Keywords: coupled oscillator, ICC, small intestine
Abstract
The interstitial cells of Cajal associated with the myenteric plexus (ICC-MP) are a network of coupled oscillators in the small intestine that generate rhythmic electrical phase waves leading to corresponding waves of contraction, yet rhythmic action potentials and intercellular calcium waves have been recorded from c-kit-mutant mice that lack the ICC-MP, suggesting that there may be a second pacemaker network. The gap junction blocker carbenoxolone induced a “pinstripe” motor pattern consisting of rhythmic “stripes” of contraction that appeared simultaneously across the intestine with a period of ~4 s. The infinite velocity of these stripes suggested they were generated by a coupled oscillator network, which we call X. In c-kit mutants rhythmic contraction waves with the period of X traveled the length of the intestine, before the induction of the pinstripe pattern by carbenoxolone. Thus X is not the ICC-MP and appears to operate under physiological conditions, a fact that could explain the viability of these mice. Individual stripes consisted of a complex pattern of bands of contraction and distension, and between stripes there could be slide waves and v waves of contraction. We hypothesized that these phenomena result from an interaction between X and the circular muscle that acts as a damped oscillator. A mathematical model of two chains of coupled Fitzhugh–Nagumo systems, representing X and circular muscle, supported this hypothesis. The presence of a second coupled oscillator network in the small intestine underlines the complexity of motor pattern generation in the gut.
NEW & NOTEWORTHY Physiological experiments and a mathematical model indicate a coupled oscillator network in the small intestine in addition to the c-kit-expressing myenteric interstitial cells of Cajal. This network interacts with the circular muscle, which itself acts as a system of damped oscillators, to generate physiological contraction waves in c-kit (W) mutant mice.
INTRODUCTION
Rhythmic waves of contraction travel down the small intestine, pushing content from the stomach to the colon. The contractions are caused by oscillatory depolarizations of the muscle cells’ membrane potential, called “slow waves.” Since the start of the twentieth century it has been observed that slow/contraction wave frequency decreases down the intestine, often discontinuously in a series of frequency “plateaus” separated by abrupt steps (14, 50). To explain this data, Bortoff suggested that slow/contraction waves were phase waves generated by a network of coupled oscillators that stretched along the small intestine’s whole length (6).
Oscillators are widespread across nature, from fireflies to Josephson junctions, from circadian gene circuits to triodes (55, 73, 75, 79). Despite this diversity, most oscillators share a few fundamental properties that result from the dynamic principles of oscillation (such as feedback with delay) rather than their specific physical realization. The most striking of these properties is synchronization (72, 74). When two oscillators are coupled (by a wire in an electric circuit or a protein in a genetic circuit), their phase difference will become fixed and tend toward to zero. If their natural frequencies (ω), the frequencies at which they oscillate in isolation, are exactly the same, then when coupled their phase difference will go to zero. If their ω are moderately different, then the oscillator with the lower ω will be “pulled” toward the frequency of the higher, but its phase will lag behind. Only when their ω are too different will synchronization fail.
When a group of oscillators are coupled together in a network, synchronization results in waves that appear to travel across the network; “appear to travel” because the waves do not “travel” or “propagate” in the usual sense of those words. They are just synchronized oscillations, “phase waves.” Phase wave velocity is the inverse of the phase lag between oscillators. With uniform ω there is zero lag, and so the phase wave has infinite velocity; it appears simultaneously across the network. Otherwise, the phase wave appears to travel down a gradient in ω. Bortoff suggested that slow/contraction waves were phase waves “traveling” down a gradient in ω. The frequency plateaus would result from frequency pulling, the steps when the difference between the pulled frequency and ω became too large and so synchronization failed. A number of experimental and modeling studies through the 1970s supported Bortoff’s hypothesis (54).
Bortoff imagined that the oscillators were muscle cells. In the 1990s it was discovered that slow/contraction waves were absent in mice that lacked expression of the c-kit tyrosine kinase receptor (“W” mutants after the chromosomal locus of the c-kit gene) or its ligand, Steel (28, 46, 49, 76, 77). This effect was traced to a population of cells, the interstitial cells of Cajal (ICC), that depended on c-kit expression for their development. A single-cell-thick network of ICC was found to cover the whole length and circumference of the intestine, sandwiched between its two muscle layers (inner circular and outer longitudinal) at the myenteric plexus. The intracellular Ca2+ and membrane potentials of myenteric ICC (ICC-MP) oscillate ahead of and independently of the muscle (25, 45). Thus the ICC-MP are Bortoff’s coupled oscillators, and the contraction/slow wave results from the muscle cells being excited by (“following”) the ICC-MP oscillations.
Recently we examined ICC-MP-generated contraction patterns in the small intestine of mice (50, 51, 53, 54, 78). We visualized contractions with diameter maps, images that show intestine diameter (a measure of contraction) as a function of time and distance along the length of the intestine. ICC-MP are coupled electrically by gap junctions, ionic channels between the cytoplasms of neighboring cells. When we added the gap junction blocker carbenoxolone to the intestine, the number of frequency steps increased so that the size of each plateau decreased (50). Instead of just traveling down the intestine, waves traveled out from the middle of each plateau in a V. Thus the entire intestine presented a chevron pattern of waves. To understand this, we created a simple mathematical model of oscillators coupled in a chain (54). The model demonstrated two things. First, there had to be variations in coupling strength along its length. Frequency steps then occurred at the points of lowest coupling. Second, there had to be noisy variation in ω, in addition to the overall gradient. Then as each plateau got smaller with carbenoxolone there was a chance that one of the oscillators at its middle had the highest ω and therefore led the rest in phase, at the apex of a V wave.
After initially causing a chevron wave pattern, higher concentrations of carbenoxolone (>40 μM) switched contractions into a very different pattern that suggested two hypotheses:
-
1.
Contractions occurred at intervals of ~4 s, outside the usual range of 1.2–1.5 s for ICC-MP oscillations. Hypothesis: The contractions were paced by cells other than the ICC-MP.
-
2.
The contractions were simultaneous across the intestine’s length, i.e., waves with an apparently infinite velocity. Hypothesis: The pacemaker cells were a network of coupled oscillators.
Here we investigate the high-carbenoxolone-induced, simultaneous wave pattern to test these hypotheses.
METHODS
Ethics Approval
All procedures were approved by and carried out in accordance with regulations of the Animal Research Ethics Board (approval no. AUP 14-12-49) of McMaster University, following the guidelines and policy statements established by the Canadian Council on Animal Care and legislation as presented in the Animals for Research Act, Ontario (1980) and administered by the Ontario Ministry of Agriculture and Food. Female mice at least 9 wk old and of various strains (see below) were obtained from Charles River Laboratories (Wilmington, MA) or Jackson Laboratories (Bar Harbor, ME) and fed ad libitum on standard chow. To obtain intestines, 60 mice were killed by cervical dislocation after induction of general anesthesia with isoflurane, following an approved standard operating procedure of the Animal Research Ethics Board.
Experimental Setup
The small intestines of mice were prepared and imaged for diameter mapping as previously described (50, 51, 53). Briefly, the organ bath consisted of a plastic wallpaper tray containing 1.5 L of Krebs solution, warmed to 36°C by a tube connected to a circulating water bath and bubbled with 95% O2-5% CO2. The Krebs solution consisted of (mM) 120 NaCl, 15.5 NaHCO3, 5.9 KCl, 1.2 NaH2PO4, 0.1 citric acid; 0.1 aspartic acid, 2.5 CaCl2, 1.2 MgCl2, and 6 glucose. At the center of the bath was a kit-kat, a length of plastic with grooved lanes 1 cm wide. A 25- to 30-cm length of small intestine was cannulated at each end, and the cannulas held the intestine in place along one of the kit-kat lanes. A linear array of 10 miniboard cameras, mounted above the bath and connected to a digital video recorder, took video of overlapping portions of the intestine (3.7 cm per camera) at a frame rate of 30 Hz.
Our experimental design varied as we optimized it to suit different goals. The major variations were as follows:
Mouse.
cd-1.
The CD-1 mouse (Charles River, Wilmington, MA; stock no. 022) is a basic inbred albino mouse.
w/wv.
The W/Wv mouse was from Jackson Laboratories (Bar Harbor, ME; WBB6F1/J-KitW/KitW-v/J; stock no. 100410). W and Wv are structural mutations in the c-kit gene (4). The lethal W mutation has to be offset by a Wv allele to produce a viable, albeit infertile, mouse. c-kit+ ICC-MP are scattered in patches through the small intestine, particularly near the mesenteric border, but c-kit+ ICC of the deep muscular plexus remain unaffected (25, 28, 32, 34, 77). The small intestine has no slow waves but does have rhythmic action potentials and calcium waves (see Table 2).
Table 2.
Frequencies in small intestine of W and Steel mutant mice
| Mutant | Activity | Stated Frequency or Interval | Interval, s | Description by Authors | Reference |
|---|---|---|---|---|---|
| Sl/Sld | AP | 11.8 ± 2.5 cpm | 5.1 | “phasic” | (76) |
| W/Wv | AP | 4–20 cpm | 3.0–15.0 | “constant frequency” | (28) |
| W/Wv | AP | 15.1 ± 2.4 cpm | 4.0 | “irregular frequency” | (48) |
| W/Wv | AP | 16–59 cpm | 1.0–3.8 | (13) | |
| Sl/Sld | AP | 17.8 ± 3.4 cpm | 3.4 | “irregular frequency” | (49) |
| W/Wv | Contraction | 4.1 ± 0.2s | 4.1 | “phasic” | (66) |
| W/Wv | “Slow wave” | 10.7 cpm | 5.6 | “rhythmic” | (26) |
| W/Wv | Ca2+ wave | 12.8 ± 3.7 cpm | 4.7 | “irregularly occurring” | (25) |
AP, action potential.
wsh/wsh.
The Wsh/Wsh mouse was from Jackson Laboratories (B6.Cg-KitW-sh/HNihrJaeBsmGlliJ; stock no. 012861). The Wsh mutation is in a regulatory region upstream of c-kit that is nonlethal and has no effect on fertility (4). The “sh” refers to the “sash” of white fur about the middle of the otherwise black heterozygote (homozygotes are black-eyed white and wild types are black). The small intestine of homozygotes completely lack c-kit+ ICC in any layer but retain neurokinin receptor 1 (NKR1)-positive “interstitial cells” in the deep muscular plexus (33). Their gut motility has not been investigated before.
c57bl/6j.
The C57BL/6J mouse (Charles River; stock no. 000664) is the genetic background to the W/Wv and Wsh/Wsh mice.
Cannulation.
perfused lumen.
Oxygenated and warmed Krebs was continually pumped into the intestine’s proximal end, and the outflow at the distal end was collected in a beaker. The outflow tube passed through a rubber window in the organ bath so that it was not raised above the level of the bath’s solution.
trendelenburg.
The proximal end was connected to a reservoir of saline (137 mM NaCl, 5.9 mM KCl) raised to give 2–4 cmH2O pressure. The distal end was connected to an open-ended tube raised above the organ bath.
Carbenoxolone application.
stepped aliquots.
Carbenoxolone was added to the bath in spaced aliquots.
continuous pumping (“ramp”).
Carbenoxolone was continuously pumped into the bath at a rate of 2 μM/min over a period of an hour or more. This procedure was adopted because the sensitivity to carbenoxolone varied from intestine to intestine. By adding carbenoxolone gradually, no transitions from one pattern to another could be missed.
The mouse, cannulation, and carbenoxolone application/concentration are given at the end of each applicable figure legend. Where carbenoxolone has been pumped, the approximate concentration at the start and end of a spatiotemporal map of changes in diameter (DMap) is indicated in the DMap by a ramp symbol followed by the concentrations. Where carbenoxolone has been stepped, this is indicated by a rectangular symbol followed by the concentration.
Diameter and Longitudinal Mapping
All diameter mapping and analysis of DMaps were carried out with custom plugins written in ImageJ. The plugin for diameter mapping, DMapLE, can be downloaded from the website of S. Parsons (www.scepticalphysiologist.com/code/code.html).
