Abstract
We examined the influence of lifelong aerobic exercise on skeletal muscle size, function, and adiposity. Young exercisers [YE; n = 20, 10 women (W), 25 ± 1 yr], lifelong exercisers (LLE; n = 28, 7 W, 74 ± 2 yr), and old healthy nonexercisers (OH; n = 20, 10 W, 75 ± 1 yr) were studied. On average, LLE exercised 5 days/wk for 7 h/wk over the past 52 ± 1 yr. The LLE men were subdivided by exercise intensity [Performance (LLE-P), n = 14; Fitness (LLE-F), n = 7]. Upper and lower leg muscle size and adiposity [intermuscular adipose tissue (IMAT)] were determined via MRI, and quadriceps isotonic and isometric function was assessed. For the quadriceps, aging decreased muscle size, isotonic and isometric strength, contraction velocity (men only), and power (P < 0.05). In women, LLE did not influence muscle size or function. In men, LLE attenuated the decline in muscle size and isometric strength by ~50% (P < 0.05). LLE did not influence other aspects of muscle function, nor did training intensity influence muscle size or function. For the triceps surae, aging decreased muscle size only in the women, whereas LLE (both sexes) and training intensity (LLE men) did not influence muscle size. In both sexes, aging increased thigh and calf IMAT by ~130% (P < 0.05), whereas LLE attenuated the thigh increase by ~50% (P < 0.05). In the LLE men, higher training intensity decreased thigh and calf IMAT by ~30% (P < 0.05). In summary, aging and lifelong aerobic exercise influenced muscle size, function, and adipose tissue infiltration in a sex- and muscle-specific fashion. Higher training intensity throughout the life span provided greater protection against adipose tissue infiltration into muscle.
NEW & NOTEWORTHY This is the first study to examine skeletal muscle size, function, and adiposity in women and men in their eighth decade of life that have engaged in lifelong aerobic exercise. The findings reveal sex and upper and lower leg muscle group-specific benefits related to skeletal muscle size, function, and adiposity and that exercise intensity influences intermuscular adiposity. This emerging cohort will further our understanding of the health implications of maintaining exercise throughout the life span.
Keywords: aging, intermuscular adipose tissue, lifelong exercise, muscle function, muscle mass
INTRODUCTION
The exercise boom in the late 1960s and early 1970s triggered a movement for women and men to engage in exercise as a hobby. More than 50 years later, some of these individuals have maintained the extraordinary commitment to exercise regularly throughout their lifetime and provide a unique opportunity to explore the effects of aging while removing physical inactivity as a major confounding factor in the aging process (8). The extent to which lifelong aerobic exercise may help preserve skeletal muscle health is unclear, but it is reasonable to consider that it may reduce the whole muscle mass losses typically observed with aging (24, 25, 40). Lifelong aerobic exercise may also impact the age-associated decline in skeletal muscle function, which is associated with decrements in cardiorespiratory fitness, increased risk of mortality, and increased intermuscular adipose tissue (IMAT) (2, 15, 19, 21, 50).
Skeletal muscle mass and its underlying cellular makeup play a central role in the health and functional status of individuals of all ages. Muscle-derived locomotion is essential for functions ranging from activities of daily living to elite athletic performance (18, 27, 28, 61, 62), and the amino acid reservoir of muscle serves as the precursor for numerous metabolic functions (17, 39, 69–71, 74). As a result, older individuals can be significantly compromised considering the typical age-associated decline in muscle mass (i.e., sarcopenia) (18, 37, 54). Short-term (e.g., 12–16 wk) exercise strategies employed later in life, such as aerobic (24, 25, 40) or resistance (5, 60, 63, 67) exercise training, show that the skeletal muscle is responsive to exercise growth stimuli into the eighth decade of life. The impact of regular exercise maintained throughout the life span on muscle mass and function is less established.
The current study examined the skeletal muscle mass, function, and adiposity of women and men that had been lifelong aerobic exercisers in their adult years (~50 yr). For comparison, we included age-matched older healthy women and men with no history of structured exercise and young exercising women and men. Our general working hypotheses were that a hierarchal pattern (young exercisers > lifelong exercisers > old healthy) would be apparent for muscle mass and function, while IMAT would show the opposite hierarchal pattern (old healthy > lifelong exercisers >young exercisers). The results reported here were part of a larger investigation, for which the framework has been previously detailed (23), that examined numerous aspects of cardiorespiratory fitness and skeletal muscle health of this unique cohort.
METHODS
Subjects
Old lifelong exercisers [LLE, n = 28, 7 women (W)], old healthy nonexercisers (OH, n = 20, 10 W), and young exercisers (YE, n = 20, 10 W) were included in this investigation (Table 1). Subjects were recruited from the greater Muncie, Indiana, area by newspaper advertisements, mailed flyers, and personal interaction. More extensive subject characteristics and more details regarding the recruitment and screening process, along with cardiovascular and skeletal muscle metabolic profiles are presented by our research team elsewhere (23). Enrolled individuals were free from acute or chronic illness (cardiac, pulmonary, liver, or kidney abnormalities, cancer, uncontrolled hypertension, insulin- or non-insulin-dependent diabetes, or other known metabolic disorders), they were free from orthopedic limitations (including any artificial joints), and they did not smoke or participate in other forms of tobacco use. The study was approved by the Institutional Review Board of Ball State University. All study procedures, risks, and benefits were explained to the subjects before their giving written consent to participate.
Table 1.
Subject characteristics
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Lifelong Exercisers |
|||||||
| YE | LLE | OH | YE | Combined | LLE-P | LLE-F | OH | |
| n | 10 | 7 | 10 | 10 | 21 | 14 | 7 | 10 |
| Age, yr | 25 ± 1 | 72 ± 2 | 75 ± 1 | 25 ± 1 | 74 ± 1 | 74 ± 1 | 75 ± 2 | 75 ± 1 |
| Height, cm | 167 ± 2 | 164 ± 2 | 157 ± 2 | 181 ± 2 | 180 ± 2 | 179 ± 2 | 182 ± 3 | 177 ± 2 |
| Weight, kg | 60 ± 2 | 61 ± 4 | 65 ± 1 | 75 ± 3 | 79 ± 2 | 77 ± 2 | 83 ± 5 | 88 ± 3* |
| BMI, kg/m2 | 21 ± 1 | 23 ± 1 | 27 ± 1* | 23 ± 1 | 24 ± 1 | 24 ± 1 | 25 ± 1 | 28 ± 1* |
| Body fat, % | 23 ± 1* | 30 ± 2† | 41 ± 2 | 18 ± 2* | 24 ± 1† | 22 ± 1‡ | 27 ± 1 | 32 ± 1 |
| Total lean mass, kg | 44 ± 1† | 40 ± 2 | 36 ± 1 | 59 ± 1 | 57 ± 1 | 57 ± 1 | 57 ± 3 | 57 ± 2 |
| V̇o2max, ml·kg−1·min−1 | 44 ± 2* | 26 ± 2† | 18 ± 1 | 53 ± 3* | 34 ± 1† | 38 ± 1‡ | 27 ± 2 | 22 ± 1 |
| Steps per day | 11,518 ± 1,404* | 7,463 ± 683 | 6,801 ± 823 | 9,404 ± 635 | 9,560 ± 619 | 9,369 ± 725 | 10,006 ± 1,265 | 5,813 ± 488* |
Values are means ± SE. V̇o2max, maximal oxygen consumption; YE, young exercisers; LLE, lifelong exercisers; LLE-P, LLE-performance; LLE-F, LLE-fitness; OH, old healthy nonexercisers.
P < 0.05 vs. main groups;
P < 0.05 vs. OH. ‡P < 0.05 LLE-P vs. LLE-F. Additional cardiovascular data, as well as details of the body fat (DEXA), V̇o2max, and steps per day measurements are presented by us elsewhere (23).
