Keywords: acinar-ductal metaplasia, metastasis, nephronectin, pancreatic intraepithelial neoplasia
Abstract
Kras mutations are associated with pancreatic ductal adenocarcinoma (PDAC). Although tobacco smoking, pancreatitis, and obesity are known environmental risk factors for PDAC, the contribution of moderate alcohol intake to PDAC remains elusive. In the present study, we tested whether a combination of risk factors or moderate alcohol intake induces PDAC development in mice. Control Pdx1Cre and Pdx1Cre;LSL-KrasG12D mutant mice were fed a Western alcohol diet containing high levels of cholesterol and saturated fat, 3.5% alcohol, and lipopolysaccharide for 5 mo. In addition, mice were treated with cerulein, for induction of pancreatitis, and nicotine every month. Treatment with all of these risk factors promoted development of advanced pancreatic neoplasia and PDAC in the Pdx1Cre;LSL-KrasG12D mice but not in the control Pdx1Cre mice. Moderate alcohol intake or Western diet feeding also significantly promoted advanced neoplasia and PDAC development in Pdx1Cre;LSL-KrasG12D mice compared with mice fed a regular chow. Alcohol, but not Western diet, increased tumor development in the liver in the Pdx1Cre;LSL-KrasG12D mice, but its origin remained elusive due to leakiness of Pdx1Cre in hepatocytes. RNA-seq analysis revealed that alcohol feeding increases expression of markers for tumors (Epcam, Krt19, Prom1, Wt1, and Wwtr1), stroma (Dcn, Fn1, and Tnc), and cytokines (Tgfb1 and Tnf) and decreases expression of Fgf21 and Il6 in the pancreatic tumor tissues. Immunostaining showed heterogeneous expression of nephronectin, S100 calcium-binding protein A6, and vascular cell adhesion molecule 1 in pancreatic tumors surrounded by podoplanin-positive stromal cells. Our data indicate that moderate alcohol drinking is a risk factor for development of PDAC.
NEW & NOTEWORTHY Heavy alcohol intake has been suspected to be a risk factor of pancreatic ductal adenocarcinoma (PDAC) in humans. However, the contribution of moderate alcohol intake to PDAC development remains elusive. In the present study, we experimentally show that moderate alcohol feeding significantly induces advanced stages of pancreatic intraepithelial neoplasia development and invasive PDAC in Pdx1Cre;LSL-KrasG12D mutant mice. Our data indicate that moderate alcohol drinking is a risk factor for PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a relatively rare cancer type but is the fourth-leading cause of cancer death in the United States (11). Because of its aggressive nature and lack of sensitive diagnostic markers, 80% of patients diagnosed with PDAC are ineligible for surgical resection (28). Although novel chemotherapy and immunotherapy have been developed for patients with PDAC, the overall 5-year survival rate remains around 8% and there is an urgent need for effective therapies.
The pancreas is an essential organ with exocrine and endocrine functions (22). During pancreas development, pancreatic and duodenal homeobox 1 (PDX1)+ endodermal progenitor cells give rise to all pancreatic epithelial cells including acinar cells, islet cells, and duct cells. PDAC exhibits a pancreatic ductal cell phenotype and has a high-metastasizing capacity (37). Cell lineage-tracing studies indicate acinar cells undergo acinar-ductal metaplasia (ADM) and give rise to an early pancreatic lesion named pancreatic intraepithelial neoplasia (PanIN) (19, 29). PanIN is graded based on its morphology and phenotype (7, 22). Early PanIN-1a lesions form a duct structure, have nuclei at the basal side, and express duct cell markers, such as cytokeratin 19 (KRT19). Cancerous epithelial cells protrude into the lumen and form a papillary structure in PanIN-1b. PanIN-2 exhibits nuclear abnormalities, loses its epithelial cell polarity, and becomes PanIN-3 that develops into PDAC.
In patients with PDAC, oncogenic gene mutations have been identified, including KRAS, TRP53, CDKN2A, SMAD4, and BRCA2 (22, 49). In particular, over 90% of patients with PDAC show oncogenic mutations of KRAS. Mice expressing oncogenic mutated KrasG12D are known to recapitulate development of an early PanIN lesion in the pancreas (23). However, its development into PDAC is rare in the Pdx1Cre;LSL-KrasG12D mouse model. By introducing additional gene mutations, such as Trp53, KrasG12D-driven PanIN lesions further develop into PDAC in mice (24).
In addition to these genetic factors, environmental risk factors have been identified for PDAC. Obesity and type II diabetes are associated with PDAC risk (21, 36, 50). Chronic pancreatitis is an established risk factor for PDAC development (5). Cerulein, a cholecystokinin analog, stimulates secretion of digestive enzymes in the pancreas (33). Injections of cerulein induce acute pancreatitis and are known to increase pancreatic tumor development in Pdx1Cre;LSL-KrasG12D mice (19, 51). Epidemiologic studies indicate tobacco smoking is a strong risk factor for PDAC (6, 36). Treatment with tobacco-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is known to enhance cerulein-induced pancreatitis in rats (1). Exposure to tobacco smoking or treatment with NNK was shown to induce advanced pancreatic cancer in Pdx1Cre;LSL-KrasG12D mice (13, 43).
Alcohol drinking has been suspected to be a risk factor for PDAC as heavy alcohol intake is associated with risk of developing chronic pancreatitis (2). Alcohol intake increases the permeability of the gut wall and translocation of lipopolysaccharide (LPS), which is known to enhance pancreatic injury (18, 46). Although earlier case-control, retrospective cohort, and prospective cohort studies failed to show association of alcohol drinking with PDAC, recent epidemiologic studies suggest association of heavy alcohol drinking with PDAC (3, 14, 15, 26, 32, 42, 54). Pdx1Cre;LSL-KrasG12D mice fed alcohol for 6 wk showed a moderate increase of PanIN lesions in the pancreas (51). However, it remains to be determined experimentally whether moderate alcohol feeding promotes pancreatic cancer development from early PanIN lesions to PDAC in mice. In the present study, we examined the effect of environmental risk factors on development of PDAC from PanIN in wild-type or Pdx1Cre;LSL-KrasG12D mice.
MATERIALS AND METHODS
Pancreatic cancer mouse models.
Pdx1Cre, LSL-KrasG12D, and Rosa26-tdTomatoflox mice were purchased from Jackson Laboratory (Bar Harbor, ME) (23, 35). Pdx1Cre;LSL-KrasG12D or control Pdx1Cre mice (2–3 mo of age) were used for testing promotion of PDAC in the pancreas. Mice were fed a regular diet (710027; Dyets, Bethlehem, PA) or Western diet (710362, Dyets: 1% wt/wt cholesterol, 21% calorie lard, and 4% calorie corn oil) containing 3.5% of ethanol. LPS is known to enhance pancreatic injury caused by alcohol in rats (46). To enhance alcohol-induced chronic pancreatic injury, we added a low dose of LPS (1 μg/ml; Sigma, St. Louis, MO) to the diet for 5 mo. Mice were also given 7 hourly injections of cerulein (50 μg/kg; Bachem, Torrance, CA) four times from the frst to fourth month (Fig. 1A). Repeated injections of NNK are known to enhance pancreatitis (1). To mimic tobacco smoking, we injected NNK (100 mg/kg, Toronto Research Chemicals, North York, ON, Canada) four times from the first to fourth month. Pdx1Cre;LSL-KrasG12D mice were also fed a Lieber-DeCarli diet containing 3.5% ethanol (710260; Dyets) or Western diet containing high-cholesterol and high-saturated fat (710142; Dyets) or were treated with cerulein for 5 mo. We changed the liquid diet and measured the consumption of the diet every day. Five months after treatment, the pancreas and blood were collected. Plasma samples prepared from the blood were used for measurement of blood alcohol levels using an AM1 Alcohol Analyzer (Analox Instruments, Stourbridge, UK). The plasma ALT values were measured using ALT reagent (Cliniqa, San Marcos, CA) and a PowerWave 200 spectrophotometer (BioTech, Winooski, VT). Pdx1Cre;Rosa26-tdTomatoflox mice were used for tracing Pdx1+ cells in the digestive tissues. All animal experiments were performed in accordance with the National Institutes of Health (NIH) guidelines under the protocol approved by the Institutional Animal Care and Use Committee at the University of Southern California.
