Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder impacting cognition, movement, and quality of life in >10 million individuals worldwide. We recently characterized and quantified a skeletal muscle pathology in PD represented by exaggerated type I myofiber grouping presumed to result from denervation-reinnervation processes. Our previous findings indicated that impaired neuromuscular junction integrity may be involved in type I grouping, which is associated with excessive motor unit activation during weight-bearing tasks. In this study, we performed transcriptional profiling to test the hypothesis that type I grouping severity would link to distinct gene expression networks. We generated transcriptome-wide poly(A) RNA-Seq data from skeletal muscle of individuals with PD [n = 12 (9 men, 3 women); 67 ± 2 yr], age- and sex-matched older adults (n = 12; 68 ± 2 yr), and sex-matched young adults (n = 12; 30 ± 1 yr). Differentially expressed genes were evaluated across cohorts. Weighted gene correlation network analysis (WGCNA) was performed to identify gene networks most correlated with indicators of abnormal type I grouping. Among coexpression networks mapping to phenotypes pathologically increased in PD muscle, one network was highly significantly correlated to type I myofiber group size and another to percentage of type I myofibers found in groups. Annotation of coexpressed networks revealed that type I grouping is associated with altered expression of genes involved in neural development, postsynaptic signaling, cell cycle regulation and cell survival, protein and energy metabolism, inflammation/immunity, and posttranscriptional regulation (microRNAs). These transcriptomic findings suggest that skeletal muscle may play an active role in signaling to promote myofiber survival, reinnervation, and remodeling, perhaps to an extreme in PD.
NEW & NOTEWORTHY Despite our awareness of the impact of Parkinson’s disease (PD) on motor function for over two centuries, limited attention has focused on skeletal muscle. We previously identified type I myofiber grouping, a novel indicator of muscle dysfunction in PD, presumably a result of heightened rates of denervation/reinnervation. Using transcriptional profiling to identify networks associated with this phenotype, we provide insight into potential mechanistic roles of skeletal muscle in signaling to promote its survival in PD.
Keywords: muscle histology, neuromuscular junction, Parkinson’s disease, RNA-Seq, type I grouping, weighted gene correlation network analysis
INTRODUCTION
Parkinson’s disease (PD) is the most common motor neurodegenerative disorder, resulting from loss of dopaminergic neurons in the substantia nigra (42). The classical symptoms of PD (e.g., tremor, bradykinesia, and dystonia) are expressed as motor control abnormalities (42). Although a substantial body of research focuses on the central nervous system effects of PD, very little remains understood about peripheral neuromuscular deficits. Our previous research led to the discovery of a unique pathological phenotype in PD skeletal muscle (36), characterized by abnormally extensive type I myofiber grouping in comparison with normal aging (37, 66). In PD patients, the degree of type I grouping is associated with abnormal motor recruitment patterns (35, 66) and symptom severity assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS), suggesting that disease burden may progress with this histopathology.
Increased type I myofiber grouping results from motor unit remodeling (46), possibly initiated by denervation of type II myofibers either centrally [i.e., death of fast (type II) alpha motoneuron] or peripherally [i.e., loss of neuromuscular junction (NMJ) integrity] (1, 8, 27). The denervated type II myofibers may then either die or be reinnervated by a branching axon of an alternate motoneuron. If the myofiber is reinnervated by a slow (type I) motor unit, it takes on at least some of the type I phenotype, including type I myosin expression (37). This process is the likely basis for the larger surviving motor units found in PD (13, 14) and aging (61, 72), as rescuing denervated myofibers from atrophy and death (60) aids in preservation of total myofiber number (66). Thus, although neuromuscular communication efficiency is hampered, motor unit remodeling that gives rise to myofiber grouping may have a protective effect against denervation-induced muscle wasting. Furthermore, increased type I myofiber grouping partially reverses with high-intensity exercise rehabilitation in PD (36), suggesting incomplete conversion to type I myofiber identity (37), transient, simultaneous innervation by both type I and type II motoneurons [evidence of which exists in animal models of denervation/reinnervation (56, 74)], and/or considerable plasticity of the NMJ and molecular environment in PD skeletal muscle.
We recently found that a targeted panel of genes and protein signaling pathways involved in NMJ integrity was altered in skeletal muscle of PD patients (36), suggesting a differential molecular environment. These findings and other evidence from animal models of denervation (43, 67) indicate that skeletal muscle actively participates in signaling to promote reinnervation. However, the underlying mechanisms remain incompletely understood. To advance the understanding of potential mediators underlying type I grouping, we performed transcriptome profiling via skeletal muscle transcriptome-wide RNA-Seq among individuals with PD and healthy old and young control subjects. Results were analyzed with the network approach weighted gene correlation network analysis (WGCNA) (44). This method identifies clusters of genes that can then be studied in relation to clinical phenotypes and functional annotations. Network-based analyses are able to take advantage of variability in gene expression across samples to illuminate regulatory relationships among transcriptional networks. Furthermore, based on their assumptions, network analyses tend to include a greater number of low-expression genes, allowing insight into potential effects and relationships between factors filtered out of single-gene analyses that may have significant biological implications (44). WGCNA was recently used to identify brain-specific gene expression patterns in aging (30), Alzheimer’s disease (47), and PD (15). The present analyses reveal novel gene expression networks in skeletal muscle that may lead to the uncovering of mechanisms of motor unit remodeling in general, as well as unique properties of muscle in PD that may help explain the extreme type I grouping and, further, serve as biomarkers for PD progression.
METHODS
Human subjects.
Twelve subjects with idiopathic PD were matched for sex against young adults (YA) and for age and sex against healthy older adults (OA). Studies for which the individuals volunteered have been published previously (35, 40, 53), and detailed recruitment and eligibility information can be found there. Briefly, all persons with PD were Hoehn and Yahr stage 2 (n = 8) or 3 (n = 4) and medication stable for at least 4 wk. Both OA and YA were nonexercising, disease-free control subjects. All volunteers provided written informed consent to have their samples stored and utilized in future studies. Each study was reviewed and approved by the University of Alabama at Birmingham Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Skeletal muscle biopsy and tissue preparation.
Skeletal muscle samples (typically 200–350 mg) were obtained from the vastus lateralis under local anesthesia with a 5-mm Bergström biopsy needle with suction and processed to remove excess fat, blood, and connective tissue. Muscle samples to be used for RNA-Seq were snap-frozen in liquid nitrogen (LN2) and stored at −80°C until use. A portion of the muscle to be used for immunohistochemistry was mounted in tragacanth gum mixed with Tissue Tek O.C.T. compound (Sakura Finetek, Torrance, CA) atop a square of cork, frozen in isopentane cooled to the temperature of LN2, and stored at −80°C.
Immunohistochemistry and myofiber grouping analysis.
Myofiber grouping parameters were assessed as previously described (37). Six-micrometer sections were cut with a microtome cryostat at −25°C (Leica, Buffalo Grove, IL) and stored at −80°C until staining. Sections were fixed with a 1:1 acetone-methanol wash, rehydrated in PBS, blocked with 5% goat serum (20 min at room temperature), and incubated with primary antibody against type I myosin heavy chain (MHC) [BA-D5; Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA] diluted to 0.9 μg/mL in 1% goat serum at 37°C for 30 min. After incubation with an Alexa Fluor 594-conjugated secondary antibody (10 μg/mL, A-21145; ThermoFisher Scientific, Waltham, MA), sections were blocked again with 5% goat serum and incubated in a cocktail of primary antibodies against laminin (5 μg/mL, MA1-06100; ThermoFisher) and MHC IIa (1.5 μg/mL, A4.74; DSHB). Conjugated secondary antibodies Alexa Fluor 488 (5 μg/mL, A-21121; ThermoFisher) and Alexa Fluor 647 (5 μg/mL, A-21247; ThermoFisher) were applied simultaneously to detect MHC IIa and laminin, respectively.
