Abstract
Sirtuin 1 (SIRT1) and general control of amino acid synthesis 5 (GCN5) regulate mitochondrial biogenesis via opposing modulation of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) acetylation status and activity. However, the combined contribution of SIRT1 and GCN5 to skeletal muscle metabolism and endurance performance in vivo is unknown. In this study, we investigated the impact of combined skeletal muscle-specific overexpression of SIRT1 and deletion of GCN5 on glucose homeostasis, skeletal muscle mitochondrial biogenesis and function, and metabolic adaptation to endurance exercise training in mice. We generated mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of SIRT1 and knockout of GCN5 (dTG) and floxed [wild type (WT)] littermates using a Cre-LoxP approach. All mice were treated with tamoxifen at 5–6 wk of age, and 4–7 wk later glucose homeostasis, skeletal muscle contractile function, mitochondrial function, and the effects of 14 days of voluntary wheel running on expression of metabolic proteins and exercise capacity were assessed. There was no difference in oral glucose tolerance, skeletal muscle contractile function, mitochondrial abundance, or maximal respiratory capacity between dTG and WT mice. Additionally, there were no genotype differences in exercise performance and markers of mitochondrial biogenesis after 14 days of voluntary wheel running. These results demonstrate that combined overexpression of SIRT1 and loss of GCN5 in vivo does not promote metabolic remodeling in skeletal muscle of sedentary or exercise-trained mice.
Keywords: acetyltransferase, deacetylase, mitochondria, peroxisome proliferator-activated receptor-γ coactivator 1α
INTRODUCTION
Acetylation regulates the activity of metabolic enzymes and transcriptional activity in skeletal muscle, thereby modulating glycolysis, fatty acid metabolism, and mitochondrial biogenesis (15, 20). Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) is a key transcriptional regulator of mitochondrial and metabolic genes (6, 27), and its activity is regulated in an opposing manner by sirtuin 1 (SIRT1) and general control of amino acid synthesis 5 (GCN5), where SIRT1 deacetylates and activates PGC-1α and GCN5 acetylates and represses PGC-1α activity (3, 6, 8, 13, 19, 21). Pharmacological activation of SIRT1 in mice increases expression of PGC-1α targets involved in fatty acid oxidation in skeletal muscle (7), and overexpression of GCN5 decreases the transcriptional activity of PGC-1α (13). SIRT1 and GCN5 are thus proposed as key opposing modulators of mitochondrial biogenesis and function, especially in skeletal muscle. Our laboratory previously generated mouse models with germline or inducible skeletal muscle-specific overexpression of SIRT1 (24–26), muscle-specific knockout of SIRT1 (19), or muscle-specific knockout of GCN5 (5). Interestingly, across these models we found no overt effects of modulating SIRT1 or GCN5 on metabolic or mitochondrial remodeling of skeletal muscle or skeletal muscle function (5, 19, 24–26). Both glucose homeostasis and skeletal muscle insulin action were comparable in SIRT1-overexpressing mice fed a standard or high-fat diet (24–26). To date, no studies have investigated the combined effects of altering SIRT1 and GCN5 activity in adult skeletal muscle. Thus, it is possible that the lack of phenotype in the ‘single’ transgenic mouse models is due to compensatory or opposing actions of the protein that is not modulated (i.e., SIRT1 or GCN5). To address this question, we generated a double-transgenic mouse model with inducible, skeletal muscle-specific overexpression of SIRT1 and knockout of GCN5. We hypothesized that by combined overexpression of SIRT1 and knockout GCN5 in skeletal muscle these mice would exhibit increased basal PGC-1α activity, leading to improved glucose homeostasis, enhanced skeletal muscle mitochondrial function, and improved exercise capacity.
MATERIALS AND METHODS
Generation of mouse model.
The double-transgenic mouse model was generated by crossing mice harboring loxP sites flanking both a stop element upstream of the Sirt1 gene (24) and exons 3–19 of Gcn5 (5) with mice carrying Cre recombinase under the control of a tamoxifen-inducible human α-skeletal actin promoter (Cre-iHSA) (16). For simplicity, we refer to this mouse model as dTG (i.e., flox/flox for both SIRT1OX and GCN5KO and Cre-iHSA positive), and their floxed but Cre-iHSA-negative littermates as wild-type (WT) controls. For all experiments, both dTG and WT mice were gavaged with tamoxifen (2 mg) at 5–6 wk of age for 5 consecutive days. Male mice were used for all experiments except for contractile function measurements, where both female and male mice were used. Mice were housed on a 12:12-h light-dark cycle and all experiments were conducted 4–7 wk after initiation of tamoxifen treatment. All experiments were approved and conducted in accordance with the Animal Care Program at the University of California, San Diego.
Tissue and blood collection and body composition.
Tissues and blood were collected from fasted (4 h) and anesthetized mice. Skeletal muscles (gastrocnemius, quadriceps, tibialis anterior, plantaris), heart, liver, and epididymal adipose tissue were rinsed in sterile saline, blotted dry, weighed, and frozen in liquid nitrogen. One tibialis anterior muscle was pinned on cork and frozen in liquid nitrogen-cooled isopentane for sectioning. Whole blood was collected with EDTA from the inferior vena cava and centrifuged at 5,000 g at 4°C for 5 min, and the plasma was isolated. All tissues and plasma were stored at −80°C for subsequent analysis. Body composition was analyzed by magnetic resonance imaging using an EchoMRI-100TM analyzer (EchoMRI Medical Systems, Houston, TX).