Longitudinal contraction has been measured in the past by creating high-contrast marks on the surface of the organ (intestine or colon) that can be tracked, either in the video (25, 42, 43) or in a DMap created from the video (23, 37). Longitudinal distances between tracked marks, relative to some reference temporal frame, are then calculated and converted into spatiotemporal maps, viz longitudinal contraction or “LMaps.” The marks have been made with a variety of materials, flame soot (42), black silk knots tied to the mesentery (23), glitter (25), and force-transducer clips (37), or natural marks have been tracked, e.g., the mesenteric arterioles (43). We used charcoal and tracked the marks in spatiotemporal maps.
Briefly, sticks of willow charcoal (PH Coate and Son, Taunton, UK) were shaved with a scalpel blade to produce dust (Fig. 1A). An applicator was made from piano wire (Fig. 1A) shaped into a curve at one end so that it would hold the intestine in place when pressed across its width. The applicator was dipped into the charcoal dust and then applied across the intestine. It was important to dry the applicator before each dip—if it was wet, the charcoal would clump onto the wire and when dipped into the organ bath most of the charcoal would come off on the bath’s meniscus (a certain amount of loss to the meniscus was always unavoidable). Applications were made about every 1 cm along the intestine’s length (Fig. 1B).
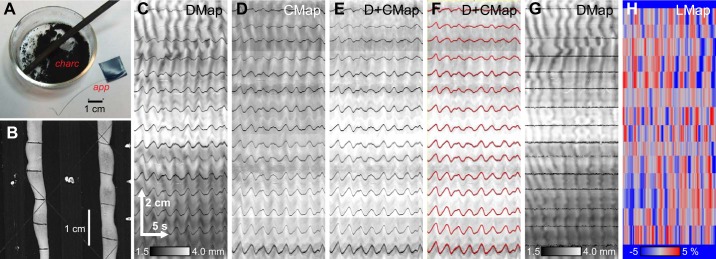
Fig. 1.
Measurement of longitudinal contraction. To measure longitudinal extension the small intestine was marked with charcoal stripes. A: a stick of willow charcoal was scraped with a scalpel to produce a dust (charc). This was applied to the small intestine with an applicator (app) made from a short length of piano wire, taped at 1 end for holding. B: charcoal marks were applied approximately every 1 cm along the length of the intestine, across 1 face. The marks are visible as dark lines in the spatiotemporal map of changes in diameter (DMap, C) and in a spatiotemporal map of the average pixel intensity across the diameter (CMap, D). The DMap and CMap were averaged (E), and the dark marker lines were tracked by a minimum-search algorithm (F, red lines). Longitudinal distances between the tracked lines, relative to a reference frame, are calculated and used to “correct” the DMap (G) and are output as a longitudinal map (LMap, H).
Two type of spatiotemporal maps were created from the marked intestines, DMaps and “cross-average” maps (CMaps) of the average pixel intensity across the measured diameter (Fig. 1, C and D). The diameter mapping algorithm detects the edges of the intestine based on an intensity threshold and from that calculates the diameter. Therefore, when the dark charcoal mark is at an edge, the mark is registered as a decrease in diameter and appears in the DMap as a dark line across time that wiggles if the intestine moves along its long axis (Fig. 1C). The charcoal mark also lowers the pixel intensity across the width of the intestine and so registers in the CMap as a dark, wiggling line across time (Fig. 1D). The charcoal marks were only made across one face of the intestine. Therefore, if the intestine rotated the amount of each mark visible changed and it might or might not reach either edge, and so the dark wiggling lines may change intensity or even disappear in the CMaps and DMaps. To counter this, a mean average of the DMap and the CMap was found most useful for tracking (Fig. 1E). If the intestine rotated, the mark might not register as clearly in one map but would in the other. Tracking was performed by a minimum-finding algorithm (Fig. 1F). Distances were measured between each pair of tracked marks over time, normalized by the average distance over the whole time period, and then converted into an LMap (Fig. 1H), which shows the percentage change in length. Longitudinal distances were used to create a DMap corrected for longitudinal displacement and contraction (Fig. 1G). LMaps were laid over corrected DMaps as a magenta color channel (see Fig. 10C) with the Make Composite function in ImageJ.
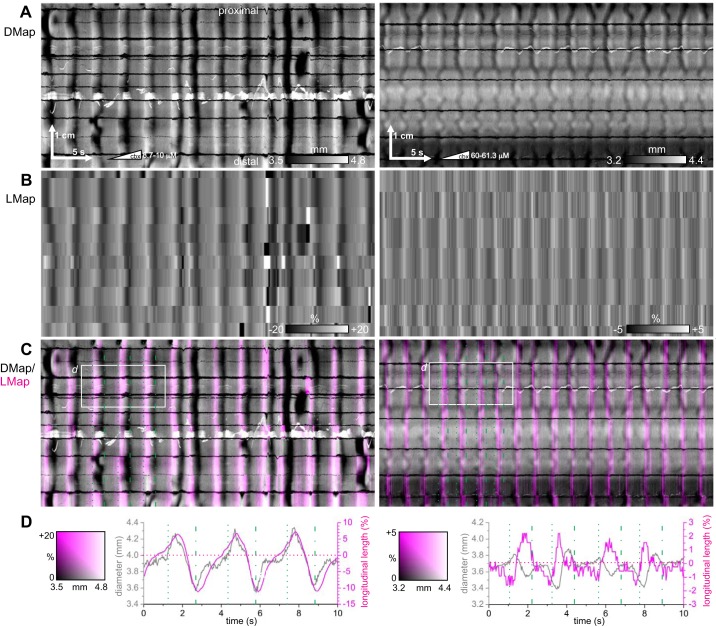
Fig. 10.
Longitudinal relaxation during stripes. Spatiotemporal maps of changes in diameter (DMaps, A) for 2 different intestines (left and right), corrected for longitudinal movement, with corresponding spatiotemporal maps of longitudinal contraction (LMaps, B), DMap/LMap grayscale/magenta overlay (C), and plots of average diameter and longitudinal extension (D) for the areas indicated in C. The intestines clearly extended (magenta stripes) at each stripe. Leading and trailing edges of each stripe are indicated by dotted and dot-dashed green lines in C and D. Left: female CD-1, perfused lumen, 2 μM/min carbenoxolone. Right: female CD-1, perfused lumen, 2 μM/min carbenoxolone. Representative of 6 experiments.
Contraction intervals were calculated from 300 s × 2 cm regions of DMap. The DMap was band-pass filtered (0.2–4 Hz, 8th-order Bessel) to remove slow variations in diameter and thresholded and intervals were calculated from the threshold crossings at every 5th pixel (0.4 mm) along the spatial axis.
Model
The Fitzhugh–Nagumo (FN) model was created as a simplified (“reduced”) version of the Hodgkin–Huxley model (17). The latter is a system of four ordinary differential equations for membrane potential; activation and inactivation of a sodium current; and activation of potassium current. In the FN these are reduced to two: membrane potential (v) and a “recovery variable” (w). The cut in the number of parameters is even greater, although as a result these parameters do not always have the same degree of physical interpretability as the Hodgkin–Huxley. Although it is much reduced in complexity, the FN produces much the same range and richness of dynamic behavior as the Hodgkin–Huxley: excitability, limit cycles, damped oscillations, anode break excitation, and threshold manifolds. This makes it an ideal “toy” model that can be used to understand the emergent principles involved in spatiotemporal pattern formation in a network of coupled excitable or oscillatory cells (52). For brief primers on nonlinear dynamics see References 52 and 18.
Our model consisted of two chains of FN systems, coupled together to form a ladder where each rung consists of two systems on opposite chains (Fig. 2). The chains are referred to as X (for an unidentified oscillator network) and CM (for the circular muscle). The system for the ith oscillator of either chain is
where d adjusts the overall timescale, so that the natural frequencies of the chains can be brought to an appropriate ratio without changing their other parameters; I is the “stimulus current,” which can be thought of energy input to the cell like an ATPase ion pump; K is the “coupling current,” the sum of inputs from neighboring systems; σ is the standard deviation of membrane potential noise from a Wiener process, W, i.e., Gaussian white noise; c separates the timescale of v and w, changing the waveform of v between relaxation and harmonic (sine wave like); a and b are arbitrary parameters; kip is the intrachain coupling strength; vi+1 and vi-1 are the membrane potentials of the neighboring systems on the same chain; kic is the interchain coupling strength; and is the membrane potential of the ith system on the opposing chain. The coupling terms on the right-hand side of the third equation represent resistive (cable-like or diffusive) coupling (52).
Fig. 2.
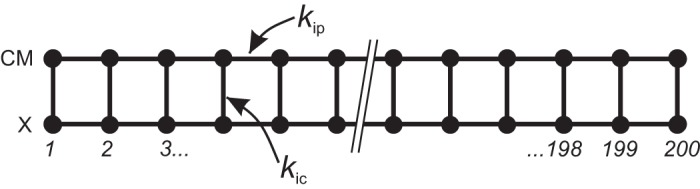
The 2-chain model. The model consisted of 2 chains [circular muscle (CM) and X], each of 200 Fitzhugh–Nagumo systems, coupled together into a ladder. Inter- and intrachain coupling were resistive (cablelike) with strengths of kip and kic, respectively.
Parameter values are given in Table 1. The parameters of the X chain give relaxation-type limit cycles about an unstable node (see Fig. 13D). The parameters of the CM chain give either excitable behavior (with a stable focus; see Fig. 13H) or limit cycle behavior (with an unstable focus; see Fig. 13D). Intrachain coupling was made stronger than interchain coupling, as might be expected because the finite thickness of the X and CM. Interchain coupling was rectifying, stronger from X to CM than vice versa. All modeling and state space plots were carried out with code written in MATLAB (MathWorks, Natick, MA). The full code is available at the Scholar’s Portal Dataverse (https://dataverse.scholarsportal.info/citation?persistentId=doi:10.5683/SP2/8LJ8UU).
Table 1.
Parameters of FN model
| Chain CM (Circular Muscle) |
|||||
|---|---|---|---|---|---|
| Parameter | Symbol | Chain X | Fig. 13, B–D | Fig. 13, F–H | Fig. 13, J–L |
| No. of FN systems | n | 200 | 200 | 200 | 200 |
| Intrachain coupling | kip | 0.4 | 0.4 | 0.4 | 0.4 |
| Interchain coupling | kic | 0.01 | 0.04 | 0.04 | 0.04 |
| Membrane potential noise | σ | 0.0 | 0.12 | 0.12 | 0.02 |
| a | 0.6 | 0.83-0.9 | 0.9 | 0.9 | |
| b | 0.1 | 0.5 | 0.5 | 0.5 | |
| Timescale separation | c | 20 | 5-8 | 7.5 | 6.01-6.02 |
| Timescale | d | 1.0 | 2.0 | 2.0 | 2.0 |
| Current | I | 0.1 | 0.5 | 0.5 | 0.5 |
Values in bold indicate varied parameters. Ranged values are linear gradients from the proximal to the distal end of the chain.
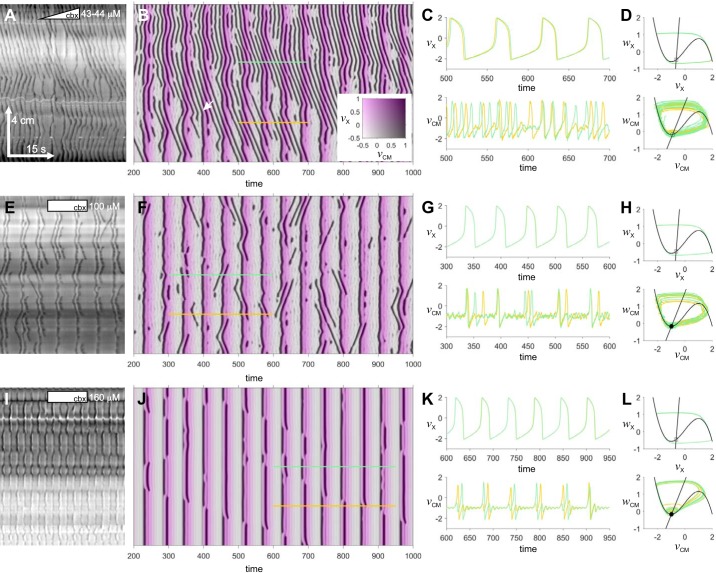
Fig. 13.