Images were captured with a fluorescence microscope (BX51; Olympus, Tokyo, Japan) at ×10 magnification with an XM10 camera (Olympus), electronically stitched together with CellSens Dimension (Olympus) to create a single image for each sample, and analyzed with Image-Pro Premier software (v9.1; Media Cybernetics, Rockville, MD). Myofiber distribution was calculated as the number of type I, IIa, and IIx myofibers relative to the total number of myofibers counted (mean ± SD 1,213 ± 464). Representative images are presented in Fig. 1 for individuals with and without PD. Myofiber grouping parameters were quantified as previously described in detail (37). Briefly, the expected mean number of like myofibers touching a given type I myofiber was calculated as the product of the total number of myofibers touching a given myofiber and type I myofiber distribution. To qualify as a myofiber group, the number of like myofibers touching a given type I myofiber must exceed the mean plus 1 SD around the mean for at least two contiguous myofibers in the core of a group. Subsequently, the number of type I myofibers touching any other type I myofiber was counted until the edge of the group or a structural barrier in the muscle (e.g., perimysium) was reached. Although this method partially controls for type I myofiber distribution, the influence is not completely removed (37). Between-group differences in histology measures were analyzed through a one-way analysis of variance (ANOVA) using SPSS Statistics (v24; IBM, Armonk, NY). Post hoc comparisons were performed with a Bonferroni correction, and significance was declared at P < 0.05.
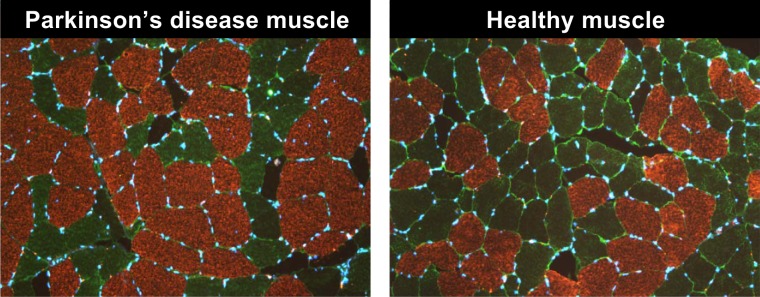
Fig. 1.
Representative images of type I myofiber grouping in skeletal muscle biopsy specimens collected from the vastus lateralis of individuals with Parkinson’s disease in comparison to healthy skeletal muscle. Our laboratory’s methodology for quantification of type I myofiber grouping is presented briefly in methods and in greater detail elsewhere (37), and we have previously investigated the potential implications of type I grouping in Parkinson’s disease (36).
RNA isolation and cDNA library synthesis.
Skeletal muscle samples (7.8 ± 2.0 mg) were homogenized in a Bead Ruptor Elite bead mill homogenizer (Omni International, Kennesaw, GA) cooled by LN2 to 10°C for 2 × 20 s at 4.2 m/s. Muscle homogenates were processed with the Agencourt RNAdvance Tissue Kit (Beckman Coulter, Indianapolis, IN) on a BioMek FXP Laboratory Automation Workstation (Beckman Coulter, Indianapolis, IN). The isolated RNA was of good concentration (130 ± 45 ng/μL), quality (260/280: 1.9 ± 0.0, 260/230: 1.4 ± 0.1), and integrity (RIN: 8.95 ± 0.31, 28s/18s: 1.6 ± 0.2), as assessed with NanoDrop and an RNA Standard Sensitivity Kit (DNF-471; Advanced Analytical Technologies, Ankeny, IA) on a Fragment Analyzer Automated CE system (Advanced Analytical Technologies). Subsequently, cDNA libraries were constructed from 250 ng of total RNA with the Universal Plus mRNA-Seq kit (NuGEN Technologies, San Carlos, CA). Library concentration (75.8 ± 16.8 ng/μL) was assessed fluorometrically with the Qubit dsDNA HS Kit (ThermoFisher), and quality (average fragment size: 336 ± 5 bp) was assessed with the Genomic DNA 50Kb Analysis Kit (DNF-467; Advanced Analytical Technologies).
RNA sequencing and preprocessing.
Preliminary sequencing of cDNA libraries (average read depth: 90,000 reads) was performed by using a MiSeq system (Illumina, San Diego, CA) to confirm library quality. Deep sequencing was subsequently performed with an S2 flow cell in a NovaSeq sequencing system (Illumina) (average read depth: 25 million pairs of 2 × 50-bp reads). Raw data were processed by using bcl2fastq Conversion Software (Illumina) to obtain FASTQ files, and the FASTQ files were aligned to the Human GENCODE hg38 genome with STAR (19). Gene expression was quantified with featureCounts (48). The raw FASTQ data and the final gene expression matrix were deposited to GEO (accession no. GSE128177).
Weighted gene correlation network analysis.
The general analysis workflow is presented in Fig. 2. Before analysis, samples were assessed for outliers in phenotypes of interest based on a Euclidean distance adjacency matrix, which resulted in no samples being discarded. Weighted gene correlation network analysis (WGCNA) was performed with Bioconductor (23) package WGCNA (44) under R version 3.4.3 (64). Gene expression was normalized to library size across samples with a variance-stabilizing transformation step. Next, transcripts were filtered for missing data, low variance in expression, or outliers with the default settings in WGCNA: transcripts were retained based on expression in at least four samples, and samples were retained based on expression of at least four transcripts. WGCNA assumes a nonrandom, scale-free topology among genes, meaning that most participate in few interactions but a small fraction (hubs) participate in many (5). Network adjacency was calculated for the remaining 42,492 transcripts with a soft-thresholding power of 12 (based on the scale-free topology of the network). Hierarchical cluster analysis and dynamic tree-cutting yielded 41 modules, which were then assigned arbitrary colors for identification (e.g., magenta, green), with no greater degree of relatedness between similarly named modules (e.g., turquoise, darkturquoise). A representative eigengene (the first principal component) was identified for each module. Pearson correlations were performed to examine the relationships between module eigengenes and type I myofiber grouping parameters, adjusted for subject age, sex, and type I myofiber distribution. Accounting for the number of WGCNA modules and controlling for a type I error rate of 0.05, a Bonferroni-corrected P value of P < 0.001 was considered statistically significant.
Fig. 2.
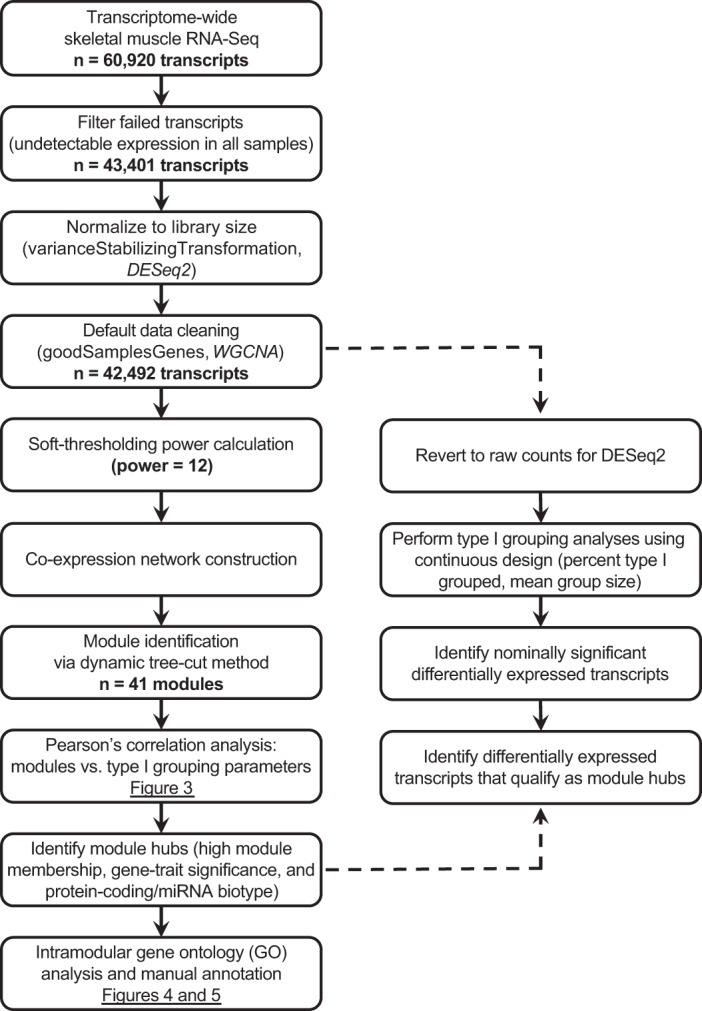
Summary of analysis workflow for transcriptome-wide RNA-Seq data generated from human skeletal muscle samples. Steps were taken to maximize the weighted gene correlation network analysis (WGCNA) method according to its assumptions (further guidance is provided in the tutorial at https://horvath.genetics.ucla.edu/html/CoexpressionNetwork/Rpackages/WGCNA/Tutorials/). When appropriate, function names and R packages used (in italics) are provided. Data were normalized to library size, and the expression network was constructed with a soft-thresholding power of 12, as indicated by our data set. From 41 modules built via dynamic tree-cutting, we further pursued 2 modules based on significant Pearson correlations with myofiber grouping parameters of interest. Genes identified as “hubs” within the modules were internally validated with a continuous design in DESeq2 from the original 42,492 genes entered into the WGCNA model. Genes were manually inspected for functional annotation by examining the available literature related to their biological roles and potential relevance to skeletal muscle, type I grouping, and/or Parkinson’s disease. miRNA, microRNA.
