Abstract
Blood pressure regulation in health and disease involves a balance between afferent and efferent signals from multiple organs and tissues. Although there are numerous reviews focused on the role of sympathetic nerves in different models of hypertension, few have revised the contribution of afferent nerves innervating adipose tissue and their role in the development of obesity-induced hypertension. Both clinical and basic research support the beneficial effects of bilateral renal denervation in lowering blood pressure. However, recent studies revealed that afferent signals from adipose tissue, in an adipose-brain-peripheral pathway, could contribute to the increased sympathetic activation and blood pressure during obesity. This review focuses on the role of adipose tissue afferent reflexes and briefly describes a number of other afferent reflexes modulating blood pressure. A comprehensive understanding of how multiple afferent reflexes contribute to the pathophysiology of essential and/or obesity-induced hypertension may provide significant insights into improving antihypertensive therapeutic approaches.
Keywords: adipose tissue, heart, kidney, sensory neurons, sympathetic activation
INTRODUCTION
Hypertension is one of the major risk factors for the development of cardiovascular disease contributing to morbidity and mortality rates worldwide (89, 128, 141, 182). In the United States, the estimated prevalence of drug-resistant hypertension is 9%, and it has been shown that these patients have a higher risk for adverse cardiovascular events, hence the need for greater efforts in improving the effectiveness of the treatments for hypertension (1, 15, 129a, 186). Targeting sympathetic nervous system (SNS) activation has been a therapeutic goal, given its major contribution to elevated blood pressure secondary to high sodium chloride intake (18, 21, 30), chronic kidney disease (83, 88, 153), obesity (2, 67–69, 102), and essential hypertension (63, 64, 189).
Excitatory sympathetic reflexes that participate in the tonic control of efferent sympathetic outflow and therefore peripheral vascular resistance of the cardiovascular system have been extensively reviewed (40, 65, 93, 151). In fact, numerous clinical studies have shown that renal nerve ablation could be an effective treatment for drug-resistant, essential hypertension (22, 74, 136, 165). However, it is still controversial whether the reduction in blood pressure is due to denervation of the sympathetic efferent nerves, the sensory afferent nerves, or both. Yet the current understanding of how the manipulation of sensory signals could be used as an approach for clinical intervention is limited. Although β-adrenergic blockers are a standard treatment for hypertension associated with obesity, there are associated adverse effects such as metabolic dysregulation and body weight gain, most likely due to the reduced capacity to dissipate calories (84, 106, 183). Notably, a rising number of preclinical studies suggest that adipose afferent signals may play a key role in modulating sympathetic outflow to key organs involved in blood pressure control (184), uncovering new potential treatments for hypertension secondary to obesity and metabolic syndrome.
The focus of this review is to highlight the contribution of afferent, sensory signals from adipose tissue in the control of cardiovascular function in health and disease. A better understanding of the underlying pathophysiological mechanisms contributing to blood pressure regulation is a critical step to improve the treatment of obesity-associated hypertension.
AFFERENT REFLEXES ARC OVERVIEW
Reflex arcs sense peripheral stimuli from sensory receptors and integrate the information in the brain, tonically modulating the sympathetic efferent outflow to organs and tissues (154). Afferent fibers with sensory receptor endings have been described in heart (117, 120, 138, 177), aorta (27, 116, 137), carotid body (37, 79, 85), pulmonary veins (118, 175), blood vessels (109, 159), adrenal glands (56, 152, 173), visceral organs (14, 70, 93, 139), kidney (54, 95, 122, 187), skeletal muscle (87, 110), and adipose tissue (7, 9, 162, 185). In physiological conditions, afferent nerve activity is critical for maintaining vascular tone, coronary vascular blood flow, water and electrolyte balance, renal microcirculation, lipolysis, and lipid redistribution.
Sensory receptors in the body are activated by temperature, mechanical deformation, and/or chemical stimuli such as changes in pH, CO2, or O2. The activation of these receptors causes an influx of ions that depolarize adjacent and nearby afferent sensory neurons transducing sensory signals into action potentials (107, 130). Certain afferent neurons have somas in the dorsal ganglion root that transmit the electrical signals to the spinal cord and subsequently to the central nervous system (CNS) modulating sympathetic outflow in physiological conditions. In the CNS, the paraventricular nucleus of the hypothalamus (PVN) is one of the major integrative centers that receives sensory signals from the periphery and participates in the control of SNS outflow and cardiovascular function via the activation of preautonomic neurons (for reviews, see 52, 68, 142). These neurons in the PVN project to the rostral ventrolateral medulla (RVLM), a key brain area that regulates basal and reflex control of sympathetic outflow, blood pressure, baroreflex, and chemo- and cardiopulmonary reflexes (66, 101, 105, 170). The PVN also projects to the nucleus tractus solitary (NTS) and spinal cord, regulating sympathetic tone and blood pressure from the hindbrain (142, 144).
Another major site for afferent signal integration is the NTS that receives sensory information from stretch-sensitive mechanoreceptors in the carotid sinus and aortic arch (aortocarotid baroreflexes), the cardiac atria and ventricles (cardiopulmonary baroreflexes), and chemo/mechanoreceptors from the viscera (3, 32, 55, 86). The NTS also receives sensory inputs from the area postrema that lacks a complete blood brain barrier and senses changes in ionic and hormonal composition of blood and cerebrospinal fluid (12, 71, 179). In turn, the NTS sends excitatory (glutamatergic) projections to the caudal and rostral ventrolateral medulla, mediating autonomic and cardiovascular regulation (32, 112, 172).
Therefore, the precise balance between sensory afferent and sympathetic efferent signals to and from the central nervous system is critical for the maintenance of physiological functions, including blood pressure regulation.
ADIPOSE AFFERENT REFLEX: AN OVERLOOKED PLAYER IN BLOOD PRESSURE REGULATION
The adipose afferent reflex (AAR) is the sympathoexcitatory reflex that initiates in adipose tissue and is activated by sensory stimulation (e.g., nociceptive, mechanoceptive, and chemoreceptive). In physiological conditions, activation of the AAR prevents fat deposition by inducing lipolysis and lipid mobilization in white adipose tissue (WAT) and promoting leptin release to reduce body weight and increase energy expenditure (13, 46, 131, 149). In response to AAR activation, WAT also secretes adiponectin that acts in the central nervous system to control appetite and energy expenditure (51, 129). Moreover, electrical or chemical stimulation of subcutaneous and visceral WAT promotes lipid mobilization as well as free fatty acid production (29, 46, 60, 147). Therefore, the AAR is a neural mechanism that, in normal conditions, contributes to body weight and fat deposition regulation by modulating sympathetic outflow to adipose tissue. Whereas the AAR in WAT is involved in lipolysis and lipid redistribution, the reflex arc in brown adipose tissue (BAT) is critical for the regulation of thermogenesis (11, 19, 103). Although the adipose afferent reflex was elegantly reviewed by Xiong et al. (185) in 2014, recent studies have shown further evidence of AAR dysfunction in the context of metabolic disease (20, 31, 44–46).
Dr. Niijima (132, 133) first described the adipose reflex arc in 1998 while studying leptin “sensors” in WAT and their role in regulating WAT metabolic functions. Leptin injections (10 ng/mL and 100 ng/mL, 0.2 mL) into epididymal WAT evoked reflex activation of sympathetic nerve activity in WAT, BAT, and other organs such as the liver and pancreas and a decrease in vagal nerve activity, suggesting reflex activation associated with metabolic functions. The sensory afferent projections from WAT contain markers for sensory innervation, such as calcitonin gene-related peptide (CGRP) and substance P (SP; 13, 160, 161). Both surgical and chemical denervation using high doses of capsaicin or resiniferatoxin (a potent capsaicin analog) selectively decreased CGRP and SP immunostaining, but not the efferent marker tyrosine hydroxylase, in both inguinal and epididymal WAT (160–162). Although it has been previously shown that CGRP is a potent vasodilator that decreases blood pressure when released from the nerve endings of sensory neurons in the proximity of blood vessels (38, 90, 148), its role in lowering blood pressure when released from adipose tissue is not well understood.
