Abstract
Signaling via the tropomyosin-related kinase receptor subtype B (TrkB) regulates neuromuscular transmission, and inhibition of TrkB kinase activity by 1NMPP1 in TrkBF616A mice worsens neuromuscular transmission failure (NMTF). We hypothesized that acute inhibition of TrkB kinase activity will impair the ability of the diaphragm muscle to produce maximal transdiaphragmatic pressure (Pdi) without impacting the ability to generate forces associated with ventilation, consistent with the greater susceptibility to NMTF in motor units responsible for higher-force nonventilatory behaviors. Adult male and female TrkBF616A mice were injected with 1NMPP1 (n = 8) or vehicle (DMSO; n = 8) 1 h before Pdi measurements during eupneic breathing, hypoxia/hypercapnia (10% O2/5% CO2), tracheal occlusion, spontaneous deep breaths (“sighs”) and during maximal activation elicited by bilateral phrenic nerve stimulation. In the vehicle-treated group, Pdi increased from ~10 cmH2O during eupnea and hypoxia/hypercapnia, to ~35 cmH2O during sighs and tracheal occlusion, and to ~65 cm H2O during maximal stimulation. There was no effect of acute 1NMPP1 treatment on Pdi generated during most behaviors, except during maximal stimulation (~30% reduction; P < 0.05). This reduction in maximal Pdi is generally similar to the worsening of NMTF previously reported with TrkB kinase inhibition in rodents. Accordingly, impaired TrkB signaling limits the range of motor behaviors accomplished by the diaphragm muscle and may contribute to neuromuscular dysfunction, primarily by impacting fatigable, higher force-generating motor units.
NEW & NOTEWORTHY TrkB signaling plays an important role in maintaining neuromuscular function in the diaphragm muscle and may be necessary to accomplish the various motor behaviors ranging from ventilation to expulsive, behaviors requiring near-maximal forces. This study shows that inhibition of TrkB kinase activity impairs maximal pressure generation by the diaphragm muscle, but the ability to generate the lower pressures required for ventilatory behaviors is not impacted.
Keywords: diaphragm muscle, motor unit, neuromuscular junction, neuromuscular transmission, neurotrophins
INTRODUCTION
The diaphragm muscle is the main inspiratory muscle and accomplishes various behaviors that require a broad range of force generation (8, 11, 67). Transdiaphragmatic pressure (Pdi) measurements constitute an indirect measurement of the forces generated by the diaphragm muscle, yet are commonly used to determine diaphragm force (1, 17, 24, 27, 31, 36–38, 42, 45, 50, 61, 67, 69). Ventilatory behaviors require generation of lower forces, ~10–30% of the maximum Pdi, and can be accomplished by recruitment of fatigue resistant slow-twitch type S and fast-twitch type FR motor units. In contrast, expulsive, nonventilatory behaviors require higher forces (near maximum Pdi obtained by bilateral phrenic nerve stimulation), and the recruitment of fast-twitch fatigable type FF motor units (27, 38, 50, 71). Motor units differ in their contractile and fatigue properties (11, 67), as well as the susceptibility to fatigue during repetitive nerve stimulation-evoked contractions (7, 33).
Neurotrophin signaling via activation of the high-affinity tropomyosin-related kinase receptor subtype B (TrkB) is an important regulator of synaptic function and plasticity at the neuromuscular junction (NMJ) (52, 54). TrkB agonists prevent force loss associated with repetitive nerve stimulation, a global measure of neuromuscular transmission failure (NMTF), in both mice (48) and rats (55), likely via increased spontaneous and evoked neurotransmitter release (14, 46). Previous studies took advantage of a chemical-genetic approach using TrkBF616A mice, which harbor a point mutation that renders TrkB kinase activity susceptible to rapid and selective inhibition by the phosphoprotein phosphatase 1 (PP1) derivative 1NMPP1, to ascertain structural and functional effects of disrupting TrkB signaling. Inhibition of TrkB kinase activity by 1NMPP1 resulted in greater NMTF with repetitive activation of phrenic nerve-diaphragm muscle preparations, but without a change in quantal content (22, 28, 54). Since NMTF reflects primarily the fatigue of type FF motor units compared with type S and FR motor units (7, 33), we hypothesized that acute inhibition of TrkB kinase activity by 1NMPP1 treatment in TrkBF616A mice will impair the ability of the diaphragm muscle to produce maximal transdiaphragmatic pressure (Pdi) without impacting the ability to generate lower forces associated with ventilation.
METHODS
Animals.
Adult male (n = 8) and female (n = 8) TrkBF616A mice at 6 mo of age were used in these experiments. TrkBF616A mice have a 129J and C57BL/6 hybrid genetic background, and were genetically modified to carry a single phenylalanine-to-alanine mutation at the ATP-binding domain of the TrkB receptor, which renders TrkB kinase activity susceptible to rapid, selective and reversible inhibition by the phosphoprotein phosphatase 1 inhibitor (PP1) 1NMPP1 (3, 49). A previous study validated a 13-fold reduction of phosphorylated TrkB in protein extracted from brain tissue after 1NMPP1 treatment (49). Animals were housed at Mayo Clinic, group caged by sex, and maintained in a 12 h light cycle with free access to food and water. All protocols were approved by the Institutional Animal Care and Use Committee, in compliance with the National Institute of Health guidelines.
Experimental treatment.