Two-chain Fitzhugh–Nagumo (FN) model. Examples of pinstripe patterns in the small intestine (A, E, and I) and in the 2-chain FN model (B, F, and J). Model parameters are given in Table 1. The model maps are color composites showing the membrane potential of the circular muscle (vCM, grayscale) and X (vX, magenta scale). The white arrow in B indicates a chevron wave suddenly changing velocity into a stripe. C, G, and K: time series of vX and vCM along the colored lines indicated in the corresponding spatiotemporal maps on left. D, H, and L, the equivalent trajectories in state space. The black lines are the nullclines of the proximal FN system (straight line = w, knee = v). Equilibria at the nullcline intersections are plotted (circle = focus; square = node; filled = stable; empty = unstable). Scale in A applies to all DMaps. A: female Wsh/ Wsh, perfused, 2 μM/min carbenoxolone (cbx). E: female W/Wv, perfused, 100 μM carbenoxolone. I: female CD-1, Trendelenburg, 160 μM carbenoxolone.
RESULTS
Carbenoxolone and the Pinstripe Pattern
In the experiments described below we used various combinations of mouse strain, apparatus, and protocol (methods). In the text we do not state the specific combination except where it made a difference to the result. At the end of each figure legend, we state the specific combination associated with the data presented there. In all experiments, except those presented in Fig. 6, the enteric nervous system was blocked by 0.5 mM lidocaine.
Fig. 6.
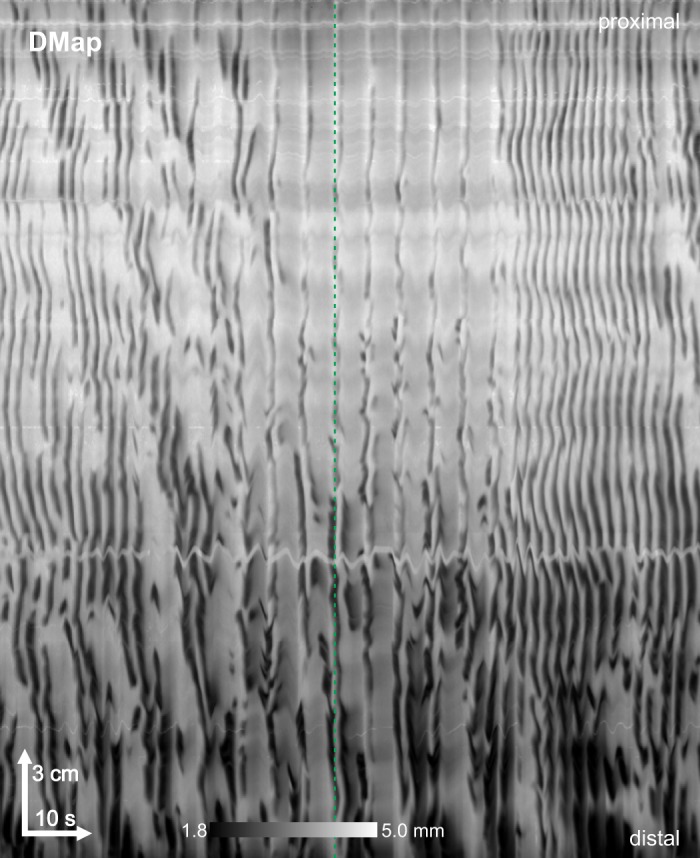
Spontaneous pinstripe pattern. On rare occasions the pinstripe pattern was seen spontaneously in wild-type mice, in the absence of carbenoxolone. Female CD-1, perfused lumen. DMap, spatiotemporal map of changes in diameter. Representative of 2 experiments.
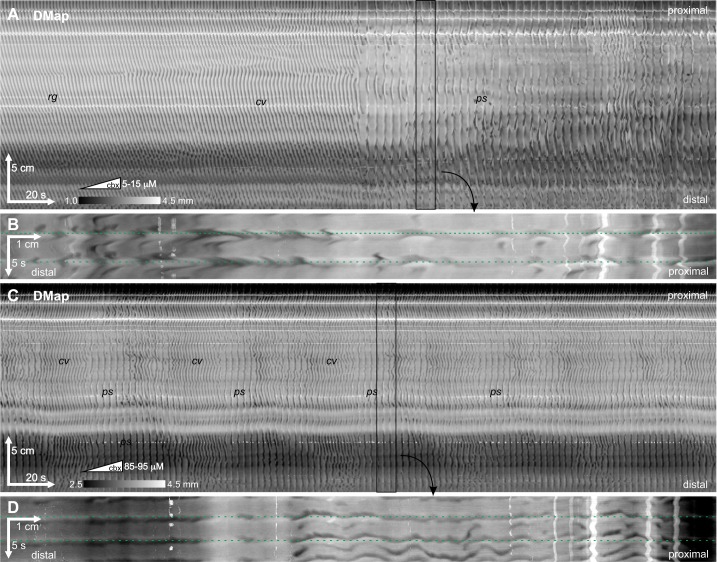
In wild-type mice (CD-1 or C57BL/6J) in the presence of lidocaine, rhythmic contraction waves traveled from the proximal to the distal end of the intestine at intervals of ~1.5 s (Fig. 3). These are ICC-MP-driven phase waves, and we refer to this as the “regular” motor pattern. Addition of carbenoxolone induced two motor patterns in all the intestines studied (n = 46): 1) The “chevron” pattern consisted of v-shaped contraction waves, with the same interval as the regular pattern (Fig. 4, A and C). 2) The “pinstripe” pattern consisted of rhythmic contractions that appeared simultaneously, but discontinuously, along the whole or part of the intestine at intervals of ~4 s (Fig. 4, A and C). Each simultaneous, intestine-long band of contraction we call a “stripe.” Sometimes contraction wave fronts along a stripe were truly simultaneous, but more often they were irregular or rippled, consisting of lots of smaller wavelets (Fig. 4, B and D). Nevertheless, the overall velocity of the stripe was infinite, with contractions occurring about a single point in time along the stripe’s length.
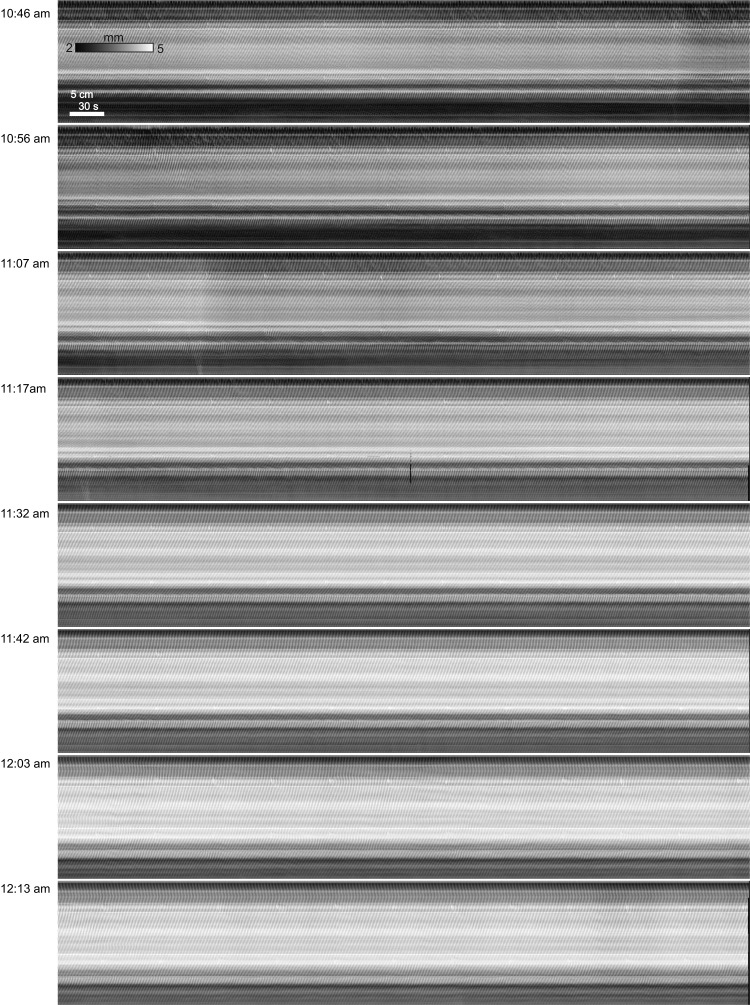
Fig. 3.
The regular pattern. Near-consecutive spatiotemporal maps of changes in diameter (DMaps) of a small intestine (start times indicated at left) over a period of almost 2 h, all in the presence of 0.5 mM lidocaine to block neural activity. Note that at 10:46 AM (start of the 1st map) the intestine had already been >40 min in the organ bath and 1.5 h had elapsed since it was removed from the mouse. The tone of the intestine decreases a little, but regular slow wave-driven contractions continue unabated. Female CD-1, 0.5 mM lidocaine, luminal perfusion. Representative of 7 experiments.
Fig. 4.
Carbenoxolone-induced pinstripe pattern. Carbenoxolone induced a series of changes in the motor pattern of the small intestine. Carbenoxolone (cbx) was pumped continuously into the organ bath at a rate of 2 μM/min [concentrations at the start and end of each spatiotemporal map of changes in diameter (DMap) are indicated by the ramp symbol (in subsequent figures a rectangle with a single concentration indicates that carbenoxolone was present at a constant concentration)]. A: the pattern changed from rhythmic, proximal to distal contraction waves with intervals of 1.5–1.6 s (regular pattern, rg) to v waves (chevron pattern, cv) to simultaneous stripes of contraction with an interval of ~3.4 s (pinstripe pattern, ps). C: 35 min after the end of A, the intestine switched back and forth between chevron and pinstripe patterns, with the latter more dominant at the distal end. B and D, zoomed and rotated insets from A and C, respectively, showing 2 stripes each. Horizontal dotted lines indicate single time points. Female C57BL/6J, perfused lumen, 2 μM/min carbenoxolone. Representative of 26 experiments.
As the concentration of carbenoxolone was increased, the intestine would usually first transition to the chevron pattern and then switch back and forth between the chevron and pinstripe patterns, often in a very rhythmic manner, before finally settling into the pinstripe pattern (Fig. 4C). Sometimes the pinstripe pattern would at first be confined to or dominant in the distal portion of the intestine (Fig. 4C). The timing and concentration dependence of pattern transitions varied substantially from intestine to intestine, even when the intestines were recorded simultaneously in the same organ bath under the exact same conditions. Sometimes the pinstripe pattern would not be seen until the concentration of carbenoxolone was upward of 60 μM; other times at concentrations of <10 μM the intestine would switch from the regular pattern directly to a pinstripe-chevron oscillation (Fig. 4A). For this reason we adopted the practice of gradually pumping carbenoxolone into the organ bath at a rate of ~2 μM/min rather than adding it in discrete aliquots. In this manner we would not miss any intermediate transitions. Where carbenoxolone has been pumped, the approximate concentration at the start and end of a DMap are indicated in the DMap by a ramp symbol followed by those concentrations. Where the concentration has been stepped, by adding discrete aliquots of carbenoxolone, this is indicated by a rectangle followed by the concentration. These additions occurred over a maximum period of 2 h, and without addition of carbenoxolone the regular pattern continued undisturbed for this period [Supplemental Fig. S1 (available at https://dataverse.scholarsportal.info/citation?persistentId=doi:10.5683/SP2/QMJHNL); see also our previous work (51, 53)].