Intramodular analysis and internal validation with DESeq2.
Within modules of interest, we identified transcripts that satisfied three conditions: intramodular connectivity (defined as kME, or module membership) above median, gene-trait significance above median (see shaded quadrants in Fig. 4A and Fig. 5A), and established biology (e.g., protein-coding mRNA, microRNA (miRNA)] based on Ensembl gene biotype (86) in biomaRt (71). From this subset, module hubs (most connected genes) were identified with a topological overlap matrix and visualized with Cytoscape v3.6.1 (68). To identify predominant biological themes within the module, hubs were assessed with both pathway-based and gene ontology (GO)-based gene set overrepresentation analyses in ConsensusPathDB (Max Planck Institute for Molecular Genetics, Munich, Germany) (33) against the comprehensive default reference set of human genes with known participation in at least one pathway or GO process. All hub genes were manually inspected for functional annotation by examining the available literature related to their biological roles and potential relevance to skeletal muscle, type I grouping, or PD (Supplemental Tables S4 and S6; all supplemental materials are available at https://doi.org/10.6084/m9.figshare.10294832).
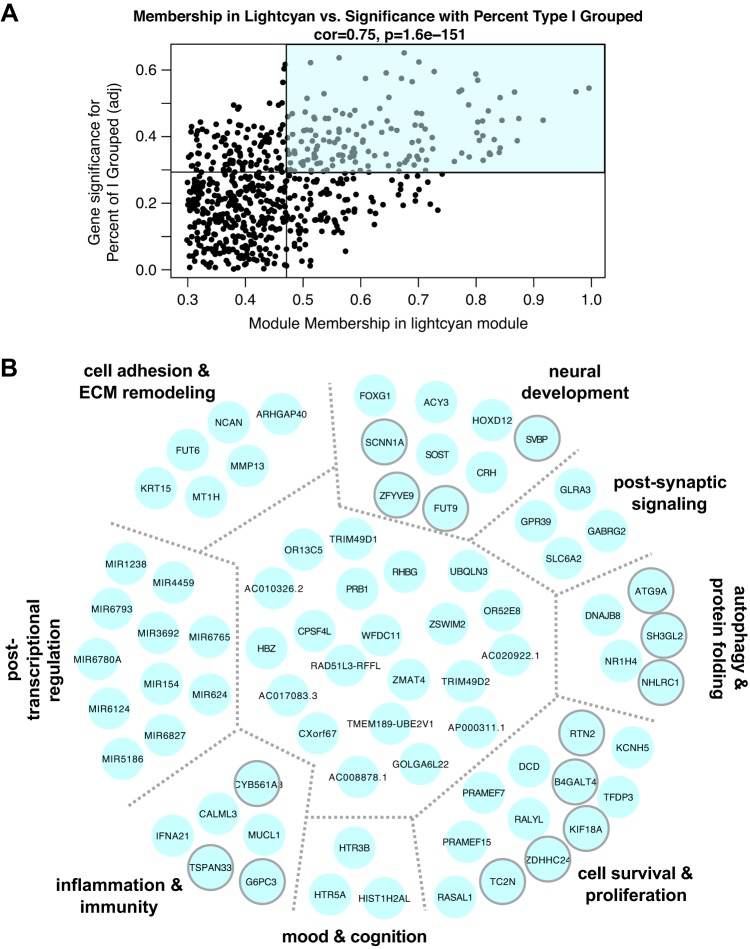
Fig. 4.
A: association for all lightcyan module members between module membership and relationship to % of type I fibers grouped across 36 samples. Lines represent median values for each parameter. Module members in the top right quadrant (shaded) were further examined for ontological relationships and common biology. B: network of genes identified as lightcyan module hubs, based on topological overlap. Manual investigation of functional annotation revealed common functions and biological phenotypes associated with clusters of genes within module (separated by gray dotted lines and further described in Supplemental Table S4). Transcripts clustered in the center (not labeled) have miscellaneous or incompletely understood functions. Transcripts outlined in gray were nominally significantly related to % of type I fibers grouped on a single-gene level (P < 0.05) with DESeq2 with a continuous variable design. ECM, extracellular matrix.
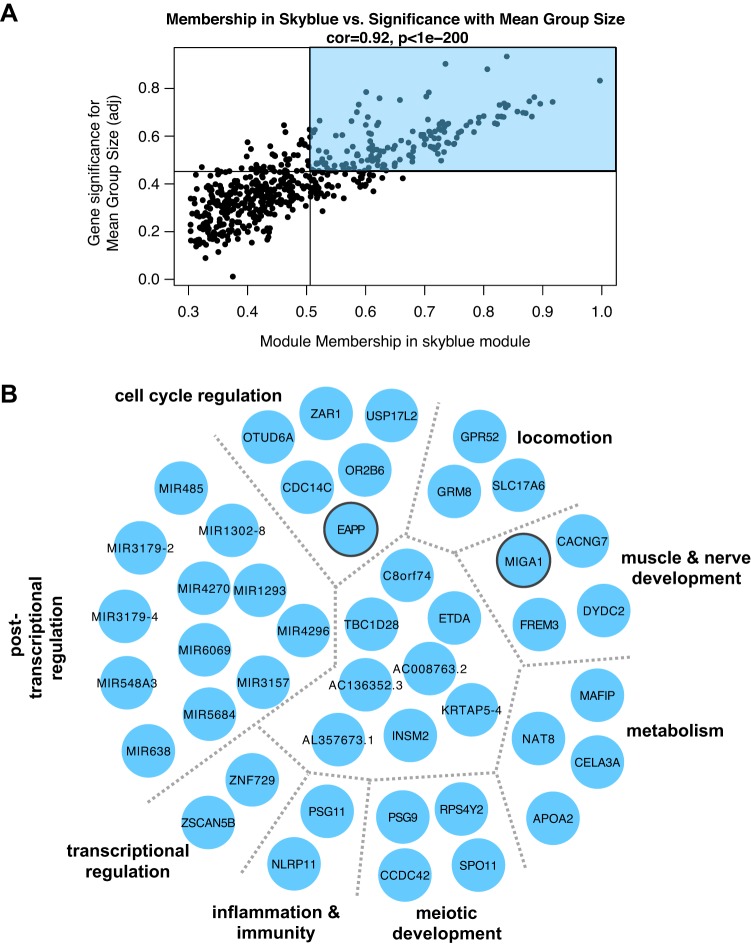
Fig. 5.
A: association for all skyblue module members between module membership and relationship to mean type I group size across 36 samples. Lines represent median values for each parameter. Module members in the top right quadrant (shaded) were further examined for ontological relationships and common biology. B: network of genes identified as skyblue module hubs, based on topological overlap. Manual investigation of functional annotation revealed common functions and biological phenotypes associated with clusters of genes within module (separated by gray dotted lines and further described in Supplemental Table S6). Transcripts clustered in the center (not labeled) have miscellaneous or incompletely understood functions. Transcripts outlined in gray were nominally significantly related to mean group size on a single-gene level (P < 0.05) with DESeq2 with a continuous variable design.
