Abstract
One exercise session can increase subsequent insulin-stimulated glucose uptake (ISGU) by skeletal muscle. Prior research on healthy muscle suggests that enhanced postexercise ISGU depends on elevated γ3-AMPK activity leading to greater phosphorylation of Akt substrate of 160 kDa (pAS160) on an AMPK-phosphomotif (Ser704). Phosphorylation of AS160Ser704, in turn, may favor greater insulin-stimulated pAS160 on an Akt-phosphomotif (Thr642) that regulates ISGU. Accordingly, we tested if exercise-induced increases in γ3-AMPK activity and pAS160 on key regulatory sites accompany improved ISGU at 3 h postexercise (3hPEX) in insulin-resistant muscle. Rats fed a high-fat diet (HFD; 2-wk) that induces insulin resistance either performed acute swim-exercise (2 h) or were sedentary (SED). SED rats fed a low-fat diet (LFD; 2 wk) served as healthy controls. Isolated epitrochlearis muscles from 3hPEX and SED rats were analyzed for ISGU, pAS160, pAkt2 (Akt-isoform that phosphorylates pAS160Thr642), and γ1-AMPK and γ3-AMPK activity. ISGU was lower in HFD-SED muscles versus LFD-SED, but this decrement was eliminated in the HFD-3hPEX group. γ3-AMPK activity, but not γ1-AMPK activity, was elevated in HFD-3hPEX muscles versus both SED controls. Furthermore, insulin-stimulated pAS160Thr642, pAS160Ser704, and pAkt2Ser474 in HFD-3hPEX muscles were elevated above HFD-SED and equal to values in LFD-SED muscles, but insulin-independent pAS160Ser704 was unaltered at 3hPEX. These results demonstrated, for the first time in an insulin-resistant model, that the postexercise increase in ISGU was accompanied by sustained enhancement of γ3-AMPK activation and greater pAkt2Ser474. Our working hypothesis is that these changes along with enhanced insulin-stimulated pAS160 increase ISGU of insulin-resistant muscles to values equaling insulin-sensitive sedentary controls.
NEW & NOTEWORTHY Earlier research focusing on signaling events linked to increased insulin sensitivity in muscle has rarely evaluated insulin resistant muscle after exercise. We assessed insulin resistant muscle after an exercise protocol that improved insulin-stimulated glucose uptake. Prior exercise also amplified several signaling steps expected to favor enhanced insulin-stimulated glucose uptake: increased γ3-AMP-activated protein kinase activity, greater insulin-stimulated Akt2 phosphorylation on Ser474, and elevated insulin-stimulated Akt substrate of 160 kDa phosphorylation on Ser588, Thr642, and Ser704.
Keywords: AMP-activated protein kinase, exercise, glucose transport, insulin resistance, TBC1D4
INTRODUCTION
Insulin resistance is a primary and essential defect in the process leading to type 2 diabetes (31). Skeletal muscle accounts for up to 85% of insulin-mediated glucose disposal (18). Because skeletal muscle is so quantitatively important for glucose disposal, it is a prime target for interventions aimed at combating insulin resistance. It is well established that exercise or muscle contractions can increase subsequent insulin-stimulated glucose uptake of skeletal muscle from either insulin-sensitive or insulin-resistant subjects (11, 13, 14, 50, 55–57, 78). Understanding how exercise regulates insulin-stimulated glucose uptake in insulin-sensitive subjects is important, but there is a more urgent need to identify the mechanisms responsible for enhanced insulin-stimulated glucose uptake after exercise during insulin resistance.
The postexercise enhancement in insulin-stimulated glucose uptake by skeletal muscle has been observed without increasing the level of insulin-stimulated signaling compared with sedentary controls at a number of insulin signaling steps in insulin-sensitive rodents and humans. A number of proximal insulin signaling steps ranging from insulin’s binding to its receptor to Akt phosphorylation have been reported to be similar in insulin-stimulated muscle from sedentary versus exercised, insulin-sensitive rodents or humans (10, 14, 19, 25, 26, 50, 67, 68, 83, 84). These results suggest that exercise might improve insulin sensitivity via regulation of a more distal insulin signaling step.
Akt substrate of 160 kDa (AS160; also known as TBC1D4) is the most distal signaling protein that has been clearly linked to insulin-stimulated glucose uptake in skeletal muscle (12). Multiple lines of evidence have suggested that altered AS160 phosphorylation is a strong candidate for mediating the improved insulin-stimulated glucose uptake by skeletal muscle following acute exercise (3, 22, 23, 71). Preventing phosphorylation of two Akt-phosphomotifs of AS160 (Thr642 to Ala642 and Ser588 to Ala588) markedly reduced insulin-stimulated GLUT4 translocation in adipocytes (59). Enhanced insulin-stimulated phosphorylation of AS160 on Thr642 and Ser588 in exercised compared with nonexercised skeletal muscle, concomitant with increased glucose uptake, has been reported in rat skeletal muscle with normal insulin sensitivity (4, 22, 23, 30, 61, 79). Acute exercise can enhance subsequent AS160 phosphorylation on Thr642 and/or Ser588 in insulin-stimulated skeletal muscles from insulin-resistant rats or humans independent of greater Akt activation (14, 50). Compelling new evidence was recently published supporting the idea that AS160 is essential for increased insulin-stimulated glucose uptake in skeletal muscle after acute contractile activity. Kjøbsted et al. (33) reported that insulin-stimulated glucose uptake by muscle several hours after in situ contraction was increased in wild-type mice, but not in AS160 muscle-specific knockout mice.
The mechanisms accounting for a sustained, postexercise increase in pAS160Thr642 and/or pAS160Ser588 of insulin-stimulated skeletal muscle are unknown. However, the phosphorylation of AS160 on Ser704, an AMPK-phosphomotif, has recently emerged as a potentially important step for enhanced insulin-stimulated glucose uptake after exercise. In human (36, 50) and rat (80) skeletal muscle with normal insulin sensitivity, the insulin-independent phosphorylation of AS160Ser704 was reported to be increased immediately postexercise and remained elevated for hours after exercise, when insulin-stimulated glucose uptake was enhanced (54, 77). Additionally, multiple studies have reported enhanced insulin-stimulated pAS160Ser704 in skeletal muscle after exercise or contraction (35, 50, 64, 76, 80). Preventing the phosphorylation of AS160Ser704 in mouse muscle by mutating Ser704 to Ala704 attenuates the insulin-stimulated phosphorylation of AS160Thr642, an Akt phosphomotif which regulates insulin-stimulated glucose uptake (37). These data suggest that the enhanced phosphorylation of AS160Ser704 after exercise may prime Thr642 to be more easily phosphorylated, a potentially critical event for enhancing insulin sensitivity. It will be important to identify exercise effects on pAS160Ser704 during insulin resistance to better understand the mechanisms which govern enhanced postexercise insulin sensitivity in insulin-resistant subjects. Previous research has not assessed prior exercise effects on Ser704 phosphorylation in insulin-resistant rat skeletal muscle. Therefore, our first major aim was to investigate the effect of acute exercise on key AS160 phosphorylation sites (Ser588, Thr642, and Ser704) in muscles from insulin-resistant rats fed a high-fat diet (HFD) after exercise, versus sedentary HFD- and low-fat diet-fed (LFD) controls.
AS160Ser704 is an AMPK phosphosite, and AMPK is a heterotrimeric complex composed of a catalytic α subunit (α1 or α2 isoform) and two regulatory subunits (β1 or β2 isoform; and γ1, γ2, or γ3 isoform). γ3-AMPK has been shown to be activated in muscle by exercise in humans (36, 72) and rats (80) or by electrically stimulated contractions in mice (35, 72). Prior incubation of isolated muscles from wild-type mice with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) resulted in enhanced insulin-stimulated glucose uptake, but this AICAR effect on insulin sensitivity was absent in muscles from γ3-AMPK knockout mice (37). Moreover, electrically stimulated ex vivo contractile activity which enhances pAS160Ser704 in muscles from wild-type (WT) mice did not increase pAS160Ser704 in γ3-AMPK knockout mice (35). These findings suggest γ3-AMPK activation and pAS160Ser704 may be critical steps for enhancing insulin-stimulated glucose uptake after AICAR or contraction in insulin-sensitive animals. However, neither prior AICAR treatment nor in situ contractions completely recapitulate in vivo exercise. Furthermore, no previously published study have evaluated either γ1- or γ3-AMPK activity in insulin-resistant rat skeletal muscle after acute exercise. Therefore, the second major aim of this experiment was to investigate γ3-AMPK activity in muscles from insulin-resistant rats fed a high-fat diet (HFD) after exercise, compared with sedentary HFD- and low-fat diet-fed (LFD) controls.
