Abstract
Increases in sympathetic nerve activity (SNA) have been implicated in obesity-induced risk for cardiovascular diseases, especially hypertension. Previous studies indicate that oxidative stress in the rostral ventrolateral medulla (RVLM), a key brain stem region that regulates sympathetic outflow to peripheral tissues, plays a pathogenic role in obesity-mediated sympathoexcitation. However, the molecular mechanisms underlying this phenomenon are not clear. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that regulates the expression of antioxidant and anti-inflammatory genes and confers cytoprotection against oxidative stress. The present study was designed to investigate whether Nrf2 dysfunction was associated with obesity-induced oxidative stress in the RVLM and sympathoexcitation. C57BL/6J mice were fed with chow or a high-fat diet (HFD) for 16 wk. Blood pressure parameters were assessed by radiotelemeters in conscious freely moving mice. SNA was measured by heart rate variability analysis and also through assessment of depressor response to ganglionic blockade. The RVLM was microdissected for gene expression and protein analysis (Western blot analysis and activity assay) related to Nrf2 signaling. Our results showed that HFD-induced obesity resulted in significant increases in SNA, although we only observed a mild increase in mean arterial pressure. Obesity-induced oxidative stress in the RVLM was associated with impaired Nrf2 signaling marked by decreased Nrf2 activity, downregulation of Nrf2 mRNA, its target genes [NAD(P)H quinone dehyrogenase 1 (Nqo1) and superoxide dismutase 2 (Sod2)], and inflammation. Our findings suggest that obesity results in Nrf2 dysfunction, which likely causes maladaptation to oxidative stress and inflammation in the RVLM. These mechanisms could potentially contribute to obesity-induced sympathoexcitation.
Keywords: hypertension, inflammation, Nrf2, obesity, oxidative stress, RVLM, sympathetic nerve activity
INTRODUCTION
Obesity is a major risk factor for cardiovascular diseases, and despite significant advances in treatment options, cardiovascular disease remains the leading cause of death in the United States (2, 7). One of the major cardiovascular consequences of obesity is development of hypertension. In fact, a recent longitudinal study involving 60,000 men and women showed that an increase in the body mass index of 5 kg/m2 led to a 2.12-mmHg increase in the mean arterial pressure over the following 10 yr (37), suggesting obesity as a strong predictor of future changes in blood pressure (BP). Furthermore, treating obesity-related cardiovascular morbidities poses a significant burden to our health care system. According to the Centers for Disease Control and Prevention, the cost to treat obesity-associated diseases in the United States is over $190.2 billion in 2012. Despite this substantial investment, as many as two-thirds of patients with obesity and hypertension still do not respond to a combination of three or more conventional antihypertensive drugs, a condition referred to as resistant hypertension. Hence, it is critical to delineate the mechanisms by which obesity contributes to cardiovascular diseases as this is an important problem from clinical, economic, and public health perspectives.
There is substantial evidence that an overactive sympathetic nervous system (SNS) plays a pathogenic role in obesity-induced increased risk for cardiovascular disease, especially hypertension. Studies utilizing direct nerve recordings of postganglionic nerve fibers have shown that obesity increases sympathetic nerve activity (SNA) to the muscle and kidneys (9, 12, 22, 38). In humans with obesity and essential hypertension, renal denervation reduces sodium retention and improves blood pressure parameters. In addition, obesity-induced hypertension was also associated with increased urinary norepinephrine excretion, whole body norepinephrine (NE) spillover, and reduced heart rate variability indicating an overactive SNS (30, 33). Some studies have also reported decreased sensitivity of the arterial baroreceptor reflex in humans with obesity (13), which augments the risk for cardiovascular diseases. Hence, it is clear that obesity-induced hypertension has a neurogenic component and it is important to understand the neural mechanisms behind obesity-induced increases in SNA.
The rostral ventrolateral medulla (RVLM) is an important brain stem region that controls basal and reflex changes in SNS activity. The neurons in the RVLM project directly to the intermediolateral cell column of the spinal cord, which then innervates sympathetic preganglionic neurons and generates sympathetic nerve activity to end organs via postganglionic neurons. Previous studies have shown that oxidative stress characterized by increased production of reactive oxygen species at the level of the RVLM contributes to the pathogenesis of neurogenic hypertension. However, the molecular mechanisms underlying obesity-induced oxidative stress in the RVLM are not clear. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) contributes to the cellular redox homeostasis by biosynthesis, utilization, and regeneration of the antioxidants and thereby controlling the production of reactive oxygen species (6, 27). Nrf2 exists in the cytoplasm in an inactive form associated with Kelch-like ECH-associated protein 1 (Keap1; 25, 42). Under basal conditions, the Nrf2-Keap1 complex is subjected to proteasomal degradation (20). In response to cellular stress, Nrf2 dissociates from Keap1, translocates to the nucleus (15), and binds to the antioxidant response elements (AREs) in the promoter region of genes encoded for multiple antioxidants, leading to upregulation of antioxidant enzymes (16, 24). A recent study by Zucker and colleagues showed that selective deletion of Nrf2 in the RVLM increases mean arterial pressure, urinary norepinephrine, and baseline renal sympathetic nerve activity in normal mice suggesting that Nrf2 is a critical modulator of redox status in the sympathetic neurons in the RVLM and contributes to sympathoregulation (11). In this study, we utilized a high-fat diet-induced obesity mouse model to investigate whether Nrf2 dysfunction in the RVLM is associated with obesity-mediated sympathoexcitation.
METHODS
Animals and treatment.
Six-week-old male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). After a 2-wk acclimatization period, the animals were divided into two groups (n = 30 mice per group) and fed either a normal diet (chow, 10% of calories from fat, cat. no. D12450B) or a high-fat diet (HFD, 60% of calories from fat, cat. no. D12492) obtained from Research Diets, Inc. (New Brunswick, NJ). The dietary treatment was performed for 16 wk. In a subset of animals (n = 4 mice per group), radiotelemeters were implanted in the 14th week of dietary treatment as described in Radiotelemeter implantation to measure hemodynamic parameters. At the end of 16 wk, the animals were euthanized by cervical dislocation. Blood was collected, and serum was separated and stored at −80°C for further analysis. Brain tissues were collected and frozen on dry ice. All the animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Oklahoma State University (IACUC no. VM-17-23) and were performed under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Radiotelemeter implantation to measure hemodynamic parameters.
