Abstract
Maternal high-fat diet (HFD) is associated with metabolic syndrome and cardiovascular diseases in adult offspring. Our previous study demonstrated that maternal HFD enhances pressor responses to ANG II or a proinflammatory cytokine (PIC), which is associated with increased expression of brain renin-angiotensin system (RAS) components and PICs in adult offspring. The present study further investigated whether inhibition of angiotensin-converting enzyme (ACE) or tumor necrosis factor-α (TNF-α) blocks sensitization of ANG II hypertension in offspring of HFD dams. All offspring were bred from dams with normal fat diet (NFD) or HFD starting two weeks before mating and maintained until weaning of the offspring. Then the weaned offspring were treated with an ACE inhibitor (captopril) or a TNF-α inhibitor (pentoxifylline) in the drinking water through the end of testing with a slow-pressor dose of ANG II. RT-PCR analyses of the lamina terminalis and paraventricular nucleus revealed upregulation of mRNA expression of several RAS components and PICs in male offspring of HFD dams when compared with age-matched offspring of NFD dams. The enhanced gene expression was attenuated by blockade of either RAS or PICs. Likewise, ANG II administration produced an augmented pressor response in offspring of HFD dams. This was abolished by either ACE or TNF-α inhibitor. Taken together, this study provides mechanistic evidence and a therapeutic strategy that systemic inhibition of the RAS and PICs can block maternal HFD-induced sensitization of ANG II hypertension, which is associated with attenuation of brain RAS and PIC expression in offspring.
Keywords: blood pressure, inflammation, maternal high-fat diet, renin-angiotensin system
INTRODUCTION
The prevalence of obesity in women of childbearing age and during pregnancy has steadily increased over the past two decades (11, 16). Accumulating evidence from epidemiologic studies and animal models indicate that maternal obesity is associated with a variety of adverse outcomes for offspring later in life, such as metabolic syndrome, behavioral disorders, and increased risk of cardiovascular disease (4, 25). Reynolds and colleagues found associations between human maternal obesity and increased risk of death from cardiovascular events during the midlife of offspring (24). Likewise, maternal high-fat diet (HFD) in animals has also been shown to elevate blood pressure (BP) and enhance the hypertensive response to systemic infusion of pressor agents such as ANG II in both male and female offspring (34, 38).
Maternal obesity is associated with increased circulating and hypothalamic proinflammatory cytokines (PICs), including tumor necrosis factor (TNF)-α, IL-1β, and IL-6, that produce adverse effects on energy balance, behavior, and BP in offspring (2, 14, 25). Dudele et al. (8) reported that exposing female mice to chronic low doses of LPS during pregnancy and lactation had similar programming effects as maternal consumption of HFD on offspring phenotype. The similarities between offspring exposed to maternal LPS or HFD strongly indicate that inflammation is a key mechanism underlying the observed programming effects. Maternal HFD also leads to activation of the renin-angiotensin system (RAS), increasing expression of several RAS components in the adiposity, liver, and brain (15, 27, 38). Zhang et al. demonstrated that maternal HFD resulted in upregulation of mRNA expression of RAS components, NADPH oxidase, and PICs in the lamina terminalis (LT) and downstream hypothalamic paraventricular nucleus (PVN) of the offspring, two critical sites that integrate humoral and metabolic signals regulating metabolism, sympathetic activity, and BP (17–19, 22, 38). This upregulated expression in the brain was associated with an enhanced pressor response to acute intracerebroventricular injections of either ANG II or TNF-α and with an augmented hypertensive response to chronic infusion of ANG II (38).
As maternal HFD and obesity are common and rapidly increasing, it is speculated that future generations will be at increased risk for metabolic syndrome, mental health disorders, and cardiovascular diseases including hypertension. Thus, it is critical to identify therapeutic strategies that effectively prevent or treat maternal HFD-induced malprogramming of BP. Studies including those from our laboratories have previously demonstrated the therapeutic actions of either an anti-inflammatory agent (pentoxifylline), angiotensin-converting enzyme (ACE) inhibitor (captopril), or ANG II receptor blocker (losartan) on adverse effects in animals induced by psychosocial stress (35) or in offspring programmed by maternal hypertension (34) or low-protein diet (30). Therefore, the present study investigated whether inhibition of ACE or TNF-α might block maternal HFD-induced sensitization of ANG II hypertension in male offspring. Furthermore, we examined the central mechanisms by which beneficial effects of systemic inhibition of RAS or inflammation were related to changes in the RAS and PICs in brain cardiovascular nuclei associated with the LT and PVN.
METHODS
Animals.
All animal procedures were reviewed and approved by the Hebei North University Institutional Animal Care and Use Committee conforming to National Institutes of Health guidelines.
Twenty female and twenty male rats (Sprague-Dawley, 10 wk old) were purchased from the Beijing Laboratory Animal Research Center (Beijing, China) and were used for breeding as described previously (38). Parents were maintained at an animal facility under barrier-sustained conditions with 12-h light/dark cycle under standard ambient conditions (temperature: 23 ± 2°C; relative humidity: 40–80%) and with free access to standard rat chow and water. Beginning two weeks before mating and continuing until weaning of the offspring at 21 days postpartum, female rats were given either a normal fat diet (NFD, 10% calories from lard, 3.85 kcal/g, cat. no.1032, HFK Bioscience CO, Beijing) or a high-fat diet (HFD, 60% calories from lard, 5.24 kcal/g, cat. no. H10060, HFK Bioscience CO, Beijing). The offspring were weighed and counted at birth, and then litter sizes during suckling were reduced to nine pups. All offspring were weaned onto the NFD and different drinking water including 1) normal drinking water; 2) drinking water with ACE inhibitor (captopril, Cap, 0.5 mg/ml, Sigma); 3) drinking water with TNF-α synthesis inhibitor [pentoxifylline (PTX), 100 mg/kg/day, Sigma] at 3 wk of age and continued until the end of the experiments. The food intake, drinking water intake, and body weight were measured one time per week. Each experimental group was composed of individual subjects that were randomly selected from different litters. Male offspring were used in all experiments.
