Abstract
Genes for the epithelial sodium channel (ENaC) subunits are expressed in a circadian manner, but whether this results in time-of-day differences in activity is not known. Recent data show that protein expression of ENaC subunits is higher in kidneys from female rats, yet females are more efficient in excreting an acute salt load. Thus, our in vivo study determined whether there is a time-of-day difference as well as a sex difference in the response to ENaC inhibition by benzamil. Our results showed that the natriuretic and diuretic responses to a single dose of benzamil were significantly greater in male compared with female rats whether given at the beginning of the inactive period [Zeitgeber time 0 (ZT0), 7 AM] or active period (ZT12, 7 PM). However, the response to benzamil was not significantly different between ZT0 and ZT12 dosing in either male or female rats. There was no difference in renal cortical α-ENaC protein abundance between ZT0 and ZT12 or males and females. Given previous reports of flow-induced stimulation of endothelin-1 (ET-1) production and sex differences in the renal endothelin system, we measured urinary ET-1 excretion to assess the effects of increased urine flow on intrarenal ET-1. ET-1 excretion was significantly increased following benzamil administration in both sexes, but this increase was significantly greater in females. These results support the hypothesis that ENaC activity is less prominent in maintaining Na+ balance in females independent of renal ET-1. Because ENaC subunit genes and protein expression vary by time of day and are greater in female rat kidneys, this suggests a clear disconnect between ENaC expression and channel activity.
Keywords: circadian, diuresis, epithelial sodium channel, natriuresis, sex differences
INTRODUCTION
The epithelial sodium channel (ENaC) localizes to the aldosterone sensitive distal nephron (35). It constitutes the final step in sodium (Na+) reabsorption and is an important contributor to the maintenance of Na+ homeostasis, blood volume, and blood pressure regulation (5). ENaC is a hetero-multimeric molecule with three homologous α-, β-, and γ-subunits that is localized primarily to principal cells of the connecting tubule (CNT) and throughout the collecting duct (cortical collecting duct, outer medullary collecting duct, and inner medullary collecting duct) (12, 26, 53). The cytoplasmic domain of these cells is rich in vesicles containing ENaC subunits that probably play a role in channel trafficking and, therefore, in the regulation of its activity (12). Activation of ENaC occurs through the proteolytic cleavage of α- and γ-subunits. The α-subunit was the first functionally identified in Xenopus laevis oocytes and was capable of expressing channel activity independently in vitro. However, maximal expression and function of ENaC requires the assembly of the three subunits (5).
ENaC is regulated at many different levels, including the transcriptional and posttranslational levels as well as by hormonal and paracrine factors modulating channel activity (13). For example, ENaC activity is reduced by high luminal Na+ levels and increased endothelin-1 (ET-1), which functions through the endothelin receptor-B (ETB) and generation of nitric oxide (NO) and cGMP (3). Overall, ENaC regulation is complex and includes a number of other factors such as vasopressin, purinergic receptors, and prostaglandins. However, the interaction of these systems and potential sex differences are poorly understood (35).
The circadian clock controls rhythmic oscillation of a large number of genes in the body. It has been shown recently that ENaC expression follows a diurnal rhythm and is under the control of clock genes. Gumz et al. (11) showed that period 1 (Per1), which is one of the core circadian clock genes, regulates ENaC mRNA expression and that knockout of Per1 in mice leads to a significant reduction in the mRNA expression of the α-subunit of the multimeric channel. Our group has also shown rhythmic oscillation in α-ENaC mRNA expression in the inner medulla of rat kidneys (48). One would expect these differences in ENaC expression to translate to functional differences, but this has not been adequately tested.
In addition to time-of-day differences in ENaC expression (23), sex differences in channel expression both at mRNA and at protein level have also been reported. Female rats were found to have higher levels of cleaved α- and γ-subunits of ENaC in their distal nephron (52). Although we know that circadian clock genes control ENaC expression and diurnal patterns in Na+ excretion (UNaV), we do not know whether there are time-of-day differences or sex differences in ENaC function. Therefore, the aim of this study was to determine whether the natriuretic and diuretic responses to ENaC inhibition are different between sexes and whether they follow a diurnal rhythm as well.
METHODS
Experimental protocol.
All protocols and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals. Both male and female Sprague-Dawley rats (12–16 wk old) used for these experiments were obtained from Envigo (Harlan Laboratories, Indianapolis, IN) and housed conventionally (2 rats/cage) in temperature and humidity-controlled conditions under a 12:12-h light-dark cycle with ad libitum access to water and regular chow. The experimental protocol began with rats placed in metabolic cages and given free access to water and normal salt diet (Teklad custom diet TD.96208, 0.49% NaCl; Envigo, Indianapolis, IN). Animals were allowed to acclimatize to the new cages for 2 days, and afterwards, baseline data were collected, where 12-h urine collections were obtained to measure baseline urine flow rate (UV) and Na+ (UNaV) and K+ excretion as well as water and food intake. On the third day after baseline collections, benzamil (Sigma-Aldrich, St. Louis, MO) was given at a dose of 1 mg/kg (ip) (2, 45, 49) either at the beginning of their inactive period/lights on [Zeitgeber time 0 (ZT0), 7 AM] or their active period/lights off (ZT12, 7 PM). At low doses, benzamil selectively blocks luminal ENaC in the aldosterone-sensitive collecting duct (1, 2). After dosing, urine was collected at 2, 6, and 12 h. Rats were then returned to their regular cages to allow them to recover for 5–7 days, after which the protocol was repeated with reversal of benzamil injection time.
Ovariectomy protocol.
Female Sprague-Dawley rats (11–12 wk old) were divided into ovariectomy (OVx) and sham (control) groups. Female rats in the OVx group were subjected to bilateral OVx through bilateral dorsal incisions, and the ovaries were located, ligated, and then removed. The muscle layer was sutured back by simple continuous sutures, whereas the overlying skin was closed by wound clips (10). The same procedure was performed on the sham group; however, the ovaries were simply exposed with no ligation or removal. Isoflurane (2%) was used for anesthesia. Rats were given pain control medication on the surgical table [buprenorphine, 0.05 mg/kg, subcutaneous injection and topical application of bupivacaine HCl (0.5%) on the closed surgical wound]. Rats were allowed to recover for 3 wk to ensure complete withdrawal of ovarian hormones before metabolic cage experiments were repeated, as previously mentioned.
Western blotting.
