Abstract
Exercise-induced increases in peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and p53 protein content in the nucleus mediate the initial phase of exercise-induced mitochondrial biogenesis. Here, we investigated whether exercise-induced increases in these and other markers of mitochondrial biogenesis were altered after 40 sessions of twice-daily high-volume, high-intensity interval training (HVT) in human skeletal muscle. Vastus lateralis muscle biopsies were collected from 10 healthy recreationally active participants before, immediately postexercise, and 3 h after a session of high-intensity interval exercise (HIIE) performed at the same absolute exercise intensity before and after HVT (pre-HVT and post-HVT, respectively). The protein content of common markers of exercise-induced mitochondrial biogenesis was assessed in nuclear- and cytosolic-enriched fractions by immunoblotting; mRNA contents of key transcription factors and mitochondrial genes were assessed by qPCR. Despite exercise-induced increases in PGC-1α, p53, and plant homeodomain finger-containing protein 20 (PHF20) protein content, the phosphorylation of p53 and acetyl-CoA carboxylase (p-p53 Ser15 and p-ACC Ser79, respectively), and PGC-1α mRNA Pre-HVT, no significant changes were observed post-HVT. Forty sessions of twice-daily high-intensity interval training blunted all of the measured exercise-induced molecular events associated with mitochondrial biogenesis that were observed pre-HVT. Future studies should determine whether this loss relates to the decrease in relative exercise intensity, habituation to the same exercise stimulus, or a combination of both.
Keywords: endurance exercise, high-intensity interval training, mitochondrial adaptations, p53, PGC-1α
INTRODUCTION
Mitochondria are responsible for the production of the majority of the energy required to sustain daily activities and are a key regulator of energy homeostasis (31). The importance of mitochondria is underlined by the links between a healthy mitochondrial pool and enhanced endurance performance (37), improved health (52), and a reduced risk of several lifestyle-related chronic diseases (6, 46). Exercise has long been known to induce mitochondrial biogenesis (34), the making of new components of the mitochondrial reticulum (22). These adaptations to exercise training have been proposed to result from the cumulative effect of transient changes in nuclear protein content (74) and mRNA expression (55) induced by each exercise session.
Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is a key regulator of exercise-induced mitochondrial biogenesis (75) [for an in-depth analysis of the effects of exercise on mitochondrial biogenesis mediated by PGC-1α (and p53) the reader is referred to some excellent reviews; see Refs. 17, 21, 35, 64]. In both rat (74) and human (24, 33, 43, 44) skeletal muscle, it has been observed that there is a postexercise increase in PGC-1α protein content in the nucleus, where PGC-1α performs its transcriptional activity (62). Changes in PGC-1α protein content (30), as well as the content of other proteins (e.g., p53; see Ref. 36), contribute to the exercise-induced upregulation of PGC-1α mRNA (58). Exercise-induced increases in the mRNA levels of PGC-1α and other genes (49, 55, 69), as well as the protein content of selected PGC-1α upstream regulators (48) and selected mitochondrial proteins and transcription factors (55, 69) measured in whole muscle lysates, have been shown to be reduced as a training intervention progresses. However, no study has investigated exercise-induced changes in the nuclear content of PGC-1α or other important proteins modulating mitochondrial biogenesis, before and after a training intervention. Given that increased PGC-1α protein content in the nucleus represents an important process that contributes to the initial phase of exercise-induced mitochondrial biogenesis (74), it is important to better understand how the response of this transcriptional cofactor changes with training.
p53 is another important regulator of exercise-induced mitochondrial biogenesis in human skeletal muscle (64). Nuclear accumulation of p53 protein has been reported immediately (24) or 3 h (70) after a single session of exercise. Although the mechanisms underlying the nuclear accumulation of p53 are complex (47, 53), they have partly been attributed to phosphorylation of p53 at serine 15 (p-p53 Ser15) (53), a posttranslational modification that enhances p53 protein stability (68) and prevents its nuclear export and cytosolic degradation (32, 53). However, once again, these molecular events have been investigated only following a single exercise session, and it is not known whether they are altered by training. Given that the majority of the p53 activity takes place in the nucleus (53), it is important to determine whether the early events of the p53-mediated exercise-induced mitochondrial biogenesis are differentially regulated in this subcellular compartment as the training intervention progresses.
Therefore, the aim of our study was to investigate whether a session of high-intensity interval exercise (HIIE) performed at the same absolute workload before and after a period of high-volume training [HVT; 40 sessions of high-intensity interval training (HIIT) performed twice-daily for 20 consecutive days] induces similar increases in the protein content of PGC-1α, p53, and p-p53 Ser15 in the nucleus. Upstream signaling, as well as the mRNA content of several genes involved in exercise-induced mitochondrial biogenesis, were also investigated before and after HVT. The same absolute workload was chosen, as this approach is often used in training studies (22), as well as in practice, where individuals regularly repeat the same exercise session. We hypothesized that 40 sessions of HIIT would result in significantly reduced exercise-induced increases in these events mediating exercise-induced mitochondrial biogenesis. Despite debate regarding how well exercise-induced molecular events can predict training-induced adaptations (22), findings from the present study will provide a better understanding of how molecular signals are altered when the same exercise stimulus is repeated. This will also improve our knowledge of the mechanisms underlying the common observation of smaller fitness gains as training progresses (42, 45) and may inform strategies to maintain the effectiveness of exercise to stimulate mitochondrial biogenesis.
MATERIALS AND METHODS
Participants
Ten healthy men (20 ± 2 yr, 180 ± 12 cm, 80 ± 15 kg, 46.2 ± 7.6 mL·min−1·kg−1), who were not regularly engaged in cycling-based sports, were moderately trained (i.e., undertaking moderate, unstructured aerobic activity for <3–4 h/wk for at least 6 mo before the study) and were nonsmokers and free of medications, volunteered to participate in this study. Upon passing an initial medical screening, participants were informed of the study requirements, risks, and benefits before giving written, informed consent. All experimental protocols and study procedures were approved by the Victoria University Human Research Ethics Committee and conformed to the standards set by the latest revision of the Declaration of Helsinki. All participants completed the study; however, due to the limited amount of muscle tissue harvested during the second biopsy trial, data from one participant were excluded (including physiological and performance data).
Study Design and Testing
This research was part of a larger, previously published study investigating the effect of different training volumes on mitochondrial adaptations (23). Therefore, the sample size, which was based on preliminary data of mitochondrial respiration obtained in human skeletal muscle in our laboratory, was determined based on the previously published larger study. The sample size was increased by 25% not only to account for dropout but also to cover for small biopsy samples and to increase statistical power to account for the larger number of analyses performed. The experimental protocol specific to the portion of the study described in this article consisted of three tests, each separated by 48 to 72 h, repeated before and after the HVT: a 20-km cycling time trial (20k-TT), a graded exercise test (GXT), and a HIIE biopsy trial (pre-HVT and post-HVT). During the 20 days of HVT participants performed HIIT twice a day (Fig. 1). Prior to beginning this phase of the larger study, participants were familiarized with the 20k-TT, the GXT, and the HIIE and completed the normal volume training (NVT) phase (12 HIIT sessions in 4 wk; Fig. 1). It has been reported that the transcriptional response to the first session of exercise (first bout effect; see Ref. 4) can differ significantly from the response to subsequent exercise sessions (4, 50, 55, 73). Thus, the NVT phase served not only to habituate participants to the rigors of twice-daily HIIT during the HVT phase but also to eliminate possible biases brought about by the “first-bout” effect (4). Finally, participants were required to refrain from vigorous exercise for the 72 h preceding each test, from alcohol for 24 h before testing, and from food and caffeine consumption for 3 h before each test. Although the lack of a “no exercise” control group could be considered a limitation of this study, it has previously been reported that markers of exercise-induced mitochondrial biogenesis (e.g., PGC-1α) do not often change when no exercise is performed (26, 50, 71).
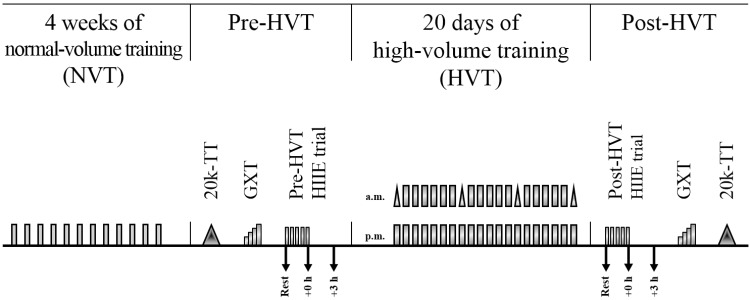
Fig. 1.