Experiment 1.
At 11 wk of age, male offspring of all groups were used to determine effects of treatment with either ACE inhibitor or TNF-α inhibitor on maternal HFD-induced sensitization of ANG II hypertension. BP and heart rate (HR) were recorded by telemetry for 5 days at baseline and then for the subsequent 14 consecutive days, and all rats received a slow-pressor ANG II infusion (120 ng·kg−1·min−1, Sigma) for 2 wk. In ACE inhibitor pretreatment experiments, the offspring comprised 4 groups (6 rats/group): 1) NFD-offspring+vehicle+ANG II; 2) NFD-offspring+Cap+ANG II; 3) HFD-offspring+vehicle+ANG II; 4) HFD-offspring+Cap+ANG II. In TNF-α synthesis inhibitor pretreatment experiments, the offspring were also composed of 4 groups (6 rats/group): 1) NFD-offspring+vehicle+ANG II; 2) NFD-offspring+PTX+ANG II; 3) HFD-offspring+vehicle+ANG II; 4) HFD-offspring+PTX+ANG II.
Experiment 2.
After 8 wk of treatment with Cap or PTX in the drinking water (11 wk old), male offspring from all groups of dams were deeply anesthetized with isoflurane. After decapitation, the brains were quickly removed and put in ice-cold saline for 1 min. Then, the brain was cut into 300-μm or so coronal sections, and the target tissues, including the LT and both sides of the PVN, were punched with a 15-gauge needle stub (inner diameter: 1.5 mm). Some immediately surrounding tissue was usually included in the punch biopsies. The structures lying along the LT include the subfornical organ (SFO), median preoptic nucleus, and organum vasculosum of the lamina terminalis (OVLT). Because each of the structures lying along the LT is very tiny and they are located in same level of brain coronal plane, we collected these structures together and analyzed their mRNA expression as a whole. mRNA expression of the RAS components [renin, angiotensinogen (AGT), ACE, ANG II type 1 receptor (AT1-R)], NADPH oxidase (NOX2) and PICs (TNF-α, IL-1β, IL-6) (n = 5 per group) were analyzed in both the LT and PVN.
Telemetry probe implantation.
Rat BP transmitters (HD-S10, DSI, St. Paul, MN) were used to directly measure arterial BP as described previously (35, 38). At 9 wk of age, the offspring were instrumented with telemetry probes (HD-S10, DSI) through the femoral artery for monitoring of BP and HR. Beginning eight days after recovery from surgery, BP and HR data collection was initiated. After 5 days baseline recording, the rats were infused with a slow pressor dose of ANG II for 2 wk (120 ng·kg−1·min−1, model 2002, Alzet).
Real-time RT-PCR analysis.
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA. mRNA levels for RAS components (renin, AGT, ACE, AT1-R), NOX2, PICs (TNF-α, IL-1β, and IL-6), and GAPDH were analyzed with SYRB Green real-time PCR. The sequences for the primers are summarized in Table 1. Real-time RT-PCR was performed with the ABI prism 7300 Sequence Detection System (Applied Biosystems, Carlsbad, CA). The values were corrected by GAPDH, and the final concentration of mRNA was calculated using the formula x = 2−ΔΔCt, where x = fold difference relative to control.
Table 1.
Primer sequences for real-time PCR
| Gene | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 |
| Renin | CTGCCACCTTGTTGTGTGAG | ACCTGGCTACAGTTCACAACG | 154 |
| AGT | TCCCTCGCTCTCTGGACTTA | AAGTGAACGTAGGTGTTGAAA | 209 |
| ACE | GTGTTGTGGAACGAATACGC | CCTTCTTTATGATCCGCTTGA | 187 |
| AT1-R | CTCAAGCCTGTCTACGAAAATGAG | GTGAATGGTCCTTTGGTCGT | 188 |
| NOX2 | CAAGATGGAGGTGGGACAGT | GCTTATCACAGCCACAAGCA | 170 |
| IL-1β | AGCAACGACAAAATCCCT GT | GAAGACAAACCGCTTTTCCA | 209 |
| IL-6 | GCCTATTGAAAATCTGCTCTGG | GGAAGTTGGGGTAGGAAGGA | 160 |
| TNF-α | GCCGATTTGCCACTTCATAC | AAGTAGACCTGCCCGGACTC | 209 |
ACE, angiotensin-converting enzyme 1; AGT, angiotensinogen; AT1-R, ANG II type 1 receptor; NOX2, NADPH oxidase 2; TNF-α, tumor necrosis factor-α.
Data analysis.
Mean arterial pressure (MAP) and HR are presented as mean daily values. Differences for MAP and HR were calculated for each animal based on the mean of a 5-day baseline subtracted from the mean of the final 5 days of ANG II treatment. Two-way ANOVA for the experimental groups was then conducted on the means of calculated differences (the factors were maternal diet and ANG II treatment). After it was established that there was a significant ANOVA, post hoc analyses were performed with Tukey multiple comparison tests between pairs of mean changes (GraphPad Prism 8.1.2). One-way ANOVAs and post hoc Tukey analyses were used to test for the differences in body weight, food and water intake, mRNA expression of the RAS and PIC components, and NADPH oxidase in the LT and PVN (GraphPad Prism 8.1.2). All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Effects of treatment with Cap on maternal HFD-induced sensitization of ANG II hypertension in the offspring.
The offspring of HFD dams showed an enhanced pressor response to 14 days ANG II infusion when compared with those offspring of NFD dams (Δ27.9 ± 3.4 vs. Δ17.9 ± 3.3 mmHg, P < 0.05, Fig. 1, A and B).