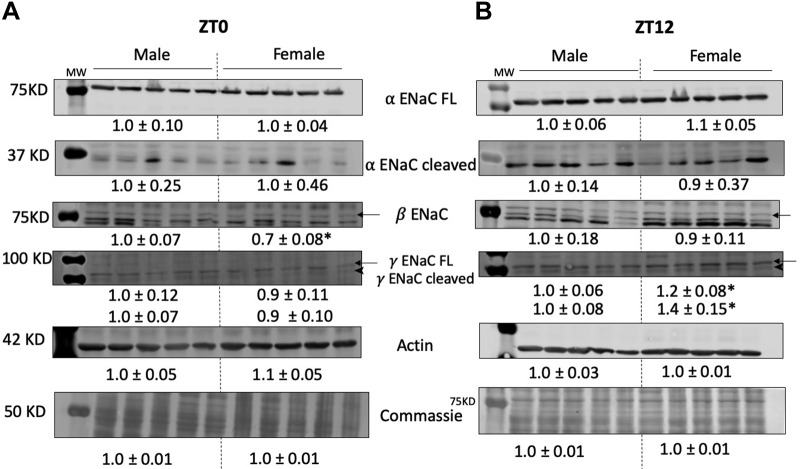
Renal cortical tissues collected from male and female SD rats at either ZT0 or ZT12 were dissected on ice and homogenized in hypotonic sucrose buffer (250 mmol/L sucrose, 30 mmol/L Tris, and 1 mmol/L EDTA at pH 7.5) and protease inhibitors (10 μmol/L leupeptin, 2 μmol/L pepstatin A, 1 mmol/L phenylmethylsulfonyl fluoride). Homogenates were centrifuged at 5,000 g for 5 min at 4°C. The pellet was discarded and the supernatant used to measure total protein concentration with Bradford assay (Quick Start, Bio-Rad, Hercules, CA). Twenty micrograms of protein were loaded and separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (18). Each membrane was probed with the antibody for a single ENaC subunit (Table 1) and incubated overnight with shaking at 4°C. Secondary antibody was added at dilutions shown in Table 1. The antibody against β-ENaC was custom produced by ProSci (Poway, CA) using the rat amino acid sequence 617-638 C-TERM (NH2-CNYDSLRLQPLDTMESDSEVEAI-COOH). Both α- and γ-ENaC antibodies were purchased from StressMarq Biosciences. All membranes were visualized with the LI-COR Infrared Odyssey Imaging System (Lincoln, NE). Signal intensity units were measured using LI-COR image studio software, and densitometry units were normalized to the male group set as the control at either ZT0 or ZT12. Membranes were probed for actin and then stained with Coomassie blue and quantified to ensure equal loading. Blots for ENaC subunits in addition to representative blots of actin and Coomassie are shown in Fig. 4.
Table 1.
Antibodies used in immunoblotting
| Antibody Target | Primary Ab Supplier | Ab Host | Dilution | Secondary Ab Supplier | Ab Host | Dilution |
|---|---|---|---|---|---|---|
| α-ENaC | StressMarq (SPC-403D) | Rabbit | 1:1,000 | Invitrogen (SA5-10036) |
Goat | 1:1,000 |
| β-ENaC | ProSci (Poway, CA) (21, 34) | Rabbit | 1:2,000 | Invitrogen (SA5-10036) |
Goat | 1:1,000 |
| γ-ENaC | StressMarq (SPC-405) | Rabbit | 1:1,000 | Invitrogen (SA5-10036) |
Goat | 1:1,000 |
| Actin | Sigma (A1978) | Mouse | 1:20,000 | Invitrogen (A21057) |
Goat | 1:10,000 |
ENaC, epithelial sodium channel.
Fig. 4.
Immunoblots of kidney cortical tissue homogenates demonstrating abundance of α-, β-, and γ-ENaC (epithelial sodium channel) subunits in both male and female Sprague-Dawley rats. Tissues collected at either Zeitgeber time (ZT) 0 or ZT12. Representative blots of actin and Coomassie are demonstrated. Densitometry signals were normalized to male rats (set to 1.0) and expressed as means ± SE. *P < 0.05 vs. male group, unpaired t-test; n = 5/group.
Analytical and statistical methods.
Na+ and K+ concentrations were measured by ion-selective electrodes (EasyLyte, Medica, Bedford, MA). Urine ET-1 concentrations were measured by immunoassay (QuantiGlo; R & D Systems, Minneapolis, MN). The manufacturer’s manual states that the assay uses a monoclonal antibody specific for ET-1 that shows 50% cross-reactivity to ET-2 isoform. However, because data published show that ET-2 is not detected in the kidney (25), we expect our data to reflect only ET-1 excretion rates. Osmolality was determined with a vapor pressure osmometer (VAPRO 5600; ELITechGroup, Logan, UT).
Data are expressed as means ± SE, with statistical significance assigned as P < 0.05. Statistical analysis for comparison of the two sexes and time-of-day effect was done mainly by repeated-measures two-way ANOVA, followed by Sidak’s multiple-comparisons test. Specific statistical tests are described in the figure captions for each data set.
RESULTS
Natriuretic response to ENaC blockade is independent of time.