RNA extraction and RT-PCR.
Equal amounts of RNA (1 μg) were used for cDNA synthesis after being extracted from snap-frozen gastrocnemius muscle using TRIzol Reagent (ThermoFisher Scientific, Waltham, MA), as previously described (24). Semiquantitative real-time PCR analysis was performed using iTaq SYBR Green Master Mix (Bio-Rad) on a CFX384 touch real-time PCR system (Bio-Rad). The ΔΔCT method was used to calculate relative levels of gene expression; TATA-binding protein (Tbp) or RNA Polymerase II Subunit A (Polr2a) was used as a normalization control. All primers have been previously described (24).
Oral glucose tolerance test and ex vivo 2-deoxyglucose uptake.
The oral glucose tolerance test (OGTT; 4 g/kg) was conducted in fasted (4 h) mice as previously described (24). Area under curve was calculated using Prism 7 (GraphPad Software Inc., La Jolla, CA) with time = 0 as the baseline. Ex vivo muscle insulin sensitivity was measured by 2-deoxyglucose (2-DOG) uptake in fasted (4 h) mice, as previously described (24); an insulin concentration of 0.36 nM was used for insulin-treated muscles. The soleus muscles used for 2-DOG uptake measurements (i.e., 50 min without or with insulin stimulation) were used to assess phosphorylation (p) of Akt on Ser473 and Thr308 (p-AktS473, p-AktT308).
Plasma insulin concentrations.
Plasma insulin was analyzed using an ELISA kit (80-INSMS-E-01; ALPCO Diagnostics, Salem, NH) per the manufacturer’s instructions.
Histological analysis.
Fiber cross-sectional area was determined in tibialis anterior muscle by fluorescent wheat germ agglutinin staining to visualize the outline of the muscle fibers, as previously described (24).
Ex vivo muscle mechanics.
Twitch- and tetanic contractile function was assessed in the fifth toe muscle of the extensor digitorum longus muscle (EDL), as previously described (12, 19, 23), except that the contraction buffer was bubbled with 100% oxygen. Muscle force was normalized to the muscle physiological cross-sectional area.
High-resolution respirometry.
High-resolution respirometry was performed using an Oroboros O2K (Oroboros Instruments, Innsbruck, Austria) in permeabilized fibers from the plantaris muscle, as previously described (24). Briefly, respiratory data were collected using a mixed fatty acid and pyruvate oxidation substrate-uncoupler-inhibitor titration: (0.5 mM malate + 0.2 mM octanoylcarnitine (M+Octc), 2.5 mM ADP (ADP), 10 μM cytochrome c (CytC), 5 mM pyruvate (Pyr), 10 mM glutamate (Glut), 10 mM succinate (Succ), 1 μM carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), 0.5 μM rotenone (Rot), and 2.5 μM antimycin A (Ama), which were added sequentially and in the order presented.
Mitochondrial enzymatic activity assays.
Citrate synthase and complex IV activity were assessed in the gastrocnemius muscle, as previously described (12).
Voluntary wheel running (VWR) and run to exhaustion treadmill testing.
Eleven-week-old mice had unlimited access to a running wheel for 14 days, as previously described (5). After 14 days, the wheel was removed, and 24 h after wheel removal, tissues were excised from as described above. Distance run, time spent running, and average speed were recorded daily throughout the training at 1400 h.
Run to exhaustion treadmill testing.
Animals were acclimatized to running on an open treadmill [voluntary wheel running (VWR)] and ran to exhaustion, as previously described (23), with minor adjustments. The treadmill incline was set to a 15° incline, and mice began running at 10 m/min for 10 min, and the speed was increased 4 m/min every 10 min until reaching 22 m/min. At 60 min, the speed was increased 1 m/min every 5 min until exhaustion. The pre-VWR time point was performed 2 wk before mice started VWR training, and the post-VWR time point was conducted on day 14 of the VWR training. The experimenter was blinded to the genotype of the mice.
Immunoblotting.
Immunoblotting was conducted on 30 μg of protein, as previously described (24). After the transfer of proteins to nitrocellulose membranes, they were reversibly stained with Ponceau S solution [0.1% (wt/vol) Ponceau S in 5% acetic acid]. The following primary antibodies were used: Cell Signaling: GCN5 (no. 3305), eukaryotic elongation factor 2 (eEF2; no. 2332), GAPDH (no. D16H11), Akt (no. 2920), p-AktS473 (no. 4058), p-AktT308 (no. 9275); MitoSciences: ATP synthase-α (ATP5A), ubiquinol-cytochrome c reductase core protein-2 (UQCRC2), mitochondrially encoded cytochrome c oxidase I (MTCO1), succinate dehydrogenase subunit B (SDHB), NADH:ubiquinone oxidoreductase subunit B8 (NDUFB8; no. MS604); Abcam: very long-chain acyl-CoA dehydrogenase (ACADVL; no. ab155138), long-chain acyl-CoA dehydrogenase (ACADL; no. ab82853); Sigma: SIRT1 (no. S5196). Densitometric analysis of immunoblots was performed using Image Laboratory Software (Bio-Rad), and a representative image is presented in each figure. Protein abundance was normalized to eukaryotic elongation factor 2 (eEF2) or Ponceau S.