Exercise history of the subjects was carefully evaluated using a comprehensive questionnaire and confirmed through personal interviews (Table 2). The LLE cohort consisted primarily of cyclists and runners that reported ~50 yr of structured exercise. LLE trained ~5 days and ~7 h per week. Exercise history of LLE subjects was extensively reviewed for frequency, duration, intensity, and athletic achievements. As such, two clear LLE subgroups of men emerged: one group that participated in lower-intensity training for physical fitness [Fitness (LLE-F), n = 7] and another group that trained more vigorously and often participated in competitive events [Performance (LLE-P), n = 14]. The LLE women were not subdivided due to the sample size (n = 7) and ranged from fitness to performance. More specifically, two of the women were performance oriented, including one that cycled ~4,000 miles the year before testing and another that was a national level Masters track and field athlete. The remaining five women were more fitness oriented with their activities (mainly running) with some occasional aerobics and cycle spinning classes. Last, although we had extensive exercise records and interviewed each subject, we were unable to make any firm conclusions regarding cross-training exercise. Due to injuries or change in exercise interests, many individuals transitioned between modes throughout their life span (mainly running and cycling). Only one of the men regularly engaged in supplementary resistance exercise, and he was in the LLE-F group.
Table 2.
Exercise training histories of female and male participants
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Lifelong Exercisers |
|||||||
| YE | LLE | OH | YE | Combined | LLE-P | LLE-F | OH | |
| Total training years | 5 ± 1 | 48 ± 2 | 5 ± 1* | 53 ± 1 | 53 ± 1 | 53 ± 3 | ||
| Competitive focus1 | Yes | Yes | Yes | No | ||||
| Lifetime average | ||||||||
| Frequency (days/wk) | 4.6 ± 0.3 | 4.5 ± 0.2 | 4.4 ± 0.2 | 4.6 ± 0.3 | ||||
| Duration (h/wk) | 6.6 ± 0.6 | 7.3 ± 0.5 | 7.6 ± 0.7 | 6.6 ± 0.9 | ||||
| Intensity2 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1‡ | 1.8 ± 0.1 | ||||
| Current decade | ||||||||
| Frequency (days/wk) | 5.4 ± 0.5 | 4.7 ± 0.4 | 5.1 ± 0.2 | 4.7 ± 0.3 | 4.5 ± 0.3 | 4.9 ± 0.7 | ||
| Duration (h/wk) | 7.3 ± 1.1 | 6.8 ± 1.0 | 7.0 ± 0.7 | 8.1 ± 1.1 | 8.5 ± 1.4 | 7.4 ± 1.9 | ||
| Intensity2 | 2.6 ± 0.1 | 2.1 ± 0.2 | 2.8 ± 0.1* | 2.0 ± 0.1 | 2.2 ± 0.1‡ | 1.5 ± 0.2 | ||
Values are means ± SE. 1Competitive focus indicates exercise training for the purpose of competition was currently or once a primary goal for the majority of the group. Lifetime average reflects current decade exercise habits for YE. 2Levels of self-reported training intensity were 1 (Light), 2 (Moderate), and 3 (Hard). In the case where a subject reported more than one training intensity, values were weighted and averaged (e.g., 80% of training at a 2 and 20% of training at a 3 resulted in an overall intensity of 2.2). YE, young exercisers; LLE, lifelong exercisers; LLE-P, LLE-performance; LLE-F, LLE-fitness; OH, old healthy nonexercisers.
P ≤ 0.05 vs. LLE Combined;
P ≤ 0.05 LLE-P vs. LLE-F. More detailed exercise training histories are presented by us elsewhere (23).
Although OH controls were not involved in any structured exercise training, participation in leisure activities (e.g., golfing, leisurely walking, and community service) was not grounds for exclusion. Young exercisers consisted of active individuals who exercised 4–6 days/wk for ~7 h/wk.
Muscle Size and IMAT
MRI.
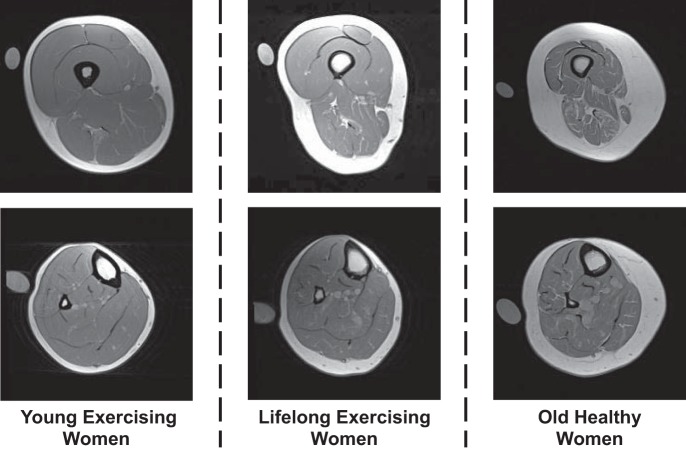
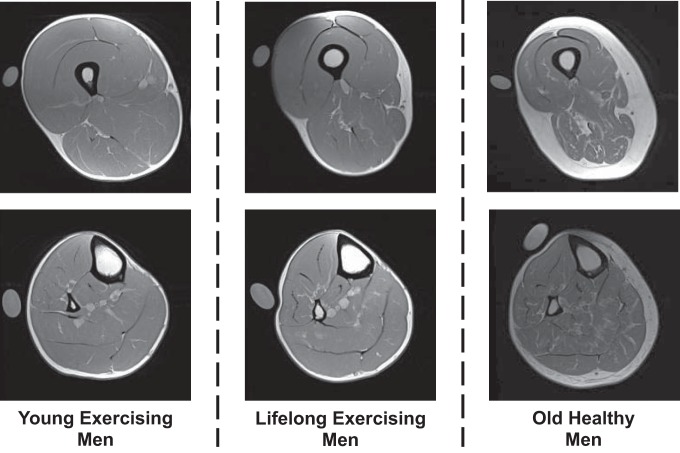
Following 1 h of supine rest to control for the influence of posture-related fluid shifts on muscle size (6), MRIs of the upper and lower leg were obtained using a nonmetallic foot restraint to control joint angle (muscle length) and scan angle and to minimize leg compression, as we have previously described (66–68). All scans were completed in the morning, and no exercise or strenuous activity was allowed on the day of scanning. Imaging was completed in a 1.5-T scanner (Siemens, Munich, Germany) using serial interleaved images 8 mm thick [TR, 2,000 ms; TE, 8 ms; 512 × 512 matrix; field of view, 480 × 480 mm (upper leg) and 350 × 350 mm (lower leg)]. Representative MRI scans for women and men are presented in Figs. 1 and 2, respectively.
Fig. 1.
Representative MRI scans for the women of the upper leg (top) and lower leg (bottom) used for analysis of muscle size and intermuscular adipose tissue content. A calibration tube with reagent-grade water is also placed in the field of view, which was not used for the analysis in the current investigation.
Fig. 2.
Representative MRI scans for the men of the upper leg (top) and lower leg (bottom) used for analysis of muscle size and intermuscular adipose tissue content. A calibration tube with reagent-grade water is also placed in the field of view, which was not used for the analysis in the current investigation.
Muscle size.
MRI images were transferred electronically from the scanner to a computer (iMac) at the Human Performance Laboratory and analyzed with NIH Image software (Image J, version 1.49). Quadriceps femoris and triceps surae muscle size was determined via manual planimetry, and a detailed description of these measurements was presented previously in our studies of aging and chronic bed rest (66, 67). Briefly, the cross-sectional area (cm2) of the muscle(s) of interest in a given slice was determined, and the muscle volume (cm3) was calculated by multiplying the cross-sectional area by the slice thickness. The right limb of each subject was used for all measurements, which were completed by the same investigator.