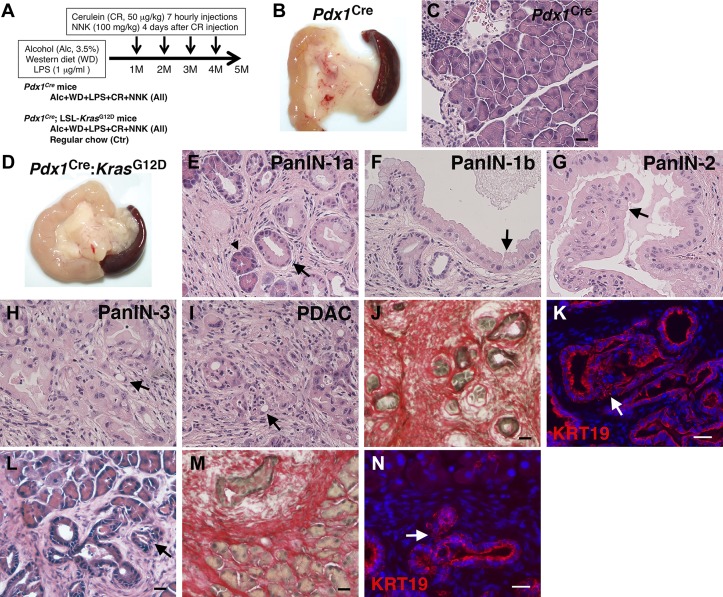
Fig. 1.
Promotion of pancreatic tumor development in Pdx1Cre;LSL-KrasG12D, but not in Pdx1Cre mice, by environmental risk factors. A: mouse models. Pdx1Cre mice and Pdx1Cre;LSL-KrasG12D mice were fed Western diet containing high cholesterol and high saturated fat, 3.5% alcohol, and LPS for 5 mo. Mice were also treated with cerulein and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) every month from 1st to 4th month. Pdx1Cre;LSL-KrasG12D mice were also fed a regular chow as a control (Ctr). B: gross appearance of the pancreas from Pdx1Cre mice treated with all insults for 5 mo. C: hematoxylin and eosin (H&E) staining of pancreas tissues from Pdx1Cre mice. D: gross appearance of the pancreas from Pdx1Cre;LSL-KrasG12D mice treated with all insults for 5 mo. E–K: H&E staining (E–I), Sirius red staining (J), and fluorescence immunostaining of cytokeratin 19 (KRT19; K) of pancreas tissues from Pdx1Cre;LSL-KrasG12D mice treated with all insults. L–N: H&E staining (L), Sirius red staining (M), and fluorescence immunostaining of KRT19 (N) of pancreas tissues from the control Pdx1Cre;LSL-KrasG12D mice fed a regular chow. An arrowhead indicates acinar cells undergoing acinar-ductal metaplasia. Arrows indicates pancreatic intraepithelial neoplasia (PanIN)-1a (E, L, and N), PanIN-1b (F), PanIN-2 (G), PanIN-3 (H and K), and pancreatic ductal adenocarcinoma (PDAC; I). Bar = 20 μm.
Histological analysis.
Pancreas tissues were fixed with 4% paraformaldehyde at 4°C overnight. Fixed tissues were incubated with 30% sucrose in PBS overnight and were embedded in freezing medium. We made cryosections (7 μm) with a Cryostat (CM1900; Leica, Buffalo Grove, IL), separated at least 10 sections from each sample, and stained with hematoxylin and eosin (H&E) or Sirius red staining as described before (4). Images were captured with a Nikon 90i microscope and DS-Fi1 digital camera (Nikon, Melville, NY). To quantify the area of tumors or stroma in the pancreas, 10 images were randomly captured from 4 sections stained with H&E or Sirius red using a ×10 objective. The tumor and pancreas areas were measured using NIS-Element software. Two observers blindly examined tumor grades in H&E-stained sections based on morphology of the tumors (7). The stroma areas stained with Sirius red were quantified by ImageJ software (NIH).
Immunofluorescence staining.
Cryosections were blocked with 5% donkey serum and 0.2% bovine serum albumin for 30 min and incubated with primary antibodies at 4°C overnight. Primary antibodies used are: E-cadherin (CDH1) conjugated with AlexaFluor 488 (100-fold dilution, 560061; BD Biosciences, San Jose, CA), doublecortin-like kinase 1 (DCLK1; 100-fold dilution, PA5-20908; Thermo Fisher Scientific, Waltham, MA), prominin-1 (PROM1; 50-fold dilution, 14-1331), S100 calcium-binding protein A6 (S100A6; 100-fold dilution, AF2377), vascular cell adhesion molecule 1 (VCAM1; 50-fold dilution, 14-1061), coxsackie virus and adenovirus receptor (CXADR; 100-fold dilution, AF2654; R&D Systems, Minneapolis, MN), nephronectin (NPNT; 100-fold dilution, AF4298), EPCAM (100-fold dilution, G88; Developmental Studies Hybridoma Bank, Iowa City, IA), KRT19 (50-fold dilution, TROMA-III), mesothelin (MSLN; 100-fold dilution, HPA017172; Sigma), and WWTR1 (50-fold dilution, HPA007415). Antibodies against α-smooth muscle actin (ACTA2), type IV collagen (COLIV), decorin (DCN), desmin (DES), podoplanin (PDPN), and vimentin (VIM) were as described previously (4). The unconjugated primary antibodies were detected with secondary antibodies conjugated with AlexaFluor 488 and 568 dyes (Thermo Fisher Scientific). The sections were counterstained with DAPI. To quantify the area of KRT19+ PanIN in the pancreas, 20 images were captured using a ×20 objective. The areas stained with KRT19 staining were quantified by NIH ImageJ software.
RNA-seq analysis.
Total RNAs were isolated from the pancreas tissues from the Pdx1Cre (n = 3) and Pdx1Cre;LSL-KrasG12D (n = 6) mice treated with all insults using an RNeasy kit (Qiagen, Germantown, MD). Library construction was carried out using the TruSeq RNA Sample Prep kit, and sequencing was performed by NextSeq500 (Illumina, San Diego, CA) at the University of Southern California Genomics Core as previously described (27). We also isolated RNAs from the Pdx1Cre;LSL-KrasG12D mouse pancreas tissues with or without alcohol feeding for 5 mo (n = 3, each group) and submitted samples to Genewiz (South Plainfield, NJ) for RNA-seq. The data were normalized with the reads per kilobase per million method and analyzed with Flow software (Partek, Chesterfield, MO). The data were deposited in the Gene Expression Omnibus database (GSE139357).
Statistical analysis.
Statistical tests for the significance of differences were assessed by a χ2 test or one-way ANOVA followed by a Tukey honestly signficant difference post hoc test. P < 0.05 was considered statistically significant.
RESULTS
No induction of pancreatic cancer by environmental risk factors in mice without KrasG12D expression.
Environmental risk factors, such as tobacco smoking, pancreatitis, and obesity, are known to increase risk of PDAC (6, 36). We first tested whether environmental risk factors initiate and promote pancreatic cancer development in wild-type mice without expression of oncogenic mutated KrasG12D. Pdx1Cre mice (10 male and 4 female) were fed a Western alcohol diet containing high-cholesterol and high-saturated fat diet and 3.5% alcohol for 5 mo (Fig. 1A). To enhance alcohol-induced pancreatic injury, we added a low dose of LPS (1 μg/ml) to the diet. To induce pancreatitis, we injected cerulein every month. We also injected NNK, a nicotine-derived nitrosamine ketone known to induce pancreatic injury (43), every month to mimic tobacco smoking. Five months after treatment with alcohol, Western diet, LPS, cerulein, and NNK, pancreata showed a normal appearance (Fig. 1B). H&E staining showed a normal pancreas morphology without any sign of tumor development (Fig. 1C), indicating that these environmental risk factors are not sufficient to initiate pancreatic tumor development without KrasG12D expression in the pancreas.
