Abstract
Glucagon-like peptide-1 (GLP-1) is an enteral peptide that contributes to the incretin effect. GLP-1 action is typically described as endocrine, but this mechanism has been questioned because rapid inactivation in the circulation by dipeptidylpeptidase 4 (DPP4) results in a short half-life, limiting the amount of the hormone that can reach the pancreatic islet. An alternative mechanism for GLP-1 to regulate insulin secretion through neuroendocrine signaling originating from sensors in the portal vein has been proposed. We hypothesized that portal infusion of GLP-1 would cause greater glucose-stimulated insulin secretion than equimolar administration into the jugular vein. To test this, hyperglycemic clamps with superimposed graded infusions of GLP-1 into the jugular or portal veins of male rats were performed. These experiments were repeated with pharmacologic DPP4 inhibition to determine the effect of GLP-1 metabolism in the jugular and portal venous beds. Contrary to our hypothesis, we found a higher insulinotropic effect with jugular compared with portal GLP-1, which was associated with higher plasma concentrations of intact GLP-1. The greater insulinotropic effect of jugular venous GLP-1 persisted even with pharmacological DPP4 inhibition. These findings do not support an important role of portal vein GLP-1 signaling for the incretin effect but highlight the hepatoportal bed as a major site of GLP-1 degradation that persists even with pharmacological inhibition. Together, these results support rapid inactivation of enterally released GLP-1 in the liver as limiting endocrine actions on the β-cell and raise questions about the conventional endocrine model of pharmacologic effects of DPP4 inhibitors.
Keywords: DPP4, glucagon-like peptide-1, incretins, insulin secretion, portal vein
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is a physiological incretin in humans and other mammalian species (7). Unlike the other known incretin glucose-dependent insulinotropic polypeptide, GLP-1 retains some of its insulinotropic effect in patients with type 2 diabetes (27). Hence, the glucose-lowering actions of GLP-1 have led to the development of incretin-based therapies for type 2 diabetes, namely GLP-1 receptor (GLP-1r) agonists and dipeptidylpeptidase 4 (DPP4) inhibitors (12).
The conventional model of GLP-1 action is endocrine, with mediation of effects on target tissues through the circulation. However, GLP-1 undergoes rapid cleavage by the ubiquitous endovascular enzyme DPP4 (24) that inactivates its insulinotropic activity (36). Metabolism by DPP4 results in a half-life of GLP-1 in the circulation of 60–90 s, and plasma concentrations of the active peptide are very low relative to glucose-dependent insulinotropic polypeptide (8) even after stimulation by meals (10, 11). The narrow range of plasma GLP-1 concentrations is contrasted by its wide dynamic range of action. In healthy humans, GLP-1 infusion reaching supraphysiologic concentrations five- to sixfold higher than postprandial levels causes an almost exponential insulinotropic effect (3). In addition, experimental data suggest that infusion of GLP-1 at a dose mimicking postprandial levels has only minimal effects to stimulate insulin secretion in a canine model (19), and a similar experiment in humans also had equivocal results (26).
The rapid metabolism of GLP-1 by DPP4 and low plasma concentrations that may be sub-stimulatory has led to a questioning of an endocrine mode of action (8, 16, 17). Several studies have proposed that a neuroendocrine signal originating in the hepatoportal region is responsible for mediating the glucose-lowering actions of GLP-1, as this venous bed is exposed to the highest GLP-1 concentrations in the circulation (4, 5, 25). We have previously reported that the GLP-1r is expressed in afferent neurons in the portal vein, and local antagonism of the GLP-1r in this region impaired glucose tolerance in rats (37). However, few studies published so far have directly compared the insulinotropic effect of portal GLP-1 to systemic administration of the peptide. In this paper, we report experiments to test two hypotheses. First, we hypothesized that portal infusion would cause greater insulin secretion than an equimolar dose of GLP-1 infused into the jugular vein. As a corollary to this hypothesis, we proposed that differences in the insulinotropic effect of portal and jugular venous GLP-1 are due to differential metabolism by DPP4.
MATERIALS AND METHODS
Animals and placement of catheters.
Experiments were performed on male Long-Evans rats with a mean body weight of 270–300 g, purchased from Harlan Laboratories Inc. (Indianapolis, IN). We have previously demonstrated expression of the GLP-1r on nerve endings in the portal vein of this strain of rat (37). The animals had ad libitum access to food and water and were fed a pelleted chow diet (Teklad; Harlan, Madison, WI). They were housed in individual cages in a vivarium with constant temperature (22°C) and were on a 12/12-h light/dark cycle. All experiments were approved by the University of Cincinnati Internal Animal Care and Use Committee and carried out in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities conforming to National Institutes of Health and U.S. Department of Agriculture regulations.
Beginning 1 wk after arrival at our facility, rats had surgery to implant vascular catheters. Polyethylene tubing (Instech Solomon, Plymouth Meeting, PA) was used for carotid catheters and silicone tubing (Braintree Scientific Inc., Braintree, MA) for cannulation of the portal and jugular veins.
Rats were anesthetized with standard isoflurane inhalation (99% Iso/mL, Abbott Laboratories, North Chicago, IL); shaved over the neck, abdomen, and back; and carotid and jugular catheters were placed as previously described (35). The portal vein was not clamped or obstructed during catheterization to avoid damage to the vessel and the surrounding nervous plexus. All three catheters were tunneled subcutaneously to the back and externalized between the shoulder blades. Catheters were flushed with heparinized saline and closed with steel rods until use.
During postsurgical recovery (8–14 days) rats were weighed and monitored daily until they reached their presurgical body weight. During that time, they were handled on a daily basis to habituate them to contact by investigators. Experiments were performed only on fully recovered, healthy rats.
Clamp experiments.
Before the clamps, rats were fasted overnight having free access to water. After weighing, the animals were brought to a room to which they had been habituated and where they were able to readjust to the new environment for >30 min. All catheters were connected and flushed with heparinized saline. The clamp procedure was only started if all 3 catheters would allow both injection and blood withdrawal. During experiments, the rats were conscious and freely moving.