In addition to leptin, several experimental studies have demonstrated that AAR can be activated by adenosine, bradykinin, protons, or low doses of capsaicin in WAT (162). Specifically, bilateral capsaicin injections in inguinal WAT and retroperitoneal WAT significantly increase renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP) within 20 min in rats. Conversely, inguinal WAT surgical denervation prevented both MAP and RSNA from capsaicin-induced activation (162, 184). Capsaicin acts via the vanilloid receptor TRPV1, a ligand-gated, nonselective cation channel receptive to the activation of nociceptive afferent neurons: unmyelinated (C fibers) and thinly myelinated axons (Aδ fibers) (113, 174). TRPV1 can be also activated by noxious heat, bradykinin, and adenosine (49, 50). In adipose tissue, small diameter, unmyelinated (C fibers) sensory nerves express TRVP1 that mediate sensory-driven negative feedback control of adiposity (9). Furthermore, TRPV1 are involved in the “sensory-effector” mechanism by releasing CGRP and SP (176). Motter and Ahern (125) demonstrated that TRPV1-null mice fed an 11% fat diet gained significantly less mass and adiposity and displayed increased thermogenic capacity when compared with wild-type animals. Thus, TRPV1 participates in promoting fat accumulation and weight gain, possibly via direct adipose afferent nerve stimulation (125).
Sensory afferent neurons from WAT project to the dorsal root ganglia (DRG) (53) and from the DRG to the hypothalamus and brainstem (for reviews, see 7, 8, 10, 185). Postganglionic sensory afferents from the fat have been extensively studied using anterograde and retrograde neuronal tracers in Siberian hamsters (150, 166). Injections of the transneuronal viral tracer H129 strain of the herpes simplex virus-1 in combination with the transneuronal retrograde tracer pseudorabies virus (PRV) in inguinal and perigonadal WAT in male hamsters showed that afferent sensory neurons project to the PVN and RVLM (9, 150, 166). These projections have a key role in autonomic and cardiovascular regulation by modulating sympathetic outflow to organs critical to metabolism and blood pressure regulation (36, 48, 142, 158).
To further investigate the AAR in normotensive rats, Shi et al. (162) lesioned the PVN with kainic acid. These lesions abolished MAP or RSNA increases in response to capsaicin injections in inguinal WAT, confirming a predominant role of the PVN as an integration center of the sensory afferent projections from WAT and thus controlling sympathetic outflow in the AAR response. Similarly, leptin injections in WAT increase the number of c-Fos-positive cells, a marker of neuronal activation, in the PVN (127). Shi et al. (163) measured activity of PVN neurons in response to capsaicin infusion in inguinal WAT and retroperitoneal WAT and were able to evoke excitatory discharges in 30% of the spontaneously active neurons in the PVN. Insulin injections into the PVN induced a greater increase in baseline sympathetic nerve activity and a further increase of RSNA and MAP after AAR stimulation in insulin-resistant rats but not in normal rats (45). Although these studies established a pivotal role for PVN regulating sympathetic outflow and blood pressure, the highly heterogeneous composition of this nucleus demands a careful study of the specific subset and localization of neurons involved in the AAR.
To determine the mechanisms that modulate the AAR in the PVN, Li et al. (104) evaluated the contribution of melanocortin receptors (MC3/4Rs) in RSNA and MAP changes after AAR stimulation with capsaicin. Microinjections of an MC3/4R agonist [melanotan II (MTII)] into the PVN enhanced RSNA and MAP, and similarly, an MC3/4R antagonist (SHU9119) or MC4R antagonist (HS024) attenuated these responses. Moreover, SHU9119 was able to abolish the increase in cAMP in the PVN in response to capsaicin infusion in inguinal WAT and in response to MTII microinjections. They also showed that AAR stimulation increases superoxide anion production and NADPH oxidase activity in the PVN (47). In these experiments, microinjections of superoxide dismutase-polyethylene glycol (PEG-SOD), tempol, and apocynin, an NADPH oxidase inhibitor, in the PVN were able to decrease RSNA and MAP in response to AAR stimulation with capsaicin in inguinal adipose tissue. These results suggest that AAR activation generates superoxide anions in the PVN that could be responsible for increased sympathetic activation and, consequently, blood pressure. Furthermore, they demonstrate that the activation of γ-aminobutyric acid (GABA) receptors GABAA and GABAB in the PVN inhibits the AAR, whereas the selective blockade of these receptors exacerbates this reflex (43). It is important to note that all of these experiments were conducted under anesthesia and the AAR was chemically stimulated. It will be extremely useful to test these mechanisms in animal models of genetic obesity where the baseline AAR could be exacerbated.
The excess adiposity is one of the major causes of sympathetic activation and increased blood pressure (48, 100, 145, 168). In the last decade, only a few studies have investigated the importance of AAR dysregulation during obesity-induced hypertension (184, 185). Xiong et al. (184) reported that a high-fat diet for 12 wk increased the AAR in response to capsaicin. Bilateral injections of capsaicin into inguinal WAT further enhanced MAP and RSNA response in obese hypertensive rats compared with normotensive rats. Plasma renin, angiotensin II, and norepinephrine levels were elevated in obese hypertensive rats after capsaicin stimulation, suggesting that the AAR activation could also increase the renin angiotensin aldosterone system (RAAS), thereby contributing to the elevated blood pressure in obese animals. Additionally, the neuronal activation of the PVN increased in all groups after capsaicin injection, with a greater increase in PVN activity in obese hypertensive animals. However, these studies do not clarify whether this increased neuronal activation is due to exacerbated afferent nerve activity or to an increased number of sensory afferent neurons projecting to the PVN. Finally, chemical inguinal WAT and retroperitoneal WAT denervation with resiniferatoxin, an ultrapotent capsaicin analog that induces selective ablation of sensory neurons (171), as well as lidocaine injections in the PVN reduced sympathetic nerve activity and blood pressure in obese rats. This decrease was greater in obese hypertensive animals compared with obese normotensive rats. Ionotropic glutamate receptors in the PVN of rats are likely responsible for sympathetic outflow in response to capsaicin-induced AAR activation (31). Therefore, microinjections of N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists in the PVN before AAR stimulation with capsaicin attenuated increased renal sympathetic nerve activity and blood pressure in response to the AAR stimulation (31).
In a follow-up study (44), the same group showed that acute injections of tumor necrosis factor α(TNFα) in the PVN increased NADPH oxidase activity and reactive oxygen species (ROS) levels, exacerbating the capsaicin-induced AAR in obese hypertensive rats. PEG-SOD and apocynin were able to decrease ROS production, NADPH oxidase activity, RSNA, and MAP after TNFα-induced enhancement of AAR. Moreover, chronic treatment with pentoxifylline, a cytokine blocker, decreased NADPH oxidase and ROS in the PVN and the elevated RSNA and blood pressure in obese hypertensive rats. These results highlight the role of inflammatory cytokines in the exacerbated AAR during obesity-induced hypertension.