Promotion of PDAC development in Pdx1Cre;LSL-KrasG12D mice fed alcohol, Western diet, and LPS and administered cerulein and NNK.
To test whether a combination of these risk factors promotes advanced PanIN and PDAC development, we treated Pdx1Cre;LSL-KrasG12D mice with them (15 male and 15 female mice) (Fig. 1A) and found massive development of tumor in the pancreas (Fig. 1D). H&E staining showed that these insults induce ADM and development of heterogeneous early pancreatic tumor grades from PanIN-1a to PanIN-3 (Fig. 1, E–H). Development of PDAC was often observed in the pancreas (Fig. 1I). PanIN lesions from Pdx1Cre;LSL-KrasG12D mice treated with all insults showed massive deposition of collagen around pancreatic tumors expressing KRT19 (Fig. 1, J and K). On the other hand, control Pdx1Cre;LSL-KrasG12D mice fed a regular diet (18 male and 18 female mice) developed only early PanIN-1a and PanIN-2 surrounded by the stroma (Fig. 1, L–N). Our data indicate that a combination of these environmental risk factors causes progressive PDAC in the Pdx1Cre;LSL-KrasG12D mouse.
Effects of alcohol, Western diet, and cerulein on pancreatic cancer development.
To test which environmental risk factor(s) promote PDAC in mice, we treated Pdx1Cre;LSL-KrasG12D mice with a combination of alcohol, Western diet, and cerulein treatment without LPS and NNK administration (8 male and 9 female mice) (Fig. 2A). H&E staining, Sirius red staining, and immunofluorescence staining of KRT19 revealed development of PDAC surrounded by the stroma similar to mice fed all insults (Fig. 2, B–D).
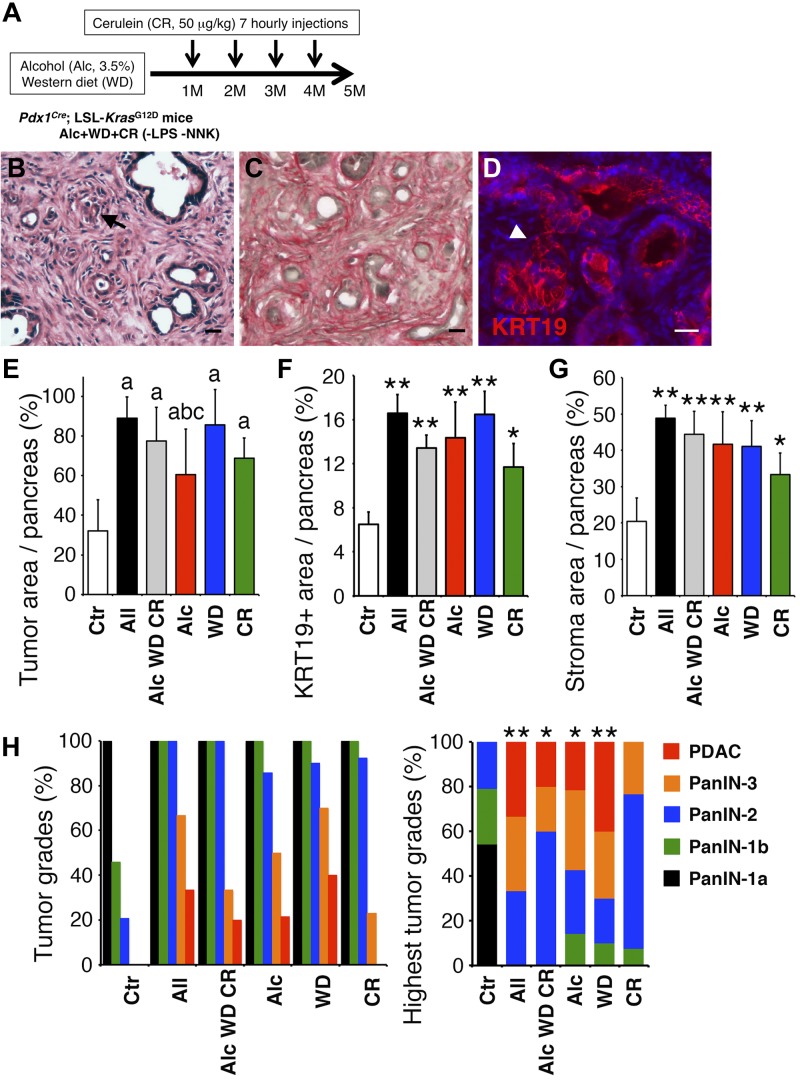
Fig. 2.
Pancreatic tumor development in Pdx1Cre;LSL-KrasG12D mice treated with different insults. A: Pdx1Cre;LSL-KrasG12D mice were fed a Western diet containing 3.5% alcohol and treated with cerulein without LPS and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) for 5 mo. B–D: pancreas sections were stained with hematoxylin and eosin (H&E; B), Sirius red (C), and cytokeratin 19 (KRT19) antibodies (D). An arrow and arrowhead indicate pancreatic ductal adenocarcinoma (PDAC) and pancreatic intraepithelial neoplasia (PanIN)-3, respectively. Bar = 20 μm. E–H: quantification and qualification of pancreatic cancer developed in Pdx1Cre;LSL-KrasG12D mice. Mice were fed a regular chow (Ctr) or treated with all insults (All); a combination of alcohol, Western diet, and cerulein (Alc WD CR); alcohol (Alc); Western diet (WD); or cerulein (CR). E: the ratio of tumor areas in Pdx1Cre;LSL-KrasG12D pancreas sections stained with H&E. aP < 0.01 against Ctr, bP < 0.01 against All, cP < 0.05 against WD. F: the ratio of KRT19+ tumor areas in the pancreas sections. G: the ratio of Sirius red-stained stroma area in the pancreas sections. H: percentage of pancreata with each tumor grade (left) and highest tumor grades (right) were determined from the H&E-stained sections. *P < 0.05 and **P < 0.01 against Ctr.
From the sections stained with H&E, Sirius red, and KRT19, we quantified the tumor and stroma areas in the pancreas tissue. In control Pdx1Cre;LSL-KrasG12D mice fed a regular chow, the tumor occupied around 32% of the pancreas (Fig. 2E, Ctr). Pdx1Cre;LSL-KrasG12D mice treated with all insults induced a pancreatic tumor that occupied around 89% of the pancreas (Fig. 2E, All). The KRT19+ tumor and stroma areas were also increased in the pancreas treated with all insults compared with the control (Fig. 2, F and G, Ctr vs. All). Compared with mice treated with all insults, those treated with alcohol, Western diet, and cerulein without LPS and NNK developed tumors and stroma at similar levels in the pancreas (Fig. 2, E–G, All vs. Alc WD CR), suggesting that the additive effects of LPS and NNK are limited on tumor development in the pancreas treated with alcohol, Western diet, and cerulein.
We further evaluated tumor grades developed in the pancreas in each group. Control Pdx1Cre;LSL-KrasG12D mice fed a regular chow developed PanIN-1a to PanIN-2 and did not develop PanIN-3 and PDAC (Fig. 2H). PDAC development was observed in 33% of mice treated with all insults (Fig. 2H). Mice treated with alcohol, Western diet, and cerulein without LPS and NNK showed a slight decrease of PDAC and PanIN-3 compared with those treated with all insults. These data suggest that treatment with all insults significantly promotes pancreatic cancer development in Pdx1Cre;LSL-KrasG12D mice.
Induction of PDAC in Pdx1Cre;LSL-KrasG12D mice fed moderate alcohol.