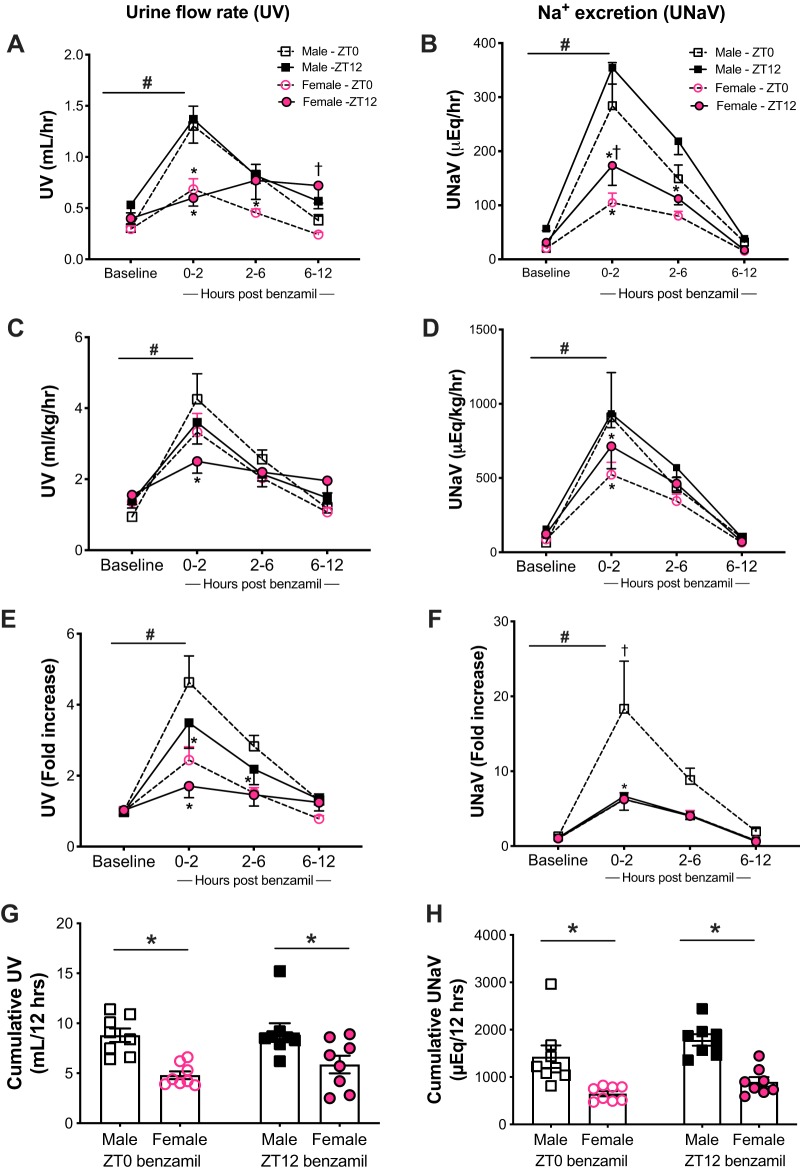
Male and female rats were each divided into two groups; one group was given benzamil (1 mg/kg), and the second was administered an equal volume of vehicle (0.9% NaCl, 1 ml/kg) to confirm that changes in UV and UNaV were due to the diuretic alone. Metabolic cage parameters were recorded during both pre- (baseline) as well as post-benzamil ip injection (Table 1). Figure 1 depicts observed changes in UV and UNaV in response to benzamil administration at either ZT0 or ZT12 in both male and female rats. Male rats given benzamil at the beginning of their inactive period (ZT0) had a significant increase in UV (from 0.3 ± 0.03 to 1.4 ± 0.2 mL/h) and UNaV (from 20.4 ± 2.2 to 284.2 ± 79.8 μEq/h), which peaked after 2 h of administration. There was a similar pattern of diuresis rising from 0.5 ± 0.1 to 1.4 ± 0.2 mL/h and natriuresis from 56.9 ± 3.9 to 354.4 ± 30.0 μEq/h when the drug was administered at ZT12 with the peak of response after 2 h of benzamil injection. However, there was no statistically significant difference in UV and UNaV when the drug was administered either at the beginning of the active or inactive periods for all three urine collection time points (P > 0.05; Fig. 1, A and B). Female Sprague-Dawley rats showed a significant increase in UV after benzamil administration from 0.3 ± 0.03 at baseline to 0.7 ± 0.10 mL/h after 2 h and a corresponding increase in UNaV from 20.5 ± 2.7 to 104.9 ± 17.6 μEq/h at ZT0. There was no difference in diuretic response to benzamil given at ZT0 and ZT12 (Fig. 1A), except for hours 6–12 postbenzamil. On the other hand, UNaV was higher at ZT12 compared with ZT0 for the 2-h collection time point (Fig. 1B).
Fig. 1.
Urine flow rate (UV) and urinary Na+ excretion (UNaV) in male and female Sprague-Dawley rats at both pre- (baseline) and post-benzamil administration at either Zeitgeber time 0 (ZT0) or ZT12 over the course of 12 h, collected at 2-, 6-, and 12-h intervals (A–F) and the 12-h cumulative UV and UNaV excretion rates (G and H). *P < 0.05 vs. males at the corresponding time point, †P < 0.05 vs. ZT0 within the same sex, and #P < 0.05 vs. baseline ± SE, repeated-measures 2-way ANOVA with Sidak’s multiple-comparisons test; n = 10–12. A, B, C, and E: ZT0 benzamil: Ptime < 0.05, Psex < 0.05, and Ptime × sex < 0.05. ZT12 benzamil: Ptime < 0.05, Psex < 0.05 and Ptime × sex < 0.05. D: ZT0: benzamil Ptime < 0.05, Psex > 0.05 and Ptime × sex > 0.05. ZT12: PTime < 0.05, PSex < 0.05 and Ptime × sex > 0.05. F: ZT0 benzamil: Ptime < 0.05, Psex < 0.05 and Ptime × sex < 0.05. ZT12 Ptime < 0.05, Psex > 0.05, and Ptime × sex > 0.05. C: females: PZT < 0.05, Ptime < 0.05, and PZT × time > 0.05. F: males: PZT < 0.05, Ptime < 0.05, and PZT × time > 0.05.
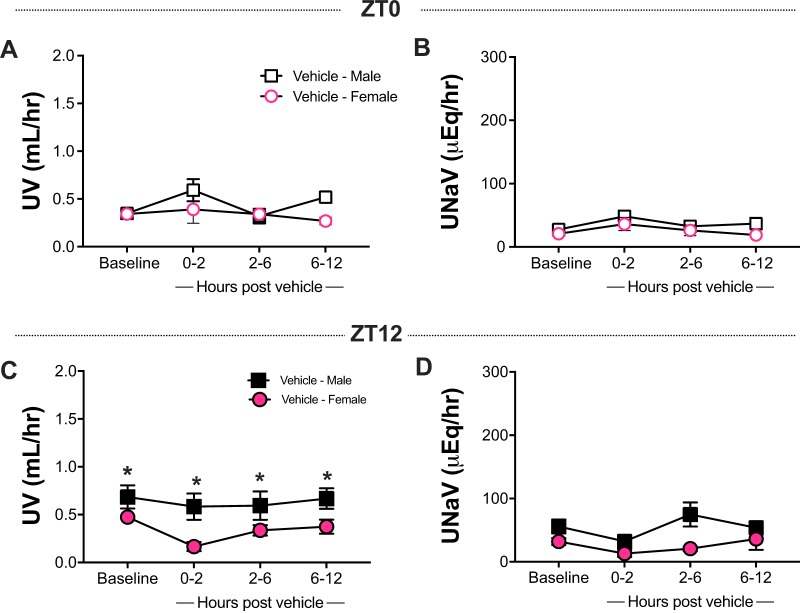
Both male and female rats subjected to vehicle injection at ZT0 showed no significant increase in UV or UNaV excretion compared with the corresponding inactive period baseline (Fig. 2, A and B). The diuretic and natriuretic responses observed at ZT12 were similar, as there was no increase in UV or UNaV after vehicle administration (Fig. 2, C and D). Baseline active period UV in male rats was higher compared with the females (Fig. 2, C and D), which correlates with higher water intake in the males during the same time frame (Table 2).
