Abstract
Nonobstructive urinary retention (NOUR) is a medical condition without an effective drug treatment, but few basic science studies have focused on this condition. In α-chloralose-anesthetized cats, the bladder was cannulated via the dome and infused with saline to induce voiding that could occur without urethral outlet obstruction. A nerve cuff electrode was implanted for tibial nerve stimulation (TNS). The threshold (T) intensity for TNS to induce toe twitch was determined initially. Repeated (6 times) application of 30-min TNS (5 Hz, 0.2 ms, 4–6T) significantly (P < 0.05) increased bladder capacity to 180% of control and reduced the duration of the micturition contraction to 30% of control with a small decrease in contraction amplitude (80% of control), which resulted in urinary retention with a low-voiding efficiency of 30% and a large amount of residual volume equivalent to 130% of control bladder capacity. This NOUR condition persisted for >2 h after the end of repeated TNS. However, lower frequency TNS (1 Hz, 0.2 ms, 4T) applied during voiding partially reversed the NOUR by significantly (P < 0.05) increasing voiding efficiency to 60% and reducing residual volume to 70% of control bladder capacity without changing bladder capacity. These results revealed that tibial nerve afferent input can activate either an excitatory or an inhibitory central nervous system mechanism depending on afferent firing frequencies (1 vs. 5 Hz). This study established the first NOUR animal model that will be useful for basic science research aimed at developing new treatments for NOUR.
Keywords: bladder, cat, stimulation, tibial, urinary retention
INTRODUCTION
Urinary retention can be caused by urethral outlet obstruction, detrusor underactivity, or both. Although the obstructive urinary retention can be resolved by surgical or pharmacological treatments to remove/reduce the urethral outlet obstruction (6, 10), the nonobstructive urinary retention (NOUR) that is mainly caused by detrusor underactivity poses a significant therapeutic challenge for clinicians (7). Currently there are very few basic science studies of NOUR when compared with the extensive research in the literature about overactive bladder (OAB). Furthermore, an effective treatment for NOUR does not exist (5, 11) leaving most patients dependent on daily intermittent self-catheterization or an indwelling catheter to empty the bladder (7, 16). Thus more basic research focusing on this disorder is needed.
The lack of research effort is partially due to the lack of an appropriate animal model of NOUR. NOUR can be myogenic, neurogenic, or idiopathic. Clinically, NOUR without an identifiable cause, i.e., no muscle/nerve disease or damage, is classified as idiopathic, which includes many NOUR patients with unidentified functional changes in the central nervous system (CNS). Although the myogenic/neurogenic animal models can be produced by bladder ischemia or pelvic nerve injury (1, 2), it has been difficult to produce an animal model of NOUR caused exclusively by CNS dysfunction. Thus the development of animal models mimicking the clinical conditions of idiopathic NOUR is needed.
Our previous studies in cats have shown that long-lasting (>2 h) poststimulation inhibition of reflex bladder activity can be induced by prolonged 5-Hz stimulation of somatic afferent axons in the tibial nerve (9, 13). Clinically, tibial nerve stimulation (TNS) applied for 30 min once a week can inhibit bladder overactivity for several weeks (12). These results indicate that TNS can activate a long-lasting inhibitory mechanism in the CNS. Therefore, in this study using anesthetized cats we explored the possibility of producing a cat model of NOUR without muscle/nerve injury by activating the long-lasting inhibitory CNS mechanism induced by TNS.
METHODS
The Animal Care and Use Committee of the University of Pittsburgh approved all protocols involving the use of animals in this study.
Surgical procedures.
A total of nine adult cats (4 males and 5 females, weight: 2.7–4.2 kg) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg iv and supplemented as needed) during data collection. The left cephalic vein was catheterized for administration of anesthetics and fluid. A tracheotomy was performed, and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen level were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue.
Through an abdominal incision, the ureters were isolated, tied, cut, and drained externally. A double lumen catheter was inserted into the bladder via a small puncture on the bladder dome and secured by a ligature, so that voiding could occur without urethral outlet obstruction. One lumen of the catheter was connected to a pump to infuse the bladder with saline, and the other lumen was attached to a pressure transducer to record intravesical pressure. A funnel was used to collect the voided fluid in a beaker that was attached to a force transducer to record the volume (14). The tibial nerve on the right side was dissected via a skin incision at the ankle. A tripolar cuff electrode (NC223pt; MicroProbe, Gaithersburg, MD) was implanted on the nerve, and then, the electrode was connected to an electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via a constant voltage stimulus isolator (SIU5; Grass Medical Instruments). After the surgery, the skin and muscle layers were closed by sutures.