IMAT.
A single slice from the midpoint of the upper leg and lower leg images was used for IMAT determination of the whole thigh and calf (triceps surae), respectively, as previously described (15, 21, 51). Briefly, MIPAV software (NIH v.7.30) was used for tracing and removal of subcutaneous adipose and osseous tissues from the image. The remaining subsuperficial fascial tissue was corrected for nonuniform intensity variances inherent in MRI images by using a MIPAV plugin that applies an N3 algorithm (43, 55). Within the corrected image, centroids form using a histogram of the three types of primary tissue intensity (dark, medium gray, and white, which refer to dense fibrous tissue, muscle, and adipose tissue, respectively). Standard cutoff values were established by the MIPAV software using the identified centroids. Volumes of tissue within each centroid and the absolute and relative proportions were determined. The right limb of each subject was used for all measurements, which were completed by the same investigator.
Muscle Function
Assessment of muscle function was distributed across three visits separated by at least 48 h. Subjects were familiarized with all testing procedures during the first visit. During the second visit, quadriceps isotonic strength [1-repetition maximum (1RM)], quadriceps maximal contraction velocity (Vmax), and quadriceps maximal isometric force (Po) were measured. During the third visit, quadriceps power and handgrip strength were measured. During these assessments, verbal encouragement was provided. For a few participants that were limited in the number of visits he or she could make to the Human Performance Laboratory, muscle function assessment was completed over two visits.
1RM.
Maximal isotonic strength (1RM) of the quadriceps (bilateral) was determined on a standard leg extension device (model 4107; Cybex Eagle, Medway, MA) outfitted with a custom range-of-motion position sensor that provided audible confirmation of a successful repetition (49, 67). After a 10-min warmup on a cycle ergometer (Monark Ergomedic 828 E; Vansbro, Sweden; 25–50 W), subjects completed two sets of five repetitions at a low load (~40% 1RM). This was followed by single attempts to lift an incrementally heavier weight starting at a load estimated to be 70–80% 1RM. Attempts were separated by a 2-min rest period, and the approach was designed to determine 1RM in two to four attempts. The heaviest weight successfully lifted was considered the 1RM.
Vmax.
Vmax of the quadriceps (unilateral) was measured with the leg unloaded on a custom-built rigid chair outfitted with fiber-optic sensors (Banner Engineering, Minneapolis, MN) positioned at 120° and 150° of knee extension and interfaced with a computer timer (LabWindows, National Instruments, Austin, TX) to record angular velocity (10, 28). Subjects were restrained in the chair to isolate the quadriceps contributions to the measurement while extending their lower leg starting at 90° of knee extension through a 90° arc and into a heavily padded bar as fast as possible. Four maximal repetitions, each separated by a rest period, were completed for the left and right legs. The highest observed value was considered Vmax.
Po.
Po of the quadriceps (unilateral) was determined at 90° of knee extension on the custom-built rigid chair using a purpose-built leg cuff that was attached to their lower leg ~3 cm proximal to the medial malleolus and interfaced to a load cell (Omegadyne, Sunbury, OH) interfaced to computer software (LabWindows) to record force (10, 28). Subjects were restrained in the chair to isolate the quadriceps contributions to the measurement. A total of three maximal repetitions, each separated by a rest period, were completed for the left and right legs. The highest observed value was considered Po.
Power.
Power output of the quadriceps (bilateral) was measured on the Cybex Eagle leg extension device outfitted with a strain gauge in a half-bridge configuration and a bridge sensor (Omega Engineering; Stamford, CT), to determine torque produced at the fulcrum of the device, and a potentiometer (Vishay Americas, Ontario, CA), to record the angular displacement, all interfaced to computer software (LabWindows) to record power output. Loads equivalent to 40% and 70% of 1RM were calculated for each subject, with 40% representing the load that should elicit near-maximal power production (28, 57, 59). After a 10-min warmup on a cycle ergometer (Monark Ergomedic 828 E), power output at these two loads was determined by having the subjects lift the load as fast as possible through a full range of motion. Three maximal repetitions, each separated by a rest period, were completed for each of the two loads. The highest observed value for each load was considered the power output.
Handgrip strength.
Maximal handgrip strength (unilateral) was assessed on a handgrip dynamometer (model DHS 176; Detecto, Webb City, MO) for both the left and right hands. Subjects stood with feet shoulder width apart and the arm held at 90° elbow flexion against their body while performing the maximal handgrip contraction. During this assessment, verbal encouragement was provided. A total of three maximal repetitions, each separated by a rest period, were completed for the left and right hands. The highest observed value was considered maximal handgrip strength.
Muscle Quality
Muscle quality was calculated based on maximal muscle force and power production relative to muscle size. Specific tension was determined by dividing Po by quadriceps cross-sectional area (N/cm2). Normalized power was determined by dividing muscle power generated at 40% of 1RM by quadriceps muscle volume (W/cm3), which was doubled, since the power measurement was completed bilaterally.
Statistical Analysis
Statistical analyses were performed using Statistical Analysis Software (SAS version 9.3; Cary, NC), and values of P ≤ 0.05 were considered statistically significant. For technical or other reasons, some data were not obtained, resulting in the following sample sizes for muscle function [women: LLE, n = 6 (power); men: LLE-P: n = 13] and for IMAT [women: YE, n = 9; OH, n = 9; men: LLE-P, n = 12; LLE-F: n = 6 (calf only)]. Intrasex comparisons between YE, OH, and LLE were conducted using a one-way ANOVA with Tukey’s post hoc test. LLE-P and LLE-F were further compared using an independent two-tailed t test with Levene’s test for equality of variance. All data are presented as means ± SE.
RESULTS
Muscle Size
Quadriceps muscle size (cross-sectional area and volume) among the groups is presented in Table 3. For the women, quadriceps size was lower in LLE (−31%) and OH (−37%) compared with YE (P < 0.05), with no differences between LLE and OH. Individual data points for the quadriceps cross-sectional area for the women are presented in Fig. 3. The quadriceps muscle size data for the men showed a hierarchical pattern among the groups (YE > LLE > OH), where LLE attenuated (P < 0.05) the age-related decline by 48% (LLE: −16%, OH: −30%; P < 0.05). Individual data points for the quadriceps cross-sectional area for the men are presented in Fig. 3. There were no differences in quadriceps size between LLE-P and LLE-F.
Table 3.
Quadriceps and triceps surae muscle size
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Lifelong Exercisers |
|||||||
| YE | LLE | OH | YE | Combined | LLE-P | LLE-F | OH | |
| Quadriceps CSA (cm2) | 59 ± 2* | 42 ± 2 | 40 ± 1 | 78 ± 3* | 67 ± 2† | 68 ± 2 | 65 ± 3 | 56 ± 3 |
| Quadriceps volume (cm3) | 920 ± 45* | 620 ± 31 | 543 ± 37 | 1,264 ± 71* | 1,050 ± 38† | 1,021 ± 46 | 1,106 ± 64 | 868 ± 58 |
| Triceps surae volume (cm3) | 464 ± 39* | 321 ± 25 | 317 ± 28 | 548 ± 30 | 466 ± 17 | 468 ± 19 | 461 ± 34 | 476 ± 34 |
Values are means ± SE. CSA, cross-sectional area; YE, young exercisers; LLE, lifelong exercisers; LLE-P, LLE-performance; LLE-F, LLE-fitness; OH, old healthy nonexercisers. Triceps surae CSA was not presented because no measurements of function or quality were assessed.
P < 0.05 vs. main groups;
P < 0.05 vs. OH.
Fig. 3.