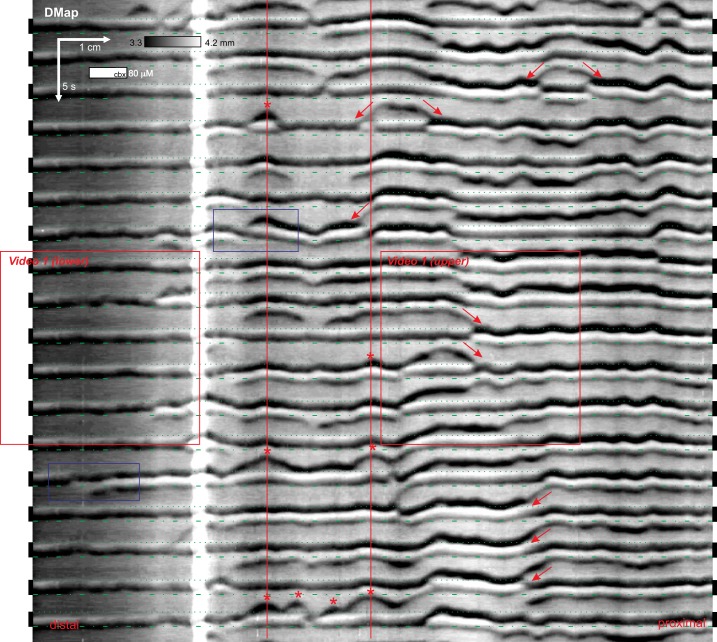
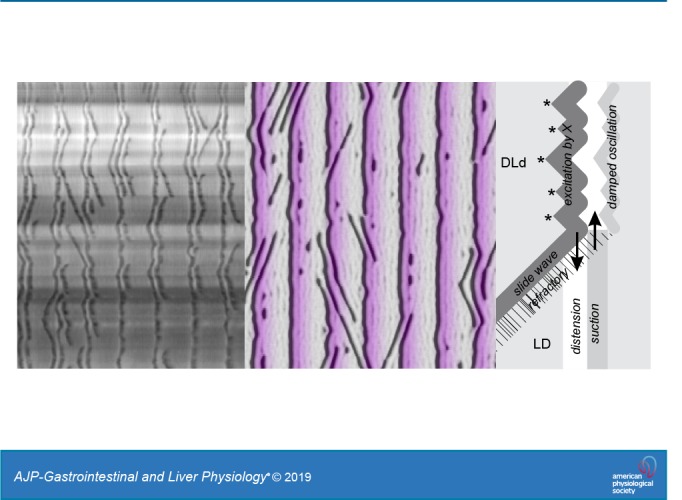
A single stripe consisted of a varying arrangement of dark (D) and light (L) bands, decreased or increased diameter relative to the baseline between stripes (Fig. 5). A light band could be followed by a dark band (LD) or vice versa (DL). A light band could be sandwiched between two dark bands (DLD), with the trailing dark band often weaker than the leading one (DLd). The dark bands were often rippled in appearance. In a DLD or DLd arrangement, rippling in the leading dark band was mirrored by the trailing dark band. Dark bands could travel across a stripe, from one stripe to another (“slide waves”), or initiate between stripes in a v wave (Fig. 5). Slide and v waves would often initiate repeatedly at the same point (Fig. 5). Where a slide or v wave hit a stripe, the band order at that point would invariably be swapped: DL in the direction of the wave and LD in its wake.
Fig. 5.
Dynamics of stripes. The pinstripe pattern had a number of characteristic features including slide waves (arrows), v waves (asterisks), and dark bands moving across a stripe (blue box). Organ bath video corresponding to the boxes marked video 1 are presented in Supplemental Video S1. The boundaries of each stripe are marked by dotted (leading edge) and dot-dashed (trailing edge) green lines. Female CD-1, Trendelenburg, 80 μM carbenoxolone (cbx). DMap, spatiotemporal map of changes in diameter. Representative of 26 experiments.
In the absence of enteric nervous system block by lidocaine, ICC-MP-driven contractions occurred in clusters up to several minutes long, separated by largely quiescent gaps, tens of seconds long (53). Under these conditions we observed, in approximately 1 of every 50 mice, the pinstripe pattern in the absence of carbenoxolone or any other drug (Fig. 6). The pinstripe appeared between clusters of ICC-MP driven contractions.
Passive and Active Bands
In the perfused lumen setup (methods) the intestine lumen and outflow tube have inside diameters of ~2 and 1.6 mm, respectively, and have a combined length of >1 m. This three orders of magnitude difference offers significant resistance to outflow such that total luminal volume will be conserved on a short timescale. Therefore, contraction along any extended part of the intestine (as during a stripe) will cause distension along the rest. In the Trendelenburg setup (methods) there is no outflow; any displaced volume has to be pushed upward, so resistance is even greater. By comparing DMaps to their corresponding videos [Fig. 5 and Supplemental Video S1 (available at https://dataverse.scholarsportal.info/citation?persistentId=doi:10.5683/SP2/A7RJKX)], it was apparent that light bands represented passive distension of the intestine in response to active contractions (dark bands).
This was most clearly seen at the distal end of the intestine. Here it was very common for the stripe to be LD (Fig. 4D and Fig. 5). Over time, mucosa tends to loosen from the inner wall of the intestine. In intestines with loose distal mucosa it was apparent from the movement of this mucosa that there was luminal flow toward the distal end during the light band (diameter increase), followed by a reverse flow during the dark band (decreased diameter) (Supplemental Video S1). The flow then stopped until the next stripe. Thus it appears that the light band was a distension in response to fluid flow into the distal end, and conversely the following dark band was a passive mechanical suction in response to fluid flow in the proximal direction. In summary, LD stripes represent a purely passive, mechanical response of the intestine. In a DLD or DL stripe the initial dark bands are active contractions and the light band is passive distension.
Myenteric ICC
The simplest explanation of a pattern of rhythmic waves (stripes) with infinite velocity would be a network of coupled oscillators with uniform ω (introduction). We call this hypothetical network X. The pinstripe pattern is distinct enough from the regular, slow-wave contraction pattern to suggest that X is not the ICC-MP. This was confirmed by examining mice with mutations at the white spotting (W) locus where c-kit and its regulatory elements reside (methods). ICC-MP are depleted in the small intestines of both W/Wv and Wsh/Wsh mutants, although other populations of ICC (intramuscular or deep muscular plexus) remain.
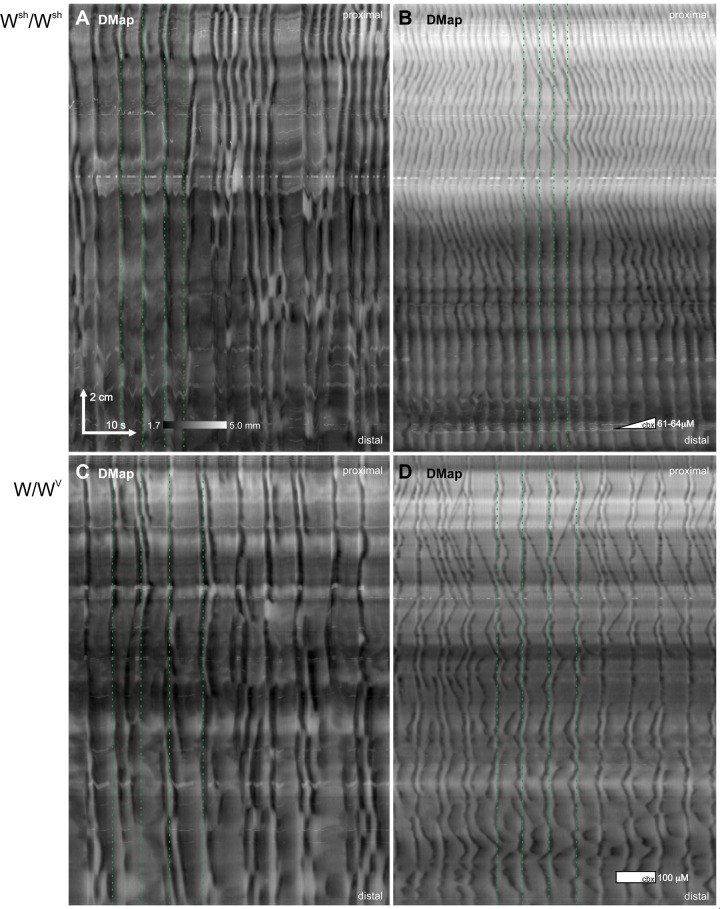
In the presence of lidocaine but the absence of carbenoxolone, the motor pattern of Wsh/Wsh and W/Wv mice resembled the pinstripe pattern of wild-type mice in the presence of carbenoxolone (n = 9 and 8, respectively; Fig. 7). Rhythmic contractions appeared clustered about lines of simultaneity with an interval of ~4 s (Fig. 7, A and C) but deviated more about that line and were more discontinuous than in the stripes of wild-type mice in the presence of carbenoxolone. Carbenoxolone induced a pinstripe pattern indistinguishable from wild-type mice in all the W mutants (n = 17; Fig. 7, B and D). As in the wild-type mice, at first there were often repeated transitions back and forth between chevron and pinstripe patterns before the latter became stable. Unlike in wild-type mice, during the chevron pattern there were often large gaps between otherwise closely spaced v waves (Fig. 7B).
Fig. 7.
Motor patterns in W mutants. In both Wsh/Wsh (A) and W/Wv (C) mutants, in the absence of carbenoxolone, contractions clustered about lines of simultaneity (dotted green lines), i.e., stripes, but deviated more from this line than in the pinstripe pattern in wild-type mice. Carbenoxolone (cbx) induced a pinstripe pattern indistinguishable from wild-type mice in both Wsh/Wsh (B) and W/Wv (D). A and B: female Wsh/Wsh, perfused lumen, 2 μM/min carbenoxolone. C and D:, female W/Wv, perfused lumen, 100 μM carbenoxolone. See Fig. 8 for n numbers and statistics.
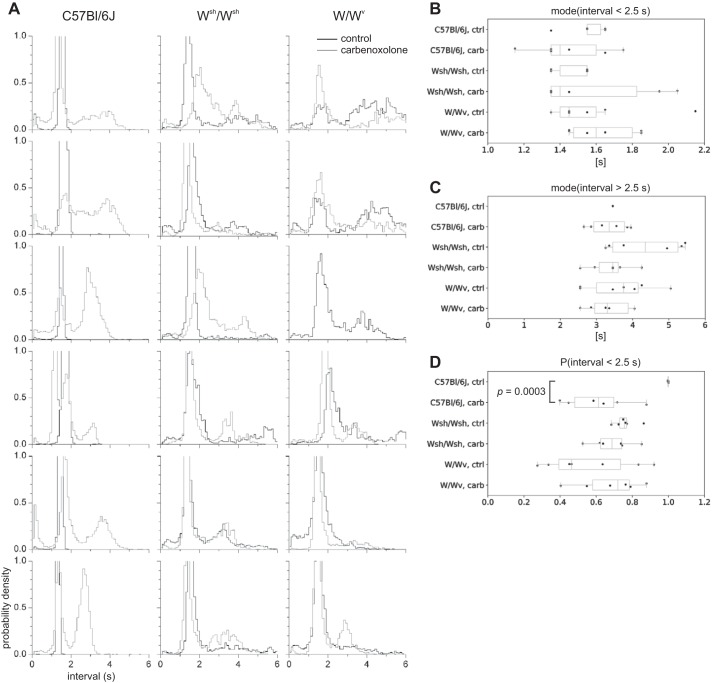
The distribution of contraction intervals in W mutants was largely unchanged by carbenoxolone (Fig. 8A). Under both conditions there were two dominant peaks near 1.5 and 4 s. The 4 s peak corresponds to the interval between stripes (i.e., the frequency of X) and is in line with the frequency of rhythmic action potentials and Ca2+ waves previously recorded in W mutants in the absence of carbenoxolone (Table 2). The 1.5 s peak corresponds to the chevron pattern and to the interval between the dark bands of a DLD or DLd stripe. In wild-type mice there was also a peak at 1.5 s, corresponding to the regular motor pattern, and this was supplemented with a 4 s peak in the presence of carbenoxolone (Fig. 8A) corresponding to the stripes.
Fig. 8.