TrkBF616A mice were assigned in randomized, masked fashion to 1NMPP1 (single dose of 5 μL, 10 mM 1NMPP1; Millipore Sigma, Burlington, MA) or vehicle (5 μL, 0.3% DMSO; Sigma-Aldrich, St Louis, MO) treatment groups. This dosage of 1NMPP1 is equivalent with previous work documenting inhibition of TrkB kinase activity in TrkBF616A mice (32, 47) as well as functional effects on neuromuscular transmission (22, 49, 54). 1NMPP1 or vehicle were administered via intraperitoneal injection 1 h before the experimental procedures. Group assignment was only revealed after data analyses were completed.
Transdiaphragmatic pressure (Pdi).
Mice were lightly anesthetized with fentanyl (0.3 mg/kg), diazepam (5 mg/kg) and droperidol (15 mg/kg) ~45 min after treatment. A level of anesthesia was maintained displaying absence of response to corneal reflex in spontaneously breathing mice (31). As in previous studies (23, 25, 27, 36, 50, 71), Pdi was calculated as the difference between the gastric and esophageal pressures. The trachea was cannulated (19G), and two pressure transducers (MikroTip catheter transducer, 3.5F, #8405249, SPR-524; Millar Instruments, Houston, TX) were placed in the stomach and esophagus, spanning the thoracic and abdominal borders of the diaphragm muscle. Correct placement of the catheters was confirmed by positive and negative pressure deflections of the gastric and esophageal transducers, respectively, across motor behaviors, with further postmortem evaluation. The abdomen was bound with a custom-made restriction device to limit abdominal excursion and thereby obtain near isometric conditions for diaphragm muscle activation. Esophageal (Pes) and abdominal (Pgas) pressures were recorded and digitized (400 Hz) with PowerLab 4/35 (ADInstruments, Colorado Springs, CO) and visualized in real-time with LabChart 8. The Pdi signal was band-pass filtered between 0.3 and 30 Hz. Data were exported, downsampled to 100 Hz, and analyzed using a custom-designed semiautomated script in MATLAB (MathWorks, Natick, MA) (18, 27, 36–38, 58). Pdi measurements were obtained during 1) quiet breathing of room air (eupnea; Eup), 2) breathing of a hypoxic-hypercapnic gas mixture (10% O2-5% CO2; hypoxia-hypercapnia) for 5 min, 3) breathing efforts against an occluded airway (Occ), and 4) maximal Pdi elicited by bilateral phrenic nerve stimulation (Stim). Supramaximal stimulation frequency at 150 Hz was used, and stimulation current was adjusted until maximum Pdi response was elicited, as previously described (27). Deep breaths (“sighs”) were defined as large inspiratory events (~2 times normal eupneic Pdi) occurring spontaneously, followed by an apneic period with elimination of at least one full breath (38, 44, 50). The animals were allowed to rest for at least 2 min between behaviors to allow Pdi amplitude to return to eupneic baseline. Heart rate and O2 saturation were monitored with a thigh pulse-oximeter (MouseOx Plus, Starr Life Sciences, Oakmont, PA). Supplemental O2 was administered when saturation fell below 80% (21) and anesthetic agents were administered (one-third of the initial dose) when heart rate increased 20% from baseline during the recovery periods.
After completing other measurements, surgical microdissection was performed in the ventral cervical region to bilaterally expose and isolate phrenic nerves before stimulation. Straight parallel bipolar electrodes (FHC, catalog #30211, Bowdoin, ME) and a Grass stimulator (Grass S88, Grass Telefactor, Warwick, RI) were employed to stimulate the phrenic nerves with 0.02-ms duration pulses at a frequency of 150-Hz supramaximal stimulation, in 330-ms-duration trains repeated every s, for 3–5 trains. During supramaximal stimulation, deflections in Pes and Pgas were verified to be in the appropriate direction, the duration of the Pdi response matched the stimulation period, and there were no obvious signs of movement artifact in the individual Pes and Pgas signals or the resulting Pdi signal (27).
Behaviors were analyzed for a 1–2 min period during eupnea, the final 2 min during hypoxia-hypercapnia, sighs recorded during eupnea and the hypoxia-hypercapnia exposure, 2–5 maximal breaths during occlusion, and 1–5 maximal stimulation events for bilateral phrenic nerve stimulation (18, 27, 36–38, 50). Baseline heart rate before placement of transducers, requirements of extra anesthetic dose, requirements of supplemental O2, and surgery duration time were recorded and showed no difference between treatment groups.
Statistical analyses.
Every statistical evaluation was performed using standard statistical software (JMP Pro 11, SAS Institute, Cary, NC), with data assessed for normality using D’Agostino and Pearson omnibus tests. Pdi amplitude and ventilatory parameters across experimental groups and motor behaviors were evaluated using a mixed linear model with behavior (eupnea, hypoxia-hypercapnia, occlusion, sigh, stimulation), treatment group (vehicle or 1NMPP1), and the behavior group interactions as fixed effects and with animal as a random effect. Based on previous reports of Pdi in mice (22, 27), we estimated that a 25% reduction in maximum Pdi following 1NMPP1 treatment would be statistically significant with 80% power and α = 0.05 using n = 6/group; thus, 8 animals were randomly assigned to each treatment to account for possible technical failures. When appropriate, post hoc analyses were conducted using Tukey-Kramer HSD test, chi-square, or t-test. Statistical significance was established at P < 0.05. All experimental data in the text of the manuscript are presented as means ± 95% confidence interval (CI) across behaviors, unless otherwise specified.
RESULTS
Animals.