Experimental protocol.
At the beginning of each experiment, uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width) were used to determine the intensity threshold (T) for TNS to induce toe twitches. Based on our recent study (9) showing that prolonged TNS of 4–6T intensity can significantly increase bladder capacity and weaken micturition contractions, strong intensity TNS (4–6T) was used in this study in an attempt to induce urinary retention.
Initially, multiple (3–5) cystometrograms (CMGs) were performed by slowly infusing (1–2 ml/min) the bladder with saline to determine the control values for bladder capacity, voided volume, residual volume, voiding efficiency, and amplitude and duration of micturition contractions. During each CMG bladder infusion was always stopped when voiding occurred or a few drops of fluid was released from the urethra. At the end of each voiding CMG, the bladder was emptied via the bladder catheter and the residual volume was recorded. Once the control urodynamic parameters were determined, TNS (5 Hz, 0.2 ms) of a 30-min duration was applied three times at 4T intensity followed by another three times at 6T intensity (9, 13) while the bladder was empty. After each 30-min TNS, a CMG without stimulation was performed to determine the changes in urodynamic parameters. After the last 30-min TNS was applied, 5 CMGs without stimulation were performed over a 2-h period to determine if the changes in urodynamic parameters were reversed with time. After the fifth CMG, two additional CMGs were performed. In the first CMG, TNS (1 Hz, 0.2 ms, 4T) was applied at the beginning of voiding and during the entire voiding episode. In the second CMG, TNS (1 Hz, 0.2 ms, 4T) was applied during the entire CMG. TNS of 1-Hz frequency was used because our previous study (15) showed that it could facilitate the micturition reflex. Finally, a control CMG was performed to determine any poststimulation effect.
Data analysis.
Repeated measurements of urodynamic parameters during the two to three control CMGs before any treatment were averaged in the same animal. Then, the urodynamic parameters (bladder capacity, voiding efficiency, maximal amplitude and duration of micturition contraction, and area under contraction curve) were measured in every CMG and normalized to their averaged control values in each cat. The residual volume was also measured in every CMG but normalized to the averaged control bladder capacity in each cat to compare the residual volume to the initial control bladder capacity. The data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures ANOVA followed by Dunnett’s multiple comparison.
RESULTS
Prolonged urinary retention induced by repeated 5-Hz TNS.
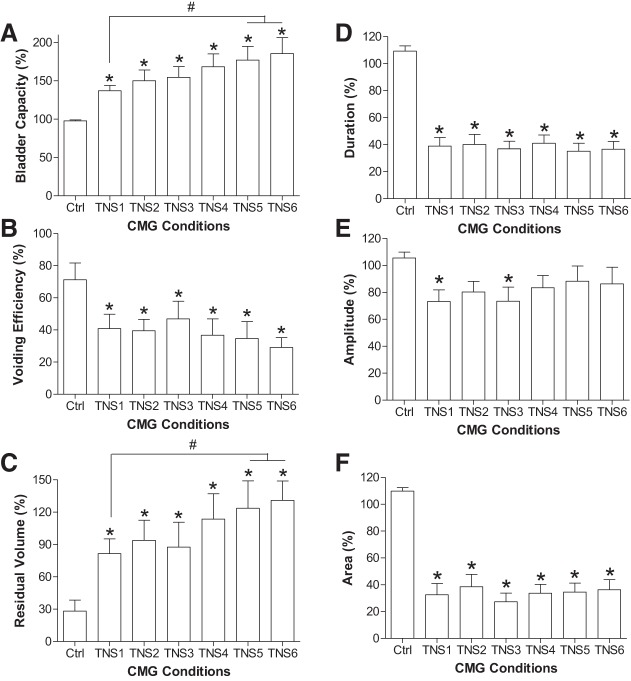
Repeated (6 times) application of 30-min TNS (5 Hz, 0.2 ms, 4–6T) gradually increased the bladder capacity and reduced the voided volume, which resulted in a large amount of residual volume in the bladder, i.e., urinary retention (Fig. 1). The control bladder capacity measured before TNS was 6.9 ± 1.2 ml (n = 9 cats) with an average voiding efficiency of 71.3 ± 10.4%. The first 30-min TNS (TNS1 in Fig. 2) significantly (P < 0.05) increased bladder capacity to 137.0 ± 6.8% of control (Fig. 2A), reduced voiding efficiency to 40.9 ± 8.9% (Fig. 2B), and resulted in a large residual volume equivalent to 81.6 ± 13.6% of the control bladder capacity (Fig. 2C). An additional five periods of 30-min TNS (TNS2–TNS6 in Fig. 2) elicited a further significant (P < 0.05) increase in bladder capacity to 185.4 ± 20.9% (Fig. 2A) and residual volume to 130.9 ± 18.0% (Fig. 2C) but did not elicit a further significant reduction in voiding efficiency (29.1 ± 6.1%, Fig. 2B). The duration of the micturition contraction was significantly (P < 0.05) reduced, but the contraction amplitude was only reduced slightly (Fig. 2, D–F).