Statistics.
Data were analyzed using an unpaired Student’s t test or two-way ANOVA (using repeated measurements where appropriate), followed by Bonferroni’s post hoc test, with significant differences at P < 0.05. Statistical analyses were performed using Prism 7 (GraphPad Software, Inc., La Jolla, CA). All data are expressed as means ± SE.
RESULTS
Validation of the dTG mouse model.
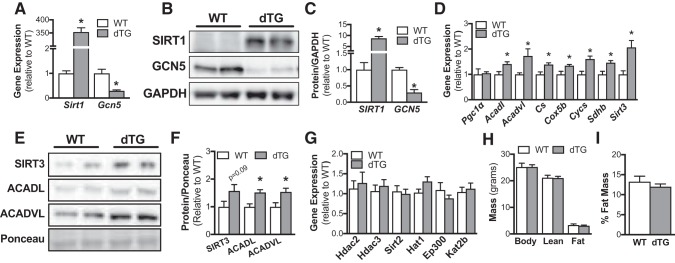
At 6 wk after initiating tamoxifen treatment, Sirt1 gene expression was increased ~350-fold and Gcn5 gene expression was reduced ~70% in skeletal muscle of dTG vs. WT mice (Fig. 1A). Similarly, SIRT1 protein abundance was elevated (~8-fold) and GCN5 protein abundance was reduced (~70%) in dTG vs. WT mice (Fig. 1, B and C). To assess PGC-1α activity in dTG mice, we measured mRNA expression of known PGC-1α target genes. Although PGC-1α mRNA levels were unchanged in dTG vs. WT mice, expression of a subset of PGC-1α target genes central to β-oxidation and mitochondrial function were significantly increased in skeletal muscle of dTG vs. WT mice (Fig. 1D). Additionally, protein abundance of SIRT3 (P = 0.09) ACADL, and ACADVL was ~50% increased in dTG vs. WT mice (Fig. 1, E and F). Since SIRT3 expression was elevated in dTG mice, we next assessed whether modulation of SIRT1 and GCN5 would alter expression of other abundant lysine acetyltransferases and deacetylases. On the basis of the relative expression data from the MuscleDB database (10) (Table 1), we assessed expressions of Hdac2, Hdac3, Sirt2, Hat1, Ep300, and Kat2b (Fig. 1G). There were no genotype differences in expression of these lysine acetyltransferases and deacetylases in WT and dTG mice.
Fig. 1.
Validation of mouse model. dTG, mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of sirtuin 1 (SIRT1) and knockout of general control of amino acid synthesis 5 (GCN5). A: transcript levels of Sirt1 and Gcn5 normalized to Tbp in wild-type (WT) and dTG skeletal muscle (n = 7/genotype). Representative blot (B) and quantification (C) of protein abundance of SIRT1 and GCN5 in skeletal muscle from WT and dTG mice. Bar graphs show protein abundance in skeletal muscle relative to GAPDH (n = 6/genotype). D: transcript levels of Pgc-1α, and its target genes Acadl, Acadvl, Cox5b, Cs, Cycs, Sdhb, and Sirt3 normalized to PolR2A in WT and dTG skeletal muscle (n = 7/genotype). Representative images (E) and quantification (F) of protein abundance of PGC-1α targets ACADVL, ACADL, and SIRT3 in skeletal muscle from WT and dTG mice. Bar graphs show protein abundance in skeletal muscle relative to Ponceau S (n = 6/genotype). G: transcript levels of Hdac2, Hdac3, Sirt2, Hat1, Ep300, and Kat2b normalized to Tbp in WT and dTG skeletal muscle (n = 7/genotype). H: body weight, lean muscle mass, and fat mass from dTG and WT mice (WT n = 4, dTG n = 7). I: fat mass expressed as percent body weight (WT n = 4, dTG n = 7). Data are reported as means ± SE. *P < 0.05 vs. WT (A–I: unpaired Student’s t test. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-α; ACADL, acyl-CoA dehydrogenase long chain; ACADVL, acyl-CoA dehydrogenase very long chain.
Table 1.