In a recent report, Cao et al. (20) showed that high-fat and high-salt diet (HFHS) impairs glucose uptake in adipose tissue and skeletal muscle and leads to insulin resistance. HFHS increased angiotensinogen expression and angiotensin II levels in adipose tissue, skeletal muscle, and the subfornical organ and PVN. Additionally, HFHS elevated efferent sympathetic nerve activity to adipose tissue and muscle. Total denervation of epididymal adipose tissue or afferent denervation with resiniferatoxin were able to downregulate RAAS components in the brain, decrease adipose inflammation and efferent sympathetic nerve activity, and improve glucose uptake in adipose tissue. Similar results were observed after tempol treatment showing that HFHS elevated efferent nerve activity increased the local production of ROS. The authors concluded that HFHS enhanced afferent signals from adipose tissue, promoting RAS and ROS activation, increased efferent nerve activity, and impaired glucose uptake leading to insulin resistance.
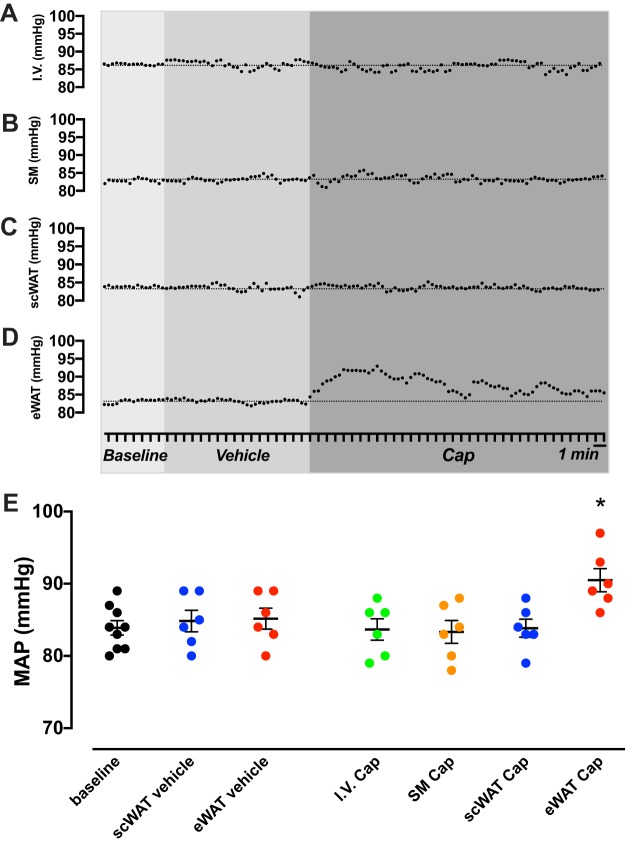
To date, all the AAR studies have been conducted in rats and Siberian hamsters. To further elucidate the mechanistic role of this reflex in different models of hypertension, we measured blood pressure changes in response to the AAR stimulation in male mice. In these experiments, we tested the MAP response to the stimulation of several tissues with capsaicin solution (5 ng capsaicin, 20 μL ethanol, 10 μL Tween 80/mL saline) or vehicle (20 μL ethanol, 10 μL Tween 80/mL saline). The AAR was induced by the injection of 1.5 pmol capsaicin (8 μL of capsaicin solution over a period of 2 min in 4 different sites, bilaterally). Capsaicin did not change MAP from baseline when infused in the blood stream via the jugular vein (Fig. 1A), intramuscular infusion into the adjacent skeletal muscle (Fig. 1B), or subcutaneous WAT (Fig. 1C). However, capsaicin induced a significant MAP increase from baseline in epidydimal WAT stimulation (Fig. 1D). Subcutaneous or epidydimal WAT stimulation with vehicle did not influence baseline MAP in either fat depot. Figure 1E shows the MAP 5-min average in response to vehicle or capsaicin solution. Thus, these data are the first to show that adipose tissue-blood pressure axis response is present in mice, which allows for the design of studies to test the contribution of the AAR in different genetic and/or experimental models of obesity-induced hypertension.
Fig. 1.
Mean arterial pressure (MAP) in response to vehicle or capsaicin (Cap) infusion in male mice in intravascular space (I.V., n = 5) (A), skeletal muscle (SM; n = 5) (B), subcutaneous white adipose tissue (scWAT; n = 5) (C), epidydimal white adipose tissue (eWAT; n = 7) (D), and scatter plot of MAP 5-min average after vehicle of Cap infusion (E). MAP was measured under anesthesia in 5- to 6-mo-old C57BL/6J male mice. The adipose afferent reflex (AAR) was induced as described previously by Shi et al. (162). The AAR was induced by the injections of Cap (8 μL of Cap 0.5 μg% over a period of 2 min in 4 different sites, bilaterally). Data are represented as mean ± SE and analyzed using SigmaPlot v.11 (Systat Software, CA). One-way analysis of variance (ANOVA) with repeated measures, followed by Tukey’s post hoc test, was used to analyze the effects of vehicle or Cap infusion. *P < 0.05 vs. baseline and vehicle infusion.
Taken together, there is compelling evidence indicating that AAR is exacerbated during obesity-induced hypertension, contributing to increased sympathetic outflow to organs involved in blood pressure control, such as the kidneys (Fig. 2). More studies are needed to determine the exact extent of the AAR contribution to hypertension, its connection with vasoactive peptides production during obesity, and its interplay with other afferent reflexes during obesity-induced hypertension. Moreover, it is imperative to investigate whether adipose afferent denervation could be a therapeutic approach to treat obese hypertensive patients. Notably, all these studies were conducted only in male rodents; therefore, it will be extremely valuable to study potential sex differences in the AAR activation and response and whether gonadal steroids are able to modulate this reflex. Finally, understanding how different subsets of PVN neurones are sensing the increased AAR, as well as the role of other brain areas such as the RVLM and NTS, will be critical for the development of therapeutic approaches within the brain.
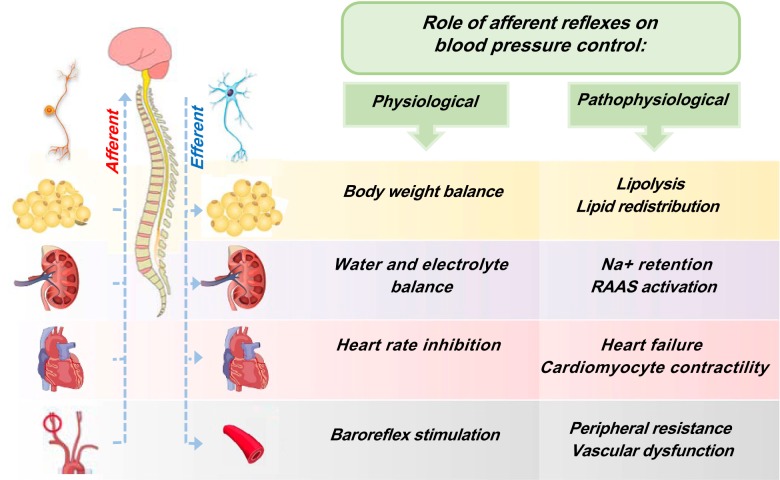
Fig. 2.
Integrated schematic diagram showing the white adipose tissue, kidney, heart, and vasculature afferent reflexes. In physiological conditions (left), these afferent reflexes work to maintain the normal physiological functions. In pathophysiological conditions (right), the overactivation of sensory signals promotes obesity, hypertension, heart failure, and vascular constriction via increase of the sympathetic outflow. In the context of metabolic disease, adipose afferent reflex (AAR) overstimulation reduces lipolysis and increases the efferent outflow to other organs involved in blood pressure control. This dysfunctional activation of AAR favors the development of obesity and hypertension. RAAS, renin-angiotensin-aldosterone system.