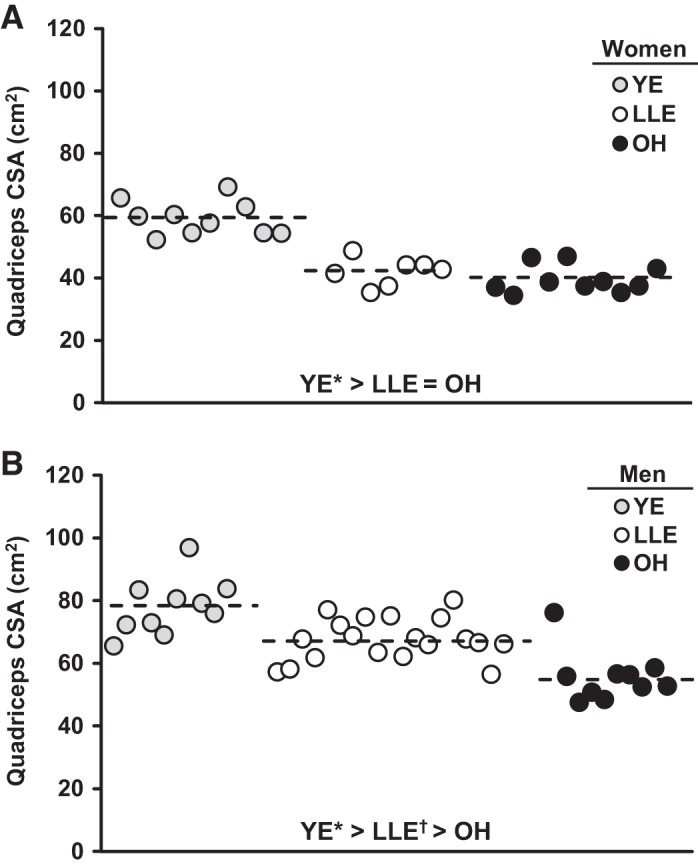
Individual and group mean (dashed line) quadriceps cross-sectional area (CSA) for the women (A) and men (B). YE, young exercisers; LLE, lifelong exercisers; OH, old healthy nonexercisers. *P < 0.05 vs. main groups; †P < 0.05 vs. OH.
Triceps surae muscle size (volume) among the groups is presented in Table 3. For the women, triceps surae size was lower in LLE (−31%) and OH (−32%) compared with YE (P < 0.05), with no difference between LLE and OH. There were no differences in triceps surae size among the three main groups of men or between the LLE-P and LLE-F.
Muscle Function
Quadriceps muscle function among the groups is presented in Table 4. Muscle function in both sexes for most measurements was lower in LLE and OH compared with YE, ranging from −18% to −64% (P < 0.05). There were no differences in quadriceps muscle function between the LLE and OH groups for the women, which was also observed for the men, with the exception of Po, where LLE attenuated (P < 0.05) the age-related decline by 44% (LLE: −20%, OH: −35%; P < 0.05).
Table 4.
Quadriceps muscle function and handgrip strength
| Men |
||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Lifelong Exercisers |
|||||||
| YE | LLE | OH | YE | Combined | LLE-P | LLE-F | OH | |
| 1RM, kg | 80 ± 6* | 54 ± 3 | 42 ± 2 | 121 ± 5* | 86 ± 3 | 86 ± 3 | 85 ± 8 | 78 ± 4 |
| Vmax, °/s | 789 ± 28 | 681 ± 36 | 715 ± 32 | 784 ± 35* | 639 ± 21 | 645 ± 28 | 629 ± 29 | 634 ± 21 |
| Po, N·m | 138 ± 10† | 105 ± 9 | 82 ± 4 | 210 ± 12* | 165 ± 6† | 166 ± 8 | 163 ± 10 | 134 ± 9 |
| Power at 40% 1RM, W | 404 ± 38* | 221 ± 20 | 146 ± 10 | 699 ± 30* | 370 ± 19 | 365 ± 13 | 377 ± 50 | 318 ± 42 |
| Power at 70% 1RM, W | 375 ± 32* | 206 ± 20 | 141 ± 7 | 641 ± 33* | 334 ± 18 | 340 ± 15 | 322 ± 46 | 294 ± 32 |
| Handgrip strength, kg | 33 ± 1* | 27 ± 2† | 20 ± 1 | 51 ± 3 | 46 ± 2 | 48 ± 3 | 43 ± 2 | 44 ± 1 |
Values are means ± SE. 1RM, 1-repetition maximum; Vmax, maximal contraction velocity; Po, maximal isometric force; YE, young exercisers; LLE, lifelong exercisers; LLE-P, LLE-performance; LLE-F, LLE-fitness; OH, old healthy nonexercisers.
P < 0.05 vs. main groups;
P < 0.05 vs. OH.
Handgrip strength in the women showed a hierarchical pattern among the groups (YE > LLE > OH), where LLE attenuated (P < 0.05) the decline by 54% (LLE: −18%, OH: −39%; P < 0.05; Table 4). There were no differences in handgrip strength among the three main groups of men or between LLE-P and LLE-F.
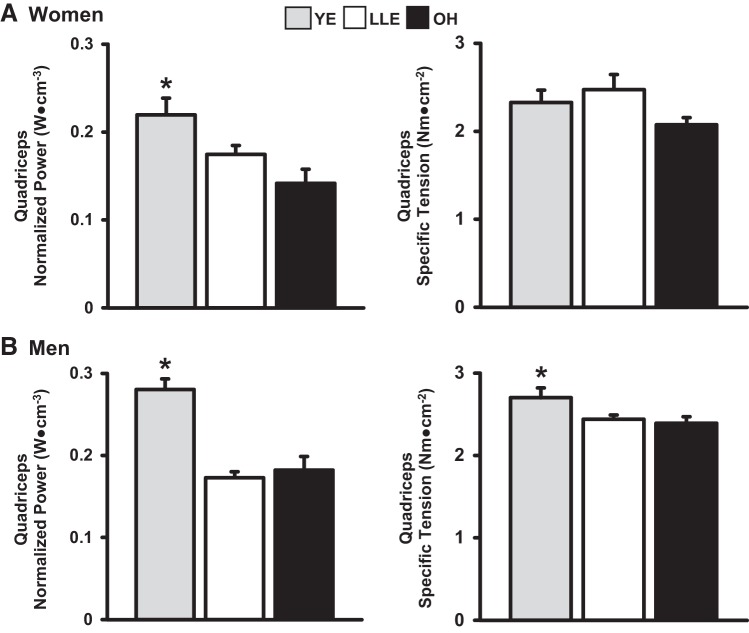
Muscle Quality
Quadriceps specific tension and normalized power for the women and men are presented in Fig. 4. For the women, there were no differences in specific tension among the three groups. Normalized power was lower in LLE (−23%) and OH (−36%) compared with YE (P < 0.05), with no difference between LLE and OH. For the men, specific tension was lower in LLE (−10%) and OH (−11%) compared with YE (P < 0.05), with no differences between LLE and OH. There was no difference in specific tension between LLE-P and LLE-F (2.41 vs. 2.50 Nm/cm2, P > 0.05). Normalized power was lower in LLE (−39%) and OH (−36%) compared with YE (P < 0.05), with no difference between LLE and OH. There was no difference in normalized power between LLE-P and LLE-F (0.18 ± 0.01 vs. 0.17 ± 0.02 W/cm3, P > 0.05).
Fig. 4.
Quadriceps specific tension and normalized power for the women (A) and men (B). YE, young exercisers; LLE, lifelong exercisers; OH, old healthy nonexercisers. *P < 0.05 vs. main groups.