Contraction interval distributions in wild type and W mutants. A: contraction interval distributions for 18 mice—6 female C57BL/6J (left), 6 female Wsh/Wsh (center), and 6 female W/Wv (right)—before (black) and after (gray) carbenoxolone. Every panel has the same interval (x) and probability density (y) scale, as indicated at bottom left. B–D: statistics of intervals for each strain, before and after carbenoxolone, shown as box-whisker plots: values for each intestine (points), median (central line), lower to upper quartile (box), and range (whiskers) are shown. B: mode of intervals < 2.5 s. C: mode of intervals > 2.5 s. D: probability of interval being <2.5 s. Comparisons were made between all categories [strain and control (ctrl) to carbenoxolone (carb)] by unpaired Student’s t test. Only the shown comparison (probability of interval > 2.5 s, from wild-type control to wild-type carbenoxolone) had P < 0.05. Note that in C C57BL/6J control n = 2, because 4 intestines had no intervals > 2.5 s.
Intervals were split into two bins separating the high-frequency peak (<2.5 s) from the low-frequency peak (>2.5 s). The modes of these bins were fairly stable (Fig. 8, B and C). There were no statistically significant changes in the modes with either mouse strain or carbenoxolone (i.e., P > 0.05 by unpaired Student’s t test). There was also no significant shift from one bin to another with carbenoxolone, with the exception of the C57BL/6J control mice. Here the probability of being in the high-frequency bin decreased from 0.99 ± 0.001 to 0.61 ± 0.16 with carbenoxolone (P = 0.0003 by unpaired Student’s t test). Otherwise there was no significant difference in the balance between bins, with the high-frequency bin accounting for 60–70% of intervals in the W mutants and, in the presence of carbenoxolone, in the wild-type mice (Fig. 8D). So during the pinstripe pattern there were ~1.5 high-frequency cycles (trailing dark stripes or chevron waves) for every cycle of X.
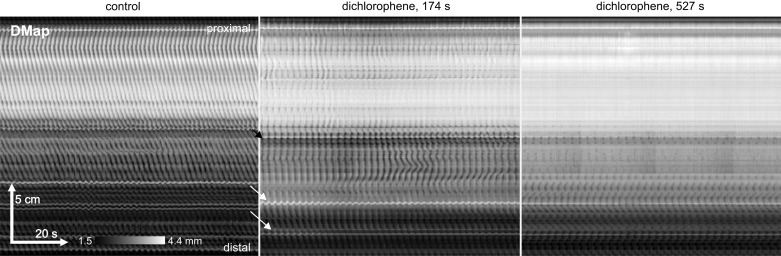
The Ano1 anion channel may play a role in slow wave generation in the small intestine. Slow waves are absent in the small intestine of neonatal Ano1-knockout mice (29) but present in adult inducible knockdowns (31). Similarly, small intestine slow waves are sensitive to Ano1 antagonists such as dichlorophene and benzbromarone, but sensitivity reduces with age (29, 31). When dichlorophene was gradually added to the organ bath in micromolar increments, reaching 10 μM after an hour, the regular motor pattern gradually decreased in amplitude until contractions completely disappeared (n = 2). However, if a supramaximal (20 μM) dose of dichlorophene was given at once, a pinstripe pattern occurred for a few minutes before itself disappearing (Fig. 9; n = 4). A supramaximal dose of benzbromarone (20 μM) blocked the regular pattern rapidly without any intervening transition to a pinstripe pattern (n = 2; not shown).
Fig. 9.
Effect of supramaximal dichlorophene. Dichlorophene is an antagonist of the Ano1 anion channel and blocks myenteric interstitial cell of Cajal oscillations. When a supramaximal concentration of dichlorophene (20 μM) was added to the bath in one go a stripe pattern was seen (center) before contractions disappeared almost completely (right). Times from dichlorophene addition are indicated at top. The intestine significantly lengthened in response to dichlorophene, as indicated by the shift in the position of mesenteric/Peyer's diameter markers (arrows). Thus we had to pull the intestine out straight (pull one end’s cannula further down the kit-kat) and so could not map the intestine continuously to register all transitional changes in the motor pattern. Female C57BL/6J, perfused lumen, no carbenoxolone. Representative of 4 experiments.
Longitudinal Movements and Inhibitory Junction Potentials
A number of investigators have recorded rhythmic action potentials (APs) in the longitudinal muscle of the small intestine and colon of various species (discussion). Could X be in the longitudinal muscle? The DMaps during the pinstripe pattern did suggest such a possibility. Peyer’s patches, fragments of mesentery that were not dissected away, or other fixed irregularities in the intestine’s diameter appear as distinct lines across the time dimension of the DMap. When those lines move, they track displacement of the intestine along its long axis. There was always some degree of longitudinal displacement synchronous with stripes (Figs. 4–7). The magnitude of displacement was often greatest immediately after induction of the pinstripe pattern (it would fade with time) and was greater than during the regular pattern. Displacement suggests longitudinal length change along some extent of the intestine that pulls it in one direction or the other. Could this have been longitudinal contraction resulting from rhythmic longitudinal APs?
To measure longitudinal contraction we marked the intestine with stripes of charcoal dust and tracked these stripes to create spatiotemporal maps of longitudinal length change, LMaps (methods). LMaps showed that during a stripe the intestine extended along its longitudinal axis by a few percent (Fig. 10). If both the circular and longitudinal muscle are mechanically passive, they should extend together, and indeed in LD (passive) stripes longitudinal length followed in phase with diameter, first increasing and then decreasing (Fig. 10, left). In contrast, in DL or DLD (active) stripes, longitudinal length was out of phase with diameter: it first increased and then decreased (Fig. 10, right).
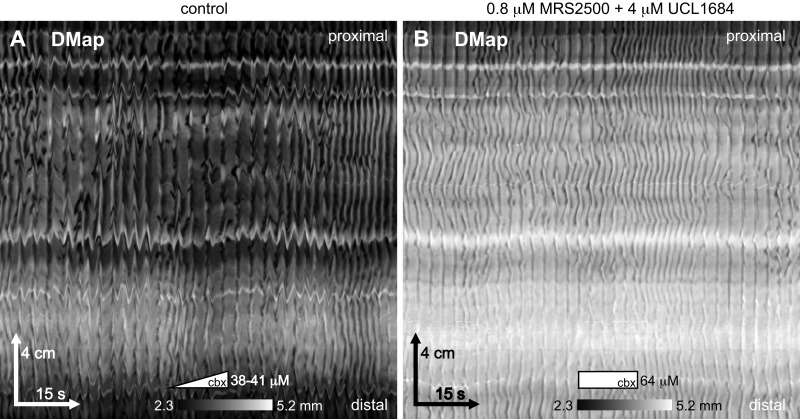
Rhythmic longitudinal extension could be a rhythmic active relaxation of the longitudinal muscle. Many investigators have recorded, from both the large and small intestine, rhythmic inhibitory junction potentials (IJPs), hyperpolarizations that might relax the muscle (discussion). These IJPs are blocked by antagonists of P2Y1 receptors and SK channels. We found that neither the P2Y1 antagonist MRS2500 (0.2–0.8 μM, n = 4) or the subsequently added SK channel antagonist UCL1694 (1–4 μM; n = 4), had any effect on the pinstripe pattern induced by carbenoxolone (Fig. 11).
Fig. 11.
Effect of antagonists of P2Y1 and SK channel. Carbenoxolone (cbx) was pumped into the bath at 2 μM/min. A: the pinstripe pattern 19 min after the start of pumping. Pumping was stopped after 32 min (at 64 μM carbenoxolone), and 0.8 μM MRS2500 (P2Y1 antagonist) was added. After a further 11 min, 4 μM UCL1684 (SK channel antagonist) was added. B: the same small intestine as in A, 31 min after the addition of MRS2500 and 20 min after the addition of UCL1684. Female CD-1, perfused lumen, 2 μM/min carbenoxolone. Representative of 4 experiments.
A Model of the Pinstripe Pattern
To explain all our data thus far we propose the following series of linked hypotheses. Together they are our mechanistic interpretation of the pinstripe pattern.
An unidentified network of coupled oscillators (X) extends the length of the small intestine. X has a near-uniform ω of ~1/4 Hz (15 cycles/min or a natural interval of 4 s).
Circular muscle (CM) cells are damped oscillators. That is, they generate a decaying series of oscillations in response to an excitatory stimulus such as the ICC-MP or X. The period of these oscillations is ~1.5 s, i.e., similar to the period of the slow wave.
X is active in the absence of carbenoxolone (Fig. 6). In wild-type mice it is usually masked by the higher-frequency pacing of ICC-MP that always excites the CM ahead of X. In W mutants X is unmasked.
The excitability of the CM is variable along the intestine’s length and stochastic because of voltage noise. Thus X excites the muscle discontinuously, leading to multiple wave initiation points that mutually annihilate, coalescing into a rippled wave front, the leading dark band of a DLD or DLd stripe (Fig. 12A).
The trailing dark band of a DLD/DLd stripe, which mirrors the ripples of the leading dark band (excited by X), is a damped oscillation of the CM (DLd; Fig. 12A).
Light bands are due to passive distension (Supplemental Video S1). In an LD stripe the light band results from distension and longitudinal extension, as fluid is forced into the segment by contraction at another segment (Fig. 12B). As the contraction is released, fluid flows out of the segment, causing suction (a weak dark band) and longitudinal compression (Fig. 12C).
The distal CM usually has low excitability, or perhaps X does not always extend to the distal intestine; hence passive LD stripes are most usual there.
Slide and v waves are propagating excitation waves in the CM. Once initiated by X they propagate through the muscle independent of X or any other oscillator network.
When a slide or v wave runs into a stripe, it annihilates with the front excited by X (the leading dark band of the stripe) and also suppresses excitation in its wake (opposite to its direction of travel) through its refractory period (Fig. 12A). Thus the DLd stripe stops where it meets the slide/v wave and is replaced by a passive LD stripe.
The chevron pattern in W mutants results from the CM changing from a damped to a limit-cycle oscillator by an Andronov–Hopf bifurcation (Fig. 2). Alternatively, there could be some remaining islands of ICC-MP in the W mutants that pace the v waves making up the chevron, but we think this unlikely (see discussion).
The natural period of ICC-MP and the damped oscillations of the CM are both ~1.5 s. This corresponds to the interval peak of the regular pattern in wild-type mice (interval determined by ICC-MP), the chevron waves in W mutants (interval determined by CM), and the leading-to-trailing dark band interval in stripes (interval determined by CM).
Fig. 12.
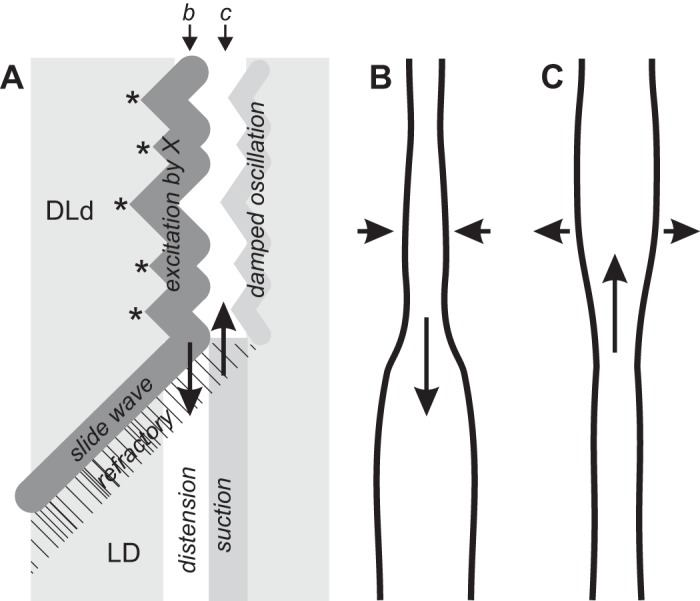
A hypothesis for stripe patternation: a cartoon of a stripe (A) and the corresponding outline of the intestine at 2 time points (B and C, as indicated). Excitation of the circular muscle by X is stochastic, due to variations in excitability and membrane potential noise. Thus excitation waves initiate at multiple points (*), resulting in a rippled dark band of contraction. A slide wave blocks excitation within its refractory wake (hatched), and fluid is forced into this segment by the excited muscle (downward arrow, A and B). As the circular muscle relaxes, fluid flows back into the previously contracted segment, increasing its diameter (a light band) and transiently reducing the diameter of the previously distended segment through suction (upward arrow, A and C). The circular muscle may then undergo a secondary damped oscillation that follows the wave front pattern of its initial excitation by X. Compare with Supplemental Video S1 and Fig. 5. DLd, trailing dark band (LD) weaker than leading LD.