Fig. 1.
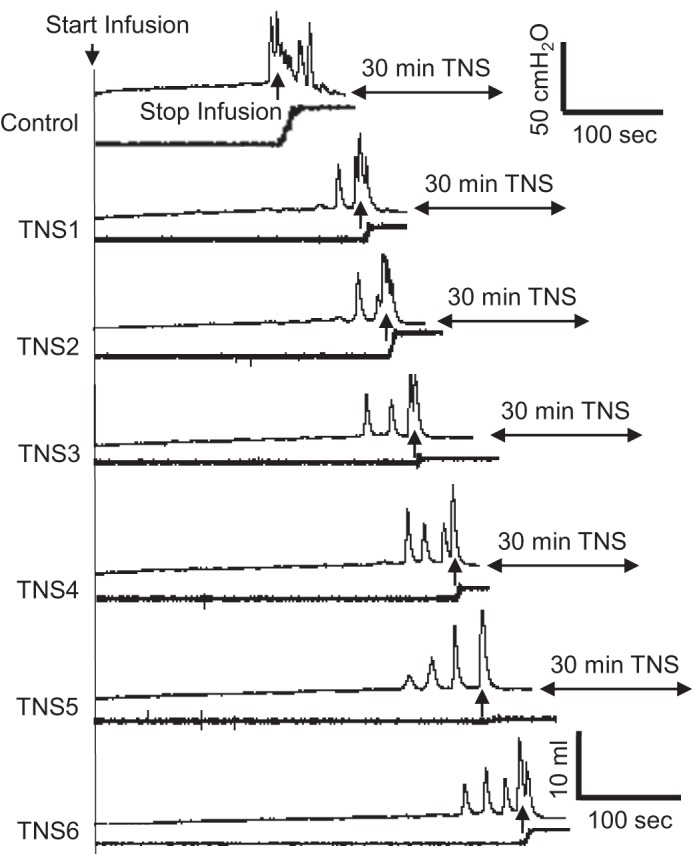
Urinary retention induced by intense (4–6T) repeated (6 times) tibial nerve stimulation (TNS). T, threshold intensity to induce toe twitch. In each voiding cystometrogram (CMG) from the control to TNS6, the bladder pressure is shown at top and the voided volume is shown at the bottom. The 5-Hz TNS of 30-min duration was applied at the end of each CMG. TNS was applied between but not during CMGs. TNS: 5 Hz, 0.2 ms, 4–6T = 2.4–3.6 V.
Fig. 2.
Effects of intense (4–6T) repeated (6 times) 30-min tibial nerve stimulation (TNS) on cystometrogram (CMG) parameters. A: bladder capacity. B: voiding efficiency. C: residual volume. D: duration of bladder contraction. E: amplitude of bladder contraction. F: area under contraction curve. Parameters in A, B, D, and E are normalized to the control values, and in C the residual volumes are normalized to the control bladder capacity. TNS: 5 Hz, 0.2 ms, 4–6T = 2.0–8.4 V. *Significantly (P < 0.05) different from the control (one-way ANOVA). #Significant difference (one-way ANOVA); n = 9 cats.
