Abstract
Leucine (Leu) and its metabolite β-hydroxy-β-methylbutyrate (HMB) stimulate mechanistic target of rapamycin (mTOR) complex 1 (mTORC1)-dependent protein synthesis in the skeletal muscle of neonatal pigs. This study aimed to determine whether HMB and Leu utilize common nutrient-sensing mechanisms to activate mTORC1. In study 1, neonatal pigs were fed one of five diets for 24 h: low protein (LP), high protein (HP), or LP supplemented with 4 (LP+HMB4), 40 (LP+HMB40), or 80 (LP+HMB80) μmol HMB·kg body wt−1·day−1. In study 2, neonatal pigs were fed for 24 h: LP, LP supplemented with Leu (LP+Leu), or HP diets delivering 9, 18, and 18 mmol Leu·kg body wt−1·day−1, respectively. The upstream signaling molecules that regulate mTORC1 activity were analyzed. mTOR phosphorylation on Ser2448 and Ser2481 was greater in LP+HMB40, LP+HMB80, and LP+Leu than in LP and greater in HP than in HMB-supplemented groups (P < 0.05), whereas HP and LP+Leu were similar. Rheb-mTOR complex formation was lower in LP than in HP (P < 0.05), with no enhancement by HMB or Leu supplementation. The Sestrin2-GATOR2 complex was more abundant in LP than in HP and was reduced by Leu (P < 0.05) but not HMB supplementation. RagA-mTOR and RagC-mTOR complexes were higher in LP+Leu and HP than in LP and HMB groups (P < 0.05). There were no treatment differences in RagB-SH3BP4, Vps34-LRS, and RagD-LRS complex abundances. Phosphorylation of Erk1/2 and TSC2, but not AMPK, was lower in LP than HP (P < 0.05) and unaffected by HMB or Leu supplementation. Our results demonstrate that HMB stimulates mTORC1 activation in neonatal muscle independent of the leucine-sensing pathway mediated by Sestrin2 and the Rag proteins.
NEW & NOTEWORTHY Dietary supplementation with either leucine or its metabolite β-hydroxy-β-methylbutyrate (HMB) stimulates protein synthesis in skeletal muscle of the neonatal pig. Our results demonstrate that both leucine and HMB stimulate mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) phosphorylation in neonatal muscle. This leucine-stimulated process involves dissociation of the Sestrin2-GATOR2 complex and increased binding of Rag A/C to mTOR. However, HMB’s activation of mTORC1 is independent of this leucine-sensing pathway.
Keywords: amino acid, growth, infant, nutrient sensing, protein synthesis
INTRODUCTION
Dietary protein is crucial for skeletal muscle growth and maintenance of muscle mass (10). The amino acid leucine not only serves as a building block for protein synthesis but also functions as a signaling molecule that stimulates protein synthesis (7). β-Hydroxy-β-methylbutyrate (HMB) is a leucine metabolite produced endogenously in small quantities from the alpha-keto acid of leucine (46) and, when taken as a supplement, can enhance protein anabolism in skeletal muscle (18, 21, 47, 48). Human and animal studies have shown that dietary supplementation with leucine or HMB can enhance lean mass and attenuate sarcopenia (2, 3, 11, 26, 50, 67, 70), although this is not a consistent finding (20, 67). Regardless, leucine and HMB continue to be used as dietary supplements to enhance skeletal muscle growth.
Despite improvements in the nutritional management of low-birth-weight infants, most experience growth faltering by the time they are discharged from the hospital (24). Some remain small into adulthood and exhibit adverse long-term outcomes, including an increased risk for developing obesity (40). Because the deficit in postnatal weight gain is largely due to reduced lean mass accretion (30), the identification of new strategies to optimize the nutritional management of infants to promote lean growth is needed. Feeding high-protein diets to neonates promote better growth but can produce azotemia and acidosis (16). Thus alternative approaches to enhance the anabolic use of dietary amino acids are required.
Using the neonatal pig as a model of the human infant, we have demonstrated that the postprandial rise in insulin and amino acids, especially leucine, independently stimulates protein synthesis in skeletal muscle by triggering the activation of insulin and nutrient signaling pathways that lead to translation initiation (15, 59, 60, 62–65). Human and animal studies demonstrate that a physiological increase in plasma leucine stimulates muscle protein synthesis via the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) pathway by promoting phosphorylation of p70 ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor (eIF)4E-binding protein 1 (4E-BP1) as well as the formation of the eIF4G-eIF4E complex that regulates mRNA translation (5, 11, 14). In neonatal pigs, supplementation of a protein-restricted diet with either leucine or its metabolite HMB stimulates muscle protein synthesis (14, 31, 68). However, whether HMB activates mTORC1 through the same pathway as leucine remains to be determined.
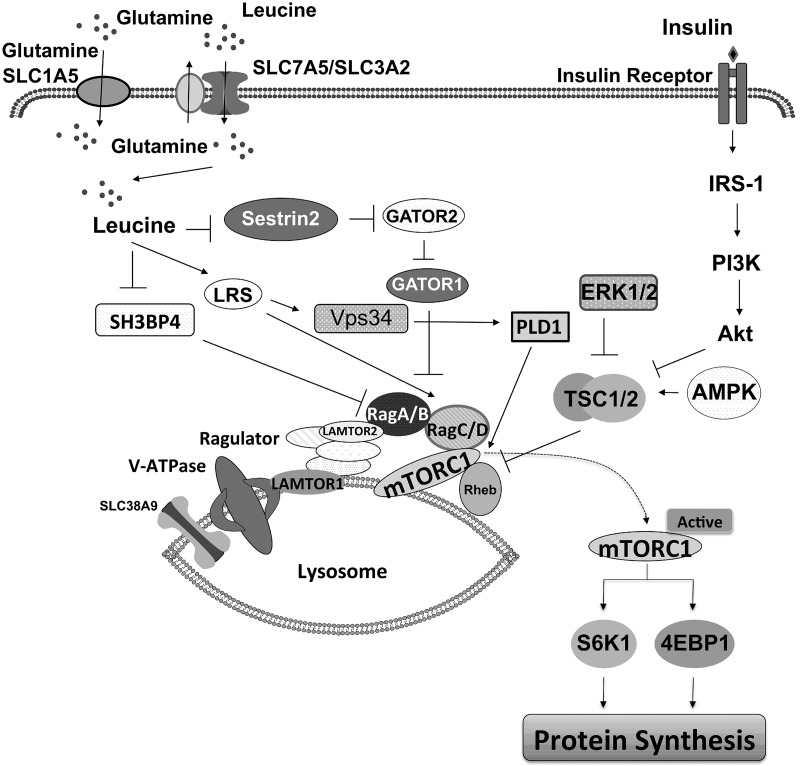
Studies performed primarily with cell cultures have identified multiple mechanisms for how leucine activates mTORC1 (Fig. 1) (23, 35, 71). Stress response protein 2 (Sestrin2) [a GTPase-activating protein (GAP) activity toward Rags-2 (GATOR2)-interacting protein] acts as a leucine sensor upstream of mTORC1 (71). Under leucine-rich conditions, leucine binds to Sestrin2, which disrupts the inhibitory Sestrin2-GATOR2 complex and ultimately results in the activation of mTORC1. Leucine also can bind to leucyl-tRNA synthetase (LRS) (an alternate leucine sensor) that can interact with either Ras-related GTPase (Rag)D (23) or vacuolar protein sorting (Vps)34 and trigger phospholipase D (PLD)1 activation, ultimately releasing the inhibition of mTOR by DEP domain-containing mTOR-interacting protein (DEPTOR) (72). Leucine can also impede the interaction of SH3 domain binding protein 4 (SH3BP4) with RagB, enabling RagB to become fully active and participate in mTORC1 activation (35).