Fig. 2.
Effect of vehicle administration on urine flow rate (UV) and urinary Na+ excretion (UNaV) as compared with baseline at both Zeitgeber time 0 (ZT0; A and B) and at ZT12 (C and D) in male and female Sprague-Dawley rats. Data shown as means ± SE, repeated-measures 2-way ANOVA with Sidak’s multiple comparison test; n = 4–6.
Table 2.
Diurnal parameters from metabolic cage studies in Sprague-Dawley rats
| Males | Females | |
|---|---|---|
| Body weight, g (n = 8) | 355.5 ± 10.9 | 225.4 ± 3.3* |
| Food intake, g/day (n = 8) | ||
| Inactive period | 1.5 ± 0.3 | 2.5 ± 0.4 |
| Active period | 14.7 ± 0.4 | 9.4 ± 0.8* |
| Water intake, mL/day (n = 8) | ||
| Inactive period | 4.3 ± 0.7 | 4.2 ± 0.6 |
| Active period | 21.7 ± 1.1 | 16.9 ± 1.4* |
| Urine osmolality, mosmol/kg H2O (n = 8) | ||
| Inactive period | 1,446 ± 140 | 1,205 ± 113 |
| Active period | 1,536 ± 119 | 1,099 ± 141 |
| UKV inactive | ||
| Baseline, µEq/h (n = 8) | 60 ± 4 | 57 ± 6 |
| 0–2 h post-ZT0 injection | 50 ± 8 | 71 ± 29 |
| 2–6 h | 22 ± 3 | 32 ± 11 |
| 6–12 h | 43 ± 6 | 43 ± 5 |
| UKV active | ||
| Baseline, µEq/h (n = 8) | 144 ± 16 | 93 ± 8* |
| 0–2 h post-ZT12 injection | 62 ± 16 | 25 ± 8 |
| 2–6 h | 32 ± 2 | 32 ± 10 |
| 6–12 h | 73 ± 16 | 63 ± 14 |
Data presented as means ± SE. UKV, urinary K+ excretion; ZT0 and ZT12, Zeitgeber time 0 and 12, respectively.
P < 0.05 vs. corresponding time point in males, 2-way ANOVA.
Food and water intake followed a diurnal rhythm in both male and female rats, with intake peaking during the dark (active) period (Table 2). However, urine osmolality showed no diurnal or sex difference. There was no difference in urinary K+ excretion (UKV) between sexes during the baseline lights-on (inactive) time period. However, females had lower baseline K+ excretion during the active period (lights-off) compared with males. The sex difference in UKV was abolished after benzamil administration.
Effect of sex on response to benzamil.
Apart from active period baseline UV being higher in males, the remainder of the UV and UNaV values were not significantly different between male and female rats. Animals were also housed in the same space in the animal house to account for environmental factors such as temperature and humidity, so we expect that dissimilarities in the response are not due to different baseline excretion rates across sexes or environmental conditions but are most likely attributed to ENaC activity and benzamil blockade efficiency. Male rats had higher UV at the 2- and 6-h marks compared with females when benzamil was given at ZT0 (Fig. 1A). This difference was abolished when excretion rates were normalized to body weights (Fig. 1C) but maintained when data were normalized to baseline excretion rates for both males and females (Fig. 1E). Female rats had blunted UNaV rates when compared with male rats at ZT0, particularly during the peak response to benzamil (Fig. 1, B, D, and F). In addition, Fig. 1 demonstrates male and female responses to benzamil administration at ZT12. Similar to the sex difference observed at ZT0 in UV, male rats had greater diuresis after 2 h compared with females (Fig. 1A). The difference in the response between sexes was preserved when UV was normalized to body weight or baseline excretion (Fig. 1, C and E). UNaV was higher in male than in female rats in the first 6 h post-injection (Fig. 1B); however, this difference was narrowed down to the first 2 h and then abolished when normalized to body weights and baseline values, respectively (Fig. 1, D and F).
To account for the difference in water and food intake between both sexes, we further normalized UV to water intake and UNaV to Na+ intake at both inactive and active periods at baseline and after benzamil administration (Table 3). UV/water intake was higher in males in response to ZT12 dose. UNaV/Na+ intake was significantly higher in male rats at 6 h after benzamil at ZT12, with an overall significant effect of sex showing higher diuretic and natriuretic responses in males compared with females. Cumulative UV and UNaV in the 12 h postbenzamil was higher in males regardless of time of day (Fig. 1, G and H).
Table 3.
UV and UNaV relative to intake
| Males | Females | 2-Way ANOVA | |
|---|---|---|---|
| UV (mL)/water intake (mL) inactive (n = 12) | |||
| Baseline | 1.01 ± 0.14 | 1.19 ± 0.16 | Psex = 0.0005, Ptime = 0.01, Pinteraction = 0.06 |
| 0–2 h Post-ZT0 injection | 1.88 ± 0.29† | 0.95 ± 0.14* | |
| 2–6 h | 2.26 ± 0.23† | 1.40 ± 0.19* | |
| 6–12 h | 1.57 ± 0.37 | 0.78 ± 0.24 | |
| UV (mL)/water intake (mL) active (n = 8) | |||
| Baseline | 0.29 ± 0.03 | 0.27 ± 0.04 | Psex = 0.04, Ptime = 0.0005, Pinteraction = 0.3 |
| 0–2 h Post-ZT12 injection | 0.57 ± 0.07† | 0.39 ± 0.05 | |
| 2–6 h | 0.64 ± 0.09† | 0.47 ± 0.11 | |
| 6–12 h | 0.37 ± 0.04 | 0.37 ± 0.05 | |
| UNaV (mEq)/Na+ intake (mg) inactive (n = 12) | |||
| Baseline | 0.30 ± 0.12 | 0.14 ± 0.05 | Psex = 0.006, Ptime < 0.0001, Pinteraction = 0.1 |
| 0–2 h Post-ZT0 injection | 1.18 ± 0.59 | 0.57 ± 0.19 | |
| 2–6 h | 2.37 ± 0.92† | 0.97 ± 0.18* | |
| 6–12 h | 0.25 ± 0.12 | 0.07 ± 0.04 | |
| UNaV (mEq)/Na+ intake (mg) active (n = 8) | |||
| Baseline | 0.02 ± 0.00† | 0.02 ± 0.00† | Psex = 0.2, Ptime < 0.0001, Pinteraction = 0.8 |
| 0–2 h Post-injection | 0.10 ± 0.01† | 0.09 ± 0.02† | |
| 2–6 h | 0.10 ± 0.02 | 0.08 ± 0.01 | |
| 6–12 h | 0.03 ± 0.01 | 0.02 ± 0.01 |
Data presented as means ± SE. UNaV, Na+ excretion; UV, urine flow rate; ZT0 and ZT12, Zeitgeber time 0 and 12, respectively.