IMAT
Whole thigh IMAT content among the groups is presented in Table 5. Whole thigh IMAT showed a hierarchical pattern in both sexes (YE < LLE < OH). For the women, lifelong aerobic exercise attenuated (P < 0.05) the age-related increase by 51% (LLE: 79%, OH: 162%; P < 0.05); in the men, lifelong aerobic exercise attenuated (P < 0.05) the age-related increase by 45% (LLE: 64%, OH: 116%; P < 0.05). Individual IMAT data for the women and men are presented in Fig. 5, respectively. LLE-P whole thigh IMAT was 32% lower than LLE-F (P < 0.05; Fig. 6).
Table 5.
Whole thigh and whole calf IMAT content
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| YE | LLE | OH | YE | LLE | OH | |
| Whole thigh | 4.7 ± 0.4* | 8.4 ± 0.9† | 12.3 ± 0.8 | 5.0 ± 0.5* | 8.2 ± 0.6† | 10.8 ± 0.8 |
| Whole calf | 4.8 ± 0.4* | 11.2 ± 1.2 | 11.4 ± 1.4 | 5.7 ± 0.4* | 13.8 ± 1.3 | 11.6 ± 0.8 |
Values are means ± SE in %. IMAT, intermuscular adipose tissue; YE, young exercisers; LLE, lifelong exercisers; OH, old healthy nonexercisers.
P < 0.05 vs. main groups;
P < 0.05 vs. OH.
Fig. 5.
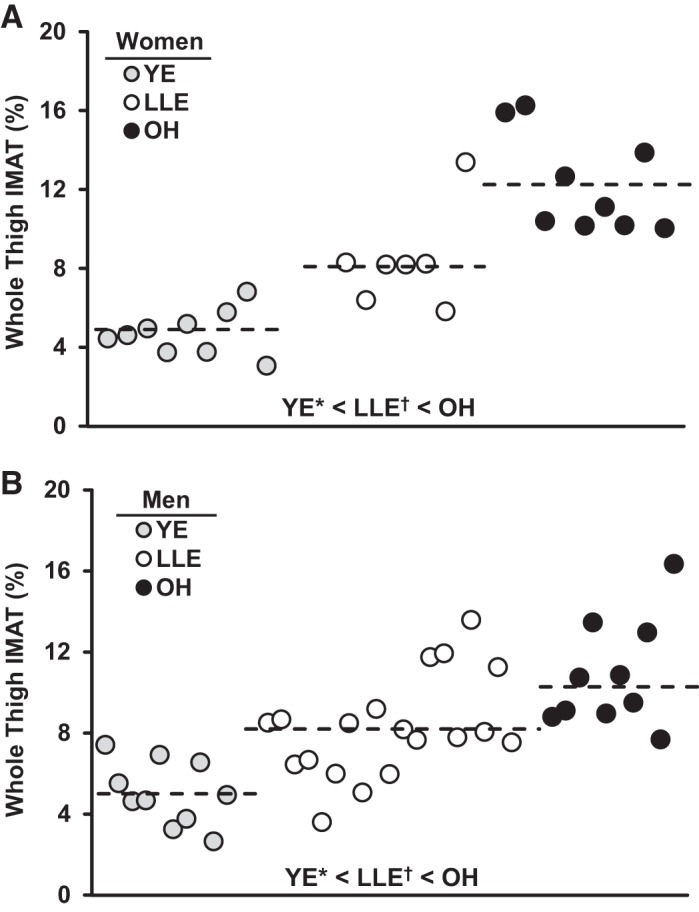
Individual and group mean (dashed line) whole thigh intermuscular adipose tissue (IMAT) content for the women (A) and men (B). YE, young exercisers; LLE, lifelong exercisers; OH, old healthy nonexercisers. *P < 0.05 vs. main groups; †P < 0.05 vs. OH.
Fig. 6.
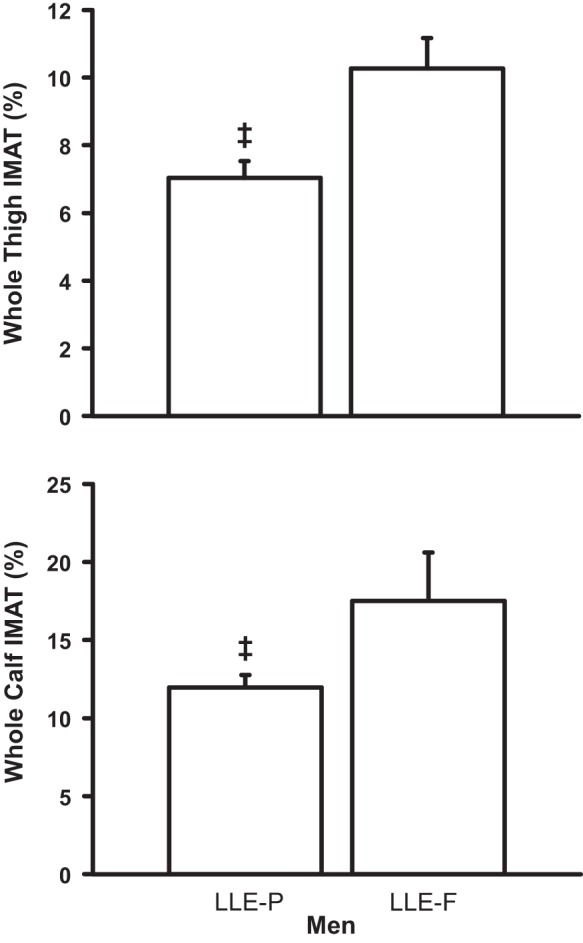
Whole thigh and calf intermuscular adipose tissue (IMAT) content for the LLE subgroups of men. LLE, lifelong exercisers; LLE-P, LLE-performance; LLE-F, LLE-fitness. ‡P < 0.05 vs. LLE-F.
Whole calf IMAT among the groups is presented in Table 5. For the women, calf IMAT was higher in LLE (133%) and OH (138%) compared with YE (P < 0.05), with no difference between LLE and OH. For the men, calf IMAT was higher in LLE (142%) and OH (104%) compared with YE (P < 0.05), with no difference between LLE and OH. LLE-P calf IMAT was 31% lower than LLE-F (P < 0.05; Fig. 6).
DISCUSSION
The exercise boom triggered a large number of women and men to engage in structured physical activity as a lifestyle. More than 50 years later, several of these individuals that remained consistent with an exercise regimen are in their eighth decade of life. This provided a unique opportunity to assess the impact of lifelong aerobic exercise on upper and lower leg skeletal muscle size, function, and adiposity. While the LLE cohort was unique in many ways, the old healthy individuals were also an extraordinary group, given their health and mobility status. The main findings were the following. 1) Lifelong aerobic exercise attenuated the age-associated quadriceps muscle size and strength loss in men by ~50% but did not similarly influence the women. 2) Lifelong aerobic exercise had no beneficial or detrimental influence on quadriceps absolute muscle power or muscle power and strength normalized to muscle size in both sexes. 3) Lifelong aerobic exercise attenuated the age-associated thigh IMAT accumulation by ~50% in both sexes. 4) Lifelong aerobic exercise had no influence on triceps surae muscle size or calf IMAT accumulation in both sexes. 5) Greater lifelong aerobic exercise training intensity was more beneficial for the IMAT profiles of the thigh and calf.
Aging results in decreased skeletal muscle size due to losses in muscle fiber size and number (37, 38, 44, 59). Compilation of the literature regarding quadriceps volume changes with aging suggests an average decline of 29%, with no apparent sex-specific difference (28, 42, 44, 68) (Fig. 7). The quadriceps volume reduction in the old healthy subjects (−36%) generally compares with these data; however, the women (−41%) appear to atrophy somewhat more than the men (−31%) (Fig. 7). The ~50% attenuation of quadriceps muscle volume loss with lifelong aerobic exercise in the men (Fig. 7) was likely due to the exercise stimulus influencing one or both of the aforementioned components of age-related muscle atrophy. Interestingly, Mikkelsen et al. (46) have shown that continuous regular aerobic exercise training for 28 years in men a decade younger than those in the current study (64 vs. 74 yr) also attenuated the typical age-associated loss of quadriceps muscle size by ~50%.