As an internal validation, we then investigated relationships between individual genes and type I grouping parameters that showed significant relationships with WGCNA networks (i.e., percent type I grouped, mean group size), using continuous variable designs in DESeq2 (49) (Fig. 2). Module hubs that reached nominal significance (P < 0.05) at the single-gene level were noted and highlighted within the network diagrams with a dotted gray outline (see Fig. 4B and Fig. 5B).
RESULTS
Subject characteristics, myofiber phenotype, and (for the PD subjects) disease stage are presented in Table 1. Myofiber distribution varied across cohorts, with PD subjects demonstrating significantly higher type I myofiber distribution (nearly half of all myofibers vs. only one-third in OA and YA; P < 0.05). Abnormal type I groups were nearly 13-fold larger in PD than in the other cohorts (P < 0.05), and this contributed to a doubling of total type I myofibers that were part of an abnormal group in PD versus OA and YA (P < 0.05).
Table 1.
Subject characteristics and skeletal muscle histology
| Parkinson’s Disease | Older Adults | Young Adults | |
|---|---|---|---|
| Subjects, n | 12 (9 M/3 F) | 12 (9 M/3 F) | 12 (9 M/3 F) |
| Age, yr | 67 ± 2 | 68 ± 2 | 30 ± 1 |
| Parkinson’s disease progression | |||
| Time since diagnosis, yr | 6 ± 1 | ||
| Hoehn and Yahr stage | 2 (n = 8), 3 (n = 4) | ||
| Levodopa equivalency dose, mg/day | 511 ± 133 | ||
| MDS-UPDRS motor score | 30 ± 3 | ||
| PDQ-39 total score | 24 ± 7 | ||
| PDQ-39 mobility subscore | 13 ± 4 | ||
| Myofiber distribution, % | |||
| Type I | 49 ± 5* | 35 ± 2 | 33 ± 2 |
| Type IIa | 36 ± 5† | 49 ± 4 | 54 ± 3 |
| Type IIx | 14 ± 4 | 17 ± 4 | 13 ± 2 |
| Type II (combined) | 51 ± 5* | 65 ± 2 | 67 ± 2 |
| Type I myofiber grouping | |||
| Mean group size, type I myofibers/group | 187 ± 73* | 14 ± 3 | 13 ± 2 |
| Standard deviation, type I myofibers/group | 121 ± 63 | 12 ± 5 | 9 ± 2 |
| Minimum group size, type I myofibers/group | 26 ± 11* | 5 ± 1 | 6 ± 1 |
| Maximum group size, type I myofibers/group | 224 ± 99* | 32 ± 10 | 34 ± 8 |
| Frequency, no. of type I groups/1,000 myofibers | 3 ± 1§ | 6 ± 1 | 7 ± 1 |
| Percentage of grouped type I myofibers, % total type I myofibers | 51 ± 10‡§ | 22 ± 4 | 28 ± 5 |
Values represent means ± SE. Parkinson’s disease progression was not assessed for healthy groups. F, female; M, male; MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale (Section III); PDQ-39, Parkinson’s Disease Questionnaire (39 items).
P < 0.05 vs. other cohorts;
P < 0.05 vs. young adults;
P < 0.05 vs. older adults;
P < 0.10 vs. young adults.
Weighted gene correlation network analysis of skeletal muscle RNA-Seq data.
Network construction by WGCNA using 42,492 transcripts identified 41 coexpression modules, for which the randomly assigned color, number of members, and average membership (kME) are presented in Supplemental Table S1. To investigate coexpression module correlation with phenotypic traits, the transcripts in each module were summarized by representative module eigengenes. The correlation analysis between each module eigengene and the average type I myofiber group size, percentage of type I myofibers in groups, and type I myofiber group frequency is shown in Fig. 3. After correction for multiple comparisons, significant module eigengene-to-trait correlations were found for percentage of type I myofibers grouped and mean group size, both of which are significantly increased in PD muscle (see Table 1). Across all samples, the representative eigengene for the lightcyan module was strongly correlated with the percentage of type I myofibers found in groups (r = 0.54, P = 8 × 10−4). The representative eigengene for the skyblue module was strongly and positively correlated with mean type I myofiber group size (r = 0.84, P = 2 × 10−10). Complete lists of transcripts classified as members of these modules are available in Supplemental Tables S2 and S3, respectively.
Fig. 3.
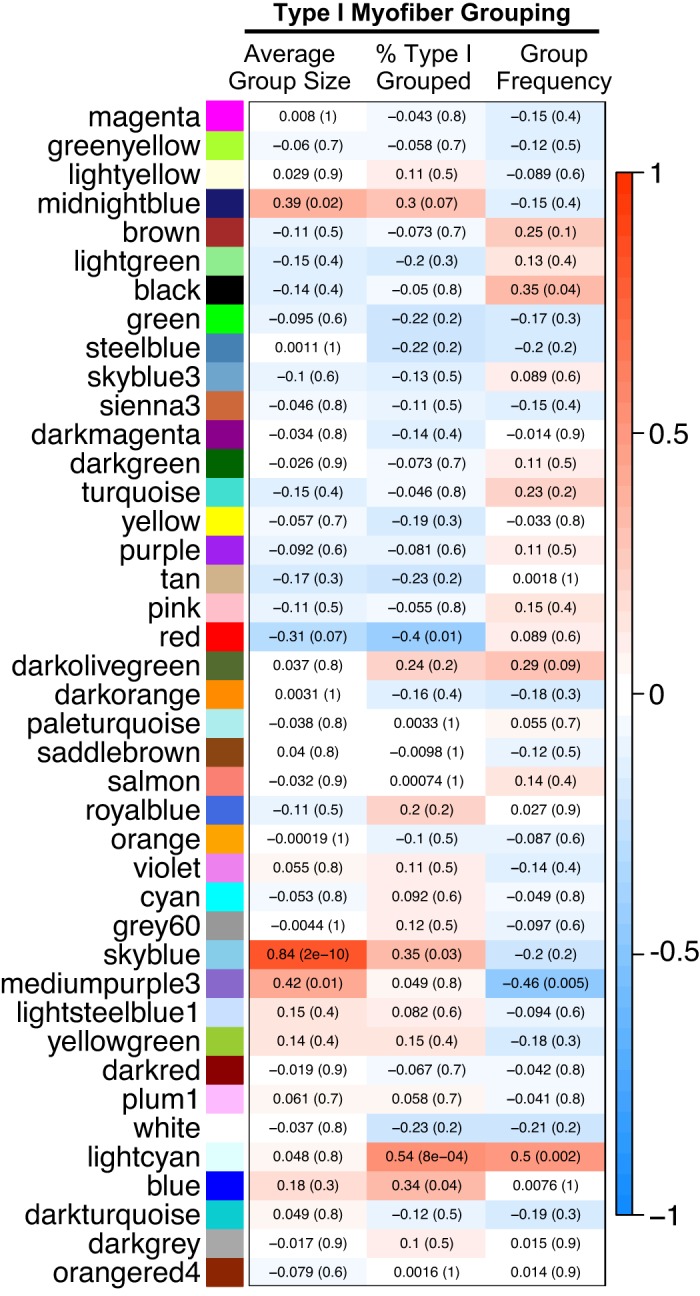
Heatmap showing Pearson correlation coefficient (and associated P value) between representative eigengenes (first principal components) for each module identified by weighted gene correlation network analysis and type I myofiber grouping traits in skeletal muscle of individuals with Parkinson’s disease (n = 12), healthy old adults (n = 12), and young adults (n = 12).
Within the two modules, we identified the transcripts most associated with the trait of interest and most connected to other module members (shaded quadrants in Fig. 4A and Fig. 5A) and then filtered for biological annotation as described in methods. Transcripts identified as lightcyan module hub genes are explored in Fig. 4B and presented with full gene name, GO classification, and relevant functional annotation in Supplemental Table S4. GO analysis revealed neurotransmitter receptor activity [GO: 0030594, adjusted P (Padj) = 0.007], and transmitter- and ligand-gated ion channel activity (GO: 0022835, Padj = 0.005 and GO: 0015276, Padj = 0.005), overrepresented as a result of GLRA3, GABRG2, HTR3B, and others. Many lightcyan transcripts play roles in signaling through neurotransmitter (e.g., GABRG2, GLRA3, HTR5A), ion (e.g., CALML3, TRIM49D1, SCNN1A), or neuroendocrine (e.g., HTR3B, HTR5A, CRH, SLC6A2) channels, and several are associated with neural development (e.g., HOXD12, FOXG1, ATG9A, NCAN) or survival (e.g., FUT9, RTN2, RALYL). Additionally, several module members were associated with neurological phenotypes in preclinical analyses (e.g., KCNH5, SH3GL2, FUT9, CRH, NHLRC1), suggesting their importance in maintenance of normal nervous system functioning. Hubs that attained nominal significance (P < 0.05) with a continuous design in DESeq2 tended to cluster together in categories related to neural development, autophagy, inflammation/immunity, and cell survival and proliferation (outlined in gray in Fig. 4B; see Supplemental Table S5 for full list).