All mice (4 male, 4 female in each treatment group) were treated with vehicle or 1NMPP1 for 1 h. Three male mice had their tracheal cannula displaced before the end of the procedure and did not complete the experiment and thus were excluded. The final analyses included six vehicle-treated (2 male, 4 female) and seven 1NMPP1-treated mice (3 male, 4 female). A significant difference in body weight across sex was observed [males: 29 ± 3 g, females: 25 ± 1 g; t(11) = 2.67, P = 0.02].
Transdiaphragmatic pressure (Pdi) measurements.
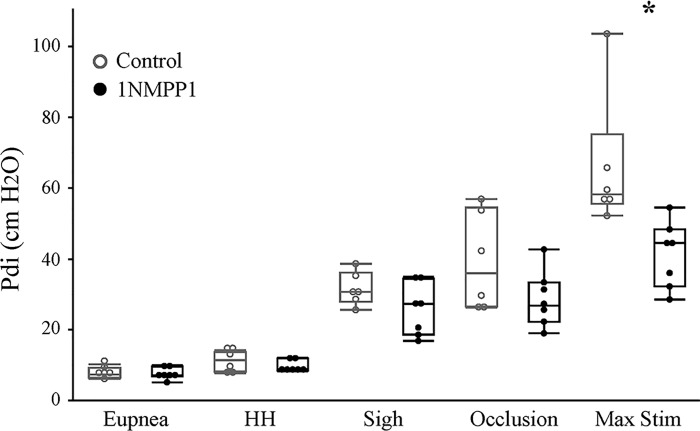
Pdi was successfully recorded for all conditions in all included 1NMPP1 animals (n = 7) or vehicle (n = 6). During each recording session, anesthetic depth was maintained across animals by continuous monitoring of heart rate, respiratory frequency, O2 saturation, and frequent assessment of the deep pain response and palpebral reflex. Mixed linear models for Pdi did not show evidence of an effect of sex, and thus all data presented reflects males and females combined. Figure 1 shows representative Pdi tracings across all behaviors and during bilateral phrenic nerve stimulation. Figure 2 shows the boxplot of mean Pdi amplitude across behaviors for each animal in both the 1NMPP1 and vehicle groups. There was a significant effect on Pdi amplitude of behavior (F4,44 = 76, P < 0.001), treatment group (F1,11 = 8, P = 0.015), and their interaction (F4,44 = 5, P = 0.002). There were no significant differences in Pdi amplitude during eupnea and hypoxia-hypercapnia between groups. Mean Pdi amplitude during eupnea was 7.7 ± 1.2 cmH2O for 1NMPP1 treatment and 7.8 ± 1.2 cmH2O for vehicle control and during hypoxia-hypercapnia was 9.7 ± 1.2 cmH2O for 1NMPP1 treatment and 11.1 ± 2.3 cmH2O for vehicle control. Pdi amplitude during occlusion and during sighs was ~3 times higher than the amplitude during eupnea. There was no effect of 1NMPP1 treatment on Pdi amplitude during occlusion (28.8 ± 5.9 cmH2O for 1NMPP1 treatment and 39.6 ± 11.0 cmH2O for vehicle control) or sighs (28.8 ± 5.4 cmH2O for NMPP1 treatment and 31.6 ± 3.8 cmH2O for vehicle control). Pdi during bilateral phrenic nerve stimulation was significantly reduced by 37% in the 1NMPP1 treatment group (41.2 ± 6.9 cmH2O) compared with vehicle control group (65.8 ± 15.2 cmH2O; P < 0.05).
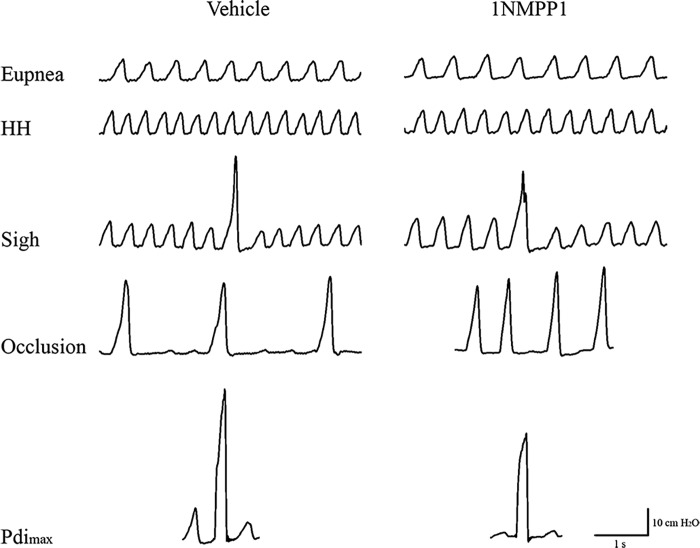
Fig. 1.
Representative transdiaphragmatic pressure (Pdi) tracings from adult 6 mo old TrkBF616A mice during eupnea (breathing room air), hypoxia-hypercapnia (HH; 10% O2-5% CO2), spontaneous deep breaths (sighs), and sustained tracheal occlusion, as well as maximal Pdi generated by bilateral phrenic nerve stimulation at 150 Hz. TrkBF616A mice were treated with vehicle or 1NMPP1 for 1 h.
Fig. 2.