Earlier research has reported that acute exercise can lead to subsequently elevated insulin-stimulated AS160 phosphorylation on key regulatory sites and glucose uptake in skeletal muscle independent of enhanced Akt phosphorylation (3, 14, 30, 50, 61). There are three isoforms of Akt (Akt1, Akt2 and Akt3) that are expressed in mammals (75), but only Akt1 and Akt2 are highly expressed in skeletal muscle (45, 60, 87). Akt2, the predominant Akt isoform expressed in human skeletal muscle (77), regulates insulin-stimulated AS160 phosphorylation (38) and insulin-stimulated glucose uptake (16, 42). Previous research using methods that cannot selectively distinguish between the phosphorylation of Akt2 and other Akt isoforms has indicated that a single exercise session does not increase subsequent insulin-stimulated pan-Akt phosphorylation in human skeletal muscle (36, 65). Because Akt2 has been reported to account for almost all of the Akt phosphorylation in insulin-stimulated muscles from sedentary humans (77), these previous results provide indirect evidence suggesting that prior exercise may not substantially increase Akt2 phosphorylation in human muscle. However, earlier studies have not used methods that exclusively assess Akt2 phosphorylation to directly test if prior exercise alters Akt2 phosphorylation in either healthy or insulin-resistant skeletal muscle of humans or rodents. Accordingly, the third major aim of the current study was to use appropriate methods to directly determine in insulin-resistant skeletal muscle the effects of acute exercise on the phosphorylation of Akt2 on its key regulatory sites, Ser474 and Thr309.
METHODS
Materials.
The reagents and apparatus for SDS-PAGE and nonfat dry milk (#170–6404) were from Bio-Rad (Hercules, CA). [3H]-2-deoxyglucose (NET328001MC), [14C]-mannitol (NEC314250UC), and [γ-32P]-ATP were from PerkinElmer (Waltham, MA). Tissue Protein Extraction Reagent, (T-PER; #PI78510), Bicinchoninic Acid Protein Assay Kit (#PI23223), MemCode Reversible Protein Stain Kit (#PI24585), and Protein G magnetic beads (#10004D), DynaMagTM-2 magnet (#12321D) were from ThermoFisher (Pittsburgh, PA). Anti-rabbit IgG horseradish peroxidase conjugate (#7074), anti-phospho Akt Ser473 (pAktSer473; #4060), anti-phospho Akt Thr308 (pAktThr308; #13038), anti-Akt (#4691), anti-phospho Akt2 Ser474 (pAkt2Ser474; #8599), anti-Akt2 (#3063; used for immunoblotting), anti-Akt3 (#3788), anti-phospho AS160 Thr642 (pAS160Thr642; #4288), anti-phospho AS160 Ser588 (pAS160Ser588; #8730), anti-phospho AMPKα Thr172 (pAMPKαThr172; #2535), anti-AMP-activated protein kinase-α (AMPKα; #2532), anti-AMP-activated protein kinase-β1 (AMPK-β1; #12063), anti-AMP-activated protein kinase-β2 (AMPK-β2; #4148), anti-acetyl CoA carboxylase (ACC; #3676), and anti-phospho ACC Ser79 (pACCSer79; #3661) were from Cell Signaling Technology (Danvers, MA). Anti-Akt1 (#AF1775) and anti-Akt2 (#AF23151; used for immunoprecipitation) were from R&D Systems (Minneapolis, MN). Skeletal muscle expresses two isoforms of ACC (ACC1 and ACC2). ACC1 has a relatively low expression in skeletal muscle and is phosphorylated by AMPK on Ser79. ACC2 has a relatively high expression in skeletal muscle and is phosphorylated by AMPK on Ser212 (1). Since the pACCSer79 antibody (#3661) detects both pACC1Ser79 and pACC2Ser212 (46), we hereafter refer to the results with this antibody as pACCSer79/212. Anti-phospho AS160Ser704 was provided by Dr. Jonas Treebak (University of Copenhagen, Denmark). Anti-AMP-activated protein kinase γ1 (γ1-AMPK; #32508) was from Abcam. Anti-AMP-activated protein kinase γ3 (γ3-AMPK) was provided by Dr. David Thomson (Brigham Young University) (27). Liquid scintillation cocktail (#111195-CS) was from Research Products International (Mount Prospect, IL). Anti-Akt substrate of 160kDa (AS160; #ABS54), anti-AMP-activated protein kinase α1 (AMPK-α1; #07–350), anti-AMP-activated protein kinase α2 (AMPK-α2; #07–363), P81 phosphocellulose squares (#20–134), and enhanced chemiluminescence Luminata Forte Western HRP Substrate (#WBLUF0100) were from EMD Millipore (Billerica, MA).
Animal treatment and muscle preparation.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (6–7 wk old; Charles River Laboratories, Boston, MA) were individually housed and provided with standard rodent chow (Laboratory Diet no. 5L0D; LabDiet, St. Louis, MO) or high-fat chow (Laboratory Diet no. D12492; ResearchDiets, New Brunswick, NJ) and water ad libitum for 2 wk until they were fasted the night before the experiment at ∼1700. Caloric intake for each rat during the 2-wk diet period was estimated based on the difference between the food provided on day 1 and the food remaining at ~1700 on the night before the experiment. Beginning at 0700 on the day of the experiment rats either remained sedentary or swam in a barrel filled with water (35°C) for four 30-min bouts with a 5-min rest between bouts. Immediately postexercise (IPEX), some rats (IPEX and Sedentary) were anesthetized (intraperitoneal sodium pentobarbital, 50 mg/kg weight), weighed, and their epitrochlearis muscles were dissected. At 3 h postexercise (3hPEX) other rats (3hPEX and Sedentary) were anesthetized, weighed, and their epitrochlearis muscle were dissected. After muscle dissections, the epididymal fat pads were dissected and weighed.
We used the same diet protocols for the LFD and HFD groups that were used in an earlier study which reported that the HFD resulted in insulin resistance compared with LFD controls (14). In the current study, sedentary LFD rats were included to confirm that the HFD protocol induced insulin resistance in this cohort of rats. In the earlier study, we used the same exercise protocol as the current study. The previous study evaluated both LFD and HFD rats. The results of the earlier study demonstrated that insulin-stimulated glucose uptake was increased at 3hPEX in each diet group compared with diet-matched controls. Furthermore, insulin-stimulated glucose uptake for the LFD-3hPEX group exceeded the values for the HFD-3hPEX group. The focus of the current study was to probe potential mechanisms for the exercise-induced increase in insulin-stimulated glucose uptake only in the HFD-fed rats. The goal was not to assess the potential mechanisms for the greater insulin-stimulated glucose uptake in the LFD-3hPEX versus HFD-3hPEX rats. Accordingly, in the current study, only HFD-fed rats were studied under exercised conditions (IPEX and 3hPEX).
Ex vivo incubations of muscles for glucose uptake.
Muscles were incubated, as previously described (47), in glass vials gassed (95% O2, 5% CO2) in a temperature controlled bath by a two-step incubation process (35°C during both steps). For the IPEX experiments, muscles were placed in vials for 10 min containing 2 ml of incubation step 1 media (Krebs Henseleit Buffer, KHB, supplemented with 0.1% bovine serum albumin, BSA, 2 mM sodium pyruvate and 6 mM mannitol). For incubation step 2 (15 min) these muscles were transferred to vials containing 2 ml of incubation step 2 media (KHB supplemented with 0.1% BSA, 0.1 mM 2-DG (2.25 mCi/mmol [3H]-2-DG), 2 mM sodium pyruvate and 6 mM mannitol (2 mCi/mmol [14C] mannitol)). For the 3hPEX experiment, paired muscles were placed in vials containing 2 ml of incubation step 1 media for 30 min with or without 100 µU/ml insulin. These muscles were then transferred to vials containing 2 ml of incubation step 2 media for 20 min with or without 100 µU/ml insulin. After step 2, whole muscles were blotted, freeze clamped, and stored at −80°C until further processing.
Whole muscle glucose uptake.