Expression of lysine acetyltransferases and deacetylases in mouse skeletal muscle
| Gene Name | Protein | EDL | GA | PLA | QUAD | SOL |
|---|---|---|---|---|---|---|
| Lysine deacetylases (KDACs) | ||||||
| Hdac1 | HDAC1 | 7.4 | 5.2 | 6.2 | 4.1 | 7.9 |
| Hdac2 | HDAC2 | 38.9 | 62.0 | 41.9 | 58.8 | 41.3 |
| Hdac3 | HDAC3 | 37.6 | 33.3 | 33.1 | 27.2 | 30.1 |
| Hdac4 | HDAC4 | 11.0 | 3.6 | 5.5 | 4.0 | 4.7 |
| Hdac5 | HDAC5 | 22.7 | 5.3 | 13.0 | 4.2 | 20.8 |
| Hdac6 | HDAC6 | 2.7 | 1.2 | 2.0 | 0.9 | 2.4 |
| Hdac7 | HDAC7 | 9.5 | 2.5 | 4.5 | 1.9 | 8.9 |
| Hdac8 | HDAC8 | 3.5 | 2.5 | 3.1 | 2.5 | 2.6 |
| Hdac9 | HDAC9 | 2.5 | 2.4 | 2.4 | 2.5 | 4.7 |
| Hdac10 | HDAC10 | 5.4 | 1.6 | 2.6 | 1.2 | 2.8 |
| Hdac11 | HDAC11 | 4.8 | 1.2 | 2.1 | 1.0 | 2.3 |
| Sirt1 | SIRT1 | 4.2 | 5.6 | 4.7 | 5.5 | 5.3 |
| Sirt2 | SIRT2 | 58.1 | 23.7 | 33.5 | 19.8 | 44.3 |
| Sirt3 | SIRT3 | 20.2 | 5.3 | 12.4 | 4.2 | 18.4 |
| Sirt4 | SIRT4 | 13.0 | 5.1 | 8.4 | 4.9 | 12.8 |
| Sirt5 | SIRT5 | 7.4 | 4.7 | 6.3 | 3.9 | 10.4 |
| Sirt6 | SIRT6 | 1.8 | 0.2 | 0.7 | 0.2 | 1.1 |
| Sirt7 | SIRT7 | 3.6 | 1.2 | 1.8 | 1.0 | 3.4 |
| Lysine acetyltransferases (KATs) | ||||||
| Atat1 | ATAT1 | 2.4 | 1.7 | 1.9 | 1.2 | 2.7 |
| Clock | CLOCK | 6.6 | 8.0 | 7.3 | 8.0 | 10.2 |
| Crebbp | CBP | 6.0 | 7.0 | 8.2 | 6.2 | 7.8 |
| Ep300 | p300 | 17.4 | 20.2 | 20.1 | 17.7 | 20.2 |
| Esco1 | ESCO1 | 6.3 | 9.1 | 6.8 | 10.2 | 7.6 |
| Esco2 | ESCO2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| Hat1 | HAT1 | 12.7 | 17.2 | 15.4 | 17.5 | 17.5 |
| Kat14 | CSRP2BP | 10.9 | 6.1 | 8.4 | 5.7 | 14.5 |
| Kat2a | GCN5 | 7.5 | 2.2 | 4.2 | 1.8 | 4.6 |
| Kat2b | PCAF | 50.2 | 73.3 | 53.9 | 83.1 | 59.2 |
| Kat5 | TIP60 | 7.4 | 4.6 | 6.8 | 4.0 | 7.1 |
| Kat6a | MOZ | 8.2 | 6.5 | 8.0 | 5.4 | 7.6 |
| Kat6b | MORF | 5.2 | 3.7 | 5.5 | 2.8 | 7.8 |
| Kat7 | HBO1 | 11.9 | 8.9 | 11.4 | 7.6 | 13.2 |
| Kat8 | MOF | 16.8 | 10.4 | 14.2 | 9.0 | 13.1 |
| Mcm3ap | MCM3AP | 11.2 | 7.4 | 10.1 | 6.3 | 10.0 |
| Naa60 | NAA60 | 23.1 | 16.4 | 17.0 | 15.5 | 16.6 |
| Taf1 | TAF1 | 5.1 | 5.7 | 5.5 | 5.6 | 7.5 |
Relative expression [fragments per kilobase of exon model per million reads mapped (FKPM)] of major lysine acetyltransferases and deacetylases in major muscle groups, from the MuscleDB database. EDL, extensor digitorum longus; GA, gastrocnemius; PLA, plantaris; Q, quadriceps; SOL, soleus.
Body composition and tissue weights were unchanged in dTG vs. WT mice.
There were no genotype differences in either body mass, body composition (lean and fat mass), percent fat mass (Fig. 1, H and I), or epididymal fat pad, skeletal muscle, liver, or heart masses (Table 2) between dTG and WT mice.
Table 2.
Tissue masses
| WT | dTG | |
|---|---|---|
| Gastrocnemius | 110 ± 6 | 101 ± 5 |
| Tibialis anterior | 48 ± 2 | 47 ± 2 |
| Quadriceps | 168 ± 9 | 167 ± 10 |
| Heart | 124 ± 5 | 112 ± 6 |
| Liver | 1,342 ± 89 | 1,317 ± 99 |
| Epididymal fat | 428 ± 49 | 442 ± 93 |
Data are means ± SE to the nearest milligram. Wild type (WT), n = 9, mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of sirtuin 1 and knockout of general control of amino acid synthesis 5 (dTG), n = 8.
Skeletal muscle insulin sensitivity was comparable in dTG and WT mice.