Hub genes of the skyblue module (associated with mean type I group size) are shown in Fig. 5B and in further detail in Supplemental Table S6. The singular GO process significantly overrepresented in this module was thiol-dependent ubiquitin-specific protease activity (GO: 0004843, Padj = 0.02), driven by OTUD6A and USP17L2. Manual annotation of skyblue transcripts revealed associations with regulation of cell cycle dynamics (e.g., OTUD6A, CDC14C, OR2B6), locomotion (e.g., GPR52, GRM8, SLC17A6), and regulation of gene expression at the transcriptional (e.g., ZSCAN5B, ZNF729) and posttranscriptional (see miRNA cluster) levels. Other transcripts played a role in regulation of neuronal survival (e.g., EAPP, CACNG7, MIGA1); both EAPP and MIGA1 attained nominal significance with a continuous design in DESeq2 (see Supplemental Table S7 for full list).
DISCUSSION
This study represents a novel investigation of the transcriptomic signatures of skeletal muscle in PD in the context of type I myofiber grouping. Through application of WGCNA, we have identified molecular networks associated with the pathology of type I myofiber grouping, a characteristic of aging muscle (37, 66) that is markedly exaggerated in PD and potentially associated with PD progression (36). We have previously shown that factors related to innervation and NMJ integrity are altered in PD at the gene and protein levels (36). It remains unclear whether the observed patterns are a primary result of denervation or an entirely separate peripheral pathology originating in skeletal muscle. Nevertheless, the present transcriptome profiling suggests that skeletal muscle capacity to signal with innervating neurons is incrementally altered with the degree of type I grouping. Furthermore, the novel WGCNA results suggest that neural development, cell cycle regulation, and protein metabolism are also implicated in this phenotype.
Evolution of assessment of type I myofiber grouping.
Myofiber grouping was described several decades ago (31, 46) and has been observed in healthy older adults (37, 66), endurance-trained Masters athletes (54, 63), and, of course, PD (20, 36, 55). Initially, myofiber groups were crudely identified as fields of myofibers enclosed by like myofibers (31). Methodology was further refined to examine all myofibers within a muscle fascicle and determine whether the abundance of enclosed myofibers within a given fascicle exceeded random chance (46). Our own laboratory’s efforts to characterize myofiber grouping (37) build further on these methods, yielding quantifiable parameters including mean group size, group frequency, and percentage of total type I fibers in groups, the latter of which incorporates both other components and provides the most comprehensive picture of the grouping phenotype.
Our pipeline is quantitative and incorporates type I distribution; thus, comparing two samples, detecting abnormal type I grouping requires passing a greater statistically defined threshold in the sample with the higher type I distribution. Based on overall type I distribution, we first calculate the expected mean of like myofibers surrounding a given fiber. As stated in methods, to qualify as an abnormal myofiber group the number of like myofibers touching a given type I myofiber must exceed the expected mean plus 1 SD for at least two contiguous myofibers in the core of a group. Unlike the enclosed fiber method (which requires all type I myofibers in the group to be enclosed by like myofibers), we count outward from the two core fibers to quantify group size, which includes the like myofibers on the periphery of the abnormal group (37).
The etiology of type I grouping is commonly understood to involve denervation and subsequent reinnervation in skeletal muscle (9, 46). In PD, the tonic nature of the metabolic/mechanical demands imposed on skeletal muscle by motor symptoms likely favors retention of low-threshold motor units (20, 36); when type II (fast) fibers lose neural input, they transition to a type I (slow) phenotype if reinnervated by a type I motoneuron (43, 59). However, although denervation/reinnervation may lead to type I grouping, not every instance of myofiber grouping may be a reflection of this process, as others have suggested that myofiber degeneration/regeneration may contribute to a similar phenotype (27). The present work provides insight into the skeletal muscle transcriptomic profile accompanying type I myofiber grouping and evidence to suggest that dynamic communicative processes between skeletal muscle and the nervous system are heightened in muscle with a greater degree of type I grouping, but continued investigation is necessary to understand whether this altered transcriptional profile confers advantages for myofiber survival and motor function in PD.
Neural development program in type I myofiber grouping.
The transcripts of several factors involved in neural development were upregulated in muscle with a higher percentage of grouped type I myofibers (lightcyan module). For instance, fucosyltransferase (FUT)9 participates in neurite outgrowth, cell adhesion, and glycosylation of a growth factor carried by neuronal cell adhesion molecule (26), an established marker of denervation in skeletal muscle (1, 36). Additionally, other factors identified may play neuroprotective roles, potentially promoting stability of newly formed NMJs: e.g., autophagy-related (ATG)9A by aiding in axon guidance (82) and corticotropin-releasing hormone (CRH) by stabilizing new connections (41). Another cluster of genes associated with extracellular matrix may also be involved in guiding and stabilizing new axonal connections with skeletal myofibers (81). For example, the axon guidance factor neurocan (NCAN) is downregulated in the substantia nigra of individuals with PD (28), and here we found its expression elevated in skeletal muscle with exaggerated type I grouping (Fig. 4B). Our previous research suggests that excessive type I grouping can be partially reversed with high-intensity exercise training (36), giving rise to the intriguing possibility that the actions of NCAN and other potential mediators of motor unit remodeling revealed in these transcript profiles may be differentially responsive to high-demand contractions requiring significant type II motor unit recruitment. We suspect that some components of these skeletal muscle coexpression networks may reveal signaling processes attempting to compensate for the peripheral consequences of a neurodegenerative phenotype. Although what contributes to neuromuscular deterioration in PD is still not well understood, the muscle itself appears to be an active participant in encouraging stability and survival of newly formed NMJs.
Neuromuscular communication.
Via this discovery approach, novel findings suggest that type I grouping is accompanied by altered skeletal muscle communication with innervating neurons. Both GABA- and glycine-driven neurotransmitter activity have been implicated in increasing motoneuron innervation in a mouse model of development (3). Here we found that expression of receptor isoforms [e.g., glycine receptor α (GLRA)3, γ-aminobutyric acid type A receptor γ (GABRG)2], along with potassium voltage-gated channel subfamily H member (KCNH)5, increased with the degree of type I grouping. KCNH5 is a nerve-specific marker that has been used to identify neuronal differentiation of progenitor cells (32). Likewise, GLRA3 expression was found to be increased during development of the cerebellar cortex (4, 34), which plays a role in motor learning and movement control (18). There is a sizable body of evidence suggesting that the cerebellum is impacted by altered communication in other parts of the brain in PD (39, 50, 70, 85). To our knowledge, these genes have not been detected at the transcript level in healthy skeletal muscle, suggesting another element of the neurodevelopmental program that may be reinitiated in skeletal muscle after denervation.
Neurotransmission through GABAergic and glycine-mediated signaling is typically inhibitory in nature (11, 52). For example, mutations in GABGR2 are associated with neuronal hyperexcitability, motor deficits, and epilepsy (78, 87). In PD, lower GABA activity in the basal ganglia is associated with higher (i.e., worse) UPDRS scores (25), and we have previously found that poor performance on the UPDRS tends to track with type I grouping (36). Presently, we found overexpression of inhibitory signaling factors in individuals with a high degree of myofiber grouping, suggesting that their expression in skeletal muscle may be a compensatory effect of deficits in the central nervous system.