P < 0.05 vs. males
and
P < 0.05 vs. baseline, Sidak’s multiple-comparisons test.
Role of ENaC blockade on endothelin-1 excretion.
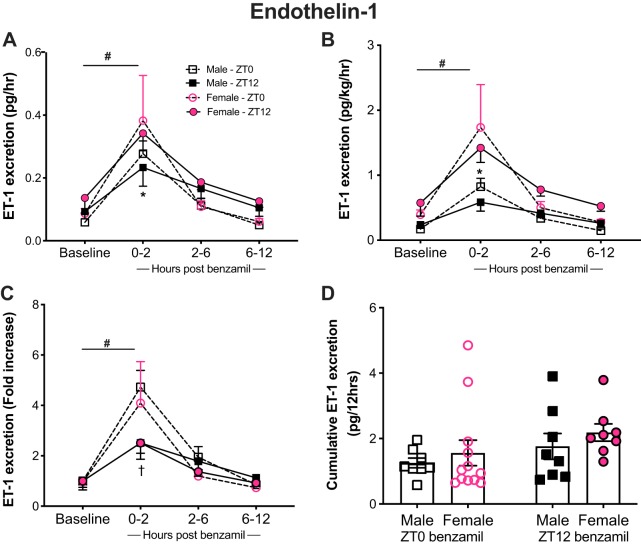
The change in ET-1 excretion, which largely reflects tubular secretion, matched UV and UNaV in response to benzamil in both male and female rats regardless of time of day. After benzamil administration, ET-1 excretion increased in both males and females compared with their corresponding baseline values reaching a maximum excretion rate of 0.28 ± 0.04 pg/h at ZT0 and 0.23 ± 0.06 pg/h at ZT12 in males and 0.38 ± 0.14 pg/h at ZT0 and 0.34 ± 0.06 pg/h at ZT12 in females after 2 h of benzamil administration (Fig. 3).
Fig. 3.
Urinary endothelin-1 (ET-1) excretion in response to benzamil administration at the beginning of the inactive [Zeitgeber time 0 (ZT0)] or active (ZT12) period in male and female Sprague-Dawley rats at baseline and over the course of 12 h postbenzamil, collected at 2-, 6-, and 12-h intervals (A–C) and 12-h ET-1 cumulative excretion rate (D). *P < 0.05 vs. males at the corresponding time point, †P < 0.05 vs. ZT0 within the same sex, and #P < 0.05 vs. baseline ± SE, repeated-measures 2-way ANOVA with Sidak’s multiple-comparisons test; n = 10–14. A and C: ZT0 benzamil: Ptime < 0.05, Psex > 0.05, and Ptime × sex > 0.05. ZT12 Ptime < 0.05, PSex > 0.05, and Ptime × sex > 0.05. B: ZT0 benzamil: Ptime < 0.05, PSex > 0.05, and Ptime × sex > 0.05. ZT12: Ptime < 0.05, Psex < 0.05, and Ptime × sex > 0.05. A and B: males: PZT > 0.05; Ptime < 0.05 and PZT × time > 0.05. Females: PZT > 0.05, PTime < 0.05, and PZT × time > 0.05. C: males: PZT > 0.05, Ptime < 0.05, and PZT × time < 0.05. Females: PZT > 0.05, PTime < 0.05, and PZT × time > 0.05.
Looking into sex differences in urine ET-1 excretion, our data showed no significant difference in excretion rates between males and females at baseline. After benzamil was given at ZT0, the rise in ET-1 excretion was comparable between both sexes. On the other hand, there was a significant sex difference in ET-1 excretion following benzamil at ZT12, which moved in an opposite direction to that observed for UV and UNaV. Here, female rats had higher ET-1 excretion that was evident at the 2-h time point compared with males (Fig. 3A). The observed sex difference was preserved when excretion rate was normalized to body weights, but not when normalized to urinary ET-1 excretion baseline values (Fig. 3, B and C). An observed effect of time at 2 h post-benzamil was evident in the male group when excretion rates were normalized to baseline excretion (Fig. 3C). This could be related to the relatively lower basal ET-1 excretion during the inactive time period.
Cortical ENaC abundance in male and female rats.
To assess whether differences in ENaC protein expression could account for our observed responses to benzamil, we measured abundance of all three ENaC subunits at the beginning of the inactive and active periods in kidneys from male and female Sprague-Dawley rats. Cortical tissue homogenates of kidneys collected at ZT0 showed no significant sex difference in the abundance of α- and γ-subunits, both the full-length and the cleaved forms, whereas the β-subunit was significantly lower in females (Fig. 4A). Protein expression of both the full-length and cleaved forms of γ-ENaC was significantly higher in females at ZT12. Expression of both α-and β-subunits were similar in kidneys from male and female rats (Fig. 4B).
Role of ovarian hormones.
Ovariectomized (OVx) and sham female Sprague-Dawley rats had similar baseline UV and UNaV for their light and dark cycles (Fig. 5). When OVx and sham rats were subjected to a single dose of benzamil at ZT0, both groups showed an increase in UNaV within 2 h of administration with no effect of ovariectomy. UV increased to 0.8 ± 0.2 ml/h in OVx and sham controls after light period benzamil injection (Fig. 5A). No diuretic effect was observed after ZT12 benzamil, as evidenced in the stable urine flow rate for both sham and OVx rats. UNaV peaked after the expected 2 h following benzamil in all groups, although the increase in the sham group was not statistically significant from baseline at ZT12; UNaV reached 112 ± 20 μEq/h in OVx and 120 ± 18 μEq/h in the sham group (Fig. 5B) at the 2-h time point at ZT0. UNaV increased to 153 ± 39 μEq/h at ZT12 in OVx females. ET-1 excretion after OVx at both baseline and with benzamil administration remained similar to the sham group regardless of time of day (Fig. 5C). Benzamil administration increased ET-1 excretion in the urine only when it was given at 7 AM for both OVx and sham rats. However, no significant increase in urinary ET-1 was observed after ZT12 administration in either group. Overall, ovariectomy increased the natriuretic of benzamil only when benzamil was given at ZT12 compared with sham controls. However, Na+ excretion values were still similar to SD females in the original protocol (Fig. 1B).