Summary results of transdiaphragmatic pressures (Pdi) generated during eupnea, hypoxia-hypercapnia (10% O2-5% CO2), sighs, tracheal occlusion and maximal stimulation in TrkBF616A mice treated with vehicle (n = 6) or 1NMPP1 (n = 7) for 1 h. Data were analyzed using a mixed linear model with animal as a random effect (see Methods). There was a significant effect on Pdi amplitude of behavior (F4,44 = 76, P < 0.001), treatment group (F1,11 = 8, P = 0.015), and their interaction (F4,44 = 5, P = 0.002). Boxplot shows the median, first and third quartiles (box), minimum and maximum values (whiskers). *Significantly different compared with vehicle-treated group in post hoc Tukey-Kramer HSD tests.
Ventilatory parameters.
Respiratory rate, inspiratory duration, and duty cycle were calculated from the Pdi signal during eupnea and hypoxia-hypercapnia and are reported in Table 1. Mixed linear models for ventilatory parameters did not show evidence of an effect of sex, and thus all data presented reflect males and females combined. There was a significant effect on respiratory rate of behavior (F1,38 = 468, P < 0.001), but not treatment (F1,10 = 0.39, P = 0.55). In both groups, respiratory rate was significantly higher during hypoxia-hypercapnia than during eupnea by ~50%. Overall, respiratory rate increased to ~175 min−1 during hypoxia-hypercapnia compared with 115 min−1 during eupnea. There was a significant effect of behavior on inspiratory duration (F1,11 = 101, P < 0.01), but not treatment (F1,11 = 0.70 P = 0.42). Inspiratory duration decreased to ~150 ms during hypoxia-hypercapnia compared with ~200 ms during eupnea. Accordingly, there was a significant effect on duty cycle of behavior (F1,35 = 93, P < 0.01), but not treatment (F1,9 = 0.43 P = 0.53).
Table 1.
Respiratory rate, inspiratory duration, and duty cycle calculated from the Pdi signal during eupnea and hypoxia-hypercapnia
| Vehicle | 1NMPP1 | |
|---|---|---|
| Respiratory rate, min−1* | ||
| Eupnea | 122.2 ± 17.4 | 111.3 ± 30.2 |
| Hypoxia-Hypercapnia | 184.3 ± 23.9 | 172.1 ± 22.1 |
| Inspiratory duration, ms* | ||
| Eupnea | 189.2 ± 10.5 | 205.4 ± 24.4 |
| Hypoxia-Hypercapnia | 152.8 ± 8.8 | 154.9 ± 12.9 |
| Duty cycle, %* | ||
| Eupnea | 38.3 ± 4.9 | 36.6 ± 5.7 |
| Hypoxia-Hypercapnia | 46.6 ± 4.3 | 43.9 ± 3.8 |
Data analyzed using a mixed linear model with animal as a random effect, and presented as means ± 95% CI.
Behavior effect was evident (P < 0.001), but no effect of treatment.
DISCUSSION
Using a genetic and chemical model that allows rapid and selective inhibition of TrkB kinase activity using PP1 derivatives such as 1NMPP1 (3, 49, 54), we found that TrkB signaling is necessary for the diaphragm muscle to generate maximal forces but not to generate lower-force ventilatory behaviors including eupnea, hypoxia-hypercapnia, sighs, and airway occlusion. These results demonstrate rapid motor effects in vivo at the whole animal level that expand on previous observations regarding the importance of TrkB kinase activity in maintaining neuromuscular function (22, 28, 48, 54, 55). Furthermore, the lack of effects on behaviors that can be accomplished by selective recruitment of fatigue resistant slow-twitch type S and fast-twitch type FR motor units in the diaphragm muscle (7, 23, 37, 50, 68, 69, 71), suggests minimal effects of TrkB kinase activity at these units. Taken together, inhibition of TrkB signaling in vivo limits the range of motor behaviors accomplished by the diaphragm muscle, and it is expected that the reduced maximal Pdi is primarily the result of inhibiting TrkB kinase activity at higher force-generating fast-twitch fatigable type FF motor units.
Motor unit composition and diaphragm muscle force generation.
The diaphragm muscle comprises different types of motor units, allowing it to accomplish various behaviors requiring a broad range of force generation. The present study used Pdi, a reliable method to evaluate diaphragm muscle force generation across species, including mice. The Pdi generated during eupnea and hypoxia-hypercapnia is ~10–20% of maximal Pdi, in accordance with previously reported data in mice (24, 27), hamsters (69), rats (17, 31, 36, 37, 50), cats (67), sheep (1, 61) and humans (45, 69). Also, consistent with previous reports, the Pdi generated during bilateral phrenic nerve stimulation was ~65 cmH2O in mice, compared with ~70 cmH2O in rabbits (6), ~75 cmH2O in sheep, ~80 cmH2O in piglets (57, 75), ~85 cmH2O in rats and ~180 cmH2O in humans (42).
The force generated by motor systems in general can be increased by either increasing the number of recruited motor units or by increasing the discharge frequency of the motor units already recruited (8, 11, 51, 53). In addition, the force generated is dependent on motor unit type (50, 67). Type S and FR motor units show lower force generation capacity when compared with type FInt motor units, and much lower when compared with type FF motor units. Across a number of species, the generation of forces necessary to accomplish behaviors associated with ventilation is possible with the recruitment of lower force generating, but fatigue resistant type S and type FR units. Other behaviors not associated with ventilation (i.e., defecation, parturition, emesis, coughing or sneezing) require higher forces (8, 27, 50, 67, 70) that can only be accomplished by the full recruitment of most motor units including the more fatigable type FInt and type FF units.