Fig. 1.
Current overview of signaling pathways leading to the activation of mechanistic target of rapamycin complex 1 (mTORC1). AKT, RAC-alpha serine/threonine-protein kinase; AMPK, AMP-activated protein kinase; ERK 1/2, extracellular signal-regulated kinases 1 and 2; GATOR1/2, GAP activity toward Rags 1/2; IRS-1, insulin receptor substrate 1; LAMTOR 1/2, late endosomal/lysosomal adaptor, MAPK and MTOR activator 1/2; LRS, leucyl-tRNA synthetase; PI3K, phosphatidylinositol-3-kinase; PLD1, phospholipase D1; Rag A/B, C/D, RAS-related GTP-binding protein A/B, C/D; Rheb, Ras homolog enriched in brain; Sestrin2, stress response protein2; SH3BP1, SH3 domain binding protein 4; SLC1A5, solute carrier family 1 member 5; SLC7A5, solute carrier family 7 member 5; SLC38A9, solute carrier family 38 member 9; V-ATPase, vacuolar H+-ATPase. Vps34, vacuolar protein sorting 34.
Although not fully delineated, alternative pathways have been proposed by which leucine activates mTORC1 to stimulate protein synthesis, and these involve protein kinase B (PKB/AKT), extracellular signal-regulated kinases 1/2 (ERK 1/2), and tuberous sclerosis complex 2 (TSC2) (1, 22, 37, 43). A study using pig primary myoblasts showed that leucine stimulated the phosphorylation of TSC2 and mTORC1 activation (22). In contrast, leucine-induced mTORC1 activation in C2C12 myoblasts was not accompanied by TSC2 phosphorylation and the association between ras homolog enriched in brain (Rheb) and mTOR (25, 28). Some studies have proposed that HMB also stimulates protein synthesis by activating AKT (1, 36, 54). HMB promoted the phosphorylation of AKT and ERK1/2 in human and chicken myoblasts under serum-starved conditions (37). However, our in vivo study and others (31, 51) showed that HMB enhances muscle protein synthesis without activation of AKT. In cell culture, HMB activates AMP-activated protein kinase (AMPK) (6, 41), but this finding is inconsistent with the notion that AMPK suppresses mTORC1 activation (4).
This study aimed to establish whether in skeletal muscles of neonatal pigs HMB utilizes nutrient-sensing mechanisms similar to leucine for the activation of mTORC1. Both the activation of insulin signaling as well as nutrient-sensing pathways that signal upstream of mTORC1 (Fig. 1) were investigated.
METHODS
Animals and Housing
Piglets (Yorkshire × Landrace × Duroc × Hampshire; Rosenbaum Farms, Burton, TX, and Texas Department of Criminal Justice, Huntsville, TX) were weaned from sows at 2 days of age, and the jugular vein and carotid catheters were surgically inserted as described previously (15). They were placed in individual steel cages and fed a milk replacement formula (Soweena; Merrik’s, Middleton, WI) ad libitum until study (31, 66). All experimental procedures were approved by the Animal Care and Use Committee of Baylor College of Medicine and were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The animals were part of studies reported previously (31, 66), and the protocols are briefly described below.
Experimental Design
Study 1.
At 5–7 days of age, overnight-fasted male (n = 20) and female (n = 18) piglets (2.2 ± 0.4 kg body wt) were randomly assigned to one of five dietary treatment groups (n = 7–9 pigs/treatment): 1) a low-protein diet without HMB supplementation (LP, n = 7); 2) LP supplemented with 4 μmol HMB·kg body wt−1·day−1 (LP+HMB4, n = 7); 3) LP supplemented with 40 μmol HMB·kg body wt−1·day−1 (LP+HMB40, n = 8); 4) LP supplemented with 80 μmol HMB·kg body wt−1·day−1 (LP+HMB80, n = 9); and 5) a high-protein diet (HP, n = 7) as previously described (31). Piglets were bolus fed (40 mL/kg body wt) for 15 min at time 0 min and every 4 h for 24 h. The LP and HP diets provided 8.3 and 18.0 g protein·kg body wt−1·day−1, respectively. The LP diet was utilized to mimic many hospitalized babies who are fed less than their optimal protein requirement because of general feeding intolerance and concerns regarding a limited ability to metabolize protein (16). The HP diet was used as a positive control. Diets were isocaloric (195 kcal·kg body wt−1·day−1) and provided the same amount of lactose. The HMB was provided in the diet twice daily in two equal doses every 12 h, at 0 and 12 h. The doses of HMB were chosen based on a previous study that showed that a plasma concentration of ~90 nmol HMB/mL was required for maximum stimulation of muscle protein synthesis (31, 68), and this was achieved with the highest dose of HMB. The lowest dose was selected to mimic the HMB concentration in human milk. The dosing frequency was based on a pilot study that determined the HMB clearance rate (31). A final feed was provided after 24 h, and a blood sample was obtained for measurement of plasma HMB and leucine concentrations at 26.25 h, when HMB concentrations were at their peak (31). Pigs were immediately euthanized, and longissimus dorsi muscles, which contain primarily fast-twitch glycolytic fibers (63), were rapidly frozen in liquid nitrogen and stored at −80°C until they were analyzed.
Study 2.
At 5 days of age, overnight-fasted male (n = 12) and female (n = 15) piglets (2.3 ± 0.1 kg body wt) were randomly assigned to one of three dietary treatment groups (n = 7–10 pigs/treatment): LP (n = 10), LP supplemented with leucine (LP+Leu, n = 10), or HP (n = 7) diets as described previously (66). Piglets were bolus fed (40 mL/kg body wt) for 15 min every 4 h for 24 h. The LP+Leu diet was designed to provide an amount of leucine equivalent to that in the HP diet (8.9, 18, and 18 mmol leucine·kg body wt−1·day−1 in LP, LP+Leu, and HP, respectively). Leucine was supplemented at each meal based on the postprandial plasma leucine profiles in preliminary and previous studies (44). The LP and HP diets provided 5 and 20 g protein·kg body wt−1·day−1, respectively. Diets contained the same amount of lactose and were isocaloric (203 kcal·kg body wt−1·day−1). For measurement of plasma leucine concentrations, blood samples were collected at 25.5 h, 1.5 h after the last feed. Piglets were immediately euthanized, and longissimus dorsi muscles were frozen in liquid nitrogen and stored at −80°C until they were analyzed.