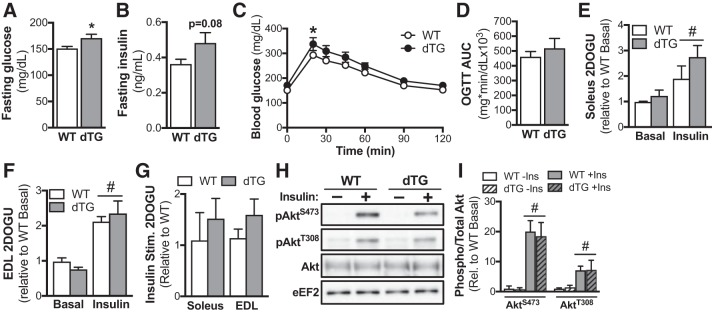
Fasted blood glucose concentration was modestly (~10%) elevated in dTG mice compared with WT littermates (Fig. 2A), and there was a trend (P = 0.08) toward higher fasted plasma insulin concentration in dTG mice (Fig. 2B). There were no genotype differences in blood glucose concentration during an OGTT, apart from slightly higher blood glucose in dTG mice compared with WT mice at 20 min post-glucose gavage (Fig. 2C). However, there were no genotype differences for area under the curve for glucose concentrations during the OGTT (Fig. 2D). We next assessed skeletal muscle insulin sensitivity in isolated soleus and EDL muscles. Although there was a main effect for insulin to significantly increased 2-DOG uptake in both EDL and soleus, there were no genotype differences in the insulin response (Figs. 2, E and F); basal glucose uptake was not different between WT and dTG mice. Furthermore, insulin-stimulated 2-DOG uptake (i.e., insulin 2-DOG uptake minus basal 2-DOG uptake) was not different between genotypes in either muscle (Fig. 2G). In line with the 2-DOG findings, there was a main effect of insulin to significantly increase the phosphorylation of AktS473 and AktT308, but there were no genotype differences (Fig. 2, H and I).
Fig. 2.
Unaltered glucose homeostasis in mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of sirtuin 1 (SIRT1) and knockout of general control of amino acid synthesis 5 (GCN5) (dTG). Blood glucose (A) and plasma insulin (B) measured after a 4-h fast (A, WT n = 16, dTG n = 10; B, WT n = 6, dTG n = 5). Blood glucose concentrations (C) and area under curve (D) of WT and dTG mice during an oral glucose tolerance test (OGTT; WT n = 16, dTG n = 10). Basal and insulin-stimulated (0.36 nmol/L) 2-deoxy-glucose uptake (2DOGU) in soleus (E) and extensor digitorum longus (EDL; F) muscles from WT and dTG mice (n = 7/genotype). G: insulin-stimulated (Insulin Stim.) 2DOGU (calculated as insulin 2DOGU – basal 2DOGU) in soleus and EDL muscles from WT and dTG mice (n = 7/genotype). H: representative blot of phospho-AktS473 (pAktS473), phospho-AktT308 (pAktT308), and total Akt in basal (−) and insulin-stimulated (+) soleus muscle from WT and dTG mice. I: quantification of pAktS473 and pAktT308 vs. total Akt in basal and insulin-stimulated soleus muscle from WT and dTG mice (n = 4/group). Data are reported as means ± SE. *P < 0.05 vs. WT; #P < 0.05 main effect of insulin. (A, B, and D, unpaired Student’s t test. C: repeated-measures (Time) 2-way ANOVA with Bonferroni’s multiple comparison test. E–I: repeated measures (Basal/Insulin) 2-way ANOVA with Bonferroni’s multiple comparison test.
Mitochondrial function is unchanged in dTG mice.
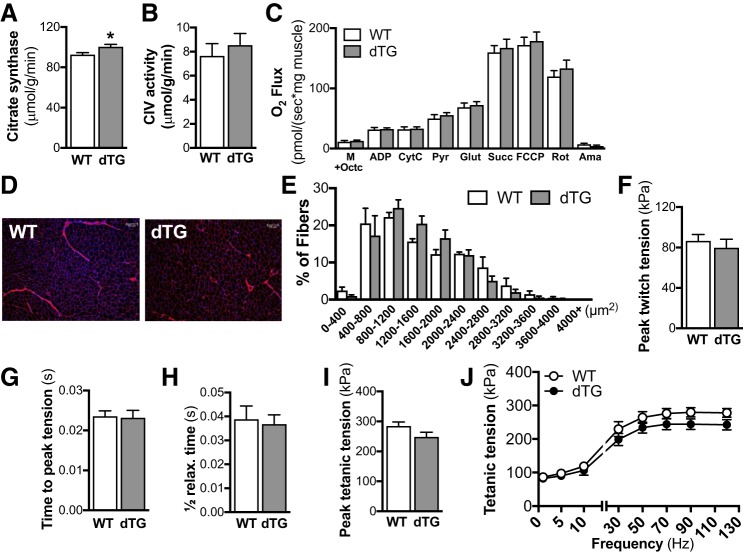
To investigate whether the increased gene expression of mitochondrial proteins (Fig. 1D) translated into functional improvements in skeletal muscle mitochondrial function, we assessed citrate synthase and complex IV activity. There was a modest (~10%) increase in citrate synthase activity in dTG vs. WT muscle (Fig. 3A), but complex IV activity was unchanged (Fig. 3B). To determine whether these differences between dTG and WT mice altered maximal respiration of skeletal muscle, we assessed mitochondrial function in permeabilized skeletal muscle fiber bundles by use of high-resolution respirometry. However, there were no differences between genotypes in skeletal muscle maximal respiratory capacity (Fig. 3C).