Frozen muscles used for 2-DG uptake were weighed and homogenized in ice-cold lysis buffer (T-PER supplemented with 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate tetrabasic decahydrate, 1 mM β-glycerophosphate, 1 µg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Homogenates were then rotated at 4°C for 1 h before centrifugation at 15,000 g for 15 min at 4°C. Aliquots (200 µl) of supernatant were added to vials along with 8 ml of scintillation cocktail. 2-[3H]-DG and 2-[14C]-mannitol disintegrations per minute were measured by scintillation counter, and then 2-DG uptake was calculated as previously described (9).
Immunoblotting.
Total protein concentrations for whole muscle lysates were determined by bicinchoninic acid assay, and equal amounts of protein for each sample were separated via SDS-PAGE, and transferred to polyvinyl difluoride membranes. After electrotransfer, the MemCode protein stain was used to confirm equal loading (2). Membranes were then blocked with 5% BSA or nonfat milk in TBST (Tris-buffered saline, pH 7.5 plus 0.1% Tween-20) for 1 h, incubated with appropriate concentrations of primary (1:1000; overnight) and secondary (1:20,000; 1 h) antibodies, and subjected to enhanced chemiluminescence to quantify protein bands by densitometry (FluorChem E Imager, AlphaView software; ProteinSimple, San Jose, CA). Individual values were normalized to the mean value of all samples on the membrane. The vendor validated that the pAkt2Ser474 antibody used in this experiment (Cell Signaling Technology #8599) is selective for this Ak2 phosphosite without cross reactivity to either Akt1 or Akt3. Therefore, it was unnecessary to isolate Akt2 by immunoprecipitation (IP) before immunoblotting with the selective pAkt2Ser474 antibody. In contrast, no commercially available phospho-antibody is currently available for the selective detection of pAkt2Thr309. Therefore, we evaluated pAkt2Thr309 using methods very similar to those that we previously described in detail (62). In brief, muscle lysates were first immunoprecipitated using an antibody (R&D Systems #AF23151) that the vendor has documented to selectively recognize recombinant Akt2, but not recombinant Akt1 or Akt3. After Akt2-IP, we immunoblotted using an antibody that has been validated by the vendor to recognize pThr309 on Akt2 (Cell Signaling Technology #13038).
We performed additional analyses using rat epitrochlearis samples to confirm that our Akt2-IP protocol effectively isolated Akt2 (results shown in Supplemental Fig. S1, available at https://doi.org/10.6084/m9.figshare.11402694). Immunoblotting was performed for both the immunoprecipitated fraction and the post-IP supernatant. The results demonstrated that the Akt2-IP protocol efficiently pelleted essentially all of the Akt2. Immunoblotting both the Akt2-IP pellet and the post-IP supernatant fractions revealed that, in contrast to Akt2, almost all of the Akt1 was in the post-IP supernatant. Akt1 was barely detectable in the pellet. Earlier research reported that both human and mouse skeletal muscle has very low Akt3 expression (40, 74). However, previous studies had not evaluated Akt3 protein abundance in rat epitrochlearis muscle. Accordingly, we analyzed rat epitrochlearis muscle. Akt3 protein was undetectable in rat epitrochlearis samples. Taken together, the results of these analyses demonstrated the efficacy of the Akt2-IP protocol for isolating Akt2 found in rat epitrochlearis muscle.
AMPK-γ1 and -γ3 isoform activity.
The specificity of γ1-AMPK and γ3-AMPK antibodies used for immunoprecipitation has been previously confirmed (80). AMPK isoform-specific activity was determined as previously described (37). Briefly, 300µg (for γ3-AMPK assay) or 600 µg (for γ1-AMPK assay) of protein from each sample was rotated at 4°C overnight with appropriate AMPK γ isoform antibody (1:1,000). Then 50 μl of protein G-magnetic beads was added to the muscle lysate/antibody mixture and rotated for 2 h at 4°C. A DynaMag-2 magnet was used to precipitate the protein G-immunocomplex. Each pellet was washed one time in buffer A [50 mmol/L NaCl, 1% Triton X-100, 50 mmol/L sodium fluoride, 5 mmol/L sodium-pyrophosphate, 20 mmol/L Tris-base (pH 7.5), 500 μmol/L PMSF, 2 mmol/L dithiothreitol (DTT), 4 μg/mL leupeptin, 4 μg/mL aprotinin, and 250 mmol/L sucrose], once in 6X assay buffer (240 mmol/L HEPES, 480 mmol/L NaCl, pH 7.0), and two times in 3X assay buffer. The activity assay was then performed in 30 μl of kinase reaction buffer [40 mmol/L HEPES, pH 7.5, 80 mmol/L NaCl, 800 μmol/L DTT, 200 μmol/L AMP, 100 μmol/L AMARA peptide, 5 mmol/L magnesium chloride (MgCl2), 200 μmol/L ATP, and 10 µCi of [γ- 32P]-ATP] for 30 min at 30°C. The reaction was stopped by the addition of 10 μl of 1% phosphoric acid and then 40 µl of supernatant was transferred to phosphocellulose paper. After 4 × 15 min washes with 1% phosphoric acid, the phosphocellulose paper was dried for 5 min and placed in the vials containing scintillation cocktail for scintillation counting. It was not feasible to assay all of the samples in a single batch. Therefore, every batch included an equal number of samples from each treatment group. The results are expressed relative to the normalized mean value for all of the samples from each batch.
Statistics.
Data are expressed as means ± standard error (SEM), with two-tailed significance levels of α < 0.05. Two-tailed t-tests were performed to compare LFD versus HFD means for body mass, fat pad mass, epitrochlearis mass, and estimated caloric intake. One-way ANOVAs were used to determine the treatment group effect of IPEX experiments. Two-way ANOVAs were used to compare means among more than two groups from 3hPEX experiments. The treatment design was not a complete factorial of diet and exercise; therefore main effect comparisons of those treatments could not be made. Instead, comparisons and conclusions were restricted to the planned comparisons of the three treatment groups (LFD-SED, HFD-SED, and either HFD-IPEX or HFD-3hPEX). The analysis evaluated the effect of exercise on rats with high-fat diets, the effect of diet in sedentary rats, and compared HFD-fed exercised rats to the LFD-SED rats. Bonferroni post hoc tests were performed to identify the source of significant differences.
RESULTS
Body mass, muscle mass, epididymal fat mass, and estimated caloric intake.
Following the 2-wk diet intervention, the HFD rats versus LFD rats had a significantly greater body mass (299 ± 4 versus 290 ± 6 g; P < 0.05), epididymal fat mass (2318 ± 128 versus 1297 ± 81 mg; P < 0.001), and estimated caloric intake (97 ± 2 versus 82 ± 2 kcal/day; P < 0.001). Epitrochlearis muscle mass did not significantly differ between LFD and HFD (59 ± 5 versus 61 ± 5 mg, respectively).
Glucose uptake.
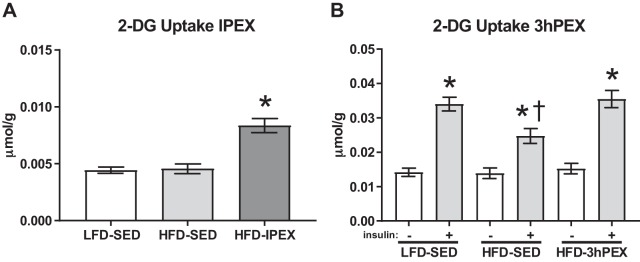
Insulin-independent glucose uptake was significantly greater in the HFD-IPEX group compared with both sedentary control groups (P < 0.001; Fig. 1A). In the 3hPEX experiment, muscles incubated with insulin had a greater glucose uptake than paired muscles incubated without insulin in all treatment groups (P < 0.001; Fig. 1B). Within the insulin-treated muscles, glucose uptake was lower in the HFD-SED group compared with either the LFD-SED or HFD-3hPEX groups (P < 0.01), but there was not a significant difference between insulin-treated LFD-SED and HFD-3hPEX muscles. There were significant main effects of diet and exercise treatment (P < 0.01) and insulin (P < 0.001), and a significant treatment × insulin interaction effect (P < 0.05) for glucose uptake.
Fig. 1.