OTHER AFFERENT REFLEXES INFLUENCING BLOOD PRESSURE REGULATION
As mentioned above, in addition to AAR, several afferent reflexes involved in blood pressure regulation have been extensively studied. We briefly review some of these afferent reflexes, including the renorenal reflex, the cardiac afferent reflex, and the arterial baroreflex.
In the kidney, electrophysiological studies have shown the existence of afferent sensory nerve activity generated from chemoreceptor and mechanoreceptor stimulation in the renal pelvis. Renal chemoreceptors are activated by changes in the intrarenal chemical (ion) composition, i.e., during renal ischemia and backflow of urine into the renal pelvis (99, 122, 146). Other agents such as potassium chloride, bradykinin, and capsaicin infusions have been reported to activate sensory neurons (122, 187). On the other hand, renal mechanoreceptors are stimulated by increases in renal arterial and venous pressure, stretching of the renal pelvis, and changes in pressure in the renal pelvis and ureters (121, 123, 124, 167). These sensory signals are discussed in relation to other visceral afferent nerves and their role in renal nociception elsewhere (122, 124). The stimulation of renal afferent sensory neurons is implicated in renorenal reflexes that “enable total renal function to be self-regulated and balanced between the two kidneys” or a “self-regulated renorenal reflex loop” (41, 42, 82, 95). Numerous studies in rats have demonstrated that during hypertension (96), congestive heart failure (97), and diabetes (25, 98), impaired responsiveness of the afferent renal mechanosensory nerves inhibits renorenal reflexes. Similarly, studies by Chen et al. (24–26) have shown that inhibition of the renorenal reflex significantly blunts natriuresis following acute saline volume expansion. Renal chemo- and mechanoreceptor afferent stimulation projects to neuronal populations within the PVN, NTS, and RVLM that regulate efferent sympathetic outflow to the kidneys (28, 153, 188). Additionally, the circumventricular organs that respond to changes in plasma osmolality and circulating angiotensin II also project to the PVN, contributing to water and electrolyte balance and blood pressure regulation (77, 91, 92, 119). Although the afferent nerves in the kidney may participate in the pathogenesis of hypertension, its contribution depends on the experimental model of hypertension. In DOCA-salt-induced hypertension (56, 80) and spontaneously hypertensive rats (81), total renal denervation delays and attenuates the development of hypertension, whereas selective ablation of the renal afferent nerves does not have a significant effect on blood pressure. Furthermore, Banek et al. (4–6) have shown that elevated afferent renal nerve activity mediates the increase in blood pressure in response to DOCA salt, suggesting an afferent neural component in the development and maintenance of hypertension. Renal afferent nerves are also critical in the development of one-kidney, one-clip and two-kidney, one-clip Goldblatt hypertension in rats; in dogs with chronic aortic coarctation hypertension (135); and in models of hypertension associated with chronic renal failure (16, 17). Both renal artery stenosis (49) and acute reductions in renal blood flow (126) activate afferent renal nerve activity, which results in systemic neurogenic hypertension. It has also been proposed that the renal afferent reflex may play a critical role in chronic renal hypertension when the baroreflex is impaired and activation of the RAAS is reduced (49).
The cardiac afferent reflex (CSAR) is a chemosensitive, sympathoexcitatory, cardiac vagal reflex that is also implicated in blood pressure regulation. In physiological conditions, CSAR works in conjunction with the baroreflex to regulate cardiac sympathetic tone (111, 115, 140, 191). Induction of the CSAR occurs via stimulation of chemoreceptors by endogenous humoral factors, including bradykinin and adenosine, or exogenous factors such as capsaicin that increase sympathetic outflow and blood pressure (58, 114, 180, 181). Afferent fibers from the subepicardium and myocardium project to the NTS in the brainstem; from there, they project to the PVN and RVLM (34, 143, 177). In normal rats, cardiac sympathetic afferents reduced arterial baroreflex sensitivity mediated by AT1 receptors (23, 59). In contrast, the enhanced effect of the CSAR initiated by the chemoreflex occurs via left ventricular electrical and capsaicin-induced stimulation of cardiac afferent reflexes. This response is abolished after bilateral microinjection of losartan into the NTS, confirming that the NTS is a key intermediary between these two reflexes (57). Spontaneously hypertensive rats displaying elevated CSAR and microRNA interference of angiotensin AT1a receptors in the PVN attenuated the hypertension in this strain (50). Similarly, the exacerbated CSAR during chronic heart failure is normalized after AT1 receptor blockade (190, 192) or resiniferatoxin afferent denervation (24, 78, 177, 178). Taken together, these studies demonstrate that angiotensin II plays a key role in the regulation of the CSAR.
Finally, the arterial baroreflex of the carotid sinus participates in acute blood pressure regulation by controlling heart rate, contractility, and systemic vascular resistance (39, 73, 75, 108). The baroreflex is a rapid sympathoinhibitory feedback loop that decreases heart rate and, consequently, blood pressure. Baroreceptors in the carotid sinus and aortic arch sense changes in blood pressure and transmit this information to the NTS to regulate sympathetic tone and blood pressure. During increases in blood pressure, the NTS activates, via glutamatergic projections, the caudal ventrolateral medulla that in turn inhibits, via GABAergic projections, the RVLM and decreases sympathetic tone and blood pressure (35, 155–157, 169). Several studies have shown a baroreflex dysfunction in clinical and experimental hypertension. In this regard, Dahl salt-sensitive and spontaneously hypertensive rats display impaired baroreflex sensitivity, similar to what has been reported in hypertensive humans (35, 61, 72, 164). In recent years, carotid baroreflex activation has been proposed as a novel therapy for resistant hypertension; however, the results are not consistent due to the lack of adequate randomized control trials and the presence of other negative conditions, such as obesity and chronic kidney disease (62, 76, 134).
CONCLUSIONS
Overall, the exacerbated activation of the afferent reflexes during experimental obesity impair blood pressure control. Given that obesity increases the risk of drug-resistant hypertension, advances in our understanding of how the AAR contributes to enhance sympathetic activation may help to develop therapeutic approaches for successfully managing blood pressure in obese patients. A comprehensive understanding of how multiple afferent reflexes contribute to uncontrolled blood pressure may provide significant insights into improving antihypertensive therapeutic approaches.
GRANTS
This study was supported by funds from the National Institutes of Health National Heart, Lung, and Blood Institute, Grant Nos. R01 HL135158 (to A. S. Loria) and R01 HL135158S1 (to J. R. Leachman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D. and A.S.L. conceived and designed research; C.D. and A.S.L. performed experiments; C.D., J.R.L., and A.S.L. analyzed data; C.D., J.L.O., and A.S.L. interpreted results of experiments; C.D. and A.S.L. prepared figures; C.D., J.R.L., J.L.O., and A.S.L. drafted manuscript; C.D., J.R.L., J.L.O., and A.S.L. edited and revised manuscript; C.D., J.R.L., J.L.O., and A.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mark Schwarcz for his editions and Thomas Dolan for his outstanding assistance on the illustration.