Fig. 1.
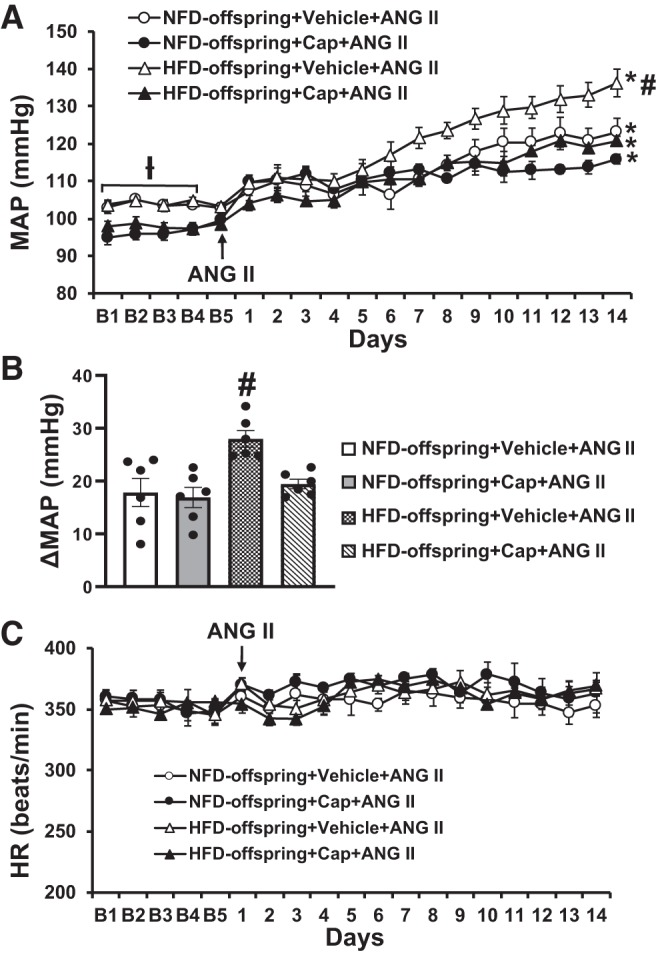
Pressor effects (A and B) and heart rate (HR) (C) changes induced by ANG II in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring with pretreatment with captopril (Cap). The enhanced pressor effect in HFD offspring was attenuated by Cap pretreatment. (n = 6/group; two-way ANOVA, *P < 0.05 vs. baseline; #P < 0.05 vs. NFD offspring or HFD offspring with Cap pretreatment). MAP, mean arterial pressure.
Cap administered in the drinking water from the time of weaning induced a slight but significant decrease in baseline MAP in both offspring of NFD (103.5 ± 1.4 to 96.7 ± 1.5 mmHg, P < 0.05, Fig. 1A) and HFD dams (104.8 ± 1.7 to 98.1 ± 1.1 mmHg, P < 0.05, Fig. 1A) but had no effect on basal HR. Furthermore, the Cap treatment significantly attenuated the enhanced hypertensive response produced by the pressor dose of ANG II as compared with animals without Cap treatment in the offspring of HFD dams (Δ27.9 ± 2.4 vs. Δ19.5 ± 1.1 mmHg, P < 0.05, Fig. 1, A and B). In Cap-treated offspring from NFD dams, a declining trend in daily MAP of the final 5 days of ANG II treatment was evident when compared with the non-Cap-treated offspring of NFD dams. However, the alteration in the ANG II-induced pressor response produced by Cap treatment was not significant (the means of difference for MAP, Δ17.9 ± 3.3 vs. Δ16.9 ± 2.4 mmHg, P > 0.05, Fig. 1, A and B). Chronic ANG II infusion had no effect on HR in all groups (P > 0.05, Fig. 1C).
Effects of treatment with PTX on maternal HFD-induced sensitization of ANG II hypertension in the offspring.
Fourteen-day ANG II infusion resulted in an augmented pressor response in offspring of HFD dams when compared with that in offspring of NFD dams (Δ27.3 ± 2.7 vs. Δ13.7 ± 3.5 mmHg, P < 0.05, Fig. 2, A and B).
Fig. 2.
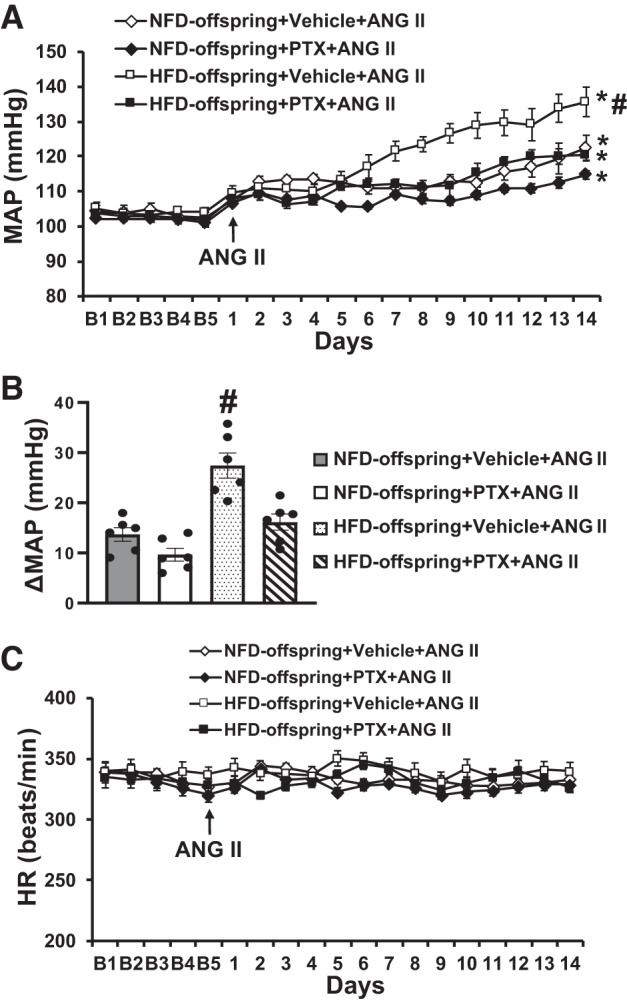
Pressor effects (A and B) and heart rate (HR) (C) changes induced by ANG II in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring with pretreatment with pentoxifylline (PTX). The enhanced pressor effect in HFD offspring was attenuated by PTX pretreatment. (n = 6/group; two-way ANOVA, *P < 0.05 vs. baseline; #P < 0.05 vs. NFD offspring or HFD offspring with PTX pretreatment). MAP, mean arterial pressure.