Motor units discharge frequencies match the steep portion of their force generating capacity (7, 11, 40, 64, 65, 67, 73), and their susceptibility to failure with repetitive nerve stimulation (33, 72). For example, the onset discharge frequencies of rat diaphragm motor units during an inspiratory burst are ~8–15 Hz for type S and FR units compared with ~25–40 Hz for type FInt and FF units (8, 9). Thus type S and FR motor units are recruited at lower discharge frequencies associated with effective and reliable neuromuscular transmission. Neuromuscular transmission failure is much more pronounced at the higher discharge rates associated with recruitment of type FInt and FF units (33, 72). Indeed, repetitive nerve stimulation resulted in greater fatigue at type IIx and/or IIb diaphragm muscle fibers than at type I and IIa muscle fibers, comprising type FInt and FF units versus type S and FR units, respectively. Of note, when higher discharge frequencies are used, more synaptic vesicles are released (4, 60). In fact, retrieval of presynaptic terminal membranes is increased at higher discharge frequencies, suggesting a greater susceptibility of type FInt and FF motor units to repetitive stimulation (20).
Previous studies evaluating the initial train of neuromuscular transmission indicate that NMTF is practically negligible at lower frequencies (10 Hz) and occurs only during high stimulation frequencies (75 Hz) (7, 10, 33). With repetitive stimulation, NMTF occurs at both frequencies, but the extent of NMTF is much larger at higher frequencies (~60–70%) than at lower frequencies (~30%) (5, 7, 10, 33, 40, 66, 72). As the higher force-generating, fatigable motor units type FInt and FF are active at the higher discharge frequencies (11, 35, 70), NMTF reflects primarily failure at type FInt and FF motor units. In agreement, maximal Pdi represents the activation of higher force generating type FInt and FF units during tetanic, maximal contractions of the diaphragm elicited by bilateral phrenic nerve stimulation (at 150 Hz).
TrkB kinase activity regulates neuromuscular transmission and Pdi generation.
Previous studies show that BDNF/TrkB signaling modulates neuromuscular transmission and plays an important role in maintaining synaptic function and structure at the adult NMJ (14, 22, 54, 55). In particular, BDNF/TrkB signaling is important for effective and reliable neuromuscular transmission. TrkB agonists improve neuromuscular transmission during repetitive phrenic nerve stimulation in various rodent models (22, 55). In fact, acetylcholine release from presynaptic terminals at individual NMJs increases after the administration of TrkB agonists such as BDNF, neurotrophin-4 (14) and 7,8-dihydroxyflavone (7,8-DHF) (48). On the other hand, TrkB kinase inhibitors such as K252a and 1NMPP1 (in TrkBF616A mice) worsens failure in various rodent models (22, 54, 55). Even though muscle fibers can express TrkB receptors, there is a consistent lack of effect of TrkB agonists or inhibitors on the contractile and fatigue properties of the diaphragm muscle ex vivo in both mice (22, 48, 54) and rats (55).
Neurotrophins are present in motor neurons (29, 39), including motor neurons in the cervical spinal cord (34), at the NMJ (15) and in skeletal muscles (12, 13). Although there is conflicting evidence, there appears to be differential expression of neurotrophic factors across muscle fiber types (62, 63, 74). In rats, full length TrkB receptor is present in the diaphragm and soleus muscles, but not in muscles predominantly comprised of fast-twitch fibers (i.e., extensor digitorum longus, gastrocnemius or tibialis anterior muscles). In agreement, expression of neurotrophin was increased soleus muscle (predominantly slow-twitch fibers) versus gastrocnemius (predominantly fast-twitch fibers) (12). Whether these differences represent motor unit type differences that are also present in the diaphragm muscle is presently not clear. Of note, previous work using aging TrkBF616A mice showed that inhibition of TrkB kinase activity with 1NMPP1 treatment for 1 wk selectively disrupts the morphology at NMJs, shifting the overall distribution of pre- and postsynaptic volumes to smaller volumes (28, 66). These results suggest that long-term disruption of TrkB kinase signaling primarily affects NMJs at type IIx muscle fibers in the mouse.
The role of BDNF/TrkB signaling on neuromotor control in vivo can be uniquely ascertained by the acute effects of inhibiting TrkB kinase activity using 1NMPP1 in TrkBF616A mice. Mice heterozygous for TrkB (TrkB+/−) showed impaired neuromuscular transmission in the soleus muscle associated with morphological changes at the NMJ (41). Postsynaptic disassembly at the NMJ was evident in rats following viral-induced overexpression of truncated TrkB receptors incapable of mediating kinase activity (19). Truncated receptor overexpression (regardless of pre- or postsynaptic localization) is expected to reduce BDNF availability at the NMJ and BDNF/TrkB signaling. The chemical-genetic approach allows selective and rapid inhibition of BDNF/TrkB signaling without confounding introduced by altered neurotrophic signaling during development (3, 22, 48, 49, 54). Taken together, these results are consistent with signaling via full-length TrkB receptors as essential for the full activation of motor units necessary for maximal force generation.
Previous studies support the high bioavailability of 1NMPP1 as it is a highly soluble molecule and is expected to cross the blood-brain barrier. Indeed, we previously reported a 13-fold reduction in the ratio of phosphorylated to total TrkB in brain tissue following 1 wk of 1NMPP1 treatment in TrkBF616A mice (49). In the present study, we did not observe any effect of 1NMPP1 on gross behaviors and/or ventilation following a 1 h systemic treatment via intraperitoneal injection, suggesting predominant 1NMPP1 effects on neuromuscular transmission (22, 54) rather than a CNS effect of 1NMPP1. Furthermore, although anesthetic agents including those used in the present study (i.e., fentanyl) can produce ventilatory depression (31, 56), previous work by our group and others document the lack of ventilatory effects at the low dose of anesthetics used (25, 30), and both treatment groups (vehicle and 1NMPP1) were otherwise studied under identical conditions. Based on their different pharmacological mechanism of action, interaction effects between 1NMPP1 and the anesthetic drugs are not expected.