On the basis of these hypotheses, we created a mathematical model consisting of two parallel chains of coupled Fitzhugh-Nagumo (FN) systems (Fig. 2). One chain models the coupled oscillator network X, and the other chain models the excitable (damped oscillator) circular muscle (CM). For a brief introduction to the FN model and nonlinear dynamics see Reference 52.
The parameters of the X chain were set to produce limit-cycle oscillations. The parameters were kept constant across the chain so that the oscillations all had the same frequency and so synchronized into infinite velocity phase waves. The parameters of the CM chain (Table 1) were varied to produce excitability with or without damped oscillations or to produce limit-cycle oscillations. By varying CM behavior we modeled a variety of patterns seen in our experiments. We examined the pattern formed by the membrane potential variable (v) of the CM chain as a proxy for intestine diameter (Fig. 13), as it is excitation of the circular muscle that causes its contraction and therefore diameter changes.
As a was increased from 0.8 to 0.9, CM behavior changed from limit-cycle oscillations to excitability through an Andronov-Hopf bifurcation (compare Fig. 13, D to H). Thus when a was graded from 0.83 at the CM chain’s proximal end to 0.9 at its distal end, there was a corresponding transition along its length from chevron to pinstripes (Fig. 13B). The stripes were synchronous with the upstroke of X (Fig. 13, B and C), i.e., they were excited by it. Clusters of chevron waves would invade the distal stripe pattern (Fig. 13B). A wave at the head of the cluster would suddenly attach to the front of X, making a sharp velocity change and becoming a stripe wave (Fig. 13B). This pattern was remarkably similar to that seen in experiment (Fig. 13A). Both the chevron and pinstripe patterns and the transition between them were stochastic because of the addition of noise to the CM chain (σ = 0.12).
When a was 0.9 across the CM (i.e., excitable), only stripes were seen (Fig. 13F). The noise in the CM generated both slide and v waves and gave the stripes themselves a rippled appearance. Damped oscillations were apparent following each CM upstroke (Fig. 13G), and in some cases these reached threshold for a secondary excitation wave that mirrored the first, like a DLD stripe (Fig. 13F).
When noise was reduced in the CM (σ from 0.12 to 0.02), the stripes were straight and there were no sliding or v waves (Fig. 13J). When c was decreased below 6, the stripes changed from being at the leading edge of X’s wave front to its trailing edge. With c balanced just near 6, the stripes alternated between edges in distinct zones, the boundaries of which gradually drifted over time (Fig. 13J). We saw this occasionally in the small intestine (Fig. 13I) and call it the chain pattern. Also, there were damped (i.e., decaying) oscillations following each excitation (Fig. 13K, bottom).
DISCUSSION
We have provided evidence for a pacemaker network (X) in the small intestine, distinct from the ICC-MP. We show that this network generates a motility pattern (“pinstripe pattern”) that can be explained if X is a network of coupled oscillators that excite the circular muscle into damped oscillations.
X: The Longitudinal Muscle?
Numerous studies have observed rhythmic spontaneous action potentials (APs) or calcium waves in the small intestine of c-kit (W or Steel) mutants (Table 2). Only one of these explicitly talked about a “pacemaker” in relation to this rhythmicity (49). Often it seems that “pacemaker activity” was reserved as a synonym for ICC-MP-generated slow waves. Nevertheless, most of the studies state quite tight interval ranges (Table 2), which cluster around 4 s, and describe the APs as either “irregular” or “constant” (Table 2). In some traces the events are highly rhythmic (low interval-to-interval variation); in others this rhythm is bursting or interrupted. By comparison to the pinstripe pattern, it must be remembered that even though the stripes are highly rhythmic, the contraction of the CM along each stripe is discontinuous and irregular, and so a recording from one point might appear “irregular” or “bursting.” So it is safe to assume we are all measuring the same thing in W mutants, the same X.
All the studies (Table 2) concluded that X was the smooth muscle cells. To a certain extent this has been the default hypothesis because “action potentials” are traditionally associated with smooth muscle cells. However, there is a long pedigree and positive evidence for such a hypothesis. Over sixty years ago Edith Bülbring produced a series of studies of rhythmic APs in the taenia coli (colonic longitudinal muscle) of the guinea pig (e.g., Refs. 7, 8). Since then, numerous investigators have observed rhythmic APs in the muscle of both the small and large intestine of various species (5, 9, 10, 12, 36, 39, 44, 64, 68). Most of these were recorded from longitudinal muscle that was dissected away from the circular muscle. This may not have been just in deference to the tradition of Bülbring. With the exception of References 69 and 71, studies that looked at both layers have remarked that APs were much more frequent and rhythmic in the longitudinal layer (9, 68, 70). In the studies in which both muscle layers were recorded from simultaneously, circular APs always followed longitudinal APs. The APs of W mutants were always recorded from the circular muscle and according to our hypothesis would represent excitation of the CM by X rather than being the depolarization of X itself.
Recent intracellular Ca2+ imaging studies have also suggested the longitudinal layer as a source of rhythmic activity (24, 25, 69–71, 80). In the guinea pig colon and small intestine and the W/Wv small intestine, rhythmic Ca2+ waves shoot down the longitudinal muscle at velocities of >10 cm/s, an order of magnitude faster than their propagation about the circumferential axis (in either the longitudinal or circular layer). This speed difference has also been observed in two-electrode AP studies (68). The circumferential spread of the Ca2+ wave is often steplike, with 100- to 200-μm bundles of muscle being activated in a saltatory fashion. The calcium waves have been measured over distances of up to a few millimeters. This spatial scale and their velocity suggest they are indeed waves, either excitation or phase waves (52), rather than just a diffusion phenomena. However, they are not so fast that they could be confused for simultaneous over distances and at temporal resolutions comparable to our diameter maps (~25 cm and 33 ms).
If X is in the longitudinal muscle, is it the muscle cells themselves or some specialized pacemaker network embedded within the longitudinal muscle such as intramuscular ICC? Occam’s razor might suggest the former, but there is also some suggestive experimental evidence that points this way. The muscle of W mutants is slightly depolarized relative to wild type (48, 76, 77). Also, rhythmic APs can be induced in a number of smooth muscles by tetraethylammonium and Ba2+, manipulations that depolarize the muscle by altering potassium channel kinetics (5, 10, 12, 39, 44, 48). Therefore it could be that a small depolarization of smooth muscle causes a bifurcation from excitable to limit-cycle behavior (25, 48). Spontaneous v waves (“ripples”) occur in the colon of embryonic mice, independent of ICC and the enteric nervous system (56, 57). Mostly the v waves appeared stochastically, so probably were noise induced, but in some DMaps they appeared quite rhythmic, suggesting the muscle cells were limit-cycle oscillators.
If X is in the longitudinal muscle or is the muscle itself, in our experiments either 1) X’s depolarization does not excite the longitudinal muscle enough to produce its contraction or 2) such contraction is negligible compared with the passive longitudinal extension caused by fluid movements in the lumen. The latter would seem more likely. The LM is thin, and so the force engendered by its contraction is weak compared with the CM.
X: The myenteric ICC?
W/Wv and Sld mice do contain patches of c-kit+ ICC-MP, especially near the mesenteric border (references in Table 2). An ultrastructural quantification gave a drop in ICC-MP from 4.3 per 100 muscle cells in wild type to 0.2 per 100 in W/Wv, i.e., 4.6% of wild type (48). We could not find any statistics for Wsh, only statements to the effect that there are no ICC-MP at all in these mice (21, 33). Nevertheless, if there are islands of ICC-MP remaining in these mice, there are many reasons to argue against these being X: 1) Rhythmic APs are recorded at almost every “stab” of the c-kit mutant muscle with a microelectrode. 2) Calcium waves in c-kit mutants propagate quickly over distances much larger than the islands. 3) In a previous study of a two-dimensional array of coupled oscillators, we showed that up to 60% of the oscillators could be removed (at random) before phase waves were noticeably disturbed (78). It is impossible that the few islands of ICC-MP in some W mutants (see above) could generate phase waves up to 30 cm long. 4) Rhythmic APs are completely different in appearance from the slow waves (or “pacemaker potentials”) associated with ICC-MP and occur at less than half the frequency. How would a reduction in ICC-MP translate to a change in their depolarization waveform and natural frequency?
In Wsh mutants there are no c-kit+ ICC of the deep muscular plexus (ICC-DMP). but nevertheless that network appears to be there by expression of neurokinin receptors (33). The possibility might be conceded that the ICC-MP network is in fact completely intact in c-kit mutants, just that the network is no longer c-kit+. Ano1 is an alternate marker for ICC, but we are not aware of anyone who has looked at its expression in c-kit mutants. If there are c-kit− ICC in the small intestine, they would also have to be altered so as to be unidentifiable by electron microscopy (28, 33, 48, 49, 76, 77).
The simplest hypothesis for X is that it is something other than the ICC-MP. This is the hypothesis that all others in the field have subscribed to thus far: there are rhythmic events in W mutants not generated by ICC-MP. The novelty of our data is that this network generates coherent, highly rhythmic phase waves on the scale of the organ that can only be explained as the output of a network of coupled oscillators. By extending the characterization of X to the organ-length scale we have provided further evidence that X is not the ICC-MP.
X: PDGFRα+ Cells or ICC-DMP?
Inhibitory junction potentials (IJPs) are transient hyperpolarizations of the muscle. Rhythmic IJPs with intervals of a few seconds have been observed by numerous investigators in the small and large intestines of various species (15, 19, 22, 35, 60, 62, 63, 65, 67). It is quite possible that IJPs could induce APs by rebound (anode break) excitation. Rhythmic IJPs are sensitive to antagonists of both P2Y1 purinergic receptors and SK small-conductance K+ channels. Some have correlated rhythmic IJPs with rhythmic bursts of APs generated by S neurons (67), suggesting that release of purinergic transmitters by these neurons acts postsynaptically on the muscle, which then generates the IJP through its SK channels. An alternative explanation is that the mechanosensitive S neurons are responding to the muscle (i.e., the signaling is the reverse). More recently it has been suggested that PDGFRα+ interstitial cells are the actual postsynaptic target of the S neuron (3, 38). PDGFRα+ interstitial cells are rich in both P2Y1 receptors and SK channels compared with the muscle (2, 59). They are also present in both circular and longitudinal muscle layers and at the myenteric plexus. They are also untouched in W mutants. Imaging has shown that PDGFRα+ interstitial cells have spontaneous, rhythmic, TTX-insensitive Ca2+ transients with intervals in the range of 3.7–30 s (2, 3). P2Y1 and SK antagonists appeared to have no effect on the pinstripe pattern (Fig. 11), but we would not rule out the possibility of X being a network of PDGFRα+ interstitial cells based purely on pharmacology.
The ICC network of the deep muscular plexus (ICC-DMP), within the circular muscle, is intact in all c-kit mutants. It was never considered a contender for X by any of the studies in Table 2. There simply was no physiological evidence at the time that ICC-DMP can generate rhythmic activity. This has changed with two recent calcium imaging studies (1, 81). Both showed that ICC-DMP spontaneously generate intracellular Ca2+ sparks and waves. The rhythmicity of these events varies considerably. In the cases where it is high (e.g., Fig. 5A in Ref. 1; Fig. 3M in Ref. 81), intervals are on the scale of 3–5 s. But there is no coordination between the ICC; they do not act as a coupled network, except under the influence of substance P (81). The latter suggests that the ICC-DMP are an “on-demand” pacemaker (27) and this might be the case for X.
Response of the Muscle Cells
The consistency of the interval distributions in W mutants, before and after carbenoxolone, suggests a consistency in mechanism, namely, coupled oscillations in X for the 4 s peak and damped oscillations in the circular muscle (in response to excitation by X) for the 1.5 s peak. We cannot find any study of damped oscillations in smooth muscle, but damped oscillations have been seen (10, 12, 13, 68, 76). There are, on average, 1.5 of the 1.5-s intervals for every 4-s interval, both before and after carbenoxolone (Fig. 8D). In W mutants in the absence of carbenoxolone, there can be clusters of several 1.5-s interval waves following each 4-s interval (Fig. 7, A and C). By definition, a pinstripe has at most a single 1.5-s interval following each excitation by X (DL followed by d). In the presence of carbenoxolone, the extra 1.5-s intervals result from the chevron pattern often seen at the proximal end of the intestine (Fig. 7B).