Mean arterial pressure (MAP) and heart rate (HR) were monitored continuously in conscious freely moving mice using radiotelemeters (HD-X11; Data Sciences International, St. Paul, MN). Twenty-two-week-old mice were anesthetized with 3% isoflurane and maintained at 1.5–2% isoflurane for the duration of the implant surgery. Radiotelemeters were implanted as described previously (1) with minor modifications. Briefly, the surgical area was shaved and disinfected with 70% isopropyl alcohol. The mice were placed on a heating pad, and the femoral artery was exposed by making an incision on the left flank region. The catheter was inserted into the femoral artery and advanced into the abdominal aorta. The transmitter body was placed subcutaneously in the left flank. The skin was closed with 3-0 silk suture. After a week of recovery, MAP and HR data were collected for a 24-h period on intermittent days for 1 wk (10 s every min with Data Sciences International Ponemah software). To assess the contribution of SNS activity to MAP and HR, a ganglionic blocker (hexamethonium; 10 mg/kg ip) was administered once in the last week of HFD treatment as described previously (1). The magnitude of the depressor response to hexamethonium injection was recorded in real time and compared between the groups. Greater depressor response was considered as an indicator of higher SNA in the animals.
Heart rate variability analysis.
To assess SNA in conscious mice, we performed blood pressure variability (BPV) and heart rate variability (HRV) analysis. Both the parameters were analyzed in the frequency domain. Briefly, multiple short-term (5-min) HRVs on the frequency domain were assessed, analyzed, and averaged using Ponemah version 6.5 software (Data Sciences International). The cutoff frequencies were set at 0.4–1.5 and 1.5–4 Hz for the low-frequency and high-frequency ranges, respectively. Power spectral analysis of heart rate was used to characterize overall HRV as well as low- and high-frequency components attributable to sympathetic and vagal influences, respectively, as described previously (4, 8).
Serum norepinephrine measurements.
Serum norepinephrine (NE) levels were measured using a commercial NE ELISA kit (Labor Diagnostika Nord, Nordhorn, Germany). Serum (30 µL) was used for NE extraction and measurements, based on the manufacturer’s instructions.
Nuclear protein extraction and protein estimation.
Nuclear protein was isolated from frozen brain stem tissue of chow- and HFD-fed mice using Nuclear Extraction Kit from Active Motif (Carlsbad, CA) as per the manufacturer’s instructions. The protein levels in nuclear extracts were estimated using a Micro BCA assay (Thermo Fisher Scientific, Rockford, IL).
Nrf2 DNA-binding capability assay.
Nuclear extracts (30 µg) were used for assessing the Nrf2 DNA-binding capability assay using a TransAM Nrf2 transcription factor assay kit (Active Motif). Briefly, nuclear extracts were incubated in a 96-well plate coated with immobilized Nrf2 ARE sequence oligonucleotides for 1 h at room temperature (RT). The captured complexes were incubated with Nrf2 antibodies (1:1,000) for 1 h and subsequently with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1,000) for 1 h. After colorimetric reaction, the absorbance was read as the optical density value at 450 nm using a Tecan Spark multimode microplate reader. The blank OD450 values were subtracted from the samples, and the data (activated Nrf2) are represented as means ± SE.
Dihydroethidium staining.
Brain stem samples from chow- and HFD-fed animals were mounted in optimum cutting temperature compound and sectioned at 20-µm thickness. Unfixed frozen brain stem sections were stained with 2 µM dihydroethidium (DHE; Thermo Fisher Scientific) diluted with DMSO and PBS in a light-protected humidified chamber for 30 min at 37°C. At the end of the incubation period, the slides were washed three times with PBS and mounted with ProLong diamond antifade mountant (Invitrogen). The fluorescence from the oxidized DHE was imaged using a Leica SP8 confocal microscope using a 488-nm excitation wavelength and a 585-nm filter. The intensity of the DHE staining was quantified using Fiji software (updated version of ImageJ).
3-Nitrotyrosine measurements.
RVLM micropunches were lysed with radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors and centrifuged at 12,000 rpm for 10 min, and the supernatant with soluble protein was isolated (n = 8 mice per group; punches from 2 mice were pooled into 1). 3-Nitrotyrosine (3-NT) content in isolated protein lysates was measured using OxiSelect Nitrotyrosine ELISA Kit purchased from Cell Biolabs (San Diego, CA). 3-NT measurements were normalized to protein concentration in the sample measured by Micro BCA assay (Thermo Fisher Scientific).
Real-time PCR.
Micropunches from the RVLM of two mice within the same treatment groups were pooled to obtain enough RNA for real-time PCR analysis. The tissue micropunches were lysed in TRIzol reagent, and RNA was extracted using Direct-zol RNA MicroPrep Kit (Zymo Research, Irvine, CA). cDNA was synthesized using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR reactions were performed using an iTaq Universal SYBR Green mix (Bio-Rad) with 10 ng cDNA per reaction. The primers for Nrf2, NAD(P)H quinone dehydrogenase 1 (NQO1), heme oxygenase 1 (HO-1), superoxide dismutase 2 (SOD2), catalase, IL-1β, monocyte chemotactic protein 1 (MCP1), TNF-α, IL-6, and the housekeeping gene, Gapdh, were designed using Primer-Basic Local Alignment Search Tool (Primer-BLAST) software and synthesized by Integrated DNA Technologies (primer sequences are listed in Table 1). Data were analyzed by the 2−ΔΔCT method (where CT is threshold cycle).
Table 1.
Primer sequences and their gene accession numbers for real-time PCR analysis
| Mouse Gene | Forward Primer | Reverse Primer | Gene Accession No. |
|---|---|---|---|
| Nrf2 | CGAGATATACGCAGGAGAGGTAAGA | GCTCGACAATGTTCTCCAGCTT | NM_010902.4 |
| Nqo1 | TATCCTTCCGAGTCATCTCTAGCA | TCTGCAGCTTCCAGCTTCTTG | NM_008706.5 |
| Hmox1 | GTCAAGCACAGGGTGACAGA | ATCACCTGCAGCTCCTCAAA | NM_010442.2 |
| Sod2 | TTAACGCGCAGATCATGCA | GGTGGCGTTGAGATTGTTCA | NM_013671.3 |
| Cat | GCTGAGAAGCCTAAGAACGCAAT | CCCTTCGCAGCCATGTG | NM_009804.2 |
| Gapdh | AAGGGCTCATGACCACAGTC | GGATGACCTTGCCCACAG | NM_001289726.1 |
| Il1b | CACAGCAGCACATCAACAAG | GTGCTCATGTCCTCATCCTG | NM_008361.4 |
| Ccl2 | GCAGTTAACGCCCCACTCA | TCCAGCCTACTCATTGGGATCA | NM_011333.3 |
| Tnf | GGAACTGGCAGAAGAGGCACTC | GCAGGAATGAGAAGAGGCTGAGAC | NM_001278601.1 |
| Il6 | ACAAGTCGGAGGCTTAATTACACAT | TTGCCATTGCACAACTCTTTTC | NM_001314054.1 |
Cat, catalase; Ccl2, chemokine (C-C motif) ligand 2 [monocyte chemotactic protein 1 (MCP1)]; Hmox1, heme oxygenase 1 (HO-1); Nqo1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid 2-related factor 2; Sod2, superoxide dismutase 2.