A: epitrochlearis 2-DG uptake immediately postexercise. Data were analyzed by one-way ANOVA. *P < 0.001, HFD-IPEX vs. both LFD-SED and HFD-SED. Values are mean ± SEM; n = 14/group. B: 2-DG uptake from paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3 h postexercise. Data were analyzed by two-way ANOVA. *P < 0.001, insulin vs no insulin. †P < 0.005, within insulin-treated muscles HFD-SED vs. both LFD-SED and HFD-3hPEX. Values are means ± SEM; n = 15/group. 2-DG, 2-deoxy-d-glucose; HFD, high-fat diet; LFD, low-fat diet; SED, sedentary; IPEX, immediately postexercise; 3hPEX, 3 h postexercise.
γ1-AMPK and γ3-AMPK activity.
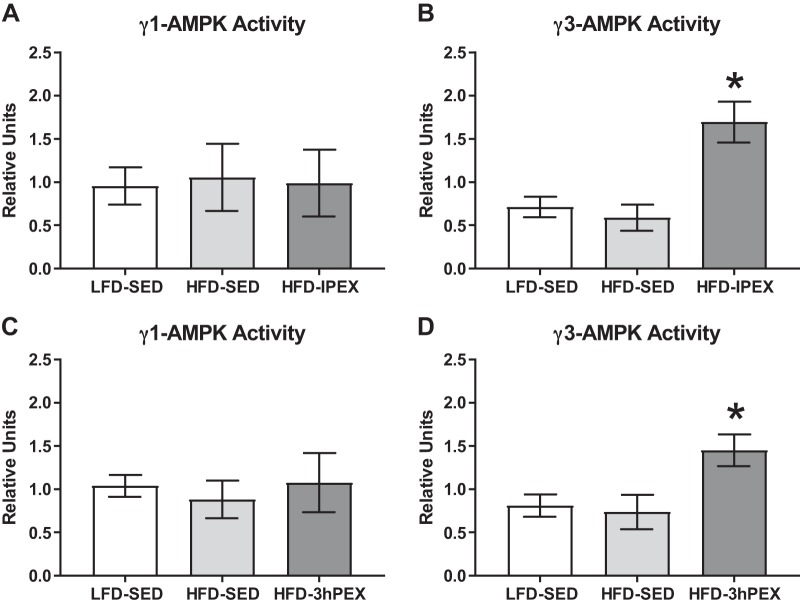
There was not a significant effect of exercise on γ1-AMPK activity in HFD-IPEX or HFD-3hPEX groups compared with either sedentary control group (Fig. 2, A and C). Increased γ3-AMPK activity was observed in both HFD-IPEX and HFD-3hPEX rats compared with both HFD-SED and LFD-SED controls (P < 0.05; Fig. 2, B and D). AMPK activity assays were performed in separate batches for the IPEX and 3hPEX groups along with their respective sedentary controls. Activity assays for γ1-AMPK and γ3-AMPK were also performed separately from each other. Therefore, it would be inappropriate to statistically compare the AMPK activity values determined either for IPEX versus 3hPEX rats or for γ1-AMPK versus γ3-AMPK.
Fig. 2.
A: γ1-AMPK activity immediately postexercise. B: γ3-AMPK activity immediately postexercise. C: γ1-AMPK activity 3 h postexercise. D: γ3-AMPK activity 3 h postexercise. For γ1-AMPK activity assays n = 5/group. For γ3-AMPK activity assays n = 8/group. Data were analyzed by one-way ANOVA. *P < 0.01, significantly greater than both other treatment groups. Values are expressed as means ± SEM. HFD, high-fat diet; LFD, low-fat diet; SED, sedentary; IPEX, immediately postexercise; 3hPEX, 3 h postexercise.
AMPK isoform abundance.
There was no significant effect of treatment group on the abundance of α1-AMPK, α2-AMPK, β1-AMPK, β2-AMPK, γ1-AMPK, or γ3-AMPK (results not shown). Because γ2-containing AMPK heterotrimers have been reported to be undetectable in skeletal muscle (69), γ2-AMPK was not measured.
AMPKα and ACC phosphorylation.
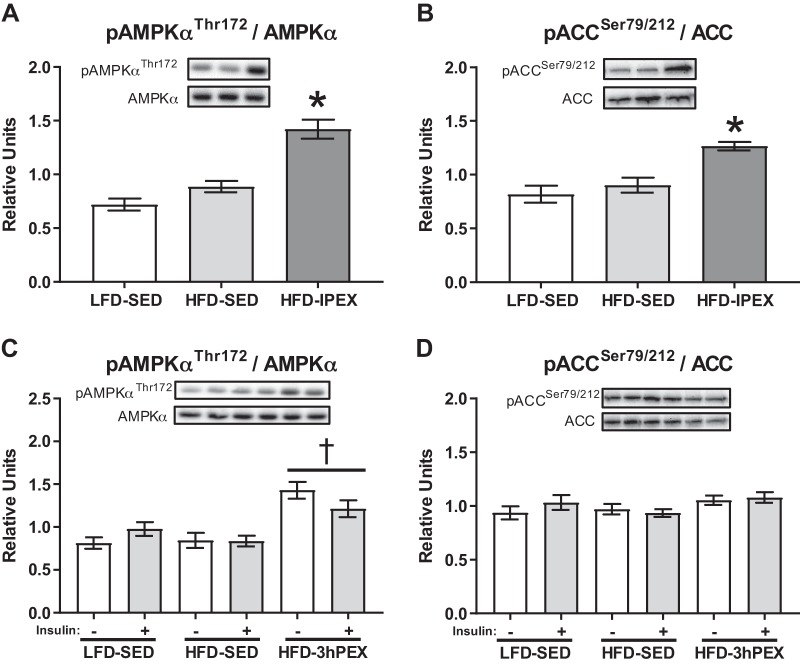
For the IPEX experiment, pAMPKαThr172 expressed relative to total AMPKα (pAMPKαThr172/ AMPKα) was significantly greater in the HFD-IPEX group compared with both SED controls (P < 0.001; Fig. 3A). At 3hPEX there was a significant main effect of exercise for increased pAMPKαThr172/ AMPKα (P < 0.001; Fig. 3C). pACCSer79/212 was significantly increased in the HFD-IPEX group compared with SED controls (P < 0.005; Fig. 3B), but there was no effect of insulin or treatment group on pACCSer79/212 at 3hPEX (Fig. 3D).
Fig. 3.
A: phosphorylated AMPKαThr172/AMPKα immediately postexercise. B: phosphorylated ACCSer79/212/ACC immediately postexercise. C: phosphorylated AMPKαThr172/AMPKα 3 h postexercise. D: phosphorylated ACCSer79/212/ACC 3 h postexercise. IPEX data were analyzed by one-way ANOVA and 3hPEX data were analyzed by two-way ANOVA. *P < 0.005, HFD-IPEX greater than both other groups. †P < 0.01, main effect of 3hPEX vs. both LFD-SED and HFD-SED. Values are expressed as means ± SEM; n = 8–17/group. HFD, high-fat diet; LFD, low-fat diet; SED, sedentary; IPEX, immediately postexercise; 3hPEX, 3 h postexercise.
AS160 phosphorylation.
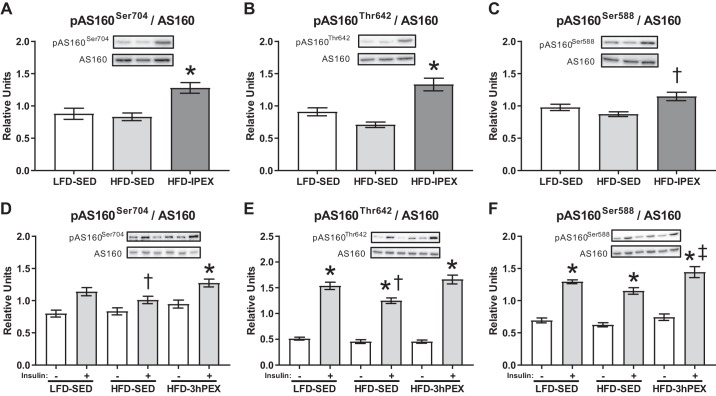
For the IPEX experiment, pAS160Ser704 and pAS160Thr642 expressed relative to total AS160 were significantly greater in the HFD-IPEX group compared with both SED controls (P < 0.005; Fig. 4, A and B). pAS160Ser588 expressed relative to total AS160 (pAS160Ser588/AS160) was significantly greater in the HFD-IPEX group compared with the HFD-SED control (P < 0.005; Fig. 4C), but not compared with the LFD-SED control. For the 3hPEX experiment, insulin-stimulated pAS160Ser704 and pAS160Thr642 were lower in the HFD-SED group compared with either LFD-SED and HFD-3hPEX (P < 0.01; Fig. 4, D and E). Insulin-stimulated pAS160Ser588 was significantly greater in the HFD-3hPEX group compared with HFD-SED control (P < 0.01; Fig. 4F), but not compared with LFD-SED control. There were significant main effects of diet and exercise treatment (P < 0.01) and insulin (P < 0.001) for pAS160Ser588, pAS160Thr642, and pAS160Ser704. There was a significant treatment × insulin interaction effect (P < 0.05) for pAS160Thr642.