Since the condition with LPS and NNK that we used in this study had a smaller effect on tumor development than if mice were treated with alcohol, Western diet, and cerulein, we focused on the sole effects of alcohol, Western diet, or cerulein on pancreatic cancer development. To test whether alcohol intake is a risk factor of PDAC, we fed Pdx1Cre;LSL-KrasG12D mice a Lieber-DeCarli diet containing 3.5% alcohol (8 male and 8 female mice) (Fig. 3A). The alcohol consumption rate was 12.9 ± 2.5 g·kg body wt−1·day−1. Five months after alcohol feeding, the blood alcohol levels increased to 76.6 ± 33.3 mg/dL in mice. We concluded that our alcohol feeding model achieves moderate alcohol intake in mice. Pdx1Cre;LSL-KrasG12D mice fed alcohol resulted in development of advanced PanIN including PanIN-3 and PDAC surrounded by the stroma (Fig. 3B). Alcohol feeding alone significantly increased the tumor and stromal areas in the pancreas compared with the control (Fig. 2, E–G, Ctr vs. Alc). Alcohol feeding also significantly promoted pancreatic tumor development, including PanIN-3 grade and PDAC, compared with the regular chow (Fig. 2H, Ctr vs. Alc). No significant difference of the ratio was observed between male and female mice (data not shown). These data indicate that moderate alcohol intake is a risk factor for promotion of PDAC.
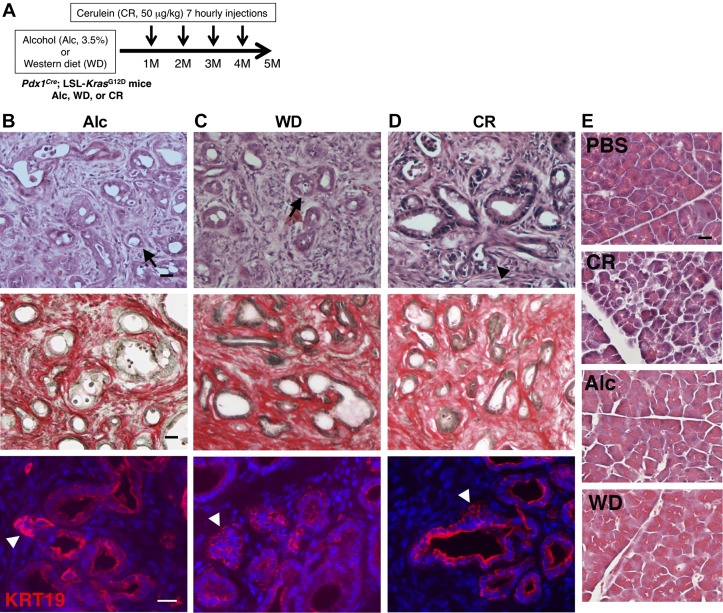
Fig. 3.
Pancreatic tumor development in Pdx1Cre;LSL-KrasG12D mice fed alcohol or Western diet or administered cerulein. A: Pdx1Cre;LSL-KrasG12D mice were fed a Lieber-DeCarli diet containing 3.5% alcohol or a Western diet for 5 mo. To induce pancreatitis, mice were injected with cerulein every month from 1st to 4th month. B–D: pancreas sections were stained with hematoxylin and eosin (H&E) (top), Sirius red (middle), and KRT19 antibodies (bottom). B and C: alcohol (Alc) or Western diet (WD) promotes pancreatic intraepithelial neoplasia (PanIN)-3 (arrowheads) and pancreatic ductal adenocarcinoma (PDAC; arrows). D: cerulein administration induces pancreatic intraepithelial neoplasia (PanIN)-3 (arrowheads) but not PDAC. E: H&E staining of the pancreata collected from control mice injected PBS or cerulein (CR). CR treatment induces acute pancreatitis. The control mice fed Alc or WD did not show injury in the pancreata. Bar = 20 μm.
Induction of PDAC in Pdx1Cre;LSL-KrasG12D mice fed Western diet but not by cerulein administration.
We examined the effect of Western diet (7 male and 8 female mice) or cerulein administration (9 male and 9 female mice) on PDAC development in Pdx1Cre;LSL-KrasG12D mice as outlined in Fig. 3A. Western diet feeding caused pancreatic tumor graded PanIN-3 and PDAC (Fig. 3C). Cerulein injections also induced the generation of advanced PanIN expressing KRT19 including PanIN-3 in the pancreas (Fig. 3D). Western diet feeding significantly increased the tumor and stroma areas in the pancreas and promoted pancreatic tumor development including PanIN-3 grade similar to that treated with all insults (Fig. 2, E–H, All vs. WD). Cerulein administration showed increased tumor and stroma areas in the pancreas compared with the control (Fig. 2, E–H, Ctr vs. CR). However, differing from the effect of alcohol or Western diet, cerulein administration did not induce PDAC (Fig. 2H). These data indicate that Western diet feeding is a strong risk factor for promotion of PDAC compared with pancreatitis induced by cerulein.
We also examined whether alcohol or Western diet induces pancreatitis in the control Pdx1Cre mice. We confirmed that mice develop acute pancreatitis 2 h after repeated injections of cerulein, but not PBS (Fig. 3E). On the other hand, the pancreata from mice fed alcohol (n = 5) or Western diet (n = 5) for 5 mo did not show signs of pancreatitis (Fig. 3E), suggesting that alcohol or Western diet used in this study promotes pancreatic tumor development without significant induction of pancreatitis in the pancreas.
Alcohol induces liver tumor formation in the Pdx1Cre;LSL-KrasG12D mouse.
Tumor formation in the liver was rare in the Pdx1Cre;LSL-KrasG12D mouse fed a regular diet, and only 1 out of 30 mice showed tumor in the liver (Fig. 4A). On the other hand, treatment with all insults resulted in liver tumor formation in 39% of Pdx1Cre;LSL-KrasG12D mouse. Interestingly, moderate alcohol feeding caused liver tumor development in 29% of Pdx1Cre;LSL-KrasG12D mice (Fig. 4, A and B). Western diet feeding resulted in liver tumor formation in 10% of the mice (Fig. 4A). Cerulein treatment did not induce liver tumor formation. H&E staining showed accumulation of undifferentiated cells with small nuclei in the Pdx1Cre;LSL-KrasG12D liver fed the alcohol diet (Fig. 4C). Immunofluorescence staining showed expression of KRT19 in these cells in the liver (Fig. 4D). The serum ALT values, which indicate liver damage, showed a tendency to increase by alcohol feeding (124 ± 92 U/L, n = 8) compared with the control (21 ± 13, P < 0.01) or Western diet-fed mice (30 ± 23 U/L, P < 0.05). These data suggest that moderate alcohol intake is the most potent promoter for liver tumor formation in the Pdx1Cre;LSL-KrasG12D mice.
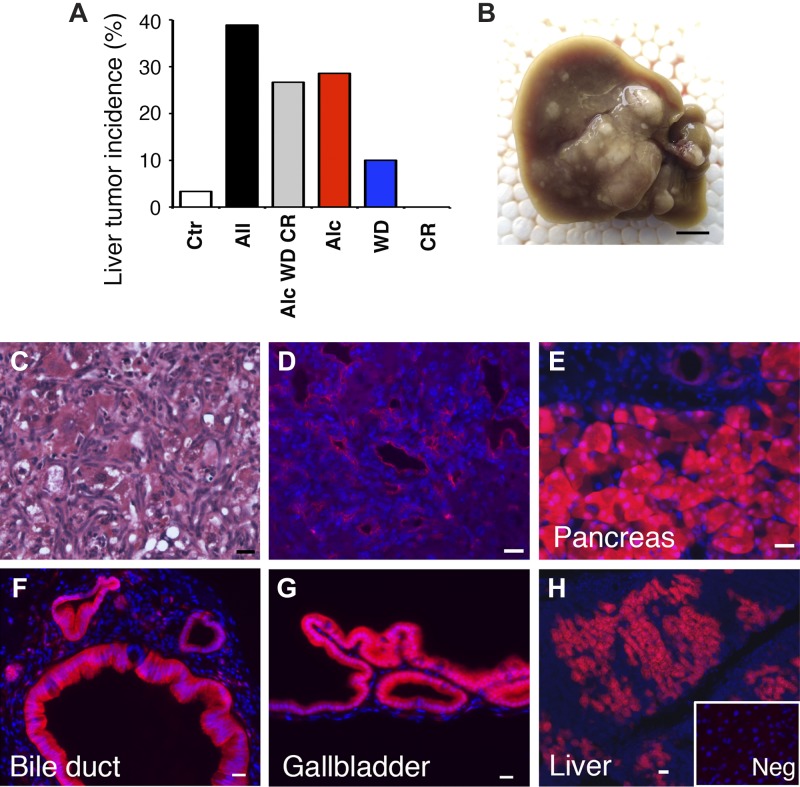
Fig. 4.