Study design. Gray rectangles indicate a high-intensity interval exercise (HIIE) session; gray triangles within high-volume, high-intensity interval training (HVT) indicate a 10-km cycling time trial; each rectangle and/or vertical pair of rectangles and/or vertical pair of rectangles and triangles, represents a training day; arrows indicate a skeletal muscle biopsy. Each test in both the pre- and post-HVT phase was separated by 48–72 h. 20k-TT, 20-km cycling time trial; GXT, graded exercise test; rest, skeletal muscle biopsy at rest; +0 h, skeletal muscle biopsy taken at the end of the HIIE session; +3 h, skeletal muscle biopsy taken 3 h after the completion of the HIIE session.
20k-TT.
Cycling time trials were performed on an electronically braked cycle ergometer (Velotron; RacerMate) after a 6-min warm-up, where participants cycled for 4 min at 66% of the power attained at the lactate threshold (ẆLT), followed by 2 min at ẆLT and 2 min of rest. During these tests, participants were allowed access only to cadence and completed distance. Heart rate was monitored (Polar-Electro) during all exercise trials and training sessions.
Graded exercise test.
A discontinuous graded exercise test (GXT) was performed on an electronically braked cycle ergometer (Lode Excalibur, version 2.0) to determine peak oxygen uptake (V̇o2peak), peak power (Ẇpeak), and ẆLT (using the modified Dmax method; see Ref. 5) and the exercise intensity for both the biopsy trial and the HVT training sessions, as previously described (25). Briefly, the test began at 60, 90, or 120 W, depending on participants’ fitness levels, and was increased by 30 W every 4 min. Stages were interspersed with 30-s breaks for the measurement of fingertip capillary blood lactate concentration using a pre-calibrated blood lactate analyzer (YSI 2300 STAT Plus; YSI). Participants were instructed to keep a cadence above 60 rpm and were allowed access only to cadence and elapsed time; the GXT was terminated when participants reached volitional exhaustion or cadence dropped below 60 rpm. The Ẇpeak was determined as the power of the last completed stage plus 7.5 W for every additional minute completed. O2 and CO2 concentrations were analyzed from expired air using a pre-calibrated gas analyzer (Moxus 2010; AEI Technologies), and V̇o2 values were recorded every 15 s. The average of the two highest consecutive 15-s values was recorded as a participant’s V̇o2peak. The same GXT was performed after 20 days of training to determine the relative exercise intensity of the post-HVT biopsy trial.
Pre- and Post-HVT HIIE biopsy trials.
Each participant performed the two biopsy trials in the morning and at the same time to avoid variations caused by circadian rhythms. Participants were provided with a standardized dinner [55 kJ/kg body mass (BM), providing 2.1 g carbohydrate/kg BM, 0.3 g fat/kg BM, and 0.6 g protein/kg BM) and breakfast (41 kJ/kg BM, providing 1.8 g carbohydrate/kg BM, 0.2 g fat/kg BM, and 0.3 g protein/kg BM) to minimize variability in muscle gene and protein expression attributable to diet, as previously described (23). While participants rested in the supine position, and after injection of local anesthetic (1% xylocaine) into the skin and fascia of the vastus lateralis muscle, three small incisions were made ∼2–3 cm apart. A resting muscle biopsy was taken (rest) using a biopsy needle with suction. Approximately 10 min later, participants were helped to an electronically braked cycle ergometer (Velotron; RacerMate) and began a warm-up consisting of cycling for 4 min at 66% of ẆLT, followed by 2 min at ẆLT and 2 min of rest, after which the Pre-HVT HIIE session began. HIIE consisted of five 4-min intervals at an exercise intensity equal to ẆLT + 0.2 (Ẇpeak − ẆLT) interspersed with 2 min of recovery at 60 W. Immediately after termination of HIIE (∼5–10 s), a second skeletal muscle biopsy was taken (+0 h), and a third one was obtained after 3 h of recovery (+3 h), during which time participants were allowed access to water ab libitum and had no access to food. Skeletal muscle samples were rapidly cleaned of excess blood, fat, and connective tissue, snap-frozen in liquid nitrogen, and later stored at −80°C for subsequent analyses. By design, the Post-HVT HIIE biopsy trial was performed at the same absolute exercise intensity used during the Pre-HVT trial and followed an identical format.
High-volume, high-intensity interval training.
The day following the pre-HVT HIIE biopsy trial, participants began HIIT twice a day for 20 consecutive days. Training sessions were performed in the morning and afternoon and consisted of either 4- or 2-min intervals interspersed with a 2- or 1-min recovery period at 60 W, respectively. To avoid stagnation, the training stimulus was progressively increased daily by virtue of increasing either the relative exercise intensity [from ẆLT + 0.3 (Ẇpeak − ẆLT) to ẆLT + 0.8 (Ẇpeak − ẆLT) for the 4-min intervals and from ẆLT + 0.5 (Ẇpeak − ẆLT) to ẆLT + 0.8 (Ẇpeak − ẆLT) for the 2-min intervals] or the number of repetitions (from 5 to 12 bouts for the 4-min intervals and from 8 to 22 bouts for the 2-min intervals) (23). As a result, single-session duration increased from 30–35 min to 70–80 min. All participants progressively increased their relative exercise intensity and number of repetitions. A 10-km cycling time trial was performed before and at regular weekly intervals during the HVT to monitor participants for signs of overreaching, as previously described (23). The intention was to prevent overreaching by reducing the training load if performance decreased by >10% (28). However, no participants experienced a performance loss throughout the entire study, and the training protocol was completed as planned. All participants completed a minimum of 36 (equivalent to 90%) training sessions; average compliance was 96.5% of the prescribed number of sessions.
Skeletal Muscle Analyses
Subcellular fractionation.
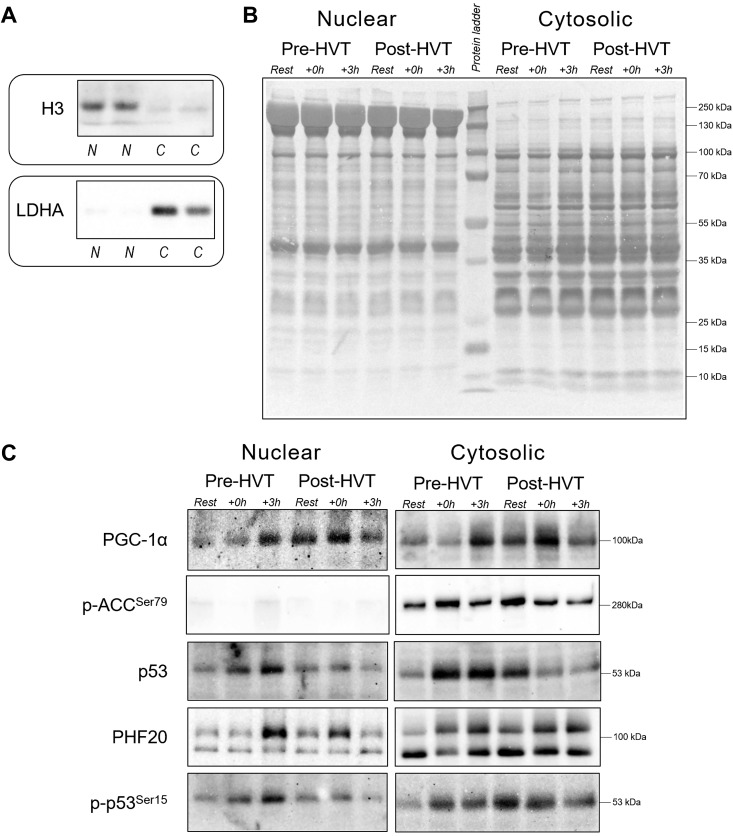
Nuclear and cytosolic fractions were prepared from 35 to 50 mg of skeletal muscle using a commercially available nuclear extraction kit (NE-PER; Pierce). Briefly, muscle samples were washed in phosphate-buffered saline (PBS), homogenized in CER-I buffer containing a protease-phosphatase inhibitor cocktail [5872; Cell Signaling Technology (CST)] and centrifuged at ∼16,000 g. The supernatant was taken as the crude cytosolic fraction. The pellet containing nuclei was washed six times in PBS to minimize cytosolic contamination, and nuclear protein was extracted by centrifugation (∼16,000 g) in a high-salt NER buffer supplemented with the same inhibitors cocktail, following the manufacturers’ instructions. Protein concentration was determined in triplicate using a commercial colorimetric assay (Bio-Rad Protein Assay kit-II; Bio-Rad, Gladesville, NSW, Australia). Nuclear and cytosolic fraction enrichment was confirmed by blotting the separated fractions against a nuclear (histone H3) and a cytosolic [lactate dehydrogenase A (LDHA)] protein; histone H3 was detected mainly in nuclear fractions, whereas LDHA was detected mainly in cytosolic fractions (Fig. 2A), indicating the subcellular fractionation enrichment was successful.
Fig. 2.