Fig. 4.
A–C: phosphorylated AS160Ser704, AS160Thr642, and AS160Ser588/AS160 immediately postexercise. Data were analyzed by one-way ANOVA. *P < 0.005, HFD-IPEX vs. both LFD-SED and HFD-SED. †P < 0.005, HFD-IPEX vs. HFD-SED. Values are expressed as means ± SEM; n = 17/group. D–F: phosphorylated AS160Ser704, AS160Thr642, and AS160Ser588/AS160 at 3 h postexercise. Data were analyzed by two-way ANOVA. *P < 0.001, insulin vs. no insulin. †P < 0.001, HFD-SED vs. both LFD-SED and HFD-3hPEX within insulin-treated muscles. ‡P < 0.01, HFD-3hPEX vs. HFD-SED within insulin-treated muscles. Values are expressed as means ± SEM; n = 10–17/group. HFD, high-fat diet; LFD, low-fat diet; SED, sedentary; IPEX, immediately postexercise; 3hPEX, 3 h postexercise.
Akt phosphorylation.
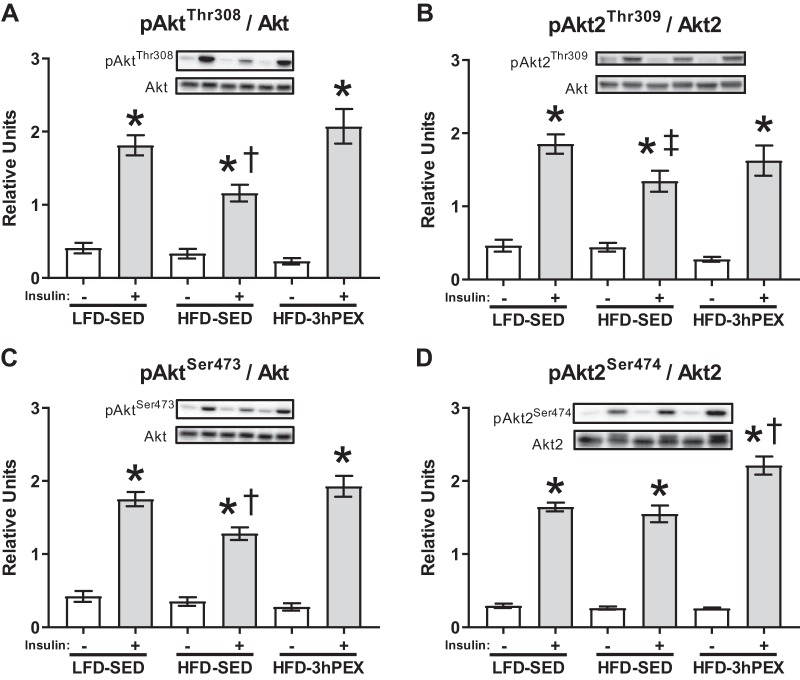
For the IPEX experiment, neither pAktThr308 nor pAktSer473 relative to total Akt (pAktThr308/Akt and pAktSer473/Akt) significantly differed among the LFD-SED, HFD-SED and HFD-IPEX rats (results not shown). Insulin-stimulated pAktThr308 and pAktSer473 relative to total Akt (pAktThr308/Akt and pAktSer473/Akt) were significantly lower in the HFD-SED group compared with the LFD-SED group and the HFD-3hPEX group (Fig. 5, A and C). Insulin-stimulated pAkt2Thr309 relative to total Akt2 (pAkt2Thr309/Akt2) was lower in HFD-SED versus LFD-SED (Fig. 5B). Insulin-stimulated pAkt2Ser474 relative to total Akt2 (pAkt2Ser474/Akt2) was greater in the HFD-3hPEX group compared with both the LFD-SED and HFD-SED control groups (Fig. 5D). There was a significant main effect of insulin (P < 0.001) for pAktThr308, pAkt2Thr309, pAktSer473, and pAkt2Ser474. For rats fed a HFD there was a significant main effect of exercise (P < 0.005) for pAktThr308, pAktSer473, pAkt2Ser474. For SED rats there was a significant main effect of diet (P < 0.005) for pAktThr308, pAktSer473, pAkt2Ser474. There was a significant treatment × insulin interaction effect (P < 0.005) for pAktThr308, pAktSer473, and pAkt2Ser474. The discovery of significant effects on pAkt2 in the current study spurred the question: Can acute exercise enhance pAkt2 in insulin-stimulated muscles from rats with normal insulin sensitivity? To answer this question, we evaluated pAkt2 in epitrochlearis muscles from LFD-SED and LFD-3hPEX rats. The muscles analyzed were from rats described in our recently published study (80) that used the same exercise protocol as the current study with LFD-fed male Wistar rats. This earlier study used the same epitrochlearis incubation protocol (±100 µU/ml insulin) as the current study. There were no differences in either basal or insulin-stimulated values between sedentary and 3hPEX muscles for pAkt2Ser474/Akt2 or pAkt2Thr309/Akt2 of LFD-fed rats (results not shown).
Fig. 5.
A–D: phosphorylated AktThr308/Akt, Akt2Thr309/Akt2, AktSer473/Akt, and Akt2Ser474/Akt2 at 3 h postexercise. Data were analyzed by two-way ANOVA. *P < 0.001, insulin vs. no insulin. †P < 0.01, significantly different from both other treatment groups within insulin-treated muscles. ‡P < 0.05, significantly different from LFD-SED within insulin-treated muscles. Values are expressed as means ± SEM; n = 10/group. HFD, high-fat diet; LFD, low-fat diet; SED, sedentary; 3hPEX, 3 h postexercise.
DISCUSSION
Because insulin resistance is a major health concern that affects millions of people, it is important to better understand the biological processes that underlie the exercise-induced improvement in insulin sensitivity of insulin-resistant skeletal muscle, the main tissue for insulin-mediated glucose disposal. The current study used a 2-wk HFD protocol that has been shown to induce skeletal muscle insulin resistance in rats (14, 48, 49). Having confirmed muscle insulin resistance based on reduced insulin-stimulated glucose uptake in the epitrochlearis in the current study (Fig. 1), we assessed key postexercise signaling events that have been proposed to be critical for enhancing insulin-stimulated glucose uptake in insulin-sensitive muscle. The main new findings of the study were: 1) γ3-AMPK activity, but not γ1-AMPK activity, in skeletal muscle was elevated both IPEX and 3hPEX compared with sedentary controls, 2) at 3hPEX insulin-stimulated pAS160Ser704 was increased, but insulin-independent pAS160Ser704 was unaltered compared with HFD-SED controls, 3) insulin-stimulated pAkt2Ser474 was elevated at 3hPEX compared with HFD-SED control, and 4) insulin-stimulated pAkt2Thr309 was decreased in sedentary muscle following a 2 wk HFD versus LFD-SED control. In addition, insulin-independent glucose uptake, pAS160Thr642, and pAS160Ser704 were elevated above sedentary (LFD and HFD) values IPEX. Furthermore, the abundance of α1, α2, β1, β2, γ1, and γ3 AMPK-subunits were unaltered by either HFD or exercise. Although a causal relationship remains to be established, these results support the idea that γ3-AMPK activity and AS160 phosphorylation are potentially part of the process leading to enhanced insulin-stimulated glucose uptake in insulin-resistant skeletal muscle following exercise.
We recently proposed a model to help elucidate the processes underlying increased insulin-stimulated glucose uptake by skeletal muscle postexercise (11). In this model, triggers are initiating events that activate downstream memory elements, which store the information that can be passed on to the mediators, which convert the memory into action that ultimately results in greater insulin-stimulated glucose uptake.