Another fascinating, yet small, cluster of transcripts in the skyblue module was associated with locomotion and movement—with obvious implications for motor disturbances associated with type I grouping in PD (36). Of these, G protein-coupled receptor (GPR)52 and glutamate metabotropic receptor (GRM)8 have both been linked to motor-based symptoms of neurological diseases (57, 84), and solute carrier family 17 member 6 (SLC17A6, also known as vesicular glutamate transporter 2) is thought to play a protective role in survival of dopaminergic neurons (73). Taken together, these data suggest that type I myofiber grouping is accompanied by increased expression of factors enabling neuromuscular communication and translation into movement, perhaps as a necessity of the lower activation threshold shared among a larger number of myofibers per motor unit (13, 14).
Self-regulation of type I myofiber group size.
Another metric of type I grouping severity, mean type I myofiber group size, was strongly associated with a network involved in ubiquitin-proteasome pathway processes (via overrepresentation analysis). Generally, ubiquitin-mediated signaling plays a role in muscle atrophy and apoptosis, which are involved in the response to denervation in skeletal muscle (6, 17) and remodeling likely required for successful reinnervation (2). Ubiquitin-specific peptidases have recently received attention in PD, and hallmark pathologies of PD in the brain (e.g., impaired mitophagy, aggregation of α-synuclein and other proteins) are directly or indirectly associated with defects in this process (7, 76). In the present investigation, ubiquitin-related genes included ubiquitin carboxy terminal hydrolase 17-like protein 2 (USP17L2) and ovarian tumor family deubiquitinase 6A (OTUD6A), both studied for their roles in regulation of apoptosis and tumor progression (10, 38, 58). In addition, several other factors known to regulate cell cycle dynamics were associated with mean type I group size. For instance, both zygote arrest (ZAR)1 and E2F-associated phosphoprotein (EAPP) have been reported to suppress neural development and survival (16, 79). Thus this transcriptional network may play a role in coordinating the complex process of denervation-reinnervation cycling.
Our study design using poly(A) pulldown to isolate muscle mRNA enables detection of primary transcript-microRNA molecules, which may later be processed into mature, biologically active microRNAs (miRNAs) to regulate posttranscriptional gene expression (80). Supporting the idea that further processing occurs, other studies have demonstrated a differential miRNA profile in the brain (45) and circulation (12, 75) of individuals with PD. This growing area of study holds promise for detection of potential biomarkers and clearly warrants further investigation. In the present study, we identified 11 miRNAs associated with increased percentage of total type I grouped and 12 associated with larger mean type I group size (see Supplemental Tables S4 and S6). Notably, a large proportion (>25%) of skyblue module hubs were miRNA transcripts. For instance, miR-638 has been shown to inhibit growth and proliferation of other cell types (69, 77) including smooth muscle. Notably, its effects on target gene DACT3 (disheveled binding antagonist of beta catenin 3) (65) suggest that miR-638 could be implicated in autophagy and neuronal development (22). Another module hub, miR-485, plays a role in muscle differentiation (62) and targets plexin A4 (PLXNA4) and beta-secretase 1 (BACE1) (21), both of which are associated with axonal development and are current therapeutic targets in neurodegenerative disease (29, 83). The fact that skeletal muscle with larger type I groups expresses miR-485, a regulator of both muscle and neural differentiation, along with several other module genes related to regulation of cell growth and death, suggests that a physiological ceiling may exist for type I myofiber grouping beyond which neuromuscular communication is too strained to allow efficient motor unit function. Interestingly, the skyblue module as a whole was not related to the percentage of type I myofibers found in groups, suggesting that group size alone may impose these limits.
Study limitations.
A limitation of this analysis is low power to detect significant differences at the transcriptome-wide level because of the small number of subjects analyzed. Our analysis was also potentially limited by the use of transcriptome-wide poly(A) RNA-Seq, which is not sensitive to differential expression of nonpolyadenylated RNA species. As described in the WGCNA methods, modules were narrowed down to transcripts that were associated with a known function. In some modules, this resulted in removal of a large number of transcripts. Although this processing step improved network visualization and ontological analysis, it is possible if not likely that some of the removed transcripts (e.g., pseudogenes, noncoding RNAs) play a yet-undocumented role of biological significance. Although genes classified as members of modules of interest were preserved whether noncoding transcripts were excluded before or after network construction, further work is clearly needed to determine the roles of such transcripts in human health and disease. Additionally, posttranscriptional (12, 75) or posttranslational (51) mechanisms of regulating phenotype may play a large role in PD pathology without being reflected by differences at the transcript level in skeletal muscle.
Several transcripts of interest identified in this study do not yet have a known biological role (at the protein level) in skeletal muscle. Rather than a limitation, we consider this a benefit of a discovery project, perhaps catalyzing future research. Finally, the skeletal muscle biopsy samples sequenced in this study represent a heterogeneous tissue that includes other cell types (24), meaning that neuronal or glial cells may have contributed to our detection of neuroactive factors such as GLRA3 and GABRG2. Future work is needed to assess whether these transcripts are translated in isolated muscle cells and subsequently whether they act in an autocrine/paracrine fashion, are degraded, or may be packaged into extracellular vesicles by skeletal muscle.
Conclusions.
This study is the first to assess the transcriptional profile in skeletal muscle of older adults with PD and expands on our previous research demonstrating that the pathology of PD, although based in neurological defects, extends to peripheral skeletal muscles (35, 36). Findings support that type I myofiber grouping is associated with an altered transcriptomic signature in skeletal muscle. WGCNA enabled identification of networks of genes that progress with this phenotype and are involved in physiological processes including neurotransmission, neural development, cell cycle regulation, and ubiquitination. Although it remains unclear which factors directly contribute to abnormal type I myofiber grouping in PD, we suspect that skeletal muscle plays an active role in signaling with reinnervating neurons and surrounding tissue to promote survival and remodeling. Continued research is needed to determine whether this motor unit remodeling continues to progress with PD severity and the mechanisms by which high-intensity exercise or other interventions may prevent and/or reverse these processes in both aging and PD.
GRANTS
This work was supported in part by National Institutes of Health Grants T32 HD-071866, R01 AG-017896, and P2C HD-086851, National Science Foundation Grant OAC-1541310, The University of Alabama at Birmingham, and the Alabama Innovation Fund. Further support was provided through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
DISCLAIMERS
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health, the National Science Foundation, The University of Alabama at Birmingham, or the Icahn School of Medicine at Mount Sinai.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.L., S.C.S., M.-L.N.M., and M.M.B. conceived and designed research; K.M.L., S.C.S., M.-L.N.M., B.M.R., K.W., V.D.N., Y.G., P.L.K., and S.T.W. performed experiments; K.M.L., S.C.S., M.-L.N.M., B.M.R., K.W., V.D.N., Y.G., P.L.K., and M.M.B. analyzed data; K.M.L., S.C.S., M.-L.N.M., B.M.R., V.D.N., Y.G., P.L.K., and M.M.B. interpreted results of experiments; K.M.L., M.-L.N.M., P.L.K., and M.M.B. prepared figures; K.M.L. drafted manuscript; K.M.L., S.C.S., M.-L.N.M., B.M.R., K.W., V.D.N., Y.G., P.L.K., S.T.W., and M.M.B. edited and revised manuscript; K.M.L., S.C.S., M.-L.N.M., B.M.R., K.W., V.D.N., Y.G., P.L.K., S.T.W., and M.M.B. approved final version of manuscript.