Subcellular enrichment, protein loading controls, and representative immunoblots. A: histone H3 and lactate dehydrogenase A (LDHA) were used as indicators of cytosolic (C) and nuclear (N) enrichment, respectively. B: whole lane Coomassie blue staining was used to verify equal loading between lanes in the nuclear and cytosolic fractions obtained from human vastus lateralis muscle biopsies before (rest), immediately postexercise (+0 h), and 3 h (+3 h) after a single session of high-intensity interval exercise (HIIE) performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume, high-intensity interval training (HVT). C: representative immunoblots of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), acetyl-CoA carboxylase phosphorylated at serine 79 (p-ACC Ser79), p53, plant homeodomain finger-containing protein 20 (PHF20; top band at 105 kDa), and p53 phosphorylated at serine 15 (p-p53 Ser15) measured in the same nuclear and cytosolic fractions. No band was detected in the nuclear fractions for p-ACC Ser79. The whole-lane Coomassie and immunoblot images in this figure were cropped to improve the conciseness and clarity of the presentation.
Immunoblotting.
Muscle lysates (10 to 50 μg) were separated by electrophoresis using SDS-PAGE gels (8–15%), as previously described (24). An internal standard was loaded in each gel, and each lane was normalized to this value to reduce gel-to-gel variability. Whole lane Coomassie blue staining (72) was performed to verify correct loading and equal transfer between lanes (Fig. 2B). The following primary antibodies were used (supplier, catalog no.): histone H3 (9715; CST), LDHA (2012; CST), p53 (2527; CST), p-acetyl-CoA carboxylase (p-ACC Ser79; 3661; CST), PGC-1α (st-1202; Calbiochem), plant homeodomain finger-containing protein 20 (PHF20; 3934; CST), and p-p53 Ser15 (9284; CST). Representative images for all target proteins are presented in Fig. 2C.
Total RNA isolation.
Total RNA was isolated from ∼15 mg of muscle tissue, as described previously (14). Briefly, samples were homogenized (FastPrep FP120 Homogenizer; Thermo Savant) in the presence of 1 g of zirconia/silica beads (1.0 mm; Daintree Scientific, St. Helens, TAS, Australia) and 800 μL of TRIzol Reagent (Invitrogen, Melbourne, Australia). Lysates were centrifuged at 13,000 rpm for 15 min at 4°C; the supernatant was collected and combined with chloroform (Sigma-Aldrich, St Louis, MO), and total RNA was extracted using the TRIzol protocol as per the manufacturer’s instructions. RNA precipitation was performed for ≥2 h at −20°C in the presence of 400 μL of isopropanol and 10 μL of 5 M NaCl (both Sigma-Aldrich). RNA concentration was determined spectrophotometrically (Nanodrop ND1000; Thermo Fisher Scientific) by measuring the absorbance at 260 (A260) and 280 (A280) nm, with A260/A280 ratios >1.8 indicating high-quality RNA (41). To ensure that RNA was free of DNA contamination, samples were DNase treated using an RQ1 RNase-free DNase kit (Promega Corporation, Madison, WI).
Real-time PCR (quantitative PCR).
First-strand cDNA synthesis was performed on 300 ng of total RNA using a thermal cycler (S1000 Thermal Cycler; Bio-Rad, Gladesville, NSW, Australia) and the commercially available iScript cDNA synthesis kit (Bio-Rad) in the presence of random hexamers and oligo(dT)s according to the manufacturer’s directions. Forward and reverse primers for all genes investigated (Table 1) were designed based on NCBI RefSeq using NCBI Primer-BLAST (www.ncbi.nlm.nih.gov/BLAST/), and specificity of the amplified product was confirmed by melting point dissociation curves. The mRNA expression of cytochrome c (cyt c), heat shock 70 kDa protein 1A (HSPA1A; usually referred to as HSP70), histone acetyltransferase KAT2A [KAT2A; usually referred to as general control of amino-acid synthesis 5 (GCN5)], nuclear respiratory factor 1 (NRF-1) and 2 (NRF-2), p53, PGC-1α, PHF20, peroxisome proliferator-activated receptor-α (PPARα), peroxisome proliferator-activated receptor-δ (PPARδ), peroxisome proliferator-activated receptor-γ (PPARγ), NAD-dependent protein deacetylase sirtuin-1 (SIRT1), and mitochondrial transcription factor A (TFAM) were quantified by quantitative real-time PCR (Mastercycler RealPlex2, Eppendorf, Germany), using SYBR Green chemistry (iTaqTM Universal SYBR Green Supermix; Bio-Rad) (10-µL PCR reaction volume). All samples were run in duplicate simultaneously with template free controls, using an automated pipetting system (epMotion 5070, Eppendorf, Germany) to reduce technical variation (41). The following PCR cycling patterns were used: initial denaturation at 95°C (3 min), 40 cycles of 95°C (15 s), and 60°C (60 s). Relative changes in mRNA content were calculated using the method. To account for the efficiency of RT and initial RNA concentration, the mRNA expression of four housekeeping genes was quantified, and their stability was determined using the BestKeeper software (56). Cyclophilin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and TATA-binging protein (TBP) were classified as stable, whereas beta-2-microglobulin (B2M) was reported as unstable, and therefore, it was excluded. These results were confirmed by the Normfinder algorithm (2).
Table 1.
Primers used for real-time PCR analyses of mRNA expression
| Gene | Primer Efficiency, % | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| ACTB | 107 | GAGCACAGAGCCTCGCCTTT | TCATCATCCATGGTGAGCTGGC |
| B2M | 98 | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| cyt c | 98.8 | GGGCCAAATCTCCATGGTCT | TCTCCCCAGATGATGCCTTT |
| GAPDH | 106 | AATCCCATCACCATCTTCCA | TGGACTCCACGACGTACTCA |
| HSPA1A | 99 | GGGCCTTTCCAAGATTGCTG | GGTGGGTCCCATAACCCTTG |
| KAT2A | 95 | TGACCCGAAGCACAAGACTC | GGTGGGTCCCATAACCCTTG |
| NRF-1 | 80.7 | CTACTCGTGTGGGACAGCAA | AGCAGACTCCAGGTCTTCCA |
| NRF-2 | 92 | AAGTGACAAGATGGGCTGCT | TGGACCACTGTATGGGATCA |
| p53 | 101.8 | GTTCCGAGAGCTGAATGAGG | TTATGGCGGGAGGTAGACTG |
| PGC-1α | 103.6 | GGCAGAAGGCAATTGAAGAG | TCAAAACGGTCCCTCAGTTC |
| PHF20 | 117.5 | GTGGGGCCGTGAGGAGAATA | AACTGGGCTCCCACTTCAAA |
| PPARα | 92.7 | GGCAGAAGAGCCGTCTCTACTTA | TTTGCATGGTTCTGGGTACTGA |
| PPARδ | 109 | CATCATTCTGTGTGGAGACCG | AGAGGTACTGGGCATCAGGG |
| PPARγ | 103.7 | CTTGTGAAGGATGCAAGGGTT | GAGACATCCCCACTGCAAGG |
| SIRT1 | 99.5 | ACGCTGGAACAGGTTGCGGGA | AAGCGGTTCATCAGCTGGGCAC |
| TBP | 99 | CAGTGACCCAGCAGCATCACT | AGGCCAAGCCCTGAGCGTAA |
| TFAM | 109.3 | CCGAGGTGGTTTTCATCTGT | GCATCTGGGTTCTGAGCTTT |
ACTB, β-actin; B2M, beta-2-microglobulin; cyt c, cytochrome c; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSPA1A, heat shock 70 kDa protein 1A (HSP70); KAT2A, histone acetyltransferase KAT2A (GCN5); NRF-1, nuclear respiratory factor 1; NRF-2, nuclear respiratory factor 2; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PHF20, plant homeodomain finger-containing protein 20; PPARα, peroxisome proliferator-activated receptor-α; PPARδ, peroxisome proliferator-activated receptor-δ; PPARγ, peroxisome proliferator-activated receptor-γ; SIRT1, NAD-dependent protein deacetylase sirtuin-1; TBP, TATA-binging protein; TFAM, mitochondrial transcription factor A.
Statistical Analysis
All values are reported as means ± SD unless otherwise specified. Outliers (defined as values outside means ± 3 SD) were first removed. Normality was assessed with a Shapiro-Wilk test; data sets that failed the normality test (P < 0.05) were log transformed, and if the data set was still nonnormal the reciprocal value was used. To investigate the influence of exercise (rest, +0 h, and +3 h) and training (pre-HVT and post-HVT) and the interaction between these two variables, two-way ANOVA with repeated measures were performed. Interactions were followed by Tukey’s honestly significant difference post hoc tests to assess differences between time points (both within and between trials). In addition, main effects of exercise were further analyzed with preplanned contrasts comparing the effect of exercise within biopsy trials only. Resting protein and mRNA content values in the pre- and post-HVT trials were also compared with a preplanned paired t-test to determine the effects of 40 sessions of HIIT. SigmaPlot 13.0 software (Jandel Scientific) was used for all statistical analyses. The level of statistical significance was set a priori at P < 0.05.