Skeletal muscle AMPK activation is a hallmark response to exercise (15, 21, 82, 85) and a potential trigger for increased insulin sensitivity. Exercise results in the reversible phosphorylation of AMPKαThr172 which markedly increases AMPK’s enzymatic activity (34). Increased pAMPKαThr172 and phosphorylation of AMPK’s substrate, acetyl CoA carboxylase (ACC) are widely used as indicators of AMPK activity (7, 28, 37, 39, 66). As expected, we observed increased pAMPKαThr172 and pACCSer79 IPEX. However, AMPK is a heterotrimeric protein complex that contains one catalytic (α) and two regulatory (β and γ) subunits, and values for pAMPKThr172 and pACCSer79 do not provide information regarding the isoform-specific activity of AMPK. The activation of various heterotrimeric combinations of AMPK in response to exercise can differ depending on the intensity and duration of the exercise bout (7, 70). In humans and rats, the effect of in vivo exercise has consistently been shown to increase γ3-containing AMPK activity (7, 36, 39, 70, 80). However, exercise-induced enhancement of γ1-AMPK activity in skeletal muscle has been less consistent. The current results, which are the first assessment of isoform-selective effects of exercise on AMPK activity in insulin-resistant rat skeletal muscle, show elevated γ3-AMPK activity, but unaltered γ1-AMPK activity, both IPEX and 3hPEX.
Earlier research provides several lines of evidence implicating γ3-AMPK activation as being potentially important for increased insulin sensitivity. Results using γ3-AMPK knockout mice revealed that γ3-AMPK is essential for the effect of prior exposure of skeletal muscle to AICAR, an AMPK-activator, on insulin-stimulated glucose uptake (37). Furthermore, γ3-AMPK activity was elevated 3 h after in situ muscle contraction along with greater insulin-stimulated glucose uptake in mouse skeletal muscle (35). Similar to the results for insulin-resistant rats in the current study, we recently reported in muscles from rats with normal insulin sensitivity that γ3-AMPK activity was increased (both IPEX and 3hPEX), and insulin-stimulated glucose uptake was enhanced 3hPEX (80).
What are possible cellular events that may serve as the memory elements that connect exercise-induced γ3-AMPK activation and insulin-stimulated glucose uptake? Increased postexercise pAS160Ser704 has emerged as a candidate memory element that is linked to AMPK because Ser704 is an AMPK phosphomotif. Furthermore, mutating AS160 Ser704 to Ala704, which prevents its phosphorylation, results in decreased insulin-stimulated pAS160Thr642 (37). Although insulin-independent pAS160Ser704 remained elevated for several hours after exercise in some studies (36, 50, 80), in the current study, pAS160Ser704 was no longer significantly increased above sedentary values at 3hPEX. Therefore, the current results do not support the idea that a sustained elevation in insulin-independent pAS160Ser704 is a likely memory element for greater insulin-stimulated glucose uptake in insulin-resistant rat skeletal muscle. It has been convincingly documented that Ser704 can be phosphorylated by AMPK (70). Phosphorylation on this site can also be increased by an insulin-dependent process (35, 50, 80) that is not mediated by AMPK (70). The insulin-regulated kinase that phosphorylates Ser704 remains to be identified. However, it has been demonstrated that insulin-stimulated Ser704 phosphorylation is inhibited by wortmannin, and is independent of either Akt or mTOR (73). It will be important for future research to identify this currently unknown, insulin-regulated kinase and to determine if it is modulated by exercise.
Given that greater activity of γ3-AMPK 3hPEX in insulin-resistant muscle was not accompanied by a sustained increase in insulin-independent pAS160Ser704, what are other possible memory elements that might rely on elevated γ3-AMPK activity? Mass spectrometry analysis identified several other sites that became phosphorylated in skeletal muscles from mice that had been injected with the AMPK-activator AICAR (73). Validated antibodies do not appear to be available for these sites, and it is unknown if they are responsive to in vivo exercise or if they have any effect on insulin-stimulated glucose uptake. However, it seems possible that AMPK-regulated phosphomotifs on AS160 other than Ser704 might influence insulin-stimulated glucose uptake in insulin-resistant muscle after exercise. Because various proteins can bind to AS160 (e.g., 14–3-3, RUVBL2, RIP140, and ClipR-59) (17, 24, 53, 86), an alternative possibility is that γ3-AMPK might phosphorylate these or other proteins, and thus indirectly regulate AS160s function. Alternatively, γ3-AMPK may lead to inhibition of protein phosphatase-1α or other phosphatases that regulate AS160 dephosphorylation (4, 63). Finally, γ3-AMPK activity remained elevated 3hPEX in insulin-resistant muscle, and we previously also observed a similar, long-lasting increase in γ3-AMPK activity in muscles from normal rats (80). Others have also reported that γ3-AMPK activity in skeletal muscle can remain elevated several hours after exercise by humans (29, 36, 65), in situ contraction (35), or ex vivo incubation with AICAR (37). Thus, it is possible that a sustained increase in muscle γ3-AMPK activity serves as a memory element that can lead to enhanced insulin sensitivity, and the mechanism may not necessarily involve AS160-dependent processes.
Insulin-stimulated phosphorylation of Akt is often reported to be unchanged following acute exercise (14, 20, 25, 30, 50, 80, 83). However, some studies have reported elevated insulin-stimulated Akt phosphorylation after exercise (3, 23, 61). Similarly, insulin-stimulated pAktSer473 and pAktThr308 were greater in insulin-stimulated muscle postexercise compared with SED controls. It is important to note that Akt2 is the isoform of Akt that is primarily responsible for insulin stimulation of both pAS160Thr642 and glucose uptake (6, 42, 58). No earlier research has evaluated acute exercise effects on the insulin-stimulated phosphorylation of Akt2 in skeletal muscle with a physiological insulin dose. Accordingly, we also measured insulin-stimulated pAkt2 and found that exercise increased insulin-stimulated pAkt2Ser474 and pAkt2Thr309 in HFD-fed rats. Increased pAkt2Ser474 is required for insulin’s full effect on Akt2 activity, pAS160Thr642, GLUT4 translocation and glucose uptake (32). In this context, our novel results suggest that the exercise-induced enhancement in insulin-stimulated pAS160Thr642 and glucose uptake may be related, at least in part, to greater phosphorylation on one or both of Akt2’s key regulatory sites. It would be valuable for future research to determine if the elevated insulin-stimulated phosphorylation on Akt2’s key regulatory sites after exercise occurs concomitant with greater Akt2 activity.
Improved insulin sensitivity after acute exercise has been reported in multiple species, including humans, rats, dogs, and sheep (41, 43, 44, 51). Humans and rats are the species that have been most frequently studied with regard to postexercise insulin sensitivity. The results for these species are similar in several important respects. The improvement in insulin sensitivity has been reported at ~1 to 4 h postexercise in both rats and humans (8, 52). Furthermore, the effect has also been observed to be sustained up to 48 h postexercise in both species (8, 52). In addition, earlier research has demonstrated that increased insulin-sensitivity can occur after acute exercise that does not result in increased total GLUT4 protein abundance in both rats and humans (14, 83). There is also evidence in both species that exercise with insulin sensitizing effects can be accompanied by greater AS160 phosphorylation on key regulatory sites (14, 50). Although many similarities have been reported between rats and humans with regard to acute exercise effects on insulin sensitivity, it remains quite possible that the specific mechanisms and features of this outcome may not be identical for all species. Nonetheless, research using various preclinical models, including rats, has substantial value for gaining insights about the potential mechanisms that underlie exercise benefits in humans.