Fig. 5.
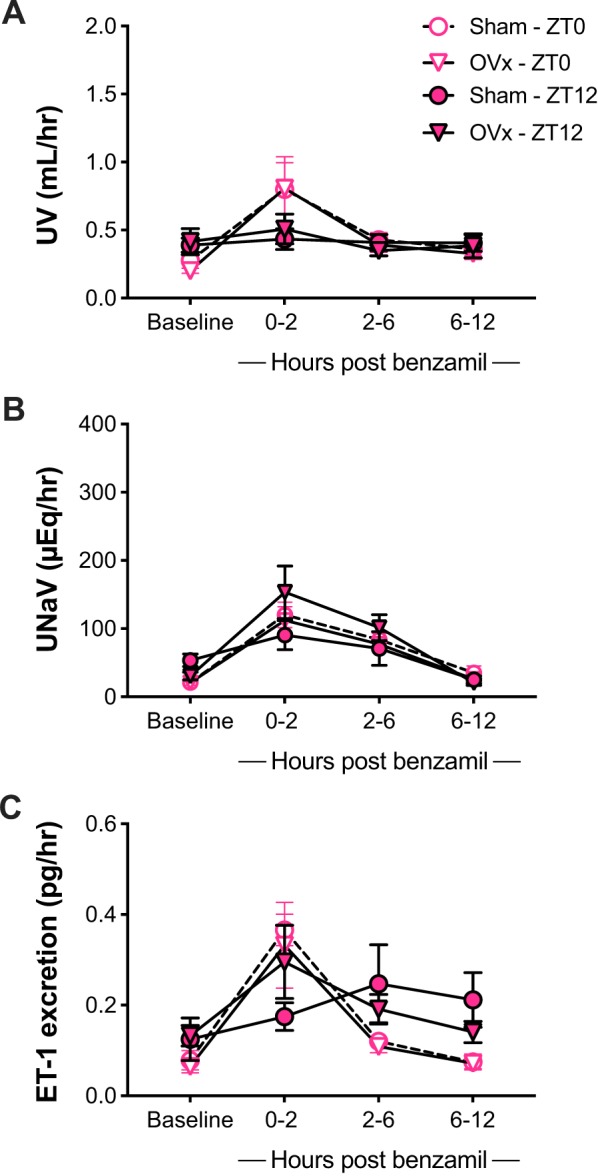
Urine flow rate (UV), urinary Na+ excretion (UNaV), and urinary endothelin-1 (ET-1) as measured in both intact (sham) and ovariectomized (OVx) female Sprague-Dawley rats at baseline and after ip benzamil administration at Zeitgeber time (ZT) 0 and ZT12 over the course of 12 h postbenzamil, collected at 2-, 6-, and 12-h intervals. Data represented as means ± SE, repeated-measures 2-way ANOVA with Sidak’s multiple comparisons test; n = 6. Ptime < 0.05, POVx > 0.05, and Ptime × OVx > 0.05.
DISCUSSION
It is important to study drug actions in females, as nearly all of the preclinical information is generated from male models, and very few sex comparisons have been reported in human studies. Female cardiovascular and kidney physiology is distinct from males, with each sex uniquely fashioned to handle sex-specific roles and physiological stressors such as reproduction. Females have lower GFR and decreased clearance of nonmetabolized drugs. Transporters and target receptor abundance and activity and different between sexes as well (51). However, there are not enough data on sex differences in drug responses attributed to transporter activity and abundance. Furthermore, doses of most antihypertensive drugs are not dosed based on body weight, which is a major determinant of volume of distribution and clearance.
Our study highlights the effect of sex on the response to benzamil, as it belongs to a family of diuretics that are widely used in treatment of hypertension (54). To our knowledge, we show here for the first time that female rats have a lower diuretic and natriuretic response to benzamil compared with their male counterparts. The peak in diuretic and natriuretic responses was achieved within 2 h of administration in both sexes. This is similar to previously published data on benzamil given the short half-life of the drug (43, 46).
Current hypertension guidelines do not consider sex in the management plans and choice of antihypertension medication, nor do they consider time-of-day dosing (54). No sufficient data currently exist to evaluate sex differences in response to diuretics and different antihypertension medications. The dosage of benzamil was calculated according to body weight to eliminate the size difference between both sexes as a confounding variable, which is not considered in regimens targeting human subjects.
Our study shows a sex difference in functional response to ENaC inhibition suggesting a more active transport in males despite the published data that female rats have higher expression levels of the cleaved forms of α- and γ-ENaC subunits in the renal cortex (52). This is consistent with our finding of the γ-subunit being higher in females at the beginning of the active period, which corresponds to the time the channel should be most active to handle the increase in filtered Na+ during active time food intake. However, there was no statistical difference in the α-subunit expression between males and females regardless of the time of day. These findings indicate that channel activity is independent of mRNA and protein expression measured in kidney tissue over the course of the day and that other factors, including channel localization and gating, are more reflective of circadian control of ENaC activity. The skin is a reservoir of osmotically inactive Na+. There are no reported sex differences in osmotically inactive Na+ storage under normal salt conditions in rats (50), and under states of balanced intake and excretion, there is no significant Na+ mobilization between compartments. The imbalance in this buffering system has been reported only in states of excessive salt intake or under conditions of hypertension and chronic kidney disease (47).
ET-1 is an upstream inhibitor of ENaC through the ETB receptor (6, 9) that is particularly active in animals on a high-salt diet (4, 8, 29). Therefore, lower levels of urinary excretion of ET-1 are typically associated with more ENaC activity. Female rats had higher ET-1 excretion rates compared with males following benzamil administration, suggesting that the weaker natriuretic response is probably due to more active ENaC in males. The relatively lower response to benzamil in females could also indicate that ENaC has reached maximum inhibition or that ENaC is under a relative state of constant inhibition induced by the relatively yet not significantly higher ET-1 at baseline conditions, which could explain the less prominent response in female rats.
Urinary ET-1 reflects ET-1 that originates from the kidney, which in large part is produced by the collecting duct (28), the same location where it functions to inhibit ENaC under high-salt conditions (8, 22, 28). Lyon-Roberts et al. (33) have shown that ET-1 production in the collecting duct can be stimulated by increases in tubular fluid flow. Thus, urinary ET-1 excretion can be increased by not only high-salt intake but also increased tubular fluid flow, such as that which occurs during diuretic treatment. We observed that the lower diuretic and natriuretic response to benzamil in females compared with male rats was accompanied by higher ET-1 excretion rates. This finding is consistent with previously published data (40) proposing dissociation of the flow-mediated ET-1 response from ENaC channel activity and supports our hypothesis that benzamil function is probably not dependent upon ET-1’s effects on ENaC activity. This is further backed up by our findings in male rats having a significant natriuretic response with a significantly lower ET-1 excretory response compared with females. It remains to be determined whether there is a sex difference in the collecting duct to produce ET-1 in response to similar changes in flow.