Fig. 7.
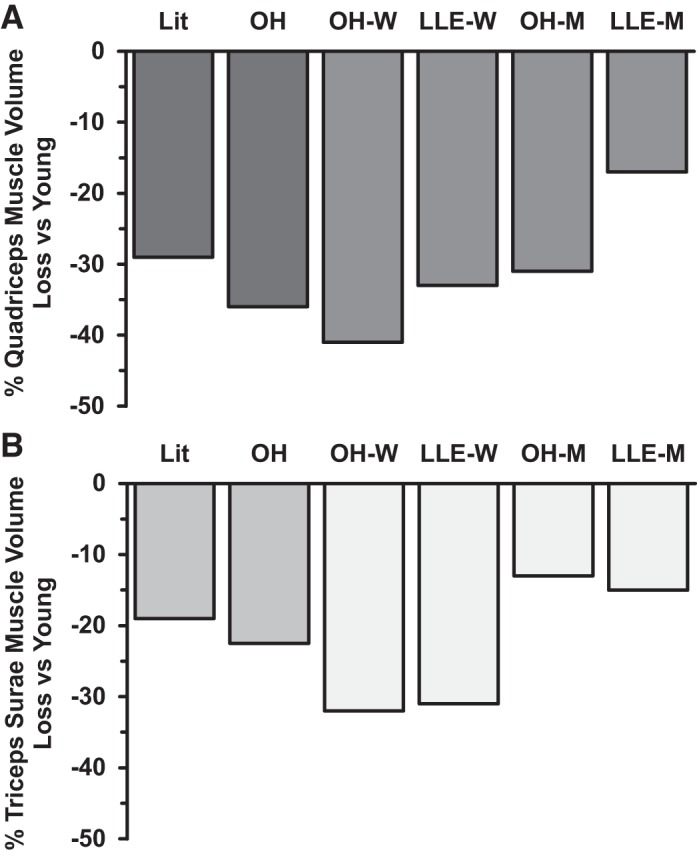
Compilation of the literature for the quadriceps muscle volume (top) changes with aging [n = 204: 89 young (23 yr), 115 old (74 yr), 101 men, 103 women] (27, 40, 42, 64) and triceps surae muscle volume (bottom) changes with aging [n = 101: 50 young (28 yr), 50 old (75 yr), 76 men, 25 women] (4, 12, 45, 52). Data from the current investigation are included for comparison. Lit, literature; OH, old healthy; LLE, lifelong exercisers; W, women; M, men. OH reflects combined data from the M and W [n = 40: 20 young (25 yr), 20 old (75 yr), 20 men, 20 women.]
Given the dearth of information on lifelong-exercising women, it is not readily apparent why the women in the current study were not similarly responsive as the men with regard to quadriceps muscle volume attenuation (Fig. 7). An obvious candidate was the difference in sex hormones, particularly testosterone, which is a known to be anabolic for skeletal muscle mass (29). Testosterone was lower in the younger women compared with the younger men, but this difference was magnified in the older cohorts (23). From an exercise standpoint, aerobic exercise training can induce muscle growth in older women and men (24, 25, 40), suggesting responsiveness to aerobic exercise in both sexes into the eighth decade of life. In terms of training programs, it is reasonable to speculate that training intensity may play a central role in regulating muscle size. However, the self-reported training intensity (along with frequency and duration) were similar between lifelong-exercising women and men (Table 2). Furthermore, the two groups of men with significantly different lifelong training intensities (LLE-P and LLE-F) did not see any differences in muscle size or strength despite differences in peak power output on the cycle ergometer (240 ± 9 vs. 185 ± 8 W) and maximal aerobic capacity (23). We also documented that the mitochondrial enzyme activity (citrate synthase) and muscle capillarity were maintained at levels similar to those of the young exercisers in both lifelong-exercising women and men (23), suggesting no sex differences in aerobic exercise regulating these components of muscle health over the life span. Last, the slightly lower step count from their younger counterparts in the LLE women compared with the LLE men, which, if reflective of long-term nonexercise activity, may have had an impact on the muscle mass results (48). Collectively, these data suggest that the regular lifelong exercise influence on muscle size appears to have a sex-specific component that needs further study.
In the current study, aging resulted in typical declines in nearly all parameters of quadriceps absolute muscle contractile function, and lifelong aerobic exercise did not appear to enhance or exacerbate the losses in strength or power, either absolute or when normalized to muscle size (Table 4 and Fig. 4). The notable exception to these findings was the benefit of lifelong exercise on quadriceps isometric strength in men, which is in line with their increased quadriceps cross-sectional area. Data from the literature on muscle strength with lifelong aerobic exercise in men are equivocal (1, 26), and, to our knowledge, are nonexistent in women. Future studies should expand upon these findings to extend these data in lifelong exercisers and include older, less functional individuals for a direct comparison.
In addition to driving the isometric strength gains in the lifelong-exercising men, the enhanced quadriceps muscle size could be expected to benefit metabolic function. In aging individuals, decreased muscle size is associated with a less favorable outcome should an infection, hospitalization, or surgical procedure arise (9, 12, 14, 33, 35, 53). Given the known hypertrophic benefits of resistance exercise training (5, 60, 63, 67), the addition of this type of training may be beneficial for numerous aspects of health and function in lifelong-exercising women and men. Indeed, across all 68 subjects there was a significant correlation (Fig. 8) between quadriceps size and maximal oxygen uptake (V̇o2max), which in turn is inversely associated with increasing comorbidities and all-cause mortality (50). It should be considered that the aerobic qualities of the skeletal muscle (e.g., capillary network, aerobic enzymes) among these aerobically conditioned individuals (23) likely contributed to the strong muscle mass-to-V̇o2max relationship and that other modes of exercise (i.e., resistance training) may yield different results.
Fig. 8.
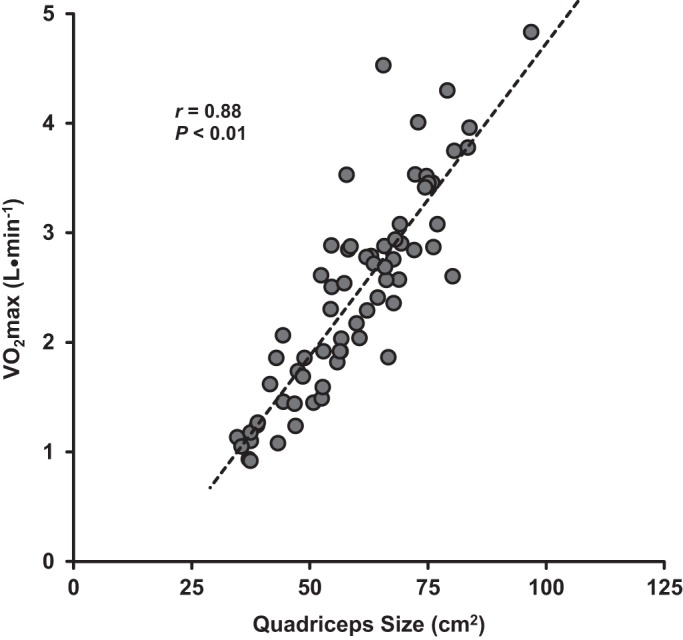
Relationship between quadriceps size (cm2) and maximum oxygen consumption (V̇o2max; L/min) among the entire cohort (n = 68).