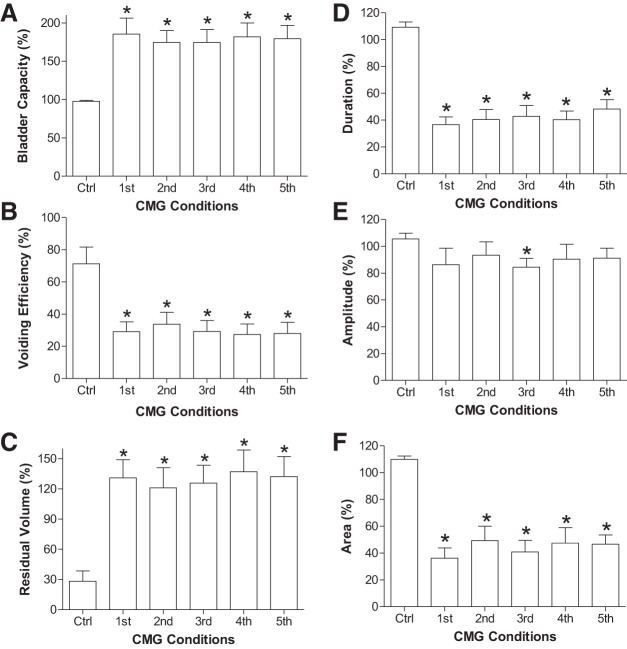
After repeated application (6 times) of 30-min TNS, the stimulation-induced urinary retention persisted during the five CMGs performed during the post-TNS 2-h period (Fig. 3). The bladder capacity was maintained at ~180% of control (Fig. 4A) with a low-voiding efficiency ~30% (Fig. 4B) and a high residual volume ~130% of control bladder capacity (Fig. 4C). During this prolonged urinary retention, the duration of the micturition contraction was significantly (P < 0.05) reduced, but the contraction amplitude was only reduced slightly (Fig. 4, D–F). Our previous studies in cats (3, 13) showed that in control experiments without TNS, cystometric parameters did not change during repeated CMGs performed at 20- to 30-min intervals over a 2-h period. This indicates that the CMG changes occurring during repeated TNS in the present experiments were due to the stimulation rather than a spontaneous change in bladder activity.
Fig. 3.
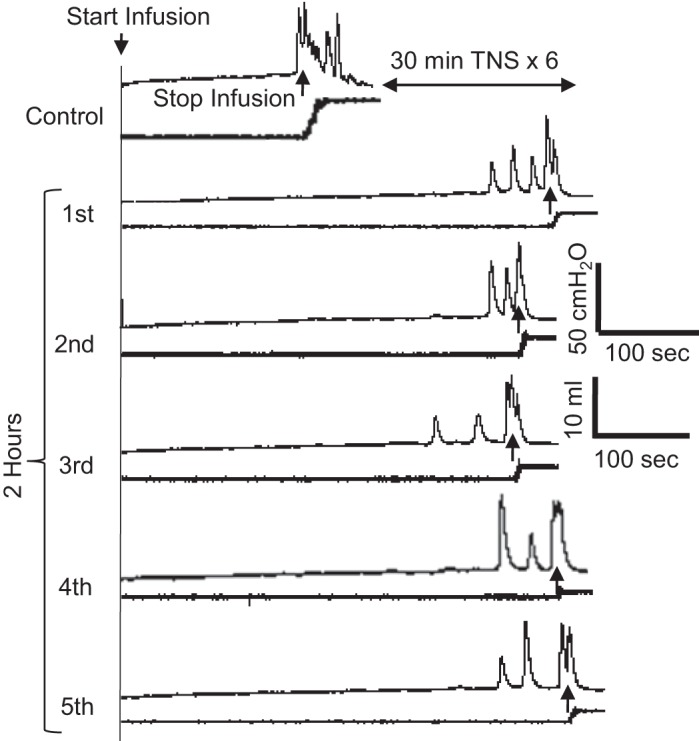
Urinary retention persists for a long period (>2 h) after ending the repeated 5-Hz tibial nerve stimulation (TNS). TNS of 30-min duration was repeated 6 times before the 1st to 5th cystometrograms (CMGs) were performed sequentially during a 2-h period. TNS was not applied during CMGs. The CMGs were obtained from the same cat as shown in Fig. 1. The control and 1st CMGs in this figure are same as the control and TNS6 CMGs in Fig. 1.
Fig. 4.