Conclusions.
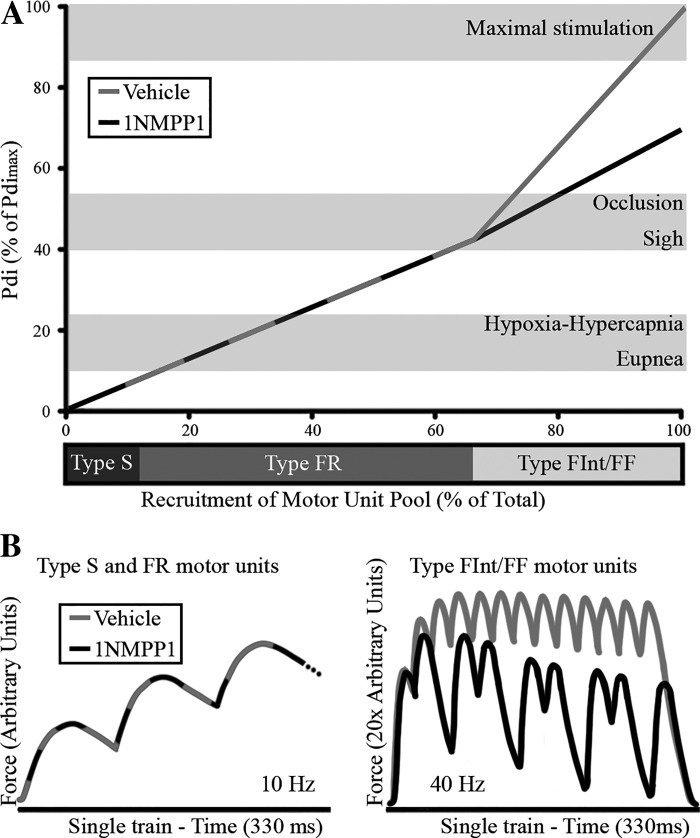
The results of the present study show that acute inhibition of the TrkB kinase activity with 1NMPP1 in TrkBF616A mice impairs the ability of the diaphragm muscle to generate maximal forces. Such a reduction in diaphragm muscle force generation is likely due to primary effects of TrkB signaling on type FInt and FF motor units (Fig. 3). Assuming an orderly recruitment of type S motor units, followed by type FR, FInt and FF units, ventilatory behaviors including eupnea, hypoxia-hypercapnia and even sighs or airway occlusion in mice can be accomplished with recruitment of more fatigue-resistant type S and FR units. Fatigable type FInt and FF units would be recruited only during behaviors that require higher forces (e.g., coughing, sneezing). In this model of motor unit recruitment in mice (24, 25, 27, 58), the proportions of each motor unit type and the forces per motor unit were estimated from previous data that reported fiber type proportions determined by myosin heavy chain isoform expression in the adult male wild-type mouse, cross-sectional areas of type-identified fibers (23, 26, 66) and differences in specific force across diaphragm muscle fiber types (16, 38, 43, 59, 71). Several studies support the predominant effect of BDNF/TrkB signaling on motor control as motor unit type specific (48, 54, 55). Inhibition of TrkB kinase activity results in reduced maximal Pdi (present study) and worsening of repetitive stimulation-induced NMTF (22, 54). If sustained, morphological changes are evident at the NMJ that reflect greater effects on type FInt and FF motor units (22). Disrupting BDNF/TrkB signaling, however, shows lack of effects on fatigue and contractile properties of the muscle. Taken together, this body of evidence supports a primary role of TrkB signaling on sustained force development at more fatigable motor units and further suggests a possible role of TrkB agonists as a therapeutic target in conditions characterized by impaired neuromuscular transmission (including neuromuscular disorders). Last, it is now clear that BDNF/TrkB signaling modulates important behavioral responses evident at the organism level (Pdi).
Fig. 3.
The diaphragm muscle accomplishes several behaviors requiring a wide range of force generation. A: in a model of motor unit recruitment, orderly recruitment of type slow (S) and fast-twitch fatigue resistant (FR) motor units generates the forces necessary for eupnea, hypoxia-hypercapnia, and even sigh or airway occlusion in mice. Fatigable type FInt and FF units are recruited only during behaviors that require higher forces such as during expulsive behaviors (e.g., coughing) or maximal stimulation. B: force development differs across motor units, with tetanic stimulation of type S and FR motor units (active at lower stimulation frequencies; left panel) resulting in substantially lower forces than stimulation of type FInt or FF motor units (active at higher frequencies; right panel) (2, 64). Inhibition of TrkB kinase activity with 1NMPP1 treatment in TrkBF616A mice results in neuromuscular transmission failure predominantly at the higher stimulation frequencies of type FInt and FF units (30–50 Hz versus 10–20 Hz at type S and FR units). Accordingly, inhibiting TrkB kinase activity worsens overall force development (B) and Pdi generation (A) in adult lightly anesthetized mice.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-AG-57052 and R01-AG-044615 and the Mayo Clinic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P.-C., H.M.G., G.C.S., and C.B.M. conceived and designed research; M.P.-C. and N.A.C. performed experiments; M.P.-C., N.A.C., and C.B.M. analyzed data; M.P.-C., N.A.C., G.C.S., and C.B.M. interpreted results of experiments; M.P.-C., H.M.G., and C.B.M. prepared figures; M.P.-C. and C.B.M. drafted manuscript; M.P.-C., H.M.G., G.C.S., and C.B.M. edited and revised manuscript; H.M.G., G.C.S., and C.B.M. approved final version of manuscript.