The chevron pattern in W mutants could be explained in several ways. The damping of the muscle could be low enough so that either 1) several oscillations occur before their decay to an amplitude below contraction threshold or 2) the coupled muscle cells can excite each other. Alternatively, the muscle cells could have passed through an Andronov–Hopf bifurcation and so have self-sustained oscillations. Another possibility is that islands of ICC-MP remaining in the W mutants (see above) act as isolated pacemaker sites and the chevron waves are muscle excitation waves originating at these sites, i.e., the chevron waves are analogous to the slide and v waves of the pinstripe pattern, just paced by ICC-MP islands rather than X.
If muscle cells are damped oscillators in wild-type mice in the absence of carbenoxolone, this could have a significant physiological function. When the frequencies of two oscillators are suitably close (that is to say they do not have to be exactly the same) they will resonate; their amplitude increases. As the damped oscillations of the circular muscle cells match the frequency of the ICC-MP, then resonance means that only a weak ICC-MP oscillation would be required to excite large oscillations in the circular muscle. Pacing the CM with a weak signal has the functional advantage that the signal can easily be switched off or decoupled if contractions need to stop. Also, it solves the problem of how a thin network of ICC can pace a thick layer of muscle cells.
We interpret light-dark (LD) bands as a purely passive response of the muscle to fluid movement in the lumen, distension followed by suction. This hypothesis would be supported by force-length analysis (11, 16) together with electrophysiological recordings to confirm that the muscle is actually passive. If true, the hypothesis suggests that X may not extend the whole length of the intestine.
Pharmacology of the Pinstripe Pattern
We hypothesize the following in regard to the effects of carbenoxolone.
1) Block of the regular (ICC-MP paced) pattern.
a) Carbenoxolone decouples the ICC-MP to the point that its phase waves (the regular pattern) lose coherence. This implies that X must have much stronger coupling (density of gap junctions) or a gap junction type with a lower sensitivity to carbenoxolone, as its phase waves (the pinstripe pattern) remain coherent until much greater concentrations of carbenoxolone. This hypothesis would be consistent with X being the longitudinal muscle cells—the longitudinal muscle is thicker in cross section than the ICC-MP and so should have greater coupling, assuming the same density of gap junctions.
b) Carbenoxolone has some effect unrelated to gap junction block (“nonspecific effect”) that blocks oscillations in the ICC-MP or makes the muscle unresponsive to their pacing, for instance, a change in polarization of the ICC-MP or muscle due to blocking of channels other than gap junctions.
2) Induction of the pinstripe (X paced) pattern.
a) X oscillates in wild-type mice in the absence of carbenoxolone but does not pace the muscle (induce a pinstripe pattern) because of the higher frequency (“dominant”) pacing by the ICC-MP. In W mutants this dominance is absent.
b) X does not oscillate in wild-type mice in the absence of carbenoxolone and is induced by an effect of carbenoxolone (e.g., a change in membrane polarization). In W mutants the same effect occurs as a result of the mutation rather than pharmacology.
Note that the hypotheses for 1 (block of the regular pattern) are independent of those for 2 (induction of the pinstripe), so we could have any combination of hypotheses for 1 and 2. We favor 1a over 1b because the chevron pattern does suggest that carbenoxolone drastically reduces coupling between ICC-MP (50, 54). However we cannot rule out the parallel operation of 1b. We favor 2b over 2a because in wild-type mice in the absence of both carbenoxolone and lidocaine the pinstripe pattern does not always appear in the gaps between the clusters of slow wave (ICC-MP)-driven contractions. Whether the ICC-MP actually stop oscillating during these gaps (which seems unlikely) or the slow waves just do not reach threshold for contraction, something must be driving the pinstripe’s (rare) appearances during gaps other than just the possible absence of pacing by ICC-MP. One possibility is depolarization beyond a given threshold that induces oscillations in X during gaps, with carbenoxolone and in W mutants. There is in fact evidence of systematic depolarization of the muscle in W mutants (48, 76, 77), and this has been used to explain the induction of action potentials in those mutants (see above).
We do not believe that the intestinal tissue is damaged or degraded by prolonged exposure to carbenoxolone or by handling during dissection and that this damage might produce patterns or individual contraction events that are pathological in nature. There are several reasons to support this belief: 1) The pinstripe pattern is induced by carbenoxolone in <10 min (see Fig. 4A). 2) Contraction amplitude is not weakened by carbenoxolone (Fig. 4A). 3) The pinstripe pattern is not so different from the motor pattern in W mutants before carbenoxolone (in terms of wave interval and velocity). Does this mean that the tissue of W mutants is in some sense “degraded” or “damaged” relative to wild-type mice? If so, what relation can this damage have to the action of carbenoxolone ? 4) When initially induced, the pinstripe pattern alternates with the chevron pattern (Fig. 4). What kind of “damage” is periodic? 5) If some local aspect of the pinstripe pattern were related to dissection or handling damage it might be expected that such damage would also correlate with some aspect of the regular motor pattern, but there is no such correlation.
All ICC, including ICC-MP of the small intestine, express the Ano1 anion channel (20, 30). Ano1-knockout mice rarely live beyond a few days and up to 20 days old show no slow waves in the stomach or small intestine (30, 61). Nevertheless, their c-kit+ ICC-MP networks are intact. ICC-MP of the knockout small intestine do have Ca2+ transients, but these are uncoordinated across the network (61). DMaps of the Ano1-knockout small intestine lacked slow wave-driven contractions (“ripples”) but did have slightly rhythmic longitudinal displacements with intervals of a few seconds (Fig. 9A in Ref. 61). To study the role of Ano1 in adult mice, inducible Ano1 knockdowns have been developed (31, 47). Their gastric slow waves are abolished by knockdown, but small intestine slow waves and ICC-MP Ca2+ transients are largely unaffected. In our study a large dose of the Ano1 inhibitor dichlorophene blocked slow-wave contractions and transiently revealed the pinstripe pattern before it also was blocked (Fig. 9). This suggests that although X is sensitive to dichlorophene, it is less sensitive than ICC-MP.
The Physiology of W mutants
W/Wv mice have a reduced life span due to anemia, impaired resistance to parasitic infection, and increased occurrence of some tumors (41, 58). Wsh/Wsh mice do not suffer from these conditions and have a normal life span (40). The small intestine is 20% shorter in W/Wv mice (13). Apart from this, no gross morphological changes to the gastrointestinal tract of W/Wv or Wsh/Wsh mice have been reported, as far as we are aware. This accords with our experience: the whole gastrointestinal tract appeared normal.
The pattern of contraction in W mutants is obviously altered by the change in pacemaker cell population. In the two in vivo studies of W/Wv mice (13, 26), pressure electrodes and barium contrast radiology showed more retrograde propulsion than in wild-type mice, resulting in longer gastric and intestinal transit times. This accords with our DMaps (Fig. 7). Still, the “contents do eventually move through the W/Wv intestine” (13), and obviously they must, because the mice are alive.
The enteric-neuronal regulation of motility appears to be intact in W/Wv mice. Migrating motor complexes still occur, albeit at a slightly lower frequency and with slow waves replaced by APs with a period of 4 s (66). Contraction amplitude during the complexes was actually greater in the mutants (66), so strength of contraction is not impaired. This accords with our own DMap data: contraction amplitude (diameter change) was no different in either W/Wv or Wsh/Wsh from controls (e.g., in the DMaps in Figs. 6 and 7, wild-type and W mutants, respectively, have almost the same diameter scale).
Conclusions
Advances in scientific approaches mean that the tools are available to make the next step behind reporting a phenomenon to locating its origin. Calcium imaging is likely the most direct way to identify X. Imaging not only identifies the what (as in intracellular recording) but also straightforwardly identifies the where. What is the function of X? X might be an “ectopic pacemaker” like the atrioventricular node in the heart: its primary (evolved) function is nonoscillatory but under rare circumstances bifurcates to oscillation. This could certainly be the case if X is the longitudinal muscle: clearly, the muscle has a primary, non-pacemaker function. In W mutants induction of X as an ectopic pacemaker “rescues” the mice. Alternatively, X might be an “on-demand” pacemaker, switched on in response to certain neuronal stimuli to generate some functionally useful motor pattern, but is otherwise rare (Fig. 6). Such a role was previously suggested for ICC-DMP (1, 27). Alternatively, X might be a constitutive pacemaker in wild-type mice, active all the time. Perhaps then X and the ICC-MP interact in their excitation of the circular muscle to generate subtle, but functionally important, effects on the “regular” motor pattern. Whether X is an ectopic, on-demand, or constitutive pacemaker, it is another layer in the supernetwork of ICC, PDGFRα+, enteric neurons and muscle that interact to produce diverse motility patterns.
GRANTS
This study was supported by Canadian Institutes of Health Research Grant 20006288 as well as Natural Sciences and Engineering Research Council Grant 2017-06243 to J. D. Huizinga. S. P. Parsons was supported in part by a research grant from the Farncombe Family Digestive Health Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.P. conceived and designed research; S.P.P. performed experiments; S.P.P. analyzed data; S.P.P. and J.D.H. interpreted results of experiments; S.P.P. prepared figures; S.P.P. drafted manuscript; S.P.P. and J.D.H. edited and revised manuscript; S.P.P. and J.D.H. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional Web site of the authors, which at the time of publication they indicate is: www.scepticalphysiologist.com/code/code.html. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the Web site address, or for any links to or from it.