RESULTS
Total Work During the Biopsy Trials
By design, the pre- and post-HVT HIIE sessions were performed at the same absolute exercise intensity (231.1 ± 33.1 W; Fig. 3) and resulted in the same total work (277.3 ± 39.8 kJ). There was an increase (9.0 ± 6.1%, P = 0.002) in ẆLT following training (215.5 ± 32.2 vs. 234.7 ± 36.8 W, pre- and post-HVT, respectively; Fig. 3), which resulted in the relative exercise intensity of the pre-HVT biopsy trial (107.4 ± 1.2% of ẆLT) being greater than the post-HVT biopsy trial (98.8 ± 5.2% of ẆLT). Following training, there was also an increase in Ẇpeak (7.8 ± 4.4%, P = 0.001, 292.5 ± 37.9 vs. 315.2 ± 42.3 W, pre- and post-HVT, respectively; Fig. 3); consequently, the exercise intensity expressed relative to Ẇpeak was also greater in the pre-HVT biopsy trial (78.9 ± 2.4% of Ẇpeak) than the Post-HVT biopsy trial (73.3 ± 3.7% of Ẇpeak). Post-HVT, there was an increase in V̇o2peak (11.7 ± 7.6%, P = 0.001, 46.2 ± 7.6 vs. 51.4 ± 7.8 mL·min−1·kg−1, pre- and post-HVT, respectively), whereas 20k-TT time was decreased (5.2 ± 2.3%, P < 0.001, 2,140.8 ± 99.9 vs. 2,028.1 ± 87.5 s, pre- and post-HVT, respectively). The participants’ BM did not change post training (0.2 ± 1.6%, P = 0.720; 80.4 ± 14.8 vs. 80.6 ± 14.5 kg, pre- and post-HVT, respectively).
Fig. 3.
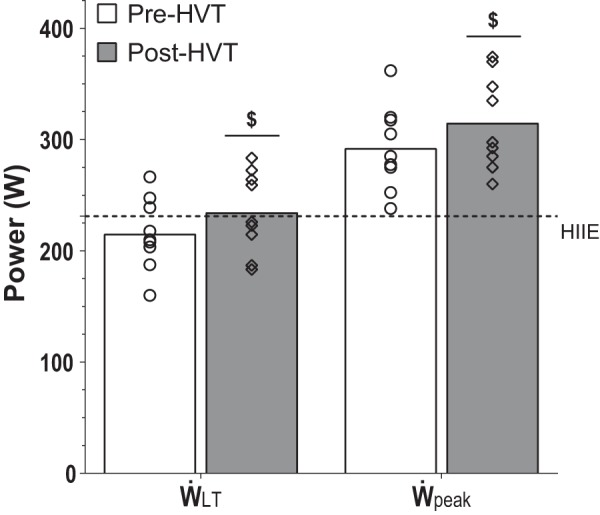
Power attained at the lactate threshold (ẆLT), peak power achieved during the graded exercise test (Ẇpeak), and mean power of the pre- and post-HVT (high-volume, high-intensity interval training) high-intensity interval exercise (HIIE) biopsy trials. ẆLT and Ẇpeak were assessed before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily HVT. ○ (Pre-HVT) and ◇ (post-HVT) represent individual values; white (pre-HVT) and gray (post-HVT) bars represent mean values; dotted line represents the mean power during the pre- and post-HVT HIIE biopsy trials; n = 9. $P < 0.05 vs. pre-HVT rest by paired t-test.
Muscle Analyses
Representative immunoblots are presented in Fig. 2C.
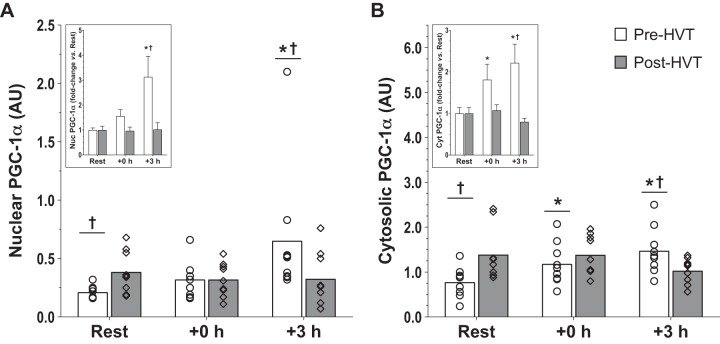
PGC-1α protein content.
There was an interaction effect in both subcellular compartments (nucleus: P = 0.044; cytosol: P = 0.004). In the nucleus (Fig. 4A), PGC-1α was increased at +3 h compared with rest during the pre-HVT (3.1-fold, P = 0.002), but not during the post-HVT (1.0-fold, P = 0.869) biopsy trial. During Pre-HVT, nuclear PGC-1α was also greater at +3 h compared with post-HVT (3.1-fold, P = 0.015). At rest, nuclear PGC-1α protein content was greater post-HVT compared with pre-HVT (1.8-fold, P = 0.013).
Fig. 4.
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) protein. Protein content of PGC-1α in nuclear (A) and cytosolic (B) subfractions before (rest), immediately postexercise (+0 h), and 3 h (+3 h) after a single session of high-intensity interval exercise (HIIE) performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume, high-intensity interval training (HVT) in the vastus lateralis muscle of young healthy men (n = 9). ○ (Pre-HVT) and ◇ (post-HVT) represent individual values; white (pre-HVT) and gray (post-HVT) bars represent mean values. *P < 0.05 vs. rest of the same group; †P < 0.05 vs. same time point of post-HVT trial by 2-way ANOVA with repeated measures, followed by Tukey’s honestly significant difference post hoc test or preplanned paired t-test for rest values between trials. To more clearly depict fold changes in postexercise values from potentially different rest values in the untrained and trained state, an inset has been added to each graph (note: significant differences between trained and untrained values at rest are not reported in insets, as both of these values are normalized to 1); the error bars represent SE. AU, arbitrary units.
In the cytosol (Fig. 4B), PGC-1α increased compared with rest both at +0 h (1.8-fold, P = 0.036) and +3 h (2.2-fold, P < 0.001) during the pre-HVT but not during the post-HVT (1.1-fold, P = 1.000 at +0 h; 0.8-fold, P = 0.070 at +3 h) biopsy trial. During the pre-HVT biopsy trial, cytosolic PGC-1α was also greater at +3 h (1.5-fold, P = 0.017) compared with the same time point of the post-HVT biopsy trial. At rest, cytosolic PGC-1α was greater post-HVT compared with pre-HVT (2.0-fold, P = 0.005).
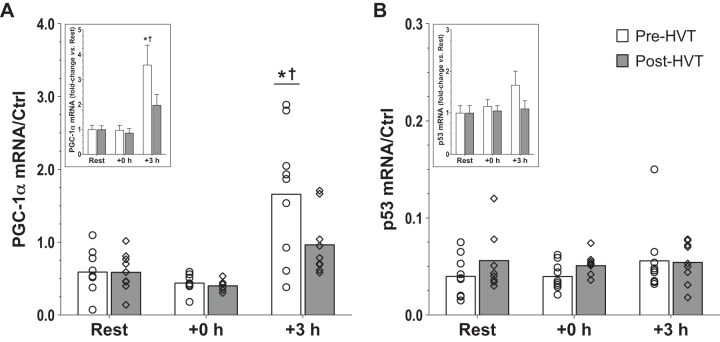
Gene expression.
There was an interaction effect for PGC-1α mRNA content (P = 0.020; Fig. 5A), which was increased at +3 h compared with rest during the pre-HVT (3.6-fold, P < 0.001) but not during the post-HVT (2.0-fold, P = 0.129) biopsy trial. During the pre-HVT biopsy trial, the mRNA content of PGC-1α at +3 h was also greater (1.9-fold, P < 0.001) compared with that recorded at the same time point during the post-HVT biopsy trial. There was no change in p53 mRNA content throughout (interaction: P = 0.425; main effect of exercise: P = 0.379; Fig. 5B). Results for the mRNA content of cyt c, GCN5, HSP70, NRF-1 and NRF-2, PHF20, PPARα, PPARδ, PPARγ, SIRT1, and TFAM are reported in Table 2.
Fig. 5.
Gene expression in whole tissue. mRNA content of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; A) and p53 (B) before (rest), immediately postexercise (+0 h), and 3 h (+3 h) after a single session of high-intensity interval exercise (HIIE) performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume, high-intensity interval training (HVT) in the vastus lateralis muscle of young healthy men (n = 9). Values are expressed relative to TATA-binging protein (TBP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and β-actin (ACTB) housekeeping genes [control (Ctrl)]. ○ (Pre-HVT) and ◇ (post-HVT) represent individual values; white (pre-HVT) and gray (post-HVT) bars represent mean values. *P < 0.05 vs. rest in the same group; †P < 0.05 vs. same time point of post-HVT trial by 2-way ANOVA with repeated measures, followed by Tukey’s honestly significant difference post hoc test or by preplanned paired t-test for rest values between trials. To more clearly depict fold changes in postexercise values from potentially different rest values in the untrained and trained state, an inset has been added to each graph; the error bars represent the SE.