REFERENCES
- 1.Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res 124: 1061–1070, 2019. doi: 10.1161/CIRCRESAHA.118.312156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002. doi: 10.1161/01.CIR.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 3.Andresen MC. Cardiovascular integration in the nucleus of the solitary tract. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston, MA: Springer, 2004. [Google Scholar]
- 4.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banek CT, Gauthier MM, Baumann DC, Van Helden D, Asirvatham-Jeyaraj N, Panoskaltsis-Mortari A, Fink GD, Osborn JW. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 314: R883–R891, 2018. doi: 10.1152/ajpregu.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol 275: R1399–R1411, 1998. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Kay Song C, Shi H, Bowers RR, Foster MT. Brain-adipose tissue cross talk. Proc Nutr Soc 64: 53–64, 2005. doi: 10.1079/PNS2004409. [DOI] [PubMed] [Google Scholar]
- 9.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol Behav 91: 343–351, 2007. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34, Suppl 1: S36–S42, 2010. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 264: H1674–H1685, 1993. doi: 10.1152/ajpheart.1993.264.5.H1674. [DOI] [PubMed] [Google Scholar]
- 13.Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 14.Brierley SM, Jones RCW III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Calhoun DA, Schiffrin EL, Flack JM. Resistant hypertension: an update. Am J Hypertens 32: 1–3, 2019. doi: 10.1093/ajh/hpy156. [DOI] [PubMed] [Google Scholar]
- 16.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25: 878–882, 1995. doi: 10.1161/01.HYP.25.4.878. [DOI] [PubMed] [Google Scholar]
- 17.Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis 26: 861–865, 1995. doi: 10.1016/0272-6386(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 18.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int 21: 371–378, 1982. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 19.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cao W, Shi M, Wu L, Li J, Yang Z, Liu Y, Wilcox CS, Hou FF. Adipocytes initiate an adipose-cerebral-peripheral sympathetic reflex to induce insulin resistance during high-fat feeding. Clin Sci (Lond) 133: 1883–1899, 2019. doi: 10.1042/CS20190412. [DOI] [PubMed] [Google Scholar]
- 21.Castiglioni P, Parati G, Lazzeroni D, Bini M, Faini A, Brambilla L, Brambilla V, Coruzzi P. Hemodynamic and autonomic response to different salt intakes in normotensive individuals. J Am Heart Assoc 5: e003736, 2016. doi: 10.1161/JAHA.116.003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro Torres Y, Katholi RE. Renal denervation for treating resistant hypertension: current evidence and future insights from a global perspective. Int J Hypertens 2013: 513214, 2013. doi: 10.1155/2013/513214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao X-Y, Zhu G-Q. Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol 96: 94–103, 2011. doi: 10.1113/expphysiol.2010.054353. [DOI] [PubMed] [Google Scholar]
- 24.Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 213: 778–794, 2015. doi: 10.1111/apha.12447. [DOI] [PubMed] [Google Scholar]
- 25.Chien CT, Chien HF, Cheng YJ, Chen CF, Hsu SM. Renal afferent signaling diuretic response is impaired in streptozotocin-induced diabetic rats. Kidney Int 57: 203–214, 2000. doi: 10.1046/j.1523-1755.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- 26.Chien CT, Fu TC, Wu MS, Chen CF. Attenuated response of renal mechanoreceptors to volume expansion in chronically hypoxic rats. Am J Physiol Renal Physiol 273: F712–F717, 1997. doi: 10.1152/ajprenal.1997.273.5.F712. [DOI] [PubMed] [Google Scholar]
- 27.Coleridge HM, Coleridge JC. Cardiovascular afferents involved in regulation of peripheral vessels. Annu Rev Physiol 42: 413–427, 1980. doi: 10.1146/annurev.ph.42.030180.002213. [DOI] [PubMed] [Google Scholar]
- 28.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 29.Correll JW. Adipose tissue: ability to respond to nerve stimulation in vitro. Science 140: 387–388, 1963. doi: 10.1126/science.140.3565.387. [DOI] [PubMed] [Google Scholar]
- 30.Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Novarini A, Castiglioni P, Di Rienzo M. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension 46: 1321–1326, 2005. doi: 10.1161/01.HYP.0000189183.50301.5c. [DOI] [PubMed] [Google Scholar]
- 31.Cui BP, Li P, Sun HJ, Ding L, Zhou YB, Wang JJ, Kang YM, Zhu GQ. Ionotropic glutamate receptors in paraventricular nucleus mediate adipose afferent reflex and regulate sympathetic outflow in rats. Acta Physiol (Oxf) 209: 45–54, 2013. doi: 10.1111/apha.12125. [DOI] [PubMed] [Google Scholar]
- 32.Cutsforth-Gregory JK, Benarroch EE. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology 88: 1187–1196, 2017. doi: 10.1212/WNL.0000000000003751. [DOI] [PubMed] [Google Scholar]
- 34.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 29: 261–268, 2002. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 35.Dampney RA. Resetting of the baroreflex control of sympathetic vasomotor activity during natural behaviors: description and conceptual model of central mechanisms. Front Neurosci 11: 461, 2017. doi: 10.3389/fnins.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Kloet AD, Herman JP. Fat-brain connections: Adipocyte glucocorticoid control of stress and metabolism. Front Neuroendocrinol 48: 50–57, 2018. doi: 10.1016/j.yfrne.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Dempsey JA, Smith CA. Update on chemoreception: influence on cardiorespiratory regulation and pathophysiology. Clin Chest Med 40: 269–283, 2019. doi: 10.1016/j.ccm.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng PY, Li YJ. Calcitonin gene-related peptide and hypertension. Peptides 26: 1676–1685, 2005. doi: 10.1016/j.peptides.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Di Rienzo M, Parati G, Radaelli A, Castiglioni P. Baroreflex contribution to blood pressure and heart rate oscillations: time scales, time-variant characteristics and nonlinearities. Philos Trans A Math Phys Eng Sci 367: 1301–1318, 2009. doi: 10.1098/rsta.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension 43: 147–150, 2004. doi: 10.1161/01.HYP.0000113047.47711.fa. [DOI] [PubMed] [Google Scholar]
- 41.DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000. doi: 10.1152/ajpregu.2000.279.5.R1517. [DOI] [PubMed] [Google Scholar]
- 42.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 43.Ding L, Gao R, Xiong XQ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ. GABA in paraventricular nucleus regulates adipose afferent reflex in rats. PLoS One 10: e0136983, 2015. doi: 10.1371/journal.pone.0136983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding L, Kang Y, Dai HB, Wang FZ, Zhou H, Gao Q, Xiong XQ, Zhang F, Song TR, Yuan Y, Liu M, Zhu GQ, Zhou YB. Adipose afferent reflex is enhanced by TNFα in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J Transl Med 17: 256, 2019. doi: 10.1186/s12967-019-2006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding L, Tong N, Feng XM, Chen D, Wang HS, Wang Y, Li Y, Zhu GQ, Zhou YB. Adipose afferent reflex response to insulin is mediated by melanocortin 4 type receptors in the paraventricular nucleus in insulin resistance rats. Acta Physiol (Oxf) 214: 450–466, 2015. doi: 10.1111/apha.12502. [DOI] [PubMed] [Google Scholar]
- 46.Ding L, Zhang F, Zhao MX, Ren XS, Chen Q, Li YH, Kang YM, Zhu GQ. Reduced lipolysis response to adipose afferent reflex involved in impaired activation of adrenoceptor-cAMP-PKA-hormone sensitive lipase pathway in obesity. Sci Rep 6: 34374, 2016. doi: 10.1038/srep34374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding L, Zhang LL, Gao R, Chen D, Wang JJ, Gao XY, Kang YM, Zhu GQ. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS One 8: e83771, 2013. doi: 10.1371/journal.pone.0083771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, de Lara Rodriguez CE, Hall JE. Obesity-induced hypertension: brain signaling pathways. Curr Hypertens Rep 18: 58, 2016. doi: 10.1007/s11906-016-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faber JE, Brody MJ. Afferent renal nerve-dependent hypertension following acute renal artery stenosis in the conscious rat. Circ Res 57: 676–688, 1985. doi: 10.1161/01.RES.57.5.676. [DOI] [PubMed] [Google Scholar]