PTX treatment had no effect on baseline MAP and HR in all groups of the offspring. However, PTX treatment significantly reduced ANG II-induced pressor response in the offspring of HFD dams (Δ27.3 ± 2.7 vs. Δ16.1 ± 1.7 mmHg, P < 0.05, Fig. 2, A and B). In the offspring of NFD dams, treatment with TNF-α inhibitor tended to attenuate ANG II–induced increase in MAP, but this was not statistically different (Δ13.7 ± 3.5 vs. Δ9.6 ± 2.2 mmHg, P > 0.05, Fig. 2, A and B). Chronic ANG II infusion had no effect on HR in all groups (P > 0.05, Fig. 2C).
Effect of Cap on maternal HFD-induced mRNA expression of RAS components, PICs, and NADPH oxidase in the brain before ANG II infusion.
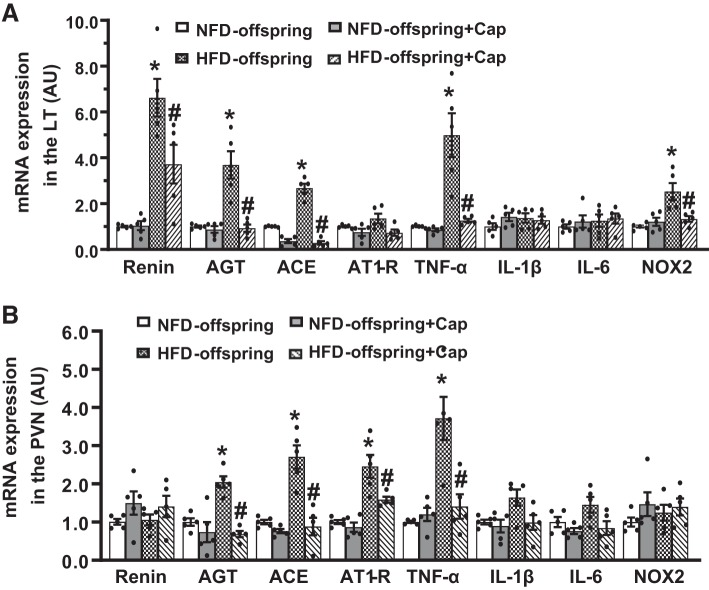
In LT tissues collected from offspring at 11 wk of age, HFD offspring exhibited increased mRNA expression of RAS components (renin, AGT, and ACE), the PICs (TNF-α), and the NOX2 when compared with NFD offspring (P < 0.05, Fig. 3A), while the mRNA expression of AT1-R, IL-1β, and IL-6 had no changes. Likewise, upregulated mRNA expression of AGT, ACE, AT1-R, and TNF-α but not renin, IL-6, and IL-1β in the PVN was evident in HFD offspring (P < 0.05, Fig. 3B). Treatment with ACE inhibitor had no effect on the gene expression in offspring of NFD but significantly attenuated the upregulated gene expression in offspring of HFD (P < 0.05, Fig. 3, A and B).
Fig. 3.
Quantitative comparison of the mRNA expression of renin-angiotensin system components, proinflammatory cytokines, and NADPH oxidase in the lamina terminalis (LT) and paraventricular nucleus (PVN) in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring with pretreatment with captopril (Cap) before ANG II infusion (A and B). (n = 5/group; one-way ANOVA, *P < 0.05 vs. NFD offspring; #P < 0.05 vs. HFD offspring without pretreatment). AGT, angiotensinogen; ACE, angiotensin-converting enzyme; AT1-R, ANG II type 1 receptor; TNF-α, tumor necrosis factor-α; IL, interleukin; NOX2, NADPH oxidase.
Effect of PTX on maternal HFD-induced mRNA expression of RAS components, PICs, and NADPH oxidase in the brain before ANG II treatment.
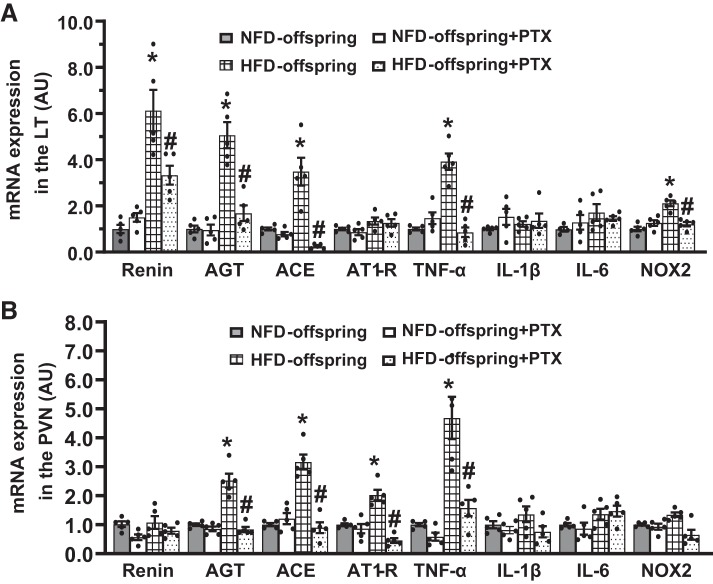
In LT tissues, the maternal HFD resulted in a significant increase in mRNA expression of renin, AGT, ACE, TNF-α, and NOX2 in their offspring when compared with the offspring of NFD dams (P < 0.05, Fig. 4A). In PVN tissues, the mRNA expression of AGT, ACE, AT1-R, and TNF-α was also upregulated in HFD-offspring (P < 0.05, Fig. 4B). Treatment with PTX significantly attenuated the enhanced gene expression in both the LT and PVN (P < 0.05, Fig. 4, A and B).