Western blot analysis.
Pooled RVLM brain stem punches (n = 8 mice per group; punches from 2 mice were pooled into 1) were lysed in RIPA buffer with protease and phosphatase inhibitors. Protein concentrations in tissue lysates were quantified by Thermo Scientific BCA assay. Equal amounts of protein (45 µg) were separated on Mini-Protean TGX precast protein gels (Bio-Rad) and transferred to a PVDF membrane using a wet transfer system (Bio-Rad). The membranes were blocked using 5% BSA in Tris-buffered saline-Tween 20 (TBST) for 1 h at RT. Then the membranes were incubated with the following primary antibodies overnight: β-actin (1:5,000, ab6276), catalase (1:1,000, ab16731), NQO1 (1:1,000, ab34173), HO-1 (1:500, sc136960), and SOD2 (1:500, sc13034,). After three washes in TBST, the membranes were incubated with the respective HRP-conjugated secondary antibodies for 1 h at RT, washed three times, and developed using SuperSignal West Pico or SuperSignal West Femto chemiluminescent substrate solutions (Thermo Fisher Scientific). Digital images were acquired using a Thermo Scientific myECL imaging system. Densitometric analysis was performed using ImageJ (Fiji) software. Precision Plus Protein Kaleidoscope prestained protein standards (Bio-Rad) were run parallel to the samples in each gel to determine the molecular weight of the proteins in our samples.
Serum and tissue cytokine and chemokine measurements.
Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead-Premixed 32 Plex kit (Millipore, Burlington, MA) was used to assess cytokine and chemokine protein levels in serum and RVLM protein extracts. The plate was read using the Bio-Plex 200 analyzer at the University of Oklahoma Health Sciences Center core facility. We diluted serum 1:2 before use in the assay. The values for RVLM samples extrapolated from the standard curve were normalized to protein concentration and expressed as picograms per microgram of protein.
Statistical analysis.
Data are expressed as means ± SE. Blood pressure and heart rate variability parameters were analyzed by two-way repeated-measures ANOVA. All the other data were analyzed by Student’s t test to assess the difference between the two groups. Because of high variability, we used the Mann–Whitney nonparametric test to analyze serum NE levels. A P value of <0.05 was considered statistically significant.
RESULTS
Obesity-induced changes in metabolic and hemodynamic parameters.
As expected, HFD treatment for 16 wk resulted in obesity in C57BL/6J mice as evident from the significant increase in body weight, average weekly food intake, and visceral adipose tissue mass compared with chow-fed animals (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.8848541.v1).
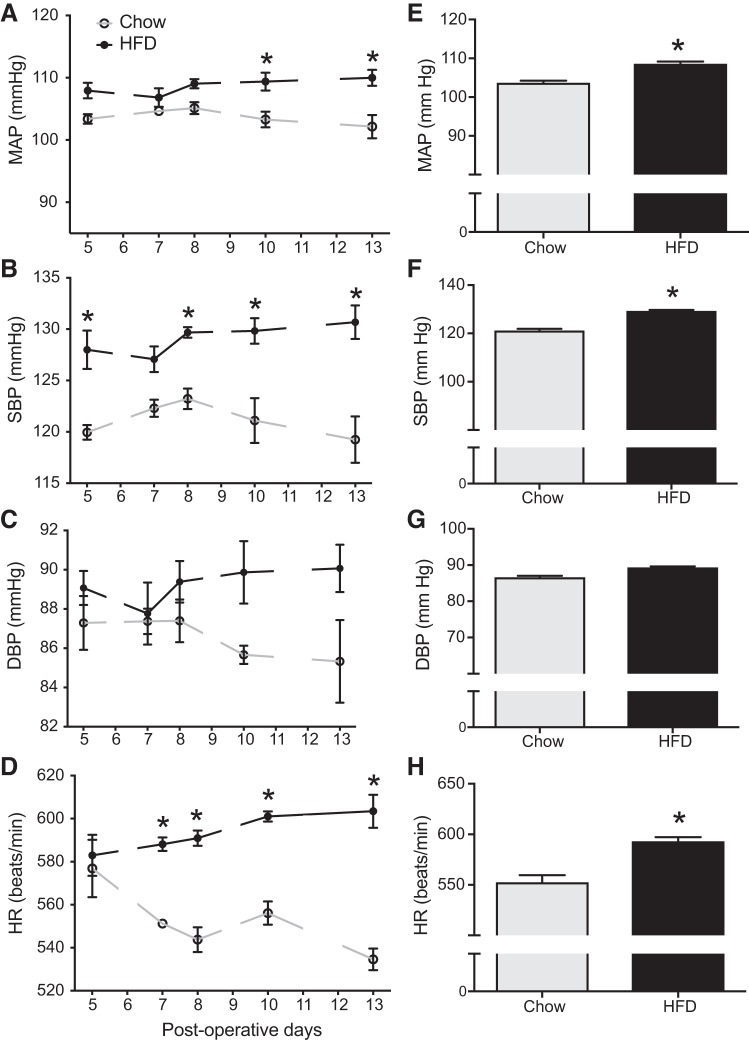
We investigated the changes in blood pressure and heart rate using radiotelemeters during the last couple of weeks of dietary treatment. Figure 1, A–D, depicts the 24-h averages of MAP, systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR recorded on intermittent days from day 5 to day 13 postsurgery in the chow and HFD groups. Figure 1, E–H, represents the overall average of the same between the groups. HFD treatment resulted in a mild but significant increase in MAP (overall average, ~6 mmHg) and SBP (overall average, ~8 mmHg) compared with the chow group (Fig. 1, E and F). We did not observe significant changes in DBP between the groups. With respect to HR, there was an ~40 beats/min increase in the HFD group compared with the chow-fed controls (P < 0.05, Fig. 1H).