- 50.Fan ZD, Zhang L, Shi Z, Gan XB, Gao XY, Zhu GQ. Artificial microRNA interference targeting AT1a receptors in paraventricular nucleus attenuates hypertension in rats. Gene Ther 19: 810–817, 2012. doi: 10.1038/gt.2011.145. [DOI] [PubMed] [Google Scholar]
- 51.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci 36: 461–470, 2015. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus—a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol Regul Integr Comp Physiol 253: R942–R944, 1987. [DOI] [PubMed] [Google Scholar]
- 54.Frame AA, Carmichael CY, Kuwabara JT, Cunningham JT, Wainford RD. Role of the afferent renal nerves in sodium homeostasis and blood pressure regulation in rats. Exp Physiol 104: 1306–1323, 2019. doi: 10.1113/EP087700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franco V, Calhoun DA, Oparil S. Pathophysiology of hypertension (1st ed.). In: Hypertension: A Companion to Braunwold’s Heart Disease, edited by Black HR, Elliot WJ. Philadelphia, PA: Saunders, 2007, p. 25–46. [Google Scholar]
- 56.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 25: 1148–1155, 2014. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J Appl Physiol (1985) 102: 37–43, 2007. doi: 10.1152/japplphysiol.00681.2006. [DOI] [PubMed] [Google Scholar]
- 58.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005. [Erratum in Hypertension 46: e25, 2005.] doi: 10.1161/01.HYP.0000168056.66981.c2. [DOI] [PubMed] [Google Scholar]
- 59.Gao L, Zhu Z, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. Am J Physiol Heart Circ Physiol 286: H1706–H1711, 2004. doi: 10.1152/ajpheart.01097.2003. [DOI] [PubMed] [Google Scholar]
- 60.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab 5: 626–634, 2016. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon FJ, Mark AL. Mechanism of impaired baroreflex control in prehypertensive Dahl salt-sensitive rats. Circ Res 54: 378–387, 1984. doi: 10.1161/01.RES.54.4.378. [DOI] [PubMed] [Google Scholar]
- 62.Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des 10: 3579–3589, 2004. doi: 10.2174/1381612043382756. [DOI] [PubMed] [Google Scholar]
- 63.Grassi G, Pisano A, Bolignano D, Seravalle G, D’Arrigo G, Quarti-Trevano F, Mallamaci F, Zoccali C, Mancia G. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta-analyses. Hypertension 72: 483–491, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11038. [DOI] [PubMed] [Google Scholar]
- 64.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10: 457–466, 2016. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 66.Guyenet PG, Stornetta RL, Holloway BB, Souza GMPR, Abbott SBG. Rostral ventrolateral medulla and hypertension. Hypertension 72: 559–566, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116: 991–1006, 2015. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol 15: 367–385, 2019. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamill RW, Shapiro RE, Vizzard MA. Peripheral autonomic nervous system (3rd ed.). In: Primer on the Autonomic Nervous System, edited by Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JF. San Diego: Academic Press, 2012, chapt 4, p. 17–26. [Google Scholar]
- 71.Hay M, Bishop VS. Interactions of area postrema and solitary tract in the nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 260: H1466–H1473, 1991. doi: 10.1152/ajpheart.1991.260.5.H1466. [DOI] [PubMed] [Google Scholar]
- 72.Head GA. Baroreflexes and cardiovascular regulation in hypertension. J Cardiovasc Pharmacol 26, Suppl 2: S7–S16, 1995. doi: 10.1097/00005344-199512020-00002. [DOI] [PubMed] [Google Scholar]
- 73.Heesch CM. Reflexes that control cardiovascular function. Am J Physiol 277: S234–S243, 1999. doi: 10.1152/advances.1999.277.6.S234. [DOI] [PubMed] [Google Scholar]
- 74.Heuser RR, Schlaich MP, Sievert H (Editors). Renal Denervation. London: Springer-Verlag, 2015, p. XIV, 201. [Google Scholar]
- 75.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55: 619–626, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 76.Hildebrandt DA, Irwin ED, Lohmeier TE. Prolonged baroreflex activation abolishes salt-induced hypertension after reductions in kidney mass. Hypertension 68: 1400–1406, 2016. doi: 10.1161/HYPERTENSIONAHA.116.08293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huber G, Schuster F, Raasch W. Brain renin-angiotensin system in the pathophysiology of cardiovascular diseases. Pharmacol Res 125: 72–90, 2017. doi: 10.1016/j.phrs.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 78.Irie T, Yamakawa K, Hamon D, Nakamura K, Shivkumar K, Vaseghi M. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am J Physiol Heart Circ Physiol 312: H392–H405, 2017. doi: 10.1152/ajpheart.00644.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res 49: 13, 2016. doi: 10.1186/s40659-016-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 81.Janssen BJ, van Essen H, Vervoort-Peters LH, Thijssen HH, Derkx FH, Struyker-Boudier HA, Smits JF. Effects of complete renal denervation and selective afferent renal denervation on the hypertension induced by intrarenal norepinephrine infusion in conscious rats. J Hypertens 7: 447–455, 1989. doi: 10.1097/00004872-198906000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 83.Jose PA, Villar VA. Chronic kidney disease and hypertension. In: Management of Hypertension: Current Practice and the Application of Landmark Trials, edited by Papademetriou V, Andreadis EA, Geladari C. Cham, Switzerland: Springer, 2019, p. 135–144. [Google Scholar]
- 84.Julius S, Valentini M, Palatini P. Overweight and hypertension: a 2-way street? Hypertension 35: 807–813, 2000. doi: 10.1161/01.HYP.35.3.807. [DOI] [PubMed] [Google Scholar]
- 85.Kara T, Narkiewicz K, Somers VK. Chemoreflexes—physiology and clinical implications. Acta Physiol Scand 177: 377–384, 2003. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 86.Kaufman JA, Jones TB. Viscerosensory pathways (5th ed.). In: Fundamental Neuroscience for Basic and Clinical Applications, edited by Haines DE, Mihailoff GA. Philadelphia, PA: Elsevier, 2018, p. 278–285. [Google Scholar]
- 87.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.RES.50.1.133. [DOI] [PubMed] [Google Scholar]
- 88.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci 18: 1682, 2017. doi: 10.3390/ijms18081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 90.Kee Z, Kodji X, Brain SD. The role of calcitonin gene related peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front Physiol 9: 1249, 2018. doi: 10.3389/fphys.2018.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinsman BJ, Browning KN, Stocker SD. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J Physiol 595: 6187–6201, 2017. doi: 10.1113/JP274537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension 69: 163–170, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koeners MP, Lewis KE, Ford AP, Paton JF. Hypertension: a problem of organ blood flow supply-demand mismatch. Future Cardiol 12: 339–349, 2016. doi: 10.2217/fca.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308: R79–R95, 2015. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kopp UC, Cicha MZ. Impaired substance P release from renal sensory nerves in SHR involves a pertussis toxin-sensitive mechanism. Am J Physiol Regul Integr Comp Physiol 286: R326–R333, 2004. doi: 10.1152/ajpregu.00493.2003. [DOI] [PubMed] [Google Scholar]
- 97.Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol 284: R116–R124, 2003. doi: 10.1152/ajpregu.00336.2002. [DOI] [PubMed] [Google Scholar]
- 98.Kopp UC, Cicha MZ, Yorek MA. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: role of angiotensin. Am J Physiol Regul Integr Comp Physiol 294: R858–R866, 2008. doi: 10.1152/ajpregu.00830.2007. [DOI] [PubMed] [Google Scholar]