Fig. 4.
Quantitative comparison of the mRNA expression of renin-angiotensin system components, proinflammatory cytokines and NADPH oxidase in the lamina terminalis (LT) and paraventricular nucleus (PVN) in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring with pretreatment with pentoxifylline (PTX) before ANG II infusion (A and B). (n = 5/group; one-way ANOVA, *P < 0.05 vs. NFD offspring; #P < 0.05 vs. HFD-offspring without pretreatment). AGT, angiotensinogen; ACE, angiotensin-converting enzyme; AT1-R, ANG II type 1 receptor; TNF-α, tumor necrosis factor-α; IL, interleukin; NOX2, NADPH oxidase.
Effects of treatment with Cap or PTX on body weight and food and water intakes.
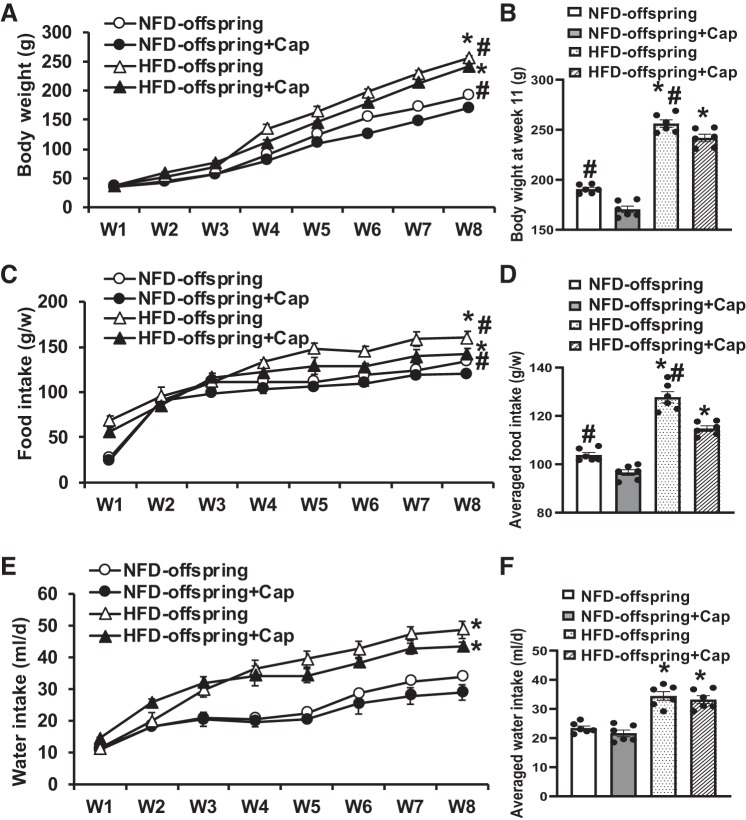
There were no differences in body weight between NFD offspring and HFD offspring at weaning. However, in the following 8 wk, the HFD offspring exhibited a significant weight gain when compared with the NFD offspring (P < 0.05, Fig. 5A). Eight weeks of Cap treatment significantly reduced body weight gain in both HFD offspring and NFD offspring (11 wk old: HFD offspring, 256.1 ± 3.7 to 241.9 ± 3.4 g; NFD offspring, 190.1 ± 3.6 to 170.6 ± 3.2 g; P < 0.05, Fig. 5B). Likewise, food intake in the HFD offspring was more than that in the NFD offspring, and Cap treatment also reduced food intake in both HFD offspring and NFD offspring (HFD offspring, 127.7 ± 2.4 to 114.8 ± 1.4 g/week; NFD offspring, 103.8 ± 1.2 to 96.6 ± 1.4 g/week; P < 0.05, Fig. 5, C and D). Similarly, water intake in the HFD offspring was more than that in the NFD offspring, and Cap treatment had no significant effect on water intake, although Cap slightly reduced water intake in both HFD offspring and NFD offspring (HFD offspring, 34.4 ± 1.9 vs. 33.2 ± 1.2 ml/day; NFD offspring, 23.5 ± 0.5 vs. 21.5 ± 1.4 g/day; P > 0.05, Fig. 5, E and F).
Fig. 5.
Changes in body weight (A and B), food intake (C and D), and drinking water intake (E and F) during pretreatment with captopril (Cap) in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring after weaning. (n = 6/group; one-way ANOVA, *P < 0.05 vs. NFD offspring; #P < 0.05 vs. HFD offspring with Cap pretreatment).