Syringe pumps (Harvard Apparatus, Holliston, MA) for the variable infusion of glucose and GLP-1 were connected to the jugular and portal catheters through a 2-channel Swivel (Instech Solomon). The GLP-1(7–36)amide infusion was prepared from a frozen stock solution with 2.5 μg/mL (Bachem, Torrance, CA) stored at −20°C and 200 μL of blood from the respective rat for protein coating of the large plastic surfaces. In previous studies, we did not observe degradation of GLP-1 when prepared in this fashion.
After removal of fasting samples (−10, 0 min) a bolus infusion of glucose (D25% Baxter, Deerfield, IL) was started into the jugular vein to create a square wave of hyperglycemia. Blood glucose was monitored every 5 min taking samples from the carotid catheter, and a variable glucose infusion was adjusted by ad hoc algorithm to maintain constant hyperglycemia of 100 mg/dL over basal. Constant hyperglycemia was maintained for a total of 120 min. In addition, a constant infusion of 4 mg·kg−1·min−1 glucose was given into the portal vein to maintain steady glycemia and comparable activation of glucose sensors (13, 18) in the hepatoportal bed to all animals.
After 60 min of constant hyperglycemia, a graded GLP-1 infusion was given into either the portal or jugular vein. The three infusion doses were 1.5 μg·kg−1·h−1 (60–80 min), 2.5 μg·kg−1·h−1 (80–100 min), and 5 μg·kg−1·h−1 (100–120 min). These doses were based on previous dose-finding experiments in our laboratory (data not shown).
Blood samples for measurement of insulin (0.3 mL) were taken at 0, 10, 30, 40, 50, 55, and 60 min during the first hour of the clamp. After starting the GLP-1 infusion, additional samples were taken at 70, 75, 80, 90, 95, 100, 110, 115, and 120 min (3 samples for each GLP-1 dose). Plasma was immediately obtained by spinning the samples for 2 min at 6,000 revolutions/min in a mini centrifuge (Research Products International Corporation, Mount Prospect, IL) and stored on ice. To avoid progressive anemia throughout the clamp, the red blood cells were resuspended with saline 0.9% and reinfused after the next blood draw.
Clamp experiments with DPP4 inhibition.
To test the hypothesis that differences in GLP-1 activation of insulin secretion was accounted for by differential DPP4 activity in the portal and systemic circulation, identical clamp experiments were performed after administration of a DPP4 inhibitor. Rats were given 10 mg vildagliptin (kindly provided by Dr. Bryan Burkey of Novartis, Cambridge, MA) suspended in 1 mL of saline intraperitoneally 60 min before the clamp.
Arterial plasma concentrations of active GLP-1(7–36) after site-specific infusion.
Because of the limited amount of blood available during the clamp experiments a separate cohort was used to measure arterial plasma concentrations of active GLP-1 during the infusions. After a baseline sample, GLP-1 was infused either into the portal or jugular vein. An infusion of GLP-1(7–36)amide of 2.5 μg·kg−1·h−1 was given for 20 min (0–20 min) followed by a rate of 5 μg·kg−1·h−1 (20–40 min). One milliliter of blood was taken at 0, 20, and 40 min of the experiment and immediately placed in chilled Eppendorf tubes prepared with a proteinase-inhibiting cocktail [100 μL per tube, EDTA (0.5 M), heparin (800 U/mL), aprotinin (0.28 mM), and diprotin A (0.066 mM)] to avoid peptide degradation. Tubes were kept on ice until the end of the experiment and then immediately spun. Plasma samples were stored at −80°C until they were assayed.
Analytical methods.
Glucose was measured in duplicate with a commercial bedside glucometer (Freestyle Flash, Abbott Diabetes Care, Alameda, CA). Insulin assays were performed using a commercially available RIA (Millipore Corporation, Billerica, MA, cat. no. HI-14K) following the manufacturer’s instructions except the use of our own specific rabbit insulin antibody, as previously described (2). GLP-1(7–36) plasma concentrations were measured using a commercially available ELISA for active GLP-1 (Millipore Corporation, cat. no. EGLP-35K) according to the manufacturer’s instructions.
Statistical analysis.
We designed the study to detect 30% lower insulin secretion with jugular compared with portal GLP-1 infusion. Based on preliminary studies with GLP-1 infusion into rats showing a standard deviation of 25% in the insulin responses, we estimated sample sizes of 10 per group with an alpha of 0.05 and 80% power. Comparison of the cohorts and the parameters of the hyperglycemic clamps were done by a Student’s t test for unpaired samples with normal variance (Table 1). The effects on hyperglycemia, glucose infusion rate, and insulin concentrations during the hyperglycemic clamp in response to the dose of GLP-1 and infusion site (portal versus jugular) were compared by two-way ANOVA for repeated measures. If there was a significant effect of the infusion site, Bonferroni post tests were performed to compare the effect of portal vein versus jugular vein infusion. A P value of <0.05 was considered statistically significant. The results are expressed as mean ± standard error (SE) for the different cohorts. Analysis and graph plotting was done using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
Table 1.
Baseline and clamp characteristics
| Portal Vein GLP-1 (n = 10) | Jugular Vein GLP-1 (n = 10) | P Value | |
|---|---|---|---|
| Body weight, g | 315.1 ± 5.9 | 314.8 ± 5.2 | 0.97 |
| Fasting glucose, mg/dL | 97.6 ± 4.9 | 95.9 ± 2.9 | 0.76 |
| Clamp glucose (average), mg/dL | 212.1 ± 3.5 | 206.3 ± 2.5 | 0.19 |
| Glucose over basal, mg/dL | 114.5 ± 6.0 | 110.4 ± 2.7 | 0.54 |
| CV: Clamp, % | 8.7 ± 0.6 | 8.8 ± 0.5 | 0.96 |
| With DPP4 Inhibition (Vildagliptin) | |||
|---|---|---|---|
| Portal Vein GLP-1 (n = 9) | Jugular Vein GLP-1 (n = 12) | P Value | |
| Body weight, g | 335.2 ± 5.8 | 319.7 ± 8.4 | 0.17 |
| Fasting glucose, mg/dL | 99.9 ± 4.8 | 98.1 ± 2.7 | 0.72 |
| Clamp glucose (average), mg/dL | 201.2 ± 1.4 | 202.7 ± 1.1 | 0.38 |
| Glucose over basal, mg/dL | 101.2 ± 1.4 | 104.6 ± 2.4 | 0.49 |
| CV: Clamp, % | 8.6 ± 0.7 | 9.4 ± 0.7 | 0.38 |
Mean ± SE for cohorts undergoing the clamp procedure; n is number of animals per group. Differences between the animals receiving portal vs. jugular vein infusion of GLP-1 were compared using a two-sided t test for unpaired cohorts with equal variances. P < 0.05 was considered statistically significant. None of the parameters differed significantly between portal and jugular vein GLP-1 infusion. CV, coefficient of variation; DPP4, diapeptidylpeptidase 4; GLP-1, glucagon-like peptide-1.