Fig. 1.
Obesity-induced changes in blood pressure parameters in C57BL/6J mice (n = 4 mice per group). A–D: line graphs depicting mean arterial pressure (MAP; mmHg), systolic blood pressure (SBP), diastolic blood pressure (DBP; mmHg), and heart rate (HR; beats/min); HFD, high-fat diet-fed mice. E–H: bar graphs showing the average values of the cardiovascular parameters over the entire observation period. *Significant difference (P < 0.05) from chow-fed mice.
Obesity-induced changes in SNA.
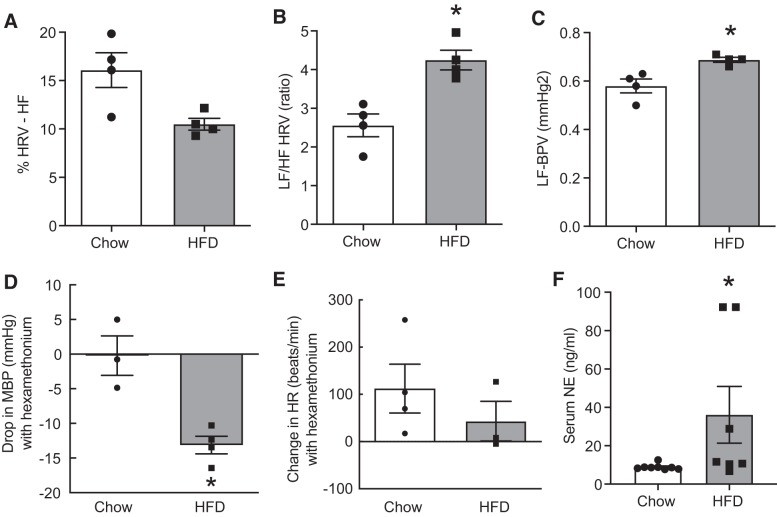
To assess SNA in conscious mice, we performed blood pressure and HRV analysis. Power spectral analysis of heart rate was used to characterize overall HRV as well as low-frequency (LF) and high-frequency (HF) components attributable to sympathetic and vagal influences, respectively, as described previously (4, 8). HRV LF-to-HF power ratio and the LF power of BPV increased significantly in HFD-fed mice compared with chow-fed mice, indicating marked elevations in cardiac and vascular sympathetic tone, respectively (Fig. 2, B and C). In addition, the normalized HRV HF power, an index of cardiac parasympathetic tone, was significantly decreased in HFD-fed animals compared with chow-fed animals indicating autonomic dysregulation in obesity (Fig. 2A). Acute depressor response (drop in MAP) to ganglionic blockade with hexamethonium has been validated as an indirect measure of whole body sympathetic activity. As shown in Fig. 2D, we have found that the magnitude of depressor response to ganglionic blockade was significantly greater in the HFD group compared with the chow group, indicating overall increases in SNA. In control animals, ganglionic blockade elicited only a small drop in MAP, which could be a result of injection-induced acute stress on hexamethonium response. We did not observe any differences in HR between the groups after ganglionic blockade (Fig. 2E), suggesting a balanced sympatho-vagal tone. We also assessed serum NE levels as an additional indicator of overall SNA. In line with other findings, we found that the HFD group had significantly higher serum NE levels compared with the control group (Fig. 2F).
Fig. 2.
Obesity-induced changes in sympathetic nerve activity. A: heart rate variability (HRV) high-frequency (HF) power, an indicator of cardiac parasympathetic tone. B: HRV low-frequency (LF)-to-HF power ratio, an index of cardiac sympathetic activity. C: blood pressure variability (BPV) LF power, an index of vascular sympathetic tone. D: depressor response of hexamethonium injection in chow- and high-fat diet (HFD)-fed mice (n = 4 mice per group). MBP, mean blood pressure. E: changes in heart rate (HR) in response to hexamethonium injection. F: serum norepinephrine (NE) levels measured using ELISA kit (n = 7–8 mice per group). Data are means ± SE (n = 4 mice per group). *Significant difference (P < 0.05) from chow-fed mice.
Obesity-associated oxidative stress is not associated with adaptive increases in Nrf2 activation in the RVLM.
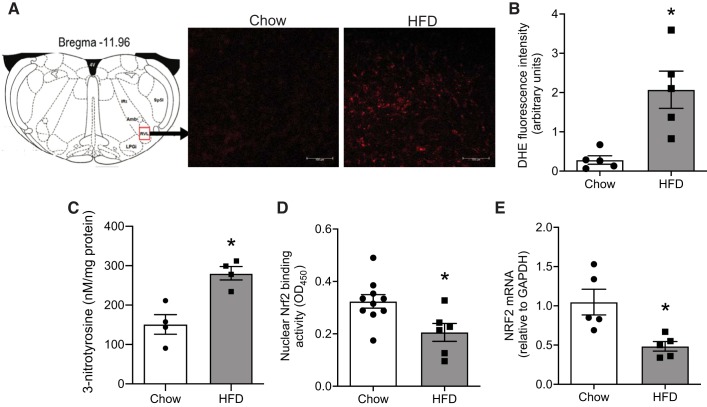
Oxidative stress was assessed by the DHE fluorometric method in fresh brain stem sections. Figure 3A depicts the representative images of stained sections showing stronger red fluorescence signals in the RVLM of HFD-fed animals compared with chow-fed controls. Quantitative analysis also showed higher fluorescence intensity in the RVLM of the HFD-fed animals indicating increased superoxide production in obesity (Fig. 3B). In addition to superoxide levels, we also assessed the levels of 3-NT in the RVLM, which is another well-known marker of oxidative stress formed by nitration of tyrosine residues in proteins by peroxynitrite. HFD-fed animals had higher 3-NT content in the RVLM compared with controls (Fig. 3C), further confirming that obesity induces oxidative stress in the RVLM.
Fig. 3.