Liver tumor formation in Pdx1Cre;LSL-KrasG12D mice and cell lineage tracing of Pdx1+ cells in the digestive organs. A: the incidence of liver tumor formation in Pdx1Cre;LSL-KrasG12D mice treated with different environmental risk factors. Alcohol induces tumor formation in the liver. Mice were fed a regular chow (Ctr) or treated with all insults (All); a combination of alcohol, Western diet, and cerulein (Alc WD CR); alcohol (Alc); Western diet (WD); or cerulein (CR). B: a gross appearance of the liver fed alcohol for 5 mo. C: hematoxylin and eosin (H&E) staining of the liver in B. Cells with small nuclei are observed in the liver. D: Immunofluorescence staining of the liver in B. Cells with small nuclei are positive for KRT19, a marker for pancreatic ductal adenocarcinoma (PDAC). E–H: cell lineage tracing of Pdx1+ cells in the digestive organs using Pdx1Cre;tdTomatoflox mice. Expression of tdTomato is observed in epithelial cells in the pancreas (E), extrahepatic bile duct (F), and gallbladder (G) and hepatocytes in the liver (H). H, inset: is a negative control using wild-type mouse livers. Nuclei were counterstained with DAPI. Bar = 0.5 cm (B) and 20 μm (C–H).
Leakiness of Cre expression in the Pdx1Cre liver.
The Pdx1Cre mouse has been used to trace Pdx1-expressing cells and descendants from Pdx1+ epithelial cells in the pancreas, duodenum, and gastric antrum. Thus the tumor in the Pdx1Cre;LSL-KrasG12D liver is expected to originate by metastasis from the KrasG12D-expressing tumor raised in these organs. To confirm the origin of the liver tumor, we crossed a Pdx1Cre mouse with a tdTomatoflox mouse and analyzed the expression of tdTomato in the digestive organs. We observed tdTomato expression in epithelial cells in the normal adult pancreas, extrahepatic bile duct, and gallbladder (Fig. 4, E–G). However, contrary to our expectation, tdTomato expression was also observed in hepatocytes of the liver (Fig. 4H), indicating leakiness of the Cre in hepatocytes. This observation raises a possibility that the liver tumor develops through overexpression of KrasG12D in hepatocytes.
RNA-seq analysis.
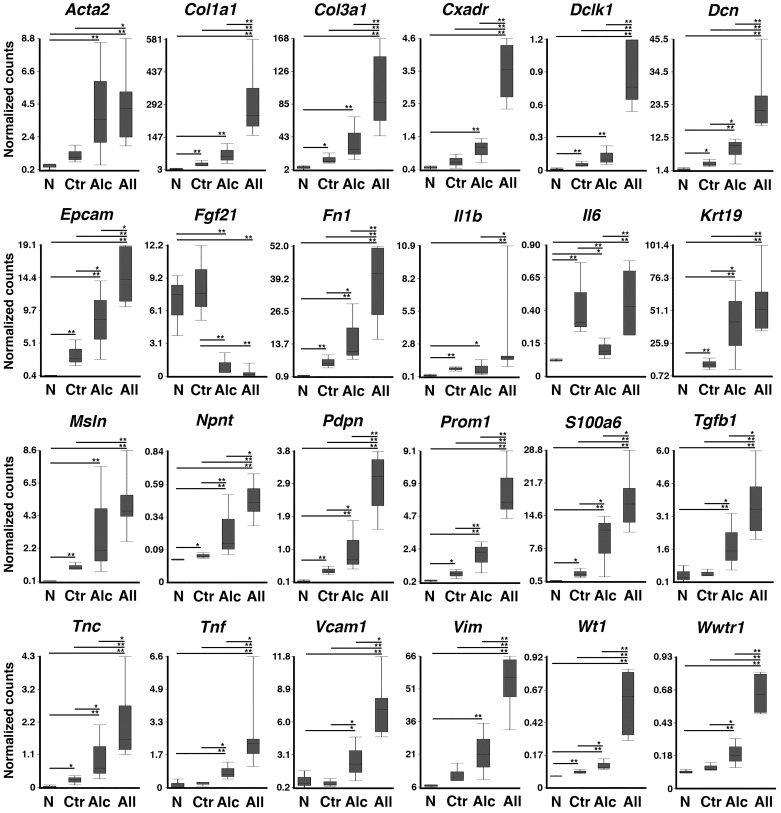
To examine how the treatment with all insults or alcohol promotes pancreatic tumor development, we analyzed gene expression profiles of the pancreas tissues collected from the Pdx1Cre (n = 3) and Pdx1Cre;LSL-KrasG12D (n = 6) mice treated with all insults and Pdx1Cre;LSL-KrasG12D mice with or without alcohol feeding (n = 3, each group) by RNA-seq. Among 21,249 genes detected by RNA-seq, 3,184 genes were upregulated over an eightfold difference (P < 0.01) in pancreas tissues from Pdx1Cre;LSL-KrasG12D mice treated with all insults compared with those from Pdx1Cre mice. Among these upregulated genes, we found markers for stromal cells (Acta2, Vim), extracellular matrices (Col1a1, Col3a1, Dcn, Fn1, Npnt, and Tnc), PDAC (Dclk1, Epcam, Krt19, Msln, and Prom1), and cytokines (Il1b, Tgfb1, and Tnf) (Fig. 5). We also found upregulation of Cxadr, Pdpn, S100a6, Vcam1, Wt1, and Wwtr1. The expression of these genes was also higher in pancreatic tumor tissues in Pdx1Cre;LSL-KrasG12D mice treated with all insults than those without treatment (Fig. 5, Ctr vs. All). Compared with the control group, the alcohol feeding group showed a significant increase of Dcn, Epcam, Fn1, Krt19, Npnt, Pdpn, Prom1, S100a6, Tgfb1, Tnc, Tnf, Vcam1, Wt1, and Wwtr1 genes in the pancreatic tumors (Fig. 5, Ctr vs. Alc).
Fig. 5.
RNA-seq analysis. RNAs from Pdx1Cre mice treated with all insults (N, n = 3) and Pdx1Cre;LSL-KrasG12D mice fed a regular chow (Ctr, n = 3), alcohol (Alc, n = 3), or treated with all insults (All, n = 6) were subjected to RNA-seq analysis. After normalization, the mean normalized counts were compared among the 4 groups. *P < 0.05, **P < 0.01.
The RNA-seq analysis showed only 182 downregulated genes over a fourfold difference (P < 0.01) in pancreas tissues from Pdx1Cre;LSL-KrasG12D mice treated with all insults compared with those from Pdx1Cre mice. Fgf21 was downregulated in tumor tissues by alcohol feeding or treatment with all insults (Fig. 5). Alcohol feeding downregulated Il6 gene expression in tumor tissues in Pdx1Cre;LSL-KrasG12D mice compared with mice without alcohol feeding or all treatment (Fig. 5).