Table 2.
mRNA content measured in whole tissue of cyt c, GCN5, HSP70, NRF-1 and -2, PHF20, PPARα, PPARδ, PPARγ, SIRT1, and TFAM measured immediately postexercise (+0 h) and 3 h (+3 h) after a single session of pre-HVT and post-HVT in the vastus lateralis muscle of young healthy men
| Gene (Interaction) | Pre-HVT | Post-HVT |
|---|---|---|
| cyt c (P = 0.079) | ||
| Rest | 2.06 ± 0.89 | 2.79 ± 1.55 |
| +0 h# | 1.63 ± 0.44 | 1.46 ± 0.67 |
| +3 h | 1.70 ± 0.64 | 2.48 ± 1.37 |
| GCN5 (P = 0.718) | ||
| Rest | 0.05 ± 0.06 | 0.09 ± 0.15 |
| +0 h | 0.06 ± 0.04 | 0.08 ± 0.07 |
| +3 h | 0.06 ± 0.08 | 0.13 ± 0.14 |
| HSP70 (P = 0.021‡) | ||
| Rest | 1.15 ± 0.84 | 1.98 ± 1.43 |
| +0 h | 4.49 ± 3.01*† | 2.00 ± 0.84 |
| +3 h | 3.87 ± 1.81* | 2.63 ± 1.11 |
| NRF-1 (P = 0.118) | ||
| Rest | 0.14 ± 0.08 | 0.13 ± 0.06 |
| +0 h | 0.19 ± 0.09 | 0.14 ± 0.04 |
| +3 h | 0.15 ± 0.05 | 0.16 ± 0.05 |
| NRF-2 (P = 0.962) | ||
| Rest | 0.23 ± 0.07 | 0.21 ± 0.06 |
| +0 h | 0.23 ± 0.09 | 0.19 ± 0.05 |
| +3 h | 0.28 ± 0.13 | 0.26 ± 0.15 |
| PHF20 (P = 0.279) | ||
| Rest | 0.34 ± 0.08 | 0.27 ± 0.04 |
| +0 h | 0.31 ± 0.08 | 0.24 ± 0.05 |
| +3 h | 0.31 ± 0.09 | 0.29 ± 0.09 |
| PPARα (P = 0.005‡) | ||
| Rest | 0.43 ± 0.20 | 0.61 ± 0.38 |
| +0 h | 0.27 ± 0.19 | 0.31 ± 0.08 |
| +3 h | 1.66 ± 0.91*† | 0.87 ± 0.40 |
| PPARδ (P = 0.766) | ||
| Rest | 0.00 ± 0.00 | 0.01 ± 0.03 |
| +0 h | 0.01 ± 0.01 | 0.01 ± 0.02 |
| +3 h | 0.02 ± 0.04 | 0.02 ± 0.02 |
| PPARγ (P = 0.096) | ||
| Rest | 0.06 ± 0.02† | 0.08 ± 0.03 |
| +0 h | 0.04 ± 0.02 | 0.05 ± 0.02 |
| +3 h | 0.04 ± 0.02 | 0.11 ± 0.11 |
| SIRT1 (P = 0.685) | ||
| Rest | 0.06 ± 0.03 | 0.07 ± 0.04 |
| +0 h# | 0.05 ± 0.03 | 0.05 ± 0.02 |
| +3 h | 0.07 ± 0.02 | 0.06 ± 0.04 |
| TFAM (P = 0.953) | ||
| Rest | 0.48 ± 0.14 | 0.44 ± 0.09 |
| +0 h | 0.40 ± 0.11 | 0.37 ± 0.08 |
| +3 h | 0.47 ± 0.13 | 0.44 ± 0.12 |
All values are means ± SD; n = 9 for nuclear respiratory factor (NRF)-1; n = 7 for plant homeodomain finger-containing protein 20 (PHF20); n = 8 for all other genes. Cyt c, cytochrome c; GCN5, histone acetyltransferase KAT2A; HSP70, heat shock 70 kDa protein 1A; PPARα, peroxisome proliferator-activated receptor-α; PPARδ, peroxisome proliferator-activated receptor-δ; PPARγ, peroxisome proliferator-activated receptor-γ; pre- and post-HVT; high-intensity interval exercise performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume high-intensity interval training (HVT); SIRT1, NAD-dependent protein deacetylase sirtuin-1; TFAM, mitochondrial transcription factor A. Values are expressed relative to TATA-binging protein, glyceraldehyde 3-phosphate dehydrogenase, and β-actin housekeeping genes.
Interaction effect (P < 0.05);
main effect of exercise (P < 0.05) vs. rest;
P < 0.05 vs. rest in the same group;
P < 0.05 vs. same time point of post-HVT trial by 2-way ANOVA with repeated measures, followed by Tukey’s honestly significant difference post hoc test or preplanned paired t-test for rest values between trials.
Phosphorylation of acetyl-CoA carboxylase at serine 79 protein content.
Phosphorylation of acetyl-CoA carboxylase (ACC) at serine 79 (p-ACC Ser79) was not detected in nuclear fractions (Fig. 2C). In the cytosol (Fig. 6), no interaction effect was reported (P = 0.774); however, there was a main effect of exercise (P < 0.001), whereby p-ACC Ser79 was greater compared with rest at +0 h (1.7-fold, P < 0.001). Preplanned comparisons within biopsy trials indicated that at +0 h cytosolic p-ACC Ser79 was greater compared with rest during the pre-HVT (2.0-fold, P = 0.013) but not during the post-HVT (1.4-fold, P = 0.114) biopsy trial.
Fig. 6.
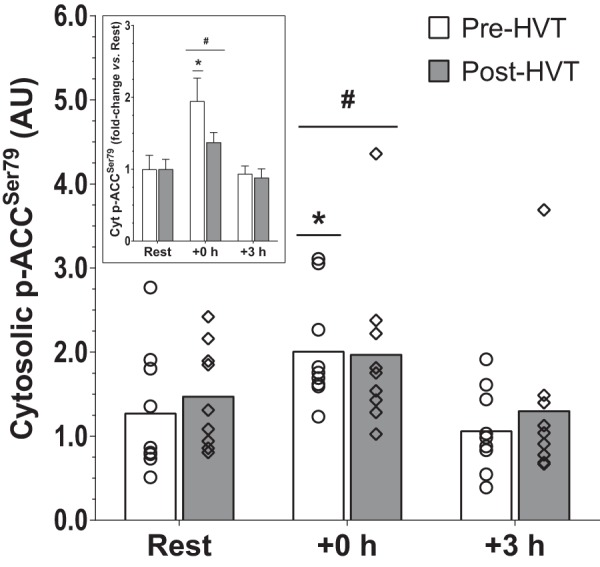
Phosphorylation of acetyl-CoA carboxylase (ACC) at serine 79 (p-ACC Ser79). Protein content of cytosolic p-ACC Ser79 before (rest), immediately postexercise (+0 h), and 3 h (+3 h) after a single session of high-intensity interval exercise (HIIE) performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume, high-intensity interval training (HVT) in the vastus lateralis muscle of young healthy men (n = 9). ○ (Pre-HVT) and ◇ (post-HVT) represent individual values; white (pre-HVT) and gray (post-HVT) bars represent mean values. #Main effect of exercise (P < 0.05) vs. rest; *P < 0.05 vs. rest in the same group by 2-way ANOVA with repeated measures, followed by Tukey’s honestly significant difference post hoc test or by preplanned paired t-test for rest values between trials. To more clearly depict fold changes in postexercise values from potentially different rest values in the untrained and trained states, an inset has been added; error bars represent the SE. AU, arbitrary units.