CMG parameters measured from the 1st to 5th cystometrograms (CMGs) performed during the prolonged (2 h) urinary retention induced by posttibial nerve stimulation (post-TNS) inhibition. A: bladder capacity. B: voiding efficiency. C: residual volume. D: duration of bladder contraction. E: amplitude of bladder contraction. F: area under contraction curve. Parameters in A, B, D, and E are normalized to the control values, and in C residual volumes are normalized to the control bladder capacity. TNS: 5 Hz, 0.2 ms, 4–6T = 2.0–8.4 V. *Significantly (P < 0.05) different from the control (one-way ANOVA); n = 9 cats.
Improving urinary retention by 1-Hz TNS.
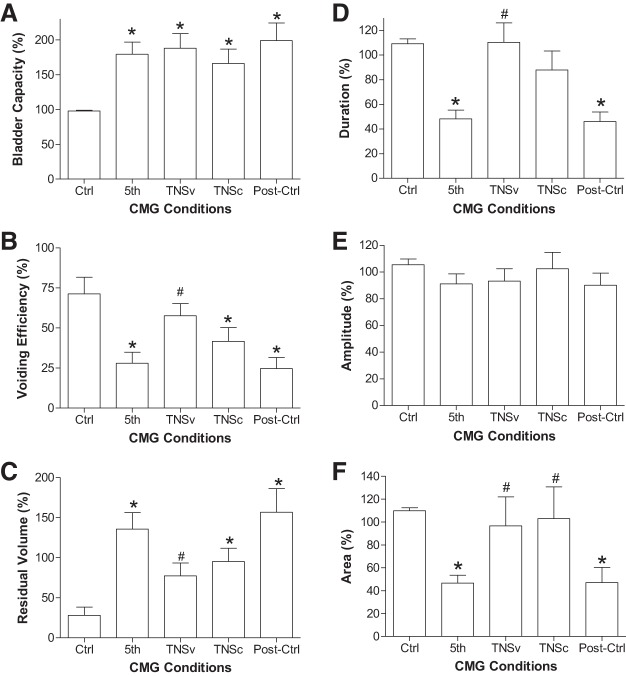
The urinary retention induced by 5-Hz TNS could be partially reversed by applying 1-Hz, 4T TNS during voiding (Figs. 5 and 6). This stimulation significantly (P < 0.05) increased the voided volume (TNSv in Fig. 5) and voiding efficiency (Fig. 6B), thereby significantly (P < 0.05) reducing residual volume (Fig. 6C). This improvement occurred with a significant (P < 0.05) increase in the duration of the micturition contraction but no increase in the contraction amplitude (Fig. 6, D–F). However, continuous application of TNS (1 Hz, 0.2 ms, 4T) during the entire CMG failed to reduce bladder capacity (TNSc in Figs. 5 and Fig. 6A) and only slightly improved the voiding efficiency or residual volume (not significant, Fig. 6, B and C).
Fig. 5.
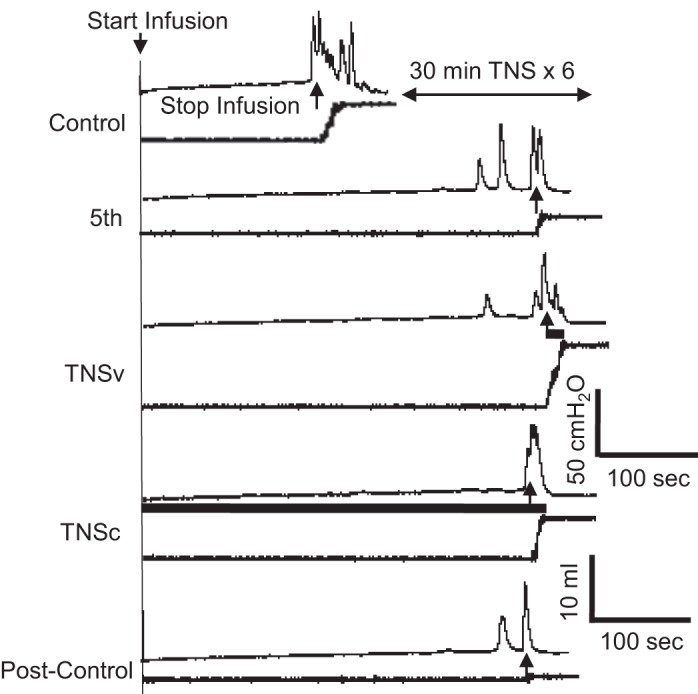
Tibial nerve stimulation (TNS) of 1-Hz frequency improved voiding efficiency but did not change bladder capacity. The 1-Hz TNS was applied only during voiding (TNSv) or continuously (TNSc) during the entire cystometrogram (CMG) The black bar under the CMG trace indicates the 1-Hz TNS duration. TNS: 1 Hz, 0.2 ms, 4T = 2.4 V. The CMGs were obtained from the same cat as shown in Fig. 3. The control and 5th CMGs in this figure are same as the control and 5th CMGs in Fig. 3.