Fig. 3.
Unchanged muscle morphology or function in mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of sirtuin 1 (SIRT1) and knockout of general control of amino acid synthesis 5 (GCN5) (dTG). Citrate synthase (A) and complex IV (CIV; B) activity in wild-type (WT) and dTG muscle (n = 7/genotype). C: respiratory flux normalized to muscle fiber weight in the presence of malate + octanoylcarnitine (M+Octc; leak respiration in the absence of adenylates), ADP, cytochrome c (CytC; mitochondrial integrity), pyruvate (Pyr), glutamate [Glut; complex I (CI) capacity], succinate [Succ; complex I + complex II (CII) capacity], carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP; uncoupled maximal respiration), rotenone (Rot; complex II capacity), and antimycin A (Ama; residual oxygen consumption) (WT n = 10, dTG n = 7). D: representative images of wheat germ agglutinin-stained tibialis anterior muscle cross sections. E: quantification of fiber cross-sectional area, presented as %fibers within each size-range (n = 4/genotype). Peak twitch tension (F), time to peak tension (G), and half-relaxation time for a twitch contraction (H) in the 5th toe (EDL) muscle from dTG and WT mice (n = 7–8/genotype). Peak tetanic tension (I) and force-frequency curve (J) in the 5th toe EDL muscle from dTG and WT mice (n = 7–8/genotype). Data are reported as means ± SE. *P < 0.05 vs. WT. A, B, F–I: unpaired Student’s t test; C, E, J: 2-way ANOVA with Bonferroni’s multiple comparison test.
Unaltered fiber size and contractile function in dTG vs. WT mice.
We next investigated whether muscle morphology or contractile function was affected in dTG mice. Skeletal muscle fiber cross-sectional area was unchanged between dTG and WT mice (Fig. 3, D and E). Ex vivo contractile function was also unchanged between dTG and WT mice, as were peak twitch tension (Fig. 3F), time to peak tension (Fig. 3G), and half-relaxation time (Fig. 3H) for twitch contractions. Peak tetanic tension (Fig. 3I) and tetanic tension across various stimulation frequencies (Fig. 3J) were also unaltered between WT and dTG mice.
Unaltered exercise capacity in dTG and WT mice.
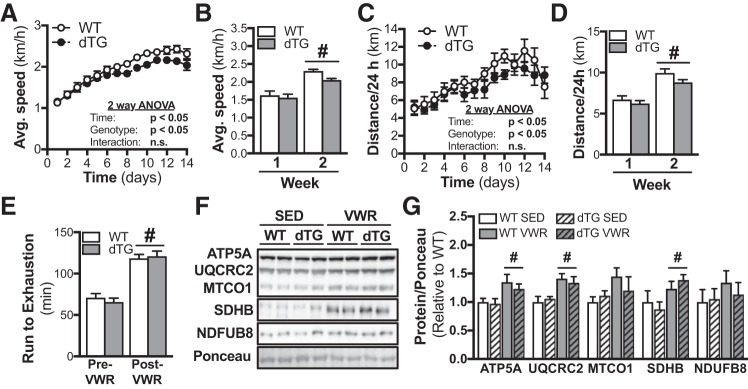
To investigate how dTG and WT mice adapt to exercise training, all mice were given free access to running wheels for 14 days. Whereas both average speed (Fig. 4, A and B) and distance run (Fig. 4, C and D) were increased during the second compared with the first week of wheel running, there were no genotype differences for either time point (Fig. 4, A–D). Exercise performance was assessed by a run-to-exhaustion treadmill test before and after the wheel running intervention. VWR increased run-to-exhaustion time to the same extent (~70%) in both dTG and WT mice (Fig. 4E). Assessing mitochondrial protein content, there was a main effect for VWR to significantly increase the protein abundance of the mitochondrial electron transport chain proteins ATP5A, cytochrome b-c1 complex subunit 2 (UQCRC2), and SDHB (Fig. 4, F and G).
Fig. 4.
Unaltered exercise adaptation in mice with combined and tamoxifen-inducible skeletal muscle-specific overexpression of sirtuin 1 (SIRT1) and knockout of general control of amino acid synthesis 5 (GCN5) (dTG). Wild-type (WT) and dTG mice were given free access to running wheels in their home cages for 14 days. Average 24-h running speed over 14 days (A) and split into average per week (B) during voluntary wheel running (VWR) in WT and dTG mice (WT n = 7, dTG n = 8);). #P < 0.05 vs. week 1. Average 24-h running distance over 14 days (C) and split into average per week (D) during VWR in WT and dTG mice (WT n = 7, dTG n = 8). #P < 0.05 vs. week 1. E: run-to-exhaustion treadmill test was performed 14 days before VWR training (pre-VWR), and at day 14 of VWR training (post-VWR) (WT n = 7, dTG n = 8). #P < 0.05 vs. Pre-VWR. Representative images (F) and quantification of protein abundances (G) of ATP synthase-α (ATP5A), ubiquinol-cytochrome c reductase core protein-2 (UQCRC2), mitochondrially encoded cytochrome c oxidase I (MTCO1), succinate dehydrogenase subunit B (SDHB), and NADH:ubiquinone oxidoreductase subunit B8 (NDUFB8). Bar graphs show protein abundance in skeletal muscle relative to Ponceau S (n = 6/group). #P < 0.05 main effect of VWR. Data are reported as means ± SE. A–D: repeated-measures (Time) 2-way ANOVA with Bonferroni’s multiple comparison test. E: repeated measures [Genotype and Time (Pre/Post-VWR)] 2-way ANOVA with Bonferroni’s multiple comparison test. G: 2-way ANOVA with Bonferroni’s multiple comparison test.