RESULTS
Test animals and hyperglycemic clamps.
Hyperglycemic clamps were performed in 10 rats with portal vein (pv) and 10 rats with jugular vein (jv) infusion of GLP-1. The body weight at the day of the clamp was similar in both cohorts (pv: 315.1 ± 5.9 g, and jv: 314.8 ± 5.2 g). Similarly, concentrations of fasting glucose, average glucose during the hyperglycemic clamp, and glucose increment over basal did not differ significantly between the cohorts (Table 1). Mean blood glucose during the clamp was 212.1 ± 3.5 mg/dL and 206.3 ± 2.5 mg/dL for the portal vein and jugular vein groups, with coefficients of variation for blood glucose over the course of the hyperglycemic clamps that were comparable (pv: 8.7 ± 0.6%, and jv: 8.8 ± 0.5%; Table 1).
The fasting and clamp parameters of rats given portal and jugular GLP-1 did not differ significantly in the experiments with vildagliptin (Table 1). Successful clamps were performed in 9 rats with infusion of GLP-1 into the portal vein and in 12 rats with infusion of GLP-1 into the jugular vein. Mean blood glucose during the clamp was 201.2 ± 1.4 mg/dL and 202.7 ± 1.1 mg/dL for the portal vein and jugular vein groups, with coefficients of variation of 8.6 ± 0.7 % and 9.4 ± 0.7 %, respectively (P = 0.38).
Portal infusion of GLP-1 is less potent to elicit insulin secretion than an equimolar jugular infusion.
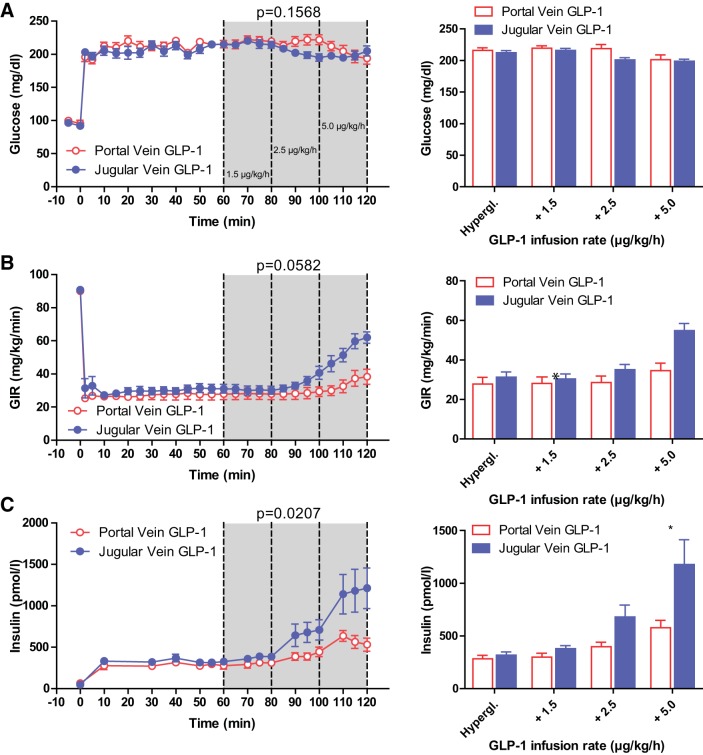
Glucose concentrations decreased significantly in both cohorts (pv 216.2 ± 4.0 mg/dL to 201.4 ± 7.4 mg/dL; jv 212.4 ± 3.2 mg/dL to 198.8 ± 3.3 mg/dL) at the end of the hyperglycemic clamp with higher doses of GLP-1 (P < 0.0001 for dose) but with no significant difference between portal and jugular vein infusion (P = 0.1568 for infusion site) (Fig. 1A). Consistent with these changes in glycemia, the glucose infusion rate (GIR) to maintain constant hyperglycemia increased significantly (pv 27.7 ± 3.4 mg·kg−1·min−1 to 34.5 ± 3.8 mg·kg−1·min−1; jv 31.2 ± 2.6 mg·kg−1·min−1 to 54.8 ± 3.6 mg·kg−1·min−1) with higher doses of GLP-1 infusion (P < 0.0001). Maintenance of the glucose clamp with portal vein GLP-1 infusion required a lower GIR than jugular vein GLP-1 infusion (P = 0.0582; Fig. 1B).
Fig. 1.
Glucose (A), glucose infusion rate (B), and arterial plasma insulin (C) during hyperglycemic clamp. Line graphs (left) depict infusion of glucagon-like peptide-1 (GLP-1) into the portal (red) or jugular (blue) veins starting at time point 60 min, with increasing doses (61–80 min 1.5 μg·kg−1·h−1; 81–100 min 2.5 μg·kg−1·h−1; 101–120 min 5.0 μg·kg−1·h−1). Bar graphs (right) depict average glucose (A), glucose infusion rate (B), and arterial plasma insulin levels (C) during infusion of portal (red) or jugular (blue) infusion of GLP-1. Hypergl. reflects the average values from 50 to 60 min before the GLP-1 infusion was started. GLP-1 infusion into the jugular vein at the highest dose had a significantly greater effect on arterial plasma insulin concentrations than portal infusion (*P < 0.05). All values are mean ± SE. GIR, glucose infusion rate.
With increasing doses of GLP-1, plasma insulin concentrations rose significantly during both portal (282 ± 33 pM to 577 ± 71 pM) and jugular vein (318 ± 29 pM to 1,178 ± 235 pM) infusion (P < 0.0001). Infusion of GLP-1 into the portal vein caused significantly lower insulin levels than GLP-1 infusion into the jugular vein (P = 0.0207). Post-test analyses revealed a significantly lower insulin concentration during infusion of GLP-1 at a dose of 5 μg·kg−1·h−1 into the portal vein when compared with infusion of the same amount into the jugular vein (P < 0.05) (Fig. 1C). Plasma insulin levels during the clamps are summarized in Table 2.