Obesity-induced changes in the expression and activity of nuclear factor erythroid 2-related factor 2 (Nrf2) in the rostral ventrolateral medulla (RVLM). A: representative images of dihydroethidium staining of brain stem sections indicating increased red fluorescence (increased superoxide levels) in the RVLM of high-fat diet (HFD)-fed mice compared with chow-fed mice. Scale bars, 100 µm. Amb, nucleus ambiguus; IRt, intermediate reticular nucleus; LPGi, lateral paragigantocellular nucleus; Sp5I, spinal trigeminal nucleus, interpolar part; 4V, fourth ventricle. B: quantitative analysis of dihydroethidium (DHE) fluorescence intensity by ImageJ analysis. C: 3-Nitrotyrosine measurements in RVLM using ELISA. D: DNA-binding ability of Nrf2 assessed by TransAM Nrf2 assay. OD450, optical density at 450 nm. E: Nrf2 mRNA levels in the RVLM measured by real-time PCR analysis. Data are means ± SE (n = 4–6 mice per group). *Significant difference (P < 0.05) from chow-fed mice.
Under normal cellular conditions, oxidative stress activates the Nrf2 pathway, wherein Nrf2 translocates to the nucleus, binds to AREs, and induces transcription of antioxidant defense enzymes. Thus, to determine whether obesity-related oxidative stress is associated with Nrf2 activation, we tested the ability of Nrf2 to bind with ARE sequences, as this provides insights about Nrf2 translocation and subsequent transcriptional activity. Nuclear protein extracted from the brain stem of HFD- and chow-fed animals was used to assess Nrf2 DNA-binding activity using the TransAM Nrf2 assay. As shown in Fig. 3D, the nuclear Nrf2-binding activity was significantly decreased in HFD-fed animals. In agreement with Nrf2 activity, RT-PCR analysis also revealed significant downregulation of Nrf2 gene expression in the RVLM of HFD-fed animals compared with chow-fed controls (Fig. 3E).
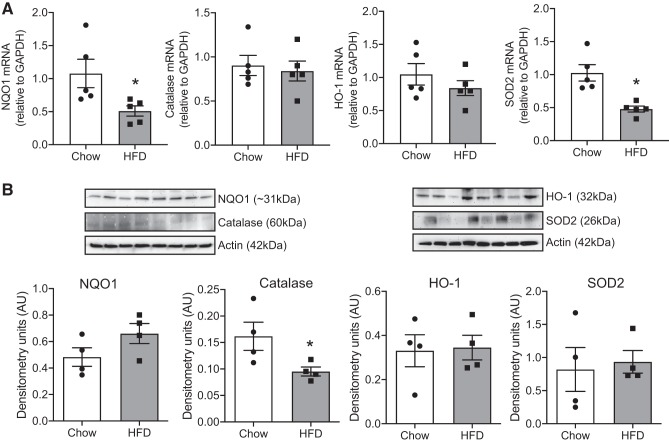
Next, we investigated the gene and protein expression of known Nrf2 targets in the RVLM of HFD-fed animals and chow-fed controls. The mRNA expression of NQO1 and SOD2 was significantly downregulated in the RVLM of HFD-fed animals (Fig. 4A). However, we did not observe any changes in protein levels of NQO1, HO-1, or SOD2 despite the presence of significant oxidative stress in the RVLM (Fig. 4B). The protein level of catalase was significantly downregulated in the RVLM of HFD-fed animals compared with chow-fed controls (Fig. 4B). Supplemental Figure S2 shows images of the whole Western blots for protein levels of Nrf2 targets that were cropped in Fig. 4 (see https://doi.org/10.6084/m9.figshare.10269503.v1).
Fig. 4.
Obesity-induced changes in the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) targets. A: changes in the mRNA levels of Nrf2 target genes, namely, NAD(P)H quinone dehydrogenase 1 (NQO1), catalase, heme oxygenase 1 (HO-1), and superoxide dismutase 2 (SOD2), in the rostral ventrolateral medulla (RVLM) of chow- and high-fat diet (HFD)-fed animals. B: Western blot analysis showing the protein levels of the same. Top: representative blots of the indicated proteins and their loading control (actin). Bottom: bar graphs of summary densitometric values after normalization to the loading control. AU, arbitrary units. Data are means ± SE (n = 4–6 mice per group). *Significant difference (P < 0.05) from chow-fed mice.
Obesity-induced Nrf2 dysfunction is associated with inflammation in the RVLM.
Oxidative stress has been shown to trigger inflammation through glial activation in the brain. We investigated whether obesity-induced oxidative stress resulted in a proinflammatory state in the RVLM during obesity. We analyzed the gene expression levels of proinflammatory cytokines and chemokines in the RVLM of HFD-fed animals and chow-fed controls. Our results showed that obesity led to significant increases in the mRNA expression of IL-1β and MCP1 and a trend toward increase in the levels of TNF-α (Fig. 5). Furthermore, we also analyzed the protein levels of various cytokines and chemokines in both protein extracts from RVLM micropunches and serum samples using Milliplex assay (Table 2). As observed at the mRNA level, the protein levels of MCP1 were also significantly higher in the RVLM after 16 wk of HFD treatment. In addition, the protein levels of proinflammatory chemokines and cytokines including keratinocyte chemoattractant [KC, or C-X-C motif chemokine 1 (CXCL1)], IL-17, IL-12p40, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were also higher in the RVLM of HFD-fed mice. Similarly, the circulating levels of MCP1 and KC were also higher in HFD-fed mice compared with controls (Table 2).
Fig. 5.
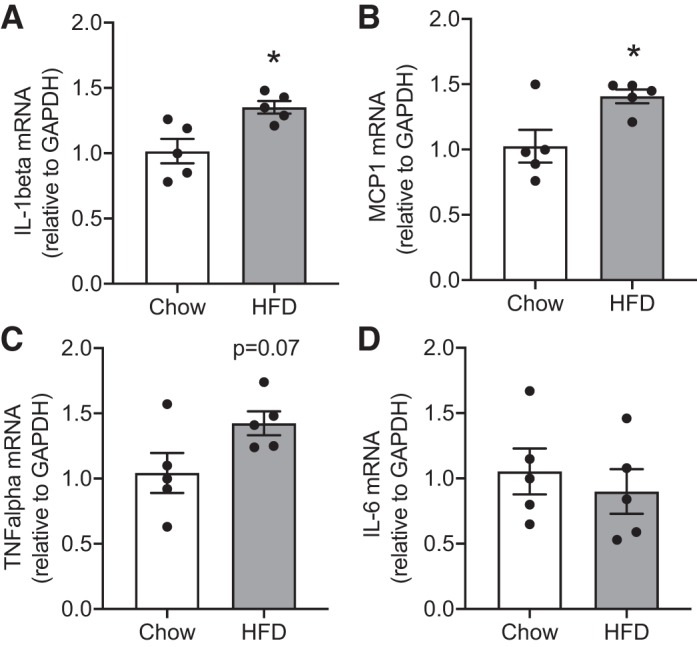
Obesity-induced changes in the expression of inflammation-related genes. A: IL-1β. B: monocyte chemotactic protein 1 (MCP1). C: TNF-α. D: IL-6. HFD, high-fat diet. Data are means ± SE (n = 4–6 mice per group). *Significant difference (P < 0.05) from chow-fed mice.