REFERENCES
- 1.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM. Spontaneous Ca2+ transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol 594: 3317–3338, 2016. doi: 10.1113/JP271699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca2+ signalling of fibroblast-like (PDGFRα+) cells in the murine gastric fundus. J Physiol 591: 6193–6208, 2013. doi: 10.1113/jphysiol.2013.264747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol 593: 1945–1963, 2015. doi: 10.1113/jphysiol.2014.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl 1993: 125–137, 1993. [PubMed] [Google Scholar]
- 5.Bolton TB. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol 216: 403–418, 1971. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortoff A. Slow potential variations of small intestine. Am J Physiol 201: 203–208, 1961. doi: 10.1152/ajplegacy.1961.201.1.203. [DOI] [Google Scholar]
- 7.Bülbring E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol 128: 200–221, 1955. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bülbring E, Kuriyama H. The effect of adrenaline on the smooth muscle of guinea-pig taenia coli in relation to the degree of stretch. J Physiol 169: 198–212, 1963. doi: 10.1113/jphysiol.1963.sp007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor JA, Kreulen D, Prosser CL, Weigel R. Interaction between longitudinal and circular muscle in intestine of cat. J Physiol 273: 665–689, 1977. doi: 10.1113/jphysiol.1977.sp012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor JA, Prosser CL, Weems WA. A study of pace-maker activity in intestinal smooth muscle. J Physiol 240: 671–701, 1974. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa M, Wiklendt L, Simpson P, Spencer NJ, Brookes SJ, Dinning PG. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea-pig colon. Neurogastroenterol Motil 27: 1466–1477, 2015. doi: 10.1111/nmo.12646. [DOI] [PubMed] [Google Scholar]
- 12.Dahms V, Prosser CL, Suzuki N. Two types of “slow waves” in intestinal smooth muscle of cat. J Physiol 392: 51–69, 1987. doi: 10.1113/jphysiol.1987.sp016769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Der-Silaphet T, Malysz J, Hagel S, Arsenault A, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114: 724–736, 1998. doi: 10.1016/S0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 14.Diamant NE, Bortoff A. Nature of the intestinal slow-wave frequency gradient. Am J Physiol 216: 301–307, 1969. doi: 10.1152/ajplegacy.1969.216.2.301. [DOI] [PubMed] [Google Scholar]
- 15.Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol 586: 4225–4240, 2008. doi: 10.1113/jphysiol.2008.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinning PG, Wiklendt L, Omari T, Arkwright JW, Spencer NJ, Brookes SJ, Costa M. Neural mechanisms of peristalsis in the isolated rabbit distal colon: a neuromechanical loop hypothesis. Front Neurosci 8: 75, 2014. doi: 10.3389/fnins.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzhugh R. Impulses and physiological states in theoretical models of nerve membrane. Biophys J 1: 445–466, 1961. doi: 10.1016/S0006-3495(61)86902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garfinkel A. A mathematics for physiology. Am J Physiol Regul Integr Comp Physiol 245: R455–R466, 1983. doi: 10.1152/ajpregu.1983.245.4.R455. [DOI] [PubMed] [Google Scholar]
- 19.Gil V, Gallego D, Grasa L, Martín MT, Jiménez M. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol 299: G158–G169, 2010. doi: 10.1152/ajpgi.00448.2009. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296: G1370–G1381, 2009. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwynne RM, Bornstein JC. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1162–G1172, 2007. doi: 10.1152/ajpgi.00441.2006. [DOI] [PubMed] [Google Scholar]
- 23.Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517: 575–590, 1999. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennig GW, Smith CB, O’Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol 543: 233–253, 2002. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennig GW, Spencer NJ, Jokela-Willis S, Bayguinov PO, Lee HT, Ritchie LA, Ward SM, Smith TK, Sanders KM. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil 22: e138–e151, 2010. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou X, Yin J, Liu J, Pasricha PJ, Chen JD. In vivo gastric and intestinal slow waves in W/WV mice. Dig Dis Sci 50: 1335–1341, 2005. doi: 10.1007/s10620-005-2783-6. [DOI] [PubMed] [Google Scholar]
- 27.Huizinga JD, Chen JH, Zhu YF, Pawelka A, McGinn RJ, Bardakjian BL, Parsons SP, Kunze WA, Wu RY, Bercik P, Khoshdel A, Chen S, Yin S, Zhang Q, Yu Y, Gao Q, Li K, Hu X, Zarate N, Collins P, Pistilli M, Ma J, Zhang R, Chen D. The origin of segmentation motor activity in the intestine. Nat Commun 5: 3326, 2014. doi: 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SJ, Basma N, Sanders KM, Ward SM. Effects of new-generation inhibitors of the calcium-activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br J Pharmacol 173: 1339–1349, 2016. doi: 10.1111/bph.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang SJ, Pardo DM, Zheng H, Bayguinov Y, Blair PJ, Fortune-Grant R, Cook RS, Hennig GW, Shonnard MC, Grainger N, Peri LE, Verma SD, Rock J, Sanders KM, Ward SM. Differential sensitivity of gastric and small intestinal muscles to inducible knockdown of anoctamin 1 and the effects on gastrointestinal motility. J Physiol 597: 2337–2360, 2019. doi: 10.1113/JP277335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131: 691–702, 2009. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 33.Iino S, Horiguchi K, Nojyo Y. Wsh/Wsh c-Kit mutant mice possess interstitial cells of Cajal in the deep muscular plexus layer of the small intestine. Neurosci Lett 459: 123–126, 2009. doi: 10.1016/j.neulet.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Iino S, Horiguchi S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal in the gastrointestinal musculature of W mutant mice. Arch Histol Cytol 70: 163–173, 2007. doi: 10.1679/aohc.70.163. [DOI] [PubMed] [Google Scholar]
- 35.Kito Y, Kurahashi M, Mitsui R, Ward SM, Sanders KM. Spontaneous transient hyperpolarizations in the rabbit small intestine. J Physiol 592: 4733–4745, 2014. doi: 10.1113/jphysiol.2014.276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi M, Nagai T, Prosser CL. Electrical interaction between muscle layers of cat intestine. Am J Physiol 211: 1281–1291, 1966. doi: 10.1152/ajplegacy.1966.211.6.1281. [DOI] [PubMed] [Google Scholar]
- 37.Kuizenga MH, Sia TC, Dodds KN, Wiklendt L, Arkwright JW, Thomas A, Brookes SJ, Spencer NJ, Wattchow DA, Dinning PG, Costa M. Neurally mediated propagating discrete clustered contractions superimposed on myogenic ripples in ex vivo segments of human ileum. Am J Physiol Gastrointest Liver Physiol 308: G1–G11, 2015. doi: 10.1152/ajpgi.00230.2014. [DOI] [PubMed] [Google Scholar]
- 38.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 307: C561–C570, 2014. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuriyama H, Osa T, Toida N. Electrophysiological study of the intestinal smooth muscle of the guinea-pig. J Physiol 191: 239–255, 1967. doi: 10.1113/jphysiol.1967.sp008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Jackson Laboratory Mouse Strain Datasheet: B6.Cg-KitW-sh/HNihrJaeBsmGlliJ. Bar Harbor, ME: The Jackson Laboratory, 2019. https://www.jax.org/strain/012861. [Google Scholar]
- 41.The Jackson Laboratory Mouse Strain Datasheet: WBB6F1/J-KitW/KitW-v/J. Bar Harbor, ME: The Jackson Laboratory, 2019. https://www.jax.org/strain/100410. [Google Scholar]
- 42.Lammers WJ, Dhanasekaran S, Slack JR, Stephen B. Two-dimensional high-resolution motility mapping in the isolated feline duodenum: methodology and initial results. Neurogastroenterol Motil 13: 309–323, 2001. doi: 10.1046/j.1365-2982.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 43.Lentle RG, Janssen PW, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y. High definition mapping of circular and longitudinal motility in the terminal ileum of the brushtail possum Trichosurus vulpecula with watery and viscous perfusates. J Comp Physiol B 177: 543–556, 2007. doi: 10.1007/s00360-007-0153-8. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Prosser CL, Job DD. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol 217: 1542–1547, 1969. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- 45.Lowie BJ, Wang XY, White EJ, Huizinga JD. On the origin of rhythmic calcium transients in the ICC-MP of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 301: G835–G845, 2011. doi: 10.1152/ajpgi.00077.2011. [DOI] [PubMed] [Google Scholar]
- 46.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development 116: 369–375, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Malysz J, Gibbons SJ, Saravanaperumal SA, Du P, Eisenman ST, Cao C, Oh U, Saur D, Klein S, Ordog T, Farrugia G. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol 312: G228–G245, 2017. doi: 10.1152/ajpgi.00363.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 271: G387–G399, 1996. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen HB, Malysz J, Huizinga JD, Thuneberg L. Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol Motil 10: 11–26, 1998. doi: 10.1046/j.1365-2982.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 50.Parsons SP, Huizinga JD. Effects of gap junction inhibition on contraction waves in the murine small intestine in relation to coupled oscillator theory. Am J Physiol Gastrointest Liver Physiol 308: G287–G297, 2015. doi: 10.1152/ajpgi.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons SP, Huizinga JD. The phase response and state space of slow wave contractions in the small intestine. Exp Physiol 102: 1118–1132, 2017. doi: 10.1113/EP086373. [DOI] [PubMed] [Google Scholar]
- 52.Parsons SP, Huizinga JD. Phase waves and trigger waves: emergent properties of oscillating and excitable networks in the gut. J Physiol 596: 4819–4829, 2018. doi: 10.1113/JP273425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parsons SP, Huizinga JD. Slow wave contraction frequency plateaux in the small intestine are composed of discrete waves of interval increase associated with dislocations. Exp Physiol 103: 1087–1100, 2018. doi: 10.1113/EP086871. [DOI] [PubMed] [Google Scholar]
- 54.Parsons SP, Huizinga JD. Spatial noise in coupling strength and natural frequency within a pacemaker network; consequences for development of intestinal motor patterns according to a weakly coupled phase oscillator model. Front Neurosci 10: 19, 2016. doi: 10.3389/fnins.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapp PE. Why are so many biological systems periodic? Prog Neurobiol 29: 261–273, 1987. doi: 10.1016/0301-0082(87)90023-2. [DOI] [PubMed] [Google Scholar]
- 56.Roberts RR, Ellis M, Gwynne RM, Bergner AJ, Lewis MD, Beckett EA, Bornstein JC, Young HM. The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. J Physiol 588: 1153–1169, 2010. doi: 10.1113/jphysiol.2009.185421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts RR, Murphy JF, Young HM, Bornstein JC. Development of colonic motility in the neonatal mouse—studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol 292: G930–G938, 2007. doi: 10.1152/ajpgi.00444.2006. [DOI] [PubMed] [Google Scholar]
- 58.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet 20: 357–459, 1979. doi: 10.1016/S0065-2660(08)60549-0. [DOI] [PubMed] [Google Scholar]
- 59.Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda) 31: 316–326, 2016. doi: 10.1152/physiol.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mulè F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol 460: 183–190, 2003. doi: 10.1016/S0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- 61.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH, Farrugia G. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592: 4051–4068, 2014. doi: 10.1113/jphysiol.2014.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith TK. Spontaneous junction potentials and slow waves in the circular muscle of isolated segments of guinea-pig ileum. J Auton Nerv Syst 27: 147–154, 1989. doi: 10.1016/0165-1838(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 63.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Syst 69: 115–121, 1998. doi: 10.1016/S0165-1838(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 64.Spencer NJ, Hennig GW, Smith TK. Electrical rhythmicity and spread of action potentials in longitudinal muscle of guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 282: G904–G917, 2002. doi: 10.1152/ajpgi.00345.2001. [DOI] [PubMed] [Google Scholar]
- 65.Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545: 629–648, 2002. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol 553: 881–893, 2003. doi: 10.1113/jphysiol.2003.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol 558: 577–596, 2004. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533: 787–799, 2001. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens RJ, Publicover NG, Smith TK. Induction and organization of Ca2+ waves by enteric neural reflexes. Nature 399: 62–66, 1999. doi: 10.1038/19973. [DOI] [PubMed] [Google Scholar]
- 70.Stevens RJ, Publicover NG, Smith TK. Propagation and neural regulation of calcium waves in longitudinal and circular muscle layers of guinea pig small intestine. Gastroenterology 118: 892–904, 2000. doi: 10.1016/S0016-5085(00)70175-2. [DOI] [PubMed] [Google Scholar]
- 71.Stevens RJ, Weinert JS, Publicover NG. Visualization of origins and propagation of excitation in canine gastric smooth muscle. Am J Physiol Cell Physiol 277: C448–C460, 1999. doi: 10.1152/ajpcell.1999.277.3.C448. [DOI] [PubMed] [Google Scholar]
- 72.Strogatz SH. From Kuramoto to Crawford: exploring the onset of synchronization in populations of coupled oscillators. Physica D 143: 1–20, 2000. doi: 10.1016/S0167-2789(00)00094-4. [DOI] [Google Scholar]
- 73.Strogatz SH. Sync: the Emerging Science of Spontaneous Order. New York: Hachette, 2004. [Google Scholar]
- 74.Strogatz SH, Stewart I. Coupled oscillators and biological synchronization. Sci Am 269: 102–109, 1993. doi: 10.1038/scientificamerican1293-102. [DOI] [PubMed] [Google Scholar]
- 75.Uriu K. Genetic oscillators in development. Dev Growth Differ 58: 16–30, 2016. doi: 10.1111/dgd.12262. [DOI] [PubMed] [Google Scholar]
- 76.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol Cell Physiol 269: C1577–C1585, 1995. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 77.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480: 91–97, 1994. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei R, Parsons SP, Huizinga JD. Network properties of interstitial cells of Cajal affect intestinal pacemaker activity and motor patterns, according to a mathematical model of weakly coupled oscillators. Exp Physiol 102: 329–346, 2017. doi: 10.1113/EP086077. [DOI] [PubMed] [Google Scholar]
- 79.Winfree AT. The Geometry of Biological Time. Heidelberg, Germany: Springer, 1980. [Google Scholar]
- 80.Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol 538: 823–835, 2002. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu YF, Wang XY, Parsons SP, Huizinga JD. Stimulus-induced pacemaker activity in interstitial cells of Cajal associated with the deep muscular plexus of the small intestine. Neurogastroenterol Motil 28: 1064–1074, 2016. doi: 10.1111/nmo.12808. [DOI] [PubMed] [Google Scholar]