Differential expression and activity of other Na+ channels along the nephron could be contributing to the weaker natriuretic response in females. NCC is reported to have higher activity in females, with higher levels of expression and phosphorylation (31, 44). This channel could be compensating for ENaC blockade and helping to maintain Na+ balance and preventing unnecessary loss of Na+ in urine since the animal model in this study is a normotensive model on a normal salt diet. Female mice had a more robust response than males to hydrochlorothiazide intravenous injection (31). This study attributed this difference to higher expression and phosphorylation levels of NCC as well as the angiotensin AT1a receptor status (31). Female rats excrete a salt load more quickly and efficiently than male rats (24, 27, 36, 52). This has been attributed mostly to lower abundance and activity of transporters in the proximal tubules of females leading to lower fractional Na+ reabsorption in that area of the nephron that is responsible for ∼65% of Na+ reabsorption along the nephron (52). However, the greater ability of male kidneys to reabsorb Na+ with salt loading could also be attributed to more ENaC activity irrespective of mRNA and protein expression. Distal nephron transporters are in charge of fine-tuning Na+ transport and the final steps of maintaining plasma salt homeostasis (7, 32, 39). The ability of female rats to excrete salt more rapidly than males could also reflect lower activity of distal tubule Na+ transporters, which enhances their ability to unload Na+ and contributes to the attenuated response to benzamil.
Our study showed that female rats had lower K+ excretion during their active period, which further supports the notion of lower ENaC activity in females and is consistent with a previous human study showing failure of amiloride to reduce blood pressure in black individuals, owed to lower ENaC activity as supported by lower K+ excretion and lower plasma aldosterone levels compared with white participants (42).
The kidneys are one of the highest organ systems expressing estrogen receptors (ER). The kidney ranks second only to the reproductive organs in the expression of ERα (19, 30). Jelinsky et al. (20) demonstrated an estrogen-induced induction of a wide array of genes in rat and mice kidneys, which suggests a direct influence of ovarian hormones on renal water and electrolyte homeostasis. ERα is significantly higher in male Sprague-Dawley rat kidneys, whereas the G protein-coupled estrogen receptor (GPER) has an opposite profile across sexes (17). This suggests differential roles of each ER in renal tubular Na+ handling that might contribute to the sexual dimorphic pattern of Na+ reabsorption. However, we observed that ovarian hormone deprivation by OVx had no effect on the female diuretic and natriuretic responses to benzamil. The lack of response to ovariectomy suggests the presence of nonfemale sex hormonal factors in the regulation of ENaC activity, at least under our standard diet conditions.
A limited number of studies have shown an improved prognosis for hypertension in patients receiving one or more bedtime antihypertensive medications compared with morning dosing. However, it is not clear whether this is due to time-of-day-dependent difference in drug response or merely owed to the control of nocturnal-type hypertension and nondipping phenotype of the disease that may vary in different populations. Nighttime antihypertensive medication is mainly concerned with the control of what is referred to as nocturnal hypertension, which has become more evident with the increased use of ambulatory blood pressure measurements (14, 16). However, there is no clear evidence on the effectiveness of diuretic administration at certain time of day on the control of essential hypertension in the absence of nocturnal blood pressure elevation specifically.
Patients, both males and females, receiving either morning or evening dosing of thiazide demonstrated adequate blood pressure control and improvement of left ventricular indices. However, the evening dosing group showed lower blood pressure values and reduction of left ventricular mass and posterior wall thickness (37). This highlights the importance of diuretic chronotherapy for better control of hypertension-associated cardiovascular disease risk factors. A number of reviews and clinical trials investigating the treatment of hypertension with chronotherapy have shown that even with comparable reduction in blood pressure indices, evening drug dosing improved cardiovascular risk and overall prognosis (15, 16, 38, 41).
Our results showed that male rats showed no time-of-day difference in diuretic and natriuretic responses to benzamil. However, females given benzamil at the beginning of their active period failed to increase their UV but not UNaV and a more robust diuretic response for inactive time dosage. This suggests that benzamil therapeutic effect is not affected by the diurnal variation of ENaC activity and expression, particularly in males. However, because we used a normotensive animal model, cardiovascular disease (CVD) risk assessment was not determined.
In summary, our finding that the magnitude of the natriuretic response to benzamil when given on the basis of body weight has a sexually dimorphic effect. This phenotype was not reversed by elimination of female sex hormones through OVx, suggesting factors other than ovarian hormones in modulating channel activity between males and females. This study also generally shows no time-of-day difference in the response to benzamil but does not address CVD risks and long-term control of blood pressure in the setting of hypertension. Taken together, this study opens the door to in-depth mechanistic studies related to sex and time of day and highlights the importance of sex-specific recommendations of antihypertensive medications.
GRANTS
This study was funded by grants from the National Heart, Lung, and Blood Institute (P01-HL-136267 and P01-HL-069999), an American Heart Association Strategically Focused Network grant (15SFRN2390002), and a University of Alabama at Birmingham School of Medicine AMC21 Reload Multi-Investigator grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.H.S. and D.M.P. conceived and designed research; R.H.S., J.G.J., E.Y.G., and C.M.T. performed experiments; R.H.S., J.G.J., E.Y.G., and C.M.T. analyzed data; R.H.S., J.G.J., E.Y.G., and D.M.P. interpreted results of experiments; R.H.S. prepared figures; R.H.S. drafted manuscript; R.H.S., J.G.J., E.Y.G., and D.M.P. edited and revised manuscript; R.H.S., J.G.J., E.Y.G., C.M.T., and D.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert assistance of Binli Tao.