Table 2.
Arterial plasma insulin levels (pmol/L) during clamp
| Portal Vein GLP-1 (n = 10) | Jugular Vein GLP-1 (n = 10) | P Value | |
|---|---|---|---|
| Hyperglycemia | 282 ± 33 | 318 ± 29 | ns |
| + GLP-1 1.5 μg·kg−1·h−1 | 300 ± 36 | 378 ± 28 | ns |
| + GLP-1 2.5 μg·kg−1·h−1 | 396 ± 44 | 679 ± 112 | ns |
| + GLP-1 5.0 μg·kg−1·h−1 | 577 ± 71 | 1,178 ± 235* | <0.05 |
| With DPP4 Inhibition (Vildagliptin) | |||
|---|---|---|---|
| Portal Vein GLP-1 (n = 9) | Jugular Vein GLP-1 (n = 12) | P Value | |
| Hyperglycemia | 543 ± 59 | 672 ± 135 | ns |
| + GLP-1 1.5 μg·kg−1·h−1 | 932 ± 168 | 1,569 ± 264 | ns |
| + GLP-1 2.5 μg·kg−1·h−1 | 1,535 ± 366 | 2,310 ± 340 | ns |
| + GLP-1 5.0 μg·kg−1·h−1 | 1,822 ± 300 | 1,788 ± 425 | ns |
Values are mean ± SE. Both the doses (P < 0.0001) of GLP-1 and the infusion site (P = 0.0207) had a significant impact on arterial plasma insulin levels when analyzed by RM two-way ANOVA. Bonferroni post tests demonstrated significantly higher insulin levels during infusion of GLP-1 into the jugular vs. portal vein at a dose of 5 μg·kg−1·h−1. With DPP4 inhibition, the dose (P < 0.0001) of GLP-1 but not infusion site (P = 0.2799) had a significant impact on arterial plasma insulin levels when analyzed by RM two-way ANOVA. All values are mean ± SE. GLP-1, glucagon-like peptide-1; RM, repeated measures; ns, not significant.
P < 0.05.
DPP4 inhibition partially protects the insulinotropic potency of portal GLP-1.
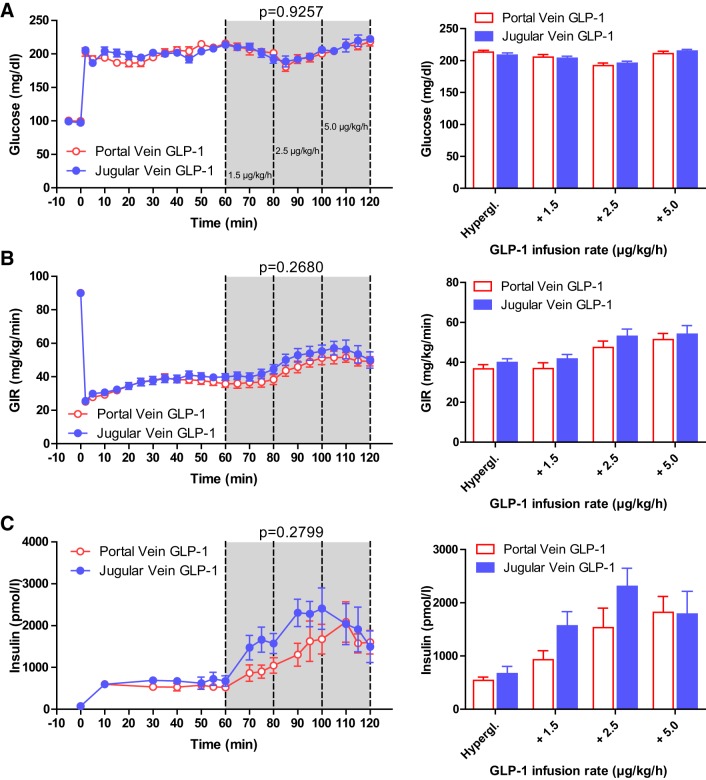
In the experiments with a preclamp dose of vildagliptin, hyperglycemia was significantly altered by GLP-1 dose (P < 0.0001) but not by infusion site (P = 0.9257). With both routes of GLP-1 infusion, there was a similar reduction of glycemia with the 2.5 μg·kg−1·h−1 GLP-1 infusion (pv 213.3 ± 2.6 mg/dL to 192.0 ± 4.3 mg/dL; jv 208.5 ± 3.4 mg/dL to 195.8 ± 3.3 mg/dL) but increased glycemia toward the end of the clamp with the highest dose of GLP-1 (pv 210.9 ± 3.8 mg/dL; jv 214.8 ± 2.4 mg/dL) (Fig. 2A). GIR increased significantly with higher doses of GLP-1 (P < 0.0001) with no difference between sites of infusion (P = 0.2680). The GIR increased steadily with each dose of GLP-1 from 36.8 ± 2.1 mg·kg−1·min−1 to 51.3 ± 3.1 mg/kg/min during portal infusion of GLP-1 and from 40.0 ± 1.9 mg·kg−1· min−1 to 54.2 ± 4.2 mg·kg−1·min−1 during jugular GLP-1 infusion (Fig. 2B).
Fig. 2.
Glucose (A), glucose infusion rate (B), and arterial plasma insulin (C) during hyperglycemic clamp with inhibition of dipeptidylpeptidase 4. Line graphs (left) depict infusion of glucagon-like peptide-1 (GLP-1) into the portal (red) or jugular (blue) veins starting at time point 60 min, with increasing doses (61–80 min 1.5 μg·kg−1·h−1; 81–100 min 2.5 μg·kg−1·h−1; 101–120 min 5.0 μg·kg−1·h−1) to rats pretreated with vildagliptin. Bar graphs (right) depict average glucose (A), glucose infusion rate (B), and arterial plasma insulin levels (C) during infusion of portal (red) or jugular (blue) infusion of GLP-1. Hypergl. reflects the average values from 50 to 60 min before the GLP-1 infusion was started. There was no significant difference between insulin concentrations with portal or jugular GLP-1 infusion. All values are mean ± SE. GIR, glucose infusion rate.