Western Blotting and Immunoprecipitation Assay (Studies 1 and 2)
These measurements were performed on the longissimus dorsi muscles collected from previous studies (31, 66). The proteins of interest were identified and quantified as previously described (59). Briefly, samples of longissimus dorsi muscle homogenates were denatured in Laemmli sample buffer, and the proteins were separated by polyacrylamide gel electrophoresis (PAGE) followed by transfer to polyvinylidene difluoride transfer membrane (Pall, Port Washington, NY). Proteins were then incubated with appropriate primary antibodies overnight at 4°C, washed, and exposed to horseradish peroxidase-tagged secondary antibodies. To analyze the total protein abundances of the respective phosphorylated target proteins, blots were stripped and reprobed by total or non-phospho-antibodies. The blots were developed with a ECL chemiluminescence kit (Bio-Rad, Hercules, CA), and bands were visualized and analyzed with a ChemiDoc-It Imaging System (UVP, Upland, CA). Antibodies against regulatory associated protein of mechanistic target of rapamycin complex 1 (Raptor) (catalog no. 2280), Rheb (catalog no. 13879), Sestrin2 (catalog no. 8487), GATOR2/Mios (catalog no. 13557), RagA (catalog no. 4357), RagC (catalog no. 9480), RagD (catalog no. 4470), and Vps34 (catalog no. 4263) were obtained from Cell Signaling Technology (Danvers, MA) and validated (59, 60). Antibodies against total TSC2 (catalog no. 4308), phosphorylated (p-)TSC2-Thr1462 (catalog no. 3617), total AMPK (catalog no. 2532), p-AMPK-Thr172 (catalog no. 2535), total ERK 1/2 (catalog no. 9102), p-ERK 1/2-Thr202/Tyr204 (catalog no. 9106), total mTOR (catalog no. 2972), and p-mTOR-Ser2481 (catalog no. 2974) and Ser2448 (catalog no. 2917) were obtained from Cell Signaling Technology (Danvers, MA) and validated (49, 61). The antibody against SH3BP4 (catalog no. 17691-1-AP) was obtained from Proteintech Group (Chicago, IL) and validated (34). The antibody against LRS (catalog no. A304-316A) was obtained from Bethyl Laboratories (Montgomery, TX) and validated (59). The phosphorylated forms were normalized to the total abundances of their respective proteins.
Immunoprecipitation Assay (Studies 1 and 2)
To determine the interaction between signaling components, longissimus dorsi muscle samples were homogenized in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer containing (in mM) 40 HEPES (pH 7.5), 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerolphosphate, 40 NaF, 1.5 sodium vanadate, 0.1 PMSF, 1 benzamidine, and 1 DTT, with 0.3% CHAPS, as previously described (59). The homogenate was mixed on a platform rocker for 30 min at 4°C and then centrifuged at 1,000 g for 10 min (4°C). Antibodies were added (2–4 μL; Sestrin2, catalog no. 8487, Cell Signaling Technology; Raptor, catalog no. 2280, Cell Signaling Technology; LRS, catalog no. A304-316A, Bethyl Laboratories; and SH3BP4, catalog no. 17691-1-AP, Proteintech Group) to the supernatant containing 500 μg of protein and mixed on a platform rocker overnight at 4°C. The next day, the immune complexes were isolated with a goat anti-mouse/donkey anti-rabbit BioMag IgG (Qiagen, Germantown, MD) bead slurry. The magnetic bead complexes were collected with a magnetic stand and washed twice with CHAPS buffer and once in CHAPS buffer containing 200 mM instead of 120 mM NaCl and 60 mM instead of 40 mM HEPES. The precipitates were resuspended with 100 μL of 1× SDS sample buffer and then boiled for 5 min and centrifuged to collect the supernatant. The samples were subjected to SDS-PAGE followed by Western blot analysis using RagA (catalog no. 4357), RagC (catalog no. 9480), RagD (catalog no. 4470), GATOR2 (catalog no. 13557), and Vps34 (catalog no. 4263) (Cell Signaling Technology) antibodies. The protein-protein interaction was normalized by the appropriate protein abundance in the precipitates.
Statistical Analysis
Data in each study are expressed as means ± SE (study 1, n = 7–9 pigs; study 2, n = 7–10 pigs). For each study, values from each treatment were analyzed with one-way ANOVA followed by Tukey post hoc multiple comparison test (Graph Pad Prism version 5; Graph Pad Software, San Diego, CA). Probability values of P < 0.05 were considered statistically significant.
RESULTS
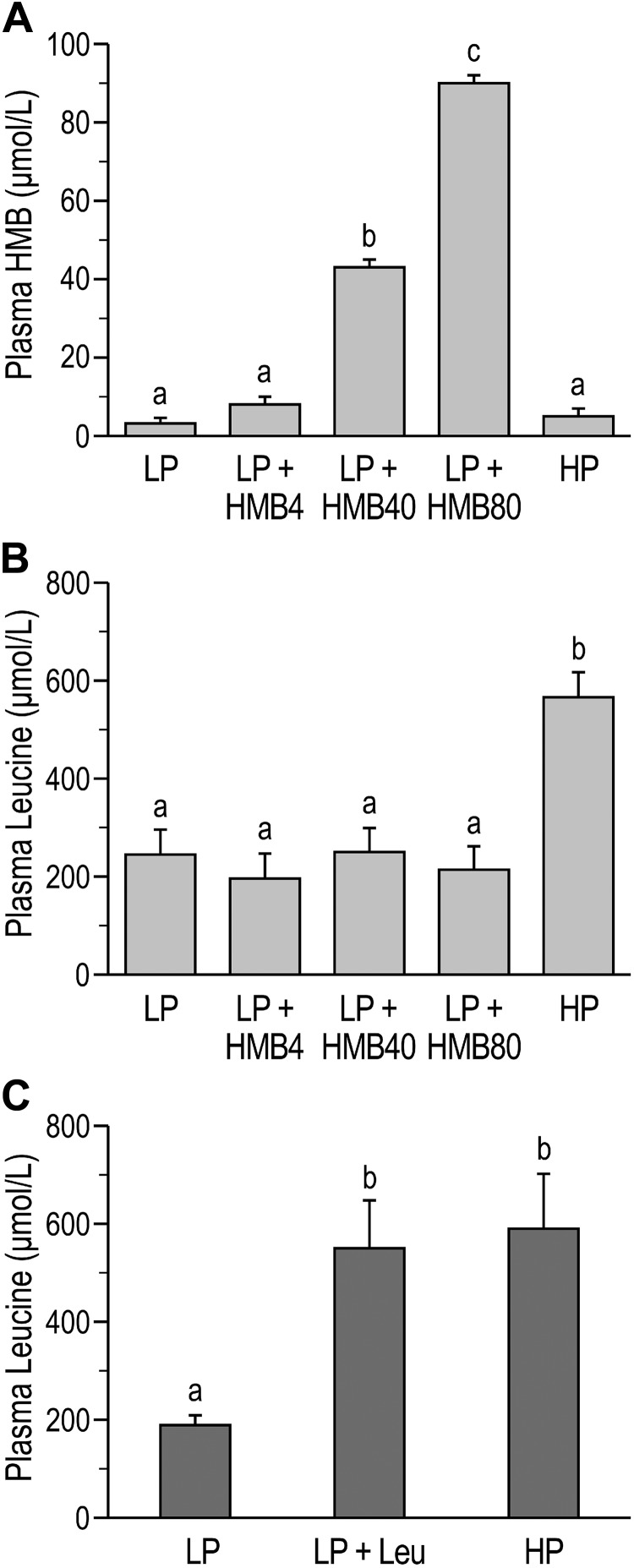
To determine whether HMB-induced mTORC1 activation in skeletal muscle of neonatal pigs occurs via the same amino acid-sensing pathways that relay the leucine signal, we compared the response to HMB (study 1) with the response to leucine (study 2) in samples obtained from previous studies (31, 66). Plasma concentrations of HMB and leucine were reported previously (31, 66) and are presented in this article for reference. Plasma concentrations of HMB in study 1 were greater in LP+HMB40 and LP+HMB80 compared with LP, LP+HMB4, and HP treatments (P < 0.05) and were highest in the LP+HMB80 group (P < 0.05; Fig. 2A) (31). The highest plasma concentration of leucine was in the HP group (P < 0.05; Fig. 2B) and was not impacted by HMB treatments (study 1) (31). Plasma leucine levels in study 2 were similar in the LP+Leu and HP groups and both were higher compared with the LP treatment (P < 0.05; Fig. 2C) (66).