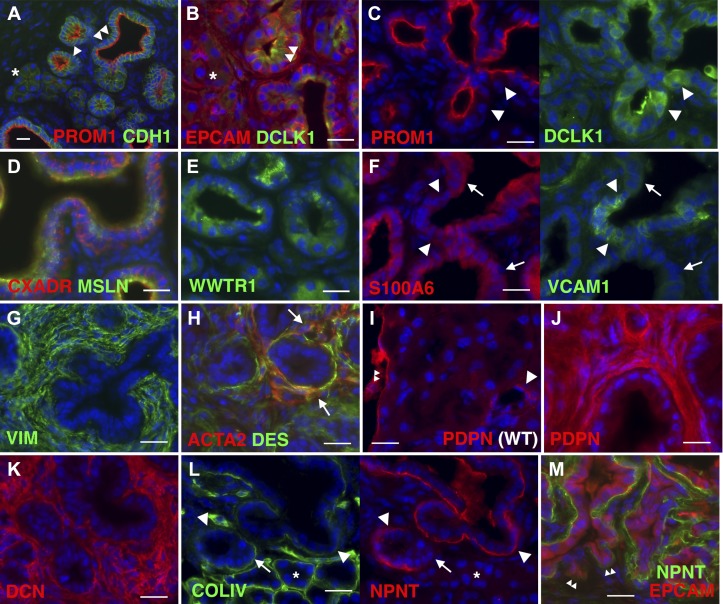
Heterogeneous expression of DCLK1, NPNT, S100A6, and VCAM1 in pancreatic tumor surrounded with PDPN+ stromal cells.
Given that many genes were shown to be upregulated in pancreas cancer, we examined their expression by immunofluorescence staining. In the cancerous pancreas tissues in Pdx1Cre;LSL-KrasG12D mice treated with all insults, normal acinar cells express CDH1 and EPCAM but not PROM1 and DCLK1 (Fig. 6, A and B). As acinar cells undergo ADM, they begin to express PROM1 on the luminal side and its expression becomes strong in PanIN-1a (Fig. 6A). Some EPCAM+ or PROM1+ PanIN express DCLK1 (Fig. 6, B and C). Tumors express CXADR, MSLN, and WWTR1/TAZ (Fig. 6, D and E). S100A6+ PanIN shows a tendency to be negative for VCAM1 (Fig. 6F). Pancreatic tumors show accumulation of tumor-associated stromal cells expressing VIM (Fig. 6G). ACTA2, a marker of myofibroblasts, is positive in some of the DES+ stromal cells adjacent to the tumor (Fig. 6H). In the normal pancreas, PDPN is expressed in mesothelial cells and lymphatic endothelial cells, but its expression is not detectable in pancreatic stellate cells (Fig. 6I). PDPN expression becomes strong in tumor-associated stromal cells in pancreatic tumors (Fig. 6J). DCN is heavily expressed in the desmoplasmic stroma around PanIN (Fig. 6K). NPNT is strongly expressed at the basal side of PanIN, but not in normal acinar cells (Fig. 6L). NPNT is first seen when acinar cells undergo ADM toward early PanIN. Basal lamina composed of COLIV is observed at the basal side of normal acinar cells (Fig. 6L). Compared with normal acinar cells, PanIN shows less staining of COLIV and increased staining of NPNT at the basal side. The advanced tumor protruding into the stroma shows less NPNT staining (Fig. 6M). These data suggest a heterogeneous nature of pancreatic cancer developed in the Pdx1Cre;LSL-KrasG12D mice.
Fig. 6.
Immunofluorescence staining of pancreatic cancer tissues. Pancreas tissues collected from Pdx1Cre;LSL-KrasG12D mice treated with all insults for 5 mo were stained with different antibodies. A: expression of prominin-1 (PROM1) and E-cadherin (CDH1) in pancreatic intraepithelial neoplasia (PanIN)-1a (double arrowheads). An arrowhead indicates acinar cells undergoing acinar-ductal metaplasia (ADM). *Normal acinar cells. B: double arrowheads indicate expression of doublecortin-like kinase 1 (DCLK1) in epithelial cell adhesion molecule positive (EPCAM+) PanIN-1a. C: heterogeneous expression of DCLK1 in PROM1+ tumor cells (arrowheads). D and E: expression of coxsackie virus and adenovirus receptor (CXADR), mesothelin (MSLN), and WW domain containing transcription regulator 1 (WWTR1) in tumors. F: heterogeneous expression of S100 calcium-binding protein A6 (S100A6; arrows) and VCAM1 (arrowheads) in tumors. G: strong expression of vimentin (VIM) in tumor-associated stromal cells. H: expression of α-smooth muscle actin (ACTA2; arrows) in some desmin-positive (DES+) stromal cells around tumors. I: podoplanin (PDPN) expression in the lymphatic vessels (arrow) and mesothelial cells (double arrowheads) in the normal pancreas (wild type). J: strong expression of PDPN in the tumor-associated stroma. K: expression of decorin (DCN) in the stroma. L: expression of nephronectin (NPNT) at the basal side of PanIN (arrowheads). Arrows indicate acinar cells that connect to early PanIN. *Normal acinar cells. M: downregulation of NPNT in EPCAM+ tumor cells that protrude into the stroma (double arrowheads). Nuclei were counterstained with DAPI. Bar = 20 μm.
DISCUSSION
The present study provides the first evidence that moderate alcohol intake promotes PDAC development from early PanIN lesions in the Pdx1Cre;LSL-KrasG12D mouse. Alcohol drinking has been suspected to be a risk factor for PDAC. Epidemiological studies suggest some association between heavy alcohol drinking and PDAC (14, 20, 47). Xu et al. (51) reported that Pdx1Cre;LSL-KrasG12D mice fed alcohol for 6 wk result in a moderate increase of PanIN development in the pancreas. However, there was no study to test the effect of moderate alcohol on the promotion of PDAC in mice. In the present study, we fed Pdx1Cre;LSL-KrasG12D mice with 3.5% (vol/vol) of ethanol for 5 mo. As compared with the standard Lieber-DeCarli diet containing 4–5% (vol/vol), the ethanol content is reduced, alcohol intake achieved by this modified diet is low, and blood alcohol levels detected in the mice fed this diet are low. Despite this moderate level of alcohol intake, not only did it induce development of PanIN with higher grades, but it also promoted development of PDAC. Our data indicate that moderate alcohol drinking is a risk factor for PDAC.
Alcohol is a risk factor for pancreatitis (2). Metabolism of alcohol by CYP2E1 generates reactive oxygen species in the pancreas (16). Oxidative metabolism of alcohol by alcohol dehydrogenase causes mitochondrial failure in the pancreas or generates acetaldehyde (41, 55). In addition, nonoxidative metabolism of alcohol generates fatty acid ethyl esters, which also induce mitochondrial dysfunction (25). Although chronic pancreatitis is a risk factor for PDAC (5), alcohol feeding alone is not sufficient to induce pancreatitis in rodents (44). We assume that alcohol feeding promotes development of PDAC from PanIN independent from pancreatitis in Pdx1Cre;LSL-KrasG12D mice.
RNA-seq analysis revealed that alcohol intake upregulates genes known to be involved in PDAC, such as Epcam, Krt19, Prom1, Wt1, and Wwtr1 (17, 40). Alcohol intake also upregulates genes related to cytokines (Tgfb1 and Tnf) and extracellular matrixes (Dcn, Fn1, and Tnc), implying induction of desmoplasia in pancreatic tumor tissues. A recent study suggests that FGF21 has a protective role in the promotion of pancreatic cancer in mice (34). Interestingly, we found that moderate alcohol feeding downregulates expression of the Fgf21 gene in the pancreatic cancer tissues. Further studies are necessary to understand how alcohol regulates the Fgf21 gene in pancreatic tumor tissues. IL6 is known to drive PDAC progression via the JAK/STAT3 pathway (30). We observed upregulation of Il6 mRNA in the pancreatic tumors from mice fed a regular chow or treated with all insults. On the other hand, moderate alcohol feeding rather downregulates its mRNA expression in the tumor tissues. The decreased IL6 expression by alcohol might be a reason the effects of alcohol on tumor development are weaker than those of all insults.
Obesity and pancreatitis are known risk factors for PDAC (50). A high-fat diet was shown to increase PDAC in Pdx1Cre;LSL-KrasG12D mice (9). Our data showed that Western diet containing high-cholesterol and high-saturated fat significantly induced PDAC development in the pancreas. Chronic pancreatitis by repeated injections of cerulein induced more PanIN development in the pancreas as previously reported (19, 51). However, we did not observe induction of PDAC by cerulein treatment. The effect of cerulein on the development of PDAC was obviously different from that achieved by alcohol or Western diet feeding.