Table 2.
Cytokine and chemokine levels measured in the serum and RVLM micropunches using multiplex assay in C57BL/6J mice after 16 wks of chow or HFD
| Serum, pg/mL |
RVLM, pg/µg protein |
|||||
|---|---|---|---|---|---|---|
| Cytokine/Chemokine | Chow | HFD | P Value | Chow | HFD | P Value |
| Eotaxin | 1,355.4 ± 136.6 | 1,348.4 ± 118.5 | 0.969 | 0.037 ± 0.003 | 0.074 ± 0.016 | 0.06 |
| G-CSF | 388.3 ± 54.9 | 329.6 ± 40.5 | 0.391 | 0.028 ± 0.003 | 0.041 ± 0.003 | 0.06 |
| GM-CSF | 0.105 ± 0.03 | 0.221 ± 0.01 | 0.01* | |||
| IFN-γ | 0.114 ± 0.02 | 0.117 ± 0.01 | 0.88 | |||
| IL-1a | 262.17 ± 56.6 | 367.92 ± 75 | 0.297 | 2.18 ± 0.29 | 2.79 ± 0.08 | 0.15 |
| IL-1b | 0.104 ± 0.02 | 0.142 ± 0.01 | 0.21 | |||
| IL-2 | 5.78 ± 1.55 | 6.82 ± 0.35 | 0.54 | |||
| IL-6 | 5.38 ± 1.7 | 14.09 ± 2.55 | 0.01* | 0.04 ± 0.01 | 0.03 ± 0.006 | 0.5 |
| IL-7 | 0.03 ± 0.004 | 0.05 ± 0.003 | 0.11 | |||
| IL-9 | 628 ± 290.99 | 260.3 ± 56.8 | 0.21 | 25.67 ± 9.6 | 23.2 ± 2.9 | 0.81 |
| IL-10 | 1.03 ± 0.20 | 1.35 ± 0.11 | 0.21 | |||
| IL-12p40 | 8.05 ± 2.64 | 12.99 ± 2.76 | 0.21 | 0.16 ± 0.01 | 0.21 ± 0.01 | 0.03* |
| IL-12p70 | 0.161 ± 0.02 | 0.25 ± 0.05 | 0.15 | |||
| IL-13 | 81.52 ± 13.8 | 75.62 ± 21.6 | 0.81 | 1.06 ± 0.07 | 1.20 ± 0.03 | 0.11 |
| IL-15 | 33.78 ± 15.4 | 50.01 ± 15.5 | 0.49 | 0.6 ± 0.08 | 0.86 ± 0.10 | 0.1 |
| IL-17 | 4.07 ± 1.31 | 7.25 ± 1.84 | 0.18 | 0.08 ± 0.01 | 0.15 ± 0.003 | 0.006* |
| IP-10 | 312.98 ± 18.5 | 471.32 ± 56.25 | 0.02* | 0.19 ± 0.03 | 0.24 ± 0.03 | 0.36 |
| KC | 369.4 ± 63.3 | 870.6 ± 131.8 | 0.005* | 0.14 ± 0.02 | 0.25 ± 0.01 | 0.004* |
| LIX | 9,718 ± 2,159 | 8,959 ± 3,036 | 0.84 | 3.51 ± 0.55 | 3.88 ± 0.15 | 0.53 |
| MCP1 | 25.12 ± 4.98 | 94.52 ± 18.94 | 0.005* | 0.19 ± 0.01 | 0.31 ± 0.03 | 0.01* |
| MCSF1 | 2.88 ± 1.53 | 20.38 ± 8.43 | 0.08 | 0.211 ± 0.03 | 0.24 ± 0.02 | 0.32 |
| MIG | 819.27 ± 129.2 | 1,320.26 ± 143.7 | 0.02* | 0.16 ± 0.04 | 0.17 ± 0.004 | 0.7 |
| MIP1a | 124.33 ± 28.8 | 100.43 ± 9.3 | 0.43 | 0.68 ± 0.15 | 0.61 ± 0.01 | 0.66 |
| MIP1b | 95.94 ± 15.94 | 94.57 ± 22.92 | 0.96 | 0.49 ± 0.01 | 0.52 ± 0.02 | 0.17 |
| MIP2 | 194.45 ± 44.43 | 275.05 ± 68.08 | 0.37 | 1.17 ± 0.06 | 1.29 ± 0.13 | 0.39 |
| RANTES | 55.88 ± 9.2 | 58.32 ± 6.4 | 0.82 | 0.18 ± 0.02 | 0.21 ± 0.02 | 0.36 |
| TNF-α | 0.04 ± 0.006 | 0.05 ± 0.008 | 0.32 | |||
| VEGF | 6.42 ± 1.5 | 4.6 ± 1.5 | 0.4 | 0.03 ± 0.005 | 0.04 ± 0.004 | 0.21 |
G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HFD, high-fat diet; IP-10, 10-kDa interferon-γ-induced protein; KC, keratinocyte chemoattractant; LIX, C-X-C motif chemokine 5; MCP1, monocyte chemotactic protein 1; MCSF1, macrophage colony-stimulating factor 1; MIG, monokine induced by interferon-γ; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normally T-expressed, and presumably secreted; RVLM, rostral ventrolateral medulla.
Significant change (P < 0.05).
DISCUSSION
Oxidative stress results from the imbalance between the production of free radicals and the ability of the antioxidant defense system to counteract the free radicals. Although low concentrations of reactive oxygen species (ROS) are beneficial for human health, excessive accumulation of ROS has been shown to induce oxidative modifications of lipids, DNA, and protein, all of which can affect the structural and functional characteristics of cells. The brain is uniquely vulnerable to oxidative stress because of its innate properties such as high energetic demands, lipid-rich content, modest antioxidant defense, and poor regenerative capability (29). The role of central ROS in the pathogenesis of cardiovascular diseases such as heart failure and hypertension is well documented (10, 17, 21, 36). However, there are very limited studies on the role of oxidative stress in obesity-induced hypertension. Even less is known about the molecular mechanisms underlying obesity-induced oxidative stress in the brain. In the present study, we present evidence that HFD-induced obesity increases mean arterial pressure and sympathetic nerve activity. In addition, we also demonstrate that obesity increases oxidative stress, which was associated with a homeostatic failure due to dysregulation of Nrf2 signaling-mediated antioxidant responses in the RVLM.