- 99.Kopp UC, Matsushita K, Sigmund RD, Smith LA, Watanabe S, Stokes JB. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am J Physiol Regul Integr Comp Physiol 275: R1780–R1792, 1998. doi: 10.1152/ajpregu.1998.275.6.R1780. [DOI] [PubMed] [Google Scholar]
- 100.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res 33: 386–393, 2010. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 101.Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, Kamayachi T, Itoh H, Saruta T. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res 35: 132–141, 2012. doi: 10.1038/hr.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50: 862–868, 2007. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 103.Lee YH, Jung YS, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med 46: e78, 2014. doi: 10.1038/emm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li P, Sun HJ, Zhang LL, Ding L, Han Y, Zhu GQ, Zhou YB. Melanocortin 4 receptors in the paraventricular nucleus modulate the adipose afferent reflex in rat. PLoS One 8: e80295, 2013. doi: 10.1371/journal.pone.0080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291: H2847–H2856, 2006. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- 106.Lithell H, Pollare T, Berne C, Saltin B. The metabolic and circulatory response to beta-blockade in hypertensive men is correlated to muscle capillary density. Blood Press 1: 20–26, 1992. doi: 10.3109/08037059209065120. [DOI] [PubMed] [Google Scholar]
- 107.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Sensory transduction (4th ed.). In: Molecular Cell Biology. New York: W. H. Freeman, 2000. [Google Scholar]
- 108.Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda) 30: 148–158, 2015. doi: 10.1152/physiol.00035.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lombard JH, Cowley AW. Neural control of blood vessels (3rd ed.). In: Primer on the Autonomic Nervous System, edited by Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JF. San Diego, CA: Academic, 2012, p. 187–191. [Google Scholar]
- 110.Longhurst JC, Mitchell JH. Reflex control of the circulation by afferents from skeletal muscle. Int Rev Physiol 18: 125–148, 1979. [PubMed] [Google Scholar]
- 111.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci 940: 179–196, 2001. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 112.Machado BH, Mauad H, Chianca Júnior DA, Haibara AS, Colombari E. Autonomic processing of the cardiovascular reflexes in the nucleus tractus solitarii. Braz J Med Biol Res 30: 533–543, 1997. doi: 10.1590/S0100-879X1997000400015. [DOI] [PubMed] [Google Scholar]
- 113.Maggi CA. The pharmacological modulation of neurotransmitter release. In: Capsaicin in the Study of Pain, edited by Wood JN. New York: Academic, 1993, p. 161–189. [Google Scholar]
- 114.Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension 39: 63–68, 2002. doi: 10.1161/hy0102.099200. [DOI] [PubMed] [Google Scholar]
- 115.Malliani A, Montano N. Sympathetic overactivity in ischaemic heart disease. Clin Sci (Lond) 106: 567–568, 2004. doi: 10.1042/CS20040068. [DOI] [PubMed] [Google Scholar]
- 116.Malliani A, Pagani M. Afferent sympathetic nerve fibres with aortic endings. J Physiol 263: 157–169, 1976. doi: 10.1113/jphysiol.1976.sp011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malliani A, Pagani M, Pizzinelli P, Furlan R, Guzzetti S. Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst 7: 295–301, 1983. doi: 10.1016/0165-1838(83)90082-6. [DOI] [PubMed] [Google Scholar]
- 118.McCormack DG, Mak JC, Coupe MO, Barnes PJ. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol (1985) 67: 1265–1270, 1989. doi: 10.1152/jappl.1989.67.3.1265. [DOI] [PubMed] [Google Scholar]
- 119.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 19: 1–6, 2004. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- 120.Mitchell JH, Schmidt RF. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. Compr Physiol. doi: 10.1002/cphy.cp020317. [DOI] [Google Scholar]
- 121.Mizutani A, Okajima K, Murakami K, Mizutani S, Kudo K, Uchino T, Kadoi Y, Noguchi T. Activation of sensory neurons reduces ischemia/reperfusion-induced acute renal injury in rats. Anesthesiology 110: 361–369, 2009. doi: 10.1097/ALN.0b013e3181942f3c. [DOI] [PubMed] [Google Scholar]
- 122.Moss NG. Electrophysiological characteristics of renal sensory receptors and afferent renal nerves. Miner Electrolyte Metab 15: 59–65, 1989. [PubMed] [Google Scholar]
- 123.Moss NG. Electrophysiological characteristics of sensory mechanisms in the kidney. Clin Exp Hypertens A 9, Suppl 1: 1–13, 1987. doi: 10.3109/10641968709160160. [DOI] [PubMed] [Google Scholar]
- 124.Moss NG. Renal function and renal afferent and efferent nerve activity. Am J Physiol 243: F425–F433, 1982. doi: 10.1152/ajprenal.1982.243.5.F425. [DOI] [PubMed] [Google Scholar]
- 125.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett 582: 2257–2262, 2008. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 304: R675–R682, 2013. doi: 10.1152/ajpregu.00599.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab 304: E1338–E1347, 2013. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 369: 448–457, 2013. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 129.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 63: 250–259, 2014. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129a.National Center for Health Statistics (NCHS) Center for Disease Control and Prevention Hypertension. https://www.cdc.gov/nchs/fastats/hypertension.htm. 2017.
- 130.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nguyen NL, Xue B, Bartness TJ. Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters. Physiol Behav 190: 28–33, 2018. doi: 10.1016/j.physbeh.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst 73: 19–25, 1998. doi: 10.1016/S0165-1838(98)00109-X. [DOI] [PubMed] [Google Scholar]
- 133.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett 262: 125–128, 1999. doi: 10.1016/S0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 134.Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ Res 116: 1074–1095, 2015. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- 135.Oparil S, Sripairojthikoon W, Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharmacol 65: 1548–1558, 1987. doi: 10.1139/y87-244. [DOI] [PubMed] [Google Scholar]
- 136.Osborn JW, Banek CT. Catheter-based renal nerve ablation as a novel hypertension therapy: lost, and then found, in translation. Hypertension 71: 383–388, 2018. doi: 10.1161/HYPERTENSIONAHA.117.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pagani M, Pizzinelli P, Bergamaschi M, Malliani A. A positive feedback sympathetic pressor reflex during stretch of the thoracic aorta in conscious dogs. Circ Res 50: 125–132, 1982. doi: 10.1161/01.RES.50.1.125. [DOI] [PubMed] [Google Scholar]
- 138.Pagani M, Pizzinelli P, Furlan R, Guzzetti S, Rimoldi O, Sandrone G, Malliani A. Analysis of the pressor sympathetic reflex produced by intracoronary injections of bradykinin in conscious dogs. Circ Res 56: 175–183, 1985. doi: 10.1161/01.RES.56.2.175. [DOI] [PubMed] [Google Scholar]
- 139.Pan HL, Deal DD, Xu Z, Chen SR. Differential responses of regional sympathetic activity and blood flow to visceral afferent stimulation. Am J Physiol Regul Integr Comp Physiol 280: R1781–R1789, 2001. doi: 10.1152/ajpregu.2001.280.6.R1781. [DOI] [PubMed] [Google Scholar]
- 140.Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JF. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med 22: 1151–1159, 2016. doi: 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 143.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol 99: 332–339, 2014. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 144.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. doi: 10.1016/S0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 145.Rahmouni K. Obesity-associated hypertension: recent progress in deciphering the pathogenesis. Hypertension 64: 215–221, 2014. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Recordati G, Moss NG, Genovesi S, Rogenes P. Renal chemoreceptors. J Auton Nerv Syst 3: 237–251, 1981. doi: 10.1016/0165-1838(81)90066-7. [DOI] [PubMed] [Google Scholar]