After DPP4 inhibition, plasma insulin concentrations increased significantly with higher doses of GLP-1 (P < 0.0001), but unlike the previous experiments without the DPP4 inhibitor, there was no significant difference between portal and jugular vein GLP-1 infusion (P = 0.2799). With infusion of GLP-1 into the portal vein, plasma insulin levels increased stepwise from 543 ± 59 pM during hyperglycemia only to 932 ± 168 pM with 1.5 μg·kg−1·h−1 GLP-1 to 1,535 ± 366 pM with 2.5 μg·kg−1·h−1 GLP-1 and ultimately 1,822 ± 300 pM with 5 μg·kg−1·h−1 of GLP-1. Similarly, with jugular vein administration, insulin concentrations increased from 672 ± 135 pM (no GLP-1) to a maximum of 2,310 ± 340 pM with the second dose of GLP-1 (2.5 μg·kg−1·h−1) but declined to 1,788 ± 425 pM with the highest dose of jugular vein infusion of GLP-1 (5 μg·kg−1·h−1) (Fig. 2C, Table 2).
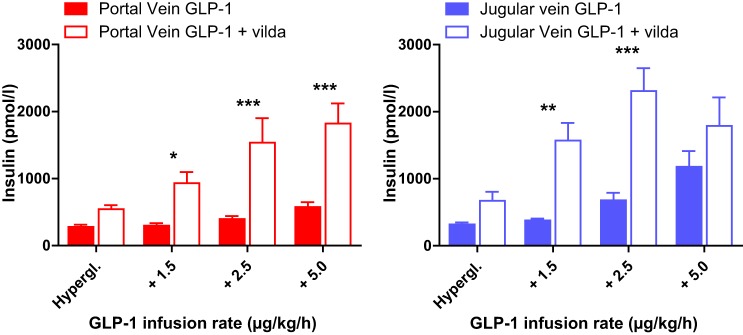
Injection of the DPP4 inhibitor vildagliptin before the clamp increased plasma insulin levels in response to the GLP-1 infusion into either site significantly [2-way ANOVA pv versus pv + vildagliptin (vilda): dose P < 0.0001, vilda P = 0.0007; jv versus jv + vilda: dose P < 0.0001, vilda P = 0.0034]. Bonferroni post test demonstrated a significant effect of vilda on insulin levels at all GLP-1 doses infused into the portal vein (P < 0.05). Infusion of 1.5 μg·kg−1·h−1 and 2.5 μg·kg−1·h−1 GLP-1 into the jugular vein resulted in significantly higher plasma insulin levels after addition of vilda (P < 0.01) but not at a rate of 5 μg·kg−1·h−1 (Fig. 3).
Fig. 3.
Arterial plasma insulin concentrations during the hyperglycemic clamp after portal (left) or jugular (right) infusion of glucagon-like peptide-1 (GLP-1)(7–36). For both infusion sites, arterial plasma insulin concentrations were significantly higher when GLP-1 was infused after dipeptidylpeptidase 4 (DPP4) inhibition by vildagliptin (empty) than without vildagliptin (filled) (repeated measures 2-way ANOVA P < 0.0001 for dose and DPP4 inhibition). Bonferroni post tests showed significantly higher arterial plasma insulin concentrations with vildagliptin (vilda) compared with native GLP-1 infusions for all concentrations except for infusion of the highest dose into the jugular vein. ***P < 0.001; **P < 0.01; *P < 0.05. All values are mean ± SE.
Plasma levels of GLP-1(7–36) during portal or jugular vein infusion.
In a separate cohort of animals, plasma concentrations of active GLP-1(7–36) were measured in arterial blood under all four conditions (pv versus jv infusion ± DPP4 inhibition). Without vildagliptin, basal GLP-1 was 2.6 ± 0.4 pM (pv experiments) and 3.3 ± 0.9 pM (jv). Both dose (P < 0.0001) and infusion site (P = 0.0001) had significant impact on the measurement of plasma GLP-1 concentration. Post-test analysis revealed that jugular vein infusion resulted in significantly higher arterial plasma GLP-1 levels than portal vein infusion both at a rate of 2.5 μg·kg−1·h−1 (P < 0.001) and at a rate of 5 μg·kg−1·h−1 (P < 0.001).
With prior administration of intraperitoneal vildagliptin basal plasma levels of active GLP-1 were similarly elevated to 7.0 ± 2.5 pM (pv) and 7.7 ± 2.2 pM (jv) in both cohorts. There were significant effects of both dose (P < 0.0001) and infusion site (P = 0.0081 for pv versus jv) on active plasma GLP-1 during the experiments with DPP4 inhibition (Table 3), with jugular vein administration giving consistently higher concentrations than portal vein infusion.
Table 3.
Arterial plasma GLP-1(7–36) concentration (pmol/L) during portal and jugular vein infusion
| Portal Vein GLP-1 (n = 5) | Jugular Vein GLP-1 (n = 5) | P Value | |
|---|---|---|---|
| Baseline | 2.6 ± 0.4 | 3.3 ± 0.9 | ns |
| 2.5 μg·kg−1·h−1 | 14.3 ± 2.4 | 43.6 ± 5.0*** | P < 0.001 |
| 5.0 μg·kg−1·h−1 | 36.6 ± 2.8 | 80.9 ± 3.4*** | P < 0.001 |
| With DPP4 Inhibition (Vildagliptin) | |||
|---|---|---|---|
| Portal Vein GLP-1 (n = 5) | Jugular Vein GLP-1 (n = 6) | P Value | |
| Baseline | 7.0 ± 2.5 | 7.7 ± 2.2 | ns |
| 2.5 μg·kg−1·h−1 | 47.1 ± 12.3 | 116.7 ± 27.9 | ns |
| 5.0 μg·kg−1·h−1 | 184.8 ± 35.7 | 443.2 ± 66.4** | P < 0.01 |
Both doses (P < 0.0001) of GLP-1 and infusion site (P < 0.0001) had a significant impact on the arterial plasma GLP-1 levels when analyzed by RM two-way ANOVA. Bonferroni post tests demonstrated significantly higher plasma GLP-1 levels after infusion of both 2.5 and 5 μg/kg/h GLP-1 into the jugular vs. portal vein (P < 0.001 for both doses). With DPP4 inhibition (vildagliptin), both doses (P < 0.0001) of GLP-1 and infusion site (P < 0.0081) had a significant impact on the arterial plasma GLP-1 levels when analyzed by RM two-way ANOVA. Bonferroni post tests demonstrated significantly higher plasma GLP-1 levels after infusion of 5 μg·kg−1·h−1 GLP-1 into the jugular vs. portal vein (P < 0.01). All values are mean ± SE. DPP4, diapeptidylpeptidase 4; GLP-1, glucagon-like peptide-1; ns, not significant; RM, repeated measures.