One of the notable benefits of lifelong aerobic exercise was the ~50% attenuation of thigh IMAT infiltration in both women and men. IMAT analysis provides an index for lipid infiltration and is associated with aging, inactivity, and muscle and mobility dysfunction (2, 15, 21, 43) and many other disorders (2, 22, 32). Thus, the beneficial effects in the current study are likely related to the regular exercise stimuli (3, 15, 21, 43, 45, 75), which is known to increase skeletal muscle lipid oxidation and the associated regulation (11, 30, 31, 34, 72). Overall energy balance and dietary intake may have also played a role in the exercise benefits on thigh IMAT (52), as total body fat mass was ~30% lower in the lifelong-exercising cohorts compared with the old healthy groups.
Although the triceps surae has been investigated with aging and sarcopenia far less than other muscles, compilation of the literature shows an average age-related reduction in triceps surae volume of −19% (4, 13, 47, 56) (Fig. 7). Unfortunately, the available data do not delineate whether there are age-associated differences in atrophy of this muscle group between women and men. The triceps surae volume reduction with aging in the old healthy groups (−23%) is in line with the literature, but there appears to be sex-specific atrophy, with the women (−32%) atrophying more than the men (−13%; Fig. 7). The literature data also suggest that this functionally important muscle group may atrophy less with aging than the quadriceps (quadriceps: −29%, triceps surae: −19%; Fig. 7). This idea is more strongly supported by the data from the old healthy men (quadriceps: −31%, triceps surae: −13%) compared with the women (quadriceps: −41%, triceps surae: −32%). Considering that the triceps surae muscle group is chronically activated for postural tasks and general daily ambulation (16, 73), a reduced amount of age-associated atrophy may be related to the “training” status of this muscle group. The triceps surae contains the soleus, a muscle with a high percentage of slow-twitch fibers (20, 58, 65) and an aerobically trained phenotype, even in individuals not completing regular exercise (41). Aging studies that analyzed the individual muscles of the triceps surae show the soleus does not significantly atrophy with aging (4) or atrophies less than the mixed-fiber type gastrocnemius muscles (47) indicating it may be less protected by postural tasks or daily ambulation.
The lack of overall effect of lifelong aerobic exercise on triceps surae size in both the women and the men (Fig. 7) may be related to the aforementioned trained status of the triceps surae. Individuals completing 25 years of regular aerobic exercise training through middle age (48 yr), at high or moderate levels of training, have similar triceps surae muscle size as nonexercisers (64). Different modes of exercise training (e.g., cycling vs. running) and the associated muscle-specific activation (7, 36) over the lifetime of training may also play a role. The lack of influence of lifelong aerobic exercise on the ~120% age-related increase in calf IMAT content in either sex supports the concept of a lack of significant additional aerobic exercise influence on this muscle group.
The subdivision of the lifelong-exercising men into performance and fitness training (Table 2) allowed for some insight into the role of intensity on whole muscle size, function, and adiposity. Whereas muscle size and function (Tables 3 and 4) were not influenced by training intensity, IMAT content in both the thigh and calf was ~30% lower in the performance (higher-intensity)-trained group compared with their fitness-trained counterparts (Fig. 6). The quadriceps (vastus lateralis) mitochondrial profiles of both groups were similar with respect to β-oxidation enzyme activity, suggesting similar lipid oxidation capacity, although some aspects of muscle capillarity were enhanced in the performance group (23). It is possible that, because the performance group was more focused on training for competition, they paid more attention to caloric intake (body weight: LLE-P: 77 ± 2 kg; LLE-F: 83 ± 5 kg), which may have contributed to their lower IMAT and body fatness (body fat: LLE-P: 21 ± 1%; LLE-F: 27 ± 1%). It is also interesting to note that higher-intensity training reduced thigh IMAT closer to the young cohort (YE: 5% vs. LLE-P: 7%; Fig. 6 and Table 5), whereas the fitness-trained group was about the same as the sedentary, old healthy group (10–11%). Conversely, the calf IMAT was maintained in the performance group compared with the sedentary, old healthy group (~12%), whereas the fitness-trained group was elevated above the other older groups of men (~18%). This provides further evidence that the triceps surae muscles are differentially influenced by exercise training and aging.
In summary, we present the first investigation into the influence of habitual aerobic exercise training throughout the life span on key upper and lower leg skeletal muscle health parameters in women and men in their eighth decade of life. There were sex- and muscle-specific benefits for muscle size as well as sex-independent benefits for adipose tissue infiltration into muscle. It is clear that more studies and overall data are needed on skeletal muscle health in the areas of aging and lifelong exercise. Although the current data provide initial insights, more muscle health data on sex differences with lifelong exercise are warranted, given the complex nature of key hormones and variations in biology among women and men across the life span. Furthermore, additional modes of exercise (e.g., resistance exercise), inclusion of older individuals with less overall functional status, and other less understood but important muscle groups such as the triceps surae muscles should be further investigated.
GRANTS
This investigation was funded by National Institute on Aging Grant AG-038576.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.R.B., U.R., G.A.L., T.A.T., and S.W.T. conceived and designed research; T.R.B., U.R., G.A.L., B.M.G., T.A.T., and S.W.T. performed experiments; T.L.C., T.R.B., and W.H.F. analyzed data; T.L.C., T.R.B., U.R., T.A.T., and S.W.T. interpreted results of experiments; T.L.C., T.A.T., and S.W.T. prepared figures; T.L.C. and T.A.T. drafted manuscript; T.L.C., U.R., T.A.T., and S.W.T. edited and revised manuscript; T.L.C., T.R.B., U.R., G.A.L., W.H.F., B.M.G., T.A.T., and S.W.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the many HPL graduate students and support staff that were involved with various aspects of data collection and interaction with the volunteers. Additionally, we are grateful to all the volunteers that graciously gave their time, energy, and support to this investigation. Finally, we thank Todd Manini, PhD for assistance in setting up the IMAT measurement in the HPL.