REFERENCES
- 1.Bazzy AR, Akabas SR, Hays AP, Haddad GG. Respiratory muscle response to load and glycogen content in type I and II fibers. Exp Neurol 101: 17–28, 1988. doi: 10.1016/0014-4886(88)90061-1. [DOI] [PubMed] [Google Scholar]
- 2.Burke RE, Levine DN, Tsairis P, Zajac FE III. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748, 1973. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron 46: 13–21, 2005. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Clayton EL, Cousin MA. The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J Neurochem 111: 901–914, 2009. doi: 10.1111/j.1471-4159.2009.06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low-dose inhaled CO. Am J Physiol Regul Integr Comp Physiol 298: R784–R789, 2010. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson GT, Irvin CG, Cherniack RM. Relationship of diaphragm glycogen, lactate, and function to respiratory failure. Am Rev Respir Dis 141: 926–932, 1990. doi: 10.1164/ajrccm/141.4_Pt_1.926. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty MJ, Gonzalez Porras MA, Mantilla CB, Sieck GC. Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol 122: 93–104, 2019. doi: 10.1152/jn.00061.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogarty MJ, Mantilla CB, Sieck GC. Breathing: motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogarty MJ, Sieck GC. Evolution and functional differentiation of the diaphragm muscle of mammals. Compr Physiol 9: 715–766, 2019. doi: 10.1002/cphy.c180012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett 125: 34–36, 1991. doi: 10.1016/0304-3940(91)90124-C. [DOI] [PubMed] [Google Scholar]
- 11.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibáñez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science 268: 1495–1499, 1995. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123: 455–465, 1993. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia N, Tomàs M, Santafé MM, Besalduch N, Lanuza MA, Tomàs J. The interaction between tropomyosin-related kinase B receptors and presynaptic muscarinic receptors modulates transmitter release in adult rodent motor nerve terminals. J Neurosci 30: 16514–16522, 2010. doi: 10.1523/JNEUROSCI.2676-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia N, Tomàs M, Santafe MM, Lanuza MA, Besalduch N, Tomàs J. Localization of brain-derived neurotrophic factor, neurotrophin-4, tropomyosin-related kinase b receptor, and p75 NTR receptor by high-resolution immunohistochemistry on the adult mouse neuromuscular junction. J Peripher Nerv Syst 15: 40–49, 2010. doi: 10.1111/j.1529-8027.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 16.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 89: 695–703, 2000. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 17.Gill LC, Gransee HM, Sieck GC, Mantilla CB. Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons. Respir Physiol Neurobiol 226: 128–136, 2016. doi: 10.1016/j.resp.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol 210: 14–21, 2015. doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron 24: 567–583, 1999. doi: 10.1016/S0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez Porras MA, Fogarty MJ, Gransee HM, Sieck GC, Mantilla CB. Frequency-dependent lipid raft uptake at rat diaphragm muscle axon terminals. Muscle Nerve 59: 611–618, 2019. doi: 10.1002/mus.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray LH, Steadman JM. Determination of the oxyhaemoglobin dissociation curves for mouse and rat blood. J Physiol 175: 161–171, 1964. doi: 10.1113/jphysiol.1964.sp007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol 593: 431–440, 2015. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greising SM, Mantilla CB, Medina-Martínez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol 309: L46–L52, 2015. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greising SM, Mantilla CB, Sieck GC. Functional measurement of respiratory muscle motor behaviors using transdiaphragmatic pressure. Methods Mol Biol 1460: 309–319, 2016. doi: 10.1007/978-1-4939-3810-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle Nerve 52: 76–82, 2015. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol 188: 56–59, 2013. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greising SM, Stowe JM, Sieck GC, Mantilla CB. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp Gerontol 72: 184–191, 2015. doi: 10.1016/j.exger.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Ruilamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 363: 266–270, 1993. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 30.Ingalls CP, Warren GL, Lowe DA, Boorstein DB, Armstrong RB. Differential effects of anesthetics on in vivo skeletal muscle contractile function in the mouse. J Appl Physiol (1985) 80: 332–340, 1996. doi: 10.1152/jappl.1996.80.1.332. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Ruiz F, Khurram OU, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB. Diaphragm muscle activity across respiratory motor behaviors in awake and lightly anesthetized rats. J Appl Physiol (1985) 124: 915–922, 2018. doi: 10.1152/japplphysiol.01004.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AW, Chen X, Crombag HS, Zhang C, Smith DR, Shokat KM, Gallagher M, Holland PC, Ginty DD. The brain-derived neurotrophic factor receptor TrkB is critical for the acquisition but not expression of conditioned incentive value. Eur J Neurosci 28: 997–1002, 2008. doi: 10.1111/j.1460-9568.2008.06383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol (1985) 75: 341–348, 1993. doi: 10.1152/jappl.1993.75.1.341. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci 20: RC77, 2000. doi: 10.1523/JNEUROSCI.20-10-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kernell D, Eerbeek O, Verhey BA. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat’s m. peroneus longus. Exp Brain Res 50: 211–219, 1983. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- 36.Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following mid-cervical contusion injury in rats. J Appl Physiol (1985) 126: 221–230, 2019. doi: 10.1152/japplphysiol.00481.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khurram OU, Fogarty MJ, Sarrafian TL, Bhatt A, Mantilla CB, Sieck GC. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep 6: e13786, 2018. doi: 10.14814/phy2.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurram OU, Sieck GC, Mantilla CB. Compensatory effects following unilateral diaphragm paralysis. Respir Physiol Neurobiol 246: 39–46, 2017. doi: 10.1016/j.resp.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10: 359–367, 1993. doi: 10.1016/0896-6273(93)90326-M. [DOI] [PubMed] [Google Scholar]