P < 0.01;
P < 0.001.
DISCUSSION
Although classically considered an incretin, and by definition a hormone, there is emerging evidence against an endocrine mechanism of GLP-1 action (16). Much of this evidence is related to the rapid rate of GLP-1 inactivation by DPP4 and the implausibility that much active peptide reaches target organs like the pancreatic islet through the circulation. Our group and others have suggested that a component of GLP-1 effects is mediated through a neurohumoral circuit initiated in the portal vein (1, 17, 28, 37). Since insulin secretion is a primary action of GLP-1, we hypothesized that an infusion of synthetic GLP-1 into the portal vein would elicit a larger insulin response than central venous administration. Contrary to this prediction, we observed that GLP-1 given into the jugular vein caused greater insulin secretion than an equimolar portal vein infusion. Consistent with the augmented effect on β-cell secretion, arterial concentrations of active GLP-1 were higher after jugular compared with portal infusion, and the differential levels of circulating peptide were not mitigated by a pharmacologic dose of the DPP4 inhibitor vildagliptin. These findings do not support significant portal mediation of insulinotropic GLP-1 activity and raise the possibility that metabolism of GLP-1 occurs in the hepatoportal bed independent of DPP4.
For this study, we chose rats as the experimental model since we had earlier demonstrated specific portal vein neural GLP-1 sensors in this model and were able to induce glucose intolerance in rats with infusion of a GLP-1r antagonist specifically into this vascular system (37). Although maintaining intact vascular cannulae in the carotid artery and jugular and portal veins is challenging, we were able to generate adequate numbers of animals to perform experiments of moderate statistical power. We used graded infusions of GLP-1 to test a range of plasma concentrations that varied from physiologic to pharmacologic levels across both experiments. The hyperglycemic clamp provided generally stable levels of glycemia from group to group and between the two experiments, allowing the effects of GLP-1 dose and site of infusion to be examined in isolation. Finally, we chose a dose of vildagliptin previously demonstrated to cause pharmacologic effects in rodents (20).
The major finding in this study was that portal venous administration of GLP-1 resulted in significantly lower arterial concentrations of active GLP-1 and lesser insulin responses than peptide infused into the jugular vein. These findings indicate that the liver or portal venous circulation has substantial capacity to metabolize GLP-1, accounting for differential concentrations of intact peptide in arterial blood. The increase in hepatoportal GLP-1 clearance was due to either amounts of DPP4 that could not be fully inhibited by the dose of vildagliptin used or another system of peptide removal not susceptible to DPP4 inhibition, which is compatible with a previous result from studies in swine (31). However, we assume that the concentrations of active GLP-1 in the portal vein, immediately downstream of the infusion catheter, were comparable with those in the jugular vein. Thus, the results of this experiment do not support specific signaling by GLP-1 through sensors located in the portal vein across a broad range of concentrations. In both experiments, there was a general correlation of plasma insulin with arterial GLP-1, suggesting that stimulation of insulin release by GLP-1 was a direct action on β-cells. Moreover, plasma insulin was similar in the portal group at the 5 μg·kg−1·h−1 dose and the jugular vein group at the 2.5 μg·kg−1·h−1 dose (Table 2), treatments that caused comparable arterial GLP-1 concentrations (Table 3). Finally, there was very little stimulation of insulin secretion in either group at the lowest dose of GLP-1, a condition we predicted to be useful for distinguishing selective sensing for the peptide in the portal vein. Taken together, our results are not compatible with an important insulinotropic action of GLP-1 mediated specifically in the portal vein of Long-Evans rats, a strain in which there is evidence for hepatoportal GLP-1 sensing (37).
Administration of vildagliptin, a potent DPP4 inhibitor, increased arterial GLP-1 levels and insulin secretion in animals that received GLP-1 through both the portal and jugular veins. However, despite using doses of vildagliptin previously demonstrated to be on the maximal portion of the dose-response curve in rats (6) or to protect intact GLP-1 comparably to DPP4 gene deletion in mice (20), we were not able to equalize the concentrations of active GLP-1 in the arterial circulation of the jugular and portal vein infusion groups. This suggests that passage of GLP-1 through the liver causes significant inactivation of GLP-1 beyond what occurs in the general circulation. The ELISA assay that we used to measure active GLP-1 is blind to the site and mechanism of GLP-1 metabolism such that the plasma levels obtained in this study do not necessarily reflect peptide cleavage by DPP4. A recent study has demonstrated substantial metabolism of GLP-1 peptides by neutral endopeptidases in mice (41) and humans (40) suggesting a potential mechanism to account for the significant removal of GLP-1 across the hepatoportal bed. Regardless, the results here support hepatic metabolism or clearance independent of DPP4, consistent with previous work indicating ~95% first-pass clearance in the liver (15). This finding has physiologic implications since intestinally released GLP-1 must traverse the hepatic circulation before reaching extrasplanchnic target organs. Furthermore, the substantial degradation of GLP-1 in the hepatoportal bed in the presence of vildagliptin suggests that a mechanism other than endocrine action accounts for the glucose lowering of DPP4 inhibition. In light of the broad use of this class of drugs for the treatment of type 2 diabetes, a better understanding of its pharmacological mechanism could improve patient care and allows individualized treatment concepts.