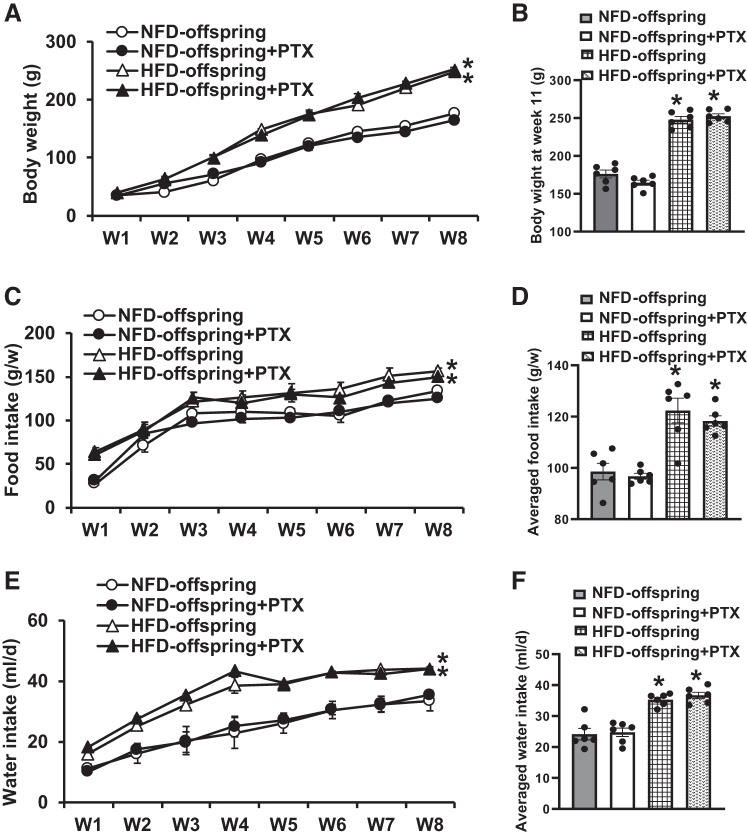
In contrast, 8 wk of PTX treatment had no effects on body weight (11 wk old: HFD offspring, 247.7 ± 4.4 to 252.3 ± 3.3 g; NFD offspring, 176.0 ± 5.1 to 164.5 ± 3.3 g; P > 0.05, Fig. 6, A and B), food intakes (HFD offspring, 122.3 ± 4.8 to 118.3 ± 2.0 g/week; NFD offspring, 98.6 ± 3.2 to 96.7 ± 1.1 g/week; P > 0.05, Fig. 6, C and D), and water intakes (HFD offspring, 35.3 ± 0.7 vs. 36.7 ± 0.9 ml/day; NFD offspring, 24.1 ± 1.7 vs. 24.7 ± 1.2 g/day; P > 0.05, Fig. 6, E and F) in both HFD offspring and NFD offspring, showing that body weight, food intake, and drinking were maintained at similar levels during PTX treatment.
Fig. 6.
Changes in body weight (A and B), food intake (C and D) and drinking water intake (E and F) during pretreatment with pentoxifylline (PTX) in maternal normal fat diet (NFD) offspring and high-fat diet (HFD) offspring after weaning. (n = 6/group; one-way ANOVA, *P < 0.05 vs. NFD offspring).
DISCUSSION
The major findings of the present study are 1) ANG II administration produced a sensitized hypertensive response in the offspring of HFD dams; 2) the enhanced hypertensive response was reversed by either Cap or PTX treatment beginning at weaning and maintained until the end of testing; 3) the offspring of HFD dams showed upregulated expression of RAS components, PICs, and NADPH oxidase in the LT and PVN when compared with the offspring of NFD dams; 4) the enhanced gene expression of RAS components, PICs, and NADPH oxidase was reduced by either Cap or PTX treatment; and 5) the offspring of HFD dams exhibited increased body weight and food and water intakes when compared with the offspring of NFD dams. Cap treatment, but not PTX treatment, attenuated the increased metabolic parameters in the offspring from both HFD and NFD dams. These results demonstrate that mothers eating HFD during pregnancy and lactation may predispose their offspring to developing metabolic disorder and hypertension through upregulation of the brain RAS and PICs. Targeting RAS and inflammatory factors is an effective strategy to prevent or reduce the incidence of cardiovascular diseases including hypertension in offspring programmed by maternal nutritional changes.
It is well established that maternal health and nutritional state during pregnancy and lactation program offspring to alter their responses to environmental challenges and have a sustained impact on metabolism and cardiovascular function later in their life (1, 7, 13, 24). Maternal low-protein diet led to increased BP and kidney damage, which was accompanied by upregulation of PICs, reactive oxygen species, and RAS components in kidney of adult male offspring (30). Similarly, maternal hypertension elicited autonomic dysfunction and sensitized ANG II–induced hypertension that was associated with enhanced mRNA expression of RAS and PIC components in brain nuclei involved in BP regulation in offspring (34). These studies further demonstrate that postweaning treatment with AT1-R blocker (losartan) or ACE inhibitor (captopril) had beneficial effects on the activity and expression of RAS, inflammatory and oxidative stress markers that reverse elevated BP, and hypertensive responses in the offspring (30, 34).
Recently, our studies showed that maternal HFD resulted in hypertensive response sensitization and upregulation of the RAS and inflammation in the brain as similar to that elicited by maternal hypertension (34, 38) and that blockade of inflammation also had beneficial effects similar to those of the inhibition of the RAS in a psychosocial stress-related hypertensive response sensitization model. Based on the effective manipulations on the RAS and inflammation to reduce increased BP in these hypertensive models, the present study therefore determined the effects of blockade of either ACE or TNF-α on maternal HFD-induced sensitization of ANG II hypertension. We confirmed that the enhanced hypertensive response by maternal HFD was reversed by treating the offspring with either ACE inhibitor or TNF-α inhibitor. Moreover, the upregulated expression of RAS and PIC components in the LT and PVN was also significantly inhibited, suggesting that systemic inhibition of RAS or inflammation abolishes maternal HFD-induced hypertensive response sensitization, at least in part, through a central mechanism.