Fig. 6.
Tibial nerve stimulation (TNS) of 1-Hz frequency improved voiding efficiency and reduced residual volume by increasing the duration of bladder contractions. A: bladder capacity. B: voiding efficiency. C: residual volume. D: duration of bladder contraction. E: amplitude of bladder contraction. F: area under contraction curve. Parameters in A, B, D, and E are normalized to the control values except in C the residual volumes are normalized to the control bladder capacity. TNS: 1 Hz, 0.2 ms, 4T = 2.0–5.6 V. *Significantly (P < 0.05) different from the control (one-way ANOVA). #Significantly (P < 0.05) different from the 5th CMG (one-way ANOVA); n = 9 cats.
DISCUSSION
This study in anesthetized cats shows that a 5-Hz stimulation of somatic afferent axons in the tibial nerve produces nonobstructive urinary retention (NOUR) (Figs. 1 and 2) and that retention can persist for at least 2 h beyond the time of stimulation (Figs. 3 and 4). In contrast to other animal models of NOUR produced by bladder ischemia and/or pelvic nerve injury (1, 2), the TNS-induced NOUR occurs without directly damaging the bladder or its neural control. These results raise the possibility that abnormal somatic afferent activity could be a pathophysiological cause of NOUR. A link between somatic afferent activity and NOUR is also supported by clinical observations in patients with Fowler’s syndrome where NOUR is believed to be caused by abnormal tonic pudendal afferent activity originating in the external urethral sphincter (4). However, continuous somatic afferent activity may not be required in all cases to produce voiding dysfunction because prolonged TNS can elicit a long-lasting reduction in voiding efficiency following the stimulation. The modulatory effects of TNS on micturition are clearly frequency dependent because the persistent urinary retention elicited by 5-Hz TNS can be partially reversed by 1-Hz TNS (Figs. 5 and 6). These findings indicate that tibial afferents have access to both inhibitory and excitatory mechanisms in the CNS that modulate the spinobulbospinal micturition reflex. Future studies of the CNS inhibitory mechanism underlying TNS-induced NOUR may provide insights into the pathophysiology of idiopathic NOUR.
In the current study, the prolonged 5-Hz TNS (6 × 30 min = 3 h) reduced the duration of the micturition contraction to ~30% of control (Fig. 2D) but only slightly reduced the contraction amplitude (Fig. 2E). However, our previous study (10) in cats showed that prolonged 5-Hz TNS (1.5–4 h) reduced contraction amplitude to ~30% of control without significantly changing the contraction duration. This difference is probably caused by the different experiment conditions. Our previous study was conducted with the urethral outlet closed by a suture, i.e., recording bladder activity under isovolumetric conditions, while the current study kept the urethral outlet open to allow voiding to occur. During isovolumetric CMGs, the micturition contraction can generate 50- to 100-cmH2O bladder pressure since the bladder contacts against the closed urethral outlet, which can further enhance the bladder afferent firing and produce a longer duration contraction. During voiding CMGs, the micturition contraction can only generate a maximal intravesical pressure of 50 cmH2O (Fig. 1) because the urethra opens. In addition, fluid flowing out of the bladder will certainly reduce the contraction duration. Therefore, the micturition contraction is smaller in amplitude and duration during voiding CMGs than during isovolumetric CMGs. These differences probably contribute to the different effects of prolonged 5-Hz TNS on bladder contraction amplitude and duration. However, in both current and previous studies the prolonged 5-Hz TNS increased bladder capacity to ~180% of control, indicating a consistent inhibition of the micturition reflex in both conditions.