The results of our study differ from the conclusions of several other groups who have studied mediation of GLP-1-stimulated insulin secretion through a portal neural reflex (1, 4, 28). The study by Nishizawa et al. is most similar to the results reported herein, because they also tested the presence of hepatoportally mediated insulin secretion by direct infusions of GLP-1 into the portal and jugular veins of rats (28). Their primary finding was that a brief low-dose portal vein infusion of GLP-1 together with portal vein glucose caused higher insulin release than infusion of glucose alone and that this effect was abolished by vagotomy. These investigators also noted that insulin concentrations were ~2-fold higher when GLP-1 was given through the jugular compared with portal vein at the same dose but did not measure plasma GLP-1 in these experiments. They concluded that in the setting of portal glucose and low-dose GLP-1, mimicking the prandial state, GLP-1 mediates insulin release mainly through a vagal signal originating in the portal vein, whereas higher doses, or administration into the jugular vein, act directly on pancreatic β-cells (28). The low dose of GLP-1 used by Nishizawa was ~7-fold less than the smallest dose infused in our study, and they infused lesser amounts of glucose with ~5-fold lower glucose-stimulated insulin secretion than the baseline we observed. These features may have increased the sensitivity of their experiments to detect an effect of portal GLP-1 sensing on insulin secretion. On the other hand, the amount of insulin stimulated through the portal neural pathway was small and did not affect glucose clearance and is at odds with the notion that GLP-1 is the major mediator of to the incretin effect and postprandial glucose clearance in rodents (22, 33) and humans (14, 32). Hence, it seems unlikely that the vagally mediated insulinotropic effect seen in the study by Nishizawa et al. is the primary mechanism by which GLP-1 mediates its insulinotropic actions and may explain why our less physiologic but more rigorous clamp design did not produce similar results. It is notable that Nishizawa et al. reported a differential effect of jugular and portal GLP-1 infusion, similar to what we observed, supporting the liver as a site of substantial clearance of GLP-1.
It has been suggested by several groups that neuroendocrine signaling through the GLP-1r originates proximal to the portal vein within the substance of the intestine (17, 23, 39) in which local GLP-1 concentrations are even higher than in the portal vein (9). Sisley and coworkers used genetic deletion of the GLP-1 receptor in nodose neurons in mice and observed only a trend toward glucose intolerance, but without formal evaluation of insulin secretion (34). However, a recent report from Krieger et al. noted that lentiviral knockdown of the GLP-1r in the nodose ganglia of rats increased postmeal hyperglycemia and reduced insulin consistent with mediation of GLP-1 effects by vagal afferent neurons (23). Veedfald et al. used a similar design to ours in pigs to test whether exogenous GLP-1 would mediate insulin secretion via intestinal vagal afferents (38). Similar to the infusion of GLP-1 into the portal vein in our study, site-specific infusion of GLP-1 into the mesenteric artery resulted in a lower insulin release than a peripheral intravenous GLP-1 infusion. However, the more proximal infusion of GLP-1 resulted in greater degradation of GLP-1 in the splanchnic and hepatoportal circulation. Although these results are compatible with the findings reported herein, it is plausible that the magnitude of stimulation by exogenous infusion of GLP-1 overshadows and obscures any insulinotropic effect via the vagus nerve (38). Future studies of splanchnic/portal GLP-1 would do well to include low as well has high doses of peptide.
An unexpected finding in our study was the drop in plasma insulin concentrations seen with the highest doses of GLP-1 in conjunction with DPP-IV inhibition. The almost exponential increase in arterial plasma concentrations of active GLP-1 with infusion of synthetic peptide into the jugular vein, protected from degradation by vildagliptin, would be expected to increase plasma insulin but instead reduced insulinemia to a level comparable with the portal infusion. One explanation for this counterintuitive response is the stimulation of the sympathetic nervous system by the massive plasma concentrations of GLP-1. We have previously observed this effect in rats given high doses of GLP-1 peripherally (30) or into the CNS (21), and other groups have reported similar findings with GLP-1 and exendin-4 (29). Although we did not measure epinephrine in this study, we have demonstrated previously that hyperglycemia and reduced plasma insulin seen in conjunction with very high doses of GLP-1 can be reversed by adrenalectomy (30). Because this unexpected drop in insulin toward the end of the high-dose GLP-1 plus vildagliptin clamp was seen consistently across the whole cohort, a random effect or technical problems with the GLP-1 infusion seem unlikely.
In summary, we were not able to show a direct insulinotropic effect through GLP-1r activation in the hepatoportal bed via vagal afferents, as we had hypothesized. This finding does not appear to be congruent with our previous demonstration that GLP-1 receptor antagonism limited to the portal vein causes glucose intolerance (37). However, we cannot exclude effects of GLP-1 to initiate noninsulin-mediated effects to lower blood glucose based on the study design presented here. A notable finding was the lower arterial GLP-1 concentrations resulting from portal compared with jugular vein administration of peptide. This finding indicates a prominent role for GLP-1 clearance in the hepatoportal bed. Altogether, our findings provide further reason to doubt a primary endocrine mechanism of action of intestinally released GLP-1 and pharmacological DPP4 inhibition.
GRANTS
This study was supported in part by National Institutes of Health Grant DK-057900 (to D. A. D’Alessio).
DISCLOSURES
R. J. Seeley: Ethicon Endo-Surgery/Johnson & Johnson Research Support >$10k/yr Ethicon Endo-Surgery/Johnson & Johnson Consultant/Scientific Advisory Board (SAB) <$10k/yr Novo Nordisk Research Support >$10k/yr Novo Nordisk Consultant/SAB <$10k/yr Sanofi Consultant/SAB <$10k/yr Janssen/Johnson & Johnson Consultant/SAB <$10k/yr Zafgen Research Support >$10k/yr Zafgen Equity <0.1% Kallyope Consultant/SAB <$10k/yr Kallyope Research Support >$10k/yr Scohia Consultant/SAB >$10k/yr Astra Zeneca Research Support >$10k/yr Ironwood Pharma Consultant/SAB >$10k/yr Redesign Health Equity Pfizer Research Support >$10k/yr GuidePoint Consultants Consultant/SAB <$10k/yr. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
B.A.A., R.J.S., and D.A.D. conceived and designed research; B.A.A. and M.P. performed experiments; B.A.A., M.P., and D.A.D. analyzed data; B.A.A., M.P., R.J.S., K.G.P., and D.A.D. interpreted results of experiments; B.A.A. prepared figures; B.A.A. and M.P. drafted manuscript; B.A.A., M.P., K.G.P., and D.A.D. edited and revised manuscript; B.A.A., M.P., R.J.S., K.G.P., and D.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kay Ellis for careful and skilled technical assistance.
All experiments and data presented in this manuscript are part of a doctoral thesis of Marta Perabo.