Several important limitations of the current study should be noted. One limitation is that a LFD exercised group was not included in this study. However, it is informative to consider together some of the key outcomes in LFD and HFD rats from this and earlier studies using the same exercise, diet, and muscle incubation protocols. In the current study, there was a 42% increase in insulin-stimulated glucose uptake of epitrochlearis muscles from HFD rats in the 3hPEX group versus HFD sedentary controls. This increase is similar to the 32% increase in HFD rats after the same exercise protocol in our earlier study (14). In both studies, exercise eliminated the HFD-induced insulin resistance for glucose uptake compared with LFD sedentary rats. In three earlier studies, we reported an ~65–70% increase in insulin-stimulated glucose uptake of epitrochlearis muscles following an identical exercise protocol in LFD-3hPEX rats versus LFD sedentary controls (4, 14, 80). When LFD-3hPEX and HFD-3hPEX rats were directly compared in a previous study, insulin-stimulated glucose uptake was significantly greater for LFD compared with HFD rats (14). Thus, the same exercise protocol elicited greater insulin-stimulated glucose uptake in LFD versus HFD rats at both relative and absolute levels. An earlier study from our laboratory evaluated the influence of the same exercise protocol on isoform-selective AMPK activity and pAS160Ser704 in LFD rats (80). The pattern for exercise effects was similar for isoform-selective AMPK activity in each diet group (increased γ3-AMPK activity IPEX and 3hPEX; unaltered γ1-AMPK at both time points). In both diet groups, pAS160Ser704 was greater in muscles sampled from rats IPEX versus sedentary controls. However, the response to exercise (3hPEX versus sedentary) diverged between LFD rats in the earlier study (80) and HFD rats in the current study with regard to pAS160Ser704 in muscles incubated without insulin. The reason for the magnitude of the exercise-induced improvement in insulin-stimulated glucose uptake was greater for LFD versus HFD rats is uncertain. However, it is notable that the two diet groups did not differ with regard to the magnitude of the: increase in insulin-independent glucose uptake, increase in AMPK phosphorylation, or decrease in glycogen in muscle (14). Accordingly, other differences between the diet groups must be responsible for the lower insulin-stimulated glucose uptake after exercise by HFD rats. The muscle pAS160Ser704 was increased at 3hPEX for the LFD-fed rats (80), but not for HFD-fed rats in the current study. It is uncertain if this difference played any role in the lesser exercise effect on insulin-stimulated glucose uptake in HFD rats. However, it is notable that for both diets, in muscles incubated with insulin, pAS160Ser704 was increased for 3hPEX versus sedentary rats eating the same diet. Earlier research has demonstrated that in healthy rats consuming a LFD, prior exercise elevates the insulin-stimulated increase in GLUT4 translocation (26). The relative magnitude of this increase was similar to the magnitude of the increase in insulin-stimulated glucose uptake. Previous research has not reported the effect of acute exercise on subsequent insulin-stimulated GLUT4 translocation in muscle from insulin-resistant rats. Our working hypothesis is that prior exercise would elevate insulin-stimulated GLUT4 translocation to a lesser extent in insulin-resistant muscle versus insulin-sensitive muscle. Another limitation of the current study is that only relatively short-term HFD was evaluated. It would be informative to perform a study under conditions that induce more profound obesity and/or insulin resistance (e.g., longer durations of dietary interventions or genetically modified animals).
In conclusion, the current results provided novel evidence that acute exercise can result in elevated γ3-AMPK activity along with greater insulin-stimulated Akt2 phosphorylation and AS160 phosphorylation on Ser704 in insulin-resistant rat skeletal muscle. Appropriate genetic models will be invaluable to test the possibility that causal relationships may exist between each of these outcomes and improved insulin-stimulated glucose uptake in insulin-resistant muscle postexercise. A recent study reported that insulin-stimulated muscle glucose uptake several hours after in situ contraction was increased in wild-type mice, but not in AS160 muscle-specific knockout mice (33). It is essential to appreciate that, although electrically stimulated contraction can be a useful model for exercise, results with this model cannot be assumed to perfectly replicate in vivo exercise. It is also relevant to recognize that there is a much more extensive literature related to the processes responsible for in vivo exercise effects on insulin-stimulated glucose uptake by skeletal muscle in rats compared with mice (11). In this context, it is important to note the recent creation of an AS160-null rat model (5). This new genetic model will enable future research aimed at testing the idea that AS160 plays a key role in the exercise-induced improvement of insulin-stimulated glucose uptake in both normal and insulin-resistant rat skeletal muscle.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-71771.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.P. and G.D.C. conceived and designed research; M.W.P., E.B.A., H.W., and X.Z. performed experiments; M.W.P. and G.D.C. analyzed data; M.W.P. and G.D.C. interpreted results of experiments; M.W.P. and G.D.C. prepared figures; M.W.P. and G.D.C. drafted manuscript; M.W.P., E.B.A., H.W., X.Z., and G.D.C. edited and revised manuscript; M.W.P., E.B.A., H.W., X.Z., and G.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Jonas Treebak for generously supplying the pAS160Ser704 antibody and Dr. David Thomson for generously supplying the γ3-AMPK antibody.
REFERENCES
- 1.Abu-Elheiga L, Almarza-Ortega DB, Baldini A, Wakil SJ. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol Chem 272: 10669–10677, 1997. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- 2.Antharavally BS, Carter B, Bell PA, Krishna Mallia A. A high-affinity reversible protein stain for Western blots. Anal Biochem 329: 276–280, 2004. doi: 10.1016/j.ab.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 3.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 4.Arias EB, Wang H, Cartee GD. Akt substrate of 160 kDa dephosphorylation rate is reduced in insulin-stimulated rat skeletal muscle after acute exercise. Physiol Res 67: 143–147, 2018. doi: 10.33549/physiolres.933591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias EB, Zheng X, Agrawal S, Cartee GD. Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model. PLoS One 14: e0216236, 2019. doi: 10.1371/journal.pone.0216236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278: 49530–49536, 2003. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 7.Birk JB, Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577: 1021–1032, 2006. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- 9.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- 10.Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol Endocrinol Metab 258: E390–E393, 1990. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- 11.Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab 309: E949–E959, 2015. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58: 19–30, 2015. doi: 10.1007/s00125-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartee GD, Arias EB, Yu CS, Pataky MW. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am J Physiol Endocrinol Metab 311: E818–E824, 2016. doi: 10.1152/ajpendo.00289.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes 63: 2297–2308, 2014. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z-P, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab 279: E1202–E1206, 2000. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 16.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292: 1728–1731, 2001. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Consitt LA, Van Meter J, Newton CA, Collier DN, Dar MS, Wojtaszewski JF, Treebak JT, Tanner CJ, Houmard JA. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes 62: 3437–3447, 2013. doi: 10.2337/db13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JS, Gao J, Han D-H, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 20.Frøsig C, Sajan MP, Maarbjerg SJ, Brandt N, Roepstorff C, Wojtaszewski JF, Kiens B, Farese RV, Richter EA. Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle. J Physiol 582: 1289–1301, 2007. doi: 10.1113/jphysiol.2007.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun 273: 1150–1155, 2000. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 22.Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab 298: E999–E1010, 2010. doi: 10.1152/ajpendo.00758.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada T, Arias EB, Cartee GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol (1985) 101: 1368–1376, 2006. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol (1985) 85: 1218–1222, 1998. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- 27.Hardman SE, Hall DE, Cabrera AJ, Hancock CR, Thomson DM. The effects of age and muscle contraction on AMPK activity and heterotrimer composition. Exp Gerontol 55: 120–128, 2014. doi: 10.1016/j.exger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 29.Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K, Færgeman NJ, Havelund JF, Parker BL, James DE, Kiens B, Richter EA, Jensen J, Wojtaszewski JFP. Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol Metab 16: 24–34, 2018. doi: 10.1016/j.molmet.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwabe M, Kawamoto E, Koshinaka K, Kawanaka K. Increased postexercise insulin sensitivity is accompanied by increased AS160 phosphorylation in slow-twitch soleus muscle. Physiol Rep 2: e12162, 2014. doi: 10.14814/phy2.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46: 3–19, 2003. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 32.Kearney AL, Cooke KC, Norris DM, Zadoorian A, Krycer JR, Fazakerley DJ, Burchfield JG, James DE. Serine 474 phosphorylation is essential for maximal Akt2 kinase activity in adipocytes. J Biol Chem 294: 16729–16739, 2019. doi: 10.1074/jbc.RA119.010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjøbsted R, Chadt A, Jørgensen NO, Kido K, Larsen JK, de Wendt C, Al-Hasani H, Wojtaszewski JFP. TBC1D4 is necessary for enhancing muscle insulin sensitivity in response to AICAR and contraction. Diabetes 68: 1756–1766, 2019. doi: 10.2337/db18-0769. [DOI] [PubMed] [Google Scholar]