DISCUSSION
SIRT1 and GCN5 regulate acetylation status and activity of PGC-1α in an opposing manner (6, 8, 21) and are in extension proposed as important regulators of skeletal muscle metabolism and mitochondrial function (6, 8). However, studies on muscle-specific modulation of either SIRT1 or GCN5 in mouse (5, 19, 24–26) or rat (2, 9) models do not support this perspective. In our present study, to better understand the combined contribution of GCN5 and SIRT1 to skeletal muscle metabolism and physiology, we generated a mouse with combined, inducible, and skeletal muscle-specific overexpression of SIRT1 and knockout of GCN5. Despite increased transcription of PGC-1α target genes, we found no differences in glucose homeostasis, mitochondrial function, exercise capacity, or metabolic adaptations to exercise training in dTG compared with WT mice. In agreement with previous work (5, 22, 24–26), these data demonstrate that SIRT1 and GCN5 are not primary mediators of skeletal muscle metabolic or mitochondrial function.
PGC-1α is a key regulator of mitochondrial gene transcription (27). In mice with whole body overexpression of SIRT1, PGC-1α expression is increased, as well as mitochondrial gene transcription and state 3 mitochondrial respiration in skeletal muscle (6). In line with this, the increased mRNA expression of established PGC-1α target genes in our dTG mice would suggest that PGC-1α activity is increased. However, this did not translate into higher mitochondrial protein content or increased mitochondrial function. For example, citrate synthase gene expression was ~40% higher in dTG vs. WT mice, whereas citrate synthase activity was only modestly increased. Furthermore, this did not noticeably affect the physiological function of skeletal muscle or exercise performance in dTG mice.
Thus, these results affirm past results from our laboratory (5, 24), as well as others’ (2, 9), demonstrating that simply changing SIRT1 and/or GCN5 activity are not sufficient to induce adaptive changes in mitochondrial function in adult mouse skeletal muscle. We interpret this finding to suggest that additional signal(s) must be necessary to coordinate, and ultimately lead to, functional changes in skeletal muscle physiology and metabolism.
Cell- or mouse-based studies have demonstrated that GCN5 and SIRT1 play important opposing roles in the regulation of glucose homeostasis and insulin sensitivity (4, 8, 13). The role of GCN5 is evident, as overexpression of GCN5 in primary skeletal muscle myotubes decreases insulin-mediated glucose uptake (11). In the present study, however, combined overexpression of SIRT1 and knockout of GCN5 did not lead to any differences in glucose tolerance or skeletal muscle insulin sensitivity compared with WT littermates. This is in line with our previous studies in SIRT1-transgenic mice, which found that skeletal muscle-specific overexpression of SIRT1 did not enhance insulin sensitivity in chow-fed mice (24–26); this overexpression also did not protect mice from high-fat diet-induced impairments in insulin sensitivity (26). This is further supported by the fact that overexpression of SIRT1 in rat skeletal muscle does not enhance insulin sensitivity or protect from glucose-induced insulin resistance (2). Importantly, previous studies have demonstrated that modulation of SIRT1 in other organs, such as the liver (14), brain, and/or pancreatic β-cells (18), can impact glucose tolerance in both aged and high-fat diet-fed mice. Additionally, overexpression of GCN5 in mouse liver decreases the gluconeogenic capacity of the liver during a pyruvate tolerance test (13). Thus, although modulating SIRT1 or GCN5 in other metabolic tissues manifests metabolic adaptations, it is clear that SIRT1 and GCN5 are not primary regulators of skeletal muscle insulin sensitivity in vivo.
Exercise training induces mitochondrial biogenesis and enhances endurance running capacity (1). To this end, we investigated whether the adaptive response to exercise training would be augmented in dTG mice. We found that, although the VWR intervention induced mitochondrial biogenesis and increased treadmill endurance capacity ~70%, this adaptation was not further enhanced in dTG mice. This finding is similar to that of previous work demonstrating that mice with muscle-specific knockout of GCN5 (5) or SIRT1 (17, 19) are comparable to their WT littermates in terms of exercise adaptation. Taken together, these data demonstrate that skeletal muscle SIRT1 and GCN5 activity are not primary mediators of exercise capacity or the adaptive response to endurance exercise in mice.