REFERENCES
- 1.Aare S, Spendiff S, Vuda M, Elkrief D, Perez A, Wu Q, Mayaki D, Hussain SN, Hettwer S, Hepple RT. Failed reinnervation in aging skeletal muscle. Skelet Muscle 6: 29, 2016. doi: 10.1186/s13395-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks GB, Kanjhan R, Wiese S, Kneussel M, Wong LM, O’Sullivan G, Sendtner M, Bellingham MC, Betz H, Noakes PG. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. J Neurosci 25: 1249–1259, 2005. doi: 10.1523/JNEUROSCI.1786-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Shira O, Maor R, Chechik G. Gene expression switching of receptor subunits in human brain development. PLOS Comput Biol 11: e1004559, 2015. doi: 10.1371/journal.pcbi.1004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barabási AL, Albert R. Emergence of scaling in random networks. Science 286: 509–512, 1999. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- 6.Baumann CW, Liu HM, Thompson LV. Denervation-induced activation of the ubiquitin-proteasome system reduces skeletal muscle quantity not quality. PLoS One 11: e0160839, 2016. doi: 10.1371/journal.pone.0160839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510: 370–375, 2014. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 8.Bishop DL, Milton RL. The effects of denervation location on fiber type mix in self-reinnervated mouse soleus muscles. Exp Neurol 147: 151–158, 1997. doi: 10.1006/exnr.1997.6605. [DOI] [PubMed] [Google Scholar]
- 9.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol 3: 1645–1687, 2013. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 10.Burrows JF, McGrattan MJ, Rascle A, Humbert M, Baek KH, Johnston JA. DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J Biol Chem 279: 13993–14000, 2004. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 11.Canto-Bustos M, Loeza-Alcocer E, Cuellar CA, Osuna P, Elias-Viñas D, Granados-Soto V, Manjarrez E, Felix R, Delgado-Lezama R. Tonically active α5GABAA receptors reduce motoneuron excitability and decrease the monosynaptic reflex. Front Cell Neurosci 11: 283, 2017. doi: 10.3389/fncel.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, Xue LJ. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett 644: 94–99, 2017. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Caviness JN, Smith BE, Clarke Stevens J, Adler CH, Caselli RJ, Hentz JG, Manfred MS, Muenter D. Motor unit number estimates in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 8: 161–164, 2002. doi: 10.1016/S1353-8020(01)00007-4. [DOI] [PubMed] [Google Scholar]
- 14.Caviness JN, Smith BE, Stevens JC, Adler CH, Caselli RJ, Reiners CA, Hentz JG, Muenter MD. Motor unit changes in sporadic idiopathic Parkinson’s disease. Mov Disord 15: 238–243, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee P, Roy D, Bhattacharyya M, Bandyopadhyay S. Biological networks in Parkinson’s disease: an insight into the epigenetic mechanisms associated with this disease. BMC Genomics 18: 721, 2017. doi: 10.1186/s12864-017-4098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Ni Y, Liu Y, Xia X, Cao J, Wang C, Mao X, Zhang W, Chen C, Chen X, Wang Y. Spatiotemporal expression of EAPP modulates neuronal apoptosis and reactive astrogliosis after spinal cord injury. J Cell Biochem 116: 1381–1390, 2015. doi: 10.1002/jcb.25096. [DOI] [PubMed] [Google Scholar]
- 17.Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM. Ubiquitin homeostasis is critical for synaptic development and function. J Neurosci 31: 17505–17513, 2011. doi: 10.1523/JNEUROSCI.2922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Zeeuw CI, Ten Brinke MM. Motor learning and the cerebellum. Cold Spring Harb Perspect Biol 7: a021683, 2015. doi: 10.1101/cshperspect.a021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edström L. Selective changes in the sizes of red and white muscle fibres in upper motor lesions and Parkinsonism. J Neurol Sci 11: 537–550, 1970. doi: 10.1016/0022-510X(70)90104-8. [DOI] [PubMed] [Google Scholar]
- 21.Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug MP, Nalls MA, Cookson MR, St-Laurent G 3rd, Wahlestedt C. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol 11: R56, 2010. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher DA, Kivimäe S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn 235: 2620–2630, 2006. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 23.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giordani L, He GJ, Negroni E, Sakai H, Law JY, Siu MM, Wan R, Corneau A, Tajbakhsh S, Cheung TH, Le Grand F. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol Cell 74: 609–621.e6, 2019. doi: 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Gong T, Xiang Y, Saleh MG, Gao F, Chen W, Edden RA, Wang G. Inhibitory motor dysfunction in parkinson’s disease subtypes. J Magn Reson Imaging 47: 1610–1615, 2018. doi: 10.1002/jmri.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouveia R, Schaffer L, Papp S, Grammel N, Kandzia S, Head SR, Kleene R, Schachner M, Conradt HS, Costa J. Expression of glycogenes in differentiating human NT2N neurons. Downregulation of fucosyltransferase 9 leads to decreased Lewis(x) levels and impaired neurite outgrowth. Biochim Biophys Acta 1820: 2007–2019, 2012. doi: 10.1016/j.bbagen.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Haddix SG, Lee YI, Kornegay JN, Thompson WJ. Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy. PLoS One 13: e0205926, 2018. doi: 10.1371/journal.pone.0205926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halbgebauer S, Öckl P, Wirth K, Steinacker P, Otto M. Protein biomarkers in Parkinson’s disease: focus on cerebrospinal fluid markers and synaptic proteins. Mov Disord 31: 848–860, 2016. doi: 10.1002/mds.26635. [DOI] [PubMed] [Google Scholar]
- 29.Han Q, Sun YA, Zong Y, Chen C, Wang HF, Tan L; Alzheimer’s Disease Neuroimaging Initiative . Common variants in PLXNA4 and correlation to CSF-related phenotypes in Alzheimer’s disease. Front Neurosci 12: 946, 2018. doi: 10.3389/fnins.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Pan J, Xin Y, Mi X, Wang J, Gao Q, Luo H. Gene expression analysis reveals novel gene signatures between young and old adults in human prefrontal cortex. Front Aging Neurosci 10: 259, 2018. doi: 10.3389/fnagi.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci 14: 259–276, 1971. doi: 10.1016/0022-510X(71)90216-4. [DOI] [PubMed] [Google Scholar]
- 32.Jeong SG, Ohn T, Kim SH, Cho GW. Valproic acid promotes neuronal differentiation by induction of neuroprogenitors in human bone-marrow mesenchymal stromal cells. Neurosci Lett 554: 22–27, 2013. doi: 10.1016/j.neulet.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 33.Kamburov A, Pentchev K, Galicka H, Wierling C, Lehrach H, Herwig R. ConsensusPathDB: toward a more complete picture of cell biology. Nucleic Acids Res 39, Suppl 1: D712–D717, 2011. doi: 10.1093/nar/gkq1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatio-temporal transcriptome of the human brain. Nature 478: 483–489, 2011. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and Parkinson’s disease on motor unit remodeling: influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SY, Kwon SK, Lee SY, Baek KH. Ubiquitin-specific peptidase 5 and ovarian tumor deubiquitinase 6A are differentially expressed in p53+/+ and p53−/− HCT116 cells. Int J Oncol 1705–1714, 2018. doi: 10.3892/ijo.2018.4302. [DOI] [PubMed] [Google Scholar]
- 39.Kishore A, Popa T. Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front Neurol 5: 157, 2014. doi: 10.3389/fneur.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 41.Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E, Remboutsika E, Karalis KP. Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: implications for neuroprotection. Mol Psychiatry 18: 300–307, 2013. doi: 10.1038/mp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord 28: 311–318, 2013. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 43.Lang F, Khaghani S, Türk C, Wiederstein JL, Hölper S, Piller T, Nogara L, Blaauw B, Günther S, Müller S, Braun T, Krüger M. Single muscle fiber proteomics reveals distinct protein changes in slow and fast fibers during muscle atrophy. J Proteome Res 17: 3333–3347, 2018. doi: 10.1021/acs.jproteome.8b00093. [DOI] [PubMed] [Google Scholar]