Excitatory bladder responses induced by 1-Hz TNS are also different during isovolumetric and voiding CMGs. During isovolumetric CMGs the 1-Hz TNS reduced bladder capacity and increased both amplitude and duration of micturition contractions during the bladder underactivity induced by prolonged 5-Hz TNS (15). During voiding CMGs, the 1-Hz TNS improved voiding (Fig. 6, B and C) by increasing contraction duration (Fig. 6D) without changing contraction amplitude (Fig. 6E) or bladder capacity (Fig. 6A). The different effects on contraction amplitude could be due to the different amplitudes of bladder contractions in isovolumetric and voiding CMGs as discussed above. The different effects on bladder capacity are probably caused by the different criteria used to define the bladder capacity. In isovolumetric CMGs, the bladder capacity is defined by the minimal bladder volume to induce a large micturition contraction, while in voiding CMGs it is defined by the fluid flowing out of the urethral orifice. Although it is difficult to directly compare the control bladder capacities in different groups of animals due to the variability between individual animals, the control capacity in this study measured during voiding CMGs was 6.9 ± 1.2 ml (n = 9 cats) while it was 9.3 ± 1.6 ml (n = 7 cats) in our previous study of isovolumetric CMGs (9). Despite these differences, the excitatory effect of 1-Hz TNS on the micturition reflex was consistently present in both previous and current studies. Our previous studies (8, 18) also showed that stimulation of the peroneal or saphenous nerve at 1 Hz could excite the bladder or facilitate the micturition reflex. Therefore, stimulation of other somatic afferent nerves to improve urinary retention should be further investigated.
The prolonged 5-Hz TNS increased bladder capacity to 180% of control (Fig. 2A) and reduced the duration of the micturition contraction to 30% of control (Fig. 2D), while the contraction amplitude was only reduced to ~80% of control (Fig. 2E). These results indicate that the TNS inhibition occurs on the sensory limb of the spinobulbospinal micturition reflex pathway to suppress the initiation of the micturition reflex, while the descending limb from the PMC that controls the magnitude of the micturition contraction is not significantly inhibited. Furthermore, it seems unlikely that the TNS-induced reduction in voiding efficiency is due to a functional obstruction of the urethral outlet caused by a tonic CNS output to the external urethral sphincter because this should produce an increase instead of a decrease in the amplitude of the bladder contraction. Thus it seems reasonable to conclude that TNS affects voiding function at least in part by targeting bladder sensory pathways in the CNS. This is supported by our recent study (17) in cats that showed 5-Hz TNS of 30-min duration can produce poststimulation inhibition of bladder-related interneurons in the sacral spinal cord, indicating that these neurons may be the origin of the ascending sensory limb of the micturition reflex and the possible site of spinal inhibition that underlies the urinary retention induced by prolonged 5-Hz TNS.
It is also possible that TNS acts at supraspinal sites to modulate bladder function. For example, the TNS-induced increase in bladder capacity or the reduction in the micturition contraction duration could be produced by a direct inhibition at the level of the PMC, while targeting the descending limb of the micturition reflex is unlikely to contribute to TNS inhibition because it would reduce the amplitude of the micturition contraction rather than delaying the occurrence of the contraction. TNS at a lower frequency (1 Hz) reduced the urinary retention elicited by 5-Hz TNS by increasing contraction duration without changing contraction amplitude or bladder capacity. If the 1-Hz TNS affected the ascending spinal pathway, it would have changed the bladder capacity, whereas if it affected the descending spinal pathway, the contraction amplitude would have been changed. Therefore, the 1-Hz TNS probably prolonged the duration of PMC excitation rather than affecting the ascending or descending limbs of the micturition reflex, indicating that 1-Hz TNS might act at a supraspinal site to improve voiding.
The urinary retention in this study occurred without physical urethral outlet obstruction or damage of bladder muscle or nerves. Therefore, it is highly likely that 5-Hz TNS activated a long-lasting inhibitory mechanism in the CNS to produce the urinary retention. To activate the central mechanism, tibial afferents must have triggered the release of some types of inhibitory neurotransmitters in the CNS. It is known that opioid receptors are involved in acute TNS inhibition of bladder overactivity induced by acetic acid irritation in cats (19). However, during saline distention of the bladder post-TNS inhibition of the micturition reflex persisted in cats after pharmacological blockade of opioid receptors by intravenous administration of a large dose of naloxone (an opioid receptor antagonist) (9, 13), indicating a minor role of opioid receptors in the urinary retention induced by prolonged 5-Hz TNS. Whether other inhibitory neurotransmitters such as GABA, glycine, or 5HT are involved still needs to be investigated.