REFERENCES
- 1.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol 35: 687–694, 2008. doi: 10.1111/j.1440-1681.2008.04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Qusairi L, Basquin D, Roy A, Rajaram RD, Maillard MP, Subramanya AR, Staub O. Renal tubular ubiquitin-protein ligase NEDD4-2 is required for renal adaptation during long-term potassium depletion. J Am Soc Nephrol 28: 2431–2442, 2017. doi: 10.1681/ASN.2016070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 6.Gallego MS, Ling BN. Regulation of amiloride-sensitive Na+ channels by endothelin-1 in distal nephron cells. Am J Physiol Renal Physiol 271: F451–F460, 1996. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- 7.Gamba G. Molecular biology of distal nephron sodium transport mechanisms. Kidney Int 56: 1606–1622, 1999. doi: 10.1046/j.1523-1755.1999.00712.x. [DOI] [PubMed] [Google Scholar]
- 8.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore ES, Stutts MJ, Milgram SL. SRC family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem 276: 42610–42617, 2001. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 10.Gohar EY, Yusuf C, Pollock DM. Ovarian hormones modulate endothelin A and B receptor expression. Life Sci 159: 148–152, 2016. doi: 10.1016/j.lfs.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of αENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim Biophys Acta 1799: 622–629, 2010. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frøkiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of α-, β-, and γ-ENaC in rat kidney. Am J Physiol Renal Physiol 280: F1093–F1106, 2001. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- 13.Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens 19: 98–105, 2010. doi: 10.1097/MNH.0b013e328332bda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: Rationale and design of the Hygia Project. Chronobiol Int 33: 906–936, 2016. doi: 10.1080/07420528.2016.1181078. [DOI] [PubMed] [Google Scholar]
- 15.Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev 59: 904–922, 2007. doi: 10.1016/j.addr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Hermida RC, Ayala DE, Smolensky MH, Fernández JR, Mojón A, Portaluppi F. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res 39: 277–292, 2016. doi: 10.1038/hr.2015.142. [DOI] [PubMed] [Google Scholar]
- 17.Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ 10: 4, 2019. doi: 10.1186/s13293-019-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyndman KA, Mironova EV, Giani JF, Dugas C, Collins J, McDonough AA, Stockand JD, Pollock JS. Collecting duct nitric oxide synthase 1β activation maintains sodium homeostasis during high sodium intake through suppression of aldosterone and renal angiotensin II pathways. J Am Heart Assoc 6: e006896, 2017. doi: 10.1161/JAHA.117.006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERα splice variants in the mouse. PLoS One 8: e70926, 2013. doi: 10.1371/journal.pone.0070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jelinsky SA, Harris HA, Brown EL, Flanagan K, Zhang X, Tunkey C, Lai K, Lane MV, Simcoe DK, Evans MJ. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology 144: 701–710, 2003. doi: 10.1210/en.2002-220728. [DOI] [PubMed] [Google Scholar]
- 21.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β- and γ-ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jin C, Speed JS, Pollock DM. High salt intake increases endothelin B receptor function in the renal medulla of rats. Life Sci 159: 144–147, 2016. doi: 10.1016/j.lfs.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston JG, Pollock DM. Circadian regulation of renal function. Free Radic Biol Med 119: 93–107, 2018. doi: 10.1016/j.freeradbiomed.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston JG, Speed JS, Jin C, Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol 311: F991–F998, 2016. doi: 10.1152/ajprenal.00103.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karet FE, Davenport AP. Localization of endothelin peptides in human kidney. Kidney Int 49: 382–387, 1996. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- 26.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol 301: F684–F696, 2011. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 14: 893–896, 2001. doi: 10.1016/S0895-7061(01)02164-1. [DOI] [PubMed] [Google Scholar]
- 28.Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011. doi: 10.1002/cphy.c100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863–870, 1997. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Hatano R, Xu S, Wan L, Yang L, Weinstein AM, Palmer L, Wang T. Gender difference in kidney electrolyte transport. I. Role of AT1a receptor in thiazide-sensitive Na+-Cl- cotransporter activity and expression in male and female mice. Am J Physiol Renal Physiol 313: F505–F513, 2017. doi: 10.1152/ajprenal.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 33.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011. doi: 10.1152/ajprenal.00530.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mironova E, Bugaj V, Roos KP, Kohan DE, Stockand JD. Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc Natl Acad Sci USA 109: 10095–10100, 2012. doi: 10.1073/pnas.1201978109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okeahialam BN, Ohihoin EN, Ajuluchukwu JN. Diuretic drugs benefit patients with hypertension more with night-time dosing. Ther Adv Drug Saf 3: 273–278, 2012. doi: 10.1177/2042098612459537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orías M, Correa-Rotter R. Chronotherapy in hypertension: a pill at night makes things right? J Am Soc Nephrol 22: 2152–2155, 2011. doi: 10.1681/ASN.2011101012. [DOI] [PubMed] [Google Scholar]
- 39.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676–687, 2015. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F541–F552, 2015. doi: 10.1152/ajprenal.00456.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portaluppi F, Smolensky MH. Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. Chronobiol Int 27: 1652–1667, 2010. doi: 10.3109/07420528.2010.510788. [DOI] [PubMed] [Google Scholar]
- 42.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol 16: 3154–3159, 2005. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 43.Riazi S, Madala-Halagappa VK, Hu X, Ecelbarger CA. Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend Med 3: 309–327, 2006. doi: 10.1016/S1550-8579(06)80219-6. [DOI] [PubMed] [Google Scholar]
- 44.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, Meade P, de Los Heros P, Arroyo-Garza I, Bernard V, Binart N, Bobadilla NA, Hadchouel J, Zambrano E, Gamba G. Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015. doi: 10.1152/ajprenal.00447.2014. [DOI] [PubMed] [Google Scholar]
- 45.Sepehrdad R, Chander PN, Singh G, Stier CT Jr. Sodium transport antagonism reduces thrombotic microangiopathy in stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol 286: F1185–F1192, 2004. doi: 10.1152/ajprenal.00355.2003. [DOI] [PubMed] [Google Scholar]
- 46.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: F1055–F1064, 2006. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 47.Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, Titze JM, Pollock DM. Diurnal pattern in skin Na+ and water content is associated with salt-sensitive hypertension in ETB receptor-deficient rats. Am J Physiol Regul Integr Comp Physiol 314: R544–R551, 2018. doi: 10.1152/ajpregu.00312.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, Pollock DM. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314: F89–F98, 2018. doi: 10.1152/ajprenal.00028.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 20: 109–117, 2007. doi: 10.1016/j.amjhyper.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 285: F1108–F1117, 2003. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 51.Ueno K, Sato H. Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens Res 35: 245–250, 2012. doi: 10.1038/hr.2011.189. [DOI] [PubMed] [Google Scholar]
- 52.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol 10: 146–157, 2014. doi: 10.1038/nrneph.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Soc Hypertens 12: 579.e1–579.e73, 2018. doi: 10.1016/j.jash.2018.06.010. [DOI] [PubMed] [Google Scholar]