In conclusion, we find that combined overexpression of SIRT1 and knockout of GCN5 in adult mouse skeletal muscle does not enhance glucose homeostasis, mitochondrial biogenesis, exercise capacity, or exercise training-induced metabolic adaptations. Our results support previous studies that found no effect of individual manipulation of skeletal muscle SIRT1 activity or GCN5 activity on in vivo metabolic and mitochondrial adaptations (5, 24–26). Taken together, this work clearly demonstrates that SIRT1 and/or GCN5 activity in skeletal muscle are not important mediators of skeletal muscle metabolism or mitochondrial biology.
GRANTS
This work was supported, in part, by US National Institutes of Health Grants R01 AG-043120 and R21 AR-069775 (to S. Schenk), T32 AR-060712 and F30 DK-115035 (to V. F. Martins), and R01 DK-095926 (to C. E. McCurdy), a UC San Diego Frontiers of Innovation Scholars Program grant (to S. Schenk), Graduate Student Research Support from the UC San Diego Institute of Engineering in Medicine and the Office of Graduate Studies (to V. F. Martins), and postdoctoral fellowships from the Swiss National Science Foundation and the American Federation of Aging Research (to K. Svensson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S., S.T., C.E.M., and S.S. conceived and designed research; K.S., S.T., V.F.M., J.R.D., A.L., N.B., and K.G. performed experiments; K.S., S.T., V.F.M., J.R.D., A.L., N.B., K.G., and C.E.M. analyzed data; K.S., S.T., V.F.M., J.R.D., A.L., N.B., K.G., C.E.M., and S.S. interpreted results of experiments; K.S. and S.T. prepared figures; K.S. and S.T. drafted manuscript; K.S., S.T., C.E.M., and S.S. edited and revised manuscript; K.S., S.T., V.F.M., J.R.D., A.L., N.B., K.G., C.E.M., and S.S. approved final version of manuscript.
REFERENCES
- 1.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 2.Brandon AE, Tid-Ang J, Wright LE, Stuart E, Suryana E, Bentley N, Turner N, Cooney GJ, Ruderman NB, Kraegen EW. Overexpression of SIRT1 in rat skeletal muscle does not alter glucose induced insulin resistance. PLoS One 10: e0121959, 2015. doi: 10.1371/journal.pone.0121959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol 8: 287–296, 2012. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- 5.Dent JR, Martins VF, Svensson K, LaBarge SA, Schlenk NC, Esparza MC, Buckner EH, Meyer GA, Hamilton DL, Schenk S, Philp A. Muscle-specific knockout of general control of amino acid synthesis 5 (GCN5) does not enhance basal or endurance exercise-induced mitochondrial adaptation. Mol Metab 6: 1574–1584, 2017. doi: 10.1016/j.molmet.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominy JE Jr, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim Biophys Acta 1804: 1676–1683, 2010. doi: 10.1016/j.bbapap.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347–358, 2008. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim S-H, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol 587: 1817–1828, 2009. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes LD, Lewis SA, Hughes ME. ExpressionDB: an open source platform for distributing genome-scale datasets. PLoS One 12: e0187457, 2017. doi: 10.1371/journal.pone.0187457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1β through lysine acetylation. J Biol Chem 284: 19945–19952, 2009. doi: 10.1074/jbc.M109.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaBarge SA, Migdal CW, Buckner EH, Okuno H, Gertsman I, Stocks B, Barshop BA, Nalbandian SR, Philp A, McCurdy CE, Schenk S. p300 is not required for metabolic adaptation to endurance exercise training. FASEB J 30: 1623–1633, 2016. doi: 10.1096/fj.15-281741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab 3: 429–438, 2006. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25: 1664–1679, 2011. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Reports 2: 419–431, 2012. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2: 8, 2012. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem 288: 6968–6979, 2013. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2: 105–117, 2005. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem 286: 30561–30570, 2011. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philp A, Rowland T, Perez-Schindler J, Schenk S. Understanding the acetylome: translating targeted proteomics into meaningful physiology. Am J Physiol Cell Physiol 307: C763–C773, 2014. doi: 10.1152/ajpcell.00399.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434: 113–118, 2005. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 22.Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson K, Dent JR, Tahvilian S, Martins VF, Sathe A, Ochala J, Patel MS, Schenk S. Defining the contribution of skeletal muscle pyruvate dehydrogenase-α1 to exercise performance and insulin action. Am J Physiol Endocrinol Metab 315: E1034–E1045, 2018. doi: 10.1152/ajpendo.00241.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svensson K, LaBarge SA, Martins VF, Schenk S. Temporal overexpression of SIRT1 in skeletal muscle of adult mice does not improve insulin sensitivity or markers of mitochondrial biogenesis. Acta Physiol (Oxf) 221: 193–203, 2017. doi: 10.1111/apha.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White AT, McCurdy CE, Philp A, Hamilton DL, Johnson CD, Schenk S. Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia 56: 1629–1637, 2013. doi: 10.1007/s00125-013-2912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AT, Philp A, Fridolfsson HN, Schilling JM, Murphy AN, Hamilton DL, McCurdy CE, Patel HH, Schenk S. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab 307: E764–E772, 2014. doi: 10.1152/ajpendo.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]