Results from the present study are in line with previously published studies where obesity resulted in mild increases in arterial pressure in C57BL/6J mice (4, 32, 40). However, obese C57BL/6J mice did develop a significant increase in cardiac and vascular sympathetic tone as shown by the power spectral analysis in Fig. 2. Furthermore, the depressor response to ganglionic blockade was also greater in obese mice indicating an overall increase in sympathetic output with obesity. Sympathetic neurons in the RVLM play a critical role in obesity-induced sympathoexcitation and hypertension (34). In particular, RVLM neurons are very susceptible to oxidative stress. Central administration of tempol (a superoxide dismutase mimetic) or an NADPH oxidase inhibitor was shown to reverse obesity-induced increase in arterial pressure and renal SNA, indicating that oxidative stress mediates the adverse effects of obesity on SNA and BP (26). In addition to obesity-induced hypertension, increased ROS in the RVLM has also been attributed to sympathoexcitation in several other animal models of hypertension (high salt intake, spontaneously hypertensive rats, and jet lag induced; 18, 19, 21) and heart failure (10). The potential source of ROS in the RVLM during obesity is not clear. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase seems to be a likely mechanism as its involvement in ANG II type 1 receptor signaling-mediated ROS production in the RVLM has been well established in hypertension and heart failure (10, 14).
Nrf2, a Cap’n’Collar basic leucine zipper transcription factor, is a master regulator of antioxidant and anti-inflammatory pathways and confers cytoprotection under stress conditions in a variety of tissues including the brain (31). The first evidence for the critical role of Nrf2 signaling in the RVLM in sympathoregulation came from the Zucker laboratory, where they demonstrated that deletion of Nrf2 gene specifically in the RVLM led to sustained hypertension, baroreflex dysfunction, and increases in renal SNA (11). On the other hand, upregulation of Nrf2 in the RVLM by gene transfer or Keap1 deletion reduced sympathetic nerve activity and improved arterial baroreflex function in coronary artery ligation-induced chronic heart failure in mice (23). Our results extend these previous studies and demonstrate that obesity-induced sympathoexcitation is associated with impaired Nrf2 signaling in the RVLM. We observed significant decreases in Nrf2 mRNA and activity in the RVLM with obesity. Despite the presence of significant oxidative stress, we did not observe any increase in the mRNA or protein levels of Nrf2 targets such as NQO1, SOD2, HO-1, and catalase indicating maladaptation to oxidative stress due to dysfunctional Nrf2 signaling in obesity. One limitation of the present study is that we did not investigate the cellular type prone to Nrf2 dysfunction and oxidative stress in the RVLM. In a recent paper by Ma et al. (23), Nrf2 overexpression specifically in glutamatergic neurons reduced SNA in chronic heart failure, suggesting the possibility that neuronal Nrf2 dysfunction plays a role in obesity-induced sympathoexcitation. However, the role of glial Nrf2 signaling should not be overlooked as the same paper also showed that global Nrf2 overexpression in the RVLM (including both neuronal and nonneuronal cells) also elicited a similar beneficial response in heart failure. Future studies comparing the effects of neuronal versus glial Nrf2 signaling in the RVLM are needed to delineate cell type-specific effects mediating obesity-induced sympathoexcitation.
The causes of obesity-induced Nrf2 dysfunction in the RVLM are likely multifaceted. First, it is possible that obesity may impair pathways that regulate activation and nuclear translocation of Nrf2 protein. In fact, we observed decreased DNA binding of Nrf2 in nuclear extracts from the RVLM of obese mice suggesting impaired nuclear translocation in the RVLM. Second, obesity may decrease the protein stability of Nrf2 through Keap1-mediated mechanisms as studies have demonstrated increased levels of Keap1 in the adipose tissue with chronic high-fat diet feeding (41). It would be interesting to know whether Keap1 levels also change in the RVLM with obesity. Finally, the adverse effects of obesity on Nrf2 could be a secondary outcome of underlying diabetes as our animals were diabetic on HFD (data not shown). Previous studies show that diabetes accelerates the 26S proteosomal degradation rate of Nrf2 (3), thus decreasing the availability of cytoplasmic Nrf2 for activation.
One of the consequences of Nrf2 dysregulation is an inflammatory response due to the well-characterized interplay between Nrf2 and NF-κB pathways (39). Nrf2 dysfunction can exacerbate NF-κB activation leading to increased cytokine and chemokine production evident from studies of Nrf2 knockout mice, which exhibited an exaggerated NF-κB-dependent inflammatory response following injury (28). This is also true in our present study, where impaired Nrf2 signaling was accompanied with a proinflammatory milieu marked by increased protein levels of MCP1, KC, and IL-17. However, the relationship between Nrf2 dysfunction and inflammation in the RVLM in our study is only correlative. Future studies involving Nrf2 manipulation by gene overexpression and pharmacological methods using Nrf2 activators are needed to directly address the causal relationship between them. In addition to inflammation, chronic oxidative stress elicited by Nrf2 dysfunction can cause significant DNA damage, which is a potential stimulus for induction of senescence in cells. In fact, our laboratory has recently reported evidence for accumulation of senescent cells in the RVLM with obesity (35). Senescent cells are known to contribute to neuroinflammation by acquisition of a senescence-associated secretory phenotype, where they secrete cytokines and chemokines (5). Furthermore, senescent cells are resistant to apoptosis and hence can sustain inflammation in the RVLM for prolonged periods of time. This can lead to chronic increases in SNA and subsequently increase the risk for cardiovascular diseases in individuals with obesity. In summary, we show that obesity leads to sympathoexcitation and mild increases in arterial pressure. We also provide evidence that Nrf2 dysfunction, marked by significant decreases in mRNA levels and transcriptional activity, promotes oxidative stress and inflammation in the RVLM in obesity.