Fig. 2.
Plasma levels of β-hydroxy-β-methylbutyrate (HMB) and leucine in neonatal pigs fed low-protein (LP) diets supplemented with HMB (31) or leucine (Leu) (66) or a high-protein (HP) diet (31, 66). A: plasma HMB from study 1. HMB4, HMB40, and HMB80, 4, 40, and 80 μmol HMB·kg body wt−1·day−1. B: plasma leucine from study 1. C: plasma leucine from study 2. Statistical analyses were performed with 1-way ANOVA followed by Tukey’s test. Values are means ± SE; n = 7–10 pigs. Means with different letters differ at P < 0.05.
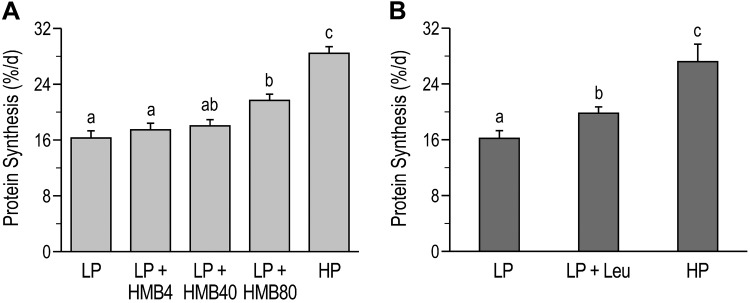
Fractional protein synthesis rates in longissimus dorsi muscles were analyzed and reported previously (31, 66). In study 1, the muscle protein synthesis rate was higher in the LP+HMB80 group compared with the LP and LP+HMB4 groups (P < 0.05; Fig. 3A) (31). Whereas the highest rate of protein synthesis was in the HP group (P < 0.05; Fig. 3A), protein synthesis did not differ significantly between the LP+HMB80 and LP+HMB40 groups. The rate of protein synthesis was higher in the LP+Leu and HP groups than in the LP group (P < 0.05; Fig. 3B) but was highest in the HP group (P < 0.05; Fig. 3B; study 2) (66).
Fig. 3.
Fractional rates of protein synthesis in longissimus dorsi muscle of neonatal pigs fed low-protein (LP) diets supplemented with β-hydroxy-β-methylbutyrate (HMB) (31) or leucine (Leu) (66) or a high-protein (HP) diet (31, 66). A: protein synthesis from study 1. HMB4, HMB40, and HMB80, 4, 40, and 80 μmol HMB·kg body wt−1·day−1. B: protein synthesis from study 2. Statistical analyses were performed with 1-way ANOVA followed by Tukey’s test. Values are means ± SE; n = 7–10 pigs. Means with different letters differ at P < 0.05.
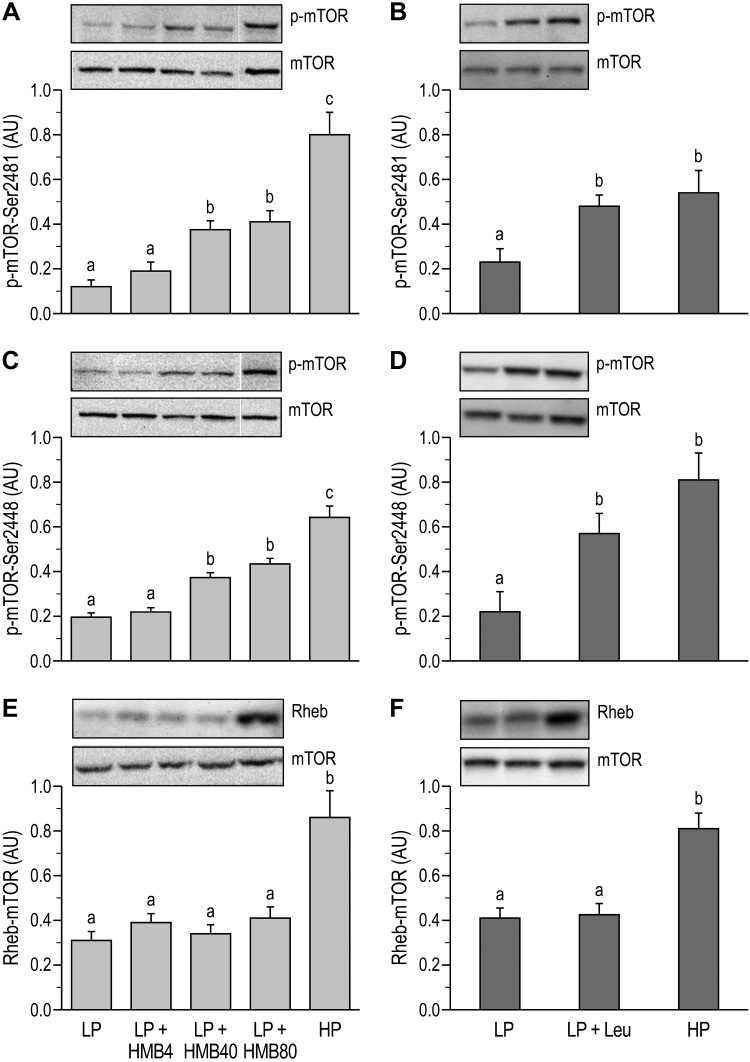
In both studies, the phosphorylation of mTOR at Ser2448 and Ser2481 was greater in the HP than in the LP group (P < 0.05; Fig. 4, A–D). HMB supplementation at the two higher doses also increased mTOR phosphorylation at Ser2448 and Ser2481 (P < 0.05) relative to the LP group, but these were lower than the HP group (Fig. 4, A and C). In study 2, leucine supplementation of LP restored mTOR phosphorylation (P < 0.05) to the same level as in the HP group (Fig. 4, B and D). The abundance of the Rheb-mTOR complex was lower in the LP groups than in the HP group (P < 0.05) and in both studies was unaffected by HMB or leucine supplementation (Fig. 4, E and F).
Fig. 4.
Phosphorylation levels of mechanistic target of rapamycin (mTOR) and abundance of the Ras homolog enriched in brain (Rheb)-mTOR complex in longissimus dorsi muscle of neonatal pigs fed low-protein (LP) diets supplemented with β-hydroxy-β-methylbutyrate (HMB) or leucine (Leu) or a high-protein (HP) diet. A: phosphorylation of mTOR (p-mTOR) at Ser2481 (study 1). B: phosphorylation of mTOR at Ser2481 (study 2). C: phosphorylation of mTOR at Ser2448 (study 1). D: phosphorylation of mTOR at Ser2448 (study 2). E: Rheb-mTOR complex abundance (study 1). F: Rheb-mTOR complex abundance (study 2). AU, arbitrary units; HMB4, HMB40, and HMB80, 4, 40, and 80 μmol HMB·kg body wt−1·day−1. On the blot images, white lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Phosphorylated forms were normalized to the total abundances of their respective proteins. Statistical analyses was performed with 1-way ANOVA followed by Tukey’s test. Values are means ± SE; n = 7–10 pigs. Means with different letters differ at P < 0.05.