REFERENCES
- 1.Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc 39: 1989–1996, 2007. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- 2.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014: 1, 2014. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang E, Tanabe K, Yokoyama N, Chijiki S, Tsuruzono T, Kuno S. Effects of daily walking on intermuscular adipose tissue accumulation with age: a 5-year follow-up of participants in a lifestyle-based daily walking program. Eur J Appl Physiol 118: 785–793, 2018. doi: 10.1007/s00421-018-3812-4. [DOI] [PubMed] [Google Scholar]
- 4.Barber LA, Barrett RS, Gillett JG, Cresswell AG, Lichtwark GA. Neuromechanical properties of the triceps surae in young and older adults. Exp Gerontol 48: 1147–1155, 2013. doi: 10.1016/j.exger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bechshøft RL, Malmgaard-Clausen NM, Gliese B, Beyer N, Mackey AL, Andersen JL, Kjær M, Holm L. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp Gerontol 92: 96–105, 2017. doi: 10.1016/j.exger.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993. doi: 10.1111/j.1748-1716.1993.tb09573.x. [DOI] [PubMed] [Google Scholar]
- 7.Bijker KE, de Groot G, Hollander AP. Differences in leg muscle activity during running and cycling in humans. Eur J Appl Physiol 87: 556–561, 2002. doi: 10.1007/s00421-002-0663-8. [DOI] [PubMed] [Google Scholar]
- 8.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey EJ. Sarcopenia in solid organ transplantation. Nutr Clin Pract 29: 159–170, 2014. doi: 10.1177/0884533613520619. [DOI] [PubMed] [Google Scholar]
- 10.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol (1985) 105: 1907–1915, 2008. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol (1985) 68: 990–996, 1990. doi: 10.1152/jappl.1990.68.3.990. [DOI] [PubMed] [Google Scholar]
- 12.Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96: 895–901, 2006. doi: 10.1017/BJN20061943. [DOI] [PubMed] [Google Scholar]
- 13.Csapo R, Malis V, Sinha U, Du J, Sinha S. Age-associated differences in triceps surae muscle composition and strength - an MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet Disord 15: 209, 2014. doi: 10.1186/1471-2474-15-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Hoogt PA, Reisinger KW, Tegels JJW, Bosmans JWAM, Tijssen F, Stoot JHMB. Functional compromise cohort study (FCCS): sarcopenia is a strong predictor of mortality in the intensive care unit. World J Surg 42: 1733–1741, 2018. doi: 10.1007/s00268-017-4386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH; Health, Aging, and Body . Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ericson MO, Nisell R, Ekholm J. Quantified electromyography of lower-limb muscles during level walking. Scand J Rehabil Med 18: 159–163, 1986. [PubMed] [Google Scholar]
- 17.Felig P. Amino acid metabolism in man. Annu Rev Biochem 44: 933–955, 1975. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- 18.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M; International Working Group on Sarcopenia . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in V̇o2 max. J Appl Physiol (1985) 65: 1147–1151, 1988. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 20.Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch 348: 247–255, 1974. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 105: 1498–1503, 2008. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71: 885–892, 2000. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 23.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 297: R1452–R1459, 2009. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985) 113: 1495–1504, 2012. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harridge S, Magnusson G, Saltin B. Life-long endurance-trained elderly men have high aerobic power, but have similar muscle strength to non-active elderly men. Aging (Milano) 9: 80–87, 1997. doi: 10.1007/BF03340131. [DOI] [PubMed] [Google Scholar]
- 27.Harridge SD, Lazarus NR. Physical activity, aging, and physiological function. Physiology (Bethesda) 32: 152–161, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985) 103: 2068–2076, 2007. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 29.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 271–277, 2004. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 31.Jordy AB, Kiens B. Regulation of exercise-induced lipid metabolism in skeletal muscle. Exp Physiol 99: 1586–1592, 2014. doi: 10.1113/expphysiol.2014.082404. [DOI] [PubMed] [Google Scholar]
- 32.Kalafateli M, Karatzas A, Tsiaoussis G, Koutroumpakis E, Tselekouni P, Koukias N, Konstantakis C, Assimakopoulos S, Gogos C, Thomopoulos K, Kalogeropoulou C, Triantos C. Muscle fat infiltration assessed by total psoas density on computed tomography predicts mortality in cirrhosis. Ann Gastroenterol 31: 491–498, 2018. doi: 10.20524/aog.2018.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirk PS, Friedman JF, Cron DC, Terjimanian MN, Wang SC, Campbell DA, Englesbe MJ, Werner NL. One-year postoperative resource utilization in sarcopenic patients. J Surg Res 199: 51–55, 2015. doi: 10.1016/j.jss.2015.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konopka AR, Wolff CA, Suer MK, Harber MP. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol Regul Integr Comp Physiol 315: R461–R468, 2018. doi: 10.1152/ajpregu.00030.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 19: 1396–1402, 2013. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lester BE, Standley RA, Lee JD, Fink WJ, Trappe SW, Trappe TA. Muscle-specific substrate use during cycle exercise at 1 G: implications for astronaut muscle health. Aviat Space Environ Med 84: 789–796, 2013. doi: 10.3357/ASEM.3440.2013. [DOI] [PubMed] [Google Scholar]
- 37.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr 98: 237–252, 2007. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 40.Lovell DI, Cuneo R, Gass GC. Can aerobic training improve muscle strength and power in older men? J Aging Phys Act 18: 14–26, 2010. doi: 10.1123/japa.18.1.14. [DOI] [PubMed] [Google Scholar]
- 41.Luden N, Hayes E, Minchev K, Louis E, Raue U, Conley T, Trappe S. Skeletal muscle plasticity with marathon training in novice runners. Scand J Med Sci Sports 22: 662–670, 2012. doi: 10.1111/j.1600-0838.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 42.Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 13: 320–328, 2013. [PubMed] [Google Scholar]
- 43.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85: 377–384, 2007. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 44.McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, Jones DA, Degens H. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol A Biol Sci Med Sci 73: 1287–1294, 2018. doi: 10.1093/gerona/gly040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkelsen UR, Agergaard J, Couppé C, Grosset JF, Karlsen A, Magnusson SP, Schjerling P, Kjaer M, Mackey AL. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: The influence of ageing. Exp Gerontol 93: 54–67, 2017. doi: 10.1016/j.exger.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, Kjær M. Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev 134: 531–540, 2013. doi: 10.1016/j.mad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 183: 291–298, 2005. doi: 10.1111/j.1365-201X.2004.01404.x. [DOI] [PubMed] [Google Scholar]
- 48.Oikawa SY, Holloway TM, Phillips SM. The impact of step reduction on muscle health in aging: protein and exercise as countermeasures. Front Nutr 6: 75, 2019. doi: 10.3389/fnut.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134: e653–e699, 2016. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 51.Ruan XY, Gallagher D, Harris T, Albu J, Heymsfield S, Kuznia P, Heshka S. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol (1985) 102: 748–754, 2007. doi: 10.1152/japplphysiol.00304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 14: 511–523, 2015. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheetz KH, Waits SA, Terjimanian MN, Sullivan J, Campbell DA, Wang SC, Englesbe MJ. Cost of major surgery in the sarcopenic patient. J Am Coll Surg 217: 813–818, 2013. doi: 10.1016/j.jamcollsurg.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, Heymsfield SB. Ethnicity-related skeletal muscle differences across the life span. Am J Hum Biol 22: 76–82, 2010. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97, 1998. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 56.Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci Med Sci 60: 1111–1117, 2005. doi: 10.1093/gerona/60.9.1111. [DOI] [PubMed] [Google Scholar]
- 57.Tihanyi J, Apor P, Fekete G. Force-velocity-power characteristics and fiber composition in human knee extensor muscles. Eur J Appl Physiol Occup Physiol 48: 331–343, 1982. doi: 10.1007/BF00430223. [DOI] [PubMed] [Google Scholar]
- 58.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol (1985) 106: 1159–1168, 2009. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 59.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 61.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trappe S, Luden N, Minchev K, Raue U, Jemiolo B, Trappe TA. Skeletal muscle signature of a champion sprint runner. J Appl Physiol (1985) 118: 1460–1466, 2015. doi: 10.1152/japplphysiol.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 64.Trappe SW, Costill DL, Goodpaster BH, Pearson DR. Calf muscle strength in former elite distance runners. Scand J Med Sci Sports 6: 205–210, 1996. doi: 10.1111/j.1600-0838.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 65.Trappe SW, Trappe TA, Lee GA, Costill DL. Calf muscle strength in humans. Int J Sports Med 22: 186–191, 2001. doi: 10.1055/s-2001-16385. [DOI] [PubMed] [Google Scholar]
- 66.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- 67.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol 90: 2070–2074, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Van Hall G, Saltin B, Wagenmakers AJ. Muscle protein degradation and amino acid metabolism during prolonged knee-extensor exercise in humans. Clin Sci (Lond) 97: 557–567, 1999. doi: 10.1042/cs0970557. [DOI] [PubMed] [Google Scholar]
- 70.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 71.Vesali RF, Klaude M, Rooyackers O, Wernerman J. Amino acid metabolism in leg muscle after an endotoxin injection in healthy volunteers. Am J Physiol Endocrinol Metab 288: E360–E364, 2005. doi: 10.1152/ajpendo.00248.2004. [DOI] [PubMed] [Google Scholar]
- 72.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297: E987–E998, 2009. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 73.Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol 67: 402–411, 1987. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 74.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 75.Wroblewski AP, Amati F, Smiley MA, Goodpaster B, Wright V. Chronic exercise preserves lean muscle mass in masters athletes. Phys Sportsmed 39: 172–178, 2011. doi: 10.3810/psm.2011.09.1933. [DOI] [PubMed] [Google Scholar]