- 147.Rosell S. Release of free fatty acids from subcutaneous adipose tissue in dogs following sympathetic nerve stimulation. Acta Physiol Scand 67: 343–351, 1966. doi: 10.1111/j.1748-1716.1966.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 148.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142, 2014. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol 306: R886–R900, 2014. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ryu V, Watts AG, Xue B, Bartness TJ. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 312: R324–R337, 2017. doi: 10.1152/ajpregu.00456.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salman IM. Major autonomic neuroregulatory pathways underlying short- and long-term control of cardiovascular function. Curr Hypertens Rep 18: 18, 2016. doi: 10.1007/s11906-016-0625-x. [DOI] [PubMed] [Google Scholar]
- 152.Sangari SK, Khatri K, Sengupta P. Sensory innervation of the suprarenal gland in the albino rat: a fluorescent tract tracer study. Clin Anat 11: 29–32, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 153.Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the sympathetic nervous system and its modulation in renal hypertension. Front Med (Lausanne) 5: 82, 2018. doi: 10.3389/fmed.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev 53: 916–947, 1973. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- 155.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 156.Seagard JL, Dean C, Hopp FA. Neurochemical transmission of baroreceptor input in the nucleus tractus solitarius. Brain Res Bull 51: 111–118, 2000. doi: 10.1016/S0361-9230(99)00235-X. [DOI] [PubMed] [Google Scholar]
- 157.Seagard JL, Dean C, Hopp FA. Properties of NTS neurons receiving input from barosensitive receptors. Ann NY Acad Sci 940: 142–156, 2001. doi: 10.1111/j.1749-6632.2001.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 158.Seravalle G, Grassi G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High Blood Press Cardiovasc Prev 23: 175–179, 2016. doi: 10.1007/s40292-016-0137-4. [DOI] [PubMed] [Google Scholar]
- 159.Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 10: 17–28, 2018. [PMC free article] [PubMed] [Google Scholar]
- 160.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol 289: R514–R520, 2005. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 161.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol 288: R1028–R1037, 2005. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 162.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol (1985) 112: 1008–1014, 2012. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 163.Shi Z, Wang YF, Wang GH, Wu YL, Ma CL. Paraventricular nucleus is involved in the central pathway of adipose afferent reflex in rats. Can J Physiol Pharmacol 94: 534–541, 2016. doi: 10.1139/cjpp-2015-0097. [DOI] [PubMed] [Google Scholar]
- 164.Sleight P. Role of the baroreceptor reflexes in circulatory control, with particular reference to hypertension. Hypertension 18, Suppl 5: III31–III34, 1991. doi: 10.1161/01.HYP.18.5_Suppl.III31. [DOI] [PubMed] [Google Scholar]
- 165.Solomonica A, Lavi S, Choudhury T, Bagur R. Renal denervation therapy beyond resistant hypertension. J Thorac Dis 10: 707–713, 2018. doi: 10.21037/jtd.2018.01.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev 71: 659–682, 1991. doi: 10.1152/physrev.1991.71.3.659. [DOI] [PubMed] [Google Scholar]
- 168.Stocker SD, Kinsman BJ, Sved AF. Recent advances in neurogenic hypertension: dietary salt, obesity, and inflammation. Hypertension 70: 474–478, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sved AF, Ito S, Madden CJ. Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull 51: 129–133, 2000. doi: 10.1016/S0361-9230(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 170.Sved AF, Ito S, Madden CJ, Stocker SD, Yajima Y. Excitatory inputs to the RVLM in the context of the baroreceptor reflex. Ann NY Acad Sci 940: 247–258, 2001. doi: 10.1111/j.1749-6632.2001.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 171.Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 255: 923–928, 1990. [PubMed] [Google Scholar]
- 172.Tjen-A-Looi S, Bonham A, Longhurst J. Interactions between sympathetic and vagal cardiac afferents in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol 272: H2843–H2851, 1997. doi: 10.1152/ajpheart.1997.272.6.H2843. [DOI] [PubMed] [Google Scholar]
- 173.Ulrich YM, Hargreaves KM, Harding-Rose CA, Bowles WR, Engeland WC. Characterization of iCGRP release from adrenal capsule primary afferent neurons. Endocr Res 24: 777–778, 1998. doi: 10.3109/07435809809032687. [DOI] [PubMed] [Google Scholar]
- 174.Vaishnava P, Wang DH. Capsaicin sensitive-sensory nerves and blood pressure regulation. Curr Med Chem Cardiovasc Hematol Agents 1: 177–188, 2003. doi: 10.2174/1568016033477540. [DOI] [PubMed] [Google Scholar]
- 175.Vaitkevicius R, Saburkina I, Rysevaite K, Vaitkeviciene I, Pauziene N, Zaliunas R, Schauerte P, Jalife J, Pauza DH. Nerve supply of the human pulmonary veins: an anatomical study. Heart Rhythm 6: 221–228, 2009. doi: 10.1016/j.hrthm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 176.Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin 26: 286–294, 2005. doi: 10.1111/j.1745-7254.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 177.Wang HJ, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. J Physiol 595: 2519–2534, 2017. doi: 10.1113/JP273764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang QP, Guan JL, Pan W, Kastin AJ, Shioda S. A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem Res 33: 2035–2043, 2008. doi: 10.1007/s11064-008-9676-y. [DOI] [PubMed] [Google Scholar]
- 180.Wang W, Ma R. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail Rev 5: 57–71, 2000. doi: 10.1023/A:1009898107964. [DOI] [PubMed] [Google Scholar]
- 181.Wang WZ, Gao L, Pan YX, Zucker IH, Wang W. Differential effects of cardiac sympathetic afferent stimulation on neurons in the nucleus tractus solitarius. Neurosci Lett 409: 146–150, 2006. doi: 10.1016/j.neulet.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.World Health Organization Hypertension 2019. https://www.who.int/news-room/fact-sheets/detail/hypertension.
- 183.Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, Hörnkvist PE, Pennert K, Tuomilehto J, Wedel H. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens 5: 561–572, 1987. doi: 10.1097/00004872-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 184.Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension 60: 1280–1286, 2012. doi: 10.1161/HYPERTENSIONAHA.112.198002. [DOI] [PubMed] [Google Scholar]
- 185.Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf) 210: 468–478, 2014. doi: 10.1111/apha.12182. [DOI] [PubMed] [Google Scholar]
- 186.Yaxley JP, Thambar SV. Resistant hypertension: an approach to management in primary care. J Family Med Prim Care 4: 193–199, 2015. doi: 10.4103/2249-4863.154630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ye C, Qiu Y, Zhang F, Chen AD, Zhou H, Wang JJ, Chen Q, Li YH, Kang YM, Zhu GQ. Chemical stimulation of renal tissue induces sympathetic activation and a pressor response via the paraventricular nucleus in rats. Neurosci Bull 36: 143–153, 2020. doi: 10.1007/s12264-019-00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci 204: 57–64, 2017. doi: 10.1016/j.autneu.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP. Impaired hypothalamic regulation of sympathetic outflow in primary hypertension. Neurosci Bull 35: 124–132, 2019. doi: 10.1007/s12264-018-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu G-Q, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 287: H1828–H1835, 2004. doi: 10.1152/ajpheart.01245.2003. [DOI] [PubMed] [Google Scholar]
- 191.Zhu GQ, Xu Y, Zhou LM, Li YH, Fan LM, Wang W, Gao XY, Chen Q. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Exp Physiol 94: 785–794, 2009. doi: 10.1113/expphysiol.2008.046565. [DOI] [PubMed] [Google Scholar]
- 192.Zhu GQ, Zucker IH, Wang W. Central AT1 receptors are involved in the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Basic Res Cardiol 97: 320–326, 2002. doi: 10.1007/s00395-002-0353-z. [DOI] [PubMed] [Google Scholar]