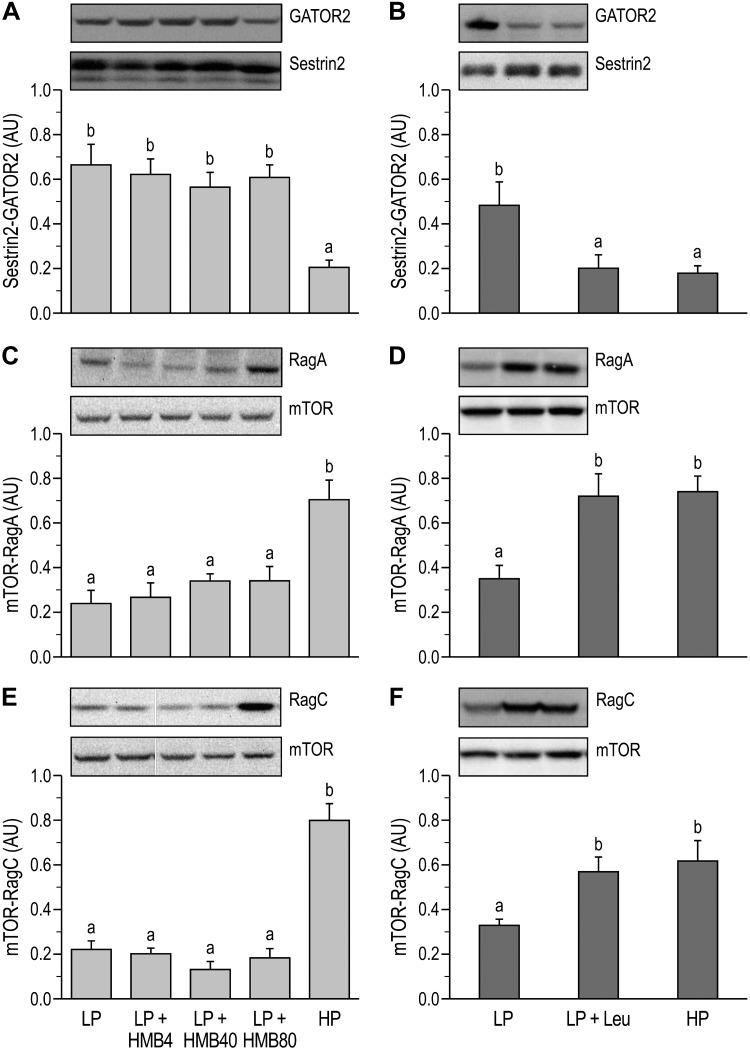
The abundance of the Sestrin2-GATOR2 complex was lower in the HP compared with the LP group in both studies (P < 0.05; Fig. 5A and B), and there was no effect of supplementing the LP diet with HMB. However, leucine supplementation of the LP diet reduced Sestrin2-GATOR2 complex formation to the same level as the HP positive control (P < 0.05; Fig. 5B). In both studies, the abundance of RagA-mTOR and RagC-mTOR complexes in the HP groups was greater than in the LP groups (P < 0.05; Fig. 5, C–F). Similar to the Sestrin-GATOR2 complex formation, HMB supplementation of the LP diet did not affect the mTOR interactions with RagA and RagC, whereas leucine supplementation of the LP diet restored levels to those observed in the HP group (P < 0.05; Fig. 5, C–F). The abundance of the RagB-SH3BP4, RagD-LRS, and Vps34-LRS complexes were similar across all dietary treatments (Table 1).
Fig. 5.
Abundance of stress response protein2 (Sestrin2)-GAP activity toward RAGs (GATOR)2, RAS-related GTP-binding protein (Rag)A-mechanistic target of rapamycin (mTOR), and RagC-mTOR complexes in longissimus dorsi muscle of neonatal pigs fed low-protein (LP) diets supplemented with β-hydroxy-β-methylbutyrate (HMB) or leucine (Leu) or a high-protein (HP) diet. A: Sestrin2-GATOR2 complex abundance (study 1). B: Sestrin2-GATOR2 complex abundance (study 2). C: RagA-mTOR complex abundance (study 1). D: RagA-mTOR complex abundance (study 2). E: RagC-mTOR complex abundance (study 1). F: RagC-mTOR complex abundance (study 2). AU, arbitrary units; HMB4, HMB40, and HMB80, 4, 40, and 80 μmol HMB·kg body wt−1·day−1. On the blot images, white lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Statistical analyses were performed with 1-way ANOVA followed by Tukey’s test. Values are means ± SE; n = 7–10 pigs. Means with different letters differ at P < 0.05.
Table 1.
Protein-protein interactions and phosphorylation of signaling proteins in longissimus dorsi muscle of neonatal pigs
|
Study 1 |
Study 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| LP | LP+HMB4 | LP+HMB40 | LP+HMB80 | HP | LP | LP+Leu | HP | |
| RagB-SH3BP4 | 0.54 ± 0.08 | 0.55 ± 0.09 | 0.62 ± 0.09 | 0.65 ± 0.09 | 0.63 ± 0.10 | 0.43 ± 0.06 | 0.52 ± 0.08 | 0.47 ± 0.09 |
| RagD-LRS | 0.63 ± 0.09 | 0.58 ± 0.07 | 0.64 ± 0.09 | 0.49 ± 0.09 | 0.61 ± 0.12 | 0.80 ± 0.13 | 0.69 ± 0.07 | 0.84 ± 0.14 |
| Vps34-LRS | 0.69 ± 0.12 | 0.61 ± 0.10 | 0.65 ± 0.06 | 0.66 ± 0.07 | 0.62 ± 0.09 | 0.55 ± 0.09 | 0.55 ± 0.09 | 0.57 ± 0.12 |
| AMPK Thr172 | 0.58 ± 0.08 | 0.63 ± 0.09 | 0.56 ± 0.06 | 0.67 ± 0.07 | 0.59 ± 0.07 | 0.51 ± 0.12 | 0.62 ± 0.09 | 0.58 ± 0.11 |
Values (in arbitrary units) are means ± SE; n = 7–9 (study 1) and 7–10 (study 2) pigs/group. Phosphorylated forms were normalized to the total abundance of their respective proteins. AMPK, AMP-activated protein kinase; HP, high protein; LP, low protein; LP+HMB4, low protein supplemented with 4 μmol β-hydroxy-β-methylbutyrate (HMB)·kg body wt–1·day–1; LP+HMB40, low protein supplemented with 40 μmol HMB·kg body wt–1·day–1; LP+HMB80, low protein supplemented with 80 μmol HMB·kg body wt–1·day–1; LP+Leu, low protein supplemented with leucine; LRS, leucyl-tRNA synthetase; Rag, RAS-related GTP-binding protein; SH3BP4, SH3 domain binding protein 4; Vps34, vacuolar protein sorting 34. There were no significant differences among treatments (1-way ANOVA followed by Tukey’s post hoc test).