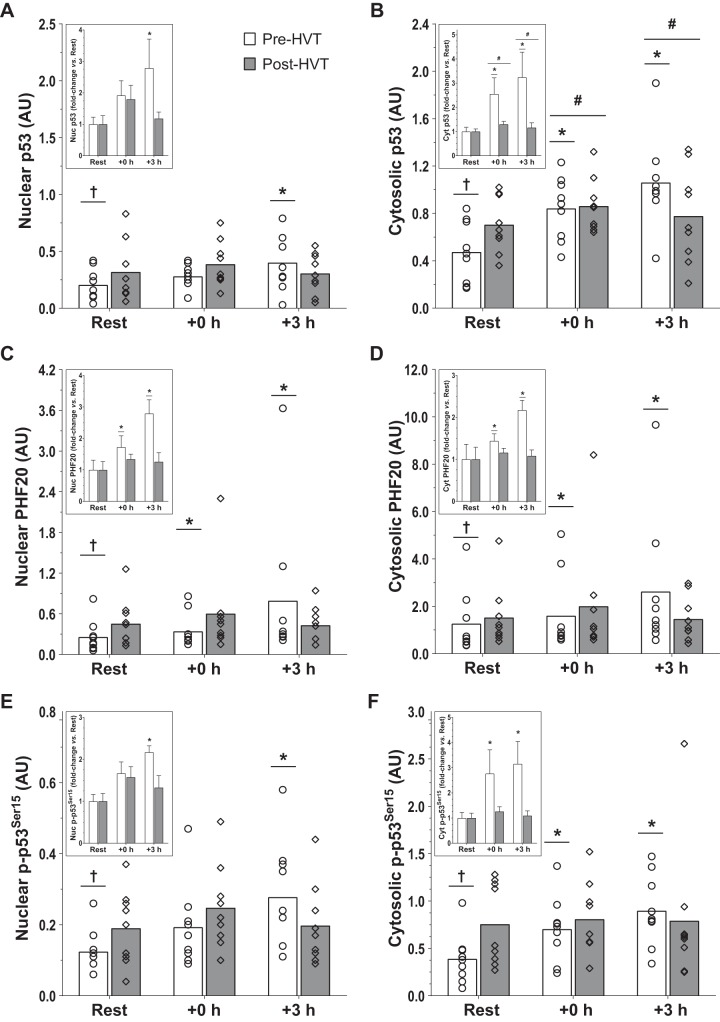
p53 protein content.
In the nucleus (Fig. 7A), there was an interaction effect (P = 0.016); nuclear p53 was increased at +3 h compared with rest during the pre-HVT (2.8-fold; P = 0.004), but not during the post-HVT (1.2-fold, P = 0.328) biopsy trial. At rest, nuclear p53 was greater post-HVT compared with pre-HVT (1.6-fold, P = 0.038).
Fig. 7.
p53 and Plant homeodomain finger-containing protein 20 (PHF20) protein. Protein content of nuclear (A) and cytosolic (B) p53, nuclear (C) and cytosolic (D) PHF20, and nuclear (E) and cytosolic (F) p-p53 Ser15 assessed before (rest), immediately postexercise (+0 h), and 3 h (+3 h) after a single session of high-intensity interval exercise (HIIE) performed at the same absolute intensity before (pre-HVT) and after (post-HVT) 40 sessions of twice-daily high-volume, high-intensity interval training (HVT) in the vastus lateralis muscle of young healthy men (n = 9). ○ (Pre-HVT) and ◇ (post-HVT) represent individual values; white (pre-HVT) and gray (post-HVT) bars represent mean values. #Main effect of exercise (P < 0.05) vs. rest; *P < 0.05 vs. rest of the same group; †P < 0.05 vs. the same time point of post-HVT trial by 2-way ANOVA with repeated measures, followed by Tukey’s honestly significant difference post hoc test or by preplanned paired t-test for rest values between trials. To more clearly depict fold changes in postexercise values from potentially different rest values in the untrained and trained states, an inset has been added to each graph (note: significant differences between trained and untrained values at rest are not reported in the insets, as both of these values are normalized to 1); the error bars represent SE. AU, arbitrary units.
In the cytosol (Fig. 7B), the interaction effect was not statistically significant (P = 0.051); however, there was a main effect of exercise (P = 0.003). Cytosolic p53 increased compared with rest both at +0 h (1.9-fold, P = 0.019) and +3 h (2.2-fold, P = 0.004). Preplanned comparisons within trials revealed that during the pre-HVT biopsy trial cytosolic p53 was greater compared with rest at both +0 h (2.6-fold, P = 0.020) and +3 h (3.2-fold, P < 0.001); however, during the post-HVT biopsy trial no differences compared with rest were reported at +0 h (1.3-fold, P = 0.440) and +3 h (1.2-fold, P = 0.835). At rest, cytosolic p53 was greater post-HVT compared with pre-HVT (1.9-fold, P = 0.015).
PHF20 protein content.
There was an interaction effect in both subcellular compartments (nucleus: P = 0.019; cytosol: P = 0.025). In the nucleus (Fig. 7C), PHF20 increased compared with rest both at +0 h (1.7-fold, P = 0.016) and +3 h (2.8-fold, P < 0.001) during the pre-HVT but not during the post-HVT (1.3-fold, P = 0.616 at +0 h; 1.3-fold, P = 0.858 at +3 h) biopsy trial. At rest, nuclear PHF20 was greater post-HVT compared with pre-HVT (1.9-fold, P = 0.004).
In the cytosol (Fig. 7D), PHF20 increased compared with rest both at +0 h (1.4-fold, P = 0.032) and +3 h (2.2-fold, P < 0.001) during the pre-HVT but not during the post-HVT (1.2-fold, P = 0.890 at +0 h; 1.1-fold, P = 0.996 at +3 h) biopsy trial. At rest, cytosolic PHF20 was greater post-HVT compared with pre-HVT (1.5-fold, P = 0.013).
p-p53 Ser15 protein content.
In the nucleus (Fig. 7E), there was an interaction effect (P = 0.021); nuclear p-p53 Ser15 was increased compared with rest at +3 h during the pre-HVT (2.2-fold; P = 0.001) but not during the post-HVT (1.3-fold, P = 0.970) biopsy trial. At rest, nuclear p-p53 Ser15 was greater post-HVT compared with pre-HVT (1.5-fold, P = 0.043).
An interaction effect (P = 0.033) was also reported in the cytosol (Fig. 7F). During pre-HVT, cytosolic p-p53 Ser15 was greater compared with rest at both +0 h (2.8-fold, P = 0.018) and +3 h (3.2-fold, P < 0.001) but not during the post-HVT (1.3-fold, P = 0.847 and 1.1-fold, P = 0.997 at +0 and +3 h, respectively) biopsy trial. At rest, cytosolic p-p53 Ser15 was greater post-HVT compared with pre-HVT (2.4-fold, P = 0.008).
DISCUSSION
We report that 40 sessions of HIIT resulted in the loss of all measured exercise-induced molecular changes recorded pre-HVT. Although training-induced blunting of specific exercise-induced molecular adaptations in whole cell lysates has previously been reported (48, 49, 55, 69), this is the first study demonstrating training-induced blunting of selected markers of mitochondrial biogenesis at the subcellular level. Despite exercise-induced increases in both the nuclear and cytosolic fractions in PGC-1α, p53, PHF20, and p-p53 Ser15 protein content before the HVT, there were no significant changes in any of these parameters when a session of HIIE was repeated at the same absolute exercise intensity posttraining. However, post-HVT there was an increase in resting values of most proteins measured in this study. In contrast to our findings, where exercise-induced upregulation of all measured parameters was blunted posttraining, previous research has reported that training-induced blunting of exercise-induced molecular changes is not universal (55, 69). These discrepancies may relate to the much greater number of training sessions in the present study [40 vs. 7 (55) and 10 (69), respectively].
We observed a significant exercise-induced increase in PGC-1α protein content in both the nuclear and cytosolic fractions Pre-HVT, consistent with most previous research (24, 33, 43, 44). However, for the first time we report that these exercise-induced increases were absent posttraining in both subcellular fractions. It has been proposed that metabolic perturbations [e.g., increases in intracellular calcium (Ca2+), adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio, oxidized nicotinamide adenine dinucleotide (NAD+) to NADH ratio, and reactive oxygen species (ROS) production] provide an important stimulus for exercise-induced mitochondrial biogenesis (17) and promote an increase in the nuclear content of PGC-1α protein (74). A possible explanation for our findings is that the lower relative exercise intensity elicited during the post-HVT session compared with pre-HVT (98.8 vs. 107.4% of ẆLT for post- and pre-HVT, respectively) may have reduced the metabolic perturbations and the subsequent molecular response posttraining. This is supported by the absence of significant changes in cytosolic p-ACC Ser79 post-HVT (as described more in detail below).
The reported increase in PGC-1α protein content in both the nucleus and the cytosol during the pre-HVT trial may be attributable at least in part to increased protein stability (63). Both p38 mitogen-activated protein kinase (MAPK) (61) and AMP-activated protein kinase (AMPK) (7) act as signaling proteins that increase PGC-1α stability via phosphorylation (and they act in similar fashion to also increase the stability of the p53 protein; see Refs. 39 and 67). Because of the limited amount of enriched lysates obtained during subcellular fractionation, we could not measure phosphorylation of p38 MAPK and/or AMPK directly. However, because of its molecular weight (∼280 kDa), when blotting for lower molecular weight proteins we were also able to measure p-ACC Ser79, a downstream target and commonly used marker of AMPK activation (9, 10, 38). As previously reported, p-ACC Ser79 was not detected in nuclear fractions (24, 44). However, we were able to measure cytosolic p-ACC Ser79 and make the novel observation that despite a postexercise increase pre-HVT, there was no significant change post-HVT. This suggests that abrogation of AMPK signaling may have contributed to the abrogation of exercise-induced increases in PGC-1α (and p53) protein content posttraining. Subcellular translocation is another factor that has been associated with increased PGC-1α protein content in the nucleus (74). Although our data do not seem to indicate cytosolic/nuclear shuttling of PGC-1α, protein translocation is a complex series of cellular processes that cannot be assessed by subcellular fractionation coupled with the immunoblotting technique (1).