Perspectives and Significance
Sympathoexcitation plays a major role in increased risk for cardiovascular diseases in obesity, and it is of utmost importance to identify potential therapeutic targets to attenuate sympathetic nerve activity in individuals with obesity. The study reported in this paper highlights a potential role for impaired Nrf2 signaling and the resultant maladaptation to oxidative stress in the RVLM in obesity-induced sympathoexcitation. Although the present findings present correlative evidence, future studies are needed to decipher the mechanistic role of Nrf2 in obesity-induced sympathoexcitation. Nevertheless, the availability of pharmacological agents to activate Nrf2 makes it a potential candidate target for the prevention or treatment of hypertension in individuals with obesity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R15-HL-148844 to M. Subramanian and Research Advisory Committee funds from the College of Veterinary Medicine, Oklahoma State University, to M. Subramanian.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B. and M.S. conceived and designed research; P.B., N.A.-J., R.M., M.K.S., D.H., and M.S. performed experiments; P.B., N.A.-J., and M.S. analyzed data; P.B. and M.S. interpreted results of experiments; P.B. and M.S. prepared figures; P.B. and M.S. drafted manuscript; P.B. and M.S. edited and revised manuscript; P.B., N.A.-J., R.M., M.K.S., D.H., and M.S. approved final version of manuscript.
REFERENCES
- 1.Asirvatham-Jeyaraj N, Fiege JK, Han R, Foss J, Banek CT, Burbach BJ, Razzoli M, Bartolomucci A, Shimizu Y, Panoskaltsis-Mortari A, Osborn JW. Renal denervation normalizes arterial pressure with no effect on glucose metabolism or renal inflammation in obese hypertensive mice. Hypertension 68: 929–936, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell BB, Rahmouni K. Leptin as a mediator of obesity-induced hypertension. Curr Obes Rep 5: 397–404, 2016. doi: 10.1007/s13679-016-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitar MS, Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab 301: E1119–E1129, 2011. doi: 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]
- 4.Chaar LJ, Coelho A, Silva NM, Festuccia WL, Antunes VR. High-fat diet-induced hypertension and autonomic imbalance are associated with an upregulation of CART in the dorsomedial hypothalamus of mice. Physiol Rep 4: e12811, 2016. doi: 10.14814/phy2.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol 68: 3–7, 2015. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88: 179–188, 2015. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, de Lara Rodriguez CE, Hall JE. Obesity-induced hypertension: brain signaling pathways. Curr Hypertens Rep 18: 58, 2016. doi: 10.1007/s11906-016-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdos B, Backes I, McCowan ML, Hayward LF, Scheuer DA. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats. Am J Physiol Heart Circ Physiol 308: H612–H622, 2015. doi: 10.1152/ajpheart.00776.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 11.Gao LZ, Zimmerman MC, Biswal S, Zucker IH. Selective Nrf2 gene deletion in the rostral ventrolateral medulla evokes hypertension and sympatho-excitation in mice. Hypertension 69: 1198–1206, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000. doi: 10.1161/01.HYP.36.4.538. [DOI] [PubMed] [Google Scholar]
- 13.Indumathy J, Pal GK, Pal P, Ananthanarayanan PH, Parija SC, Balachander J, Dutta TK. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity. Metabolism 64: 1704–1714, 2015. doi: 10.1016/j.metabol.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8: 1583–1596, 2006. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 15.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 17.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004. doi: 10.1161/01.CIR.0000128695.49900.12. [DOI] [PubMed] [Google Scholar]
- 18.Kishi T, Hirooka Y, Ogawa K, Konno S, Sunagawa K. Calorie restriction inhibits sympathetic nerve activity via anti-oxidant effect in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Clin Exp Hypertens 33: 240–245, 2011. doi: 10.3109/10641963.2011.583969. [DOI] [PubMed] [Google Scholar]
- 19.Kishi T, Sunagawa K. Experimental “jet lag” causes sympathoexcitation via oxidative stress through AT1 receptor in the brainstem. Conf Proc IEEE Eng Med Biol Soc 2011: 1969–1972, 2011. doi: 10.1109/IEMBS.2011.6090555. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24: 7130–7139, 2004. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koga Y, Hirooka Y, Araki S, Nozoe M, Kishi T, Sunagawa K. High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens Res 31: 2075–2083, 2008. doi: 10.1291/hypres.31.2075. [DOI] [PubMed] [Google Scholar]
- 22.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50: 862–868, 2007. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 23.Ma A, Hong J, Shanks J, Rudebush T, Yu L, Hackfort BT, Wang H, Zucker IH, Gao L. Upregulating Nrf2 in the RVLM ameliorates sympatho-excitation in mice with chronic heart failure. Free Radic Biol Med 141: 84–92, 2019. doi: 10.1016/j.freeradbiomed.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278: 21592–21600, 2003. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 26.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm 2012: 217580, 2012. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37: 768–778, 2016. doi: 10.1016/j.tips.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am J Med 75: 249–258, 1983. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79: 298–306, 2014. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sipe LM, Yang C, Ephrem J, Garren E, Hirsh J, Deppmann CD. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr Diabetes 7: e260, 2017. doi: 10.1038/nutd.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol 590: 1787–1801, 2012. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian M, Csiszar A, Ungvari Z, Balasubramanian P. Cellular senescence in the rostral ventrolateral medulla (RVLM): novel implications for obesity-induced sympathoexcitation (Abstract). FASEB J 33, Suppl 1, 563.3, 2019. [Google Scholar]
- 36.Tai MH, Wang LL, Wu KL, Chan JY. Increased superoxide anion in rostral ventrolateral medulla contributes to hypertension in spontaneously hypertensive rats via interactions with nitric oxide. Free Radic Biol Med 38: 450–462, 2005. doi: 10.1016/j.freeradbiomed.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Van Hemelrijck M, Ulmer H, Nagel G, Peter RS, Fritz J, Myte R, van Guelpen B, Föger B, Concin H, Häggström C, Stattin P, Stocks T. Longitudinal study of body mass index, dyslipidemia, hyperglycemia, and hypertension in 60,000 men and women in Sweden and Austria. PLoS One 13: e0197830, 2018. doi: 10.1371/journal.pone.0197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997. doi: 10.1161/01.CIR.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 39.Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43: 621–626, 2015. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30: 769–778, 2003. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, Fantus IG, Jin T. Oltipraz upregulates the nuclear factor 2 alpha subunit (NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54: 922–934, 2011. [Erratum in Diabetologia 54: 989, 2011.] doi: 10.1007/s00125-010-2001-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23: 8137–8151, 2003. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]