Numerous studies have demonstrated that maternal HFD is linked to brain inflammation (i.e., the arcuate nucleus, PVN, and hippocampus) in offspring via increased microglial activation and enhanced PICs expression such as TNF-α, IL-1β, and IL-6 (2, 4, 8, 25, 26). Moreover, the offspring of maternal HFD also have increased circulating cytokines (liver, fat, and serum) that can be transferred to the offspring brain including hypothalamus and hippocampus, thereby impacting metabolism and behavior (3, 14). Similarly, maternal HFD is associated with increased RAS activity and expression in the periphery and the brain including the LT and PVN (15, 27, 38). The activated RAS components and enhanced inflammation appear to work in concert to exacerbate maternal HFD-induced adverse effects in offspring. However, how the peripheral RAS and inflammatory factors reciprocally crosstalk with central ones is not well understood. Forebrain structures along with the LT (i.e., the SFO, median preoptic nucleus and OVLT) and the hypothalamus (particularly the PVN) play important roles in the long-term regulation of BP and metabolism. LT structures, lacking a normal blood-brain barrier, are involved in both sensing and processing input derived from humoral factors (e.g., ANG II and inflammatory cytokines) and transmitting this information to the PVN, which in turn projects to hindbrain and spinal cord cardiovascular control structures (22). Experimental ablations of the SFO or OVLT can interrupt the course of development or maintenance of high BP produced by the systemic infusion of ANG II or cytokine (29, 31, 32). In the present study, we found that besides the PVN, the LT structures also exhibited upregulated expression of the RAS and PIC components which was significantly inhibited by systemic administration of either ACE or TNF-α inhibitor in offspring of HFD dams. Based on the aforementioned studies and our results, it can be speculated that the LT structures are key sites that connect the peripheral and central signaling of the RAS and inflammatory factors, which plays a critical role in sensitization of ANG II–induced hypertension in offspring of HFD dams in the present study. Studies to test roles of brain RAS activation and inflammation versus circulating RAS and inflammation in mediating the maternal HFD-induced sensitization of ANG II hypertension are warranted in the future.
It should be noted that the crosstalk between RAS and inflammatory signaling within the brain increases sympathetic output and cardiovascular dysfunction in a positive feedback manner has been well established. This is likely to contribute to the development and progression of hypertension (5, 37). In the present study, the selected doses for captopril (0.5 mg/L) and PTX (100 mg·kg−1·day−1) in the drinking water have been demonstrated to effectively block the ACE and PIC production in the periphery and brain (23, 28). Blockade of either ACE or TNF-α production reduced all maternal HFD-induced upregulation of gene expression of RAS and PIC components. In other words, blockade of RAS activation can attenuate PIC production and vice versa. Therefore, it is likely that the downregulated RAS and PICs by either ACE inhibitor or TNF-α inhibitor will attenuate hypertensive response sensitization produced by maternal HFD. Second, besides the sensitized hypertensive response, increased food and water intakes and elevated body weight were evident in the offspring of HFD dams when compared with the offspring of NFD dams. This suggests that maternal HFD also induced a metabolic disorder in the offspring, which is consistent with many other studies on the effect of maternal HFD on the offspring (6, 9, 21). Systemic administration of ACE inhibitor (Cap), but not TNF-α inhibitor (PTX), resulted in a slight decrease in water intake and a significant decrease in food intake, thereby body weight reduction in the offspring of both HFD and NFD dams. This might be due to an aversive taste in the drinking water produced by the dose of Cap that made animals drink and eat less and gain less body weight (10, 12). Nevertheless, we are unaware of data that support an influence of food intake or growth rate on the maternal HFD-induced sensitization of hypertension as well as the beneficial effects of blockade of RAS activation and of cytokines production. Third, sex-specific effects are widely observed in perinatal developmental programming studies including maternal HFD (9, 20). Our previous studies have also shown that there are sex differences in low dose of ANG II-induced sensitization of hypertension (36) and in the maternal hypertension-induced sensitization of ANG II hypertension (33). Female sex hormones account for the sex-specific sensitization of the hypertensive response to ANG II, which may be associated with estrogen upregulation of the RAS antihypertensive components and with downregulation of the RAS prohypertensive components in the brain. Whether similar mechanisms underlying sex-specific sensitization in offspring of HFD dams need to be studied in the future.
Perspectives and Significance
Epidemiological studies and animal experiments demonstrate an association between maternal HFD and metabolic syndrome and cardiovascular diseases, in which the RAS activation and inflammation contribute to compromised cardiovascular health in the offspring later life. The present study further demonstrates that systemic blockade of either RAS or inflammation in offspring abolished upregulated brain RAS and PICs and sensitization of the hypertensive response to ANG II produced by maternal HFD. Our studies may provide a potential therapeutic option to reduce the impact of maternal obesity or HFD on adverse outcomes, especially hypertension, in the offspring.
GRANTS
This work was supported by the funds for major program of Hebei North University (ZD201316 to J. D. Li) and the National Institutes of Health (HL-139575 to A. K. Johnson and B. Xue).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.P., A.K.J., and B.X. conceived and designed research; X.-F.W., J.-D.L., Y.-L.H., Y.-P.Z., Z.-Q.F., and H.-P.W. performed experiments; X.-F.W., J.-D.L., Y.-L.H., and B.X. analyzed data; W.P. and B.X. interpreted results of experiments; X.-F.W., J.-D.L., and Y.-L.H. prepared figures; W.P. drafted manuscript; W.P., A.K.J., and B.X. edited and revised manuscript; X.-F.W., J.-D.L., Y.-L.H., Y.-P.Z., Z.-Q.F., H.-P.W., W.P., A.K.J., and B.X. approved final version of manuscript.