We found that alcohol feeding induced tumor development in the Pdx1Cre;LSL-KrasG12D liver. Interestingly, liver tumor formation was not enhanced by Western diet or cerulein injection. Alcohol feeding in our model moderately induced liver injury as revealed by the increased serum ALT value compared with Western diet. Thus liver injury caused by alcohol metabolism might provide a niche for survival and growth of metastasizing PDAC from the pancreas. Although Pdx1Cre mice have been widely used to delete or activate genes in pancreatic epithelial cells, we detected unexpected Cre recombination in hepatocytes. Overexpression of KrasG12D in hepatocytes by AlbuminCre mice showed development of hepatocellular carcinoma or cholangiocarcinoma (38, 52). These studies raise a possibility that the liver tumor observed in the present study was caused by the KrasG12D expression in hepatocytes in Pdx1Cre;LSL-KrasG12D mice rather than metastasis from the pancreas. Use of specific Cre mouse lines for acinar cells is necessary to determine the origin of the liver tumor.
Pancreatic tumors developed in the Pdx1Cre;LSL-KrasG12D mice treated with all insults express CXADR and MSLN. Although MSLN is known to promote PDAC (10), the function of CXADR remains elusive in PDAC. Immunofluorescence staining revealed the heterogeneous expression of DCLK1, S100A6, and VCAM1 in the pancreatic tumor. The previous study reported that DCLK1 is expressed in progenitor cells in pancreatic cancer (48). The expression of S100A6 was reported in PDAC, and its high expression was shown to be associated with poor survival of patients (39, 45). We found that S100A6 is negative in early PanIN developing from acinar cells. S100A6+ tumor cells showed a tendency to be negative for VCAM1. A recent study showed the expression of VCAM1 is associated with disease progression of PDAC (53). It remains to be clarified how these heterogeneous PanIN cells contribute to PDAC development.
Immunostaining shows strong expression of PDPN in tumor associated stromal cells in which only some of them express ACTA2, as recently reported (8). We also found that DCN and NPNT are upregulated in the tumor-bearing pancreas. NPNT is a component of the basal lamina and plays an essential role in epithelial-mesenchymal interaction in the developing kidney (31), but its expression was not known to be in the pancreas tumor. Our data showed that normal acinar cells are surrounded by a basement membrane composed of COLIV. Upon differentiation to PanIN, the basement membrane at the basal side shows the presence of NPNT and the expression of COLIV becomes weak. Although PanIN is surrounded by NPNT at the basal side, pancreatic tumors showing invasion to the stroma show less deposition of NPNT, suggesting that NPNT may be an early marker for PanIN.
The present study showed no pancreatic cancer development in wild-type mice treated with Western diet, alcohol, cerulein, LPS, and NNK, underscoring the importance of oncogenic Kras mutation in the initiation of pancreatic cancer development. Differing from wild-type mice, Pdx1Cre;LSL-KrasG12D mice treated with those risk factors significantly promoted tumor development in the pancreas. It has been known that LPS and NNK enhance pancreatitis (1, 46). However, Pdx1Cre;LSL-KrasG12D mice treated with alcohol, Western diet, and cerulein without LPS and NNK still developed advanced PanIN and PDAC similar to those treated with all insults. Since we treated mice with alcohol, Western diet, and cerulein for 5 mo, we injected NNK less frequently (once a month for 4 mo) and used a low dose of LPS (1 μg/ml) in mice in the present study. This might be a reason why we did not observe significant additive effects of NNK and LPS in Pdx1Cre;LSL-KrasG12D mice treated with alcohol, Western diet, and cerulein. Tobacco smoking is an established risk factor for PDAC (6, 36). Tobacco smoke contains more than 4,000 chemicals and more than 60 are considered potential carcinogens (12). In addition to NNK, other chemicals in tobacco smoke could still be detrimental in Pdx1Cre;LSL-KrasG12D mice treated with alcohol, Western diet, and cerulein.
In conclusion, our results are the first to demonstrate that moderate alcohol drinking is an independent risk factor for PDAC. Our alcohol feeding mouse model will provide a unique opportunity to examine molecular and cellular mechanisms of alcohol-promoted development of PDAC.
GRANTS
This work was supported by pilot project funding from National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant P50-AA-011999 (to K. Asahina), University of Southern California Dean’s pilot project program (to K. Asahina), NIAAA Grant K08-AA-025112 (to K. Lai), American Cancer Society Grant IRG-58-007-54 (to K. Lai), a Rose Hills summer research fellowship (to E. Moon), a Lee summer research fellowship (to E. Wan), the Dornsife summer undergraduate research fund (to E. Wan), and a Student Opportunities for Academic Research award (to E. Wan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A., K.L., and H.T. conceived and designed research; K.A., S.B., E.H., E.M., E.W., K.S., J.F., J.R., Q.Y., and K.L. performed experiments; K.A., Y.C., and S.W.F. analyzed data; K.A., S.W.F., and H.T. interpreted results of experiments; K.A. prepared figures; K.A. drafted manuscript; K.A., S.B., and H.T. edited and revised manuscript; K.A., S.B., E.H., E.M., E.W., K.S., Y.C., J.F., J.R., Q.Y., K.L., S.W.F., and H.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Raul Lazaro, Yuchang Li, Ingrid Lua, Tomohiro Ogawa, and Jeffery Zhou for maintaining the mouse model.
REFERENCES
- 1.Alexandre M, Uduman AK, Minervini S, Raoof A, Shugrue CA, Akinbiyi EO, Patel V, Shitia M, Kolodecik TR, Patton R, Gorelick FS, Thrower EC. Tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone initiates and enhances pancreatitis responses. Am J Physiol Gastrointest Liver Physiol 303: G696–G704, 2012. doi: 10.1152/ajpgi.00138.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte MV, Pirola RC, Wilson JS. Molecular mechanisms of alcoholic pancreatitis. Dig Dis 23: 232–240, 2005. doi: 10.1159/000090170. [DOI] [PubMed] [Google Scholar]
- 3.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112: 580–593, 2015. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balog S, Li Y, Ogawa T, Miki T, Saito T, French SW, Asahina K. Development of capsular fibrosis beneath the liver surface in humans and mice. Hepatology hep.30809, 2019. doi: 10.1002/hep.30809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology 109: 247–251, 1995. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 6.Barone E, Corrado A, Gemignani F, Landi S. Environmental risk factors for pancreatic cancer: an update. Arch Toxicol 90: 2617–2642, 2016. doi: 10.1007/s00204-016-1821-9. [DOI] [PubMed] [Google Scholar]
- 7.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T; Baltimore Consensus Meeting . A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 39: 1730–1741, 2015. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9: 282–301, 2019. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang HH, Moro A, Takakura K, Su HY, Mo A, Nakanishi M, Waldron RT, French SW, Dawson DW, Hines OJ, Li G, Go VL, Sinnett-Smith J, Pandol SJ, Lugea A, Gukovskaya AS, Duff MO, Rosenberg DW, Rozengurt E, Eibl G. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One 12: e0184455, 2017. doi: 10.1371/journal.pone.0184455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep 3: 1870, 2013. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiaravalli M, Reni M, O’Reilly EM. Pancreatic ductal adenocarcinoma: state-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 60: 32–43, 2017. doi: 10.1016/j.ctrv.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Edderkaoui M, Thrower E. Smoking and pancreatic disease. J Cancer Ther 4: 34–40, 2013. doi: 10.4236/jct.2013.410A005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edderkaoui M, Xu S, Chheda C, Morvaridi S, Hu RW, Grippo PJ, Mascariñas E, Principe DR, Knudsen B, Xue J, Habtezion A, Uyeminami D, Pinkerton KE, Pandol SJ. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget 7: 7747–7760, 2016. doi: 10.18632/oncotarget.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gapstur SM, Jacobs EJ, Deka A, McCullough ML, Patel AV, Thun MJ. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch Intern Med 171: 444–451, 2011. doi: 10.1001/archinternmed.2010.536. [DOI] [PubMed] [Google Scholar]