REFERENCES
- 1.Ahrén B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol 286: R269–R272, 2004. doi: 10.1152/ajpregu.00423.2003. [DOI] [PubMed] [Google Scholar]
- 2.Aulinger BA, Vahl TP, Prigeon RL, D’Alessio DA, Elder DA. The incretin effect in obese adolescents with and without type 2 diabetes: impaired or intact? Am J Physiol Endocrinol Metab 310: E774–E781, 2016. doi: 10.1152/ajpendo.00496.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J Clin Endocrinol Metab 100: 2489–2496, 2015. doi: 10.1210/jc.2014-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol 279: R1449–R1454, 2000. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 5.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50: 1720–1728, 2001. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- 6.Burkey BF, Li X, Bolognese L, Balkan B, Mone M, Russell M, Hughes TE, Wang PR. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther 315: 688–695, 2005. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- 7.Creutzfeldt W. The incretin concept today. Diabetologia 16: 75–85, 1979. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 8.D’Alessio D. Is GLP-1 a hormone: whether and when? J Diabetes Investig 7, Suppl 1: 50–55, 2016. doi: 10.1111/jdi.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293: R2163–R2169, 2007. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 10.Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res 36: 761–765, 2004. doi: 10.1055/s-2004-826160. [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27: 740–756, 2018. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705, 2006. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 13.Edgerton DS, Kraft G, Smith MS, Moore LM, Farmer B, Scott M, Moore MC, Nauck MA, Cherrington AD. Effect of portal glucose sensing on incretin hormone secretion in a canine model. Am J Physiol Endocrinol Metab 317: E244–E249, 2019. doi: 10.1152/ajpendo.00100.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 48: 86–93, 1999. doi: 10.2337/diabetes.48.1.86. [DOI] [PubMed] [Google Scholar]
- 15.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140: 5356–5363, 1999. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 16.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 17.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48: 612–615, 2005. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 18.Ionut V, Hucking K, Liberty IF, Bergman RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48: 967–975, 2005. doi: 10.1007/s00125-005-1709-3. [DOI] [PubMed] [Google Scholar]
- 19.Ionut V, Liberty IF, Hucking K, Lottati M, Stefanovski D, Zheng D, Bergman RN. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab 291: E779–E785, 2006. doi: 10.1152/ajpendo.00106.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jessen L, Aulinger BA, Hassel JL, Roy KJ, Smith EP, Greer TM, Woods SC, Seeley RJ, D’Alessio DA. Suppression of food intake by glucagon-like peptide-1 receptor agonists: relative potencies and role of dipeptidyl peptidase-4. Endocrinology 153: 5735–5745, 2012. doi: 10.1210/en.2012-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jessen L, Smith EP, Ulrich-Lai Y, Herman JP, Seeley RJ, Sandoval D, D’Alessio D. Central nervous system GLP-1 receptors regulate islet hormone secretion and glucose homeostasis in male rats. Endocrinology 158: 2124–2133, 2017. doi: 10.1210/en.2016-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolligs F, Fehmann HC, Göke R, Göke B. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9-39) amide. Diabetes 44: 16–19, 1995. doi: 10.2337/diab.44.1.16. [DOI] [PubMed] [Google Scholar]
- 23.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 65: 34–43, 2016. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 24.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829–835, 1993. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol Endocrinol Metab 271: E808–E813, 1996. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, El-Ouaghlidi A. The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia 48: 608–611, 2005. doi: 10.1007/s00125-005-1704-8. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307, 1993. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishizawa M, Nakabayashi H, Uehara K, Nakagawa A, Uchida K, Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am J Physiol Endocrinol Metab 305: E376–E387, 2013. doi: 10.1152/ajpendo.00565.2012. [DOI] [PubMed] [Google Scholar]
- 29.Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 50: 583–589, 2001. doi: 10.1053/meta.2001.22519. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Tilve D, González-Matías L, Aulinger BA, Alvarez-Crespo M, Gil-Lozano M, Alvarez E, Andrade-Olivie AM, Tschöp MH, D’Alessio DA, Mallo F. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab 298: E1088–E1096, 2010. doi: 10.1152/ajpendo.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 48: 1882–1890, 2005. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 32.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 101: 1421–1430, 1998. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2: 1254–1258, 1996. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 34.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 124: 2456–2463, 2014. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahl TP, Aulinger BA, Smith EP, Drazen DL, Ulrich-Lai Y, Seeley RJ, Woods SC, D’Alessio DA. Meal feeding improves oral glucose tolerance in male rats and causes adaptations in postprandial islet hormone secretion that are independent of plasma incretins or glycemia. Am J Physiol Endocrinol Metab 307: E784–E792, 2014. doi: 10.1152/ajpendo.00339.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7–36)NH2, GLP-1-(7–37), and GLP-1-(9–36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 88: 1772–1779, 2003. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 37.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D’Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 38.Veedfald S, Hansen M, Christensen LW, Larsen SA, Hjøllund KR, Plamboeck A, Hartmann B, Deacon CF, Holst JJ. The insulinotropic effect of exogenous glucagon-like peptide-1 is not affected by acute vagotomy in anaesthetized pigs. Exp Physiol 101: 895–912, 2016. doi: 10.1113/EP085692. [DOI] [PubMed] [Google Scholar]
- 39.Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, Sulpice T, Holst JJ, Drucker DJ, Magnan C, Burcelin R. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 152: 3018–3029, 2011. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- 40.Wewer Albrechtsen NJ, Mark PD, Terzic D, Hansen LH, Andersen UO, Hartmann B, Carr RD, Gustafsson F, Deacon CF, Holst JJ, Goetze JP, Plomgaard P. Sacubitril/valsartan augments postprandial plasma concentrations of active GLP-1 when combined with sitagliptin in men. J Clin Endocrinol Metab 104: 3868–3876, 2019. doi: 10.1210/jc.2019-00515. [DOI] [PubMed] [Google Scholar]
- 41.Windeløv JA, Wewer Albrechtsen NJ, Kuhre RE, Jepsen SL, Hornburg D, Pedersen J, Jensen EP, Galsgaard KD, Winther-Sørensen M, Ørgaard A, Deacon CF, Mann M, Kissow H, Hartmann B, Holst JJ. Why is it so difficult to measure glucagon-like peptide-1 in a mouse? Diabetologia 60: 2066–2075, 2017. doi: 10.1007/s00125-017-4347-7. [DOI] [PubMed] [Google Scholar]