PGC-1α has been shown to be activated via deacetylation by SIRT1 (7, 63). Although previous research has reported exercise-induced increases in SIRT1 mRNA in human skeletal muscle following both low-intensity continuous (13) and high-intensity interval (15) exercise, our results indicate a small significant decrease at +0 h (0.8-fold change). SIRT1 activity rather than protein content seems to regulate mitochondrial biogenesis in humans (27); however, due to limited tissue availability, we were not able to perform this measurement. It has also been reported that SIRT1 deacetylase activity may not be required for exercise-induced mitochondrial biogenesis or PGC-1α deacetylation and that changes in the acetyltransferase activity and subcellular location of GCN5, a negative regulator of PGC-1α (18), may be more important factors regulating exercise-induced PGC-1α activity (57). Consistent with previous findings (15), we report no change in GCN5 mRNA content either pre- or post-HVT. Limited skeletal muscle availability precluded us from assessing GCN5 activity or protein content in different subcellular fractions.
The PGC-1α protein itself has been reported to stimulate PGC-1α transcriptional activity via an autoregulatory loop that requires coactivation of the myocyte enhancer factor-2 protein (30). The exercise-induced increase in PGC-1α mRNA content observed pre-HVT is consistent with previous findings investigating HIIE (12, 15, 49, 51, 55, 59, 60) and with the notion that increased nuclear PGC-1α protein content and stability are associated with greater PGC-1α transcriptional activity (3). No exercise-induced increase in PGC-1α mRNA content was reported post-HVT, suggesting that 20 days of HVT also blunted the exercise-induced increase in PGC-1α transcription. However, previous studies have reported a reduction (rather than complete loss) of the exercise-induced upregulation of PGC-1α mRNA content posttraining compared with pretraining when the exercise session was repeated at the same relative (55) or absolute (49, 69) exercise intensity. This discrepancy may relate to the much greater number of sessions performed between exercise biopsy trials in our study compared with these three previous studies (40 vs. 7–12, respectively) and a likely greater reduction in the relative exercise intensity between the pre- and post-HVT trials. Moreover, in contrast to the three previous studies, our participants were habituated to HIIE; this raises the possibility that the greater molecular response recorded pretraining in the previous studies may be partly attributable to the “first bout” effect (4, 50).
To better characterize the effect of 40 sessions of HIIT on exercise-induced mitochondrial adaptations to HIIE, we measured the mRNA content of nuclear (NRF-1 and NRF-2; see Ref. 65) and mitochondrial (TFAM; see Ref. 66) transcription factors regulating mitochondrial biogenesis that are transcriptionally controlled by PGC-1α (75). The mRNA content of cyt c (a gene under the regulation of PGC-1α and NRF-1; see Ref. 75), p53 (a transcriptional regulator of PGC-1α gene expression; see Ref. 36), and PHF20 (a protein that stabilizes and activates p53; see Ref. 11) was also measured. In addition, we also assessed the mRNA content of three PPAR genes, which are involved in fatty acid metabolism and transport (19), and HSP70, a chaperone protein required for the import and folding of mitochondrial proteins (40). Both HSP70 and PPARα were increased following exercise during the pre-HVT trial, but not during the post-HVT trial, following a similar response to the majority of the molecular events linked with exercise-induced mitochondrial biogenesis measured in our study. Aside from a decrease in cyt c mRNA content at +0 h in both HIIE trials, we observed no exercise-induced changes in any of the other genes either pre- or post-HVT. It is important to note that a possible explanation for the lack of exercise-induced upregulation of some of these mRNAs (at least pre-HVT) may relate to the biopsy timings chosen postexercise, as there is evidence that the exercise-induced upregulation of some of these genes peak more than 3 h postexercise (8, 12, 16, 21, 29).
Similarly to our results for PGC-1α protein, we observed an exercise-induced increase in p53 protein content pretraining in the nuclear and cytosolic fractions, as previously demonstrated (24, 70). However, this exercise-induced increase in both subcellular fractions was blunted following 40 training sessions. No other study has investigated exercise-induced changes in p53 protein content pre- and post-training in subcellular fractions. Nonetheless, our results are consistent with findings showing reduced/blunted exercise-induced mitochondrial adaptations (e.g., PGC-1α mRNA, PGC-1α protein in whole muscle lysates) when the same exercise session is repeated posttraining both at the same absolute (49, 69) or relative (55) exercise intensity.
A possible factor contributing to the lack of exercise-induced changes in nuclear p53 protein content post-HVT is that 40 sessions of HIIT increased the resting values of p53 in both fractions. A second factor relates to a possible decrease in subcellular shuttling (20); however, simply immunoblotting subcellular enriched fractions for p53 (or PGC-1α) protein is not a valid technique to demonstrate p53 (similar to PGC-1α) nuclear/cytosolic shuttling, a process requiring an intricate and tightly synchronized series of events (20, 47). Nonetheless, a further novel observation is that there was a concomitant increase in p53 and PHF20 protein content in both subcellular fractions pre-HVT but not post-HVT. In this regard, PHF20 has been reported to increase p53 protein stability (53) by disrupting the murine double minute-2 (MDM2)-p53 interaction (11) responsible for p53 protein degradation (32, 53). Although we were not able to measure the interaction between these two proteins due to limited lysate availability, it is plausible that our findings may indicate greater p53-PHF20 and reduced p53-MDM2 interaction pre- versus post-HVT.
A second important event disrupting the p53-MDM2 interaction and promoting p53 stability is phosphorylation of p53 at serine 15 (68). Pre-HVT, and consistent with this notion, both nuclear and cytosolic p-p53 Ser15 increased in parallel with the increase in p53 protein content, as previously reported (24), suggesting that phosphorylation of p53 at serine 15 may indeed be involved in the regulation of the p53 protein stability during exercise in human skeletal muscle. In contrast, we report for the first time that there were no exercise-induced changes in p-p53 Ser15 in either the nuclear or cytosolic fractions after a period of training (i.e., post-HVT); the increase in resting p-p53 Ser15 Post-HVT may be a contributing factor for the lack of exercise-induced changes in p-p53 Ser15 after 40 sessions of HIIT.
This research adds novel information regarding the early molecular events regulating the exercise-induced mitochondrial adaptations in subcellular fractions and how these are altered by an exercise training intervention. We provide evidence that 40 sessions of HIIT blunted the exercise-induced increases recorded pretraining in both nuclear and cytosolic-enriched subcellular fractions in all of the molecular parameters measured. Although training has previously been shown to blunt some of the exercise-induced adaptations in whole muscle lysates (55), this is the first study to report training-induced blunting of protein changes in the nucleus, where the majority of transcriptional activity takes place, and where an early increase in PGC-1α protein content has been reported to constitute the initial phase of the exercise-induced adaptive response (74). Future studies should investigate whether the loss (or reduction) of the exercise-induced increases in markers of mitochondrial adaptations posttraining relates solely to the decrease in relative exercise intensity and/or whether this is exacerbated by the continuous repetition of the same exercise stimulus during the training intervention. Well-designed experiments comparing exercise sessions repeated pre- and post-training at the same relative exercise intensity and at different time points during the training intervention (even after only 1 or 2 training sessions to determine the role, if any, of the “first bout effect”) should provide valuable insight into the mechanisms driving this phenomenon.
GRANTS
This study was funded by a grant from the ANZ-MASON Foundation provided to D. J. Bishop and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to J. P. Little.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.G. and D.J.B. conceived and designed research; C.G. and R.S.F.O. performed experiments; C.G., R.S.F.O., J.P.L., and D.J.B. analyzed data; C.G., R.S.F.O., J.P.L., and D.J.B. interpreted results of experiments; C.G. prepared figures; C.G., J.P.L., and D.J.B. drafted manuscript; C.G., R.S.F.O., J.P.L., and D.J.B. edited and revised manuscript; C.G., R.S.F.O., J.P.L., and D.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for time, effort, and commitment to this study. We acknowledge Elise Brentnall and Maarten Missinne for valuable help in data collection and biochemical analyses, respectively.