- 15.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, Hankinson SE, Horn-Ross PL, Leitzmann M, Männistö S, Marshall JR, McCullough ML, Miller AB, Reding DJ, Robien K, Rohan TE, Schatzkin A, Stevens VL, Stolzenberg-Solomon RZ, Verhage BA, Wolk A, Ziegler RG, Smith-Warner SA. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev 18: 765–776, 2009. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol 35: 205–211, 2005. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology 151: 526–539, 2016. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu H, Fortunato F, Bergmann F, Büchler MW, Whitcomb DC, Werner J. Alcohol exacerbates LPS-induced fibrosis in subclinical acute pancreatitis. Am J Pathol 183: 1508–1517, 2013. doi: 10.1016/j.ajpath.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11: 291–302, 2007. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Wang F, Holly EA, Bracci PM. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: a population-based study. Cancer Causes Control 21: 1047–1059, 2010. doi: 10.1007/s10552-010-9533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R, Jiao L, Li D. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol 102: 2696–2707, 2007. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20: 1218–1249, 2006. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 23.Hingorani SR, Petricoin EF 3rd, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4: 437–450, 2003. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 24.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7: 469–483, 2005. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, Armstrong JA, Dingsdale H, Cash N, Li Y, Greenhalf W, Mukherjee R, Kaphalia BS, Jaffar M, Petersen OH, Tepikin AV, Sutton R, Criddle DN. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut 63: 1313–1324, 2014. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao L, Silverman DT, Schairer C, Thiébaut AC, Hollenbeck AR, Leitzmann MF, Schatzkin A, Stolzenberg-Solomon RZ. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 169: 1043–1051, 2009. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, Llorente C, Pan SQ, Yang Q, Li Y, Lazaro R, Ansong C, Smith RD, Bataller R, Morgan T, Schnabl B, Tsukamoto H. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 67: 1737–1753, 2018. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers 2: 16022, 2016. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 29.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JP IV, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22: 737–750, 2012. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19: 456–469, 2011. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin α8β1-mediated stimulation of Gdnf expression. Development 134: 2501–2509, 2007. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT, Bosetti C, Li D, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham E, Bamlet WR, Holly EA, Gao YT, Negri E, Hassan M, Cotterchio M, Su J, Maisonneuve P, Boffetta P, Duell EJ. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 23: 374–382, 2012. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lugea A, Nan L, French SW, Bezerra JA, Gukovskaya AS, Pandol SJ. Pancreas recovery following cerulein-induced pancreatitis is impaired in plasminogen-deficient mice. Gastroenterology 131: 885–899, 2006. doi: 10.1053/j.gastro.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y, Yang Y, Liu M, Wang D, Wang F, Bi Y, Ji J, Li S, Liu Y, Chen R, Huang H, Wang X, Swidnicka-Siergiejko AK, Janowitz T, Beyaz S, Wang G, Xu S, Bialkowska AB, Luo CK, Pin CL, Liang G, Lu X, Wu M, Shroyer KR, Wolff RA, Plunkett W, Ji B, Li Z, Li E, Li X, Yang VW, Logsdon CD, Abbruzzese JL, Lu W. Oncogenic KRAS reduces expression of FGF21 in acinar cells to promote pancreatic tumorigenesis in mice on a high-fat diet. Gastroenterology 157: 1413–1428.e11, 2019. doi: 10.1053/j.gastro.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol 44: 186–198, 2015. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 37.Morris JP IV, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 10: 683–695, 2010. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. KrasG12D and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res 72: 1557–1567, 2012. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohuchida K, Mizumoto K, Yu J, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. S100A6 is increased in a stepwise manner during pancreatic carcinogenesis: clinical value of expression analysis in 98 pancreatic juice samples. Cancer Epidemiol Biomarkers Prev 16: 649–654, 2007. doi: 10.1158/1055-9965.EPI-06-0157. [DOI] [PubMed] [Google Scholar]
- 40.Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, Yamamoto H, Sakon M, Nezu R, Kawano K, Nishida S, Ikegame K, Kawakami M, Tsuboi A, Oka Y, Yoshikawa K, Aozasa K, Monden M, Sugiyama H. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci 95: 583–587, 2004. doi: 10.1111/j.1349-7006.2004.tb02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalbueva N, Mareninova OA, Gerloff A, Yuan J, Waldron RT, Pandol SJ, Gukovskaya AS. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 144: 437–446.e6, 2013. doi: 10.1053/j.gastro.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soler M, Chatenoud L, La Vecchia C, Franceschi S, Negri E. Diet, alcohol, coffee and pancreatic cancer: final results from an Italian study. Eur J Cancer Prev 7: 455–460, 1998. doi: 10.1097/00008469-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan S, Totiger T, Shi C, Castellanos J, Lamichhane P, Dosch AR, Messaggio F, Kashikar N, Honnenahally K, Ban Y, Merchant NB, VanSaun M, Nagathihalli NS. Tobacco carcinogen-induced production of GM-CSF activates CREB to promote pancreatic cancer. Cancer Res 78: 6146–6158, 2018. doi: 10.1158/0008-5472.CAN-18-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukamoto H, Towner SJ, Yu GS, French SW. Potentiation of ethanol-induced pancreatic injury by dietary fat. Induction of chronic pancreatitis by alcohol in rats. Am J Pathol 131: 246–257, 1988. [PMC free article] [PubMed] [Google Scholar]
- 45.Vimalachandran D, Greenhalf W, Thompson C, Lüttges J, Prime W, Campbell F, Dodson A, Watson R, Crnogorac-Jurcevic T, Lemoine N, Neoptolemos J, Costello E. High nuclear S100A6 (Calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res 65: 3218–3225, 2005. doi: 10.1158/0008-5472.CAN-04-4311. [DOI] [PubMed] [Google Scholar]
- 46.Vonlaufen A, Phillips PA, Xu Z, Zhang X, Yang L, Pirola RC, Wilson JS, Apte MV. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 60: 238–246, 2011. doi: 10.1136/gut.2010.211250. [DOI] [PubMed] [Google Scholar]
- 47.Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer 16: 212, 2016. doi: 10.1186/s12885-016-2241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, May R, Cho Y, Asfaha S, Worthley DL, Hayakawa Y, Urbanska AM, Quante M, Reichert M, Broyde J, Subramaniam PS, Remotti H, Su GH, Rustgi AK, Friedman RA, Honig B, Califano A, Houchen CW, Olive KP, Wang TC. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell 18: 441–455, 2016. doi: 10.1016/j.stem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology 144: 1292–1302, 2013. doi: 10.1053/j.gastro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and pancreatic cancer: overview of epidemiology and potential prevention by weight loss. Pancreas 47: 158–162, 2018. doi: 10.1097/MPA.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S, Chheda C, Ouhaddi Y, Benhaddou H, Bourhim M, Grippo PJ, Principe DR, Mascariñas E, DeCant B, Tsukamoto H, Pandol SJ, Edderkaoui M. Characterization of mouse models of early pancreatic lesions induced by alcohol and chronic pancreatitis. Pancreas 44: 882–887, 2015. doi: 10.1097/MPA.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye H, Zhang C, Wang BJ, Tan XH, Zhang WP, Teng Y, Yang X. Synergistic function of Kras mutation and HBx in initiation and progression of hepatocellular carcinoma in mice. Oncogene 33: 5133–5138, 2014. doi: 10.1038/onc.2013.468. [DOI] [PubMed] [Google Scholar]
- 53.Ye H, Zhou Q, Zheng S, Li G, Lin Q, Wei L, Fu Z, Zhang B, Liu Y, Li Z, Chen R. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis 9: 453, 2018. doi: 10.1038/s41419-018-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye W, Lagergren J, Weiderpass E, Nyrén O, Adami HO, Ekbom A. Alcohol abuse and the risk of pancreatic cancer. Gut 51: 236–239, 2002. doi: 10.1136/gut.51.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, Kawamoto T. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact 188: 367–375, 2010. doi: 10.1016/j.cbi.2010.08.005. [DOI] [PubMed] [Google Scholar]