- 44.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leggio L, Vivarelli S, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Marchetti B, Iraci N. microRNAs in Parkinson’s disease: from pathogenesis to novel diagnostic and therapeutic approaches. Int J Mol Sci 18: 2698, 2017. doi: 10.3390/ijms18122698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 47.Liang JW, Fang ZY, Huang Y, Liuyang ZY, Zhang XL, Wang JL, Wei H, Wang JZ, Wang XC, Zeng J, Liu R. Application of weighted gene co-expression network analysis to explore the key genes in Alzheimer’s disease. J Alzheimers Dis 65: 1353–1364, 2018. doi: 10.3233/JAD-180400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 49.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu MK, Chen JC, Chen CM, Duann JR, Ziemann U, Tsai CH. Impaired cerebellum to primary motor cortex associative plasticity in Parkinson’s disease and spinocerebellar ataxia type 3. Front Neurol 8: 445, 2017. doi: 10.3389/fneur.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majd S, Power JH, Grantham HJ. Neuronal response in Alzheimer’s and Parkinson’s disease: the effect of toxic proteins on intracellular pathways. BMC Neurosci 16: 69, 2015. doi: 10.1186/s12868-015-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCracken LM, Lowes DC, Salling MC, Carreau-Vollmer C, Odean NN, Blednov YA, Betz H, Harris RA, Harrison NL. Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proc Natl Acad Sci USA 114: E7179–E7186, 2017. doi: 10.1073/pnas.1703839114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosole S, Carraro U, Kern H, Loefler S, Zampieri S. Use it or lose it: tonic activity of slow motoneurons promotes their survival and preferentially increases slow fiber-type groupings in muscles of old lifelong recreational sportsmen. Eur J Transl Myol 26: 5972, 2016. doi: 10.4081/ejtm.2016.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG; Arizona Parkinson’s Disease Consortium . Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol 71: 520–530, 2012. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obongo R, Bon-Mardion N, Duclos C, Strunski V, Guerout N, Marie JP. Dual innervation may occur in a partially denervated muscle. Muscle Nerve 59: 108–115, 2019. doi: 10.1002/mus.26323. [DOI] [PubMed] [Google Scholar]
- 57.Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, Pilc A. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids 32: 179–188, 2007. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- 58.Pereg Y, Liu BY, O’Rourke KM, Sagolla M, Dey A, Komuves L, French DM, Dixit VM. Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol 12: 400–406, 2010. doi: 10.1038/ncb2041. [DOI] [PubMed] [Google Scholar]
- 59.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50: 500–509, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 60.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol 596: 1627–1637, 2018. doi: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594: 4525–4536, 2016. doi: 10.1113/JP271087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polesskaya A, Degerny C, Pinna G, Maury Y, Kratassiouk G, Mouly V, Morozova N, Kropp J, Frandsen N, Harel-Bellan A. Genome-wide exploration of miRNA function in mammalian muscle cell differentiation. PLoS One 8: e71927, 2013. doi: 10.1371/journal.pone.0071927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: can age-related deficits be outrun? J Appl Physiol (1985) 121: 1013–1020, 2016. doi: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 65.Ren Y, Chen Y, Liang X, Lu Y, Pan W, Yang M. MiRNA-638 promotes autophagy and malignant phenotypes of cancer cells via directly suppressing DACT3. Cancer Lett 390: 126–136, 2017. doi: 10.1016/j.canlet.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7: e29082, 2012. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Y, Chen H, Gao L, Zhang W, He J, Yang X, Qin L, Xue X, Guo Z. MiR-638 acts as a tumor suppressor gene in gastric cancer. Oncotarget 8: 108170–108180, 2017. doi: 10.18632/oncotarget.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simioni AC, Dagher A, Fellows LK. Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson’s disease. Neuroimage Clin 10: 54–62, 2016. doi: 10.1016/j.nicl.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J, Arnaiz O, Awedh MH, Baldock R, Barbiera G, Bardou P, Beck T, Blake A, Bonierbale M, Brookes AJ, Bucci G, Buetti I, Burge S, Cabau C, Carlson JW, Chelala C, Chrysostomou C, Cittaro D, Collin O, Cordova R, Cutts RJ, Dassi E, Di Genova A, Djari A, Esposito A, Estrella H, Eyras E, Fernandez-Banet J, Forbes S, Free RC, Fujisawa T, Gadaleta E, Garcia-Manteiga JM, Goodstein D, Gray K, Guerra-Assunção JA, Haggarty B, Han DJ, Han BW, Harris T, Harshbarger J, Hastings RK, Hayes RD, Hoede C, Hu S, Hu ZL, Hutchins L, Kan Z, Kawaji H, Keliet A, Kerhornou A, Kim S, Kinsella R, Klopp C, Kong L, Lawson D, Lazarevic D, Lee JH, Letellier T, Li CY, Lio P, Liu CJ, Luo J, Maass A, Mariette J, Maurel T, Merella S, Mohamed AM, Moreews F, Nabihoudine I, Ndegwa N, Noirot C, Perez-Llamas C, Primig M, Quattrone A, Quesneville H, Rambaldi D, Reecy J, Riba M, Rosanoff S, Saddiq AA, Salas E, Sallou O, Shepherd R, Simon R, Sperling L, Spooner W, Staines DM, Steinbach D, Stone K, Stupka E, Teague JW, Dayem Ullah AZ, Wang J, Ware D, Wong-Erasmus M, Youens-Clark K, Zadissa A, Zhang SJ, Kasprzyk A. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res 43: W589–W598, 2015. doi: 10.1093/nar/gkv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stålberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry 45: 870–878, 1982. doi: 10.1136/jnnp.45.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinkellner T, Zell V, Farino ZJ, Sonders MS, Villeneuve M, Freyberg RJ, Przedborski S, Lu W, Freyberg Z, Hnasko TS. Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J Clin Invest 128: 774–788, 2018. doi: 10.1172/JCI95795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA 107: 14863–14868, 2010. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallelunga A, Ragusa M, Di Mauro S, Iannitti T, Pilleri M, Biundo R, Weis L, Di Pietro C, De Iuliis A, Nicoletti A, Zappia M, Purrello M, Antonini A. Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front Cell Neurosci 8: 156, 2014. doi: 10.3389/fncel.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walden H, Muqit MM. Ubiquitin and Parkinson’s disease through the looking glass of genetics. Biochem J 474: 1439–1451, 2017. doi: 10.1042/BCJ20160498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Yao H, Yi B, Kazama K, Liu Y, Deshpande D, Zhang J, Sun J. MicroRNA-638 inhibits human airway smooth muscle cell proliferation and migration through targeting cyclin D1 and NOR1. J Cell Physiol 234: 369–381, 2019. doi: 10.1002/jcp.26930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Shen D, Xia G, Shen W, Macdonald RL, Xu D, Kang JQ. Differential protein structural disturbances and suppression of assembly partners produced by nonsense GABRG2 epilepsy mutations: implications for disease phenotypic heterogeneity. Sci Rep 6: 35294, 2016. doi: 10.1038/srep35294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watanabe Y, Ishizuka Y, Hirano T, Nagasaki-Maeoka E, Hoshi R, Yoshizawa S, Uekusa S, Kawashima H, Sugito K, Shinohara K, Fukuda N, Nagase H, Soma M, Koshinaga T, Fujiwara K. ZAR1 knockdown promotes the differentiation of human neuroblastoma cells by suppression of MYCN expression. Med Oncol 34: 158, 2017. doi: 10.1007/s12032-017-0999-x. [DOI] [PubMed] [Google Scholar]
- 80.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234, 2009. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 81.Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev Neurobiol 71: 1040–1053, 2011. doi: 10.1002/dneu.20958. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi J, Suzuki C, Nanao T, Kakuta S, Ozawa K, Tanida I, Saitoh T, Sunabori T, Komatsu M, Tanaka K, Aoki S, Sakimura K, Uchiyama Y. Atg9a deficiency causes axon-specific lesions including neuronal circuit dysgenesis. Autophagy 14: 764–777, 2018. doi: 10.1080/15548627.2017.1314897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan XX, Ma C, Gai WP, Cai H, Luo XG. Can BACE1 inhibition mitigate early axonal pathology in neurological diseases? J Alzheimers Dis 38: 705–718, 2014. doi: 10.3233/JAD-131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao Y, Cui X, Al-Ramahi I, Sun X, Li B, Hou J, Difiglia M, Palacino J, Wu ZY, Ma L, Botas J, Lu B. A striatal-enriched intronic GPCR modulates huntingtin levels and toxicity. eLife 4: e05449, 2015. doi: 10.7554/eLife.05449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35: 222–233, 2007. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res 46: D754–D761, 2018. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou F, McWalter K, Schmidt L, Decker A, Picker JD, Lincoln S, Sweetser DA, Briere LC, Harini C, Marsh E, Medne L, Wang RY, Leydiker K, Mower A, Visser G, Cuppen I, van Gassen KL, van der Smagt J, Yousaf A, Tennison M, Shanmugham A, Butler E, Richard G, McKnight D; Members of the Undiagnosed Diseases Network . Expanding the phenotypic spectrum of GABRG2 variants: a recurrent GABRG2 missense variant associated with a severe phenotype. J Neurogenet 31: 30–36, 2017. doi: 10.1080/01677063.2017.1315417. [DOI] [PMC free article] [PubMed] [Google Scholar]