- 40.Kuei JH, Shadmehr R, Sieck GC. Relative contribution of neurotransmission failure to diaphragm fatigue. J Appl Physiol (1985) 68: 174–180, 1990. doi: 10.1152/jappl.1990.68.1.174. [DOI] [PubMed] [Google Scholar]
- 41.Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol (1985) 111: 844–852, 2011. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- 42.Laporta D, Grassino A. Assessment of transdiaphragmatic pressure in humans. J Appl Physiol (1985) 58: 1469–1476, 1985. doi: 10.1152/jappl.1985.58.5.1469. [DOI] [PubMed] [Google Scholar]
- 43.Lewis MI, Sieck GC. Effect of acute nutritional deprivation on diaphragm structure and function. J Appl Physiol (1985) 68: 1938–1944, 1990. doi: 10.1152/jappl.1990.68.5.1938. [DOI] [PubMed] [Google Scholar]
- 44.Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature 530: 293–297, 2016. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisboa C, Paré PD, Pertuzé J, Contreras G, Moreno R, Guillemi S, Cruz E. Inspiratory muscle function in unilateral diaphragmatic paralysis. Am Rev Respir Dis 134: 488–492, 1986. doi: 10.1164/arrd.1986.134.3.488. [DOI] [PubMed] [Google Scholar]
- 46.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363: 350–353, 1993. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Ji Y, Ganesan S, Schloesser R, Martinowich K, Sun M, Mei F, Chao MV, Lu B. TrkB as a potential synaptic and behavioral tag. J Neurosci 31: 11762–11771, 2011. doi: 10.1523/JNEUROSCI.2707-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve 45: 274–276, 2012. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol 261: 190–195, 2014. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985) 104: 1818–1827, 2008. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol 164: 252–262, 2008. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol (1985) 117: 910–920, 2014. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29: 381–386, 2004. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- 56.Marini RP, Hurley RJ, Avison DL, Lipman NS. An evaluation of three neuroleptanalgesic combinations in rabbits. Lab Anim Sci 43: 338–345, 1993. [PubMed] [Google Scholar]
- 57.Mayock DE, Standaert TA, Watchko JF, Woodrum DE. Effect of aminophylline on diaphragmatic contractility in the piglet. Pediatr Res 28: 196–198, 1990. doi: 10.1203/00006450-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Medina-Martínez JS, Greising SM, Sieck GC, Mantilla CB. Semi-automated assessment of transdiaphragmatic pressure variability across motor behaviors. Respir Physiol Neurobiol 215: 73–81, 2015. doi: 10.1016/j.resp.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol (1985) 89: 563–572, 2000. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- 60.Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol 98: 478–487, 2007. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- 61.Sadoul N, Bazzy AR, Akabas SR, Haddad GG. Ventilatory response to fatiguing and nonfatiguing resistive loads in awake sheep. J Appl Physiol (1985) 59: 969–978, 1985. doi: 10.1152/jappl.1985.59.3.969. [DOI] [PubMed] [Google Scholar]
- 62.Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li YJ, Kaneda S, Sorimachi Y, Yoshimoto K, Yasuhara M, Totsuka T. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res 907: 1–19, 2001. doi: 10.1016/S0006-8993(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 63.Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol 2011: 201696, 2011. doi: 10.1155/2011/201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316, 2014. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol 180: 88–96, 2012. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988. [PubMed] [Google Scholar]
- 68.Sieck GC. Neural control of the inspiratory pump. Physiology 6: 260–264, 1991. doi: 10.1152/physiologyonline.1991.6.6.260. [DOI] [Google Scholar]
- 69.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- 70.Sieck GC, Fournier M. Metabolic profile of muscle fibers in the fetal cat diaphragm. In: Sudden Infant Death Syndrome, Risk Factors, and Basic Mechanisms, edited by Harper RM, Hoffman HJ. New York: PMA Publishing, 1988, p. 361–378. [Google Scholar]
- 71.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 72.Sieck GC, Prakash YS. Fatigue at the neuromuscular junction: branch point vs. presynaptic vs. postsynaptic mechanisms. In: Neural and Neuromuscular Aspects of Muscle Fatigue, edited by Stuart DG, Gandevia S, Enoka RM, McComas AJ, Thomas CK. New York: Plenum, 1995, p. 83–100. [PubMed] [Google Scholar]
- 73.Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- 74.Walker UA, Schon EA. Neurotrophin-4 is up-regulated in ragged-red fibers associated with pathogenic mitochondrial DNA mutations. Ann Neurol 43: 536–540, 1998. doi: 10.1002/ana.410430421. [DOI] [PubMed] [Google Scholar]
- 75.Watchko JF, Mayock DE, Standaert TA, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res 20: 658–661, 1986. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]