, October 18-23, 2011.
References
- Cervellione K.L., Burdick K.E., Cottone J.G., Rhinewine J.P., Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(7):867–878. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Emsley R., Rabinowitz J., Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am. J. Psychiatry. 2006;163(4):743–745. doi: 10.1176/ajp.2006.163.4.743. [DOI] [PubMed] [Google Scholar]
- Emsley R., Berwaerts J., Eerdekens M., Kramer M., Lane R., Lim P., Hough D., Palumbo J. Efficacy and safety of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 52-week open-label studies. Int. Clin. Psychopharmacol. 2008;23(6):343–356. doi: 10.1097/YIC.0b013e328314e1f3. [DOI] [PubMed] [Google Scholar]
- Findling R.L., Johnson J.L., McClellan J., Frazier J.A., Vitiello B., Hamer R.M., Lieberman J.A., Ritz L., McNamara N.K., Lingler J., Hlastala S., Pierson L., Puglia M., Maloney A.E., Kaufman E.M., Noyes N., Sikich L. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(6):583–594. doi: 10.1016/j.jaac.2010.03.013. (quiz 632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Giuliano A.J., Johnson J.L., Yakutis L., Youngstrom E.A., Breiger D., Sikich L., Findling R.L., McClellan J., Hamer R.M., Vitiello B., Lieberman J.A., Hooper S.R. Neurocognitive outcomes in the treatment of early-onset schizophrenia spectrum disorders study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(5):496–505. doi: 10.1016/j.jaac.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Calkins M.E., Satterthwaite T.D., Ruparel K., Bilker W.B., Moore T.M., Savitt A.P., Hakonarson H., Gur R.E. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71(4):366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Haas M., Eerdekens M., Kushner S., Singer J., Augustyns I., Quiroz J., Pandina G., Kusumakar V. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: double-blind study. Br. J. Psychiatry J. Ment. Sci. 2009;194(2):158–164. doi: 10.1192/bjp.bp.107.046177. [DOI] [PubMed] [Google Scholar]
- Harrington C.A., English C. Tolerability of paliperidone: a meta-analysis of randomized, controlled trials. Int. Clin. Psychopharmacol. 2010;25(6):334–341. doi: 10.1097/YIC.0b013e32833db3d8. [DOI] [PubMed] [Google Scholar]
- Juuhl-Langseth M., Holmen A., Thormodsen R., Oie M., Rund B.R. Relative stability of neurocognitive deficits in early onset schizophrenia spectrum patients. Schizophr. Res. 2014;156(2–3):241–247. doi: 10.1016/j.schres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Bilder R.M., Davis S.M., Harvey P.D., Palmer B.W., Gold J.M., Meltzer H.Y., Green M.F., Capuano G., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Davis C.E., Hsiao J.K., Lieberman J.A. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Ohlsson H., Mezuk B., Sundquist K., Sundquist J. A Swedish National prospective and co-relative study of school achievement at age 16, and risk for schizophrenia, other nonaffective psychosis, and bipolar illness. Schizophr. Bull. 2016;42(1):77–86. doi: 10.1093/schbul/sbv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravariti E., Morris R.G., Rabe-Hesketh S., Murray R.M., Frangou S. The Maudsley Early-Onset Schizophrenia Study: cognitive function in adolescent-onset schizophrenia. Schizophr. Res. 2003;65(2–3):95–103. doi: 10.1016/s0920-9964(03)00067-7. [DOI] [PubMed] [Google Scholar]
- Kremen W.S., Vinogradov S., Poole J.H., Schaefer C.A., Deicken R.F., Factor-Litvak P., Brown A.S. Cognitive decline in schizophrenia from childhood to midlife: a 33-year longitudinal birth cohort study. Schizophr. Res. 2010;118(1–3):1–5. doi: 10.1016/j.schres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe J.H., Wicks S., Lofving S., David A.S., Berndtsson A., Gustafsson J.E., Allebeck P., Dalman C. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70(3):261–270. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- Marder S.R., Kramer M., Ford L., Eerdekens E., Lim P., Eerdekens M., Lowy A. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol. Psychiatry. 2007;62(12):1363–1370. doi: 10.1016/j.biopsych.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., Bobo W.V., Nuamah I.F., Lane R., Hough D., Kramer M., Eerdekens M. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. The Journal of Clinical Psychiatry. 2008;69(5):817–829. doi: 10.4088/jcp.v69n0515. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Bearden C.E., Johnson J.K., McKinley M., Loewy R., O’Brien M., Nuechterlein K.H., Green M.F., Cannon T.D. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr. Res. 2006;84(1):100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Rapoport J.L., Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. International Journal of Developmental Neuroscience: the official journal of the International Society for Developmental Neuroscience. 2011;29(3):251–258. doi: 10.1016/j.ijdevneu.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A., Caspi A., Harrington H., Houts R., Keefe R.S., Murray R.M., Poulton R., Moffitt T.E. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz A., Lane R., Nuamah I., Singh J., Hough D., Gopal S. Long-term safety of paliperidone extended release in adolescents with schizophrenia: an open-label, flexible dose study. Journal of Child and Adolescent Psychopharmacology. 2015;25(7):548–557. doi: 10.1089/cap.2014.0130. [DOI] [PubMed] [Google Scholar]
- Savitz A.J., Lane R., Nuamah I., Gopal S., Hough D. Efficacy and safety of paliperidone extended release in adolescents with schizophrenia: a randomized, double-blind study. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(2):126–137.e121. doi: 10.1016/j.jaac.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Singh J., Robb A., Vijapurkar U., Nuamah I., Hough D. A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol. Psychiatry. 2011;70(12):1179–1187. doi: 10.1016/j.biopsych.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Gen K., Inoue Y., Hibino H., Mikami A., Matsumoto H., Mikami K. The influence of switching from risperidone to paliperidone on the extrapyramidal symptoms and cognitive function in elderly patients with schizophrenia: a preliminary open-label trial. Int. J. Psychiatry Clin. Pract. 2014;18(1):58–62. doi: 10.3109/13651501.2013.845218. [DOI] [PubMed] [Google Scholar]