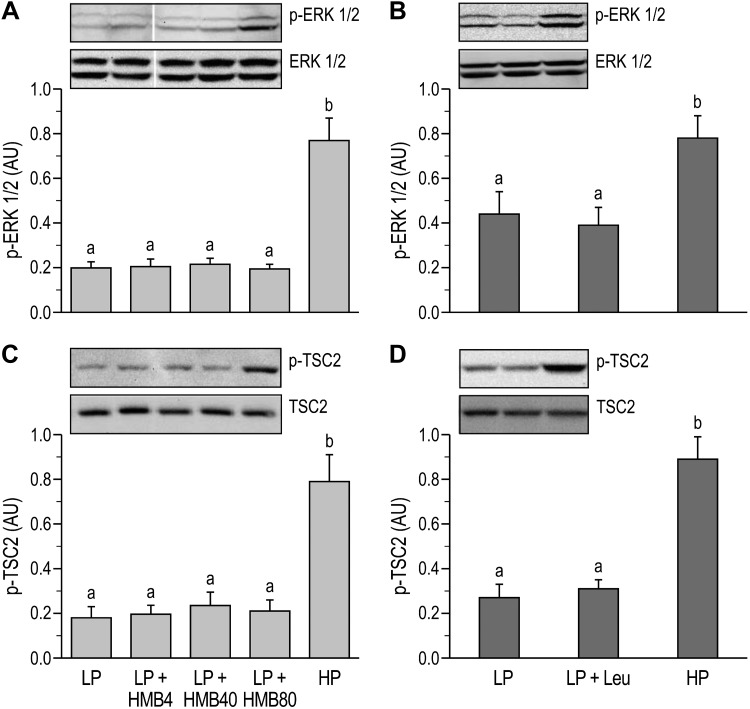
The phosphorylation of ERK1/2 in skeletal muscles was greater in the HP groups compared with the LP groups (P < 0.05; Fig. 6, A and B) in both studies. However, neither HMB nor leucine supplementation of the LP diet promoted phosphorylation of ERK 1/2. The phosphorylation of AMPK was unaffected by the protein content of the diet or supplementation with HMB or leucine (Table 1). In both studies, the phosphorylation of TSC2 was lower in the LP than HP groups (P < 0.05) and was unaffected by HMB or leucine supplementation (Fig. 6, C and D).
Fig. 6.
Extracellular signal-regulated kinases 1 and 2 (ERK 1/2) and tuberous sclerosis complex 2 (TSC2) activation in longissimus dorsi muscle of neonatal pigs fed low-protein (LP) diets supplemented with β-hydroxy-β-methylbutyrate (HMB) or leucine (Leu) or a high-protein (HP) diet. A: phosphorylation of ERK 1/2 (p-ERK 1/2) (study 1). B: phosphorylation of ERK 1/2 (study 2). C: phosphorylation of TSC2 (p-TSC2) (study 1). D: phosphorylation of TSC2 (study 2). AU, arbitrary units; HMB4, HMB40, and HMB80, 4, 40, and 80 μmol HMB·kg body wt−1·day−1. On the blot images, white lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Phosphorylated forms were normalized to the total abundances of their respective proteins. Statistical analyses were performed with 1-way ANOVA followed by Tukey’s test. Values are means ± SE; n = 7–10 pigs. Means with different letters differ at P < 0.05.
DISCUSSION
Studies conducted in cell culture, as well as in animal models and humans, have demonstrated that leucine and its metabolite HMB can promote anabolic processes (10, 44, 53, 70), although this is not a consistent finding (52, 69). Both leucine and HMB activate mTORC1 downstream effectors (S6K1 and 4E-BP1) that regulate translation initiation (14, 15, 31, 68), but unlike with leucine (22, 32, 71), how HMB activates nutrient-sensing pathways upstream of mTORC1 is unknown. Thus, using our current knowledge of the mechanisms of leucine signaling we sought to establish whether the same molecular mechanisms are employed by leucine and HMB to promote mTORC1 activation in vivo.
The signaling function of mTORC1 is regulated in part by the phosphorylation of mTOR at Ser2448 and Ser2481 and the formation of the Rheb-mTOR complex (Fig. 1) (42, 72). Although S6K1 phosphorylates mTOR at Ser2448 via a feedback loop mechanism (9, 27), its contribution to mTORC1 activation remains uncertain (17). In contrast, insulin- or amino acid-induced mTOR autophosphorylation at Ser2481 is associated with mTOR-specific catalytic or kinase activity (58). Our present results are in agreement with previous reports, which demonstrated that HMB (19) and leucine (57) stimulation of translation initiation are mediated by phosphorylation of mTOR at Ser2448 and its downstream target, S6K1. This response is further substantiated by the demonstration that rapamycin completely blocked leucine-induced phosphorylation of mTOR at Ser2448 and Ser2481 as well as the phosphorylation of 4E-BP1 and S6K1 (62). Consistent with the notion that leucine and HMB do not engage mediators of growth factor signaling pathways in the activation of mTORC1 (74), the Rheb-mTOR association did not change with leucine or HMB supplementation of the LP diet, unlike the response that occurred when the nutrient-rich HP diet was fed.
Leucine’s activation of mTORC1 is initiated by binding to Sestrin2 that disrupts the Sestrin2-GATOR2 complex, thereby allowing activation of mTORC1 (Fig. 1) (33, 39, 55, 71). Our results showed that leucine, but not HMB, diminished the abundance of the inhibitory Sestrin2-GATOR2 complex with a reciprocal increase in both RagA-mTOR and RagC-mTOR complex formation. These findings suggest that the mechanism of mTORC1 activation by HMB is independent of the Sestrin2-Rags axis. Such a mechanism has been observed for glutamine, in which glutamine can stimulate mTORC1 independently of the Rags (32) and employs ADP ribosylation factor 1 (Arf-1) GTPase and the lysosomal H+-ATPase (v-ATPase) for glutamine-induced lysosomal translocation and activation of mTORC1 (32).
LRS is a purported leucine sensor involved in the leucine-induced activation of mTORC1 (Fig. 1) (23). In the presence of leucine, LRS interacts with Vps34 (a lipid kinase), causing the activation of Vps34 and mTORC1 via a Vps34-PLD1-mTORC1 interaction (73). Leucine may also facilitate the formation of a LRS and RagD complex that functions as a GAP for Rag GTPase to activate mTORC1 (23). In agreement with results from a human study that showed that leucine enhanced mTOR phosphorylation without altering the LRS-mTORC1 complex (8), our data showed that LRS-Vps34 or LRS-RagD association was not affected by protein intake, leucine, or HMB. LRS gene knockdown that did not affect mTOR phosphorylation and cell hypertrophy in mouse myotubes (13) suggests that LRS is not necessary for mTORC1 activation and is consistent with our present findings.
SH3BP4, a Rag GTPase-binding protein, has been reported to be an important component in leucine-induced activation of mTORC1 (Fig. 1) (35). In HEK293T cells, it was demonstrated that in leucine-deprived conditions SH3BP4 binds to RagC or RagB, resulting in the inhibition of mTORC1 (35). Our results showed that the formation of the RagB-SH3BP4 complex was not affected by protein intake, leucine, or HMB. To our knowledge, after this initial finding there have been no other reports to support the validity of this mechanism of leucine action.
In both studies, the HP diet was included as a positive control and resulted in the highest postprandial fractional rate of muscle protein synthesis (31, 66). The reduction in protein synthesis rate in response to the LP diet was partially mitigated when the diet was supplemented with either leucine or HMB (at the highest dose). The extent of the mTORC1 response, however, differed in that leucine, but not HMB, was able to restore mTORC1 activation to the same level as in the HP diet. This difference in the mTORC1 response to leucine versus HMB may reflect the contribution of the amino acid-sensing components, i.e., Sestrin2-GATOR2, mTOR-RagA, and mTOR-RagC complexes in the presence of leucine when either the HP diet or the leucine supplement were fed, but was absent in the HMB group. Nonetheless, the higher rate of protein synthesis in the HP group than in the leucine-supplemented group suggests greater activation of other mTORC1-independent pathways that regulate protein synthesis.