- 34.Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz M-N, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J 32: 1741–1777, 2018. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjøbsted R, Munk-Hansen N, Birk JB, Foretz M, Viollet B, Björnholm M, Zierath JR, Treebak JT, Wojtaszewski JF. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 66: 598–612, 2017. doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 36.Kjøbsted R, Pedersen AJ, Hingst JR, Sabaratnam R, Birk JB, Kristensen JM, Højlund K, Wojtaszewski JF. Intact regulation of the AMPK signaling network in response to exercise and insulin in skeletal muscle of male patients with type 2 diabetes: illumination of AMPK activation in recovery from exercise. Diabetes 65: 1219–1230, 2016. doi: 10.2337/db15-1034. [DOI] [PubMed] [Google Scholar]
- 37.Kjøbsted R, Treebak JT, Fentz J, Lantier L, Viollet B, Birk JB, Schjerling P, Björnholm M, Zierath JR, Wojtaszewski JF. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 64: 2042–2055, 2015. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 38.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen DE, Albers PH, Prats C, Baba O, Birk JB, Wojtaszewski JF. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol 593: 2053–2069, 2015. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masure S, Haefner B, Wesselink JJ, Hoefnagel E, Mortier E, Verhasselt P, Tuytelaars A, Gordon R, Richardson A. Molecular cloning, expression and characterization of the human serine/threonine kinase Akt-3. Eur J Biochem 265: 353–360, 1999. doi: 10.1046/j.1432-1327.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 41.McConell GK, Kaur G, Falcão-Tebas F, Hong YH, Gatford KL. Acute exercise increases insulin sensitivity in adult sheep: a new preclinical model. Am J Physiol Regul Integr Comp Physiol 308: R500–R506, 2015. doi: 10.1152/ajpregu.00466.2014. [DOI] [PubMed] [Google Scholar]
- 42.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005. doi: 10.2337/diabetes.54.5.1349. [DOI] [PubMed] [Google Scholar]
- 43.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab 254: E248–E259, 1988. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 44.Nagasawa J, Sato Y, Ishiko T. Time course of in vivo insulin sensitivity after a single bout of exercise in rats. Int J Sports Med 12: 399–402, 1991. doi: 10.1055/s-2007-1024701. [DOI] [PubMed] [Google Scholar]
- 45.Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (protein kinase B γ) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun 257: 906–910, 1999. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- 46.O’Neill HM, Lally JS, Galic S, Pulinilkunnil T, Ford RJ, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Skeletal muscle ACC2 S212 phosphorylation is not required for the control of fatty acid oxidation during exercise. Physiol Rep 3: e12444, 2015. doi: 10.14814/phy2.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pataky MW, Arias EB, Cartee GD. Measuring both glucose uptake and myosin heavy chain isoform expression in single rat skeletal muscle fibers. Methods Mol Biol 1889: 283–300, 2019. doi: 10.1007/978-1-4939-8897-6_17. [DOI] [PubMed] [Google Scholar]
- 48.Pataky MW, Wang H, Yu CS, Arias EB, Ploutz-Snyder RJ, Zheng X, Cartee GD. High-fat diet-induced insulin resistance in single skeletal muscle fibers is fiber type selective. Sci Rep 7: 13642, 2017. doi: 10.1038/s41598-017-12682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pataky MW, Yu CS, Nie Y, Arias EB, Singh M, Mendias CL, Ploutz-Snyder RJ, Cartee GD. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. Am J Physiol Endocrinol Metab 316: E695–E706, 2019. doi: 10.1152/ajpendo.00482.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pehmøller C, Brandt N, Birk JB, Høeg LD, Sjøberg KA, Goodyear LJ, Kiens B, Richter EA, Wojtaszewski JF. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes 61: 2743–2752, 2012. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pencek RR, James F, Lacy DB, Jabbour K, Williams PE, Fueger PT, Wasserman DH. Interaction of insulin and prior exercise in control of hepatic metabolism of a glucose load. Diabetes 52: 1897–1903, 2003. doi: 10.2337/diabetes.52.8.1897. [DOI] [PubMed] [Google Scholar]
- 52.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 53.Ren W, Cheema S, Du K. The association of ClipR-59 protein with AS160 modulates AS160 protein phosphorylation and adipocyte Glut4 protein membrane translocation. J Biol Chem 287: 26890–26900, 2012. doi: 10.1074/jbc.M112.357699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol Endocrinol Metab 246: E476–E482, 1984. doi: 10.1152/ajpendo.1984.246.6.E476. [DOI] [PubMed] [Google Scholar]
- 55.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol (1985) 66: 876–885, 1989. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 57.Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577: 997–1007, 2006. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab 291: E1031–E1037, 2006. doi: 10.1152/ajpendo.00204.2006. [DOI] [PubMed] [Google Scholar]
- 59.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 60.Schultze SM, Jensen J, Hemmings BA, Tschopp O, Niessen M. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch Physiol Biochem 117: 70–77, 2011. doi: 10.3109/13813455.2010.539236. [DOI] [PubMed] [Google Scholar]
- 61.Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol (1985) 113: 1852–1861, 2012. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma N, Arias EB, Bhat AD, Sequea DA, Ho S, Croff KK, Sajan MP, Farese RV, Cartee GD. Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. Am J Physiol Endocrinol Metab 300: E966–E978, 2011. doi: 10.1152/ajpendo.00659.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma P, Arias EB, Cartee GD. Protein phosphatase 1-α regulates AS160 Ser588 and Thr642 dephosphorylation in skeletal muscle. Diabetes 65: 2606–2617, 2016. doi: 10.2337/db15-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sjøberg KA, Frøsig C, Kjøbsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66: 1501–1510, 2017. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 65.Steenberg DE, Jørgensen NB, Birk JB, Sjøberg KA, Kiens B, Richter EA, Wojtaszewski JFP. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J Physiol 597: 89–103, 2019. doi: 10.1113/JP276735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437–443, 2000. doi: 10.1042/bj3450437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism 56: 1719–1728, 2007. doi: 10.1016/j.metabol.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Thong FS, Derave W, Kiens B, Graham TE, Ursø B, Wojtaszewski JF, Hansen BF, Richter EA. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 51: 583–590, 2002. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- 69.Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. A-769662 activates AMPK β1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol 297: C1041–C1052, 2009. doi: 10.1152/ajpcell.00051.2009. [DOI] [PubMed] [Google Scholar]
- 70.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 71.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treebak JT, Pehmøller C, Kristensen JM, Kjøbsted R, Birk JB, Schjerling P, Richter EA, Goodyear LJ, Wojtaszewski JF. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol 592: 351–375, 2014. doi: 10.1113/jphysiol.2013.266338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh H-J, Xie J, Feener EP, Wojtaszewski JF, Hirshman MF, Goodyear LJ. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol 298: C377–C385, 2010. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132: 2943–2954, 2005. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 75.Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol 347: 105–133, 2010. doi: 10.1007/82_2010_66. [DOI] [PubMed] [Google Scholar]
- 76.Vendelbo MH, Møller AB, Treebak JT, Gormsen LC, Goodyear LJ, Wojtaszewski JF, Jørgensen JOL, Møller N, Jessen N. Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise. J Appl Physiol (1985) 117: 289–296, 2014. doi: 10.1152/japplphysiol.00044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vind BF, Birk JB, Vienberg SG, Andersen B, Beck-Nielsen H, Wojtaszewski JF, Højlund K. Hyperglycaemia normalises insulin action on glucose metabolism but not the impaired activation of AKT and glycogen synthase in the skeletal muscle of patients with type 2 diabetes. Diabetologia 55: 1435–1445, 2012. doi: 10.1007/s00125-012-2482-8. [DOI] [PubMed] [Google Scholar]
- 78.Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol (1985) 65: 909–913, 1988. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Arias EB, Oki K, Pataky MW, Almallouhi JA, Cartee GD. Fiber type-selective exercise effects on AS160 phosphorylation. Am J Physiol Endocrinol Metab 316: E837–E851, 2019. doi: 10.1152/ajpendo.00528.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Arias EB, Pataky MW, Goodyear LJ, Cartee GD. Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am J Physiol Endocrinol Metab 315: E859–E871, 2018. doi: 10.1152/ajpendo.00020.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab 270: E299–E304, 1996. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 83.Wojtaszewski JF, Hansen BF, Gade J, Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49: 325–331, 2000. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 84.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes 46: 1775–1781, 1997. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 85.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol 528: 221–226, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie X, Chen Y, Xue P, Fan Y, Deng Y, Peng G, Yang F, Xu T. RUVBL2, a novel AS160-binding protein, regulates insulin-stimulated GLUT4 translocation. Cell Res 19: 1090–1097, 2009. doi: 10.1038/cr.2009.68. [DOI] [PubMed] [Google Scholar]
- 87.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B α/Akt1 regulates placental development and fetal growth. J Biol Chem 278: 32124–32131, 2003. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]