Perspectives and Significance
This study established an animal model of NOUR by prolonged TNS and revealed an inhibitory mechanism in the CNS that may contribute to the clinical disorder termed idiopathic NOUR. More detailed information about the neurochemical mechanisms underlying TNS-induced urinary retention in this model may facilitate the development of new treatments for NOUR.
GRANTS
This study is supported by the National Institutes of Diabetes, Digestive and Kidney Diseases Grants DK-121698 and DK-111382.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. conceived and designed research; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; S.L., J.B., K.T., T.Y., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Aizawa N, Igawa Y. Pathophysiology of the underactive bladder. Investig Clin Urol 58, Suppl 2: S82–S89, 2017. doi: 10.4111/icu.2017.58.S2.S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deruyver Y, Weyne E, Dewulf K, Rietjens R, Pinto S, Van Ranst N, Franken J, Vanneste M, Albersen M, Gevaert T, Vennekens R, De Ridder D, Voets T, Everaerts W. Intravesical activation of the cation channel TRPV4 improves bladder function in a rat model for detrusor underactivity. Eur Urol 74: 336–345, 2018. doi: 10.1016/j.eururo.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol 309: F242–F250, 2015. doi: 10.1152/ajprenal.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby RS, Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 297: 1436–1438, 1988. doi: 10.1136/bmj.297.6661.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juszczak K, Drewa T. Pharmacotherapy in detrusor underactivity: a new challenge for urologists and pharmacologists (from lab to clinic). Pharmacol Rep 68: 703–706, 2016. doi: 10.1016/j.pharep.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Kalejaiye O, Speakman MJ. Management of acute and chronic retention in men. Eur Urol 8: 523–529, 2009. doi: 10.1016/j.eursup.2009.02.002. [DOI] [Google Scholar]
- 7.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 5: 657–666, 2008. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Li X, Theisen K, Browning J, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Saphenous nerve stimulation normalizes bladder underactivity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 315: F247–F253, 2018. doi: 10.1152/ajprenal.00422.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Theisen K, Browning J, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Bladder underactivity after prolonged stimulation of somatic afferent axons in the tibial nerve in cats. Neurourol Urodyn 37: 2121–2127, 2018. doi: 10.1002/nau.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negro CL, Muir GH. Chronic urinary retention in men: how we define it, and how does it affect treatment outcome. BJU Int 110: 1590–1594, 2012. doi: 10.1111/j.1464-410X.2012.11101.x. [DOI] [PubMed] [Google Scholar]
- 11.Osman NI, Chapple CR. Are there pharmacotherapeutic options for underactive bladder? Eur Urol Focus 4: 6–7, 2018. doi: 10.1016/j.euf.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Peters KM, Carrico DJ, Wooldridge LS, Miller CJ, MacDiarmid SA. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 189: 2194–2201, 2013. doi: 10.1016/j.juro.2012.11.175. [DOI] [PubMed] [Google Scholar]
- 13.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011. doi: 10.1152/ajprenal.00526.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn 26: 879–886, 2007. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theisen K, Browning J, Li X, Li S, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Frequency dependent tibial neuromodulation of bladder underactivity and overactivity in cats. Neuromodulation 21: 700–706, 2018. doi: 10.1111/ner.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uren AD, Cotterill N, Harding C, Hillary C, Chapple C, Klaver M, Bongaerts D, Hakimi Z, Abrams P. Qualitative exploration of the patient experience of underactive bladder. Eur Urol 72: 402–407, 2017. doi: 10.1016/j.eururo.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Yecies T, Li S, Zhang Y, Cai H, Shen B, Wang J, Roppolo J, de Groat W, Tai C. Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats. Exp Neurol 308: 100–110, 2018. doi: 10.1016/j.expneurol.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, Uy J, Jiang X, Li X, Jones C, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. An excitatory reflex from the superficial peroneal nerve to the bladder in cats. Am J Physiol Renal Physiol 313: F1161–F1168, 2017. doi: 10.1152/ajprenal.00265.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Slater RC, Ferroni MC, Kadow BT, Lyon TD, Shen B, Xiao Z, Wang J, Kang A, Roppolo JR, de Groat WC, Tai C. Role of µ, κ, and δ opioid receptors in tibial inhibition of bladder overactivity in cats. J Pharmacol Exp Ther 355: 228–234, 2015. doi: 10.1124/jpet.115.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]