Studies have suggested that alternative pathways are involved in HMB-induced muscle protein anabolism (56). Some studies using human, chicken, and mouse muscle cells report that HMB stimulates hypertrophy in vitro by activating MAPK/ERK and AKT pathways (36). Both pathways activate mTORC1 through inhibition of TSC2 (41) and the consequent stimulation of Rheb binding to mTOR. In the present study, not only did we find no evidence of ERK 1/2 activation by HMB or leucine supplementation but we also found that the supplements did not affect the phosphorylation of TSC2 or the abundance of the Rheb-mTOR complex, although their activation was impacted by the HP diet. Thus neither leucine nor HMB appear to utilize the MAPK/ERK-AKT-TSC2 axis in vivo to regulate translation initiation in skeletal muscle.
As a master regulator of energy homeostasis in cells, AMPK regulates the restoration of energy balance under conditions of metabolic stress (38). Hence, AMPK activates TSC2, thereby reducing mTORC1 activation (29). In vitro studies show conflicting data, with leucine reducing (12) and HMB inducing (41) AMPK activation. Our present results show no effect of leucine or HMB supplementation on AMPK phosphorylation, an anticipated outcome as the pigs were all fed the same energy intake. Moreover, in our previous studies, short-term variations in feeding status, insulin, or amino acid intake did not influence AMPK activity in skeletal muscle of neonatal pigs (61, 63).
We acknowledge that there are some potential limitations to these studies. One shortcoming is that tissue was only collected at one time point after feeding, and we cannot discount the possibility that activation of the MAPK/ERK pathway by HMB had dissipated before sampling. This is highly unlikely, however, because even after 2 h the plasma levels of HMB and leucine were still elevated (31). A second potential concern for both studies 1 and 2 is that the analyses were done on tissues that had been stored long-term and were not performed in parallel with the measurements of protein synthesis published previously (31, 66). However, consideration of the observed responses suggests that it is highly unlikely that the integrity of the samples was compromised to an extent that would invalidate our conclusions. Specifically, we observed the anticipated decrease in Sestrin2-GATOR2 abundance when the HP or leucine-supplemented diets were fed. We have reported a similar change in muscles of term piglets after feeding that was analyzed after short-term storage (45). The dose response of mTOR phosphorylation to HMB supplementation reflected the phosphorylation level of S6K1 that was measured previously (31). We documented an increase in p-ERK1/2 only in the HP groups in both studies, which in both cases was accompanied by anticipated increases in p-TSC2 and Rheb-mTOR interaction.
Given that mTORC1 plays a pivotal role in regulating muscle protein synthesis, a full understanding of how anabolic agents such as leucine and HMB regulate mTORC1 activation is essential and may help to develop a therapeutic approach for enhancing lean mass accretion during the neonatal period, especially when there has been growth faltering. The results from our studies support the notion that leucine regulates the Sestrin2-GATOR1/2-Rags axis to facilitate mTORC1 activation in neonatal muscle. However, HMB’s action on mTORC1 appears to be independent of these signaling pathways. Other proposed modes of action involving LRS and SH3BP4 or the PI3K/Akt and MAP/ERK signaling pathways seem to be unaffected by leucine or HMB supplementation. Thus alternative pathways need to be explored to develop a better understanding of the regulation of muscle protein synthesis by HMB.
GRANTS
These studies were supported by National Institutes of Health Grants AR-044474 (T. A. Davis), HD-072891 (T. A. Davis), HD-085573 (T. A. Davis), and HD-099080 (T. A. Davis and M. L. Fiorotto), National Institute of Food and Agriculture Grant 2013-67015-20438 (T. A. Davis), and Agricultural Research Service Grant 3092-51000-060-01S (T. A. Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S., M.L.F., and T.A.D. conceived and designed research; A.S. and M.R. performed experiments; A.S. and M.R. analyzed data; A.S., M.R., M.L.F., and T.A.D. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., M.R., M.L.F., and T.A.D. edited and revised manuscript; A.S., M.R., M.L.F., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hanh Nguyen and Rosemarie Parada for technical assistance.
REFERENCES
- 1.Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol 48: 973–984, 2013. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 3.Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care 13: 265–270, 2010. doi: 10.1097/MCO.0b013e328336f6b8. [DOI] [PubMed] [Google Scholar]
- 4.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 5.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 134: 1704–1710, 2004. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 6.Bruckbauer A, Zemel MB. Synergistic effects of polyphenols and methylxanthines with leucine on AMPK/sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS One 9: e89166, 2014. doi: 10.1371/journal.pone.0089166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest 56: 1250–1261, 1975. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlin MB, Tanner RE, Agergaard J, Jalili T, McClain DA, Drummond MJ. Skeletal muscle Ras-related GTP binding B mRNA and protein expression is increased after essential amino acid ingestion in healthy humans. J Nutr 144: 1409–1414, 2014. doi: 10.3945/jn.114.196691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490, 2005. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 10.Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47: 259–270, 2015. doi: 10.1007/s00726-014-1866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci 85: 919–927, 2007. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- 13.Durán RV, Hall MN. Leucyl-tRNA synthetase: double duty in amino acid sensing. Cell Res 22: 1207–1209, 2012. [Erratum in Cell Res 22: 1509, 2012.] doi: 10.1038/cr.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140: 1418–1424, 2010. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288: E914–E921, 2005. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- 16.Fenton TR, Premji SS, Al-Wassia H, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev 4: CD003959, 2014. doi: 10.1002/14651858.CD003959.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiredo VC, Markworth JF, Cameron-Smith D. Considerations on mTOR regulation at serine 2448: implications for muscle metabolism studies. Cell Mol Life Sci 74: 2537–2545, 2017. doi: 10.1007/s00018-017-2481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of β-hydroxy-β-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 20: 445–451, 2004. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Girón MD, Vílchez JD, Salto R, Manzano M, Sevillano N, Campos N, Argilés JM, Rueda R, López-Pedrosa JM. Conversion of leucine to β-hydroxy-β-methylbutyrate by α-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J Cachexia Sarcopenia Muscle 7: 68–78, 2016. doi: 10.1002/jcsm.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girón MD, Vílchez JD, Shreeram S, Salto R, Manzano M, Cabrera E, Campos N, Edens NK, Rueda R, López-Pedrosa JM. β-Hydroxy-β-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PLoS One 10: e0117520, 2015. doi: 10.1371/journal.pone.0117520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ham DJ, Caldow MK, Lynch GS, Koopman R. Leucine as a treatment for muscle wasting: a critical review. Clin Nutr 33: 937–945, 2014. doi: 10.1016/j.clnu.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Dev 75: 810–817, 2008. doi: 10.1002/mrd.20832. [DOI] [PubMed] [Google Scholar]
- 23.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149: 410–424, 2012. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Harding JE, Derraik JG, Berry MJ, Jaquiery AL, Alsweiler JM, Cormack BE, Bloomfield FH. Optimum feeding and growth in preterm neonates. J Dev Orig Health Dis 4: 215–222, 2013. doi: 10.1017/S2040174412000736. [DOI] [PubMed] [Google Scholar]