REFERENCES
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell (5th ed.). New York: Garland Science, 2007. [Google Scholar]
- 2.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250, 2004. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RM, Barger JL, Edwards MG, Braun KH, O’Connor CE, Prolla TA, Weindruch R. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 7: 101–111, 2008. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop DJ, Botella J, Genders AJ, Lee MJ-C, Saner NJ, Kuang J, Yan X, Granata C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology (Bethesda) 34: 56–70, 2019. doi: 10.1152/physiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 5.Bishop D, Jenkins DG, McEniery M, Carey MF. Relationship between plasma lactate parameters and muscle characteristics in female cyclists. Med Sci Sports Exerc 32: 1088–1093, 2000. doi: 10.1097/00005768-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol (1985) 88: 774–787, 2000. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 7.Cantó C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z-P, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab 279: E1202–E1206, 2000. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52: 2205–2212, 2003. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- 11.Cui G, Park S, Badeaux AI, Kim D, Lee J, Thompson JR, Yan F, Kaneko S, Yuan Z, Botuyan MV, Bedford MT, Cheng JQ, Mer G. PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53. Nat Struct Mol Biol 19: 916–924, 2012. doi: 10.1038/nsmb.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 294: E607–E614, 2008. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 13.Dumke CL, Mark Davis J, Angela Murphy E, Nieman DC, Carmichael MD, Quindry JC, Travis Triplett N, Utter AC, Gross Gowin SJ, Henson DA, McAnulty SR, McAnulty LS. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol 107: 419–427, 2009. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 14.Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci 7: 191–196, 2018. doi: 10.1016/j.jshs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgett BA, Foster WS, Hankinson PB, Simpson CA, Little JP, Graham RB, Gurd BJ. Dissociation of increases in PGC-1α and its regulators from exercise intensity and muscle activation following acute exercise. PLoS One 8: e71623, 2013. doi: 10.1371/journal.pone.0071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan B, O’Connor PL, Zierath JR, O’Gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One 8: e74098, 2013. doi: 10.1371/journal.pone.0074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 26: 1913–1923, 2007. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilde AJ, Van Bilsen M. Peroxisome proliferator-activated receptors (PPARS): regulators of gene expression in heart and skeletal muscle. Acta Physiol Scand 178: 425–434, 2003. doi: 10.1046/j.1365-201X.2003.01161.x. [DOI] [PubMed] [Google Scholar]
- 20.Gottifredi V, Prives C. Molecular biology. Getting p53 out of the nucleus. Science 292: 1851–1852, 2001. doi: 10.1126/science.1062238. [DOI] [PubMed] [Google Scholar]
- 21.Granata C, Jamnick NA, Bishop DJ. Principles of exercise prescription, and how they influence exercise-induced changes of transcription factors and other regulators of mitochondrial biogenesis. Sports Med 48: 1541–1559, 2018. [Erratum in: Sports Med 48: 1991.] doi: 10.1007/s40279-018-0894-4. [DOI] [PubMed] [Google Scholar]
- 22.Granata C, Jamnick NA, Bishop DJ. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med 48: 1809–1828, 2018. doi: 10.1007/s40279-018-0936-y. [DOI] [PubMed] [Google Scholar]
- 23.Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J 30: 3413–3423, 2016. doi: 10.1096/fj.201500100R. [DOI] [PubMed] [Google Scholar]
- 24.Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Sprint-interval but not continuous exercise increases PGC-1α protein content and p53 phosphorylation in nuclear fractions of human skeletal muscle. Sci Rep 7: 44227, 2017. doi: 10.1038/srep44227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J 30: 959–970, 2016. doi: 10.1096/fj.15-276907. [DOI] [PubMed] [Google Scholar]
- 26.Groennebaek T, Jespersen NR, Jakobsgaard JE, Sieljacks P, Wang J, Rindom E, Musci RV, Bøtker HE, Hamilton KL, Miller BF, de Paoli FV, Vissing K. Skeletal muscle mitochondrial protein synthesis and respiration increase with low-load blood flow restricted as well as high-load resistance training. Front Physiol 9: 1796, 2018. doi: 10.3389/fphys.2018.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurd BJ, Yoshida Y, McFarlan JT, Holloway GP, Moyes CD, Heigenhauser GJF, Spriet L, Bonen A. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 301: R67–R75, 2011. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 28.Halson SL, Bridge MW, Meeusen R, Busschaert B, Gleeson M, Jones DA, Jeukendrup AE. Time course of performance changes and fatigue markers during intensified training in trained cyclists. J Appl Physiol (1985) 93: 947–956, 2002. doi: 10.1152/japplphysiol.01164.2001. [DOI] [PubMed] [Google Scholar]
- 29.Hammond KM, Impey SG, Currell K, Mitchell N, Shepherd SO, Jeromson S, Hawley JA, Close GL, Hamilton LD, Sharples AP, Morton JP. Postexercise high-fat feeding suppresses p70S6K1 activity in human skeletal muscle. Med Sci Sports Exerc 48: 2108–2117, 2016. doi: 10.1249/MSS.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 30.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci USA 100: 7111–7116, 2003. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 32.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299, 1997. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 33.Heesch MW, Shute RJ, Kreiling JL, Slivka DR. Transcriptional control, but not subcellular location, of PGC-1α is altered following exercise in a hot environment. J Appl Physiol (1985) 121: 741–749, 2016. doi: 10.1152/japplphysiol.01065.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 35.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34: 465–472, 2009. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 36.Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS One 3: e3614, 2008. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 114: 344–350, 2013. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 38.Jäger S, Handschin C, St.-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA 104: 12017–12022, 2007. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18: 283–293, 2005. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, Park ASD, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuang J, Yan X, Genders AJ, Granata C, Bishop DJ. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS One 13: e0196438, 2018. doi: 10.1371/journal.pone.0196438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med 32: 53–73, 2002. doi: 10.2165/00007256-200232010-00003. [DOI] [PubMed] [Google Scholar]
- 43.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 44.Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298: R912–R917, 2010. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- 45.Londeree BR. Effect of training on lactate/ventilatory thresholds: a meta-analysis. Med Sci Sports Exerc 29: 837–843, 1997. doi: 10.1097/00005768-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci USA 91: 8731–8738, 1994. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchenko ND, Hanel W, Li D, Becker K, Reich N, Moll UM. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-α3 binding. Cell Death Differ 17: 255–267, 2010. doi: 10.1038/cdd.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568: 665–676, 2005. doi: 10.1113/jphysiol.2005.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89: 852–862, 2015. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 50.Murton AJ, Billeter R, Stephens FB, Des Etages SG, Graber F, Hill RJ, Marimuthu K, Greenhaff PL. Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training. J Appl Physiol (1985) 116: 113–125, 2014. doi: 10.1152/japplphysiol.00426.2013. [DOI] [PubMed] [Google Scholar]
- 51.Nordsborg NB, Lundby C, Leick L, Pilegaard H. Relative workload determines exercise-induced increases in PGC-1α mRNA. Med Sci Sports Exerc 42: 1477–1484, 2010. doi: 10.1249/MSS.0b013e3181d2d21c. [DOI] [PubMed] [Google Scholar]
- 52.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem 274: 36031–36034, 1999. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 55.Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper — Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515, 2004. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 57.Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J Biol Chem 286: 30561–30570, 2011. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858, 2003. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popov D, Zinovkin R, Karger E, Tarasova O, Vinogradova O. Effects of continuous and intermittent aerobic exercise upon mRNA expression of metabolic genes in human skeletal muscle. J Sports Med Phys Fitness 54: 362–369, 2014. [PubMed] [Google Scholar]
- 60.Popov DV, Zinovkin RA, Karger EM, Tarasova OS, Vinogradova OL. The effect of aerobic exercise on the expression of genes in skeletal muscles of trained and untrained men. Hum Physiol 39: 190–195, 2013. doi: 10.1134/S0362119713020126. [DOI] [PubMed] [Google Scholar]
- 61.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8: 971–982, 2001. doi: 10.1016/S1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 62.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 63.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 α and SIRT1 pathways. FEBS Lett 582: 46–53, 2008. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saleem A, Carter HN, Iqbal S, Hood DA. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc Sport Sci Rev 39: 199–205, 2011. doi: 10.1097/JES.0b013e31822d71be. [DOI] [PubMed] [Google Scholar]
- 65.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576: 1–14, 2002. doi: 10.1016/S0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 66.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 67.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res 61: 1604–1610, 2001. [PubMed] [Google Scholar]
- 68.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91: 325–334, 1997. doi: 10.1016/S0092-8674(00)80416-X. [DOI] [PubMed] [Google Scholar]
- 69.Stepto NK, Benziane B, Wadley GD, Chibalin AV, Canny BJ, Eynon N, McConell GK. Short-term intensified cycle training alters acute and chronic responses of PGC1α and Cytochrome C oxidase IV to exercise in human skeletal muscle. PLoS One 7: e53080, 2012. doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tachtsis B, Smiles WJ, Lane SC, Hawley JA, Camera DM. Acute endurance exercises induces nuclear p53 abundance in human skeletal muscle. Front Physiol 7: 144, 2016. doi: 10.3389/fphys.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? FASEB J 19: 94–96, 2005. doi: 10.1096/fj.04-2084fje. [DOI] [PubMed] [Google Scholar]
- 72.Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res 10: 1416–1419, 2011. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein sub-fraction synthesis in humans. Am J Physiol Heart Circ Physiol 306: E571–E579, 2014. doi: 10.1152/ajpendo.00650.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem 282: 194–199, 2007. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 75.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]