REFERENCES
- 1.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol 5: 997–1025, 2015. doi: 10.1002/cphy.c140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahari H, Caruso V, Morris MJ. Late-onset exercise in female rat offspring ameliorates the detrimental metabolic impact of maternal obesity. Endocrinology 154: 3610–3621, 2013. doi: 10.1210/en.2013-1059. [DOI] [PubMed] [Google Scholar]
- 3.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24: 2104–2115, 2010. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 4.Bolton JL, Bilbo SD. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialogues Clin Neurosci 16: 307–320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κBin the paraventricular nucleus. Hypertension 59: 113–121, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol 211: 237.e1–237.e13, 2014. doi: 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong M, Zheng Q, Ford SP, Nathanielsz PW, Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J Mol Cell Cardiol 55: 111–116, 2013. doi: 10.1016/j.yjmcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Dudele A, Hougaard KS, Kjølby M, Hokland M, Winther G, Elfving B, Wegener G, Nielsen AL, Larsen A, Nøhr MK, Pedersen SB, Wang T, Lund S. Chronic maternal inflammation or high-fat-feeding programs offspring obesity in a sex-dependent manner. Int J Obes 41: 1420–1426, 2017. doi: 10.1038/ijo.2017.136. [DOI] [PubMed] [Google Scholar]
- 9.Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 102: 514–519, 2009. doi: 10.1017/S000711450820749X. [DOI] [PubMed] [Google Scholar]
- 10.Evered MD, Robinson MM, Richardson MA. Captopril given intracerebroventricularly, subcutaneously or by gavage inhibits angiotensin-converting enzyme activity in the rat brain. Eur J Pharmacol 68: 443–449, 1980. doi: 10.1016/0014-2999(80)90419-7. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491–497, 2012. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 12.Fregly MJ. Effect of the angiotensin converting enzyme inhibitor, captopril, on NaCl appetite of rats. J Pharmacol Exp Ther 215: 407–412, 1980. [PubMed] [Google Scholar]
- 13.Gademan MG, van Eijsden M, Roseboom TJ, van der Post JA, Stronks K, Vrijkotte TG. Maternal prepregnancy body mass index and their children’s blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension 62: 641–647, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01511. [DOI] [PubMed] [Google Scholar]
- 14.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151: 1622–1632, 2010. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guberman C, Jellyman JK, Han G, Ross MG, Desai M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol 209: 262.e1–262.e8, 2013. doi: 10.1016/j.ajog.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. Int J Obes 34: 420–428, 2010. [Erratum in: Int J Obes 34: 1353, 2010.] 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AK, Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol 14: 750–766, 2018. doi: 10.1038/s41581-018-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AK, Zhang Z, Clayton SC, Beltz TG, Hurley SW, Thunhorst RL, Xue B. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1309–R1325, 2015. doi: 10.1152/ajpregu.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. doi: 10.1161/01.HYP.0000047511.97879.FC. [DOI] [PubMed] [Google Scholar]
- 21.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 153: 2823–2830, 2012. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- 22.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav 121: 96–102, 2013. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Moe KE, Weiss ML, Epstein AN. Sodium appetite during captopril blockade of endogenous angiotensin II formation. Am J Physiol Regul Integr Comp Physiol 247: R356–R365, 1984. doi: 10.1152/ajpregu.1984.247.2.R356. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Bhattacharya S, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347: f4539, 2013. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93: 1–8, 2011. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo JD, Morris MJ, Jones NM. Maternal obesity increases inflammation and exacerbates damage following neonatal hypoxic-ischaemic brain injury in rats. Brain Behav Immun 63: 186–196, 2017. doi: 10.1016/j.bbi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Tiao MM, Lin YJ, Yu HR, Sheen JM, Lin IC, Lai YJ, Tain YL, Huang LT, Tsai CC. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis 17: 178, 2018. doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vakili A, Mojarrad S, Akhavan MM, Rashidy-Pour A. Pentoxifylline attenuates TNF-α protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res 1377: 119–125, 2011. doi: 10.1016/j.brainres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J Physiol Regul Integr Comp Physiol 299: R1564–R1571, 2010. doi: 10.1152/ajpregu.00034.2010. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe IKM, Jara ZP, Volpini RA, Franco MDC, Jung FF, Casarini DE. Up-regulation of renal renin-angiotensin system and inflammatory mechanisms in the prenatal programming by low-protein diet: beneficial effect of the post-weaning losartan treatment. J Dev Orig Health Dis 9: 530–535, 2018. doi: 10.1017/S2040174418000296. [DOI] [PubMed] [Google Scholar]
- 31.Wei SG, Yu Y, Felder RB. Blood-borne interleukin-1β acts on the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 314: R447–R458, 2018. doi: 10.1152/ajpregu.00211.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 65: 1126–1133, 2015. doi: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue B, Beltz TG, Guo F, Johnson AK. Sex differences in maternal gestational hypertension-induced sensitization of angiotensin II hypertension in rat offspring: the protective effect of estrogen. Am J Physiol Regul Integr Comp Physiol 314: R274–R281, 2018. doi: 10.1152/ajpregu.00216.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue B, Yin H, Guo F, Beltz TG, Thunhorst RL, Johnson AK. Maternal gestational hypertension-induced sensitization of angiotensin II hypertension is reversed by renal denervation or angiotensin-converting enzyme inhibition in rat offspring. Hypertension 69: 669–677, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue B, Yu Y, Wei SG, Beltz TG, Guo F, Felder RB, Johnson AK. Stress-induced sensitization of angiotensin II hypertension is reversed by blockade of angiotensin-converting enzyme or tumor necrosis factor-α. Am J Hypertens 32: 909–917, 2019. doi: 10.1093/ajh/hpz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol 307: H191–H198, 2014. doi: 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Wei SG, Weiss RM, Felder RB. Angiotensin II type 1a receptors in the subfornical organ modulate neuroinflammation in the hypothalamic paraventricular nucleus in heart failure rats. Neuroscience 381: 46–58, 2018. doi: 10.1016/j.neuroscience.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YP, Huo YL, Fang ZQ, Wang XF, Li JD, Wang HP, Peng W, Johnson AK, Xue B. Maternal high-fat diet acts on the brain to induce baroreflex dysfunction and sensitization of angiotensin II-induced hypertension in adult offspring. Am J Physiol Heart Circ Physiol 314: H1061–H1069, 2018. doi: 10.1152/ajpheart.00698.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]