- 25.Heard JJ, Fong V, Bathaie SZ, Tamanoi F. Recent progress in the study of the Rheb family GTPases. Cell Signal 26: 1950–1957, 2014. doi: 10.1016/j.cellsig.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle 8: 529–541, 2017. doi: 10.1002/jcsm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280: 26089–26093, 2005. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 28.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol 302: C1557–C1565, 2012. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 130: e640–e649, 2012. doi: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- 31.Kao M, Columbus DA, Suryawan A, Steinhoff-Wagner J, Hernandez-Garcia A, Nguyen HV, Fiorotto ML, Davis TA. Enteral β-hydroxy-β-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 310: E1072–E1084, 2016. doi: 10.1152/ajpendo.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol 21: 63–71, 2019. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park H, Cho US, Wang W, Guan KL, Karin M, Lee JH. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci Rep 5: 9502–9511, 2015. [Erratum in Sci Rep 5: 14029, 2015.] doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KH, Lee TR, Cho EG. SH3BP4, a novel pigmentation gene, is inversely regulated by miR-125b and MITF. Exp Mol Med 49: e367, 2017. doi: 10.1038/emm.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YM, Stone M, Hwang TH, Kim YG, Dunlevy JR, Griffin TJ, Kim DH. SH3BP4 is a negative regulator of amino acid-Rag GTPase-mTORC1 signaling. Mol Cell 46: 833–846, 2012. doi: 10.1016/j.molcel.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura K, Cheng XW, Inoue A, Hu L, Koike T, Kuzuya M. β-Hydroxy-β-methylbutyrate facilitates PI3K/Akt-dependent mammalian target of rapamycin and FoxO1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ-induced MuRF-1 expression in C2C12 cells. Nutr Res 34: 368–374, 2014. doi: 10.1016/j.nutres.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. β-hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1793: 755–763, 2009. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmøller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, Ferry A, Wojtaszewski JF, Foretz M, Viollet B. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 28: 3211–3224, 2014. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Bodmer R, Bier E, Karin M. Sestrins at the crossroad between stress and aging. Aging (Albany NY) 2: 369–374, 2010. doi: 10.18632/aging.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Wang W, Wu G, Wang J. Nutritional support for low birth weight infants: insights from animal studies. Br J Nutr 117: 1390–1402, 2017. doi: 10.1017/S000711451700126X. [DOI] [PubMed] [Google Scholar]
- 41.Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab 2014: 239750, 2014. doi: 10.1155/2014/239750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 280: 23433–23436, 2005. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 43.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk. Implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140: 2145–2152, 2010. doi: 10.3945/jn.110.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naberhuis JK, Suryawan A, Nguyen HV, Hernandez-Garcia A, Cruz SM, Lau PE, Olutoye OO, Stoll B, Burrin DG, Fiorotto ML, Davis TA. Prematurity blunts the feeding-induced stimulation of translation initiation signaling and protein synthesis in muscle of neonatal piglets. Am J Physiol Endocrinol Metab 317: E839–E851, 2019. doi: 10.1152/ajpendo.00151.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nissen S, Van Koevering M, Webb D. Analysis of β-hydroxy-β-methyl butyrate in plasma by gas chromatography and mass spectrometry. Anal Biochem 188: 17–19, 1990. doi: 10.1016/0003-2697(90)90522-B. [DOI] [PubMed] [Google Scholar]
- 47.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC Jr. β-Hydroxy-β-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 130: 1937–1945, 2000. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 48.Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem 8: 300–311, 1997. doi: 10.1016/S0955-2863(97)00048-X. [DOI] [Google Scholar]
- 49.Orellana RA, Suryawan A, Kimball SR, Wu G, Nguyen HV, Jefferson LS, Davis TA. Insulin signaling in skeletal muscle and liver of neonatal pigs during endotoxemia. Pediatr Res 64: 505–510, 2008. doi: 10.1203/PDR.0b013e318183fd4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr 94: 809–818, 2011. doi: 10.3945/ajcn.111.017061. [DOI] [PubMed] [Google Scholar]
- 51.Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Oller do Nascimento CM, de Mello MT, Tufik S, Santos RV. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab (Lond) 8: 11, 2011. doi: 10.1186/1743-7075-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiao X, Zhang HJ, Wu SG, Yue HY, Zuo JJ, Feng DY, Qi GH. Effect of β-hydroxy-β-methylbutyrate calcium on growth, blood parameters, and carcass qualities of broiler chickens. Poult Sci 92: 753–759, 2013. doi: 10.3382/ps.2012-02341. [DOI] [PubMed] [Google Scholar]
- 53.Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 136: 1466–1471, 2006. doi: 10.1093/jn/136.6.1466. [DOI] [PubMed] [Google Scholar]
- 54.Salto R, Vílchez JD, Girón MD, Cabrera E, Campos N, Manzano M, Rueda R, López-Pedrosa JM. β-Hydroxy-β-methylbutyrate (HMB) promotes neurite outgrowth in Neuro2a cells. PLoS One 10: e0135614, 2015. doi: 10.1371/journal.pone.0135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in Cell 169: 361–371, 2017.] doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slater GJ, Jenkins D. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med 30: 105–116, 2000. doi: 10.2165/00007256-200030020-00004. [DOI] [PubMed] [Google Scholar]
- 57.Smith GI, Yoshino J, Stromsdorfer KL, Klein SJ, Magkos F, Reeds DN, Klein S, Mittendorfer B. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 64: 1555–1563, 2015. doi: 10.2337/db14-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285: 7866–7879, 2010. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suryawan A, Davis TA. Amino acid- and insulin-induced activation of mTORC1 in neonatal piglet skeletal muscle involves Sestrin2-GATOR2, Rag A/C-mTOR, and Rheb-mTOR complex formation. J Nutr 148: 825–833, 2018. doi: 10.1093/jn/nxy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol (1985) 109: 1448–1454, 2010. doi: 10.1152/japplphysiol.00428.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 291: E849–E859, 2006. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- 62.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295: E868–E875, 2008. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suryawan A, O’Connor PM, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 37: 97–104, 2009. doi: 10.1007/s00726-008-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suryawan A, O’Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134: 24–30, 2004. doi: 10.1093/jn/134.1.24. [DOI] [PubMed] [Google Scholar]
- 65.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71: 324–331, 2012. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szcześniak KA, Ostaszewski P, Fuller JC Jr, Ciecierska A, Sadkowski T. Dietary supplementation of β-hydroxy-β-methylbutyrate in animals—a review. J Anim Physiol Anim Nutr (Berl) 99: 405–417, 2015. doi: 10.1111/jpn.12234. [DOI] [PubMed] [Google Scholar]
- 68.Wheatley SM, El-Kadi SW, Suryawan A, Boutry C, Orellana RA, Nguyen HV, Davis SR, Davis TA. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 306: E91–E99, 2014. doi: 10.1152/ajpendo.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wibert GJ, Layman DK, Hong SO. An in vivo examination of the effects of leucine on skeletal muscle protein synthesis in the fasting rat. Nutr Res 11: 1155–1166, 1991. doi: 10.1016/S0271-5317(05)80693-1. [DOI] [Google Scholar]
- 70.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591: 2911–2923, 2013. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48, 2016. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yonezawa K, Yoshino KI, Tokunaga C, Hara K. Kinase activities associated with mTOR. Curr Top Microbiol Immunol 279: 271–282, 2004. doi: 10.1007/978-3-642-18930-2_16. [DOI] [PubMed] [Google Scholar]
- 73.Yoon MS, Son K, Arauz E, Han JM, Kim S, Chen J. Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep 16: 1510–1517, 2016. doi: 10.1016/j.celrep.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshida S, Pacitto R, Inoki K, Swanson J. Macropinocytosis, mTORC1 and cellular growth control. Cell Mol Life Sci 75: 1227–1239, 2018. doi: 10.1007/s00018-017-2710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]